- 1School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Rehabilitation, Zhongjiang County Hospital of Traditional Chinese Medicine, Deyang, China
- 3Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Diabetic gastroparesis is a common complication in patient with diabetes. Dietary intervention has been widely used in the treatment of diabetic gastroparesis. The aim of this study is to evaluate the role of diet in the treatment of diabetic gastroparesis.
Methods: This systematic review was conducted a comprehensive search of randomized controlled trials using dietary interventions for the treatment of diabetic gastroparesis up to 9 November 2023. The primary outcomes were gastric emptying time and clinical effect, while fasting blood glucose, 2-hour postprandial blood glucose and glycosylated hemoglobin were secondary outcomes. Data analysis was performed using RevMan 5.4 software, and publication bias test was performed using Stata 15.1 software.
Results: A total of 15 randomized controlled trials involving 1106 participants were included in this review. The results showed that patients with diabetic gastroparesis benefit from dietary interventions (whether personalized dietary care alone or personalized dietary care+routine dietary care). Compared with routine dietary care, personalized dietary care and personalized dietary care+routine dietary care can shorten the gastric emptying time, improve clinical efficacy, and reduce the level of fasting blood glucose, 2-hour postprandial blood glucose and glycosylated hemoglobin.
Conclusions: Limited evidence suggests that dietary intervention can promote gastric emptying and stabilize blood glucose control in patients with diabetic gastroparesis. Dietary intervention has unique potential in the treatment of diabetic gastroparesis, and more high-quality randomized controlled trials are needed to further validate our research results.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023481621.
1 Introduction
Diabetes gastroparesis (DGP) is a common complication of diabetes. The symptoms and objective evidence of DGP suggest food retention in the stomach, delayed gastric emptying without mechanical obstruction, and clinical manifestations mainly include postprandial satiety, nausea, vomiting and epigastric pain (1). According to the data of the International diabetes Federation (IDF), the prevalence rate of diabetes is 10.5%, showing an increasing trend year by year, by 2030, there will be 643 million adults with diabetes (2). The prevalence of DGP is positively related to the prevalence of diabetes, and DGP will become more and more common with the increase of diabetes patients (3). Research has shown that DGP patients have poor quality of life and blood sugar control (4), and long-term treatment is needed to effectively control symptoms (5). In addition, compared with diabetes patients without gastroparesis, the mortality of DGP patients will increase due to the occurrence of cardiovascular events (6). Therefore, DGP has become a serious social public health issue that affects the health and quality of life of patients.
At present, the treatment of DGP includes drug therapy, gastric electrical stimulation, endoscopic therapy, surgical treatment, and dietary therapy, all of which can improve gastric emptying (7). However, clinicians mainly focus on gastric prokinetic drugs for the treatment of DGP, such as domperidone, metoclopramide, and mosapride citrate (8, 9). Although these drugs can help alleviate gastrointestinal discomfort in DGP patients, long-term use of them may also cause adverse reactions such as tardade dyskinesia (1). Some DGP patients also choose gastric electrical stimulation therapy, which can improve gastric emptying but can lead to weight gain (10). In addition, endoscopic or surgical treatment is the last choice for DGP patients, used for some refractory DGP (11, 12), but the long-term effects are not satisfactory and increase the economic burden on patients and their families.
As a green, safe, simple and effective alternative therapy, dietary therapy plays an important role in preventing people with impaired fasting blood glucose or impaired glucose tolerance from developing type 2 diabetes (13, 14), and has been widely used in the treatment of DGP. Studies (15, 16) have shown that dietary adjustments are beneficial for DGP patients. The American College of Gastroenterology (ACG) guidelines for gastroparesis also recommend dietary management for DGP patients to correct their nutritional status, increase the likelihood of symptom relief, enhance gastric emptying, and improve their blood sugar control (1). However, patients receive dietary interventions based on physiological principles rather than clinical evidence (17). Therefore, it is particularly important to study the role of diet in DGP treatment based on clinical evidence. At present, it seems that no researchers have summarized and analyzed the clinical evidence of dietary intervention in DGP.
Therefore, it is necessary to improve the understanding of the available evidence by integrating and analyzing the existing clinical evidence of dietary therapy for DGP to help recommend the best dietary interventions for the treatment of DGP. This study included randomized controlled trials of using diet to intervene for patients with DGP and conducted a systematic review and meta-analysis of them, aiming to observe the changes in gastric emptying time (GET), clinical effect (CE), fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hPBG), and glycosylated hemoglobin (HbA1c) levels in patients with DGP after dietary intervention, and to explore the effects of dietary intervention on gastric emptying and blood glucose control in patients with DGP.
2 Methods
2.1 Registration
This review was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (18), and the format was followed the Preferred Reporting Items for Systematic reviews and Meta-analyses (19). We mentioned the reference to the PRISMA flow chart (Figure 1). The registered study protocol of this review was published in PROSPERO (registration number: CRD42023481621).
