- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, West China Second University Hospital, Sichuan University, Chengdu, China
Objective: To assess the effect of intravenous immunoglobulin (IVIG) therapy on unexplained recurrent spontaneous abortion (URSA).
Methods: We retrieved all randomized controlled trials (RCTs) related to the effect of IVIG therapy on URSA in the following databases: PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials before April 30, 2023, according to the PRISMA statement. The therapeutic effect of IVIG was measured by live birth rates. Quality assessment was conducted independently by two reviewers, based on the Newcastle‐Ottawa scale. For the meta-analysis, we used odds ratios (random effects model and fixed effects model). The between-study heterogeneity was assessed by the Q test. Publication bias was assessed by funnel plots.
Results: A total of 12 studies with 751 participants were included in this meta-analysis. There was no statistical significance [OR = 1.07, 95%CI (0.65, 1.75), P=0.80] between the IVIG group and the non-IVIG group, including low molecular weight heparin (LMWH) plus low-dose aspirin (LDA), intralipid, multivitamins, albumin, and normal saline. A subgroup analysis was conducted according to the different treatment regimens of the non-IVIG group. Compared to the placebo group, including multivitamins, albumin, and saline, the live birth rate of the IVIG group is superior, but there was no statistical significance [OR =1.43, 95%CI (0.99, 2.07), P=0.05]. Another subgroup analysis was performed according to URSA with positive for antiphospholipid antibodies (aPLs). Results showed the live birth rate of IVIG on URSA with positive for aPLs is inferior to that of LMWH plus LDA [OR = 0.25, 95%CI (0.11, 0.55), P=0.0007].
Conclusions: IVIG didn’t increase the live birth rate of URSA compared to placebo. Conversely, compared with the IVIG, the LMWH plus LDA treatment schedule can increase the live birth rate of URSA with positive for aPLs.
1 Introduction
As reported, the prevalence of recurrent spontaneous abortion (RSA) is 1%-5% (1) and is increasing year by year (2). What’s more, RSA affects around 1%-2% of couples with fertility needs (3). However, the definition of RSA is still debated, with the main points of contention being the number of miscarriages and gestational age (4). The British College of Obstetricians and Gynaecologists (RCOG) defines RSA as three or more spontaneous abortions before the 24th week of gestation (5). The American Society for Reproductive Medicine criteria (ASRM) defines RSA as two or more spontaneous abortions, without limiting the gestational age (6). The European Society of Human Reproduction and Embryology (ESHRE) Guideline Group on RPL defines RSA as two or more miscarriages before the 24th week of gestation (3). And, it is estimated that nearly 5% of women will experience two consecutive miscarriages, and only 1% experience three or more times (7). Etiological screening of RSA patients with two previous miscarriages and those with three or more previous miscarriages found no significant difference, which supports the inclusion of patients with two or more prior miscarriages in RSA management (8). Therefore, in our meta-analysis, RSA is defined as two or more consecutive miscarriages before the 24th week of gestation.
The etiology of RSA (7) is complex, including chromosomal or genetic abnormalities, anatomical abnormalities, autoimmune diseases, the prethrombotic state (PTS), endocrine system disorders, infectious causes, male causes, environmental psychological causes, and so on. However, a large proportion of RSA patients (approximately 40%-75%) have no known cause, known as the unexplained RSA (URSA) (5, 7). According to reports, 8%-46% of patients with RSA have positive for antiphospholipid antibodies (aPLs) (7). It is worth emphasizing to URSA patients that the chance of a successful pregnancy in the future may exceed 50% to 60% (7). Therefore, exploring an effective treatment for URSA may greatly improve the live birth rate of RSA patients.
It is recorded that the occurrence of URSA is related to the imbalance of maternal-fetal immunity (5, 9). In recent years, many treatments for maternal-fetal immune imbalance have emerged in clinical practice. For example, lymphocyte immunotherapy (LIT), intravenous immunoglobulin (IVIG), tumor necrosis factor α (TNF-α), granulocyte colony-stimulating factor (G-CSF), intralipid therapy, progesterone therapy, anticoagulation therapy, immune-suppressant (mainly including glucocorticoids and cyclosporine A), traditional Chinese medicine therapy (10), low molecular weight heparin (LMWH) plus low-dose aspirin (LDA) (11) and so on. However, most of these treatments are laboratory-based, and their efficacy and safety in clinical are still controversial.
Accumulating evidence suggests that, in vitro and in vivo models, IVIG plays a potential role in immune modulation and inflammation regulation by upregulation of Receptor I for the Fc Region of Immunoglobulin G (FcγRI) and Receptor III for the Fc Region of Immunoglobulin G (FcγRIII) with downregulation of Receptor II B for the Fc Region of Immunoglobulin G (FcγRIIB) receptors, neutralizing autoantibodies, triggering the expansion of the regulatory T (Treg) cells, and reducing natural killer (NK) cell levels and activity (12). At present, there are also some updates on the mechanism of IVIG in URSA with immune imbalance. The possible mechanisms of IVIG preventing URSA are as follows (11–13): down-regulating the function of B cells, inhibiting the anti-idiotypic effect of autoantibody, reducing the phagocytosis induced by Fc receptor, increasing the regulation of T cells, reducing the complement activation system, and inhibiting the expression and function of cytokines. However, the exact mechanism of IVIG’s effect on URSA has not yet been fully investigated.
To date, numerous clinical trials have been conducted to explore the efficacy of IVIG for URSA. A double-blind randomized trial (14) in 1994 indicated there was no conclusive evidence that IVIG could prevent further miscarriage in women with URSA. However, a randomized controlled trial (RCT) (15) conducted by Hideto Yamada in 2022 showed that IVIG increased ongoing pregnancy and live birth rates in 50 patients with URSA who had 4 or more miscarriages. The effect of IVIG on URSA is a lack of consistency. Therefore, this meta-analysis aims to evaluate the effect of IVIG on URSA by synthesizing all randomized controlled trials (RCTs) published on April 30, 2023.
2 Materials and methods
2.1 Data source and search strategy
Two researchers separately retrieved the following databases: PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials. Combinations of MeSH terms, “Abortion, Habitual”, “Recurrent abortion”, “Immunoglobulin” and “Immunoglobulins, Intravenous” and their entry terms were used. Queries were limited to human studies. The systematic search strategy is outlined in Appendix S1. Publications dated before April 30, 2023.
2.2 Study selection
Two researchers respectively screened the full text, the title, and the abstract of the search results. First of all, we removed the repeated articles. Then, we respectively selected articles based on the following inclusion and exclusion criteria. As for debate articles, we discussed with the third researcher to get a result. Two reviewers (Q.L. and J.F.X.) independently evaluated the titles and abstracts. Duplications were removed using ENDNOTE online software and manually. Disagreements were resolved by discussion among authors; if required, a third investigator (B.P.) was involved to resolve the disagreement between evaluators.
The inclusion criteria were: 1) two or more URSA before the 24th week of gestation; 2) intervention groups only received IVIG; 3) no IVIG in control groups; 4) outcomes of the trials included live birth rate; 4) RCTs. The exclusion criteria were: 1) duplicated articles; 2) Case series, case reports, book chapters, review articles, letters to editors, conference reports, cross-sectional studies, case-control studies, cohort studies, and observational prospective studies; 3) full manuscripts not accessible; 4) non-human studies; 5) participants with infectious, genetic, endocrine or anatomical abnormality; 6) intervention group received a combination of IVIG and another drug or no IVIG; 7) the outcome wasn’t live birth rate; 8) non-English language.
2.3 Outcomes
The outcome was the number of live births or the live birth rate.
