
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 17 June 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1380929
This article is part of the Research TopicUnderstanding and Managing Diabetic Neuropathy: Current Perspectives and Future DirectionsView all 9 articles
 Aysegul Atmaca1*
Aysegul Atmaca1* Aysegul Ketenci2
Aysegul Ketenci2 Ibrahim Sahin3
Ibrahim Sahin3 Ihsan Sukru Sengun4
Ihsan Sukru Sengun4 Ramazan Ilyas Oner5
Ramazan Ilyas Oner5 Hacer Erdem Tilki6
Hacer Erdem Tilki6 Mine Adas7
Mine Adas7 Hatice Soyleli8
Hatice Soyleli8 Tevfik Demir9
Tevfik Demir9The proposed expert opinion aimed to address the current knowledge on conceptual, clinical, and therapeutic aspects of diabetic peripheral neuropathy (DPN) and to provide a guidance document to assist clinicians for the best practice in DPN care. The participating experts consider the suspicion of the disease by clinicians as a key factor in early recognition and diagnosis, emphasizing an improved awareness of the disease by the first-admission or referring physicians. The proposed “screening and diagnostic” algorithm involves the consideration of DPN in a patient with prediabetes or diabetes who presents with neuropathic symptoms and/or signs of neuropathy in the presence of DPN risk factors, with careful consideration of laboratory testing to rule out other causes of distal symmetric peripheral neuropathy and referral for a detailed neurological work-up for a confirmative test of either small or large nerve fiber dysfunction in atypical cases. Although, the first-line interventions for DPN are currently represented by optimized glycemic control (mainly for type 1 diabetes) and multifactorial intervention (mainly for type 2 diabetes), there is a need for individualized pathogenesis-directed treatment approaches for DPN. Alpha-lipoic acid (ALA) seems to be an important first-line pathogenesis-directed agent, given that it is a direct and indirect antioxidant that works with a strategy targeted directly against reactive oxygen species and indirectly in favor of endogenous antioxidant capacity for improving DPN conditions. There is still a gap in existing research in the field, necessitating well-designed, robust, multicenter clinical trials with sensitive endpoints and standardized protocols to facilitate the diagnosis of DPN via a simple and effective algorithm and to track progression of disease and treatment response. Identification of biomarkers/predictors that would allow an individualized approach from a potentially disease-modifying perspective may provide opportunities for novel treatments that would be efficacious in early stages of DPN, and may modify the natural course of the disease. This expert opinion document is expected to increase awareness among physicians about conceptual, clinical, and therapeutic aspects of DPN and to assist them in timely recognition of DPN and translating this information into their clinical practice for best practice in the management of patients with DPN.
The International Diabetes Federation 2021 report considers diabetes a fast-growing global pandemic of the 21st century, estimating an increase in the prevalence from 10.5% (536.6 million people) in 2021 to 12.2% (783.2 million people) in 2045 (1).
The continuing rise in diabetes and prediabetes burden worldwide is expected to manifest itself also in the form of increasing prevalence of chronic diabetes-related complications, such as diabetic nephropathy, within the next decades (2–5). Diabetic neuropathy is one of the most common and troublesome complications of both type 1 diabetes (T1D) and type 2 diabetes (T2D), while patients with prediabetes may also develop diabetic neuropathy, which ultimately advances upon transition to frank T2D (2–5).
Neuropathy in diabetes constitutes a heterogeneous group of disorders that differ with respect to clinical presentations and the pathophysiological background, which can be categorized as “diffuse or symmetrical” neuropathies (distal symmetrical polyneuropathy, autonomic and acute sensory neuropathy) and “focal or multifocal” neuropathies (radiculoplexus neuropathy, entrapment syndromes, cranial palsies and other mononeuropathies) (2–4). Still, patients with diabetes may also develop non-diabetic neuropathies (pressure palsies, acute treatment-induced painful small fiber neuropathies and chronic inflammatory demyelinating polyneuropathy) (2, 6).
By far, the most common diabetic neuropathy is distal symmetric polyneuropathy (also known as typical diabetic neuropathy), which we will primarily focus on and refer to as diabetic peripheral neuropathy (DPN) in this review (3, 7). Accounting for approximately 75% of all diabetic neuropathies, DPN affects up to 50% of T1D and T2D patients and at least 10% of prediabetic patients, while there is heterogeneity and wide variation in prevalence estimates depending on the applied diagnostic methodology (4, 8–12). DPN refers to damage to peripheral nerves including characteristic glove and stocking-like presentation of distal sensory or motor function loss with or without neuropathic pain (3, 4, 7, 9, 13, 14).
Untreated DPN has substantial effects on patient morbidity and quality of life (i.e., loss of limb sensation, falls, and increased risk of foot ulcers and lower limb amputations), while diabetic patients with DPN are also at higher risk of all-cause and cardiovascular mortality than those without DPN (3, 5, 7, 15–17).
Accordingly, early recognition and preventive measures are essential in DPN practice (3, 5, 7).
However, in contrast to other major diabetes complications (i.e., retinopathy and nephropathy), no single gold standard diagnostic test exists for DPN, along with diagnostic challenges particularly for diagnosing DPN early in the disease course (3).
Besides, the management of DPN primarily relies on improved glycemic control, which is more effective in T1D than in T2D, lifestyle and multifactorial risk interventions (mainly in T2D), while the symptomatic management in painful DN enables sufficient pain relief in less than one-third of patients (3, 4, 18). Hence, there is a need for specific disease-modifying treatments and standardized validated tools to identify risk groups in terms of sub-clinical neuropathy, disease mechanisms and treatment response that would enable the implementation of personalized treatment/mechanism-based approaches (4, 18).
Therefore, the proposed expert opinion aims to address the current knowledge on conceptual, clinical, and therapeutic aspects of DPN and to provide a practical guidance document to assist clinicians in the best practice in recognition, diagnosis, and management of the disease.
The present expert panel of four different specialties involved in the management of DPN (endocrinology, neurology, internal medicine and physical medicine and rehabilitation) with at least 15 years of experience in the management of DPN, convened a meeting to develop a consensus opinion on the conceptual, clinical and therapeutic aspects of DPN. The panel critically analyzed recommendations from existing guidelines and data from systematic reviews, meta-analyses and literature reviews of articles published on DPN and agreed on a series of statements supported by scientific evidence and expert clinical opinion to assist clinicians on best practices in recognition, diagnosis, and management of DPN. The proposed expert opinion planned to provide a practical and implementable guidance document addressing DPN in terms of:
a) definition, pathophysiology and risk factors,
b) clinical manifestations and diagnosis (current challenges, a proposed screening and diagnostic algorithm),
c) screening for early diagnosis and symptom progression, and
d) management of disease in terms of the optimal diabetes treatment (intensive glycemic control, lifestyle modification and multifactorial risk intervention), the pathogenetically oriented pharmacotherapy (with special emphasis on alpha lipoic acid), and the symptomatic pain relief (in painful DPN).
DPN is considered a symmetrical, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvascular alterations due to chronic hyperglycemia exposure (diabetes) and cardiovascular risk covariates (13, 19). A simpler definition for clinical practice can be ‘the presence of symptoms and/or signs of neuropathy that develops in the context of prediabetes or diabetes after the exclusion of other causes of peripheral neuropathy (20–22).
DPN progresses gradually with increasing duration of diabetes and in relation to glycemic control in both T1D and T2D (23, 24). Notably, while the prevalence of DPN is considered to be rather low within the first five years of disease onset in T1D, slowing of nerve conduction velocity may be one of the earliest neuropathic abnormalities in T2D, often present at diagnosis (23, 24). Hence, DPN should be suspected in all patients with prediabetes and T2D and those who have had T1D for more than five years (24, 25).
Although the exact pathophysiology of DPN remains unknown, the role of various signaling cascades has been proposed such as advanced glycation end-product (AGE) pathway, polyol pathway, protein kinase C (PKC) pathway, hexosamine pathway and poly (ADP ribose) polymerase (PARP) pathway (9, 26–29). Endothelial health is compromised by multiple factors such as glycosylation, hyperlipidemias, hyperhomocysteinemia, hypertension, excessive platelet activity, reduced nitric oxide and excessive generation of reactive oxygen species (ROS) (30, 31).
Nonetheless, chronic hyperglycemia is the main underlying cause of DPN, which leads to damage at the vascular level and the free passage of glucose to neurons and Schwann cells, resulting in an imbalance between nerve damage and nerve fiber repair (20, 32). All the proposed pathways in pathogenesis are activated with hyperglycemia, and they can directly or indirectly impair the redox capacity of the cell and increase the production of ROS (9, 26–29). With the accumulation of ROS (oxygen free radicals such as superoxide anion radical and hydroxyl radical), the endogenous antioxidant defense system (i.e., superoxide dismutase, catalase, glutathione ascorbate) fails to counteract ROS generation, resulting in an increase in oxidative stress (28, 33). The increased oxidative stress leads to impaired neural function, gradually heading to apoptosis in neurons and supporting glial cells (i.e., Schwann cells and satellite glial cells) of the peripheral nervous system (9, 26–29).
The causes of DPN are thought to be a multi-factorial metabolic process that increasingly deteriorates tissues. Hyperglycemia, increased sorbitol and protein kinase C, elevated homocysteine, reduced nitric oxide and excessive Reactive Oxygen Species (ROS) damage endothelial tissue and produce a rheological change that increases vascular resistance and reduces blood flow to the nerve
Diabetes is the strongest risk factor for DPN, along with certain disease characteristics such as diabetes duration and poor glycemic control in terms of fasting blood glucose (FBG), glycated hemoglobin (HbA1c; >7%), postprandial hyperglycemia and the glycemic variability (4, 7, 25, 34–39). Poor glycemic control is the most critical and principal modifiable risk factor for DPN (4, 5, 7, 25). Presence of symptoms and/or signs of distal symmetric polyneuropathy have also been reported in prediabetes although prevalence is variable among studies (40–42). A growing body of evidence supports an association between prediabetes and early small-fiber symptoms and the likelihood of neuropathy to be already developed in the prediabetic stage (7, 12). Given the stronger association of neuropathy with impaired glucose tolerance (IGT) rather than the impaired fasting glucose (IFG), post-load hyperglycemia seems to be the key mechanism for inducing increased oxidative stress, endothelial dysfunction, and activation of both PKC and the polyol pathway, leading to impaired neuronal metabolism and DNA damage in prediabetic stage (37, 43).
Other risk factors increasing the prevalence of DPN include the non-modifiable risk factors such as older age (>50 years), height (directly related to the length of the axon), female gender (particularly for painful DPN), comorbid diabetic retinopathy/nephropathy and positive HLA-DR3/4 genotype, and the modifiable risk factors including the hypertension, dyslipidemia, smoking, alcohol use, obesity, and vitamin D deficiency, vitamin B12 deficiency and low C-peptide levels (4, 7, 25, 32–37, 44–49) (Table 1). The recognition of risk factors is of critical importance in the timely identification of subclinical/early DPN for effective disease control and prevention of hazards (i.e., ulcer, gangrene, and amputation) and social burden (2, 32, 50).