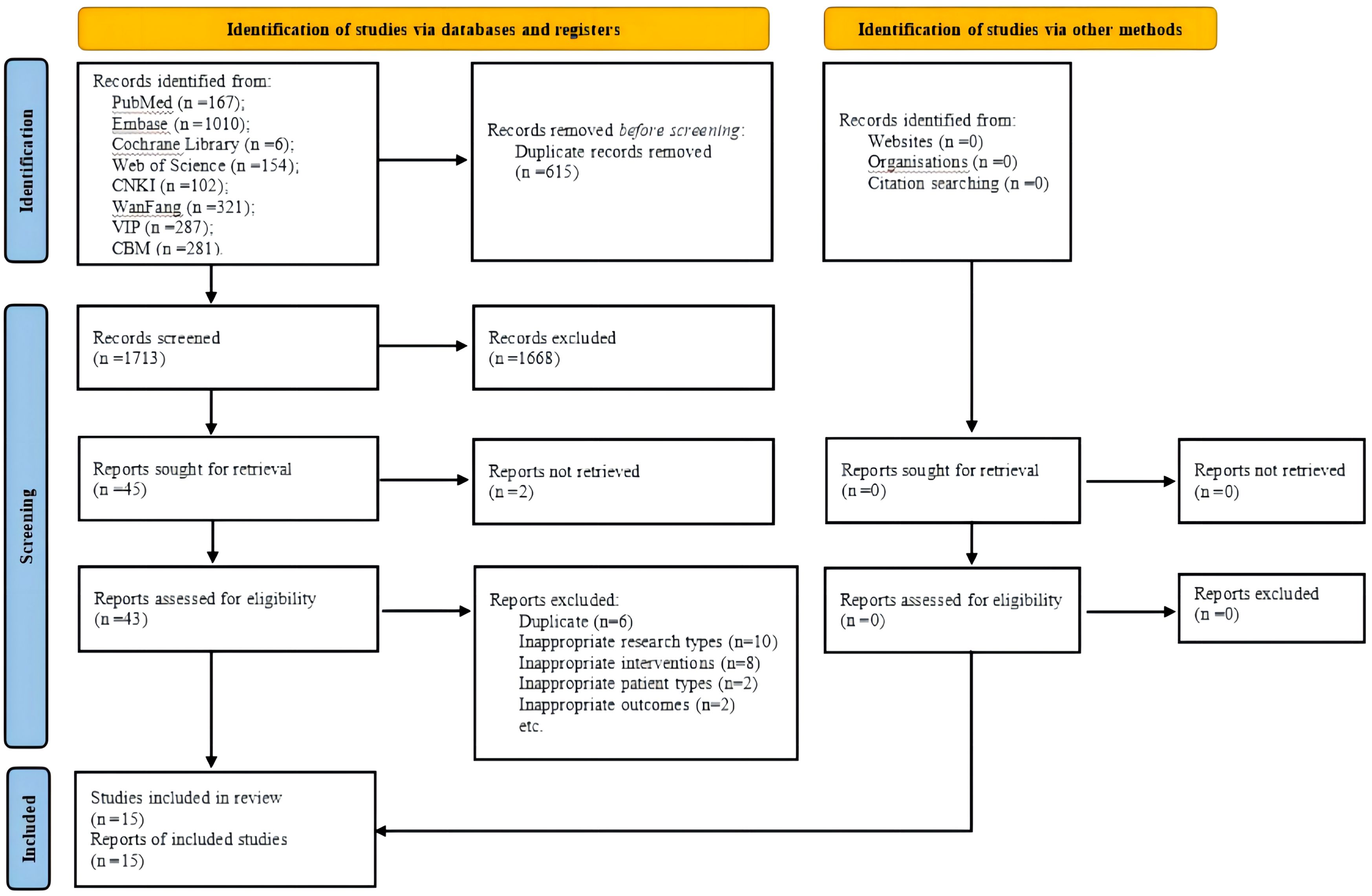
Figure 1 PRISMA flow chart. CNKI, China National Knowledge Infrastructure; VIP, Chongqing VIP Database; CBM, Chinese Biomedical Literature Database.