2.4 Data Extraction and Risk of Bias Assessment
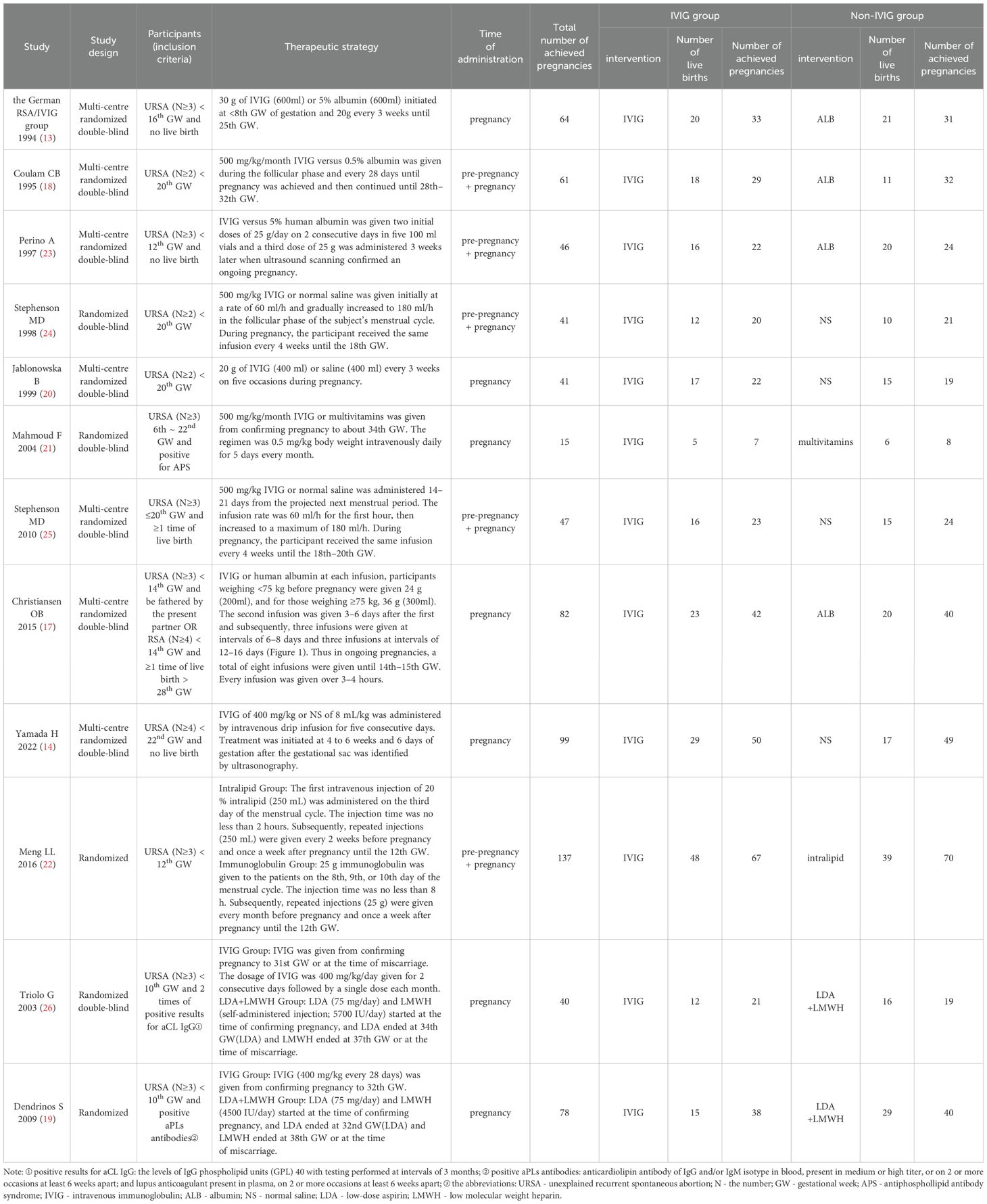
Two researchers respectively extracted baseline information from every included study, which included the first author, year of publication, inclusion criteria of participants, intervention therapeutic regimen, study design, and the live birth rate as treatment outcome.
We used the risk of bias assessment tables from the Cochrane RevMan software of Cochrane Centre (5.4.7) to evaluate the risk of bias in the included studies. Two researchers respectively evaluate the methodological quality of each included RCTs.
2.5 Data Synthesis
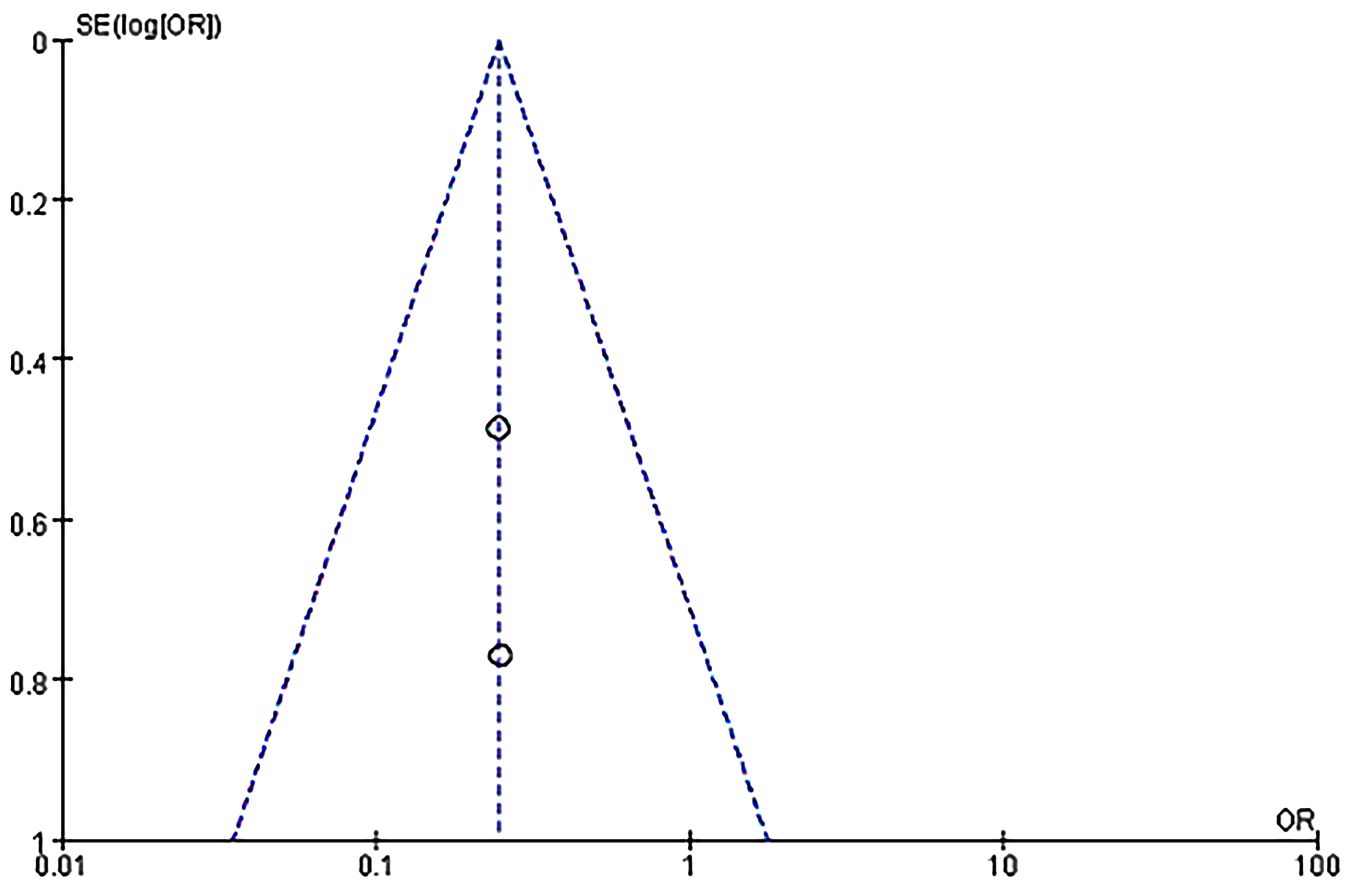
We followed the MOOSE checklist and PRISMA guidelines for this systematic review (16, 17). We used odds ratio (OR) and 95% confidence interval (95%CI) to calculate binary data outcomes. Data analysis was conducted using Cochrane RevMan software of Cochrane Centre (5.4.7). The heterogeneity of the meta-analysis was assessed by the chi-squared method. The random-effect model was used in case of significant heterogeneity among studies (P ≥0.05, I2 >50%). Subgroup analysis was conducted to assess the potential sources of heterogeneity. Forest plots were used to represent the statistical data graphically. Publication bias was assessed by funnel plots. When a funnel chart is symmetric, then publication bias is less likely to exist and vice versa.