DPN usually manifests as a length-dependent, distal-symmetrical, sensorimotor polyneuropathy (22, 51). Its onset is generally insidious, and the course is chronic and progressive without treatment (23). Symptoms at presentation can be either “positive”, including neuropathic pain (described as deep aching, burning, and sharp stabbing sensations), paresthesia and hyperesthesia, or “negative”, including loss of sensations (hypoesthesia), including different sensory modalities relating to small fiber (temperature, pain) and large fiber (touch, pressure, vibration, position) functions and ataxic gait (6, 20, 22, 52, 53).
Alternatively, some patients may be completely asymptomatic, and signs may be only revealed by a detailed neurological examination (6, 22, 23), while some others may present with rare, atypical diabetic neuropathies with distinct features and underlying mechanisms, as well as with neuropathies attributed to causes other than diabetes (6, 54).
In accordance with the higher vulnerability of lower-limb long axons to injury, DPN usually develops first in the feet and then progresses proximally involving the upper limbs (dying-back type of axonal degeneration), and patients typically present with a “stocking-glove” like distribution of neuronal dysfunction (20, 22, 51).
Overall, up to 50% of affected subjects do not report symptoms, and up to one fourth of patients develop painful DPN which is particularly associated with physical and psychosocial impairment, disability, and reduced health-related quality of life (20–22, 24, 25, 55).
Most patients show a “mixed” neuropathy with both large and small nerve fiber damage and all patients with DPN are considered to be at increased risk of neuropathic complications such as foot ulceration and Charcot’s neuroarthropathy as well as the falls and fractures (20, 23–25).
Despite its major impact on morbidity and mortality, DPN is frequently underdiagnosed and underestimated in clinical practice (22, 23, 56, 57). The factors considered responsible for the challenges in recognition and timely diagnosis of DPN include:
a) the insidious course of disease manifesting with non-specific symptoms and signs mimicking many other diseases,
b) a lack of consensus on optimal screening and diagnostic procedures,
c) the absence of a well-established diagnostic scrutiny, and
d) the poor acceptance of guidelines and insufficient awareness of the disease among physicians (22, 23, 25, 30, 56–58).
DPN is a diagnosis by exclusion, and its recognition is mainly based on clinical suspicion and the effort put into finding it (22, 23). However, there is insufficient physician awareness regarding the recognition of DPN, even when the neuropathy is symptomatic (22, 23, 30, 57, 58). The population-based studies reported that painful DPN and painless DPN were previously undiagnosed in almost 60% and 80% of T2D patients, respectively (59–61).
The current practice of DPN care is also considered inadequate in terms of patient journey which includes multiple visits to different clinicians having no specialist training to assess the level of risk, to provide advice, or to appropriately refer the patients for further investigation and appropriate interventions (62, 63).
Accordingly, the participating experts consider the suspicion of the disease by clinicians as the key factor, emphasizing the awareness of the disease during the patient journey by the first-admission or referring physicians. In this regard, improved awareness of DPN among internal medicine, family medicine and physical medicine and rehabilitation specialists besides the neurology and endocrinology specialists as the most consulted specialists, seems to be the key factor in consideration of DPN in differential diagnosis of patients presenting with suspected neuropathic symptoms. Increase in familiarity of non-specialist physicians with recognition of DPN seems also important to reduce the unnecessary referrals among several different specialists before a correct diagnosis is reached.
DPN is considered to appear in approximately half of all diabetic individuals at some stage in their lives, emphasizing the utility of screening in early diagnosis of DPN (9, 64). Data from the MONICA/KORA Augsburg Surveys from Germany revealed the varying prevalence of DPN and painful DPN across the spectrum of diabetes, pre-diabetic states and normoglycemia, including known diabetes (28% and 13%, respectively), IGT (13% and 9%), IFG (11% and 4%) and normal glucose tolerance (NGT; 7% and 1%) (40, 65).
In a multicenter study with 1,113 diabetic patients from Turkey, the prevalence of DPN was found to be 40.4% based on the clinical examination alone and increased to 62.2% by combining nerve conduction studies with clinical examination, while the neuropathic pain prevalence was 14.0% (66). In another study from Turkey in 100 newly diagnosed prediabetic individuals who were screened for microvascular and macrovascular diabetic complications, microvascular complications were found in 12% of the participants (neuropathy: 4%, nephropathy: 8%) and 19% had macrovascular complications (67).
Accordingly, given the accumulating evidence on the increasing risk of DPN in patients with prediabetes and with the duration of the disease in those with known diabetes (40, 65, 68), early screening for DPN in the setting of prediabetes and diabetes is important to prevent and delay the occurrence of DPN (20, 22, 34). Screening for early detection and subsequent follow-up of progression is also important given that DPN is already well-established by the time its symptoms and/or clinical signs develop, impeding the benefits of intensified multifactorial intervention at an early stage of disease trajectory (3, 63, 69, 70).
The newly updated “American Diabetes Association (ADA) Standard of Care in Diabetes 2024” for Neuropathy Screening Guidelines states that patients with T2D at the time of diagnosis and those with T1D five years after diagnosis should be evaluated for DPN by obtaining a careful medical history and physical examination (sensory assessment and foot examination), and subsequently they should be evaluated every year (71).
In addition, given the likely association between different microvascular complications, as well as between micro and macrovascular complications of diabetes, when a patient is diagnosed to have one diabetes complication, it is important to screen for others (72, 73).
Clinical history and examination are the mainstays of clinical diagnosis, and when medical history and basic neurological examination (simple semi-quantitative bedside instruments and inspection of feet) reveals the corresponding neuropathic symptoms and/or signs in the absence of other potential causes of distal symmetric peripheral neuropathy, this supports the diagnosis of DPN (4, 6, 7, 23, 74).
Accordingly, in most cases, the diagnosis of DPN is made by ruling out other potential causes of distal symmetric peripheral neuropathy, such as alcohol use, nutritional deficiencies (i.e., vitamins B6, B12, and E, thiamine, folate, copper, phosphate), hypothyroidism/hyperthyroidism, autoimmune diseases (i.e., systemic lupus erythematosus, rheumatoid arthritis), malignancy (i.e., multiple myeloma), infections (HIV/AIDS, Lyme disease), chemotherapy and toxin exposure (4, 11, 20, 49, 72). This necessitates a careful medical history and a screening laboratory test for complete blood count, comprehensive metabolic profile, fasting blood glucose, thyroid-stimulating hormone, and vitamin B12 levels, as well as the serum protein electrophoresis with immunofixation to exclude other causes of peripheral neuropathy (6, 20, 22, 25, 56, 63, 75, 76).
Neuropathic symptoms include the pain (characteristically described as burning, painful cold, lancinating, tingling, stabbing or shooting) and the non-painful neuropathic symptoms such as paresthesias (tingling, prickling or ant-like sensations), dysesthesias (unpleasant abnormal sensation whether spontaneous or evoked), sensory ataxia (ataxic gait) or numbness (often described as “wrapped in wool” or like “walking on thick socks”) (20, 22) (Table 2; Figure 1).
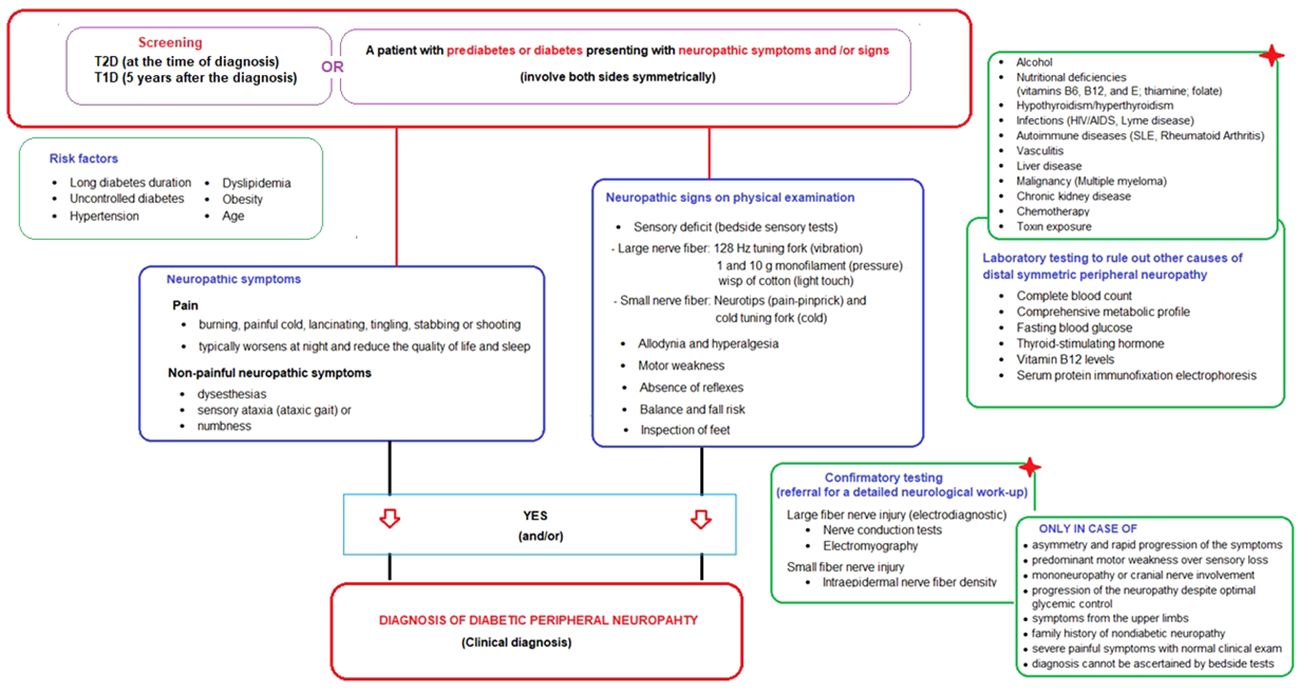
Figure 1 Screening and diagnostic algorithm for diabetic peripheral neuropathy in prediabetic and diabetic patients presenting with neuropathic symptoms and/or signs.
Neuropathic signs detected on basic neurological examination including bedside sensory tests and foot inspection include (a) sensory deficit in large nerve fiber (via 128 Hz tuning fork for vibration sensation, 1 and 10 g monofilament for pressure sensation and wisp of cotton for light touch sensation), (b) sensory deficit in small nerve fiber (via neurotips for pain-pinprick sensation) and cold tuning fork for cold sensation), (c) allodynia (pain triggered by normally non-painful stimuli such as the contact of socks, shoes, or bedclothes), (d) hyperalgesia (exaggerated response to painful stimuli), (e) motor weakness (extension of the big toe, ankle dorsiflexion, and walking on heels), (f) absence of reflexes (ankle and patellar deep tendon reflexes), (g) balance and fall risk (Romberg test, normal gait, and tandem gait), and (h) inspection of feet (for deformities, ulcers, fungal infection, muscle wasting, hair distribution or loss, and the presence or absence of pulses) (20, 22, 23) (Table 2; Figure 1).