2.2 Search strategy
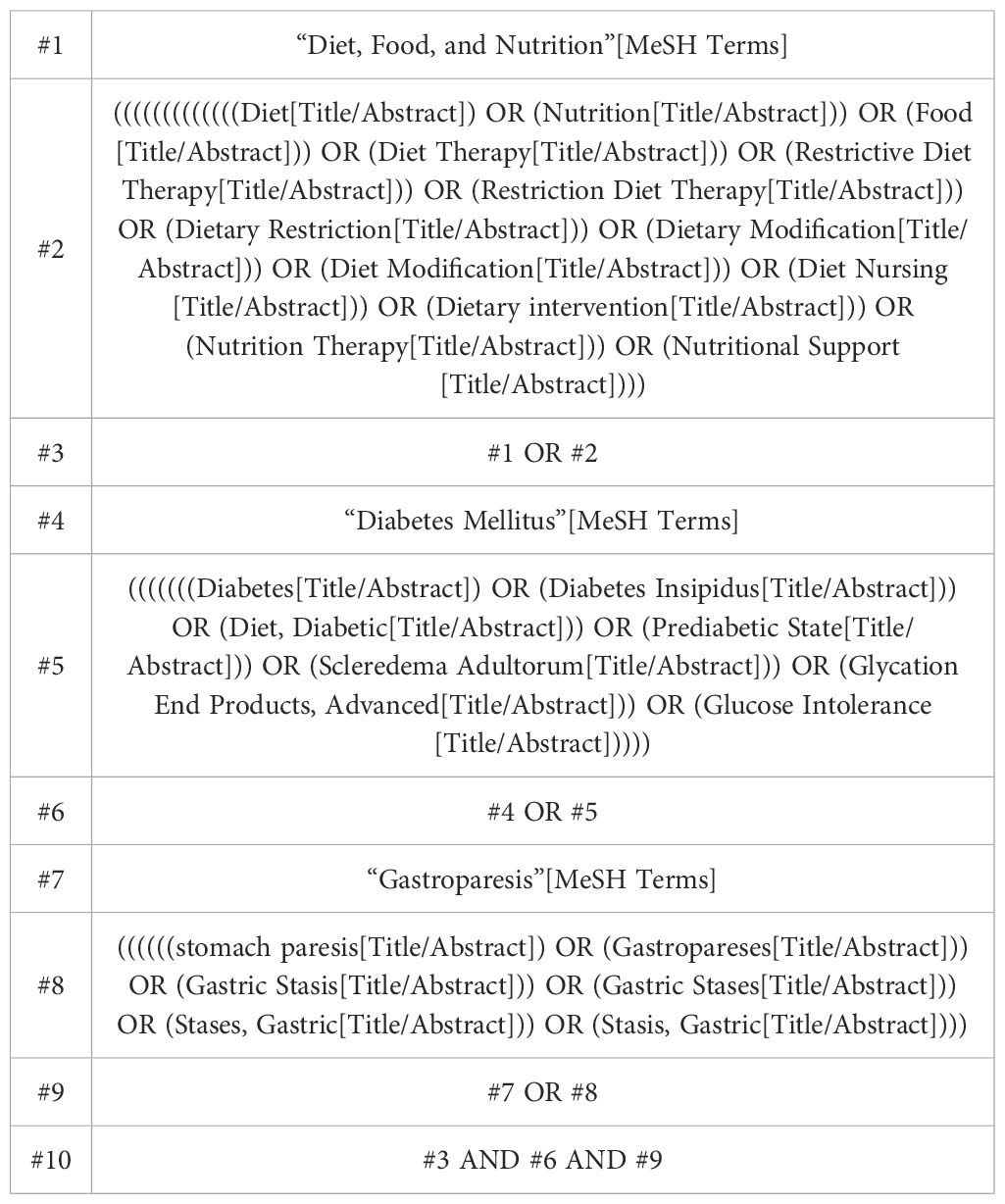
Two authors (HW and YO) systematically searched four English language databases (PubMed, Cochrane Library, EMBASE, and Cochrane Library, and Web of Science) and four Chinese databases (China National Knowledge Infrastructure, Chongqing VIP Database, Wanfang Database, and Chinese Biomedical Literature Database). The eight databases were searched for studies published from the time of database establishment until 9 November 2023. The search strategy combined the main keywords such as “diabetes mellitus”, “gastroparesis”, and “diet, food, and nutrition”. The strategy used to search PubMed databases is shown in Table 1, which has been modified to optimize the search of other databases. The authors also used the reference list of related articles and review articles to find cited articles that were not found in the database search.
2.3 Study selection
2.3.1 Eligibility criteria
1. Patients with DGP over the age of 18 years (No restriction on the type of diabetes).
2. Intervention (s): Personalized dietary intervention, dietary patterns, or personalized dietary care (PDC) combined with routine dietary care (RDC).
3. Comparator(s)/control: All interventions, placebo or routine care.
4. Outcomes: Primary outcomes are GET and CE. Secondry outcomes included FBG, 2hPBG, and HbA1c.
5. Study design: Randomized controlled trials of using diet to intervene for patients with DGP.
2.3.2 Exclusion criteria
1. Studies with repeated data or secondary analysis.
2. Study type does not match (including animal studies, master and doctoral dissertations, books, protocols, conference abstracts, case reports, correspondence, overview, or systematic review).
3. Non-DGP.
4. The therapy of the intervention group was non-personalized dietary intervention, dietary patterns, or personalized dietary intervention combined with routine dietary intervention.
5. Outcome indicators do not match.
6. Language is not Chinese or English.
2.4 Data extraction
Two investigators (LL, QZ and JY) independently extracted data from the included studies using a self-defined standardized extraction format. The extracted data included the name of the primary investigator, publication year, diabetes type, patient age, the country in which the study was performed, study design, dietary intervention mode, type of control intervention, the sample size of each group, intervention duration, randomization, allocation concealment and blinding methods, clinical variables, etc. Any disagreements were solved by consensus between the two investigators. In the event that a consensus could not be reached, a third investigator (DP) made the final judgment. When information was missing from a study, the corresponding author was contacted if the information necessary for correspondence was available.
2.5 Risk-of-bias assessment
Based on the RevMan 5.4 software built-in risk bias assessment tool provided by the Cochrane Collaboration (20), two researchers (YO and LL) evaluated the methodological quality of the included studies. The following seven aspects were chiefly included: 1) random sequence generation (selection bias); 2) allocation concealment (selection bias); 3) performance bias: blinded implementation (including participants, investigators, and outcome assessors); 4) detection bias: blinded evaluation of study results; 5) attrition bias: outcome data integrity; 6) reporting bias: selective reporting of results; 7) other biases. All the above biases were assessed and classified as low, unclear, or high risk of bias. Disagreements were discussed between the two reviewers, and if disagreements were not resolved between the two reviewers, a third reviewer (SP) participated in the discussion until a consensus was reached.
