- 1Department of Diabetes and Endocrine Medicine, Kagoshima City Hospital, Kagoshima, Japan
- 2Department of Diabetes and Endocrine Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan
This study aimed to determine the efficacy of assessing the severity of diabetic polyneuropathy (DPN) in patients with untreated diabetes. Seventy-two patients with untreated type 2 diabetes who were hospitalized for glycemic control were enrolled and divided into the following two groups: patients who had no prior diagnosis and patients who were unattended or had discontinued treatment. Electrophysiological criteria consistent with Baba’s classification were used to diagnose and assess the severity of DPN. The patients were divided into three subgroups: no DPN (stage 0), mild DPN (stage 1), and moderate or more-severe DPN (stages 2–4). Intergroup comparisons were performed for the clinical characteristics and the results of the nerve conduction studies. Twenty-two (30%), 25 (35%), and 25 (35%) patients were categorized into the no DPN, mild DPN, and moderate or more-severe DPN subgroups, respectively. The number of patients who were unattended or had discontinued treatment in the moderate or more-severe DPN subgroup was significantly higher than that in the no DPN subgroup. The patients in the moderate or more-severe DPN subgroup had an increased risk of developing diabetic retinopathy and nephropathy, with odds ratios of 19.5 and 11.0 for advanced stages of retinopathy and nephropathy, respectively. Thus, the assessment of the severity of DPN could aid in the prediction of the risk of developing diabetic complications in patients with untreated diabetes.
1 Introduction
The prevalence of type 2 diabetes has been increasing in Japan, with a national survey estimating that approximately 10 million individuals in Japan have diabetes (1). Approximately 23% of patients with diabetes (21.3% of men and 25.9% of women) are untreated (1). Patients with type 2 diabetes often experience few subjective symptoms; consequently, diabetes often remains undetected, and diabetic complications tend to progress without being detected (2, 3). A review of the clinical background of patients with advanced diabetic retinopathy at presentation revealed that 107 individuals, including 65 patients with untreated diabetes (22 were unattended, 20 had discontinued treatment, and 23 had no prior diagnosis) (4), had pre-proliferative or proliferative diabetic retinopathy.
Diabetic polyneuropathy (DPN) is the most prevalent chronic complication of diabetes that occurs during the earliest stages of the disease (5, 6). The early stages of DPN are associated with few subjective symptoms, with generally only mild numbness or abnormal sensations below the knee and in both legs; however, as DPN progresses, it becomes a serious complication that causes neuropathic pain, autonomic neuropathy, diabetic foot, and sudden death. Diabetic bladder dysfunction caused by autonomic neuropathy, occurs with relatively frequency. This can present as incomplete bladder emptying, an increased postvoid residual, decreased peak urinary flow rate, bladder overdistention, and urine retention, which can lead to urinary tract infections (7, 8). Severe DPN has a significant impact on the quality of life and prognosis of patients with diabetes. Baba et al. proposed the use of a combination of sural sensory nerve action potential (SNAP) amplitude, tibial compound muscle action potential (CMAP) amplitude, and velocity system indices [such as tibial F-wave latency, tibial motor nerve conduction velocity (MCV), and sural sensory nerve conduction velocity (SCV) in the lower limb] for the diagnosis of the severity of DPN (9). The severity of DPN diagnosed according to this classification was associated with the prognosis of patients with diabetes in a recent 5-year prospective study (10).
The association between diabetic retinopathy and diabetic neuropathy is correlated with the severity of DPN and alterations in retinal thickness (11). Thus, evaluating the severity of DPN at the initial visit may be essential in all diabetic patients. Evaluating the severity of DPN may be especially useful in predicting the prognosis of patients with untreated diabetes at high risk of developing diabetic complications. Therefore, this study aimed to investigate the severity of DPN in patients with untreated diabetes and examine the association between the development of diabetic complications and the severity of DPN.
