- 1Department of Neurology & Neuro-ophthalmology, The First Hospital of Xi’an (The First Affiliated Hospital of Northwestern University), Xi’an, China
- 2Xi’an Key Laboratory for Innovation and Translation of Neuroimmunological Diseases, Xi’an, China
Background: Central retinal artery occlusion (CRAO) is a medical condition characterized by sudden blockage of the central retinal artery, which leads to a significant and often irreversible loss of vision. Observational studies have indicated that diabetes mellitus is a risk factor for CRAO; however, there is no research on the causal relationship between diabetes mellitus, particularly type 2 diabetes, and CRAO. This study aimed to perform Mendelian randomization (MR) analysis to clarify the causal relationship between type 2 diabetes and CRAO.
Methods: Genetic variants associated with type 2 diabetes were selected from two different datasets. A recent genome-wide association study of CRAO conducted using the FinnGen database was used as the outcome data. A two-sample MR was performed to evaluate the causal relationship between type 2 diabetes and CRAO. Inverse variance weighting was the primary method, and MR-Egger, maximum likelihood, and median weighting were used as complementary methods. A multivariate MR (MVMR) analysis was performed to further evaluate the robustness of the results. Cochran’s Q test, MR-Egger intercept test, and MR-PRESSO global test were used for the sensitivity analyses.
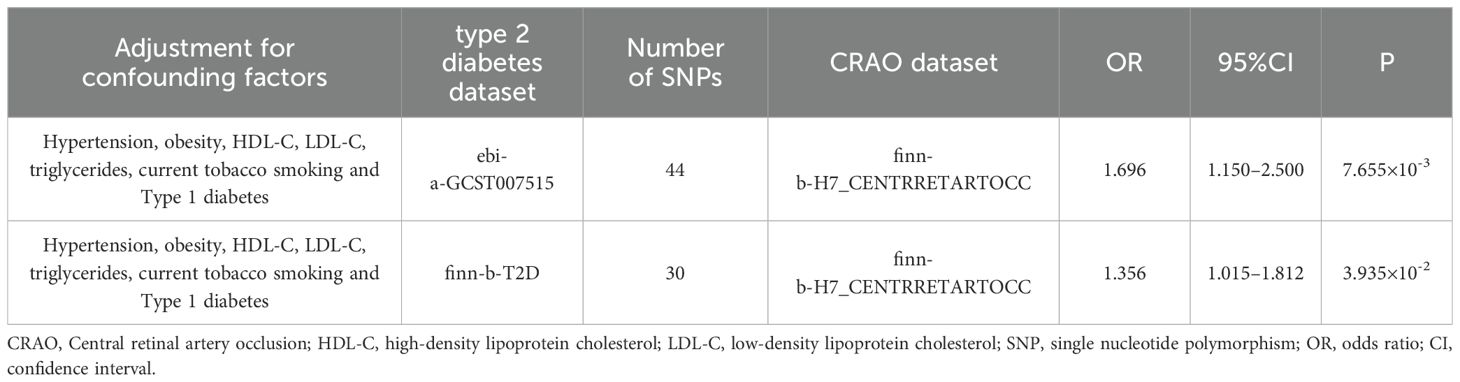
Results: Genetically predicted type 2 diabetes was causally associated with CRAO(odds ratio [OR] =2.108, 95% confidence interval [CI]: 1.221–3.638, P=7.423×10-3), which was consistent with the results from the validation dataset (OR=1.398, 95%CI: 1.015–1.925, P=0.040). The MVMR analysis suggested that type 2 diabetes may be an independent risk factor for CRAO (adjusted OR=1.696; 95%CI=1.150–2.500; P=7.655×10-3), which was assumed by the validation dataset (adjusted OR=1.356; 95%CI=1.015–1.812; P=0.039).
Conclusion: Our results show that genetically predicted type 2 diabetes may be causally associated with CRAO in European populations. This suggests that preventing and controlling type 2 diabetes may reduce the risk of CRAO.
1 Introduction
Central retinal artery occlusion (CRAO) is caused by the obstruction of the central retinal artery by thromboembolism or vasospasm, with or without retinal ischemia (1). The incidence of CRAO is estimated at 1–2/100,000 people per year (2). Clinically, CRAO is typically characterized by a painless and sudden loss of vision (3). Despite over 150 years of research involving intravenous or intra-arterial thrombolysis and conservative treatments such as regulating intraocular pressure or vasodilating retinal vasculature, an optimal management plan for CRAO has not been clearly defined (1).
Previous studies have indicated that CRAO is associated with ipsilateral internal carotid artery stenosis and various cardiovascular risk factors such as obesity, hypertension, tobacco usage, hypercholesterolemia, and diabetes mellitus (1, 4–6). A study by Dziedzic et al. revealed that 81.7% of patients with CRAO were affected by obesity or were overweight as an atherosclerotic risk factor (4). Furthermore, a retrospective analysis of patients with non-inflammatory ocular vascular conditions found that 79.8% of those with CRAO had hypertension (5). In a Polish single-center case-control study, the CRAO group displayed significantly higher risk factors such as hypertension, diabetes, hypercholesterolemia, and smoking compared to the control group (6).
Type 2 diabetes, also known as non-insulin-dependent diabetes mellitus, is a chronic metabolic disorder characterized by hyperglycemia (7). Several studies have examined the clinical characteristics and outcomes of diabetes mellitus in patients with CRAO. Sawada et al. indicated diabetes mellitus as an etiological factor of CRAO in young patients (8). Further, research by Glueck et al. and the European Assessment Group for Lysis in the Eye Study reported diabetes mellitus prevalence rates among CRAO patients of 16% and 14%, respectively (9, 10). Studies on retinal artery occlusion (RAO) patients in the United States indicate that nearly one-quarter of these patients suffer from diabetes (11, 12).
Research on the relationship between type 2 diabetes and CRAO is limited, predominantly comprising observational studies that may have been influenced by confounding factors. To date, no research has investigated the causal relationship between type 2 diabetes and CRAO at the genetic level. Mendelian randomization (MR) is a reliable method for estimating causal relationships and is less susceptible to conventional confounding issues (13). Our study aimed to elucidate the causal relationship between genetic predisposition to type 2 diabetes and CRAO using MR analysis.
2 Methods
2.1 Study design
The Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization guidelines were followed in this MR study (13).
