Abstract
Background:
Inappropriate management of blood sugar in patients with diabetes mellitus leads to micro-vascular and macro-vascular complications, subsequently leading to high morbidity and mortality rates. In addition, diabetes independently increases the occurrence of cognitive impairment complicated by dementia. Scientific evidence on the magnitude of cognitive impairment will provide a sound basis for the determination of healthcare needs and the planning of effective healthcare services. Despite this, there are no comprehensive data on the prevalence and associated factors of cognitive impairment among patients with diabetes in Africa.
Methods:
To identify relevant articles for this review, we searched PubMed, Cochrane Library, Science Direct, African Journals Online, and Google Scholar. After extraction, the data were imported into Stata software version 11 (Stata Corp., TX, USA) for further analysis. The random-effects model, specifically the DerSimonian and Laird (D+L) pooled estimation method, was used due to the high heterogeneity between the included articles. Begg’s and Egger’s regression tests were used to determine the evidence of publication bias. Sub-group analyses and sensitivity analyses were also conducted to handle heterogeneity.
Results:
The pooled prevalence of cognitive impairment among patients with diabetes in Africa is found to be 43.99% (95% CI: 30.15–57.83, p < 0.001). According to our analysis, primary level of education [pooled odds ratio (POR) = 6.08, 95% CI: 3.57–10.36, I2 = 40.7%], poorly controlled diabetes mellitus (POR = 5.85, 95% CI: 1.64–20.92, I2 = 87.8%), age above 60 years old (POR = 3.83, 95% 95% CI: 1.36–10.79, I2 = 63.7%), and diabetes duration greater than 10 years (POR = 1.13; 95% CI: 1.07–1.19, I2 = 0.0%) were factors associated with cognitive impairment among patients with diabetes.
Conclusion:
Based on our systematic review, individuals with diabetes mellitus exhibit a substantial prevalence rate (43.99%) of cognitive impairment. Cognitive impairment was found to be associated with factors such as primary level of education, poorly controlled diabetes mellitus, age above 60 years, and diabetes duration greater than 10 years. Developing suitable risk assessment tools is crucial to address uncontrolled hyperglycemia effectively.
Systematic review registration:
https://www.crd.york.ac.uk/prospero, identifier CRD42024561484.
Introduction
Diabetes mellitus (DM) is a group of metabolic disorders characterized by elevated levels of glucose in the blood resulting from defects in insulin secretion, insulin action, or both (1). The burden of DM is increasing worldwide, especially in developing countries (2). In 2014, the global prevalence of diabetes was 422 million, and by 2040, this number is expected to rise to more than 642 million. The healthcare costs for DM reached 162 billion dollars in 2019 and will be 185 billion dollars in 2045 (3, 4). Inappropriate management of blood sugar in patients with DM leads to micro-vascular and macro-vascular complications, subsequently leading to high morbidity and mortality rates. In addition, diabetes independently increases the occurrence of cognitive impairment (CI), which is complicated by dementia (5–7).
CI is defined as a disturbance in memory, acquiring knowledge, focusing, or making decisions that have a negative impact on activities of daily life (8). Patients with DM are more likely to develop cognitive problems and dementia than patients without DM (9). DM can lead to the accumulation of waxy protein in the neuron by decreasing its excretion through cerebrospinal fluid, ultimately resulting in cognitive decline (10). Extended exposure of nerve cells to high levels of glucose weakens the connections between neurons, leading to their distortion, a condition directly linked to cognitive dysfunction (7).
Different studies have suggested that the exact pathophysiology of CI in diabetic patients may be due to blood vessel abnormality, insulin transmission disturbance in the cerebrum, recurrent attack of nerve cells with hyperglycemia and hypoglycemia, and accumulation of waxy protein in the neuron (11, 12). The global prevalence of CI is 45% (13) with the lowest and highest prevalence being 21.8% and 67.5%, respectively (14, 15). The global prevalence of complications of CI (dementia) in 2010 was 35.6 million, which is expected to rise to 65.7 million by 2030 (16). The overall prevalence of CI in sub-Saharan African countries among the general population has been estimated to be between 6.3% and 25% (17). The comorbidity of DM and CI is the major challenge for the long-term management of DM. DM not only causes CI but also induces the complications of CI (18). The global healthcare cost of CI complicated by dementia is 1.5 times higher than that of patients without dementia (19).
An increase in the magnitude of CI with the high cost of healthcare will impose a serious social, medical, and economic burden, causing a major challenge to the already strained healthcare system of African countries. CI is a major problem that affects the effective long-term management of diabetes. Early diagnosis of CI in patients with diabetes is important for the recovery of cognitive function and the delay of cognitive decline, as well as improving medication adherence in people with diabetes. Scientific evidence on the magnitude of CI will provide a sound basis for the determination of healthcare needs and the planning of effective healthcare services.