2 Materials and methods
2.1 Patients
This study was conducted at the Kagoshima City Hospital, Kagoshima, Japan, between June 2015 and May 2021. Among the 3370 patients with type 2 diabetes who visited our department for the first time, 649 patients were untreated. Among these 649 patients, 117 were hospitalized for the management of hyperglycemia during the study period, and 72 (28 women and 44 men) participated in the current study. Forty-five patients, comprising 38 patients who did not undergo nerve conduction studies (NCSs), six patients aged >75 years, and one patient with a neuromuscular disease, were excluded from the study. All patients underwent routine biochemical and hematological tests and screening for diabetic complications. In addition, drug treatment was also analyzed after hospitalization. The untreated patients were divided into two groups: patients with no prior diagnosis and patients who were unattended or had discontinued treatment. Patients classified as having no prior diagnosis comprised those who i) had not previously been diagnosed with diabetes following any physical examination or hospital visit and ii) those who had not undergone a physical examination and were diagnosed with diabetes for the first time on visiting our department. Unattended patients were defined as patients diagnosed with diabetes at a medical checkup who did not initiate treatment. Discontinuation of treatment was defined as a failure to visit the hospital for ≥1 year after commencing treatment.
2.2 Nerve conduction study
All patients underwent conventional sensory and motor NCSs. The median, ulnar, tibial, and sural nerves of the upper and lower extremities were examined. NCSs were performed using a standard electromyographic device with stimulating and recording electrodes (Viking Select; Nicolet Biomedical Japan, Tokyo, Japan). The skin temperature was maintained above 32°C and at 31°C on the forearm and mid-leg, respectively. The American Academy of Electrodiagnostic Medicine defines DPN as a distal symmetric sensorimotor polyneuropathy. Patients with abnormalities in any attribute of nerve conduction in two separate nerves, one of which must be the sural nerve, were diagnosed with DPN (12). The classification proposed by Baba was also used as a diagnostic reference in addition to the authorized diagnostic criterion. DPN was classified into five stages based on the severity classification of DPN in this classification system: stage 0, normal with no NCS abnormalities; stage 1, mild neuropathy with tibial MCV of <42 m/s, sural SCV of <42 m/s, tibial minimal F-wave latency (> [12.8 + 0.22 × height (cm)] ms), or the presence of an A wave; stage 2, moderate neuropathy with a sural SNAP amplitude of <5 µV; stage 3, moderate-to-severe neuropathy with a sural SNAP amplitude of <5 µV and a tibial CMAP amplitude ranging between ≥2 and <5 mV; and stage 4, severe neuropathy with a sural SNAP amplitude of <5 µV and a tibial CMAP amplitude of <2 mV (13). The patients were further divided into the following three subgroups according to these grades: no DPN (stage 0), mild DPN (stage 1), and moderate or more-severe DPN (stages 2–4). Intergroup comparisons were performed for the clinical characteristics of the patients and the NCS results.
2.3 Statistical analysis