Two-sample magnetic resonance imaging MR and multivariate MR (MVMR) were performed to evaluate the causal relationship between type 2 diabetes and CRAO. Summary statistics were obtained from a genome-wide association study (GWAS). The foundational assumptions for our MR inference included as follows: Assumption 1, that instrument single nucleotide polymorphisms (SNPs) were strongly associated with type 2 diabetes (P<5.0×10-8); assumption 2, the instrumental variable genetic variants were not associated with any potential confounders; and assumption 3, genetic variants influenced CRAO solely through the selected exposure. A brief overview of the research process and foundational assumptions for our MR study is presented in Figure 1. While our study utilized publicly available data that did not require formal ethical approval, we recognize the significant importance of potential risks related to data privacy in genetic research. Since the data are anonymized and publicly accessible, they do not directly involve or impact individual participants. Nevertheless, we maintained rigorous ethical standards in data handling, analysis, and reporting to uphold integrity and privacy respect in studies using public database information.
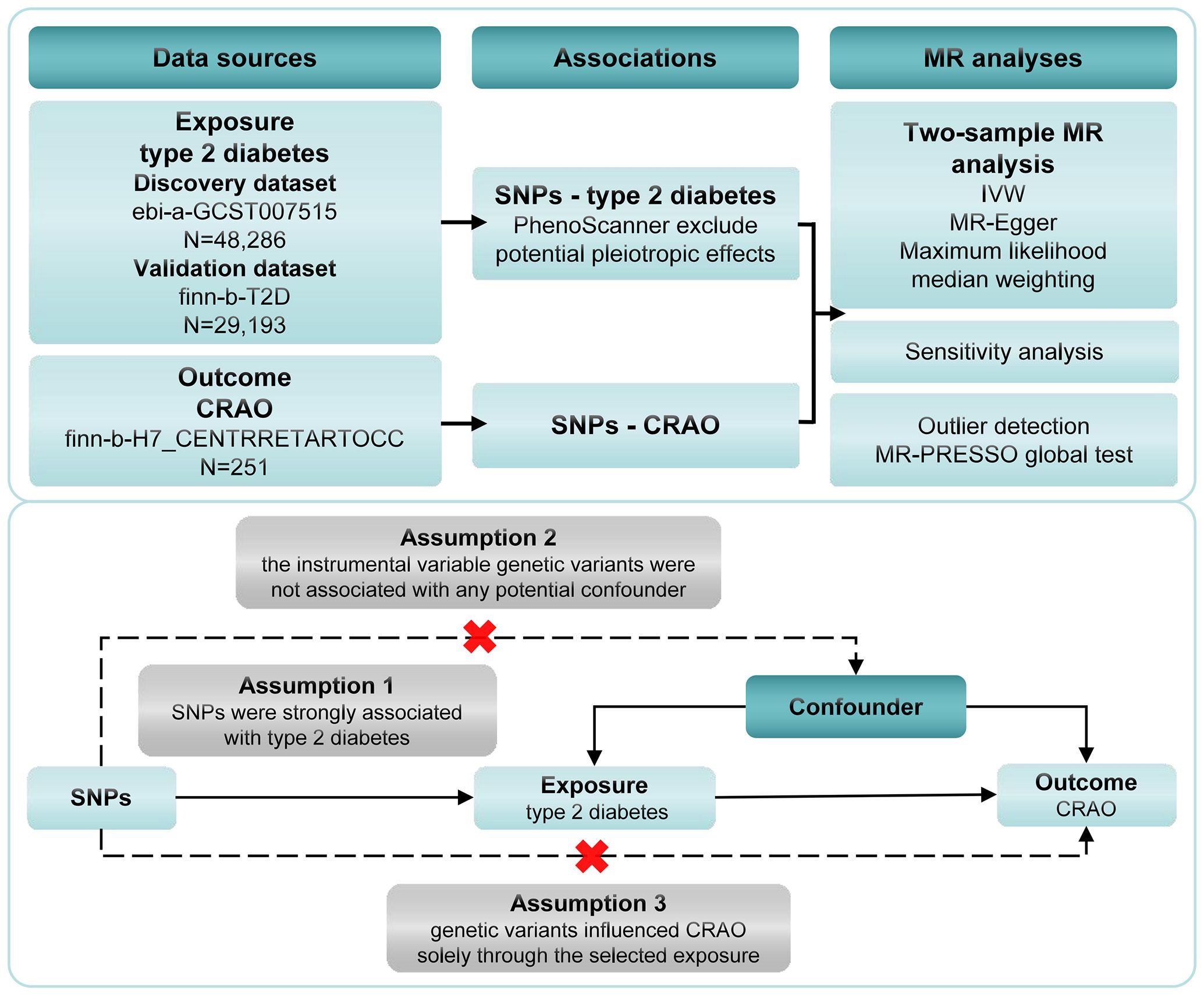
Figure 1. Framework for the Mendelian randomization analysis of type 2 diabetes and CRAO. CRAO, central retinal artery occlusion; SNPs, instrument single nucleotide polymorphisms; IVW, inverse variance weighting.