Despite this, there are no comprehensive data on the prevalence and associated factors of CI among patients with diabetes in Africa. We therefore designed this review to assess the prevalence and associated factors of CI among patients with diabetes in Africa.
Materials and methods
The international Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used (20) to report the findings of this systematic review and meta-analysis ( Supplementary Material 1 ).
Publication search strategy
To identify pertinent articles for this review, we conducted searches in several databases, including PubMed, Cochrane Library, Science Direct, African Journals Online, and Google Scholar. These searches were performed by two authors (WCT and AMZ) between 10 November 2023 and 10 January 2024. We used the following MeSH terms while searching from the above electronic databases: “cognitive dysfunction” OR “cognitive impairment” OR “neurocognitive disorder” OR “cognitive decline” AND “diabetes” OR “diabetes mellitus” OR “diabetes mellitus type 2” OR “diabetes mellitus type II” OR “type II diabetes mellitus” OR “type 2 diabetes mellitus” OR “type 2 diabetes” OR “diabetes mellitus type 1” OR “diabetes mellitus type I” OR “type I diabetes mellitus” OR “type 1 diabetes mellitus” OR “type 1 diabetes”. The snowball technique from the searched articles was also used to obtain additional articles.
Eligibility criteria
Inclusion criteria
We included the following types of primary studies: cross-sectional, case–control, and cohort studies that reported the prevalence of CI among patients with diabetes; peer-reviewed studies published in English; studies conducted inside Africa among patients with diabetes; moderately and highly qualified studies; and freely accessible studies.
Exclusion criteria
The review excluded studies that did not involve patients with diabetes, those that did not provide data on CI prevalence, case reports, low-quality studies, or studies that were published in languages other than English.
Outcome of interest
The outcome of this review is the prevalence of CI and associated factors among patients with diabetes. CI was assessed using the Mini-Mental State Examination (MMSE) in the primary studies.
Article selection and data extraction
Duplicate articles were deleted after importing all articles into the EndNote version X7 software. Then, two authors (YAF and AMZ) screened the articles critically for eligibility criteria. The corresponding author, publication year, study setting, study design, study population, sampling procedure, total sample size, response rate (participant), associated factors, and prevalence of CI among patients with diabetes were extracted by two authors (YAF and AMZ) using a standardized Microsoft Excel data extraction format. Associated factors were extracted based on the following eligibility criteria: having a similar categorization, having a similar operational definition, having been reported with a similar statistical measure (odds ratio), having a similar direction of association, and having been associated with two or more articles.
Quality assessment
Two authors (WCT and YAF) assessed the quality of articles using tools assessing the risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement developed by Hoy and Brooks (21). The tool consists of 10 items addressing four domains of bias plus a summary risk of bias assessment. The 10 items are representativeness, sampling frame/procedure, random selection, non-response bias, direct data collection from patients, acceptability of case definition, study tool reliability and validity, same mode of data collection, appropriate length of prevalence period, and appropriateness of numerator and denominator. Uncertain or unclear items were considered to have a high risk of bias. After summarizing the high risk of bias, the overall risk of bias was evaluated as low (≤2), moderate (3–5), and high (≥5) ( Supplementary Material 2 ).
Statistical analysis
After extraction, the data were imported to Stata software version 11 (Stata Corp LLC., TX, USA) for further analysis. Heterogeneity between the included articles was assessed using Cochran’s Q chi-square test at a significance level of less than 0.05 and inverse variance (I² index). Values of 0%–40%, 40%–60%, 60%–90%, and 90%–100% indicated low, medium, substantial, and high heterogeneity, respectively (22). The random-effects model, specifically the DerSimonian and Laird (D+L) pooled estimation method, was used due to the high heterogeneity among the included articles (23). Sub-group analyses and sensitivity analyses were also conducted to handle heterogeneity. Egger’s and Begg’s regression tests and visual inspection of the funnel plot were utilized to assess the evidence of publication bias.
Results
Study selection
Our systematic search found a total of 613,277 articles from five databases: [Google Scholar (18,200), PubMed (588,989), Cochrane Library (402), Science Direct (5352), and African Journal Online (334)]. After importing all articles into Endnote, 433,547 articles were removed due to duplication. Of the remaining articles, 178,868 were excluded after title screening. The abstract text of 862 articles was assessed for eligibility criteria; finally, 13 articles ultimately met the inclusion criteria and were included in the review. A summary of the steps involved in the screening process and the reasons for the exclusion of articles is provided (Figure 1).
Figure 1

PRISMA flow diagram of the selection of publications for a systematic review and meta-analysis on the prevalence and associated factors of cognitive impairment among patients with diabetes in Africa (N = 13).