Data are presented as the mean and standard deviation. Excel 2021 (Microsoft, Redmond, WA, USA) with the add-in software Statcel 3 (OMS, Tokyo, Japan) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) were used to perform all statistical analyses (14). Significant differences among the three groups were compared using one-way analysis of variance and the Tukey–Kramer or Steel–Dwass post-hoc test. The adjusted odds ratios with the 95% confidence intervals (CIs) were calculated using the logistic regression model to determine the risk of developing diabetic retinopathy and diabetic nephropathy. A P-value of <0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics of the patients
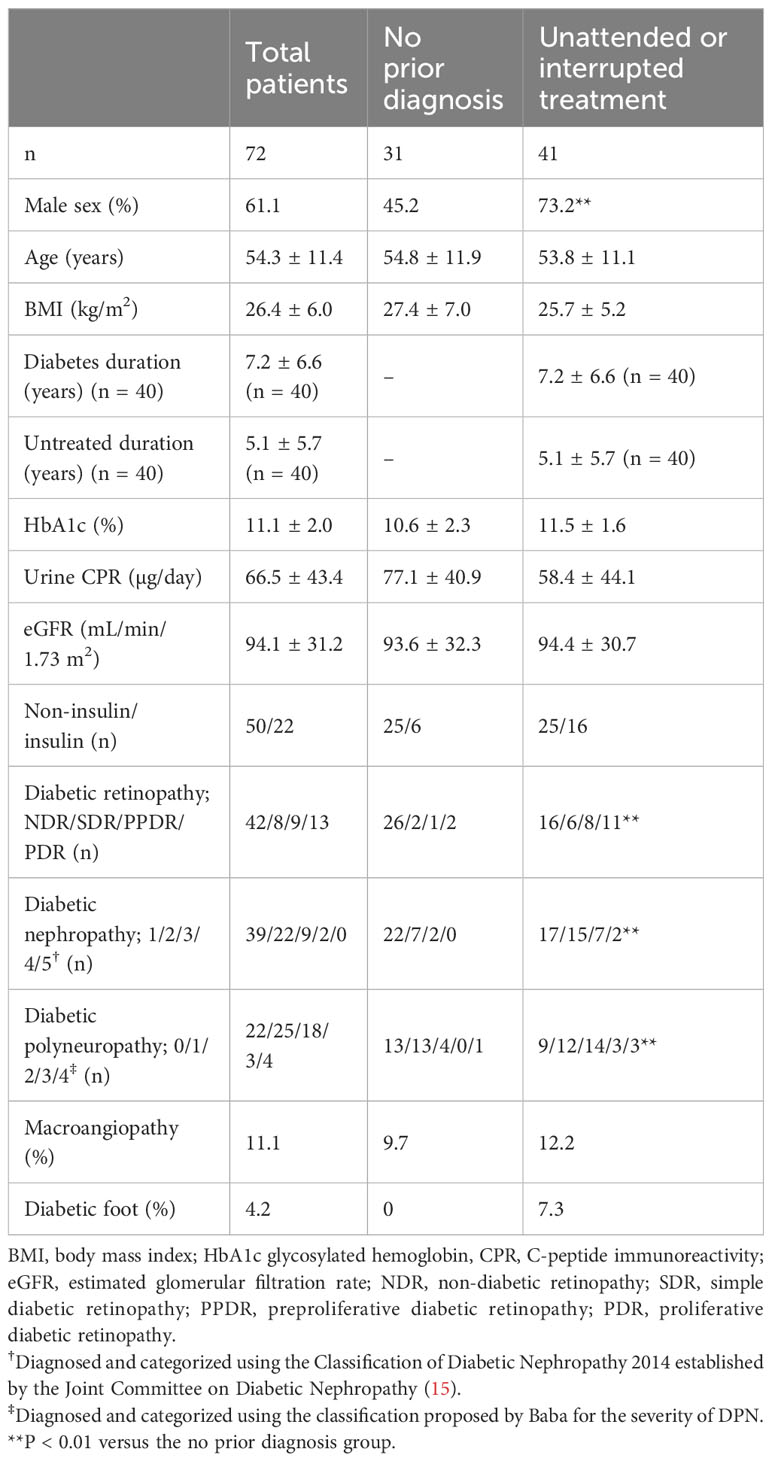
Table 1 presents the demographic data of the 72 participants. The mean age of the participants was 54.3 ± 11.4 years. The mean duration of diabetes was 7.2 ± 6.6 years. Thirty-one of the 72 participants had no prior diagnosis of diabetes. The remaining 41 were unattended or had discontinued treatment. The mean glycosylated hemoglobin (HbA1c) level was 11.1% ± 2.0%. Retinopathy, nephropathy, and macroangiopathy were detected in 30 (42%), 33 (46%), and eight (11%) patients, respectively. Three (4%) patients had diabetic foot. Twenty-two (30%), 25 (35%), 18 (25%), three (4%), and four (6%) patients were categorized as having no DPN (stage 0), mild DPN (stage 1), moderate DPN (stage 2), moderate to severe DPN (stage 3), and severe DPN (stage 4), respectively. The proportion of males among patients who were unattended or had discontinued treatment was significantly higher. The participants who were unattended or had discontinued had more advanced stages of diabetic retinopathy, diabetic nephropathy, or DPN than the participants with no prior diagnosis (all P < 0.01).
3.2 Clinical characteristics of the patients stratified according to the classification proposed by Baba
Table 2 presents the clinical characteristics of the 72 patients stratified according to the classification proposed by Baba (9, 13). The diagnosis was consistent with the authorized diagnostic criteria and the classification proposed by Baba in all cases. No significant differences were observed among the three subgroups in terms of the estimated glomerular filtration rate or the prevalence of macroangiopathy or diabetic foot. The body mass index and lower urinary C-peptide immunoreactivity of the patients with moderate or more-severe DPN were significantly lower than those of the patients without DPN (both P < 0.01). The HbA1c values of the patients with moderate or more-severe DPN were significantly higher than those in the no DPN subgroup (P < 0.05). The proportion of patients who were unattended or had interrupted treatment in the moderate or more-severe-DPN group was significantly higher than that in the no-DPN group (P < 0.05). The use of insulin was similar among all three subgroups. The stages of diabetic retinopathy and diabetic nephropathy in the moderate or more-severe-DPN subgroup were significantly more advanced than those in the no-DPN and mild-DPN subgroups (retinopathy: both P < 0.01, nephropathy: P < 0.01 for no DPN and P < 0.05 for mild DPN). Other than age and HbA1c values, no significant differences were observed between the no DPN and mild-DPN subgroups in terms of clinical presentation.