2.6 Evidence quality assessment of included studies
The Grading of Recommendations Assessment, Development, and Evaluations (GRADE) approach was used to rate the overall quality of evidence (21). The GRADE guideline provides evidence ratings for five aspects of included studies: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The GRADE was divided into four levels of gradings for evidence quality: high, moderate, low, and very low. Two researchers (DL and HW) independently performed the assessment, and a third researcher (SP) then reviewed the evaluation. Any disagreement was resolved by discussion with a professional.
2.7 Data analysis
Statistical analysis was performed using RevMan (version 5.4). Continuous variables were evaluated using the mean difference (MD) with a 95% confidence interval (CI) when the measurement methods and units of the outcome indicators included in the study were the same, and the odds ratio (OR) values were used to evaluate the dichotomous variables. Evaluate the heterogeneity among included studies through I2. If I2 was ≤ 50% in the results, use a fixed-effects model. If I2 was > 50% in the results, there is significant heterogeneity between a group of studies, a random-effects model should be adopted to pool the data, and the sources of heterogeneity should be explored. Sensitivity analysis was performed by excluding each RCT sequentially from a group of studies, and comparing the model characteristics to test the robustness of the result. A funnel plot was used to evaluate the publication bias if more than nine trials were included in the meta-analysis. The asymmetry of the funnel plots was evaluated using Egger’s tests, and a P-value of < 0.05 represented significant publication bias (22).
3 Results
3.1 Search results
A total of 2328 potentially eligible articles were identified. After removing 615 duplicates and screening the 1713 remaining articles, 43 candidate studies were isolated. Review of the full text of each shortlisted study based on the eligibility criteria excluded 28 studies. Eventually, 15 studies (23–37) were included in this meta-analysis (Figure 1).
3.2 Study characteristics
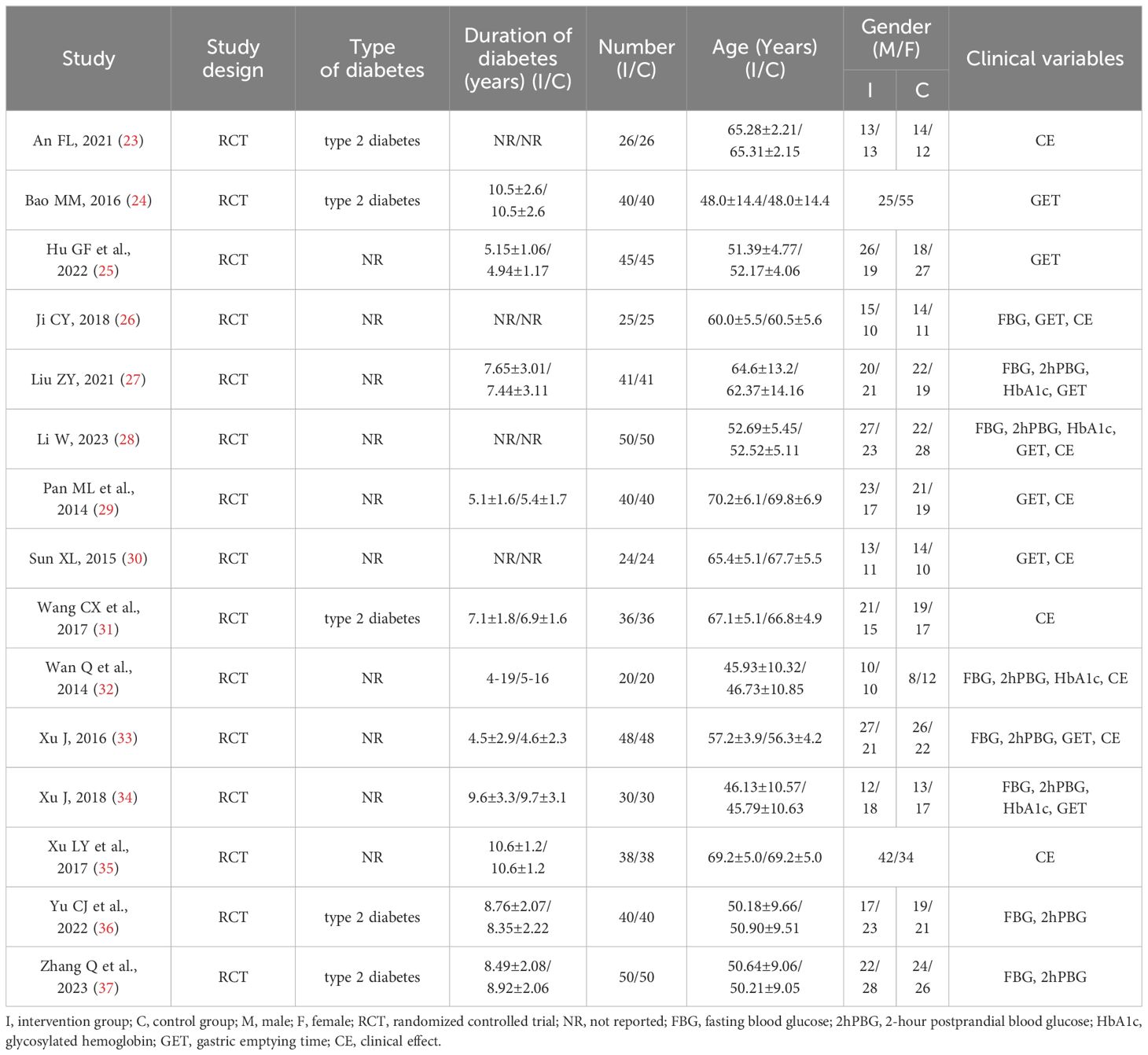
The main characteristics of the 15 included studies are summarized in Table 2.