Baseline characteristics of the included publications
The review analyzed the results of 13 articles (11 studies are institutional-based cross-sectional, and the remaining 2 are cohort and case–control studies). The included articles were conducted in different countries in Africa; three from Ethiopia (24–26), four from Nigeria (27–30), three from Egypt (31–33), and the remaining from Cameroon, Congo, and Morocco (34–36). Detailed baseline characteristics of the included articles are presented in Table 1.
Table 1
| Author | Publication year | Country | Study design | Study population | Sample size | Prevalence (%) | Sampling method |
|---|---|---|---|---|---|---|---|
| Ashebir et al. (24) | 2024 | Ethiopia | IBCS | Patients with DM | 421 | 56.3 | Systematic random |
| Mulugeta et al. (25) | 2013 | Ethiopia | IBCS | Patients with DM | 384 | 45 | Simple random |
| Abba et al. (34) | 2017 | Cameroon | IBCS | Patients with DM | 223 | 14.8 | Consecutive |
| Eze et al. (27) | 2015 | Nigeria | IBCS | Patients with DM | 113 | 40 | Systematic random |
| Abdellatif et al. (31) | 2020 | Egypt | IBCS | Patients with DM | 200 | 34 | Consecutive |
| Williams et al. (28) | 2020 | Nigeria | IBCS | Patients with DM | 485 | 33.4 | Consecutive |
| Dagnew et al. (26) | 2017 | Ethiopia | IBCS | Patients with DM | 210 | 53.3 | Consecutive |
| Mohamed et al. (32) | 2023 | Egypt | IBCS | Patients with DM | 400 | 50 | Simple random |
| Anwar et al. (33) | 2018 | Egypt | IBCS | Patients with DM | 100 | 18 | Consecutive |
| Bashir and Yarube (29) | 2022 | Nigeria | IBCS | Patients with DM | 93 | 88.5 | Systematic random |
| Ossou et al. (35) | 2019 | Congo | Case control | Patients with DM | 200 | 57 | Consecutive |
| Tlemcani et al. (36) | 2022 | Morocco | Cohort | Patients with DM | 100 | 47.5 | Consecutive |
| Adebayo et al. (30) | 2022 | Nigeria | IBCS | Patients with DM | 274 | 27 | Systematic random |
Baseline characteristics of the included articles on the prevalence and associated factors of cognitive impairment among patients with diabetes in Africa (N = 13).
*IBCS, institutional-based cross-sectional study.
Quality of the included studies
Based on the quality assessment results, 11 studies (84.61%) of the included articles have a low risk of bias, and 2 have a moderate risk of bias. The detailed results of the quality assessment of the articles are provided in the Supplementary File (Supplementary File 2).
Publication bias
Begg’s and Egger’s regression tests were used to determine the evidence of publication bias. Based on our results, there is no significant publication bias indicated with Egger’s regression test p-value >0.05 (p = 0.532) and symmetrical inspection of the funnel plot (Figure 2).
Figure 2

Funnel plot showing the absence of publication bias in the systematic review and meta-analysis of prevalence and associated factors of cognitive impairment among patients with diabetes in Africa (N = 13).
Sub-group analysis
Sub-group analysis was done based on sampling techniques. The results showed that the highest prevalence of CI was reported in articles that used systematic random sampling [53.52% (CI: 23.41, 83.62)] and there was no heterogeneity in articles that used simple random sampling (Figure 3).
Figure 3

Forest plot of the sub-group analysis showing the prevalence of cognitive impairment among patients with diabetes based on sampling technique in Africa (N = 13).
Meta-analysis
The pooled prevalence of CI among patients with diabetes in Africa is found to be 43.99% (95% CI: 30.15–57.83, p < 0.001). The analysis showed a high heterogeneity between the included articles (I2 = 92.7%, p < 0.001). As a result, a random-effects model, specifically the DerSimonian and Laird (D+L) pooled estimation method, was used to estimate the pooled prevalence of CI (Figure 4).
Figure 4

Forest plot showing the prevalence of cognitive impairment among patients with diabetes in Africa (N = 13).
Sensitivity analysis
Sensitivity analysis was also conducted using the random-effects model, and the results showed that no single study influenced the pooled prevalence of CI among patients with diabetes (Figure 5).