Small and large nerve fiber damage most frequently coexist in DPN, and thus testing small and large nerve fiber function with appropriate semi-quantitative bedside tests is equally important (22, 51). In the painful DPN, intensity of pain is evaluated via numeric rating scale [NRS] or a visual analogue scale [VAS]), while the pain typically worsens at night and may interfere with daily activities and reduce the quality of life and sleep and characteristics (20, 22, 23).
The ADA definition does not require an abnormal electrodiagnostic test for clinical neuropathy diagnosis, as also supported by studies indicating that electrodiagnostic tests rarely change the etiology and/or management of patients meeting a clinical DPN definition (3, 77, 78). However, when evaluating a person with diabetes and signs and/or symptoms of neuropathy, it is important to remember that DPN is not the only possible cause of these signs and/or symptoms and that rare diabetic neuropathies or even neuropathies of other etiologies may be present (6, 24). Hence, the confirmatory testing (detailed neurological work-up) is not usually needed for clinical diagnosis but is used in clinical research and is helpful in the diagnosis of patients with atypical presentations (6, 7). These tests include the large fiber nerve injury tests (electrodiagnostic tests: nerve conduction tests and electromyography) and the small fiber nerve injury (intraepidermal nerve fiber density) (6, 7, 74) (Figure 1).
The atypical presentations that should alert the physician to consider non-diabetic causes of neuropathy via referral for a detailed neurological work-up (confirmatory tests) include: (1) asymmetry and rapid progression of the symptoms, (2) predominant motor weakness over sensory loss, (3) mononeuropathy or cranial nerve involvement, (4) progression of the neuropathy despite optimal glycemic control, (5) symptoms from the upper limbs, (6) family history of nondiabetic neuropathy, (7) severe painful symptoms in the feet whilst clinical examination is normal, 8) diagnosis of DPN cannot be ascertained by clinical examination with the semi-quantitative bedside tests (6–8, 20, 22, 74) (Figure 1).
Accordingly, the present expert panel suggests that DPN should be considered in a patient with prediabetes or diabetes who presents with neuropathic symptoms (involve both sides symmetrically) and/or signs of neuropathy in the presence of DPN risk factors (i.e., advancing age, obesity, hypertension, dyslipidemia, poor glycemic control), with careful consideration of laboratory testing to rule out other causes of distal symmetric peripheral neuropathy and referral for a detailed neurological work-up for a confirmative test of either small or large nerve fiber dysfunction only in atypical cases. The proposed “screening and diagnostic” algorithm is provided in Figure 1.
Currently, there is no single gold standard test for objective assessment and early identification of DPN in routine clinical practice (28, 63), while there is considerable inter-physician variability in judgement and weighing of neuropathic symptoms/signs to draw a clinical diagnosis (3, 79).
Bedside sensory tests are operator-dependent tests that tend to diagnose DPN when it is already well-established, while their implementation is challenging by general practitioners or at busy diabetes clinics given the time constraints (28, 63, 80). Nevertheless, ADA recommends pinprick and temperature sensation tests for small fiber dysfunction; and lower extremity reflexes (particularly Achilles reflex) and vibration sensation with 128 Hz tuning fork for large fiber dysfunction (71). The expert panel also encourage the use of these tests (Figure 1).
Since the clinical-electrophysiological dissociation occurs regularly in the early stage of DPN, it is not uncommon to encounter diabetic patients with normal clinical but abnormal electrophysiological features (subclinical neuropathy) or those with abnormal clinical and normal electrophysiological features (early DPN; clinically defined DPN) (81).
Although the ADA definition does not require an abnormal electrodiagnostic test for clinical neuropathy diagnosis, nerve conduction studies are often needed to confirm the diagnosis and document the severity of DPN. Another essential clinical point is that the presence of DM in a patient with neuropathy does not prove that diabetes is the cause of polyneuropathy since it has been observed that neuropathy is due to causes other than diabetes in 2% of type 1 diabetics and 6% of type 2 diabetics (82). Moreover, nerve conduction studies could predict foot ulcers and even mortality (83).
DPN is the strongest risk factor for foot ulcers and extremity amputations, which lead to labor loss and have very high individual and social costs. Since DPN is a length-dependent process that occurs with sensory system dysfunction, examination of the dorsal sural and medial plantar nerves, whose responses are recorded more distally, their conduction studies are found to be much more sensitive than sural nerve conduction studies used in daily routine electrophysiological examination in detecting polyneuropathy (84, 85). Recently the abnormality of RR interval, another electrophysiological test, has been shown to provide early information about cardiac autonomic neuropathy, even without clinical abnormalities (86).
Nerve conduction tests and electromyography, provide higher sensitivity than clinical examinations in the evaluation of peripheral symmetrical polyneuropathies and are the least variable noninvasive measure of neuropathy and its progression (63, 81, 83, 87, 88). However, nerve conduction studies are labor-intensive, costly and impractical to implement in routine clinical care (22, 63). Moreover, DPN symptoms often occur with subclinical dysfunction of nerves. Skin biopsy is also used for diagnosing peripheral neuropathy on the basis of intraepidermal nerve fiber density (IENFD), while it is impractical for monitoring symptom progression or assessing treatment efficacy in clinical settings (28, 89).
The point-of-care devices (POCD), which are developed as rapid and non-invasive tests for diagnosis of subclinical neuropathy, include corneal confocal microscopy (CCM; for assessing small-fiber neuropathy and progression to large-fiber neuropathy), SUDOSCAN (for assessing sudomotor function), quantitative sensory testing (QST), NeuroQuick and NeuroPAD (28, 63, 90–93). Although the current evidence suggests the likelihood of PCODs to meet the criteria of an ideal screening test (safe, quick and sufficiently simple to provide objective measures), the cost of using these tests for screening purposes in all patients with diabetes and their convenience for busy diabetic clinics also need to be carefully appraised (63).
Over the years, a wide range of clinical scales, which often combine symptom assessments with bedside evaluations of clinical DPN signs, have been proposed as standardized objective and quantitative measures for screening and grading of severity of DPN (3, 52, 63). The most used ones are Michigan Neuropathy Screening Instrument (MNSI), Toronto Clinical Neuropathy Score (TCNS), United Kingdom Screening Test (UKST), Utah Early Neuropathy Score (UENS) and Neuropathy Impairment score for Lower Limbs (NIS-LL) (52, 63). However, their assessment remains subjective and heavily reliant on the examiners’ interpretations in addition to concerns regarding their validity, and they are considered particularly useful for epidemiological studies investigating the prevalence of DPN in larger populations (3, 52, 63, 94).
To improve clinical outcomes in DPN, there is an urgent need to diagnose DPN early before overt clinical signs are apparent and to assess disease progression accurately in order to effectively reduce morbidity and to reliably inform patients of their underlying risk of foot ulceration (63). Hence, rapid sensitive and specific tests that do not require specialty training but provide a reliable and sensitive cost-effective method to screen for DPN will ultimately be needed (3, 63, 79) (Box 1).
Box 1. Key points - Screening tests in DPN (3, 22, 28, 52, 63, 79, 81–93).
● There is no gold-standard test or specific simple markers for early detection of DPN in routine clinical practice.
● Bedside sensory tests are operator-dependent tests that tend to diagnose DPN when it is already well established and their implementation is challenging by general practitioners or at busy diabetic clinics given the time constraints.
● It is not uncommon to encounter diabetic patients with normal clinical but abnormal electrophysiological features (subclinical neuropathy) or with abnormal clinical and normal electrophysiological features (early DPN; clinically defined DPN).
● Nerve conduction tests and electromyography, are the least variable noninvasive measure of neuropathy and its progression, but they are labor-intensive, time-consuming, costly and impractical to implement in routine clinical care, and are unsuitable for evaluating the small-fiber neuropathy.
● Skin biopsy is used for diagnosing peripheral neuropathy on the basis of intraepidermal nerve fiber density (IENFD); however, it is impractical for monitoring symptom progression or assessing treatment efficacy in clinical settings.
● Pint-of-care devices (POCD), such as CCM (assessing the small-fiber neuropathy and progression to large-fiber neuropathy), SUDOSCAN (assessing the sudomotor function) and QST, are rapid and non-invasive tests for diagnosis of subclinical neuropathy. However, the cost of using these tests for screening purposes in all patients with diabetes and their convenience for busy diabetic clinics also need to be carefully appraised.
● The clinical scales are standardized objective and quantitative measures for screening and grading of severity of DPN but their results remain subjective and heavily reliant on the examiners’ interpretations in addition to concerns regarding their validity.
● There is an urgent need for rapid sensitive and specific tests that do not require specialty training to diagnose DPN early before overt clinical signs are apparent, to assess disease progression accurately in order to effectively reduce morbidity.
DPN is best managed thorough by multidisciplinary support provided by an interprofessional team including endocrinology, internal medicine, neurology, physical therapy and rehabilitation, nephrology and ophthalmology specialists, a dedicated diabetic nurse, a dietician, an exercise specialist, a podiatrist, and a psychologist to ensure the provision of the available standard of care with minimal morbidity (8, 44, 63, 95, 96).
First-line interventions for DPN is currently represented by optimized glycemic control (mainly for T1D) and multifactorial intervention (mainly for T2D), along with lifestyle optimization and weight management (3, 97). There is a need for personalized treatment/mechanism-based approaches for DPN, which includes the targeted interventions based on the comorbid risk factors and underlying disease mechanisms specific to each patient, besides the optimal diabetes treatment (4, 18). Overall, the management of DPN is based three principles including (10, 22, 49, 97, 98):
(1) optimal diabetes treatment via intensive glycemic control, lifestyle modification and multifactorial risk intervention,
(2) pathogenetically oriented pharmacotherapy, and
(3) symptomatic pain relief
Glycemic control early in the course of the disease is considered the most effective prophylactic treatment to delay the emergence of DPN, because hyperglycemia is the primary underlying pathophysiologic insult that contributes to DPN (9, 99).
However, while strict glycemic control slows DPN progression (78% relative risk reduction) in patients with T1D (100, 101), it has generally modest impact on DPN (5–9% relative risk reduction) in the setting of T2D, as reported by the large-scale studies (Steno 2, ADVANCE, ACCORD and VADT) and meta-analyses (102–107). This is considered in favor of the independent contribution of metabolic syndrome and its components (obesity, dyslipidemia, and hypertension), besides the hyperglycemia, to the onset and progression of DPN in T2D (5, 9, 25, 33, 45, 107, 108). However, since the DPN was not a primary outcome in the above-mentioned clinical trials, most of these studies relied on insensitive measures of neuropathy (9, 63, 106). Notably, the studies using more sensitive clinical endpoints (i.e., corneal confocal microscopy) revealed a benefit of strict glycemic control (HbA1c < 6.5%) also in T2D patients (63, 109, 110).
Moreover, the BARI 2D trial demonstrated a significantly lower cumulative incidence of DPN with the use of insulin-sensitizing agents (metformin, thiazolidinediones) rather than the insulin-providing (sulfonylurea and insulin) strategy (111). Hence, while glycemic control is the mainstay approach to preventing and managing diabetes-related complications, including DPN, the degree to which glycemic control can prevent or reverse DPN may differ depending on the choice of anti-diabetic agents used to achieve targets, type of diabetes and the duration of the disease as well as the glucose variability and glycemic excursions (4, 111, 112).