Patient characteristics: The 15 RCTs (23–37) included 1106 patients diagnosed with DGP, 547 of whom were male and 559 of whom were female. In terms of type of diabetes, five studies (23, 24, 31, 36, 37) included patients with type 2 diabetes, and the other 10 studies did not mention the classification of diabetes. In terms of geographical distribution, all participants are from China.
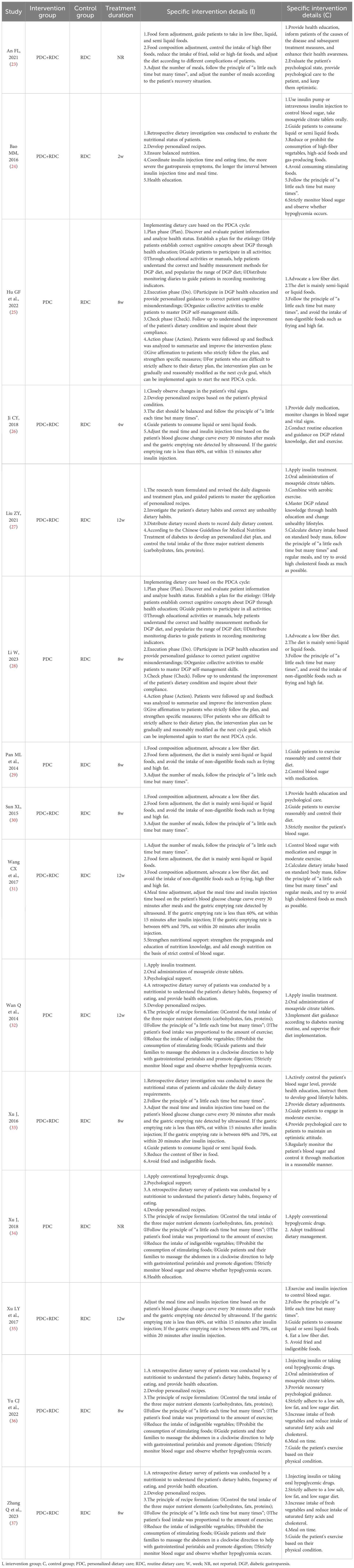
Intervention characteristics: The intervention group involves two dietary related therapies, namely PDC (25, 28, 29, 32, 34) and PDC combined with RDC (23, 24, 26, 27, 30, 31, 33, 35–37). Although there are differences between each study on PDC, there are the following consensus: 1) Retrospective dietary investigation was conducted to evaluate the nutritional status of DGP patients, and to educate them on diet and nutrient-related health knowledge; 2) Develop personalized recipes based on the patient’s physical condition; 3) Adjust food composition: advocate a low-fiber diet; 4) Adjust food form: the diet is mainly semi-liquid or liquid foods, and avoid the intake of non-digestible foods such as frying and high fat; 5) Adjust the number of meals: follow the principle of “a little each time but many times”; 6) Adjust the meal time and insulin injection time based on the patient’s postprandial blood glucose changes and gastric emptying. The specific intervention details of the intervention group are shown in Table 3.
Control characteristics: The control group only involved RDC. RDC mainly includes: 1) Injecting insulin or taking oral hypoglycemic drugs to control blood sugar levels; 2) Oral administration of mosapride citrate tablets; 3) Conduct routine education and guidance on DGP related knowledge, diet and exercise; 4) Provide psychological care to patients to maintain an optimistic attitude. The specific intervention details of the control group are shown in Table 3.
Outcomes: The role of diet in the treatment of DGP was evaluated by subjective or objective outcome indicators such as GET, CE, FBG, 2hPBG and HbA1c.
3.3 Risk of bias
Figure 2A summarizes the risk of bias in the included studies from seven domains. In terms of random sequence generation, four studies (27, 29, 31, 36) explicitly reported low-risk randomization methods. None of the studies mentioned allocation concealment and blinding (including blinding to participants, researchers, and outcome assessors). All included RCTs reported complete outcome measures were rated as low risk. In the field of selective reporting, four trials (27, 33, 34, 37) were evaluated as having a low risk of bias. Regarding the other bias, none of the included studies had any other risk of bias. Figure 2B shows the risk of bias for each included trials.