3 Results
3.1 Study selection
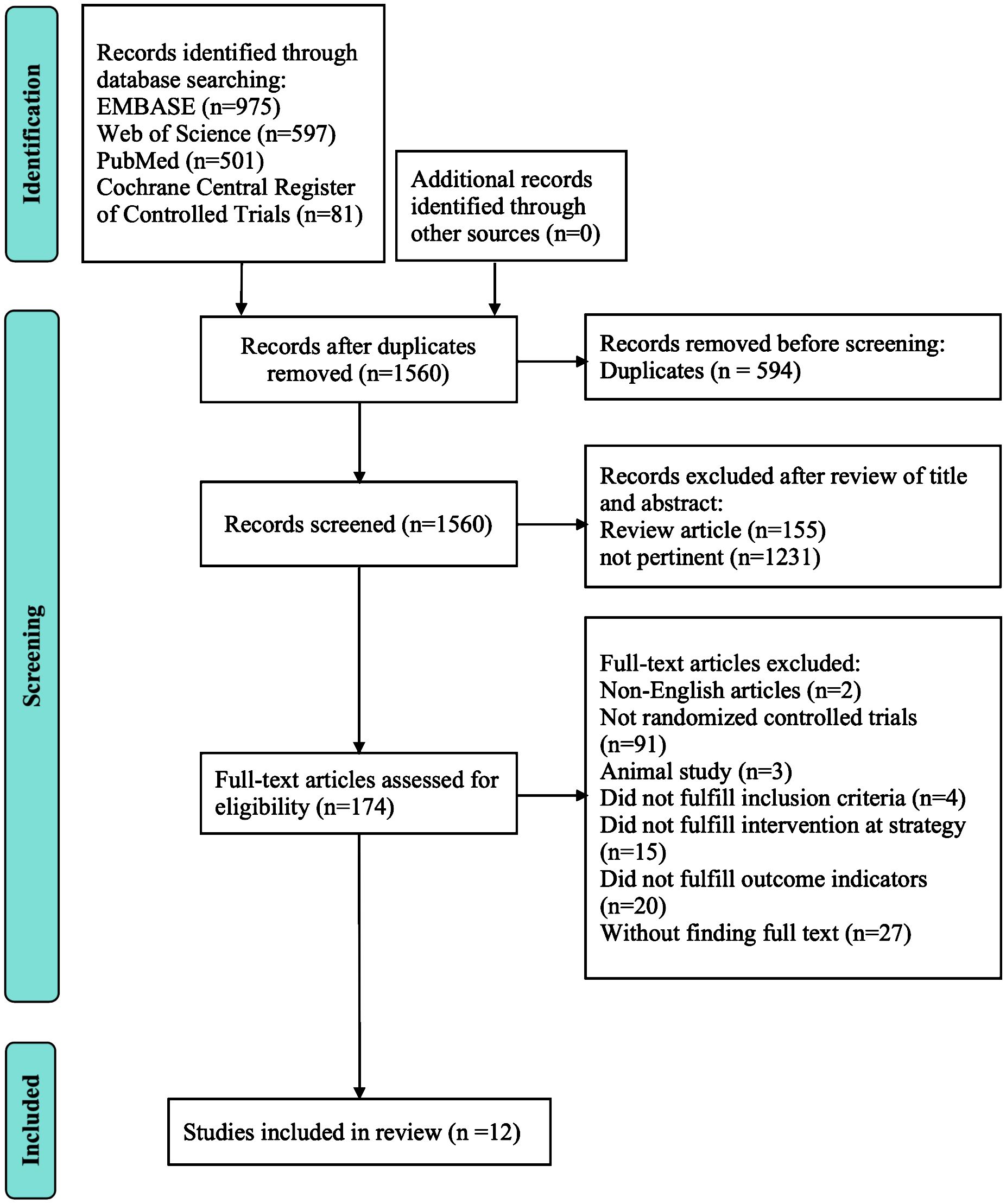
A primary retrieval found 2154 relevant articles, including 975 articles from Embase, 597 articles from the Web of Science, 501 articles from PubMed, and 81 articles from Cochrane Central Register of Controlled Trials. Firstly, we excluded 594 duplicated articles. Then, we excluded 155 review articles and 1231 unrelated articles by reviewing the title and abstract. After that, we respectively carefully perused the remaining 174 articles in full text. Twelve RCTs (14, 15, 18–27) with 751 patients were selected for detailed assessment. Table 1 depicts the characteristics of the included studies. Figure 1 depicts the review flow diagram.
3.2 Study characteristics and Quality assessment
Using the risk of bias assessment tool to evaluate the methodology of each included trial. Overall, all the included studies (12 articles) were lowly risky, but 6 studies had an uncertain risk of biases in the following aspects: random sequence generation, allocation concealment, blinding of participants and intervention providers, and other unknown source of bias (Figures 2A, B).