2.2 Selection of data sources
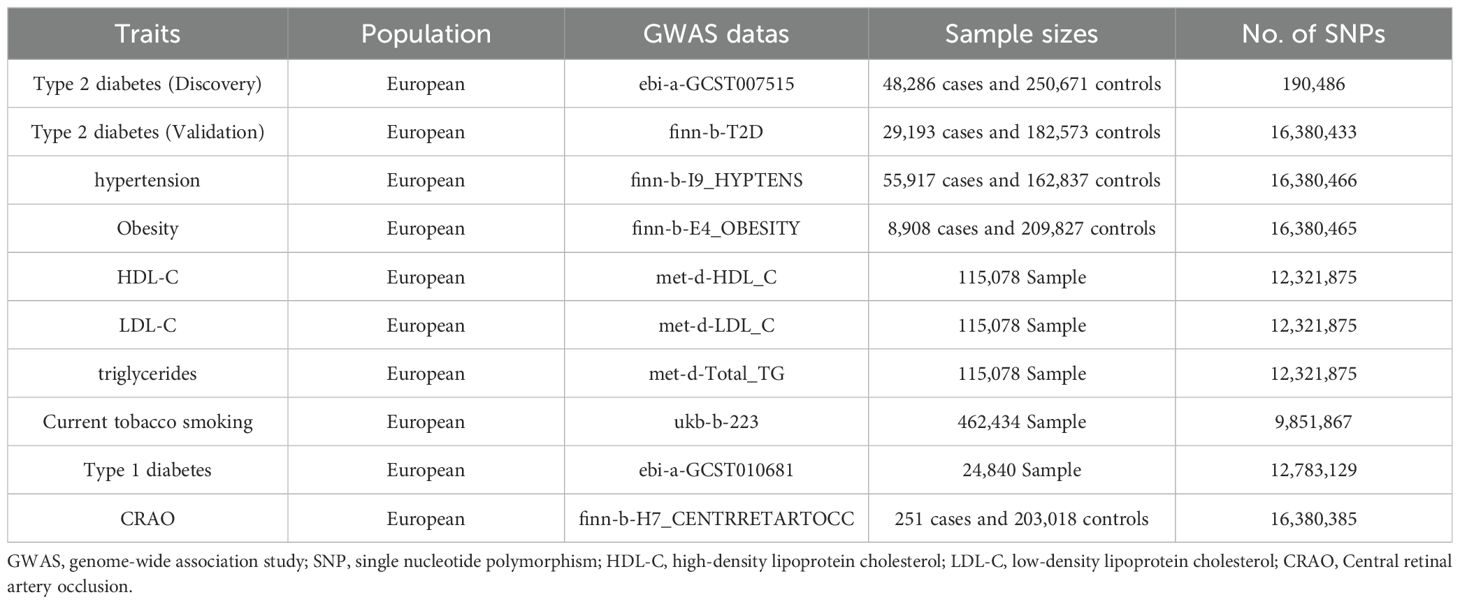
Genetic variants associated with type 2 diabetes were selected from two different datasets. The discovery dataset, pulled from a meta-analysis conducted by Mahajan et al. (14), encompasses SNPs from 48,286 patients and 250,671 controls, all of European ancestry. Similarly, the validation dataset, also based on patients of European ancestry, includes SNPs from 29,193 cases and 182,573 controls. We based the outcome data, on a recent GWAS on CRAO employing the FinnGen database, which comprised 251 cases and 203,018 control participants, all of European ancestry. Due to the low incidence rate of CRAO, sample size estimation was not conducted using conventional methods (2). Table 1 presents the datasets employed for these summary statistics.
We selected SNPs (P<5.0×10−8, linkage disequilibrium R2 < 0.001, clumping window = 10,000), discarded palindromic SNPs, and obtained the primary instrumental variables of type 2 diabetes. Reasons for threshold choices included ensuring that only SNPs with the strongest statistical evidence of association with type 2 diabetes were included, thus minimizing false positives and enhancing result reliability (15). This approach also aimed to reduce the risk of confounding due to neighboring SNPs and improve the validity of causal inference. Additionally, the thresholds were set to reduce redundancy and potential biases from closely linked variants, ultimately providing a more representative set of genetic instruments for studying type 2 diabetes (16).
Each primary instrumental variable was searched using PhenoScanner V2 to identify potential confounders. Any instrumental variable found to be associated with these potential confounders, or those that were not researched, were deleted to exclude potential pleiotropic effects (17). Hypertension, obesity, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and current tobacco smoking have been identified as potential confounders (1). We also removed SNPs associated with type 1 diabetes to enhance rigor. Although research has demonstrated a heightened risk of diabetes mellitus associated with CRAO, distinctions between type 1 and type 2 diabetes have not been elucidated. Conversely, type 1 diabetes is linked with significantly elevated risks of stroke, likely related to CRAO (1, 18). F-statistics were utilized to evaluate the robustness of the instrumental variables for the analyzed SNPs (19).
2.3 Statistical analysis
We ensured that the effect alleles were harmonized across the type 2 diabetes and CRAO datasets. In the two-sample MR analysis, the inverse variance weighting (IVW) method was the main analytical method used to assess the potential pleiotropy of instrument SNPs. MR-Egger, maximum likelihood, and median weighting were used to complement the IVW estimate (13). The MR-PRESSO global test was used to identify and remove outliers (20). Statistical power was calculated with a two-sided significance test (https://shiny.cnsgenomics.com/mRnd/, α=0.05) (21).
In order to enhance the robustness of the results, we utilized MVMR to adjust for potential confounding variables, including hypertension, obesity, HDL-C, LDL-C, triglycerides, current tobacco smoking, and type 1 diabetes (1, 18).
The analysis was conducted using Package TwoSampleMR (version 0.5.7), PRESSO (version 1.0), and MVMR (version 0.4), all of which were executed within the R software environment (version 4.3.1).
2.4 Sensitivity Analyses
Various methods were used for the sensitivity analyses. Cochran’s Q test was utilized to assess the heterogeneity (22). The MR-Egger intercept test was used to evaluate the average directional pleiotropy, and the MR-PRESSO global test was used to adjust for the horizontal pleiotropy (23). Leave-one-out sensitivity analyses was used to detect single SNPs that caused disproportionate. Forest and funnel plots were generated to illustrate pleiotropy directly.