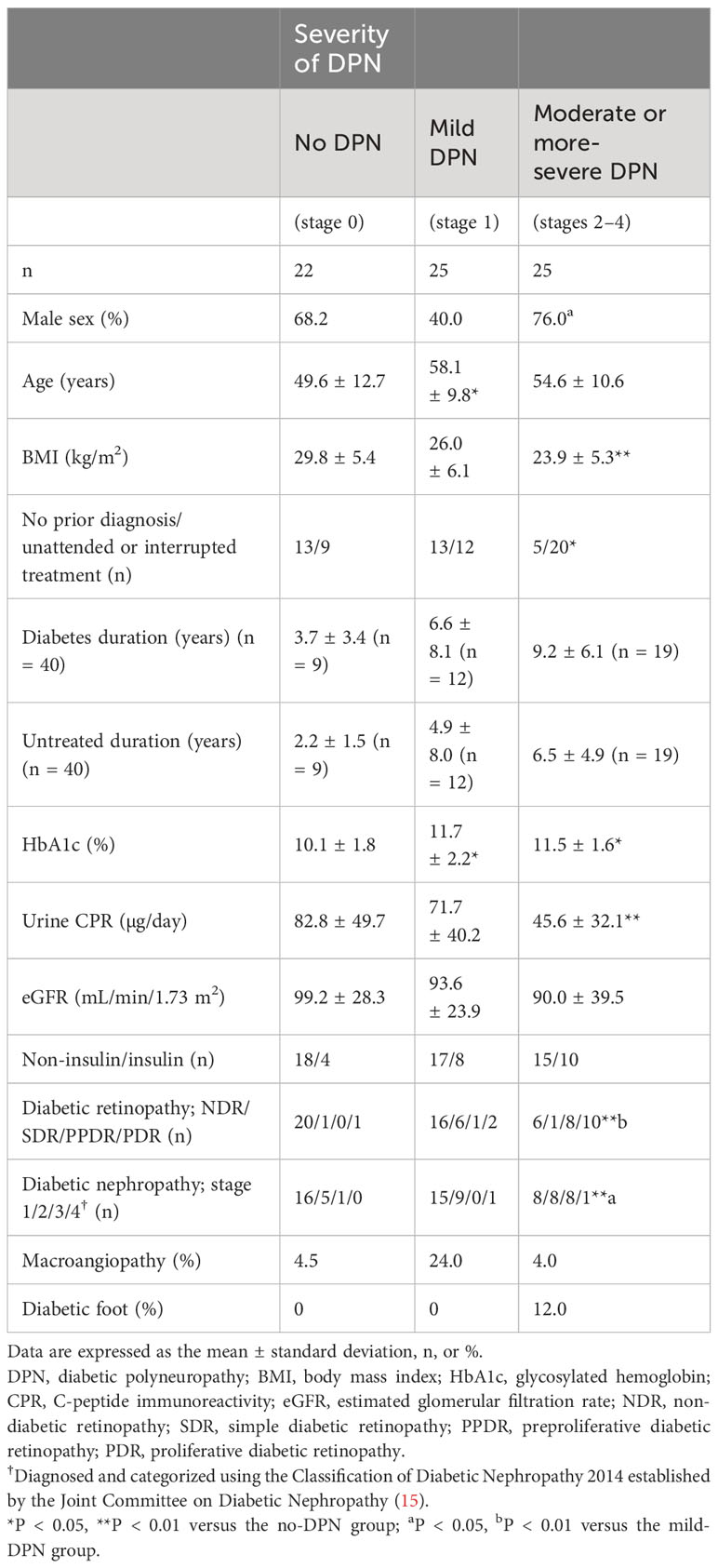
Table 2 Clinical characteristics of the patients stratified according to the classification proposed by Baba.