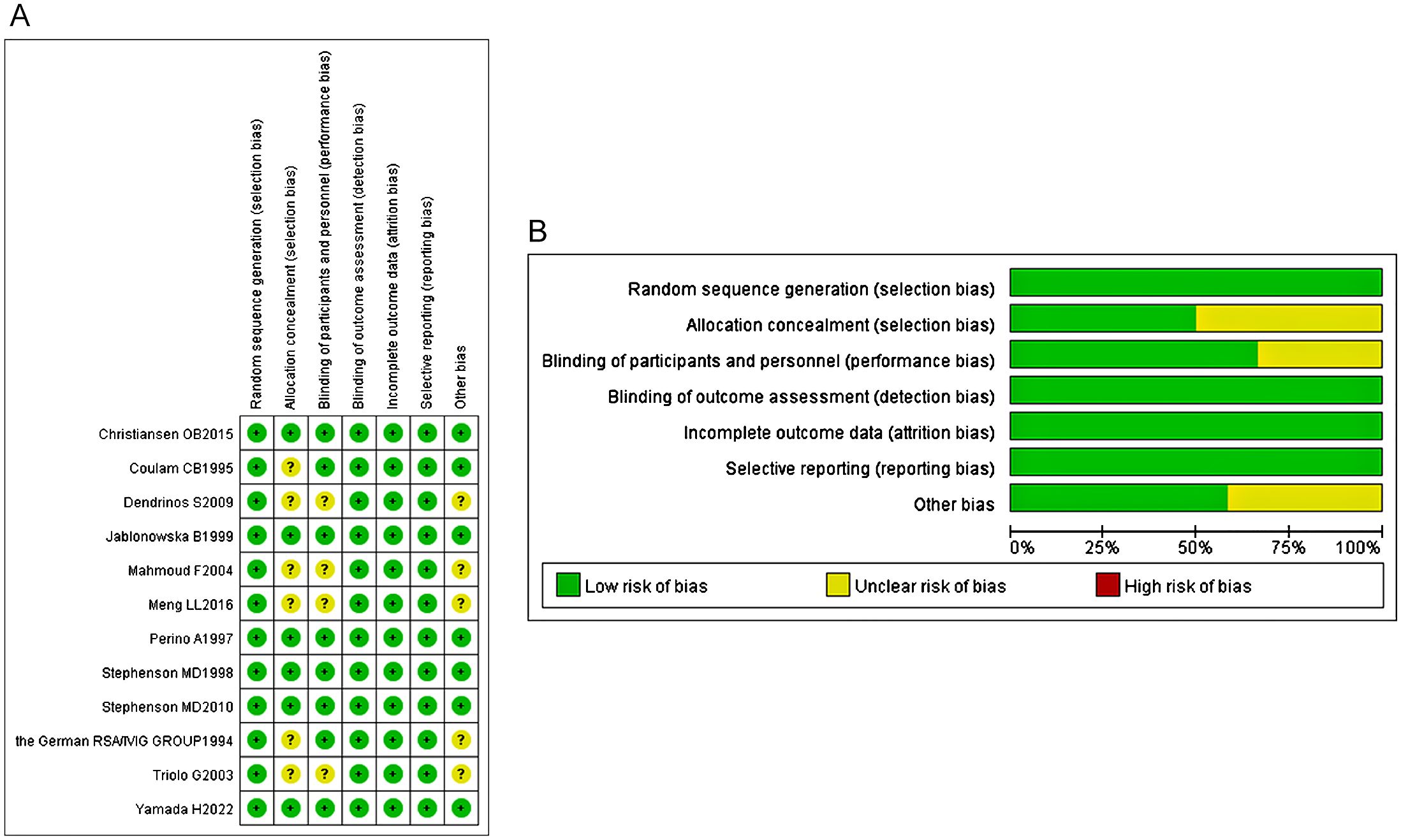
Figure 2. (A) The risk of bias assessment of each included study. (B) The risk of bias assessment of each included study.
3.3 Effect of IVIG on URSA
3.3.1 Live birth rate between the IVIG group and the non-IVIG group
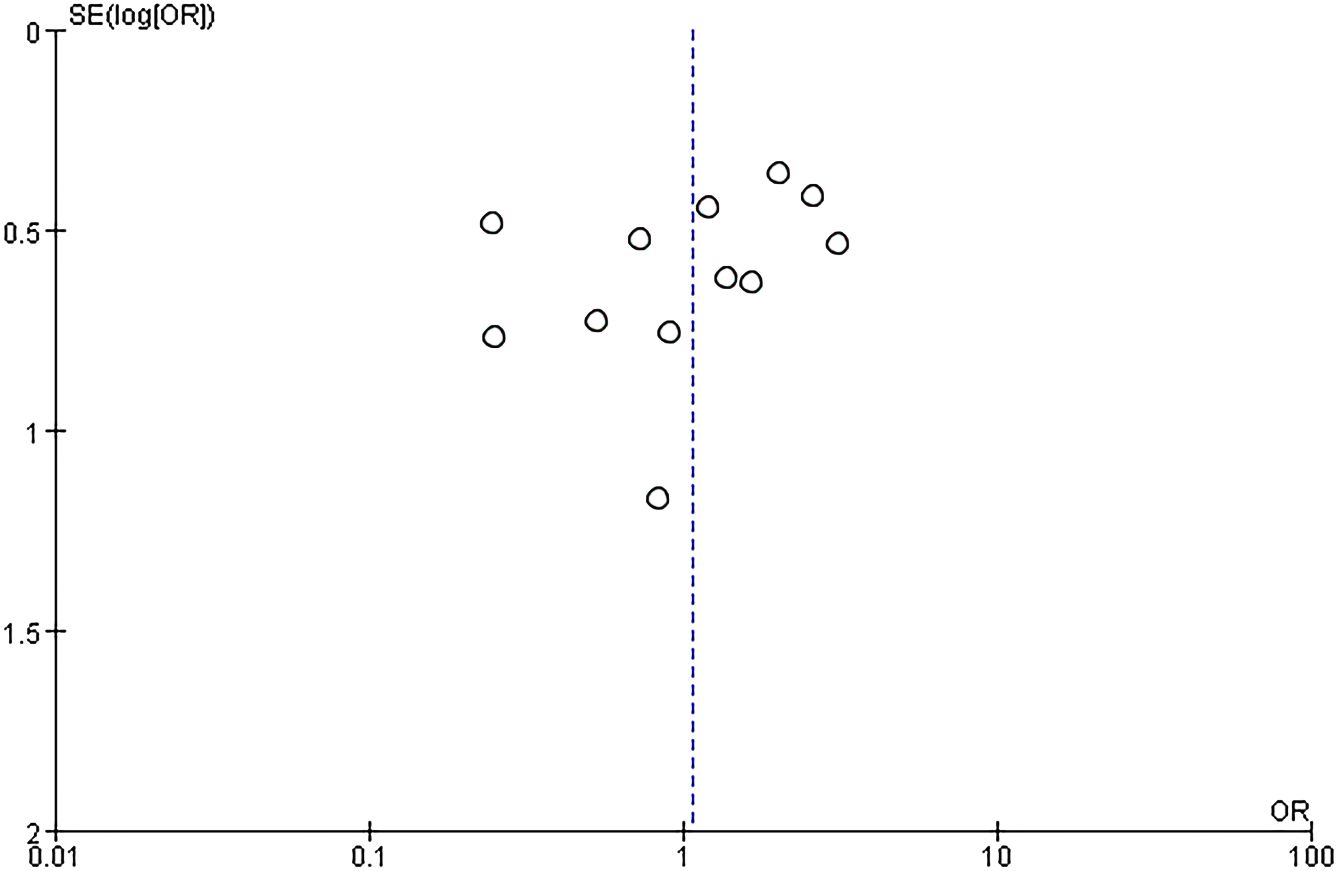
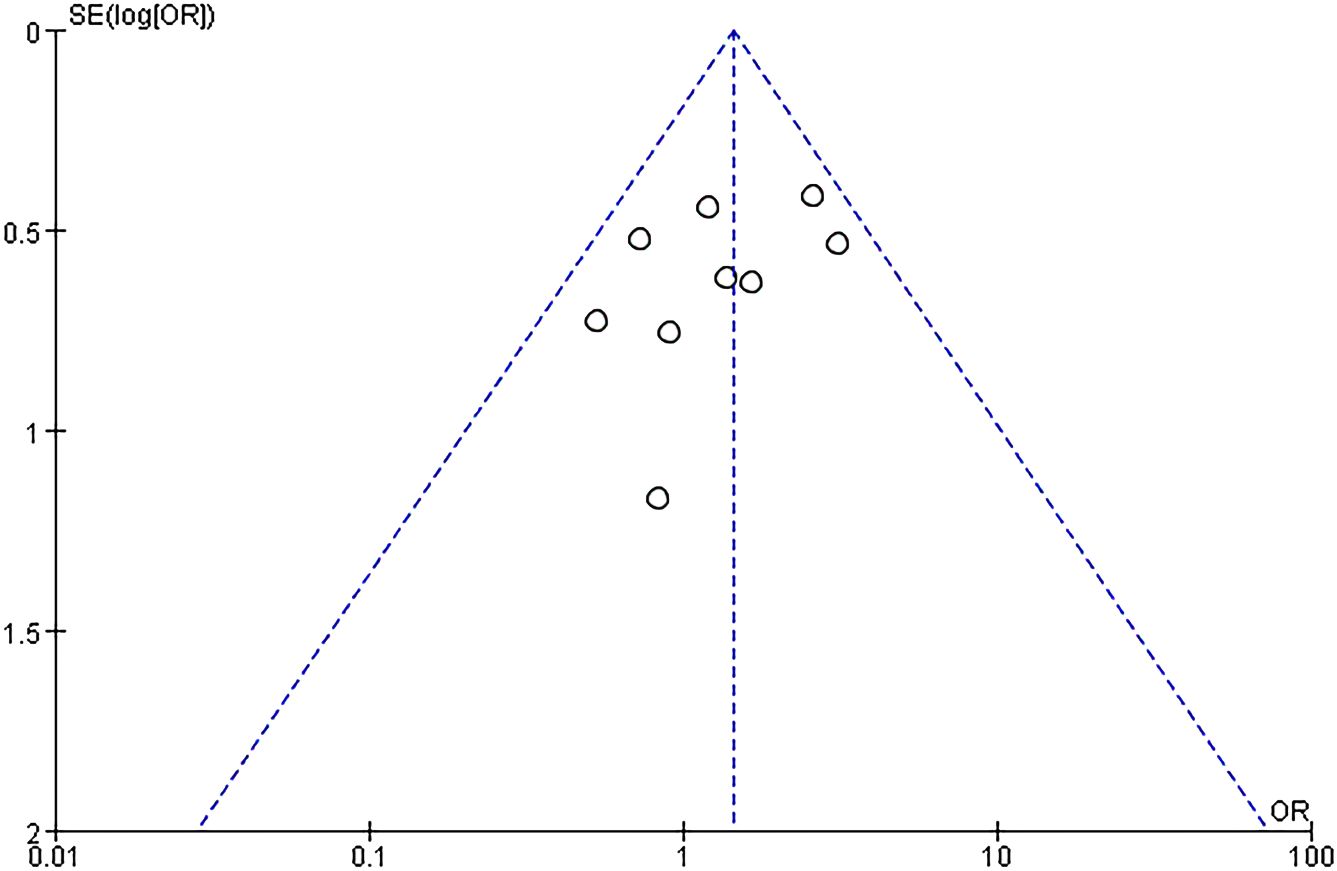
Twelve RCTs (751 patients) were included, with 374 IVIG users and 377 non-IVIG users. The non-IVIG group refers to the no use of IVIG at the time of treatment, including low molecular weight heparin (LMWH) plus low-dose aspirin (LDA), intralipid, multivitamins, albumin, and normal saline in this meta-analysis. Some heterogeneity was detected among the studies (P = 0.006, I2 = 58%). Thus, the random effects model was used to conduct data analysis. Results showed that the live birth rate of the IVIG group is higher in comparison with the non-IVIG group, but there was no statistical significance [OR = 1.07, 95%CI (0.65, 1.68), P=1.75] (Figure 3). All plots fall in the funnel figure and are almost symmetric (Figure 4). Therefore, no significant publication bias was identified.