Figure 5
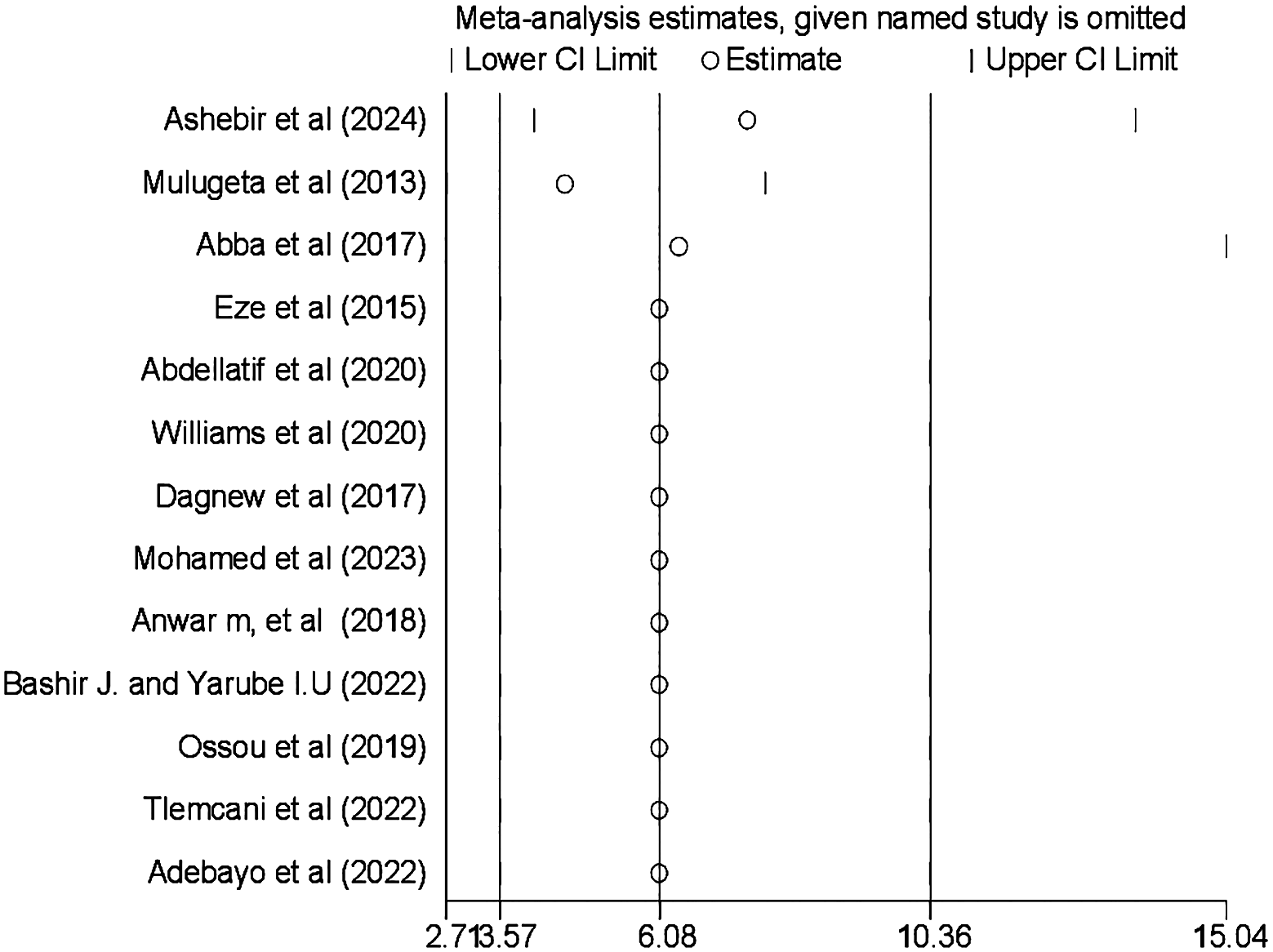
Result of the sensitivity analysis on the prevalence and associated factors of cognitive impairment among patients with diabetes in Africa (N = 13).
Associated factors of cognitive impairment
Four factors are associated with CI among patients with diabetes, based on extracted factors from the primary articles. They are primary educational level, uncontrolled DM, age greater than 60 years old, and duration of DM greater than 10 years. According to our analysis, the random pooled odds ratio of developing CI was 6.08 times (POR = 5.85, 95% CI: 1.64–20.92, I2 = 87.8%) higher among patients with diabetes who completed primary education compared to college and post-college education(Figure 6). The random pooled odds ratio of developing CI was 5.85 times higher (POR = 3.83, 95% CI: 1.36–10.79, I2 = 63.7%) among patients with DM whose blood glucose was uncontrolled as compared to patients with DM whose blood sugar was controlled (Figure 7). Patients with diabetes whose age was above 60 years old were 3.83 times more likely to develop CI (POR: 1.13; 95% CI: 1.07–1.19, I2 = 0.0%) than patients whose age was lower than or equal to 60 years old (Figure 8). The review also found that patients with DM who had diabetes for more than 10 years were 1.13 times more likely to develop CI (POR: 1.13; 95% CI: 1.07–1.19, I2 = 0.0%) than patients with DM for less than 10 years (Figure 9).