3 Results
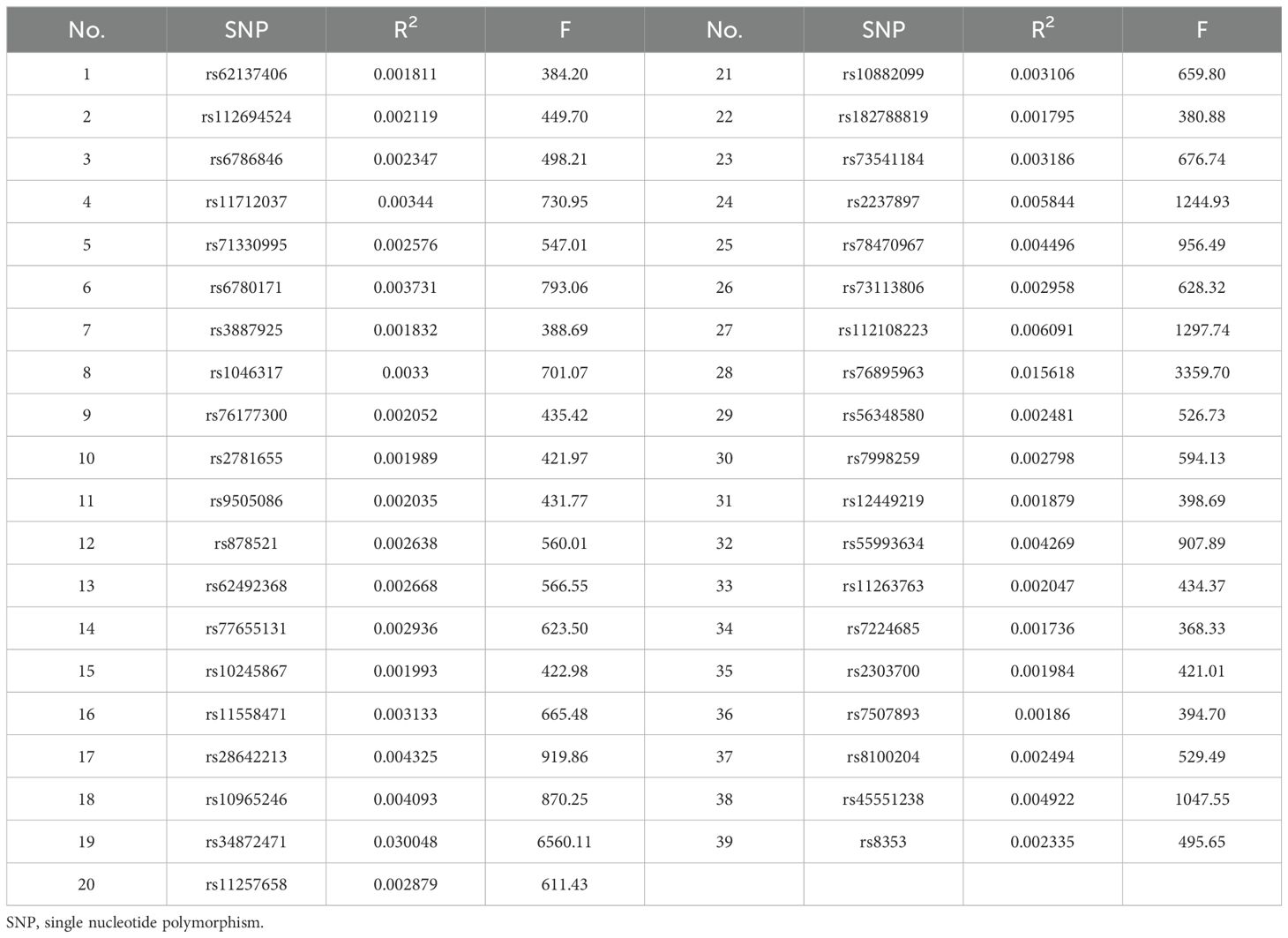
We identified 67 SNPs from the discovery dataset (ebi-a-GCST007515) and 55 SNPs from the validation dataset (finn-b-T2D) as primary instrumental variables (Supplementary Datasets 1, 2). After excluding potential pleiotropic effects (Supplementary Datasets 3, 4), 38 SNPs were screened in the discovery dataset, and the variances ranged from 0.041% to 0.466%. All the F-statistics exceeded the empirical threshold of 10, suggesting that the presence of weak instrument bias was highly unlikely. This indicates that the study has a high capacity to detect real effects. In the validation dataset, the variance explained by the 39 SNPs ranged from 0.174% to 3.005%, and the F-statistics were all greater than 10. The screened SNPs and F-statistics for each dataset are presented in Tables 2, 3.
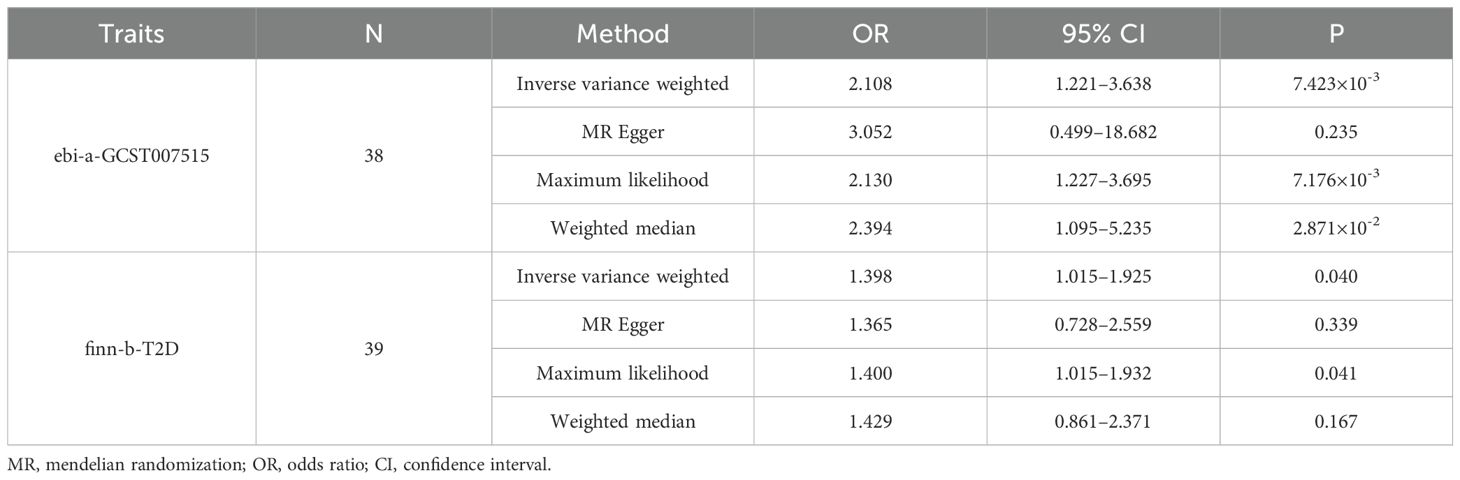
Evaluation of the association between type 2 diabetes and CRAO risk is shown in Table 4. The results of the IVW method indicated that type 2 diabetes was causally associated with a heightened risk of CRAO (odds ratio [OR] =2.108, 95% confidence interval [CI]:1.221–3.638, P=7.423×10-3). This effect was supported by the maximum likelihood (OR=2.130, 95%CI:1.227–3.695, P=7.176×10-3) and weighted median (OR=2.394, 95%CI:1.095–5.235, P=2.871×10-2) methods. The validation dataset confirming the causal effect of type 2 diabetes yielded consistent conclusions. Moreover, the MR-PRESSO global test did not detect outliers in any of the instrumental variables used.