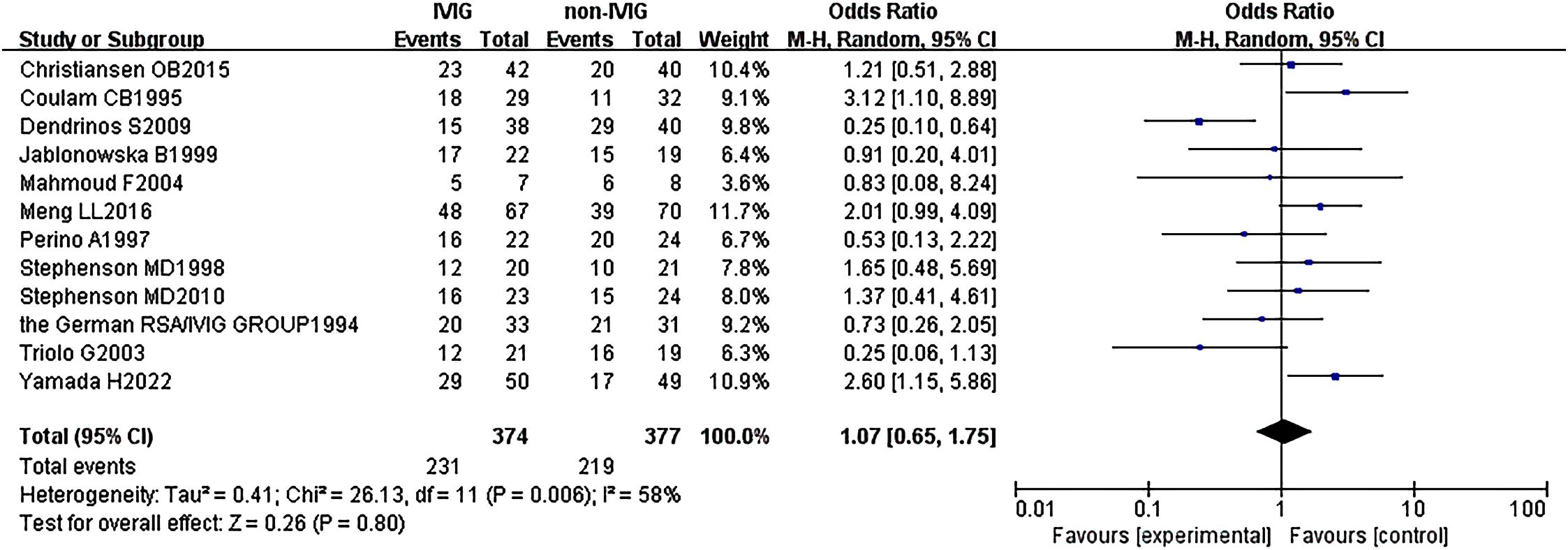
Figure 3. Live birth rate between the IVIG group and the non-IVIG group in patients with recurrent spontaneous abortion.
3.3.2 Live birth rate between the IVIG group and the placebo group
A subgroup analysis was conducted according to the different treatment regimens of the non-IVIG group. Placebo group refers to the use of no-effect reagents on URSA at the time of treatment, including multivitamins, albumin, and saline in this meta-analysis. Nine studies with 496 patients reported the live birth rate of IVIG therapy (248 patients) and placebo group (248 patients). There was no heterogeneity among the studies (P = 0.39, I2 = 5%). Therefore, the fixed effects model was used to get a total summary. There was no significant difference in the live birth rate between the IVIG group and the placebo group [OR = 1.43, 95%CI (0.99, 2.07), P=0.05] (Figure 5). All plots fall in the funnel figure and are almost symmetric (Figure 6). Therefore, no significant publication bias was identified.