Accumulating evidence indicates the multifactorial risk reduction strategies that involve adopting a healthy lifestyle incorporating a balanced diet (i.e., a low-fat, low-calorie diet, or a Mediterranean diet) and regular aerobic and weight-resistance physical activities as the best way to control the risk factors and prevent the development and progression of DPN (4, 7, 63, 113, 114).
RCTs in patients with DPN indicated the association of a 12-week exercise and lifestyle intervention with a significant reduction in DPN severity based on Modified Toronto Clinical Neuropathy Score [mTCNs] (115), and the association of a 2.5-hour, weekly supervised treadmill exercise and dietary intervention program with significant improvement in markers (intraepithelial nerve fiber density and regenerative capacity) of DPN (116).
Some authors also suggested that DPN in T2D is pathogenetically different from T1D and should be rather managed in the context of the metabolic syndrome (63, 97, 117). Nonetheless, several large intervention studies targeting multiple risk factors (UKPDS, Steno-2, ADDITION) failed to show a reduction in DPN despite clear benefits in renal and retinal complications (118–120). This, once again, emphasizes the importance of using sensitive, robust methods used to diagnose and quantify DPN (63, 97).
In this regard, further research is needed to re-examine the impact of multifactorial interventions upon DPN using more reliable, reproducible and sensitive measures of DPN (63). Given the growing need for personalized treatment/mechanism-based approaches that can consider individual differences in disease mechanisms, severity, and response to treatment (4, 18), early identifications of subjects with sub-clinical or early neuropathy using validated, yet novel non-invasive POCDs may allow investigation of the effect of a targeted intensified cardiometabolic risk factor control on prevention of clinical DPN or reversal of disease progression in larger studies (63).
In addition, while some studies indicate the association of angiotensin-converting enzyme (ACE) inhibitors with improvements in DPN based on clinical and nerve conduction parameters (121, 122), conflicting data have been reported on the impact of dyslipidemia treatment on DPN (45, 63, 97). Some studies found that treatment with either statin or fibrate was associated with a reduced risk of new-onset DPN and decreased the risk of neuropathy (43, 123, 124), while others indicated no association of statin therapy with a reduction of DPN risk (125) or even significantly higher prevalence of DPN among statin-users compared with non-users (126, 127). Nonetheless, the association of statins therapy with symptoms of DPN in diabetic patients remains a controversial subject, given that statin therapy is given for persons with a very prominent hazard of DPN, and there are also accessible substitutes of statins that are not associated with DPN (127).
Upstream inhibition of key glycolytic enzymes by oxidative stress activates major pathways (polyol, hexosamine, PKC, PARP and AGE) implicated in the development of DPN (22, 128). Ideally, a targeted intervention is expected to address the underlying pathogenetic mechanisms and to alter the natural trajectory of the disease (97). Several compounds are available which target these major pathways implicated in the pathogenesis of DPN, such as aldose reductase inhibitors (sorbinil, tolrestat, ponalrestat, fidarestat, epalrestat, zenarestat) acting on polyol pathway, PKC inhibitors (ruboxiastaurin), benfotiamine acting on hexosamine pathway, agents acting on AGE pathway (minocycline, aminoguanidine), actovegin acting as PARP inhibitor and ROS inhibitors (alpha-lipoic acid, coenzyme Q10, nicotinamide, resveratrol, taurine) (52, 128).
Amongst them, alpha-lipoic acid (ALA) has a special place in the current DPN treatment paradigm, given that it is licensed as a drug for the treatment of DPN in many countries. Thus, its unique and wide-range antioxidant effects as a favorable agent aimed at the pathogenesis of DPN have been extensively investigated in countries where ALA is widely used, such as Germany, Eastern Europe and Far East (28, 31, 52, 99, 129).
All of the pathways involved in DPN lead to a unique result of enhanced cellular oxidative stress caused by ROS generation and decreased antioxidant defense, which makes antioxidant response as an attractive drug target in DPN (130–132). ALA is considered a promising first-line disease-modifying antioxidant therapy for DPN in this regard, given its ability to prevent early development and progression of DPN by exerting direct (inhibition of ROS generation) and indirect (increase of endogenous antioxidant glutathione) antioxidant effects, which subsequently manifests as a notable clinical improvement of DPN (132–135) (Figure 2).Also, serum homocysteine level is considered a modifiable risk for DPN with significant contribution of hyperhomocysteinemia to endothelial tissue damage according to microvascular hypothesis (30, 136). ALA has been reported to ameliorate the hyperhomocysteinemia- induced endoplasmic reticulum stress and oxidation in human aortic endothelial cells (137). Hence, ALA may also be effective pathogenesis-directed agent in the setting of microvascular hypothesis in DPN, as a potentially homocysteine-lowering (30, 136).
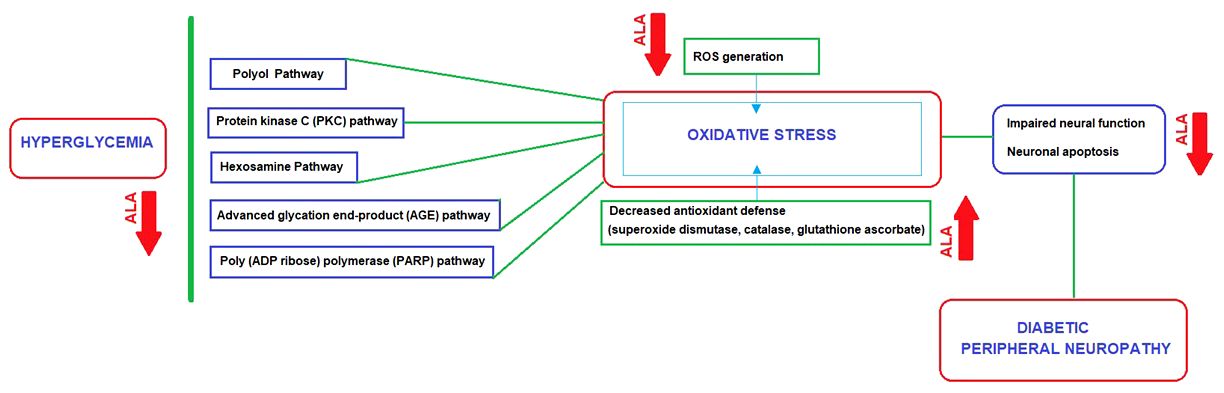
Figure 2 ALA as a disease-modifying agent: Direct and indirect antioxidant effects in relation to main pathways implicated in the DPN pathogenesis.
In the ALADIN II (a-Lipoic Acid in Diabetic Neuropathy) trial, two years of initially intravenous and then oral administration of ALA was associated with improvement in motor and sensory nerve conduction velocity and amelioration of neuropathic symptoms (138).
In the SYDNEY 2 trial, oral 600 mg once daily treatment with ALA for 5 weeks improved neuropathic symptoms (stabbing pain, burning pain, paresthesia, and asleep numbness of the feet) and deficits in DPN as assessed by Neuropathy Symptoms and Change (NSC) and Neuropathy Impairment Score (NIS), providing the optimum risk-to-benefit ratio (139).
In a meta-analysis of three RCT trials in ALA-treated patients with symptomatic DPN, 600 mg ALA either orally or intravenously was shown to reduce significantly neuropathic symptoms (140). Another meta-analysis of four RCTs (ALADIN I, ALADIN III, SYDNEY, NATHAN I; in 1258 patients) demonstrated that 3 weeks of ALA therapy significantly improved the total symptom score (TSS; relative difference vs placebo: 24.1% with significant effect on pain, burning, and numbness), and the NIS-LL (relative difference versus placebo: 16.0% with significant effect on ankle reflexes, pin-prick and touch-pressure sensation) (141). In a meta-analysis of 19 RCTs in 1242 patients with DPN, the efficacy of oral ALA (600, 1200, and 1800 mg/day) was evaluated based on TSS, neurological disability score (NDS), neuropathy impaired score (NIS), NIS-LL, vibration perception threshold (VPT), nerve conduction study (NCS) results, and global satisfaction (28). ALA treatment produced favorable results for TSS (a dose-related trend was observed), NDS, and the global satisfaction score but not for VAS, VPT, NIS-LL, and NCS (28). Also, in a small cohort of 20 patients, ALA improved night pain, paresthesia, muscle atrophy and difficulty in walking (142).
In the NATHAN 1 (The Neurological Assessment of Thioctic Acid in Diabetic Neuropathy) trial, neuropathic deficits were improved after 4 years of ALA treatment in patients with mild to moderate largely asymptomatic DPN (143). In a post-hoc analysis of the NATHAN 1 trial, the use of ALA (vs. placebo) was associated with better outcome in terms of NIS-LL following 4-year treatment in patients with mild-to-moderate DPN, which was predicted by higher age, lower BMI, male sex, normal blood pressure, history of cardiovascular disease (CVD), insulin treatment, longer duration of diabetes and neuropathy, and higher neuropathy stage (144). Thus, ALA is considered effective in the amelioration of neuropathic impairments in elderly insulin-treated patients with history of CVD in whom weight and blood pressure are well controlled, as well as in those with more severe stages of diabetes and neuropathy, and HbA1c levels ≥7% (144). Although there is little evidence from studies, the participating experts consider that poor glycemic control leads to higher rate and a worse prognosis of macro- and micro-vascular complications. Without achieving the glycemic control, it is impossible to prevent complications and to slow progression in any patient. Therefore, it is not possible for any of the agents used in treatment of neuropathy to prevent complications as long as the glucolipotoxicity persists.
In a meta-analysis of 9 studies of DPN, compared to placebo, oral ALA treatment revealed a reduction in the NIS (muscle weakness, reflex loss, touch pressure, vibration, joint position, and motion), NIS-LL (motor nerve function and reflexes) and NDS (cranial nerve damage, muscle strength, reflex loss, and loss of sensation), besides the TSS (145). Accordingly, oral ALA treatment is considered a favorable option for DPN in terms of improving pain symptoms, and motor and nerve damage along with excellent safety profile (145–147). In a real-world cohort of 443 diabetic patients with chronic painful neuropathy who were treated with ALA 600 mg qd orally for a mean period of 5 years, long-term therapy with ALA acid was considered a safe and effective treatment option in outpatients with DPN (148). Notably, stopping ALA after 5 years of treatment yielded the recurrence of symptoms after 2 weeks, while switching from long-term treatment with ALA to central analgesic drugs such as gabapentin was associated with considerably higher rates of side effects, frequencies of outpatient visits, and daily costs of treatment (148). These findings were considered to indicate that treatment of DPN is a long term treatment, even at symptom-free intervals, which requires a drug with pathogenetic properties like ALA (148).
In addition, patients with diabetes were reported to have decreased levels of circulating ALA, supporting the beneficial effects of ALA supplementation in the management of DPN (132, 144).