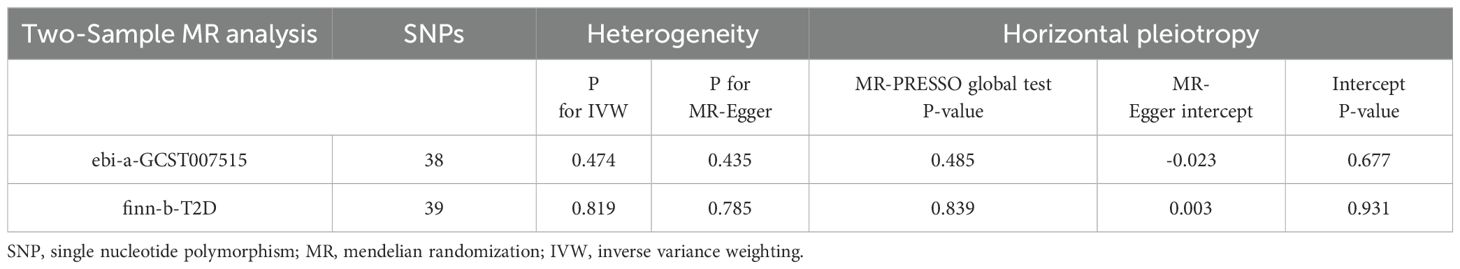
Sensitivity analyses demonstrated no presence of heterogeneity and horizontal pleiotropy (Table 5). In the discovery dataset, the Cochran’s Q test derived P was 0.474 for IVW and 0.435 for MR-Egger, indicating no heterogeneity. The MR-Egger intercept test showed no evidence of a significant intercept (intercept=-0.023, P=0.677). In addition, the MR-PRESSO global test did not identify any evidence of horizontal pleiotropy (P=0.485). Moreover, sensitivity analyses of the validation dataset was consistent with those of the discovery dataset. The scatter plots depicted the causal relationship between type 2 diabetes and CRAO (Figures 2, 3). Leave-one-out analysis indicated that no individual genetic variant had strong influence on the association between type 2 diabetes and CRAO (Supplementary Figures).
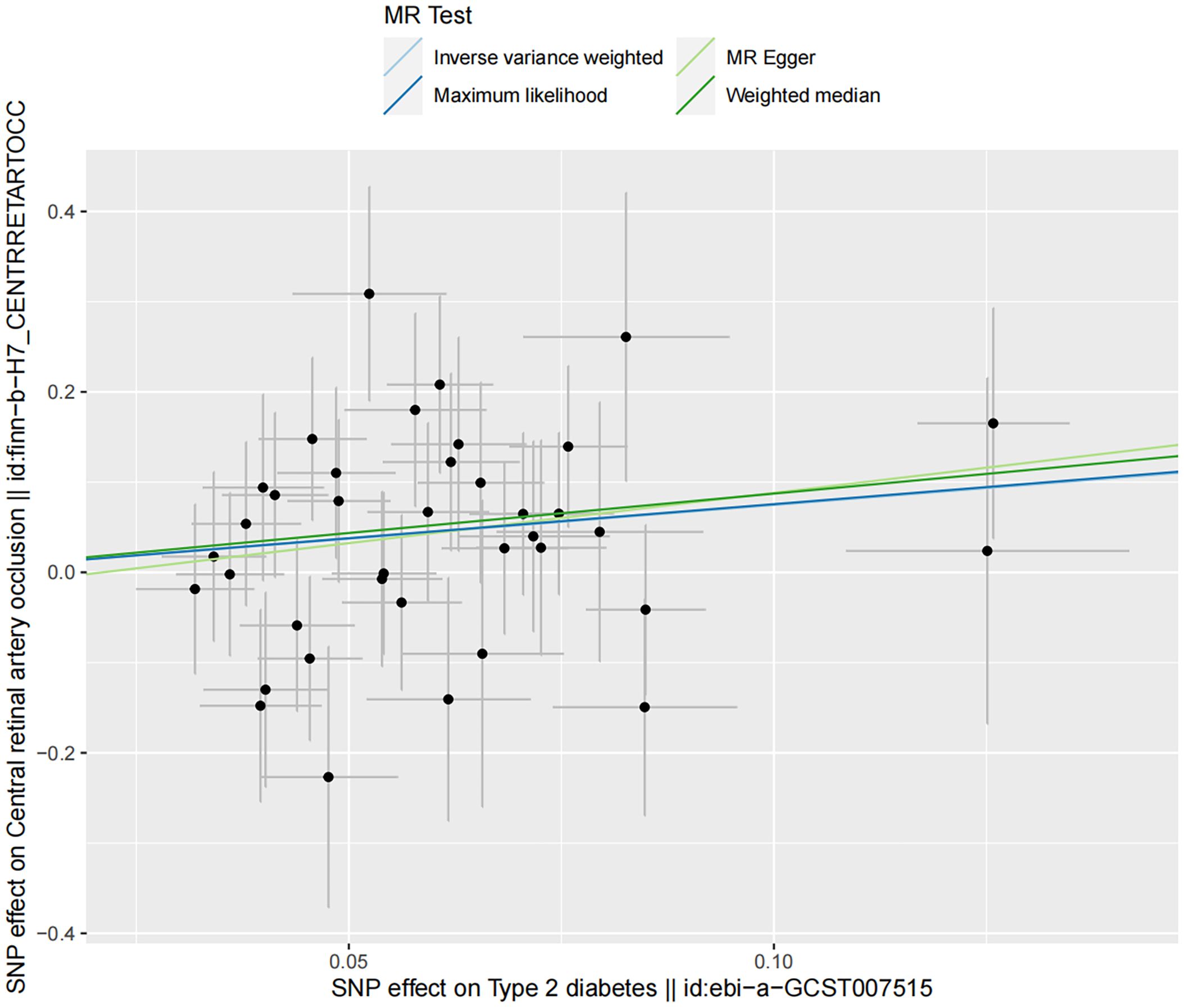
Figure 2. Scatter plot illustrating the risk of CRAO associated with type 2 diabetes, derived from the dataset ebi-a-GCST007515, analyzed using IVW, MR-Egger, Maximum Likelihood, and Median Weighting methods.