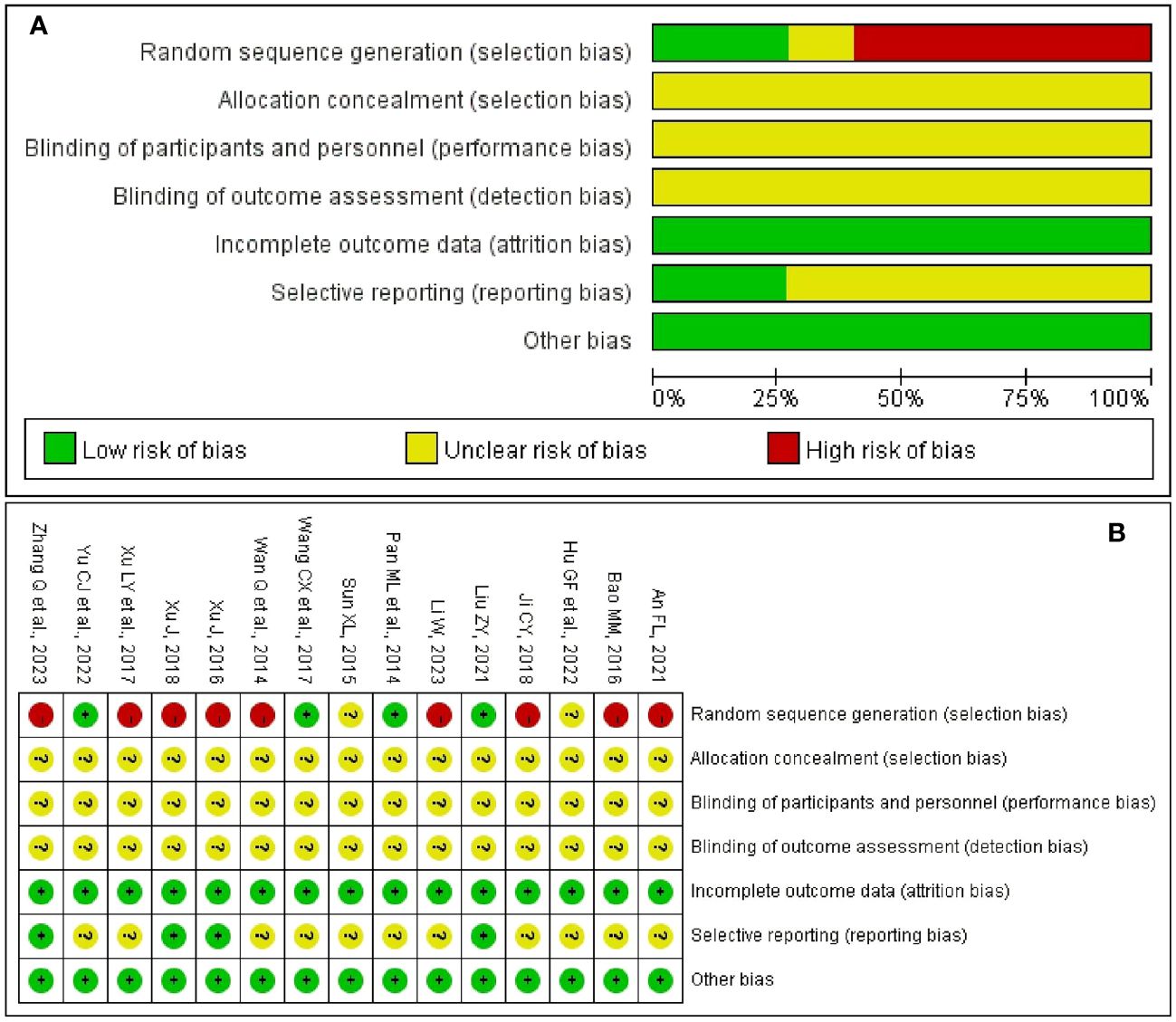
Figure 2 Risk of bias. (A) Risk of bias graph of included trials. (B) Risk of bias summary of included trials.
3.4 Safety
Only one study (24) reported adverse events of diarrhea and constipation in the dietary intervention group, it may be related to the individual physical condition of the participants. No adverse events were reported in the remaining 14 trials (23, 25–37), it may be related to the fact that most of the included studies closely monitor blood sugar to prevent the occurrence of hypoglycemia.
3.5 Meta-analysis
3.5.1 PDC vs. RDC
GET: Four studies (25, 28, 29, 34) included 330 participants and reported the changes in GET. Comprehensive analysis showed that PDC better shortened GET compared to RDC. (MD = -0.51; 95% CI: -0.67 to -0.35; P < 0.00001; I2 = 0%; Figure 3A).