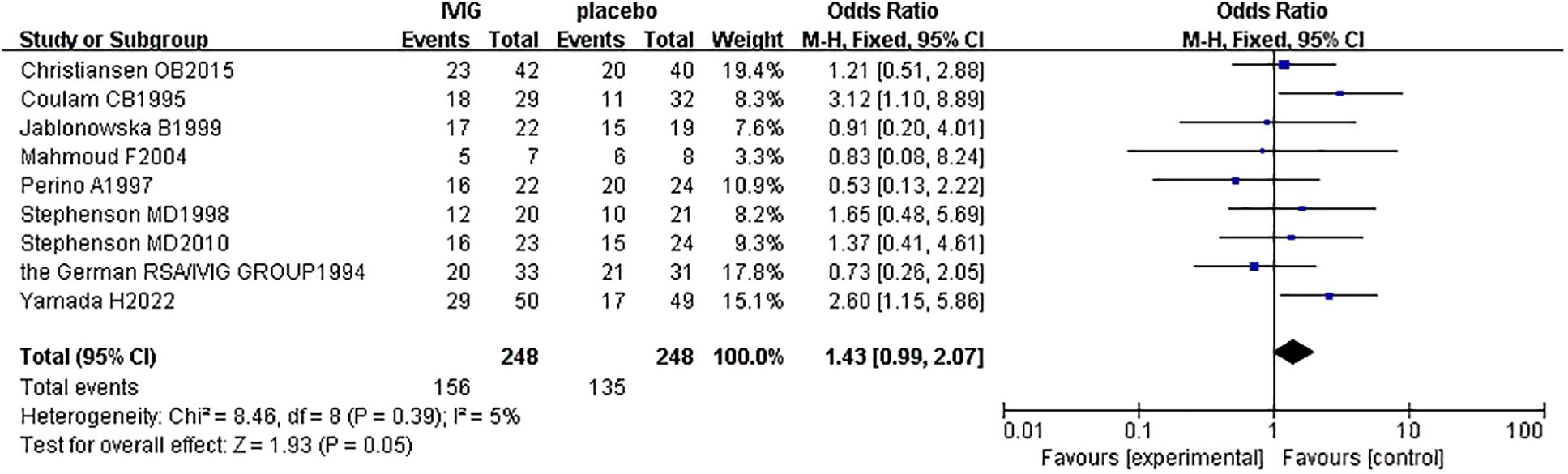
Figure 5. Live birth rate between the IVIG group and the placebo group in patients with recurrent spontaneous abortion.
3.3.3 Live birth rate between the IVIG group and low molecular weight heparin plus low-dose aspirin group
Another subgroup analysis was performed between IVIG treatment and LMWH plus LDA treatment. And the participants are positive for antiphospholipid antibodies (aPLs). Two studies (118 patients) reported the difference in live birth rate between IVIG treatment (59 patients) and LMWH plus LDA treatment (59 patients). There was no statistical heterogeneity among the studies (P = 0.99, I2 = 0%). There was a significant difference in the live birth rate between the IVIG group and the LMWH plus LDA group [OR = 0.25, 95%CI (0.11, 0.55), P=0.0007] (Figure 7). All plots fall in the funnel figure and are almost symmetric (Figure 8). Therefore, no significant publication bias was identified.
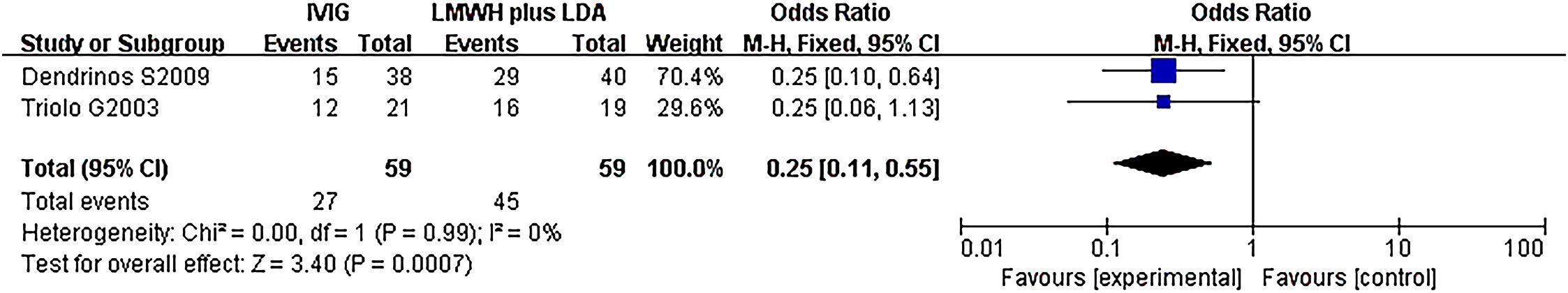
Figure 7. Live birth rate between the IVIG group and the LMWH plus LDA group in recurrent spontaneous abortion patients with positive for antiphospholipid antibodies (aPLs).
4 Discussion
Although the exact mechanism of IVIG is not well elucidated, IVIG has been widely used as a non-specific immunosuppressant in the clinical treatment of many complex immune diseases, such as URSA (28). The U.S. Food and Drug Administration (FDA) has approved the use of IVIG as a first-line treatment for autoimmune thrombocytopenia, while other conditions such as RSA, antiphospholipid antibody syndrome, and repeated unexplained IVF failures remain off-label indications for this blood product (29, 30). There are no international guidelines that explicitly recommend IVIG for the routine treatment of RSA (3, 31). The effect of IVIG on URSA is controversial.
In our study, there is no significant difference between the IVIG group and the non-IVIG group [OR = 1.07, 95%CI (0.65, 1.75), P=0.80]. Considering that there are 5 different therapy regimens in the non-IVIG group, including LMWH plus LDA, intralipid, multivitamins, albumin, and normal saline, they may have different efficacy for URSA. This may be the reason why no statistical significance between the IVIG group and the non-IVIG group. Therefore, we performed a three-subgroup analysis according to the different therapy regimens in the non-IVIG group. Firstly, the placebo group refers to the use of no-effect reagents on URSA at the time of treatment in the non-IVIG group, including multivitamins, albumin, and saline. Secondly, the LMWH plus LDA group refers to the use of LWMH plus LDA at the time of treatment in the non-IVIG group. Thirdly, the intralipid group refers to the use of only intralipid at the time of treatment in the non-IVIG group.
The subgroup meta-analysis between the IVIG group and the placebo group suggested IVIG treatment can’t improve the live birth of URSA. Meanwhile, Hutton et al. indicated that IVIG has no positive effect on URSA in improving live birth rates, based on a systematic review and meta-analysis of 7 studies (28). However, A systematic review and meta-analysis (32) of 11 studies published in 2016 showed that IVIG can improve the live birth rate of URSA. Another systematic meta-analysis (33) of 13 RCTs published in 2022 concluded that, compared to placebo, IVIG can significantly increase the live birth rate of RSA. However, our result is inconsistent with the above two published meta-analyses (32, 33). It is worth considering whether IVIG should be used to treat URSA.
The second subgroup analysis was performed (20, 27) between the IVIG group and the LMWH plus LDA group. The result indicated the curative efficacy of the IVIG therapy group was inferior to the combination of LMWH and LDA treatment. Noteworthy, participants of two studies included in our meta-analysis were positive for aPLs. The aPLs go down as one of the most closely relevant pathogenic factors in URSA with autoimmune diseases (7). In clinical, 5% to 20% of URSA patients are positive for aPLs (7). According to the result of our subgroup analysis, IVIG was found to have a lower live birth rate compared with LDA+LMWH in our meta-analysis. However, the study conducted by Mahmoud F et al (22) showed that the IVIG group reduced the rate of preterm birth and miscarriage in people with RSA with APS, compared with placebo. Thus, the effectiveness of IVIG against URSA with positive for aPLs is controversial. It is well known the use of LMWH plus LDA has been regarded as the first-line treatment for prevention of URSA in pregnant women with positive for aPLs (34–36). Moreover, some researchers reported that IVIG can be used as a second-line treatment for URSA with positive for aPLs (36). There are currently few studies focusing on the effect of IVIG in URSA patients with positive for aPLs. Therefore, more studies are needed to show the effects of IVIG on URSA patients with positive for aPLs.
The third subgroup analysis was conducted between the IVIG group and the intralipid group. It only included one study, including 87 patients, with 48 in the IVIG group and 39 in the intralipid group, compared the effect of IVIG versus intralipid on URSA (37). The study suggested that IVIG tends to improve the live birth rate, compared to intralipid. But there was no statistical significance [OR=2.01, 95%CI (0.99,4.09)]. An article (38) published by Coulam CB in 2021 proved that intravenous infusion of intralipid significantly increases the live birth rate in patients with URSA who have increased peripheral blood NK cell activity or intimatal NK cell density. A case-series report (39) published by Plaçais L is consistent with the standpoint of the Coulam CB, which suggests an intravenous infusion of intralipid increases live birth rates and reduces miscarriage rates in patients with URSA. So intralipid can be an option for clinical trials in selected URSA patients who have failed conventional therapy and have certain immune abnormalities (elevated NK cell activity). Therefore, more studies are needed to prove the effect of intralipid on URSA in the future.