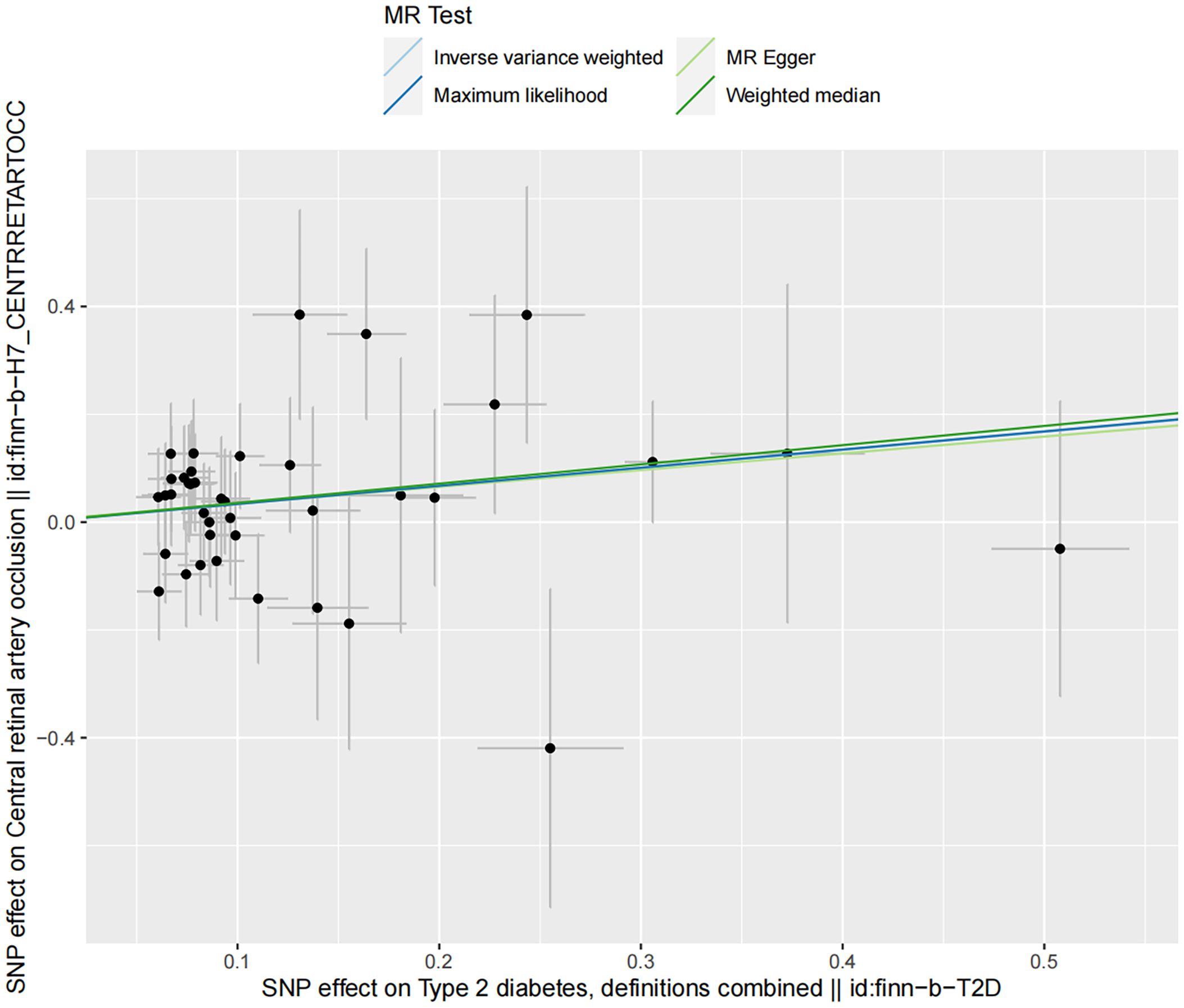
Figure 3. Scatter plot illustrating the risk of CRAO associated with type 2 diabetes, derived from the dataset finn-b-T2D, analyzed using IVW, MR-Egger, Maximum Likelihood, and Median Weighting methods.
For the discovery dataset, the calculated power value was 98%, indicating a strong ability to detect real effects and determine whether type 2 diabetes acted as a risk factor for CRAO. In the validation dataset, the obtained power value of 70% somewhat supported the findings.
The results of the MVMR analysis showed that type 2 diabetes was associated with a significantly increased risk of CRAO (Table 6).
4 Discussion
MR analysis has been a popular method for establishing causal relationships and has unearthed new perspectives into disease mechanisms. Our study evaluated and suggested the causal relationship between type 2 diabetes and CRAO using patient data based on European ancestry with a two-sample MR.
So far, most studies have focused on the risk of diabetes mellitus on RAO. Furthermore, an analysis of the English national linked Hospital Episode Statistics indicated a significant association between diabetes mellitus and an increased risk of RAO (rate ratio=9.31, 95%CI: 8.26–10.50) based on the population of England (24). Additionally, Calway et al. investigated potential risk factors for perioperative RAO in cardiac surgery from the United States National Inpatient Sample. They observed that diabetes mellitus with ophthalmic complications was a risk factor for RAO during cardiac surgery, while type 2 diabetes was associated with a lower risk (25). However, these studies did not specifically explore RAO subtypes related to diabetes mellitus.
The association between CRAO and diabetes mellitus has rarely been investigated in population-based studies. Hayreh et al. classified RAO into CRAO and branch artery occlusion in a cohort study, demonstrating a significantly higher prevalence of diabetes mellitus compared to that in a matched US population (P< 0.0001) (12, 18). A cohort study encompassing 482,392 participants (mean age, 55.06 years), indicated a 2.28-fold increased risk of CRAO in Taiwanese patients with diabetes mellitus (188 patients with CRAO and diabetes mellitus, 86 patients in the control group) (26). According to a Scientific Statement From the American Heart Association, the incidence of CRAO is heightened with the presence of vascular risk factors such as hypertension, hyperlipidemia, diabetes mellitus, tobacco exposure, and obesity, as supported by some studies (1). Notably, the current study did not differentiate between diabetes type 1 and 2 in its analysis. Moreover, most risk factors were not consistently adjusted for in the epidemiological data.
Our two-sample MR analysis using the IVW method suggested a significant causal association between type 2 diabetes and CRAO (discovery dataset: OR=2.108, 95%CI: 1.221–3.638, P=7.423×10-3; validation dataset: OR=1.398, 95%CI: 1.015–1.925, P=0.040). This association was confirmed using sensitivity analyses. SNPs associated with potential confounders were removed to reveal the direct effects of type 2 diabetes on CRAO. MVMR analysis suggested that type 2 diabetes was an independent risk factor for CRAO (ebi-a-GCST007515: adjusted OR=1.696, 95%CI=1.150–2.500, P=7.655×10-3; finn-b-T2D: adjusted OR=1.356, 95%CI=1.015–1.812, P=0.039).