3.3 NCS parameters
Table 3 presents the results of the lower limb NCS for the 72 participants. The tibial MCV and sural SCV of the moderate or more-severe-DPN subgroup were significantly lower than those of the no-DPN and mild-DPN subgroups (P < 0.01). The tibial F-wave latency of the moderate or more-severe-DPN subgroup was significantly longer than those of the no-DPN and mild-DPN subgroups (P < 0.01). The amplitudes of the tibial CMAP and sural SNAP in the moderate or more severe-DPN subgroup were significantly smaller than those in the no-DPN and mild-DPN groups (CMAP: P < 0.01 for no DPN and P < 0.05 for mild DPN, SNAP: both P < 0.01). The tibial MCV was significantly lower, the tibial F-wave latency was significantly longer, and the sural SNAP was smaller in the mild-DPN group than those in the no-DPN group (all P < 0.01).
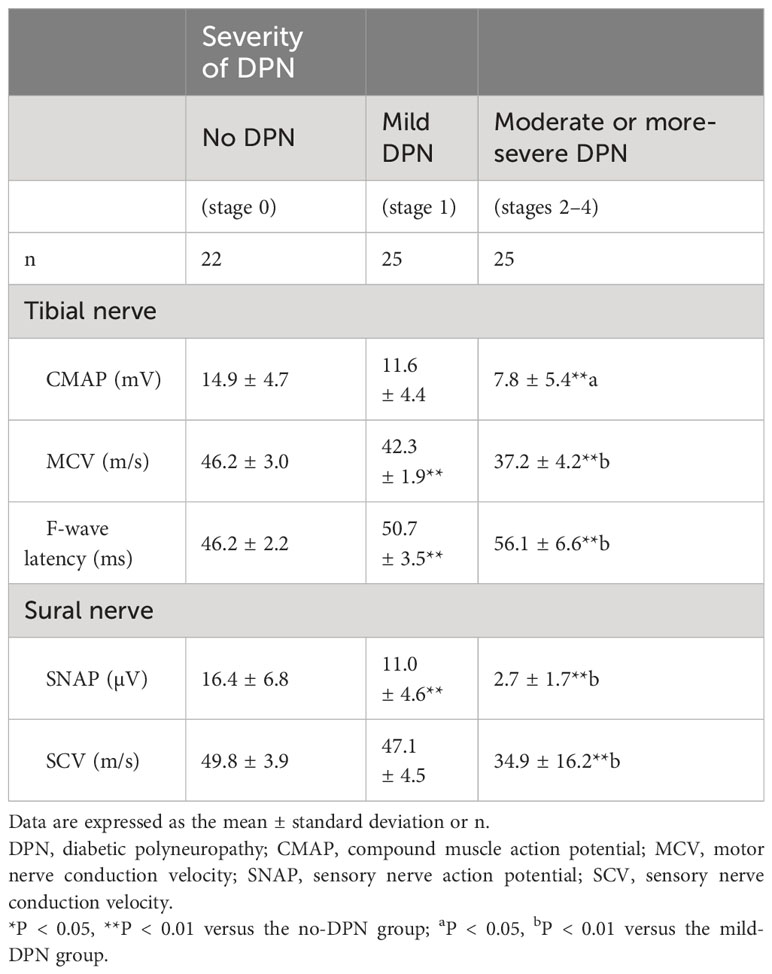
Table 3 Comparison of the results of the lower limb nerve conduction studies in patients stratified according to the classification proposed by Baba.
3.4 Risk of developing retinopathy or nephropathy in multivariate logistic regression analysis
The risk of developing diabetic retinopathy and diabetic nephropathy among patients who were unattended or had discontinued treatment was calculated as odds ratios after adjusting for the potential confounder of diabetes duration (Table 4). The risk of developing diabetic retinopathy was higher in the moderate or more-severe-DPN subgroup, with an adjusted odds ratio of 7.56 (95% CI: 1.590–36.0, P = 0.011). The moderate or more-severe DPN subgroup also showed significant associations with the risk of progression of retinopathy and nephropathy, with adjusted odds ratios of 19.5 (95% CI: 3.660–104.0, P < 0.001) and 11.0 (95% CI: 1.150–106.0, P = 0.037) for retinopathy and nephropathy, respectively. The duration of diabetes showed no associations with the risk of developing diabetic retinopathy or diabetic nephropathy.