Our study had several strengths and shortcomings. Compared with the three previous systematic reviews and meta-analyses (28, 32, 33), our meta-analysis added another four RCTs (15, 20, 23, 27), and had a more rigorous assessment of the quality of the included studies, which makes our results more reliable. Furthermore, we excluded four articles (13, 40–43) that were included in the previous meta-analysis (33), whose study types were not RCTs after screening and serious discussion. Furthermore, we excluded two RCTs (44, 45) that were included in the previous meta-analysis (28, 33) because the abortion gestational age of the participants included in this RCT was after the 24th week of gestation. Therefore, our meta-analysis had a smaller sample size but higher reliability. However, our findings only relate to the possibility that IVIG can improve the live birth rate of URSA patients. What is lacking is that the studies we included did not investigate the risk of adverse obstetric complications, such as premature birth, gestational hypertension, preeclampsia, eclampsia, placental abruption, and adverse fetal outcome, such as the weight and height of birth, fetal teratogenicity and so on. Due to the lack of consistency in the dose and timing of IVIG (pre-pregnancy or pregnancy) in the included RCTs, we were unable to further explore the most effective dose and optimal administration timing of IVIG for URSA.
It is important to consider whether IVIG may cause other serious complications in pregnant women and fetuses. Besides, IVIG is a blood product derived from human plasma, which theoretically carries the risk of transmitting blood-borne diseases. There have been some reports on the safety of IVIG. A study (46), that included 370 women with reproductive failures who used IVIG during their pregnancy, proved that the use of IVIG during pregnancy did not increase obstetric complications, mainly including preterm births, gestational diabetes, preeclampsia, placental abruption, placenta previa, and placenta accrete, and fetal teratogenicity. Some articles indicated that the common side effects of IVIG include headache, fever, chills, dizziness, nausea, vomiting, muscle pain, and so on (33, 46). However, these side effects often occur early before IVIG treatment. Reducing the infusion rate can alleviate the side effects (33). These will serve as available evidence for the maternal and fetal safety of IVIG use during pregnancy. Furthermore, IVIG is quite expensive, and it has been reported that a course of treatment for adults usually costs more than $10,000 (28). Because of the limited efficacy and safety of IVIG, and its high cost, whether IVIG can be used as a first-line treatment for URSA is worth exploring and considering.
5 Conclusion
According to our 12 high-quality, low-risk, randomized controlled trials, we may conclude that URSA patients who were defined as two or more URSA before the 24th week of gestation in our meta-analysis might not benefit from IVIG treatment compared to placebo. Besides, the treatment of LMWH plus LDA would be better than IVIG treatment in URSA patients with positive for aPLs. In the future, more RCTs are needed to explore the effect of IVIG on URSA with or without positive for aPLs. Also, more large-scale clinical trials must be conducted to investigate the optimal dosage and the best time for IVIG administration on URSA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
QL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YT: Writing – original draft. DC: Formal analysis, Validation, Writing – review & editing. CM: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. BP: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Sichuan Province (2022NSFSC0701, 2023NSFSC1456), the National Natural Science Foundation of China (82200084), the Postdoctoral Science Foundation funded project of Sichuan Province (TB2023047), and the Sichuan University postdoctoral interdisciplinary Innovation Fund (0020404153020).
Acknowledgments
We appreciate that Furong Tang and Jianfeng Chen give us guidance about making charts and tables.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1381461/full#supplementary-material
References
1. Deng T, Liao X, Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol survey. (2022) 77:355–66. doi: 10.1097/ogx.0000000000001033
2. Green DM, O'Donoghue K. A review of reproductive outcomes of women with two consecutive miscarriages and no living child. J Obstet Gynaecol. (2019) 39:816–21. doi: 10.1080/01443615.2019.1576600
3. Atik RB, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. (2022) 7:hoad002. doi: 10.1093/hropen/hoad002
4. La X, Wang W, Zhang M, Liang L. Definition and multiple factors of recurrent spontaneous abortion. Adv Exp Med Biol. (2021) 1300:231–57. doi: 10.1007/978-981-33-4187-6_11
5. Yao Y, Ye YQ, Chen J, Zhang M, Cai XY, Zheng CH. Maternal-fetal immunity and recurrent spontaneous abortion. Am J Reprod Immunol. (2024) 91:e13859. doi: 10.1111/aji.13859
6. Amer Soc Reprod M. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility Sterility. (2020) 113:533–5. doi: 10.1016/j.fertnstert.2019.11.025
7. Amer Soc Reprod M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertility Sterility. (2012) 98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048
8. van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. (2020) 26:356–67. doi: 10.1093/humupd/dmz048
9. Xu J, Zhou F, Wang X, Mo C. Role of ferroptosis in pregnancy related diseases and its therapeutic potential. Front Cell Dev Biol. (2023) 11:1083838. doi: 10.3389/fcell.2023.1083838
10. Shigemi D, Hashimoto Y, Michihata N, Yasunaga H. Effect of Japanese herbal Kampo medicines on live birth rate in women with recurrent pregnancy loss. Int J Gynecol Obstet. (2021) 153:489–95. doi: 10.1002/ijgo.13477
11. Lin Q-D, Qiu L-H. Pathogenesis, diagnosis, and treatment of recurrent spontaneous abortion with immune type. Front Med China. (2010) 4:275–9. doi: 10.1007/s11684-010-0101-y
12. Yang XH, Meng T. Is there a role of intravenous immunoglobulin in immunologic recurrent pregnancy loss? J Immunol Res. (2020) 2020:6672865. doi: 10.1155/2020/6672865
13. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Afkham A, Danaii S, et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed Pharmacother. (2017) 92:1095–102. doi: 10.1016/j.biopha.2017.06.001
14. The German RSA/IVIG GROUP. Intravenous immunoglobulin in the prevention of recurrent miscarriage. The German RSA/IVIG Group. Br J Obstet Gynaecol. (1994) 101:1072–7. doi: 10.1111/j.1471-0528.1994.tb13584.x
15. Yamada H, Deguchi M, Saito S, Takeshita T, Mitsui M, Saito T, et al. Intravenous immunoglobulin treatment in women with four or more recurrent pregnancy losses: A double-blind, randomised, placebo-controlled trial. Eclinicalmedicine. (2022) 50:101527. doi: 10.1016/j.eclinm.2022.101527
16. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology - A proposal for reporting. Jama-Journal Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