Although the biological mechanisms through which diabetes mellitus increases the susceptibility to CRAO remain unclear, several possible mechanisms have been proposed (27). Firstly, the most common cause of CRAO is the obstruction of the central retinal artery due to emboli that originate from the ipsilateral internal carotid artery, aortic arch, or heart (1). Individuals diagnosed with type 2 diabetes show a higher presence of lipid-rich necrotic core (LRNC). In these patients, carotid plaques containing LRNC% > 22.0% are defined as a risk factor for the emergence of acute cerebral infarction lesions restricted to the carotid region (28). Secondly, from a gene-specific perspective, atrial fibrillation patients carrying clonal haematopoiesis of indeterminate potential (CHIP) gene mutations have a higher prevalence of diabetes, and they are also at a higher risk of developing composite clinical events, including ischemic stroke (29). This highlights the genetic connections influencing both cardiovascular and ocular health in diabetic patients. Thirdly, concerning inflammatory, diseases like giant cell arteritis (GCA) that affect the proximal ophthalmic artery can induce simultaneous ischemia of the retina and the optic nerve head (1, 30). Diabetes increases the likelihood of developing GCA, and the subsequent necessity for glucocorticoid therapy in these patients can further elevate the risk of diabetes onset (31–33). Diabetes mellitus also impacts the ocular inflammatory mediators and immune-competent cells (32, 34). Dysregulation of the innate immune system is associated with the occurrence and progression of GCA (35). In cases of poorly controlled diabetes, certain immune responses may lead to dysbiosis of the ocular surface microbiome and an increase in harmful bacteria (32, 36). Additionally, alterations in the gut microbiome of patients with type 2 diabetes could potentially increase the risk of CRAO, suggesting a novel area for further investigation into the systemic conditions affecting ocular health (37).
The incidence of CRAO in patients with diabetes mellitus calls for an urgent, interdisciplinary approach, underscoring the necessity for tight collaboration between neuro-ophthalmologists and endocrinologists. Neuro-ophthalmologists are integral in addressing acute retinal ischemia and enhancing visual outcomes. Their responsibilities include conducting prompt ophthalmological examinations, comprehensive neurological evaluations, and brain computed tomography scans; orchestrating diagnostic procedures; recommending treatments, such as intravenous tissue plasminogen activator (tPA), intra-arterial tPA, or conservative methods; and meticulously monitoring the patient’s condition during treatment (1, 38). Endocrinologists, aware of the severe visual complications associated with CRAO, should offer pertinent health advice to patients with diabetes mellitus, encouraging emergency referrals and advocating for early preventive interventions. Strengthened collaboration between endocrinologists and neuro-ophthalmologists is pivotal in diminishing the risk of CRAO and lessening visual impairment in patients with diabetes mellitus.
Our study possesses several strengths. First, an association between genetic susceptibility to type 2 diabetes and CRAO by MR analysis is reported for the first time. Second, genetic variants associated with type 2 diabetes were selected from two different datasets, and consistent results were obtained. Third, to avoid traditional confounding factors and inverse causality, MVMR was performed to confirm the results of the two-sample MR.
However, our study has some limitations. First, MR analysis using genetic variants as instrumental variables cannot avoid biases caused by population stratification and compensatory processes during development. As our data were derived solely from patients of European ancestry, this may not be representative of other populations. Future studies should employ diverse datasets to explore the causal relationship between type 2 diabetes and CRAO more broadly. Second, the lower power value in the validation dataset likely resulted from its smaller sample size. Future efforts should focus on acquiring larger datasets to enhance statistical robustness and more effectively validate the relationship between type 2 diabetes and CRAO. Third, although we have adjusted for common risk factors for CRAO in our analysis, the influence of other unexplored factors cannot be completely ruled out. Our team is actively collecting case data from CRAO patients. Upcoming research will utilize longitudinal studies and genomics to address the current limitations of our study.
5 Conclusions
In summary, our result show that genetically predicted type 2 diabetes is causally associated with CRAO in two datasets based on patients of European ancestry. This suggests that prevention and management of type 2 diabetes may have the potential to mitigate the risk of CRAO.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
TL: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation. SW: Writing – review & editing, Validation, Supervision. QL: Writing – review & editing, Validation, Supervision. ZL: Writing – review & editing, Validation. XML: Writing – review & editing, Supervision. LP: Writing – review & editing, Validation. XPL: Writing – review & editing, Validation. WG: Writing – review & editing, Validation. PL: Writing – review & editing, Validation. NZ: Writing – review & editing, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Science and Technology Program of Shaanxi Province (2022SF-381; 2022SF-507; 2023-YBSF-048; 2023-YBSF-052); The Project of Shaanxi Administration of Traditional Chinese Medicine (2022-SLRH-LJ-013); The Science and Technology Program of Xi’an City (22YXYJ0061; 22YXYJ0074; 23YXYJ0005); and the Scientific Research Project of the Xi’an Health Commission (2024yb15).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1379549/full#supplementary-material
References
1. Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, et al. Management of central retinal artery occlusion: A scientific statement from the american heart association[J/OL]. Journal. (2021) 52:e282–94. doi: 10.1161/STR.0000000000000366
2. Chodnicki KD, Tanke LB, Pulido JS, Hodge DO, Klaas JP, Olsen TW, et al. Stroke Risk before and after Central Retinal Artery Occlusion: A Population-based Analysis. Ophthalmology. (2022) 129:203–8. doi: 10.1016/j.ophtha.2021.07.017
3. Lu Q, Han X, Liu P, Lin X, Wu S. Central retinal artery occlusion caused by radiation-induced carotid stenosis. Acta Neurol Belg. (2024) 124:371–3. doi: 10.1007/s13760-023-02346-3
4. Dziedzic R, Zareba L, Iwaniec T, Kubicka-Trzaska A, Romanowska-Dixon B, Bazan-Socha S, et al. High prevalence of thrombophilic risk factors in patients with central retinal artery occlusion. Thromb J. (2023) 21:81. doi: 10.1186/s12959-023-00525-z
5. Schmidt D, Hetzel A, Geibel-Zehender A, Schulte-Monting J. Systemic diseases in non-inflammatory branch and central retinal artery occlusion–an overview of 416 patients. Eur J Med Res. (2007) 12:595–603. Available at: https://www.researchgate.net/publication/5823451_Systemic_diseases_in_non-inflammatory_branch_and_central_retinal_artery_occlusion_An_overview_of_416_patients.