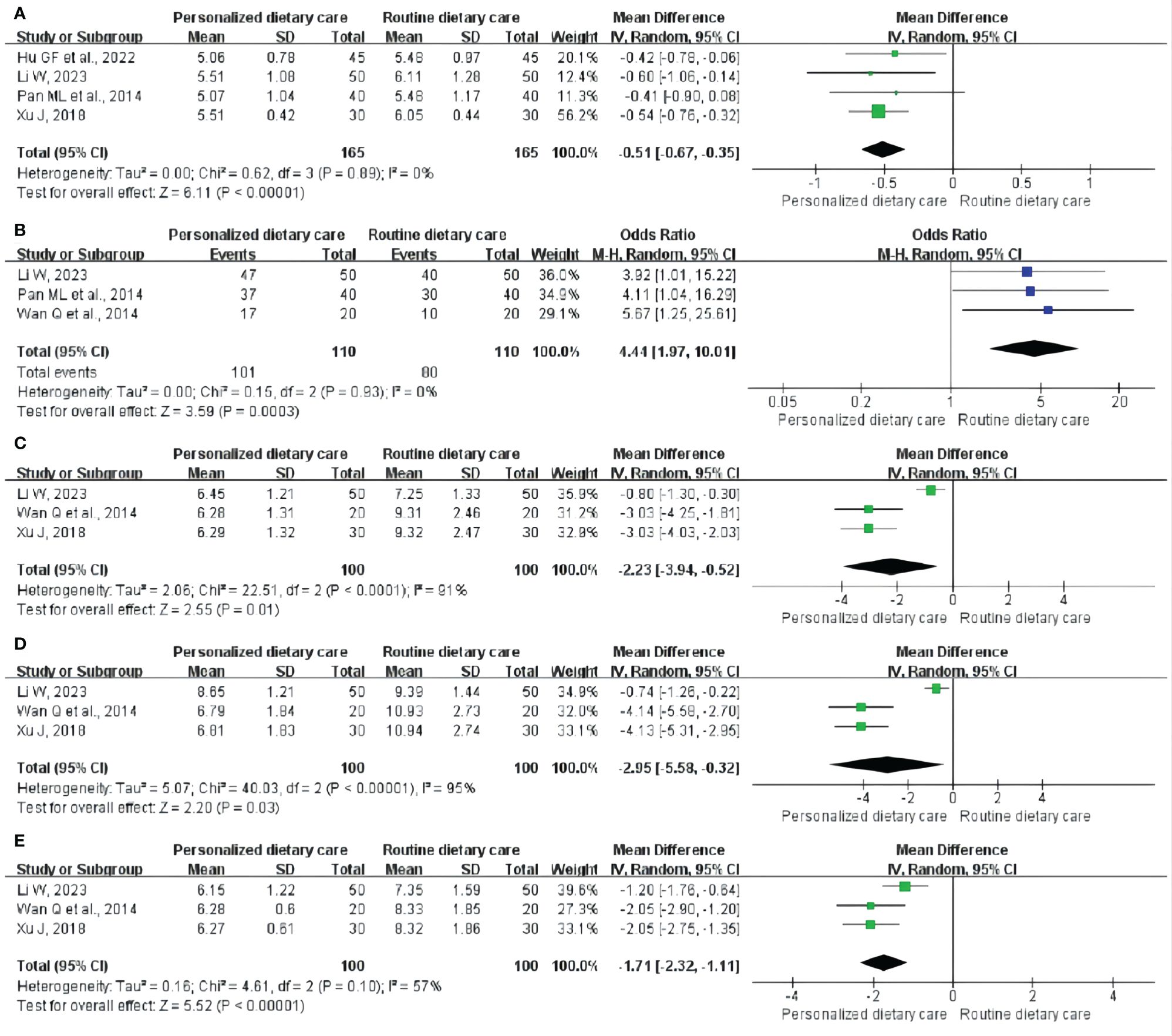
Figure 3 Meta-analysis of PDC versus RDC. (A) Meta-analysis of PDC versus RDC for the GET. (B) Meta-analysis of PDC versus RDC for the CE. (C)Metaanalysis of PDC versus RDC for the FBG. (D) Meta-analysis of PDC versus RDC for the 2hPBG. (E) Meta-analysis of PDC versus RDC for the HbA1c. PDC, personalized dietary care; RDC, routine dietary care; GET, gastric emptying time; CE, clinical effect; FBG, fasting blood glucose; 2hPBG, 2-hour postprandial blood glucose; HbA1c, glycosylated hemoglobin.
CE: CE was observed in three trials (28, 29, 32) involving 220 participants. The analysis showed that compared with RDC, PDC could significantly improve CE in DGP treatment. (MD = 4.44; 95% CI: 1.97 to 10.01; P = 0.0003; I2 = 0%; Figure 3B).
FBG: Three RCTs (28, 32, 34) including 200 participants reported the results, showing that PDC reduced FBG levels in DGP patients compared with RDC. (MD = -2.23; 95% CI: -3.94 to -0.52; P = 0.01; I2 = 91%; Figure 3C).
2hPBG: Three studies (28, 32, 34) involving 200 participants reported 2hPBG. Compared with RDC, PDC could reduce the 2hPBG level in patients with DGP. (MD = -2.95; 95% CI: -5.58 to -0.32; P = 0.03; I2 = 95%; Figure 3D).
HbA1c: Three trials (28, 32, 34) included 200 participants and measured HbA1c levels. Compared to RDC, PDC reduced HbA1c levels in patients with DGP. (MD = -1.71; 95% CI: -2.32 to -1.11; P < 0.00001; I2 = 57%; Figure 3E).
3.5.2 PDC+RDC vs. RDC
GET: Five studies (24, 26, 27, 30, 33) included 356 participants and reported the changes in GET. Comprehensive analysis showed that PDC+RDC better shortened GET compared to RDC. (MD = -0.64; 95% CI: -0.92 to -0.36; P < 0.00001; I2 = 8%; Figure 4A).