Notably, in a recent meta-analysis of 24 RCTs in patients with metabolic diseases, ALA was also found to improve glucose homeostasis (decreased FBG, insulin, HOMA-IR and HbA1c) and lipid profile (decreased triglycerides, total cholesterol and LDL-cholesterol) (149). The potential mechanisms underlying its ability to improve glucose homeostasis have been demonstrated in some studies, mainly based on ALA-mediated increase in glucose uptake in peripheral tissues (i.e., muscles) in obese animals (150, 151) and stimulation of glucose uptake by translocation of glucose transporters to plasma membrane and increasing the tyrosine phosphorylation of insulin receptor substrate-1 (152).
Also, its effect on DPN is considered to be greater when used as part of a conventional treatment (i.e. gliclazide, SGLT2i, metformin, and GLP 1 analogs) in T2D patients experiencing neuropathic pain (153, 154) (Box 2).
Box 2. Key points – ALA as a pathogenesis-directed antioxidant therapy for DPN (132–135, 138–147, 153, 154).
● ALA is a first-line disease-modifying antioxidant therapy for DPN, given that it is a direct and indirect antioxidant that works with a strategy targeted directly against ROS and indirectly in favor of endogenous antioxidant capacity (glutathione) for improving DPN conditions.
● Its efficacy has been consistently demonstrated in many clinical trials, real-word studies and meta-analyses in the setting of DPN, in terms of improvement in motor and sensory nerve conduction velocity and amelioration of neuropathic symptoms (stabbing pain, burning pain, paresthesia, and asleep numbness of the feet).
● Specifically, ALA treatment was associated with a reduction in the NIS (muscle weakness, reflex loss, touch pressure, vibration, joint position, and motion), NIS-LL (motor nerve function and reflexes) and NDS (cranial nerve damage, muscle strength, reflex loss, and loss of sensation), besides the reduction in the TSS
● Long-term therapy with ALA is considered a safe and effective treatment option in patients with DPN.
● The recurrence of symptoms after 2 weeks of treatment discontinuation in patients who were on ALA therapy for 5 years, and the association of switching from long-term treatment with ALA to central analgesic drugs such as gabapentin with considerably higher rates of side effects, frequencies of outpatient visits, and daily costs of treatment, indicate that treatment of DPN is a long term treatment, even at symptom-free intervals, which requires a drug with pathogenetic properties like ALA.
● ALA seems to be helpful in improving glucose homeostasis (decreased FBG, insulin, HOMA-IR and HbA1c) and lipid profile (decreased triglycerides, total cholesterol and LDL-cholesterol), and its effect on DPN is considered to be greater when used with conventional treatment in T2D patients experiencing neuropathic pain.
For pharmacological treatment of painful DPN, serotonin-norepinephrine reuptake inhibitors (SNRIs: duloxetine and venlafaxine), tricyclic agents (TCAs: amitriptyline, imipramine) and gamma-aminobutyric acid (GABA) analogues (gabapentin or pregabalin) are considered as the first-line agents, while the combination therapy (antidepressant combined with gabapentin), topical treatments and opioids are recommended as the second-line or third-line agents (97, 128, 155–157). Recently, mirogabalin, a third member of gabapentinoids with distinct binding characteristics of voltage-gated calcium channels, was approved for use in DPN in Japan, Korea, Taiwan, and China (158).
The antioxidant ALA (IV form in particular) is also an effective treatment in amelioration of neuropathic pain, and even in reducing the use of rescue drugs (i.e., pregabalin, duloxetine, and tapedantol) (8, 52, 129, 135, 138, 139, 143–145, 159). Notably, in a retrospective analysis of the nationwide database in Hungary, a comparison of propensity matched cohorts of DPN patients treated with ALA vs. those treated with symptomatic analgesic pharmacotherapies revealed the lower occurrence of cardiovascular and cerebrovascular morbidity (acute myocardial infarction, stroke and hospitalization for heart failure), cancer events and all-cause mortality in patients treated with pathogenetically oriented ALA, while no significant change was observed in hazard for lower limb amputation (146). The duration of follow up treatment was also significantly longer with ALA than with symptomatic pharmacotherapies (146, 147).
When choosing a particular agent for treating painful DPN, certain factors besides the potential efficacy of the drug should be considered, such as existing comorbidities, potentials side effects of the medication, drug interactions and cost (8, 49, 97, 155, 160). The commonly prescribed drugs for painful DPN are summarized in Table 3, with regard to doses and comorbidity-based contraindications (8, 97, 155, 160).
However, despite these options, suboptimal pain relief is common with response to analgesic monotherapy achieved only by 50% of patients with painful DPN (8, 22, 98, 155, 161). Hence, insufficient response to a fist-line agent is not uncommon, necessitating a second first-line drug or a combination pharmacotherapy (gabapentinoids and antidepressants in particular) in those with treatment failure despite careful dose titration and adequate duration of first-line therapy (22, 49, 97, 154, 159).
Because there is no entirely satisfactory pharmacotherapy of painful DSPN, non-pharmacological treatments such as psychological support, acupuncture, transcutaneous electrical nerve stimulation (TENS), percutaneous electrical nerve stimulation (PENS), low-intensity laser therapy and for severe resistant cases, electrical spinal cord stimulation and high-frequency repeated transcranial magnetic stimulation (rTMS) of the motor cortex can also be used, despite the relatively low level of evidence (4, 8, 22, 97, 162, 163).
Psychological interventions such as mindfulness-based stress reduction and cognitive behavioral therapy can also have a significant beneficial effect on pain severity, pain interference, depressive symptoms, and quality of life in patients with DPN (164).
Diabetic patients with chronic use of metformin and proton pump inhibitors (PPIs) are at increased risk of vitamin B12 deficiency, emphasizing the need for annual assessment of the vitamin B12 status in people with diabetes treated with metformin or PPIs (99, 165, 166).
The screening for vitamin B12 status in T2D patients with chronic metformin use is based on categories of low (<200 pg/mL), borderline (200–300 pg/mL) and normal (> 300 pg/mL) vitamin B12 levels, in accordance with normal values derived from one of the largest studies in the Diabetes Prevention Program Outcomes Study (48, 167).
Vitamin B12 supplementation in deficient patients with DPN was reported to be effective in reducing neurophysiological parameters, pain intensity and sudomotor function, and improving the quality of life (168–170). However, it should be noted that excessive vitamin B6 (pyridoxine) ingestion in the form of vitamin B1–6-12 combination tablets may also cause irreversible neurotoxicity (171).
While vitamin B12 deficiency is suggested to develop in a dose- and time-dependent manner in T2D patients on chronic metformin treatment (172), in a meta-analysis metformin use was not found to correlate with a high incidence of neuropathy (165). Likewise, in a longitudinal 9-year follow-up study assessing the interaction between vitamin B12 deficiency, DPN, and metformin exposure in diabetes, people with vitamin B12 deficiency (vs. those without deficiency) had an increased hazard of developing DPN, while people with DPN (vs. those without DPN) had increased hazard of developing vitamin B12 deficiency (173). Metformin significantly increased the risk of DPN in patients with vitamin B12 deficiency, whereas metformin treatment in preexisting DPN patients did not increase but rather reduced the risk of vitamin B12 deficiency (173).
Hence, further prospective studies addressing the incidence of DPN in relation to the degree of vitamin B12 deficiency per daily and cumulative doses of metformin in each therapeutic period are necessary to clarify the complex relationship between metformin treatment, vitamin B12 deficiency, and DPN occurrence in T2D (173) (Box 3).
Box 3. Key points – Treatment options and future perspectives (3, 4, 18, 97, 127, 131–135, 173).
● First-line interventions for DPN is currently represented by optimized glycemic control (mainly for T1D) and multifactorial intervention (mainly for T2D), along with lifestyle optimization and weight management.
● There is a need for personalized treatment/mechanism-based approaches for DPN, which includes the targeted interventions based on the comorbid risk factors and underlying disease mechanisms specific to each patient, besides the optimal diabetes treatment
● ALA is an effective first-line disease-modifying antioxidant therapy for long-term management of DPN.
● There is still a gap in existing research in the field, necessitating well-designed, robust, multicenter clinical trials, with sensitive endpoints and standardized protocols to facilitate the diagnosis of DPN via a simple and effective algorithm and to track progression of disease and treatment response.
● Identification of novel risk and prognostic biomarkers may enable an individualized treatment pathway for patients with DPN from a potentially disease-modifying perspective, and provide onward opportunities for novel treatments that would be efficacious in early stages of DPN, and alter the natural course of the disease.
The participating experts consider the suspicion of the disease by clinicians as the key factor, emphasizing the improved awareness of the disease during the patient journey by the first admission or referring physicians as well as the increased familiarity of non-specialist physicians with recognition of DPN. The proposed screening and diagnostic algorithm involves the consideration of clinical DPN in a patient with prediabetes or diabetes who presents with neuropathic symptoms and/or signs of neuropathy in the presence of DPN risk factors, with careful consideration of laboratory testing to rule out other causes of distal symmetric peripheral neuropathy and referral for a detailed neurological work-up for a confirmative test of either small or large nerve fiber dysfunction only in atypical cases. There is still a gap in existing research in the field, necessitating well-designed, robust, multicenter clinical trials with sensitive endpoints and standardized protocols to facilitate the diagnosis of DPN via a simple and effective algorithm and to track the progression of disease and treatment response. Although, the first-line interventions for DPN are currently represented by optimized glycemic control (mainly for T1D) and multifactorial intervention (mainly for T2D), along with lifestyle optimization and weight management, there is a need for individualized pathogenesis-directed approaches targeting specific underlying mechanisms involved in the disease. ALA seems to be an important first-line disease-modifying and pathogenesis-directed agent in this regard, given that it is a direct and indirect antioxidant that works with a strategy targeted directly against ROS and indirectly in favor of endogenous antioxidant capacity (glutathione) for improving DPN conditions. Identification of biomarkers/predictors that would allow an individualized approach from a potentially disease-modifying perspective may provide onward opportunities for novel treatments that would be efficacious in early stages of DPN and alter the natural course of the disease.
In this regard, this expert opinion document is expected to increase awareness among physicians about conceptual, clinical, and therapeutic aspects of DPN and to assist them in timely recognition of DPN and translating this information into their clinical practice for best practice in the management of patients with DPN.
AA: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. IS: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. ISS: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. RO: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. HE: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MA: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. HS: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. TD: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported by Abdi Ibrahim Pharmaceuticals Turkey which played a role in organization of expert panel meetings including invitation of participants and compensation for the time and transport expenses of the experts. Abdi Ibrahim Pharmaceuticals Turkey had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Cagla Ayhan, MD from KAPPA Training Consultancy Research LLC, Izmir, Turkey who provided editorial support funded by Abdi Ibrahim Pharmaceuticals Turkey.
Author HS was employed by the company Abdi Ibrahim Turkey.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. International Diabetes Federation. IDF Diabetes Atlas(2021). Available online at: https://diabetesatlas.org/atlas/tenth-edition/ (Accessed February 3, 2023).
2. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. (2012) 11:521–34. doi: 10.1016/S1474–4422(12)70065–0
3. Jensen TS, Karlsson P, Gylfadottir SS, Andersen ST, Bennett DL, Tankisi H, et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain. (2021) 144:1632–45. doi: 10.1093/brain/awab079
4. Zaino B, Goel R, Devaragudi S, Prakash A, Vaghamashi Y, Sethi Y, et al. Diabetic neuropathy: Pathogenesis and evolving principles of management. Dis Mon. (2023) 69:101582. doi: 10.1016/j.disamonth.2023.101582
5. Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, et al. New perspectives in diabetic neuropathy. Neuron. (2023) 111:2623–41. doi: 10.1016/j.neuron.2023.05.003
6. Samakidou G, Eleftheriadou I, Tentolouris A, Papanas N, Tentolouris N. Rare diabetic neuropathies: It is not only distal symmetrical polyneuropathy. Diabetes Res Clin Pract. (2021) 177:108932. doi: 10.1016/j.diabres.2021.108932
7. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. (2022) 21:922–36. doi: 10.1016/S1474–4422(22)00188–0
9. Kaur M, Misra S, Swarnkar P, Patel P, Das Kurmi B, Das Gupta G, et al. Understanding the role of hyperglycemia and the molecular mechanism associated with diabetic neuropathy and possible therapeutic strategies. Biochem Pharmacol. (2023) 215:115723. doi: 10.1016/j.bcp.2023.115723
10. Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. (2021) 17:400–20. doi: 10.1038/s41574–021-00496-z
11. Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: A review. JAMA. (2015) 314:2172–81. doi: 10.1001/jama.2015.13611
12. Kirthi V, Perumbalath A, Brown E, Nevitt S, Petropoulos IN, Burgess J, et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open Diabetes Res Care. (2021) 9:e002040. doi: 10.1136/bmjdrc-2020–002040
13. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10–1303
14. Gorson KC, Ropper AH. Additional causes for distal sensory polyneuropathy in diabetic patients. J Neurol Neurosurg Psychiatry. (2006) 77:354–8. doi: 10.1136/jnnp.2005.075119
15. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in US adults: a prospective cohort study. Ann Intern Med. (2021) 174:167–74. doi: 10.7326/M20–1340
16. Burgess J, Frank B, Marshall A, Khalil RS, Ponirakis G, Petropoulos IN, et al. Early detection of diabetic peripheral neuropathy: A focus on small nerve fibres. Diagnostics (Basel). (2021) 11:165. doi: 10.3390/diagnostics11020165
17. Akbar M, Wandy A, Soraya GV, Goysal Y, Lotisna M, Basri MI. Sudomotor dysfunction in diabetic peripheral neuropathy (DPN) and its testing modalities: A literature review. Heliyon. (2023) 9:e18184. doi: 10.1016/j.heliyon.2023.e18184
18. Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. (2017) 93:1296–313. doi: 10.1016/J.NEURON.2017.02.005
19. Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels GJ, Bril V, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. (2011) 27:620–8. doi: 10.1002/dmrr.1226
20. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care. (2017) 40:136–54. doi: 10.2337/dc16–2042
21. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43:S135–51. doi: 10.2337/dc20-S011
22. Ziegler D, Tesfaye S, Spallone V, Gurieva I, Al Kaabi J, Mankovsky B, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: International expert consensus recommendations. Diabetes Res Clin Pract. (2022) 186:109063. doi: 10.1016/j.diabres.2021.109063
23. Vinik AI, Casellini C, Névoret ML. Alternative quantitative tools in the assessment of diabetic peripheral and autonomic neuropathy. Int Rev Neurobiol. (2016) 127:235–85. doi: 10.1016/bs.irn.2016.03.010
24. Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. (2013) 42:747–87. doi: 10.1016/j.ecl.2013.06.001
25. Yavuz DG. “Classification, risk factors, and clinical presentation diabetic neuropathy”. In: Takavoli M, editor. Diabetic Neuropathy. Netherlands, United Kingdom, United States: Elsevier Inc. (2022). p. 1–9. doi: 10.1016/B978–0-12–820669–0.00014–1
26. Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. (2014) 80:21–35. doi: 10.1016/j.phrs.2013.12.005
27. Leinninger GM, Edwards JL, Lipshaw MJ, Feldman EL. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol. (2006) 2:620–8. doi: 10.1038/ncpneuro0320
28. Hsieh RY, Huang IC, Chen C, Sung JY. Effects of oral alpha-lipoic acid treatment on diabetic polyneuropathy: A meta-analysis and systematic review. Nutrients. (2023) 15:3634. doi: 10.3390/nu15163634
29. Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, Miranda-Díaz AG. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J Diabetes Res. (2017) 2017:1673081. doi: 10.1155/2017/1673081
30. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, Allende-Vigo MZ, Duconge J. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. (2011) 6:260–73. doi: 10.2174/157488411798375967
31. Muresan M, Micle O, Antal L, Dobjanschi L, Antonescu A, Vicas L, et al. Correlation between reactive oxygen species and homocysteine levels in normal pregnancy. Farmacia. (2011) 59:179–90.
32. Méndez-Morales ST, Pérez-De Marcos JC, Rodríguez-Cortés O, Flores-Mejía R, Martínez-Venegas M, Sánchez-Vera Y, et al. Diabetic neuropathy: Molecular approach a treatment opportunity. Vascul Pharmacol. (2022) 143:106954. doi: 10.1016/j.vph.2022.106954
33. Viana MDM, Lauria PSS, Lima AA, Opretzka LCF, Marcelino HR, Villarreal CF. Alpha-lipoic acid as an antioxidant strategy for managing neuropathic pain. Antioxidants (Basel). (2022) 11:2420. doi: 10.3390/antiox11122420
34. Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: A meta-analysis. PloS One. (2019) 14:e0212574. doi: 10.1371/journal.pone.0212574
35. Callaghan BC, Gao L, Li Y, Zhou X, Reynolds E, Banerjee M, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. (2018) 5:397–405. doi: 10.1002/acn3.531
36. Zhang X, Yang X, Sun B, Zhu C. Perspectives of glycemic variability in diabetic neuropathy: a comprehensive review. Commun Biol. (2021) 4:1366. doi: 10.1038/s42003–021-02896–3
37. Cheng Y, Cao W, Zhang J, Wang J, Liu X, Wu Q, et al. Determinants of diabetic peripheral neuropathy and their clinical significance: A retrospective cohort study. Front Endocrinol (Lausanne). (2022) 13:934020. doi: 10.3389/fendo.2022.934020
38. Darivemula S, Nagoor K, Patan SK, Reddy NB, Deepthi CS, Chittooru CS. Prevalence and its associated determinants of diabetic peripheral neuropathy (DPN) in individuals having type-2 diabetes mellitus in rural South India. Indian J Community Med. (2019) 44:88–91. doi: 10.4103/ijcm.IJCM_207_18
39. Pai YW, Tang CL, Lin CH, Lin SY, Lee IT, Chang MH. Glycaemic control for painful diabetic peripheral neuropathy is more than fasting plasma glucose and glycated haemoglobin. Diabetes Metab. (2021) 47:101158. doi: 10.1016/j.diabet.2020.04.004
40. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. (2009) 10:393–400. doi: 10.1111/j.1526–4637.2008.00555.x
41. Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Diabetes Care. (2015) 38:793–800. doi: 10.2337/dc14–2585
42. Dyck PJ, Clark VM, Overland CJ, Davies JL, Pach JM, Dyck PJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care. (2012) 35:584–91. doi: 10.2337/dc11–1421
43. Papanas N, Ziegler D. Polyneuropathy in impaired glucose tolerance: is postprandial hyperglycemia the main culprit? A mini-review. Gerontology. (2013) 59:193–8. doi: 10.1159/000343988
44. Bodman MA, Varacallo M. Peripheral diabetic neuropathy. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023).
45. Chang KC, Pai YW, Lin CH, Lee IT, Chang MH. The association between hyperlipidemia, lipid-lowering drugs and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus. PloS One. (2023) 18:e0287373. doi: 10.1371/journal.pone.0287373
46. Shen J, Liu M, Liu X, Xu X, Gong H, Liu H, et al. Relationship between postprandial C-peptide level and diabetic peripheral neuropathy in Chinese type 2 diabetes patients. Int J Clin Exp Pathol. (2016) 9:9318–24.
47. Wang W, Ji Q, Ran X, Li C, Kuang H, Yu X, et al. Prevalence and risk factors of diabetic peripheral neuropathy: A population-based cross-sectional study in China. Diabetes Metab Res Rev. (2023) 39:e3702. doi: 10.1002/dmrr.3702
48. Alvarez M, Sierra OR, Saavedra G, Moreno S. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: a cross-sectional study. Endocr Connect. (2019) 8:1324–29. doi: 10.1530/EC-19–0382
49. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:41. doi: 10.1038/s41572-019-0092-1
50. Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab (Seoul). (2016) 31:230–8. doi: 10.3803/EnM.2016.31.2.230
51. Gylfadottir SS, Weeracharoenkul D, Andersen ST, Niruthisard S, Suwanwalaikorn S, Jensen TS. Painful and non-painful diabetic polyneuropathy: Clinical characteristics and diagnostic issues. J Diabetes Investig. (2019) 10:1148–57. doi: 10.1111/jdi.13105
52. Sharma S, Rayman G. Frontiers in diagnostic and therapeutic approaches in diabetic sensorimotor neuropathy (DSPN). Front Endocrinol (Lausanne). (2023) 14:1165505. doi: 10.3389/fendo.2023.1165505
53. Apfel SC, Asbury AK, Bril V, Burns TM, Campbell JN, Chalk CH, et al. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. J Neurol Sci. (2001) 189:3–5. doi: 10.1016/s0022–510x(01)00584–6
54. Freeman R. Not all neuropathy in diabetes is of diabetic etiology: differential diagnosis of diabetic neuropathy. Curr Diabetes Rep. (2009) 9:423–31. doi: 10.1007/s11892–009-0069–7
55. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. (2014) 126:3–22. doi: 10.1016/B978–0-444–53480-4.00001–1
56. Malik RA, Andag-Silva A, Dejthevaporn C, Hakim M, Koh JS, Pinzon R, et al. Diagnosing peripheral neuropathy in South-East Asia: A focus on diabetic neuropathy. J Diabetes Investig. (2020) 11:1097–103. doi: 10.1111/jdi.13269
57. Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. (2005) 28:1480–1. doi: 10.2337/diacare.28.6.1480
58. Diabetes Canada Clinical Practice Guidelines Expert Committee, Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. (2018) 42 Suppl 1:S10–5. doi: 10.1016/j.jcjd.2017.10.003
59. Ziegler D, Landgraf R, Lobmann R, Reiners K, Rett K, Schnell O, et al. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract. (2018) 139:147–54. doi: 10.1016/j.diabres.2018.02.043
60. Ponirakis G, Elhadd T, Chinnaiyan S, Dabbous Z, Siddiqui M, Al-Muhannadi H, et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig. (2019) 10:1558–64. doi: 10.1111/jdi.13037
61. Ponirakis G, Elhadd T, Chinnaiyan S, Hamza AH, Sheik S, Kalathingal MA, et al. Prevalence and risk factors for diabetic neuropathy and painful diabetic neuropathy in primary and secondary healthcare in Qatar. J Diabetes Investig. (2021) 12:592–600. doi: 10.1111/jdi.13388
62. Paisey RB, Abbott A, Levenson R, Harrington A, Browne D, Moore J, et al. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the South-West of England. Diabetes Med. (2018) 35:53–62. doi: 10.1111/dme.13512
63. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/S2213–8587(19)30081–6
64. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diabetes Rep. (2019) 19:86. doi: 10.1007/s11892–019-1212–8
65. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. (2008) 31:464–9. doi: 10.2337/dc07–1796
66. Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M, TURNEP Study Group. Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol. (2011) 28:51–5. doi: 10.1097/WNP.0b013e3182051334
67. Genç S, Evren B, Çankaya C, Tecellioğlu M, Bozbay A, Yavuz AÖ, et al. Vascular complications and associated comorbidities in newly diagnosed pre-diabetes: is it the tip of the iceberg? Eur Rev Med Pharmacol Sci. (2023) 27:7557–68. doi: 10.26355/eurrev_202308_33407
68. Papanas N, Ziegler D. Prediabetic neuropathy: does it exist? Curr Diabetes Rep. (2012) 12:376–83. doi: 10.1007/s11892–012-0278–3
69. Ziegler D, Strom A, Lobmann R, Reiners K, Rett K, Schnell O. High prevalence of diagnosed and undiagnosed polyneuropathy in subjects with and without diabetes participating in a nationwide educational initiative (PROTECT study). J Diabetes Complications. (2015) 29:998–1002. doi: 10.1016/j.jdiacomp.2015.09.008
70. Weisman A, Bril V, Ngo M, Lovblom LE, Halpern EM, Orszag A, et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PloS One. (2013) 8:e58783. doi: 10.1371/journal.pone.0058783
71. American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S231–43. doi: 10.2337/dc24-S012
72. Nabrdalik K, Kwiendacz H, Moos J, Moos Ł, Kulpa J, Brzoza Z, et al. Diabetic peripheral neuropathy is associated with diabetic kidney disease and cardiovascular disease: the Silesia diabetes-heart project. Curr Probl Cardiol. (2023) 48:101726. doi: 10.1016/j.cpcardiol.2023.101726
73. Issar T, Arnold R, Kwai NCG, Walker S, Yan A, Borire AA, et al. Relative contributions of diabetes and chronic kidney disease to neuropathy development in diabetic nephropathy patients. Clin Neurophysiol. (2019) 130:2088–95. doi: 10.1016/j.clinph.2019.08.005
74. Castelli G, Desai KM, Cantone RE. Peripheral neuropathy: evaluation and differential diagnosis. Am Fam Physician. (2020) 102:732– 9.