6. Dropinski J, Dziedzic R, Kubicka-Trzaska A, Romanowska-Dixon B, Iwaniec T, Zareba L, et al. Central retinal artery occlusion is related to vascular endothelial injury and left ventricular diastolic dysfunction. J Clin Med. (2022) 11:2263. doi: 10.3390/jcm11082263
7. Mambiya M, Shang M, Wang Y, Li Q, Liu S, Yang L, et al. The play of genes and non-genetic factors on type 2 diabetes. Front Public Health. (2019) 7:349. doi: 10.3389/fpubh.2019.00349
8. Sawada T, Harino S, Ikeda T. Central retinal artery occlusion in a patient with fibromuscular dysplasia. Retina. (2004) 24:461–4. doi: 10.1097/00006982-200406000-00023
9. Glueck CJ, Hutchins RK, Jurantee J, Khan Z, Wang P. Thrombophilia and retinal vascular occlusion. Clin Ophthalmol. (2012) 6:1377–84. doi: 10.2147/OPTH.S34627
10. Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. (2015) 122:1881–8. doi: 10.1016/j.ophtha.2015.05.044
11. Schorr EM, Rossi KC, Stein LK, Park BL, Tuhrim S, Dhamoon MS. Characteristics and outcomes of retinal artery occlusion: nationally representative data. Stroke. (2020) 51:800–7. doi: 10.1161/STROKEAHA.119.027034
12. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. (2009) 116:1928–36. doi: 10.1016/j.ophtha.2009.03.006
13. Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
14. Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. (2018) 50:559–71. doi: 10.1038/s41588-018-0084-1
15. International HapMap C. A haplotype map of the human genome. Nature. (2005) 437:1299–320. doi: 10.1038/nature04226
16. Luo P, Yuan Q, Wan X, Yang M, Xu P. A two-sample Mendelian randomization study of circulating lipids and deep venous thrombosis. Sci Rep. (2023) 13:7432. doi: 10.1038/s41598-023-34726-3
17. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
18. Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses' Health Study. Diabetes Care. (2007) 30:1730–5. doi: 10.2337/dc06-2363
19. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
20. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Wang Y, Gao L, Lang W, Li H, Cui P, Zhang N, et al. Serum calcium levels and parkinson's disease: A mendelian randomization study. Front Genet. (2020) 11:824. doi: 10.3389/fgene.2020.00824
22. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
23. Huang J. Mendelian randomization indicates a causal contribution of type 2 diabetes to retinal vein occlusion. Front Endocrinol (Lausanne). (2023) 14:1146185. doi: 10.3389/fendo.2023.1146185
24. Huisingh C, McGwin G Jr. Risk of selected eye diseases in people admitted to hospital for hypertension or diabetes mellitus: record linkage studies. Br J Ophthalmol. (2012) 96:1274. doi: 10.1136/bjophthalmol-2012-302033
25. Calway T, Rubin DS, Moss HE, Joslin CE, Beckmann K, Roth S. Perioperative retinal artery occlusion: risk factors in cardiac surgery from the United States national inpatient sample 1998-2013. Ophthalmology. (2017) 124:189–96. doi: 10.1016/j.ophtha.2016.10.025
26. Chang YS, Ho CH, Chu CC, Wang JJ, Tseng SH, Jan RL. Risk of retinal artery occlusion in patients with diabetes mellitus: A retrospective large-scale cohort study[J/OL]. Journal. (2018) 13:e0201627. doi: 10.1371/journal.pone.0201627
27. Cheung CY, Ikram MK, Klein R, Wong TY. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. (2015) 58:871–85. doi: 10.1007/s00125-015-3511-1
28. Sun B, Li X, Liu X, Ge X, Lu Q, Zhao X, et al. Association between carotid plaque characteristics and acute cerebral infarction determined by MRI in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. (2017) 16:111. doi: 10.1186/s12933-017-0592-9
29. Ahn HJ, An HY, Ryu G, Lim J, Sun C, Song H, et al. Clonal haematopoiesis of indeterminate potential and atrial fibrillation: an east Asian cohort study. Eur Heart J. (2024) 45:778–90. doi: 10.1093/eurheartj/ehad869
30. Zhang Y, Xing Z, Deng A. Unveiling the predictive capacity of inflammatory and platelet markers for central retinal artery occlusion. Thromb Res. (2023) 232:108–12. doi: 10.1016/j.thromres.2023.11.004
31. Abel AS, Yashkin AP, Sloan FA, Lee MS. Effect of diabetes mellitus on giant cell arteritis. J Neuroophthalmol. (2015) 35:134–8. doi: 10.1097/WNO.0000000000000218
32. Monti S, Milanesi A, Klersy C, Tomelleri A, Dagna L, Campochiaro C, et al. Age at diagnosis influences the clinical phenotype, treatment strategies and outcomes in patients with giant cell arteritis: results from the observational GCAGE study on a large cohort of 1004 patients. Ann Rheum Dis. (2023) 82:1098–106. doi: 10.1136/ard-2023-223895
33. Esen I, Arends S, Dalsgaard Nielsen B, Therkildsen P, Hansen I, van 't Ende A, et al. Metabolic features and glucocorticoid-induced comorbidities in patients with giant cell arteritis and polymyalgia rheumatica in a Dutch and Danish cohort. RMD Open. (2023) 9:e002640. doi: 10.1136/rmdopen-2022-002640
34. Tandon M, Sheemar A, Bhatnagar K, Meena S, Shakrawal J. Central retinal artery occlusion in rhino-orbital-cerebral mucormycosis: an inflammatory-prothrombotic state. Asia Pac J Ophthalmol (Phila). (2023) 12:16–20. doi: 10.1097/APO.0000000000000593
35. Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. (2019) 10:1744. doi: 10.3389/fimmu.2019.01744
36. Amorim M, Martins B, Fernandes R. Immune fingerprint in diabetes: ocular surface and retinal inflammation. Int J Mol Sci. (2023) 24:9821. doi: 10.3390/ijms24129821
37. Lincke JB, Christe L, Unterlauft JD, Zinkernagel MS, Zysset-Burri DC. Microbiome and retinal vascular diseases. Am J Pathol. (2023) 193:1675–82. doi: 10.1016/j.ajpath.2023.02.017
Keywords: central retinal artery occlusion, type 2 diabetes, Mendelian randomization, causal relationship, risk factor
Citation: Liu T, Lu Q, Liu Z, Lin X, Peng L, Lu X, Guo W, Liu P, Zhang N and Wu S (2024) Causal association of type 2 diabetes with central retinal artery occlusion: a Mendelian randomization study. Front. Endocrinol. 15:1379549. doi: 10.3389/fendo.2024.1379549
Received: 31 January 2024; Accepted: 25 July 2024;
Published: 08 August 2024.
Edited by:
James Hurley, University of Washington, United StatesReviewed by:
Changzheng Chen, Renmin Hospital of Wuhan University, ChinaJan Kubicek, VSB-Technical University of Ostrava, Czechia
Copyright © 2024 Liu, Lu, Liu, Lin, Peng, Lu, Guo, Liu, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songdi Wu, d3Vzb25nZGlAZ21haWwuY29t
 Tong Liu1,2
Tong Liu1,2 Zhongzhong Liu
Zhongzhong Liu Xuemei Lin
Xuemei Lin Pei Liu
Pei Liu Songdi Wu
Songdi Wu