Figure 6

Forest plot showing the association between primary education level and cognitive impairment among patients with diabetes in Africa (N = 13).
Figure 7

Forest plot showing the association between uncontrolled DM and cognitive impairment among patients with diabetes in Africa (N = 13).
Figure 8

Forest plot showing the association between age greater than 60 years old and cognitive impairment among patients with diabetes in Africa (N = 13).
Figure 9

Forest plot showing the association between the duration of DM greater than 10 years and cognitive impairment among patients with diabetes in Africa (N = 13).
Discussion
According to the review, the pooled prevalence of CI among patients with diabetes in Africa is found to be 43.99% (95% CI: 30.15–57.82, p < 0.001). This result is in line with the findings of studies conducted in China, 45.0% (95% CI: 36.0–54.0) (13); India, 33.73% (37); Korea, 31.5% (38); Philippines, 45% (39); and Poland, 32.7% (40).
However, the finding is higher than the result of studies done in Japan, 26% (41); China, 21.8% (14) and China, 13.5% (42); USA, 25.6% (43); and New York, 28% (44). The elevated prevalence observed in our review could stem from several factors. First, in the setting of the aforementioned study, hospitalized patients may typically experience better plasma glucose management as physicians prioritize close monitoring of their levels, potentially leading to a lower prevalence in this group. Conversely, in our study setting, the study population tends to be of lower socioeconomic status, which is linked to poorer cognitive function due to limited resources and healthcare access. Additionally, variations in the study populations, such as the inclusion of individuals with advanced DM in the Nigerian study, may significantly contribute to the occurrence of complications and cognitive decline. Finally, the substandard quality of healthcare services in African contexts may also play a role in the higher prevalence observed. The other interesting reason for this discrepancy is that carriers of apolipoprotein E (APOE) ϵ4 are at high risk for cognitive decline and Alzheimer’s disease. The ϵ4 allele is more common in Black than white individuals. The ϵ4 allele is associated with a risk of cognitive decline and dementia (45).
However, the pooled prevalence of CI in our review is lower than the findings of studies done in India, 58.29% (46) and 64.86% (47) and Pakistan, 67.3% (15). A potential explanation could be the variation in the age demographics of the subjects studied. The research conducted in Pakistan involved older patients with DM, who are more prone to age-related cognitive decline. Conversely, studies in India focused on patients with chronic DM, who are at higher risk for various complications, including CI.
According to our analysis, the random pooled odds ratio of CI among patients with diabetes who completed primary education was 6.08 times higher as compared to those whose educational level was college or above. The finding is similar to the results of studies conducted in China and Germany (48, 49). This may be due to the fact that individuals with higher education tend to have a larger “cognitive reserve,” meaning that they have a greater capacity for mental processing and can better compensate for age-related declines in brain function. This reserve may be built through years of stimulating mental activity, including learning new information, problem solving, and engaging in complex tasks.
The random pooled odds ratio of developing CI among patients with DM whose blood glucose was uncontrolled was 5.85 times higher compared to patients with DM whose blood sugar was controlled. The finding is consistent with the results of studies done in New York and India (38, 50). This is explained by the fact that elevated blood sugar levels in diabetes can directly damage neurons through an increased polyol pathway, advanced glycation end products, protein kinase C (PKC) activation, and increased production of free radicals (highly reactive molecules that damage cells and DNA) (51).
Patients with diabetes whose age was above 60 years old were 3.83 times more likely to develop CI than patients whose age was lower than or equal to 60 years old. This is in line with the findings of a study done in India (52). The possible justification for this might be that as age increases, the brains naturally undergo changes that can contribute to cognitive decline, including neuronal loss (gradual death of neurons throughout the brain, particularly in areas critical for memory, learning, and reasoning), synaptic dysfunction (weakening of connections between neurons, hindering communication and information processing), and neuro-inflammation (chronic low-grade inflammation in the brain, which can damage neurons and impair cognitive function) (48). The other justification for this may be due to a decline in processing speed, working memory, and episodic memory (recalling specific events) with age (52). Another reason for this may be due to early-onset familial Alzheimer’s disease (EOFAD), which has some distinctive features including early age at onset, positive family history, a variety of non-cognitive neurological symptoms and signs, and a more aggressive course. There is marked phenotypic heterogeneity among different mutations in EOFAD.
The review also identified that patients with DM who had diabetes for more than 10 years were more than 1.13 times more likely to develop CI than patients with diabetes duration of less than 10 years. The finding is in line with the studies done in China, Mexico, and London (42, 53, 54). This is justified by the long duration of DM as an atherogenic factor; it may increase the risk of cognitive dysfunction through well-recognized associations with stroke, causing cerebral disease and cerebral infarction (55).