75. Doughty CT, Seyedsadjadi R. Approach to peripheral neuropathy for the primary care clinician. Am J Med. (2018) 131:1010–6. doi: 10.1016/j.amjmed.2017.12.042
76. Willison HJ, Winer JB. Clinical evaluation and investigation of neuropathy. J Neurol Neurosurg Psychiatry. (2003) 74 Suppl 2:ii3–8. doi: 10.1136/jnnp.74.suppl_2.ii3
77. Callaghan BC, Kerber KA, Lisabeth LL, Morgenstern LB, Longoria R, Rodgers A, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. (2014) 71:1143–9. doi: 10.1001/jamaneurol.2014.1279
78. Rosenberg NR, Portegies P, de Visser M, Vermeulen M. Diagnostic investigation of patients with chronic polyneuropathy: Evaluation of a clinical guideline. J Neurol Neurosurg Psychiatry. (2001) 71:205–9. doi: 10.1136/jnnp.71.2.205
79. Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. (2010) 42:157–64. doi: 10.1002/mus.21661
80. Tan LS. The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract. (2010) 90:1–7. doi: 10.1016/j.diabres.2010.06.021
81. Shabeeb D, Najafi M, Hasanzadeh G, Hadian MR, Musa AE, Shirazi A. Electrophysiological measurements of diabetic peripheral neuropathy: A systematic review. Diabetes Metab Syndr. (2018) 12:591–600. doi: 10.1016/j.dsx.2018.03.026
82. Mauermann ML, Burns TM. The evaluation of chronic axonal polyneuropathies. Semin Neurol. (2008) 28:133–51. doi: 10.1055/s-2008–1062270
83. Carrington AL, Shaw JE, Van Schie CH, Abbott CA, Vileikyte L, Boulton AJ. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. (2002) 25:2010–5. doi: 10.2337/diacare.25.11.2010
84. Løseth S, Mellgren SI, Jorde R, Lindal S, Stålberg E. Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev. (2010) 26:100–6. doi: 10.1002/dmrr.1049
85. Ince H, Taşdemir HA, Aydin M, Ozyürek H, Tilki HE. Evaluation of nerve conduction studies in obese children with insulin resistance or impaired glucose tolerance. J Child Neurol. (2015) 30:989–99. doi: 10.1177/0883073814550188
86. Serhiyenko VA, Serhiyenko LM, Sehin VB, Serhiyenko AA. Pathophysiological and clinical aspects of the circadian rhythm of arterial stiffness in diabetes mellitus: A minireview. Endocr Regul. (2022) 56:284–94. doi: 10.2478/enr-2022–0031
87. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetrical polyneuropathy: A definition for clinical research. A report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Arch Phys Med Rehabil. (2005) 86:167–74. doi: 10.1016/j.apmr.2004.09.011
88. Aso Y. Updates in diabetic neuropathy: A call for new diagnostic and treatment approaches. J Diabetes Investig. (2022) 13:432–34. doi: 10.1111/jdi.13711
89. Lauria G, Lombardi R. Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ. (2007) 334:1159–62. doi: 10.1136/bmj.39192.488125.BE
90. Petropoulos IN, Ponirakis G, Ferdousi M, Azmi S, Kalteniece A, Khan A, et al. Corneal confocal microscopy: A biomarker for diabetic peripheral neuropathy. Clin Ther. (2021) 43:1457–75. doi: 10.1016/j.clinthera.2021.04.003
91. Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. (2013) 154:1807–19. doi: 10.1016/j.pain.2013.05.047
92. Papanas N, Ziegler D. New vistas in the diagnosis of diabetic polyneuropathy. Endocrine. (2014) 47:690–8. doi: 10.1007/s12020–014-0285-z
93. Bilen H, Atmaca A, Akcay G. Neuropad indicator test for diagnosis of sudomotor dysfunction in type 2 diabetes. Adv Ther. (2007) 24:1020–7. doi: 10.1007/BF02877707
94. Gewandter JS, Gibbons CH, Campagnolo M, Lee J, Chaudari J, Ward N, et al. Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology. (2019) 93:346–60. doi: 10.1212/WNL.0000000000007974
95. Ferraresi R, Casini A, Caminiti M, Losurdo F, Clerici G. [Multidisciplinary approach to diabetic foot: a challenge of expertises]. G Ital Cardiol (Rome). (2018) 19:495–503. doi: 10.1714/2951.29668
96. Jiménez S, Rubio JA, Álvarez J, Lázaro-Martínez JL. Analysis of recurrent ulcerations at a multidisciplinary diabetic Foot unit after implementation of a comprehensive Foot care program. Endocrinol Diabetes Nutr (Engl Ed). (2018) 65:438.e1–10. doi: 10.1016/j.endinu.2018.03.012
97. Cernea S, Raz I. Management of diabetic neuropathy. Metabolism. (2021) 123:154867. doi: 10.1016/j.metabol.2021.154867
98. Javed S, Alam U, Malik RA. Treating diabetic neuropathy: present strategies and emerging solutions. Rev Diabetes Stud. (2015) 12:63–83. doi: 10.1900/RDS.2015.12.63
99. Didangelos T, Karlafti E, Kotzakioulafi E, Kontoninas Z, Margaritidis C, Giannoulaki P, et al. Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin B12, and carnitine for 12 months in patients with diabetic neuropathy. Nutrients. (2020) 12:3254. doi: 10.3390/nu12113254
100. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. (1993) 329:977–86. doi: 10.1056/NEJM199309303291401
101. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. (1995) 38:869–80. doi: 10.1002/ana.410380607
102. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. (2003) 348:383–93. doi: 10.1056/NEJMoa021778
103. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. doi: 10.1056/NEJMoa0802987
104. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. (2010) 376:419–30. doi: 10.1016/S0140–6736(10)60576–4
105. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. (2009) 360:129–39. doi: 10.1056/NEJMoa0808431
106. Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. (2020) 72:863–72. doi: 10.1111/jphp.13241
107. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. (2012) 6:CD007543. doi: 10.1002/14651858.CD007543.pub2
108. Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Res. (2016) 5:F1000 Faculty Rev–738. doi: 10.12688/f1000research.7898.1
109. Ishibashi F, Taniguchi M, Kosaka A, Uetake H, Tavakoli M. Improvement in neuropathy outcomes with normalizing hbA1c in patients with type 2 diabetes. Diabetes Care. (2019) 42:110–8. doi: 10.2337/dc18–1560
110. Nishikawa T, Sasahara T, Kiritoshi S, Sonoda K, Senokuchi T, Matsuo T, et al. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. (2003) 26:1507–12. doi: 10.2337/diacare.26.5.1507
111. Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. (2013) 36:3208–15. doi: 10.2337/dc13–0012
112. Pan J, Yan X, Li F, Zhang Y, Jiang L, Wang C. Association of glycemic variability assessed by continuous glucose monitoring with subclinical diabetic polyneuropathy in type 2 diabetes patients. J Diabetes Investig. (2022) 13:328–35. doi: 10.1111/jdi.13652
113. Callaghan BC, Reynolds EL, Banerjee M, Akinci G, Chant E, Villegas-Umana E, et al. Dietary weight loss in people with severe obesity stabilizes neuropathy and improves symptomatology. Obes (Silver Spring). (2021) 29:2108–18. doi: 10.1002/oby.23246
114. Zilliox LA, Russell JW. Physical activity and dietary interventions in diabetic neuropathy: a systematic review. Clin Auton Res. (2019) 29:443–55. doi: 10.1007/s10286–019-00607-x
115. Ghavami H, Radfar M, Soheily S, Shamsi A, Khalkhali HR. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, result of a randomized clinical trial. Agri. (2018) 30:165–70. doi: 10.5505/agri.2018.45477
116. Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. (2015) 77:146–53. doi: 10.1002/ana.24310
117. Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. (2020) 25:76–84. doi: 10.1111/jns.12387
118. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. (1998) 317:703–13.