18. Christiansen OB, Larsen EC, Egerup P, Lunoee L, Egestad L, Nielsen HS. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double-blind, placebo-controlled trial. Bjog-an Int J Obstet Gynaecol. (2015) 122:500–8. doi: 10.1111/1471-0528.13192
19. Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol. (1995) 34:333–7. doi: 10.1111/j.1600-0897.1995.tb00960.x
20. Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynecol Obstet. (2009) 104:223–5. doi: 10.1016/j.ijgo.2008.11.010
21. Jablonowska B, Selbing A, Palfi M, Ernerudh J, Kjellberg S, Lindton B. Prevention of recurrent spontaneous abortion by intravenous immunoglobulin: a double-blind placebo-controlled study. Hum Reprod. (1999) 14:838–41. doi: 10.1093/humrep/14.3.838
22. Mahmoud F, Diejomaoh M, Omu A, Abul H, Haines D. Effect of IgG therapy on lymphocyte subpopulations in the peripheral blood of Kuwaiti women experiencing recurrent pregnancy loss. Gynecol Obstet Invest. (2004) 58:77–83. doi: 10.1159/000078154
23. Meng LL, Lin JZ, Chen LB, Wang ZH, Liu ML, Liu YK, et al. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch Gynecol Obstet. (2016) 294:29–39. doi: 10.1007/s00404-015-3922-8
24. Perino A, Vassiliadis A, Vucetich A, Colacurci N, Menato G, Cignitti M, et al. Short-term therapy for recurrent abortion using intravenous immunoglobulins: results of a double-blind placebo-controlled Italian study. Hum Reprod. (1997) 12:2388–92. doi: 10.1093/humrep/12.11.2388
25. Stephenson MD, Dreher K, Houlihan E, Wu V. Prevention of unexplained recurrent spontaneous abortion using intravenous immunoglobulin: A prospective, randomized, double-blinded, placebo-controlled trial. Am J Reprod Immunol. (1998) 39:82–8. doi: 10.1111/j.1600-0897.1998.tb00339.x
26. Stephenson MD, Kutteh WH, Purkiss S, Librach C, Schultz P, Houlihan E, et al. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod. (2010) 25:2203–9. doi: 10.1093/humrep/deq179
27. Triolo G, Ferrante A, Ciccia F, Acarda-Palumbo A, Perino A, Castelli A, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis Rheumat. (2003) 48:728–31. doi: 10.1002/art.10957
28. Hutton B, Sharma R, Fergusson D, Tinmouth A, Hebert P, Jamieson J, et al. Use of intravenous immunoglobulin for treatment of recurrent miscarriage: a systematic review. Bjog-an Int J Obstet Gynaecol. (2007) 114:134–42. doi: 10.1111/j.1471-0528.2006.01201.x
29. Christiansen OB, Nielsen HS, Pedersen B. Active or passive immunization in unexplained recurrent miscarriage. J Reprod Immunol. (2004) 62:41–52. doi: 10.1016/j.jri.2003.09.003
30. Omwandho COA, Gruessner SEM, Roberts TK, Tinneberg HR. Intravenous immunoglobulin (IVIG): modes of action in the clinical management of recurrent pregnancy loss (RPL) and selected autoimmune disorders. Clin Chem Lab Med. (2004) 42:359–70. doi: 10.1515/cclm.2004.065
31. Atik RB, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018:13. doi: 10.1093/hropen/hoy004
32. Wang SW, Zhong SY, Lou LJ, Hu ZF, Sun HY, Zhu HY. The effect of intravenous immunoglobulin passive immunotherapy on unexplained recurrent spontaneous abortion: a meta-analysis. Reprod Biomed Online. (2016) 33:720–36. doi: 10.1016/j.rbmo.2016.08.025
33. Shi YM, Tan DM, Hao BZ, Zhang XR, Geng W, Wang YY, et al. Efficacy of intravenous immunoglobulin in the treatment of recurrent spontaneous abortion: A systematic review and meta-analysis. Am J Reprod Immunol. (2022) 88:e13615. doi: 10.1111/aji.13615
34. Yu XM, He L. Aspirin and heparin in the treatment of recurrent spontaneous abortion associated with antiphospholipid antibody syndrome: A systematic review and meta-analysis. Exp Ther Med. (2021) 21:57. doi: 10.3892/etm.2020.9489
35. Zhang TY, Ye XF, Zhu TT, Xiao X, Liu YZ, Wei X, et al. Antithrombotic treatment for recurrent miscarriage bayesian network meta-analysis and systematic review. Medicine. (2015) 94:e1732. doi: 10.1097/md.0000000000001732
36. Yang ZY, Shen XL, Zhou CQ, Wang M, Liu Y, Zhou L. Prevention of recurrent miscarriage in women with antiphospholipid syndrome: A systematic review and network meta-analysis. Lupus. (2021) 30:70–9. doi: 10.1177/0961203320967097
37. Li L, Dou LX, Leung PC, Chung TKH, Wang CC. Chinese herbal medicines for unexplained recurrent miscarriage. Cochrane Database Systemat Rev. (2016) 45:Cd010568. doi: 10.1002/14651858.CD010568.pub2
38. Coulam CB. Intralipid treatment for women with reproductive failures. American Journal of Reproductive Immunology. American Journal of Reproductive Immunology (2021) 85(5):. doi: 10.1111/aji.13290
39. Plaçais L, Kolanska K, Ben Kraiem Y, Cohen J, Suner L, Bornes M, et al. Intralipid therapy for unexplained recurrent miscarriage and failure: Case-series and literature review. Eur J Obstet Gynecol Reprod Biol. (2020) 252:100–4. doi: 10.1016/j.ejogrb.2020.06.017
40. Ahmadi M, Aghdam SA, Nouri M, Babaloo Z, Farzadi L, Ghasemzadeh A, et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: Non randomized, open-label clinical trial. Immunol Lett. (2017) 192:12–9. doi: 10.1016/j.imlet.2017.10.003
41. Carp HJA, Toder V, Gazit E, Ahiron R, Torchinski A, Mashiach S, et al. Further experience with intravenous immunoglobulin in women with recurrent miscarriage and a poor prognosis. Am J Reprod Immunol. (2001) 46:268–73. doi: 10.1034/j.1600-0897.2001.d01-12.x
42. Ahmadi M, Ghaebi M, Abdolmohammadi-Vahid S, Abbaspour-Aghdam S, Hamdi K, Abdollahi-Fard S, et al. NK cell frequency and cytotoxicity in correlation to pregnancy outcome and response to IVIG therapy among women with recurrent pregnancy loss. J Cell Physiol. (2019) 234:9428–37. doi: 10.1002/jcp.27627
43. Ramos-Medina R, García-Segovia A, Gil J, Carbone J, de la Cruz AA, Seyfferth A, et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am J Reprod Immunol. (2014) 71:458–66. doi: 10.1111/aji.12217
44. Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, et al. Placebo-controlled trial of treatment of unexplained secondary recurrent spontaneous-abortions and recurrent late spontaneous-abortions with iv immunoglobulin. Hum Reprod. (1995) 10:2690–5. doi: 10.1093/oxfordjournals.humrep.a135769
45. Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod. (2002) 17:809–16. doi: 10.1093/humrep/17.3.809
Keywords: intravenous immunoglobulin, unexplained recurrent spontaneous abortion, antiphospholipid syndrome, treatment, meta-analysis
Citation: Ling Q, Xu J, Tian Y, Chen D, Mo C and Peng B (2024) Effect of IVIG therapy on pregnant women with unexplained recurrent spontaneous abortion: a systematic review and meta-analysis. Front. Endocrinol. 15:1381461. doi: 10.3389/fendo.2024.1381461
Received: 13 February 2024; Accepted: 29 July 2024;
Published: 14 August 2024.
Edited by:
Johannes Ott, Medical University of Vienna, AustriaReviewed by:
Keiichi Matsubara, Ehime University, JapanTatiana Panafidina, V.A.Nasonova Research Institute of Rheumatology, Russia
Fatemeh Rezaei-Tazangi, Fasa University of Medical Sciences, Iran
Copyright © 2024 Ling, Xu, Tian, Chen, Mo and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Peng, cGVuZ2JpbmdAc2N1LmVkdS5jbg==; Chunheng Mo, Y2h1bmhlbmdtb0BnbWFpbC5jb20=; Y2h1bmhlbmdtb0BzY3UuZWR1LmNu
†These authors share first authorship
 Qiao Ling
Qiao Ling Jinfeng Xu
Jinfeng Xu Yuan Tian1,3
Yuan Tian1,3 Chunheng Mo
Chunheng Mo Bing Peng
Bing Peng