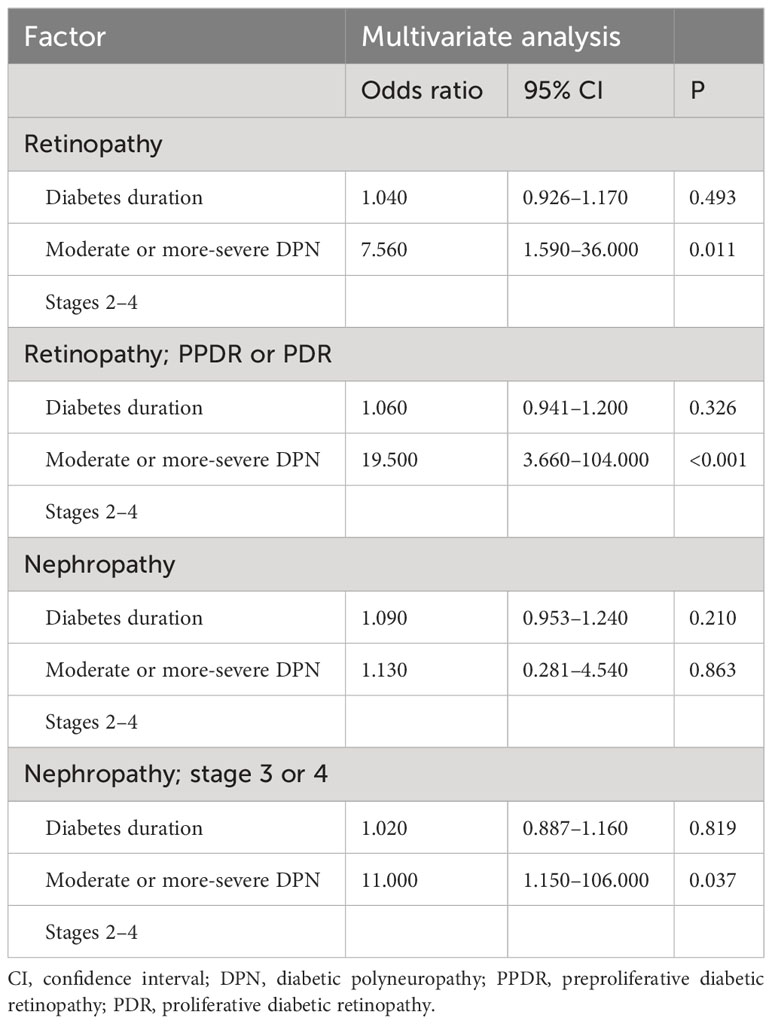
Table 4 Risk of developing retinopathy or nephropathy in the multivariate logistic regression analysis.
4 Discussion
To the best of our knowledge, this study is the first to investigate the validity of assessing the severity of DPN for predicting the risk of developing diabetic complications in patients with untreated diabetes. The present study revealed that the risk of developing diabetic complications was related to the DPN severity classification system proposed by Baba. Patients with moderate or more-severe DPN had a higher risk of developing advanced stages of diabetic retinopathy and diabetic nephropathy.
The Michigan Neuropathy Screening Instrument (16) and the Toronto Consensus (17) have been used for the diagnosis of DPN worldwide. The use of the simplified diagnostic criteria of the Japanese Study Group on Diabetic Neuropathy (18) has been recommended in routine practice in Japan. DPN was detected in 35.8% of patients with type 2 diabetes in Japan according to these criteria in a previous study (19). However, these criteria comprise the physical signs and symptoms of peripheral neuropathy and cannot be used to determine the severity of DPN.
NCSs provide the most objective and quantitative method for diagnosing DPN (20). DPN was detected in 70% of patients with poorly controlled untreated diabetes via NCSs in the present study. The prevalence of mild DPN was 35%. The prevalence of moderate or more-severe DPN was also 35%. The number of patients with stage 3 and 4 disease was small; however, the frequency of severe DPN among the participants was comparable with that reported in other Japanese studies (21, 22). This finding indicates the high prevalence of DPN among patients with untreated diabetes and suggests that NCS shows excellent sensitivity for the detection of DPN. However, NCSs require expensive equipment and an advanced examination technique. Moreover, they are only available in limited facilities at present. Consequently, a point-of-care device, DPNCheck™ (NeuroMetrix Inc., Waltham, MA, USA), was developed to test only the sural nerve to overcome the lack of versatility of NCSs (23, 24). The results of DPNCheck™ are highly reproducible and correlate well with a standard electromyographic system (25, 26). Kamiya et al. developed a multiple regression model to predict the severity of DPN based on the classification system proposed by Baba that used sural nerve conduction data in DPNCheck™ (21). The model could effectively diagnose moderate or more-severe DPN (stages 2–4) (21). The use of this model may facilitate the assessment of the severity of DPN in facilities where NCSs cannot be performed.