Limitations
This review has some limitations. The sub-group analysis did not indicate adequate factors to explain the observed high heterogeneity; important factors associated with CI were not analyzed in our review because of the lack of information in the primary studies and inconsistent categorization, and the number of included articles was not adequate (N = 13), which may affect the representativeness of the results.
Conclusion and recommendation
Based on our systematic review, individuals with DM exhibit a substantial prevalence rate (43.99%) of CI. CI was found to be associated with factors such as primary level of education, poorly controlled DM, age above 60 years, and diabetes duration greater than 10 years. Developing suitable risk assessment tools is crucial to address uncontrolled hyperglycemia effectively. Healthcare professionals in Africa ought to prioritize the monitoring of cognitive function in patients with DM. Early identification of CI among patients with DM is beneficial for both restoring cognitive function and slowing down cognitive decline. Healthcare institutions need to create diagnostic and treatment plans tailored to individuals with chronic diabetes to address cognitive issues, with a particular emphasis on the elderly population. Additional interventional research endeavors focused on mitigating cognitive decline in DM, particularly those targeting novel risk factors in primary care settings, are recommended.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WC: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. YF: Data curation, Writing – original draft. AZ: Data curation, Writing – original draft.
Funding
The author(s) that no declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the authors of the included studies, which were used as the data source for the conduct of this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DM, diabetes mellitus; CI, cognitive impairment; IBCS, institutional-based cross-sectional study; POR, pooled odds ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
References
1
WhitingDRGuariguataLWeilCShawJ. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. (2011) 94:311–21. doi: 10.1016/j.diabres.2011.10.029
2
FederationID. IDF diabetes atlas 8th edition. International diabetes federation. (2017), 905–11.
3
SaeediPPetersohnISalpeaPMalandaBKarurangaSUnwinNet al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
4
LovicDPiperidouAZografouIGrassosHPittarasAManolisA. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:104–9. doi: 10.2174/1570161117666190405165911
5
KodlCTSeaquistER. Cognitive dysfunction and diabetes mellitus. Endocrine Rev. (2008) 29:494–511. doi: 10.1210/er.2007-0034
6
RobertsROKnopmanDSMielkeMMChaRHPankratzVSChristiansonTJet al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. (2014) 82:317–25. doi: 10.1212/WNL.0000000000000055
7
RobertsROGedaYEKnopmanDSChristiansonTJPankratzVSBoeveBFet al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. (2008) 65:1066–73. doi: 10.1001/archneur.65.8.1066
8
LopezOL. Mild cognitive impairment. Continuum: Lifelong Learn Neurol. (2013) 19:411. doi: 10.1212/01.CON.0000429175.29601.97
9
MukherjeePMazumdarSGoswamiSBhowmikJChakrobortySMukhopadhyaySet al. Cognitive dysfunction in diabetic patients with special reference to age of onset, duration and control of diabetes. Activitas Nervosa Superior. (2012) 54:67–75. doi: 10.1007/BF03379585
10
SasakiNFukatsuRTsuzukiKHayashiYYoshidaTFujiiNet al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol. (1998) 153:1149–55. doi: 10.1016/S0002-9440(10)65659-3
11
RiedererPKorczynADAliSSBajenaruOChoiMSChoppMet al. The diabetic brain and cognition. J Neural Transmission. (2017) 124:1431–54. doi: 10.1007/s00702-017-1763-2
12
EhtewishHArredouaniAEl-AgnafO. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int J Mol Sci. (2022) 23:6144. doi: 10.3390/ijms23116144
13
YouYLiuZChenYXuYQinJGuoSet al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. (2021) 58:671–85. doi: 10.1007/s00592-020-01648-9
14
LiWSunLLiGXiaoS. Prevalence, influence factors and cognitive characteristics of mild cognitive impairment in type 2 diabetes mellitus. Front Aging Neurosci. (2019) 11:180. doi: 10.3389/fnagi.2019.00180
15
AtifMSaleemQScahillS. Depression and mild cognitive impairment (MCI) among elderly patients with type 2 diabetes mellitus in Pakistan: possible determinants. Int J Diabetes Develop Countries. (2018) 38:312–20. doi: 10.1007/s13410-017-0600-3
16
PrinceMBryceRAlbaneseEWimoARibeiroWFerriCP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s dementia. (2013) 9:63–75. e2. doi: 10.1016/j.jalz.2012.11.007
17
MavrodarisAPowellJThorogoodM. Prevalences of dementia and cognitive impairment among older people in sub-Saharan Africa: a systematic review. Bull World Health Organization. (2013) 91:773–83. doi: 10.2471/BLT.13.118422
18
GroeneveldOReijmerYHeinenRKuijfHKoekkoekPJanssenJet al. Brain imaging correlates of mild cognitive impairment and early dementia in patients with type 2 diabetes mellitus. Nutrition Metab Cardiovasc Diseases. (2018) 28:1253–60. doi: 10.1016/j.numecd.2018.07.008
19
VagelatosNTEslickGD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiologic Rev. (2013) 35:152–60. doi: 10.1093/epirev/mxs012
20
ShamseerLMoherDClarkeMGhersiDLiberatiAPetticrewMet al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. (2015) 349. doi: 10.1136/bmj.g7647
21
HoyDBrooksPWoolfABlythFMarchLBainCet al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65:934–9. doi: 10.1016/j.jclinepi.2011.11.014
22
HigginsJPThompsonSG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
23
GeorgeBJAbanIB. An application of meta-analysis based on DerSimonian and Laird method. Springer (2016) p. 690–2.