119. Gæde P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. (2016) 59:2298–307. doi: 10.1007/s00125–016-4065–6
120. Sandbæk A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screendetected diabetes: a randomised controlled trial: the ADDITION-Europe study. Diabetes Care. (2014) 37:2015–23. doi: 10.2337/dc13–1544
121. Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension. (2011) 58:776–83. doi: 10.1161/HYPERTENSIONAHA.111.174474
122. Didangelos T, Tziomalos K, Margaritidis C, Kontoninas Z, Stergiou I, Tsotoulidis S, et al. Efficacy of administration of an angiotensin converting enzyme inhibitor for two years on autonomic and peripheral neuropathy in patients with diabetes mellitus. J Diabetes Res. (2017) 2017:6719239. doi: 10.1155/2017/6719239
123. Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. (2014) 2:894–900. doi: 10.1016/S2213–8587(14)70173–1
124. Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: The Fremantle Diabetes Study. Diabetologia. (2008) 51:562–6. doi: 10.1007/s00125–007-0919–2
125. Kristensen FP, Christensen DH, Callaghan BC, Kahlert J, Knudsen ST, Sindrup SH, et al. Statin therapy and risk of polyneuropathy in type 2 diabetes: A danish cohort study. Diabetes Care. (2020) 43:2945–52. doi: 10.2337/dc20–1004
126. Tierney EF, Thurman DJ, Beckles GL, Cadwell BL. Association of statin use with peripheral neuropathy in the U.S. population 40 years of age or older. J Diabetes. (2013) 5:207–15. doi: 10.1111/1753–0407.12013
127. Hammad MA, Syed Sulaiman SA, Alghamdi S, Mangi AA, Aziz NA, Mohamed Noor DA. Statins-related peripheral neuropathy among diabetic patients. Diabetes Metab Syndr. (2020) 14:341–6. doi: 10.1016/j.dsx.2020.04.005
128. Bönhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev. (2019) 40:153–92. doi: 10.1210/er.2018–00107
129. Papanas N, Ziegler D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother. (2014) 15:2721–31. doi: 10.1517/14656566.2014.972935
130. Vincent AM, Edwards JL, Sadidi M, Feldman EL. The antioxidant response as a drug target in diabetic neuropathy. Curr Drug Targets. (2008) 9:94–100. doi: 10.2174/138945008783431754
131. Sadeghiyan Galeshkalami N, Abdollahi M, Najafi R, Baeeri M, Jamshidzade A, Falak R, et al. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sci. (2019) 216:101–10. doi: 10.1016/j.lfs.2018.10.055
132. Dugbartey GJ, Alornyo KK, N’guessan BB, Atule S, Mensah SD, Adjei S. Supplementation of conventional anti-diabetic therapy with alpha-lipoic acid prevents early development and progression of diabetic nephropathy. BioMed Pharmacother. (2022) 149:112818. doi: 10.1016/j.biopha.2022.112818
133. Jeffrey S, Samraj PI, Raj BS. The role of alpha-lipoic acid supplementation in the prevention of diabetes complications: a comprehensive review of clinical trials. Curr Diabetes Rev. (2021) 17:e011821190404. doi: 10.2174/1573399817666210118145550
134. Bast A, Haenen GR. Lipoic acid: a multifunctional antioxidant. Biofactors. (2003) 17:207–13. doi: 10.1002/biof.5520170120
135. Capece U, Moffa S, Improta I, Di Giuseppe G, Nista EC, Cefalo CMA, et al. Alpha-lipoic acid and glucose metabolism: A comprehensive update on biochemical and therapeutic features. Nutrients. (2022) 15:18. doi: 10.3390/nu15010018
136. Guo H, Chen X, Zhang H, Zhang X. Serum homocysteine levels and diabetic neuropathy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Clin Exp Med. (2016) 9:19588–94.
137. Hu H, Wang C, Jin Y, Meng Q, Liu Q, Liu K, et al. Alpha-lipoic acid defends homocysteine-induced endoplasmic reticulum and oxidative stress in HAECs. BioMed Pharmacother. (2016) 80:63–72. doi: 10.1016/j.biopha.2016.02.022
138. Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Möller W, et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic Res. (1999) 31:171–9. doi: 10.1080/10715769900300721
139. Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. (2006) 29:2365–70. doi: 10.2337/dc06–1216
140. Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. (2012) 2012:456279. doi: 10.1155/2012/456279
141. Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabetes Med. (2004) 21:114–21. doi: 10.1111/j.1464–5491.2004.01109.x
142. Ibrahimpasic K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med Arch. (2013) 67:7–9. doi: 10.5455/medarh.2013.67.7–9
143. Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. (2011) 34:2054–60. doi: 10.2337/dc11–0503
144. Ziegler D, Low PA, Freeman R, Tritschler H, Vinik AI. Predictors of improvement and progression of diabetic polyneuropathy following treatment with α-lipoic acid for 4 years in the NATHAN 1 trial. J Diabetes Complications. (2016) 30:350–6. doi: 10.1016/j.jdiacomp.2015.10.018
145. Cassanego G, Rodrigues P, De Freitas Bauermann L, Trevisan G. Evaluation of the analgesic effect of α-lipoic acid in treating pain disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2022) 177:106075. doi: 10.1016/j.phrs.2022.106075
146. Jermendy G, Rokszin G, Fábián I, Kempler P, Wittmann I. Morbidity and mortality of patients with diabetic neuropathy treated with pathogenetically oriented alpha-lipoic acid versus symptomatic pharmacotherapies - A nationwide database analysis from Hungary. Diabetes Res Clin Pract. (2023) 201:110734. doi: 10.1016/j.diabres.2023.110734
147. Fogacci F, Rizzo M, Krogager C, Kennedy C, Georges CMG, Knežević T, et al. Safety evaluation of α-lipoic acid supplementation: a systematic review and meta-analysis of randomized placebo-controlled clinical studies. Antioxidants (Basel). (2020) 9:1011. doi: 10.3390/antiox9101011
148. Ruessmann HJ, German Society of out patient diabetes centres AND (Arbeitsgemeinschaft niedergelassener diabetologisch tätiger Arzte e.V.). Switching from pathogenetic treatment with alpha-lipoic acid to gabapentin and other analgesics in painful diabetic neuropathy: a real-world study in outpatients. J Diabetes Complications. (2009) 23:174–7. doi: 10.1016/j.jdiacomp.2008.02.002
149. Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Khatibi SR, et al. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metabolism. (2018) 87:56–69. doi: 10.1016/j.metabol.2018.07.002
150. Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol. (2009) 54:391–8. doi: 10.1097/fjc.0b013e3181be7554
151. Eason RC, Archer HE, Akhtar S, Bailey CJ. Lipoic acid increases glucose uptake by skeletal muscles of obese-diabetic ob/ob mice. Diabetes Obes Metab. (2002) 4:29–35. doi: 10.1046/j.1463–1326.2002.00171.x
152. Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, et al. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. (2001) 50:1464–71. doi: 10.2337/diabetes.50.6.1464
153. Karalis DT, Karalis T, Karalis S, Kleisiari AS, Malakoudi F, Maimari KE. The effect of alpha-lipoic acid on diabetic peripheral neuropathy and the upcoming depressive disorders of type II diabetics. Cureus. (2021) 13:e12773. doi: 10.7759/cureus.12773
154. Abubaker SA, Alonazy AM, Abdulrahman A. Effect of alpha-lipoic acid in the treatment of diabetic neuropathy: A systematic review. Cureus. (2022) 14:e25750. doi: 10.7759/cureus.25750
155. Ziegler D, Papanas N, Schnell O, Nguyen BDT, Nguyen KT, Kulkantrakorn K, et al. Current concepts in the management of diabetic polyneuropathy. J Diabetes Investig. (2021) 12:464–75. doi: 10.1111/jdi.13401
156. Azmi S, Alam U, Burgess J, Malik RA. State-of-the-art pharmacotherapy for diabetic neuropathy. Expert Opin Pharmacother. (2021) 22:55–68. doi: 10.1080/14656566.2020.1812578
157. Paul A, Kumar M, Das P, Guha N, Rudrapal M, Zaman MK. Drug repurposing - A search for novel therapy for the treatment of diabetic neuropathy. BioMed Pharmacother. (2022) 156:113846. doi: 10.1016/j.biopha.2022.113846
158. Baba M, Matsui N, Kuroha M, Wasaki Y, Ohwada S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. (2019) 10:1299–306. doi: 10.1111/jdi.13013
159. Jang HN, Oh TJ. Pharmacological and nonpharmacological treatments for painful diabetic peripheral neuropathy. Diabetes Metab J. (2023) 47:743–56. doi: 10.4093/dmj.2023.0018
160. Neuropathic pain in adults: pharmacological management in non-specialist settings. London: National Institute for Health and Care Excellence (NICE (2020).
161. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:162–73. doi: 10.1016/S1474–4422(14)70251–0
162. Amato Nesbit S, Sharma R, Waldfogel JM, Zhang A, Bennett WL, Yeh HC, et al. Nonpharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin. (2019) 35:15–25. doi: 10.1080/03007995.2018.1497958
163. Fan Q, Gordon Smith A. Recent updates in the treatment of diabetic polyneuropathy. Fac Rev. (2022) 11:30. doi: 10.12703/r/11–30
164. Racaru S, Sturt J, Celik A. The effects of psychological interventions on diabetic peripheral neuropathy: A systematic review and meta-analysis. Pain Manag Nurs. (2021) 22:302–11. doi: 10.1016/j.pmn.2020.11.001
165. Yang W, Cai X, Wu H, Ji L. Associations between metformin use and vitamin B12 levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J Diabetes. (2019) 11:729–43. doi: 10.1111/1753–0407.12900
166. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. (2013) 310:2435–42. doi: 10.1001/jama.2013.280490
167. Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. (2016) 101:1754–61. doi: 10.1210/jc.2015–3754
168. Didangelos T, Karlafti E, Kotzakioulafi E, Margariti E, Giannoulaki P, Batanis G, et al. Vitamin B12 supplementation in diabetic neuropathy: A 1-year, randomized, double-blind. Placebo-Controlled Trial Nutrients. (2021) 13:395. doi: 10.3390/nu13020395
169. Sun Y, Lai MS, Lu CJ. Effectiveness of vitamin B12 on diabetic neuropathy: systematic review of clinical controlled trials. Acta Neurol Taiwan. (2005) 14:48–54.
170. Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast. (2013) 2013:424651. doi: 10.1155/2013/424651
171. Kulkantrakorn K. Pyridoxine-induced sensory ataxic neuronopathy and neuropathy: revisited. Neurol Sci. (2014) 35:1827–30. doi: 10.1007/s10072–014-1902–6
172. Gupta K, Jain A, Rohatgi A. An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabetes Metab Syndr. (2018) 12:51–8. doi: 10.1016/j.dsx.2017.08.014
Keywords: diabetic peripheral neuropathy, expert panel, multidisciplinary approach, pathogenesis, awareness, screening, clinical diagnosis, pathogenetically-oriented pharmacotherapy
Citation: Atmaca A, Ketenci A, Sahin I, Sengun IS, Oner RI, Erdem Tilki H, Adas M, Soyleli H and Demir T (2024) Expert opinion on screening, diagnosis and management of diabetic peripheral neuropathy: a multidisciplinary approach. Front. Endocrinol. 15:1380929. doi: 10.3389/fendo.2024.1380929
Received: 02 February 2024; Accepted: 15 May 2024;
Published: 17 June 2024.
Edited by:
Keiichiro Susuki, Wright State University, United StatesReviewed by:
Kazunori Sango, Tokyo Metropolitan Institute of Medical Science, JapanCopyright © 2024 Atmaca, Ketenci, Sahin, Sengun, Oner, Erdem Tilki, Adas, Soyleli and Demir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aysegul Atmaca, YXRtYWNhX2F5c2VndWxAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.