The prevalence of severe diabetic complications has been increasing among patients who do not visit a physician owing to social and economic reasons. A lack of understanding of treatment priorities, lack of awareness regarding the disease, and financial burdens have been identified as reasons for discontinuing treatment (27). Severe complications from proliferative diabetic retinopathy, such as tractional retinal detachment, tend to develop in patients who discontinue treatment (28). More effective medical care and guidance can be provided by identifying untreated patients at a high risk of diabetic complications at the initial visit. Assessing the severity of DPN aids in predicting the risk of developing diabetic complications and preventing the progression of complications and the development of diabetic foot. However, owing to the difficulty in implementing NCSs, their use for the prediction of retinopathy or nephropathy is not feasible. Thus, objective assessment of the severity of DPN using DPNCheck™ in various medical settings could aid in the detection of high-risk patients and the prevention of severe diabetes in the future.
This study is limited by its single-center design and relatively small sample size. The number of patients with stage 3 and 4 disease was insufficient to facilitate statistical analyses; consequently, differences among stages 0, 1, and 2–4 were analyzed. In addition, the analysis could not distinguish moderate DPN from moderate-to-severe or severe DPN. Participants with moderate-to-severe or severe DPN may be at increased risk of developing diabetic complications, including diabetic foot. Therefore, the generalizability of these findings may be limited, and additional data must be accumulated on participants with advanced DPN. Further studies with large sample sizes are required to determine the relevance of the findings of the present study more accurately.
In conclusion, assessment of the severity of DPN aids in the prediction of the risk of developing diabetic complications in patients with untreated diabetes. These findings could be used as guidelines to identify patients with untreated diabetes at high risk of developing diabetic complications at the initial visit to the hospital.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Kagoshima City Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SH: Writing – review & editing, Writing – original draft. TD: Writing – review & editing. MM: Writing – review & editing. AI: Writing – review & editing. YK: Writing – review & editing. YN: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ministry of Health. Labour and Welfare (Japan) (2016). Available online at: https://www.mhlw.go.jp/bunya/kenkou/eiyou/h28-houkoku.html (Accessed August 8, 2018).
2. Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. (1992) 15:815–9. doi: 10.2337/diacare.15.7.815
3. Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care. (1993) 16:642–52. doi: 10.2337/diacare.16.4.642
4. Gemma R, Kitahara K, Kono E, Imoto M, Ikeya A, Iwaki H, et al. Clinical background factors of patients with advanced diabetic retinopathy at the first presentation. J Japan Diabetes Soc. (2015) 58:192–7. doi: 10.11213/tonyobyo.58.192
5. Dyck PJ, Herrmann DN, Staff NP, Dyck PJ. Assessing decreased sensation and increased sensory phenomena in diabetic polyneuropathies. Diabetes. (2013) 62:3677–86. doi: 10.2337/db13-0352
6. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. (2017) 40:136–54. doi: 10.2337/dc16-2042
7. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. (2003) 26:1553–79. doi: 10.2337/diacare.26.5.1553
8. Micle O, Antal L, Naghi P, Tica O, Zaha DC, Zdrîncă MM, et al. The prevalence of urinary tract infections in pregnancy and implications on foetal development. Farmacia. (2020) 68:463–9. doi: 10.31925/farmacia.2020.3.11
9. Baba M, Suzuki C. Electrophysiological grading of diabetic polyneuropathy by nerve conduction study. J Clin Exp Med. (2013) 41:143–50. doi: 10.11422/jscn.41.143
10. Baba M, Suzuki C, Ogawa Y. Severity grading system of diabetic neuropathy in type-2 diabetes by nerve conduction study: five-year prospective study on occurrence of diabetic foot, macroangiopathic events, and eventual death. Jpn J Clin Neurophysiol. (2018) 46:71–7. doi: 10.11422/jscn.46.71