24
AshebirNHailesilassieHGirmaSNigusuEEzoE. Prevalence of cognitive impairment and associated factors among diabetes mellitus patients attending follow-up treatment at fiche general hospital, north Ethiopia. SAGE Open Nursing. (2024) 10:23779608241227752. doi: 10.1177/23779608241227752
25
MulugetaTDessalegnMBehailuS. Cognitive impairment among type 2 diabetes mellitus patients in Ethiopia. Int J Of Med And Appl Sci. (2013) 2:40–54.
26
DagnewBADWMossieA. Cognitive impairment among type 2 diabetes mellitus patients at Jimma University Specialized Hospital, Southwest Ethiopia. (2019). doi: 10.1186/s13104-019-4550-3
27
EzeCEzeokpoBKaluUOnwuekweI. The prevalence of cognitive impairment amongst type 2 diabetes mellitus patients at Abakaliki South-East Nigeria. J Diabetes Metab Syndr Disord. (2015) 1. doi: 10.24966/DMD-201X
28
WilliamsUEUhegbuVMOkpaHOOparahSKEnangOEEssienOE. Cognitive dysfunction and mood disorders in patients with diabetes mellitus: experience from southern Nigeria. World J Med Sci. (2020) 17:59–68.
29
BashirJYarubeI. Occurrence of mild cognitive impairment with hyperinsulinaemia in Africans with advanced type 2 diabetes mellitus. IBRO Neurosci Rep. (2022) 12:182–7. doi: 10.1016/j.ibneur.2022.02.003
30
AdebayoRYOdeighaLOAlabiANMohammedAObalowuIAAdemolaCO. Body mass index, blood pressure, and cognitive impairment among type 2 diabetic patients in a primary care setting, North-Central Nigeria. Ann Afr Med Res. (2022) 5. doi: 10.4081/aamr.2022.158
31
AbdellatifGAHassanAMGabalMSHemedaSAEl-ChamiNHSalamaII. Mild cognitive impairment among Type II diabetes mellitus patients attending university teaching hospital. Open Access Macedonian J Med Sci. (2020) 8:105–11. doi: 10.3889/oamjms.2020.4245
32
MohamedMMNour-EldeinHSaudiRA. Prevalence of cognitive impairment among type 2 diabetes mellitus patients attending family medicine clin-ic in suez canal university hospital. Suez Canal Univ Med J. (2023) 26:51–63. doi: 10.21608/scumj.2023.324133
33
AnwarMDarwishEsMohamedAWalaaAm. Cognitive impairment in patients with diabetes mellitus on insulin therapy. Med J Cairo Univ. (2018) 86:2605–14. doi: 10.21608/mjcu.2018.58064
34
AbbaZIMboue-DjiekaYMapoureYNNkouonlackCLumaHNChoukemS-P. Prevalence and risk factors of cognitive dysfunction in patients with type 2 diabetes mellitus receiving care in a reference hospital in Cameroon: a cross-sectional study. Int J Diabetes Develop Countries. (2018) 38:158–64. doi: 10.1007/s13410-017-0565-2
35
Ossou-NguietPMDoungouNDMpandzouGANgoumaYAPEObondzo-AlobaKBanzouziLFet al. Troubles cognitifs et diabete de type 2 au Congo. Afr J Neurol Sci. (2019) 38:78–90.
36
TlemçaniFZRElamariSMotaibILaidiSAlidrissiNAhidSet al. Factors associated with mild cognitive impairment in patients with type 2 diabetes: A cohort study. Cureus. (2022) 14.
37
KhullarSKaurGDhillonHSharmaRMehtaKSinghMet al. The prevalence and predictors of cognitive impairment in type 2 diabetic population of Punjab, India. J Soc Health Diabet. (2017) 5:047–53.