11. Yamada Y, Himeno T, Tsuboi K, Shibata Y, Kawai M, Asada-Yamada Y, et al. Alterations of retinal thickness measured by optical coherence tomography correlate with neurophysiological measures in diabetic polyneuropathy. J Diabetes Investig. (2021) 12:1430–41. doi: 10.1111/jdi.13476
12. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. (2005) 64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA
13. Himeno T, Kamiya H, Nakamura J. Lumos for the long trail: strategies for clinical diagnosis and severity staging for diabetic polyneuropathy and future directions. J Diabetes Investig. (2020) 11:5–16. doi: 10.1111/jdi.13173
14. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
15. Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, et al. A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig. (2015) 6:242–6. doi: 10.1111/jdi.12319
16. Feldman EL, Stevens MU, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. (1994) 17:1281–9. doi: 10.2337/diacare.17.11.1281
17. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10-1303
18. Yasuda H, Sanada M, Kitada K, Terashima T, Kim H, Sakaue Y, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract. (2007) 77:S178–83. doi: 10.1016/j.diabres.2007.01.053
19. Satoh J, Baba M, Yagihashi S, Suda T, Tominaga M, Daimon M, et al. Frequency of diabetic polyneuropathy (DPN) and clinical significance of Achilles tendon reflex in diagnosis of DPN -survey of 15,000 patients in Tohoku, Japan. J Japan Diabetes Soc. (2007) 50:799–806. doi: 10.11213/tonyobyo.50.799
20. Kohara N, Kimura J, Kaji R, Goto Y, Ishii J, Takiguchi M, et al. F-wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia. (2000) 43:915–21. doi: 10.1007/s001250051469
21. Kamiya H, Shibata Y, Himeno T, Tani H, Nakayama T, Murotani K, et al. Point-of-care nerve conduction device predicts the severity of diabetic polyneuropathy: A quantitative, but easy-to-use, prediction model. J Diabetes Investig. (2021) 12:583–91. doi: 10.1111/jdi.13386
22. Mikura K, Kodama E, Iida T, Imai H, Hashizume M, Kigawa Y, et al. Association between sarcopenia and the severity of diabetic polyneuropathy assessed by nerve conduction studies in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. (2022) 13:1357–65. doi: 10.1111/jdi.13788
23. Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care. (2006) 29:2023–7. doi: 10.2337/dc08-0500
24. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PloS One. (2014) 9:e86515. doi: 10.1371/journal.pone.0086515
25. Hirayasu K, Sasaki H, Kishimoto S, Kurisu S, Noda K, Ogawa K, et al. Difference in normal limit values of nerve conduction parameters between Westerners and Japanese people might need to be considered when diagnosing diabetic polyneuropathy using a point-of-care sural nerve conduction device (NC-stat®/DPNCheck™). J Diabetes Investig. (2018) 9:1173–81. doi: 10.1111/jdi.12818
26. Shibata Y, Himeno T, Kamiya T, Tani H, Nakayama T, Kojima C, et al. Validity and reliability of a point-of-care nerve conduction device in diabetes patients. J Diabetes Investig. (2019) 10:1291–8. doi: 10.1111/jdi.13007
27. Japanese Practice Guidance to Improve Patients’ Adherence to Appointments for Diabetes Care (2019). Available online at: https://human-data.or.jp/wp/wp-content/uploads/2018/07/dm_jushinchudan_guide43_e.pdf (Accessed July 15, 2019).
28. Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. (2019) 126:407–13. doi: 10.1016/j.ophtha.2018.07.027
Keywords: diabetic polyneuropathy, Baba’s classification, nerve conduction study, diabetic complications, untreated diabetes
Citation: Horinouchi S, Deguchi T, Mukai M, Ijuin A, Kawamoto Y and Nishio Y (2024) Assessment of the severity of diabetic polyneuropathy aids in predicting the risk of developing diabetic complications in patients with untreated diabetes. Front. Endocrinol. 15:1380970. doi: 10.3389/fendo.2024.1380970
Received: 02 February 2024; Accepted: 04 March 2024;
Published: 15 March 2024.
Edited by:
Xuebin Fu, Ann & Robert H. Lurie Children’s Hospital of Chicago, United StatesCopyright © 2024 Horinouchi, Deguchi, Mukai, Ijuin, Kawamoto and Nishio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuji Horinouchi, c2h1aG9yaTUwMUB5YWhvby5jby5qcA==
 Shuji Horinouchi
Shuji Horinouchi Takahisa Deguchi
Takahisa Deguchi Miki Mukai1
Miki Mukai1