38
MoheetAMangiaSSeaquistER. Impact of diabetes on cognitive function and brain structure. Ann New York Acad Sci. (2015) 1353:60–71. doi: 10.1111/nyas.12807
39
BlanquiscoLAbejeroJEBunoIIBTrajano-AcampadoLCeninaASantiagoD. Factors associated with mild cognitive impairment among elderly filipinos with type 2 diabetes mellitus. J ASEAN Fed Endocrine Societies. (2017) 32:145. doi: 10.15605/jafes
40
Gorska-CiebiadaMSaryusz-WolskaMCiebiadaMLobaJ. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res. (2014) 2014. doi: 10.1155/2014/179648
41
MurataYKadoyaYYamadaSSankeT. Cognitive impairment in elderly patients with type 2 diabetes mellitus: prevalence and related clinical factors. Diabetol Int. (2017) 8:193–8. doi: 10.1007/s13340-016-0292-9
42
GaoYXiaoYMiaoRZhaoJCuiMHuangGet al. The prevalence of mild cognitive impairment with type 2 diabetes mellitus among elderly people in China: a cross-sectional study. Arch Gerontol geriatrics. (2016) 62:138–42. doi: 10.1016/j.archger.2015.09.003
43
CallaghanBCReynoldsELBanerjeeMChantEVillegas-UmanaEGardnerTWet al. The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Diabetes Care. (2020) 43:683–90. doi: 10.2337/dc19-1642
44
LuchsingerJAReitzCPatelBTangM-XManlyJJMayeuxR. Relation of diabetes to mild cognitive impairment. Arch Neurol. (2007) 64:570–5. doi: 10.1001/archneur.64.4.570
45
BorensteinARMortimerJAWuYJureidini-WebbFMFallinMDSmallBJet al. Apolipoprotein E and cognition in community-based samples of African Americans and Caucasians. Ethnicity Dis. (2006) 16:9–15.
46
LalithambikaCVArunCSSaraswathyLABhaskaranR. Cognitive impairment and its association with glycemic control in type 2 diabetes mellitus patients. Indian J Endocrinol Metab. (2019) 23:353. doi: 10.4103/ijem.IJEM_24_19
47
KatariaLPandyaHShahSShahHGergR. Prevalence and pattern of cognitive dysfunction in type 2 diabetes mellitus. Int J Med Appl Sci. (2013) 2:240–6.
48
ZhangMKatzmanRSalmonDJinHCaiGWangZet al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol: Off J Am Neurol Assoc Child Neurol Society. (1990) 27:428–37. doi: 10.1002/ana.410270412
49
SattlerCToroPSchönknechtPSchröderJ. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. (2012) 196:90–5. doi: 10.1016/j.psychres.2011.11.012
50
MuthiahSKanagamuthuRSaravanan MohanrajSLK. Assessment of memory and cognitive functions in controlled and uncontrolled Type 2 diabetes mellitus patients. Natl J Physiology Pharm Pharmacol. (2019) 9:140–. doi: 10.5455/njppp.
51
VijayakumarTSirishaGBegamFDhanarajuM. Mechanism linking cognitive impairment and diabetes mellitus. Eur J Appl Sci. (2012) 4:01–5.
52
SenguptaPBenjaminAISinghYGroverA. Prevalence and correlates of cognitive impairment in a north Indian elderly population. WHO South-East Asia J Public Health. (2014) 3:135–43. doi: 10.4103/2224-3151.206729
53
RobertsROKnopmanDSChaRHMielkeMMPankratzVSBoeveBFet al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. (2014) 55:759–64. doi: 10.2967/jnumed.113.132647
54
TuligengaRHDugravotATabákAGElbazABrunnerEJKivimäkiMet al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. (2014) 2:228–35. doi: 10.1016/S2213-8587(13)70192-X
55
VermeerSEDen HeijerTKoudstaalPJOudkerkMHofmanABretelerMM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. (2003) 34:392–6. doi: 10.1161/01.STR.0000052631.98405.15
Summary
Keywords
cognitive impairment, diabetes mellitus, systematic review, meta-analysis, Africa
Citation
Chekol Tassew W, Ferede YA and Zeleke AM (2024) Cognitive impairment and associated factors among patients with diabetes mellitus in Africa: a systematic review and meta-analysis. Front. Endocrinol. 15:1386600. doi: 10.3389/fendo.2024.1386600
Received
15 February 2024
Accepted
21 June 2024
Published
17 July 2024
Volume
15 - 2024
Edited by
Joana Nicolau, Son Llatzer Hospital, Spain
Reviewed by
Inha Jung, Korea University Ansan Hospital, Republic of Korea
Dorota Formanowicz, Poznan University of Medical Sciences, Poland
Updates
Copyright
© 2024 Chekol Tassew, Ferede and Zeleke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Worku Chekol Tassew, workukid16@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.