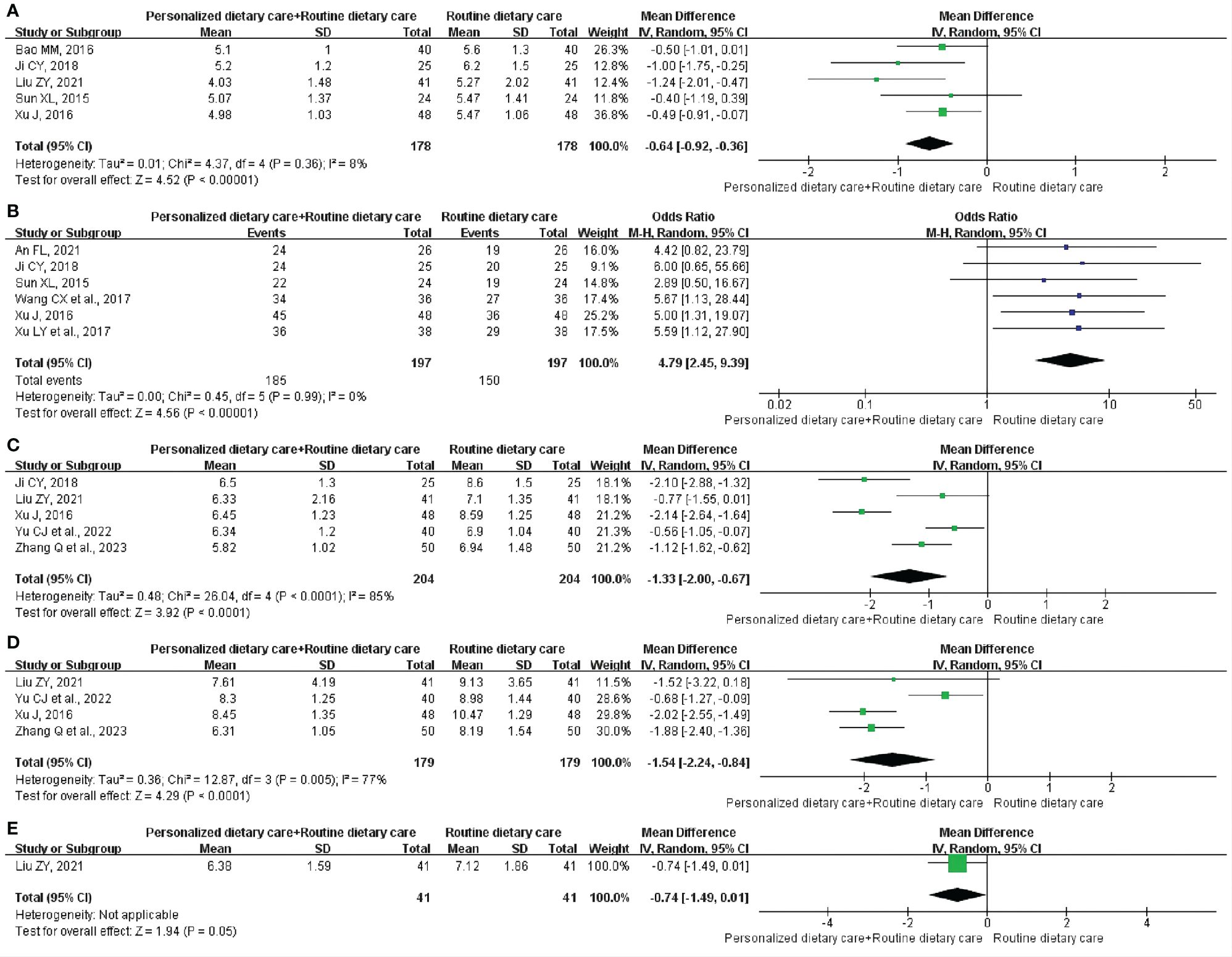
Figure 4 Meta-analysis of PDC+RDC versus RDC. (A) Meta-analysis of PDC+RDC versus RDC for the GET.(B) Meta-analysis of PDC+RDC versus RDC for the CE. (C) Metaanalysis of PDC+RDC versus RDC for the FBG. (D) Meta-analysis of PDC+RDC versus RDC for the 2hPBG. (E) Meta-analysis of PDC+RDC versus RDC for the HbA1c. PDC, personalized dietary care; RDC, routine dietary care; GET, gastric emptying time; CE, clinical effect; FBG, fasting blood glucose; 2hPBG, 2-hour postprandial blood glucose; HbA1c, glycosylated hemoglobin.
CE: CE was observed in six trials (23, 26, 30, 31, 33, 35) involving 394 participants. The analysis showed that compared with RDC, PDC+RDC could significantly improve CE in DGP treatment. (MD = 4.79; 95% CI: 2.45 to 9.39; P < 0.00001; I2 = 0%; Figure 4B).
FBG: Five RCTs (26, 27, 33, 36, 37) including 408 participants reported the results, showing that PDC+RDC reduced FBG levels in DGP patients compared with RDC. (MD = -1.33; 95% CI: -2.00 to -0.67; P < 0.0001; I2 = 85%; Figure 4C).
2hPBG: Four studies (27, 33, 36, 37) involving 358 participants reported 2hPBG. Compared with RDC, PDC+RDC could reduce the 2hPBG level in patients with DGP. (MD = -1.54; 95% CI: -2.24 to -0.84; P < 0.0001; I2 = 77%; Figure 4D).
HbA1c: One trial (27) included 82 participants and measured HbA1c levels. Compared to RDC, PDC+RDC reduced HbA1c levels in patients with DGP. (MD = -0.74; 95% CI: -1.49 to 0.01; P = 0.05; Figure 4E).
3.6 Sensitivity analysis
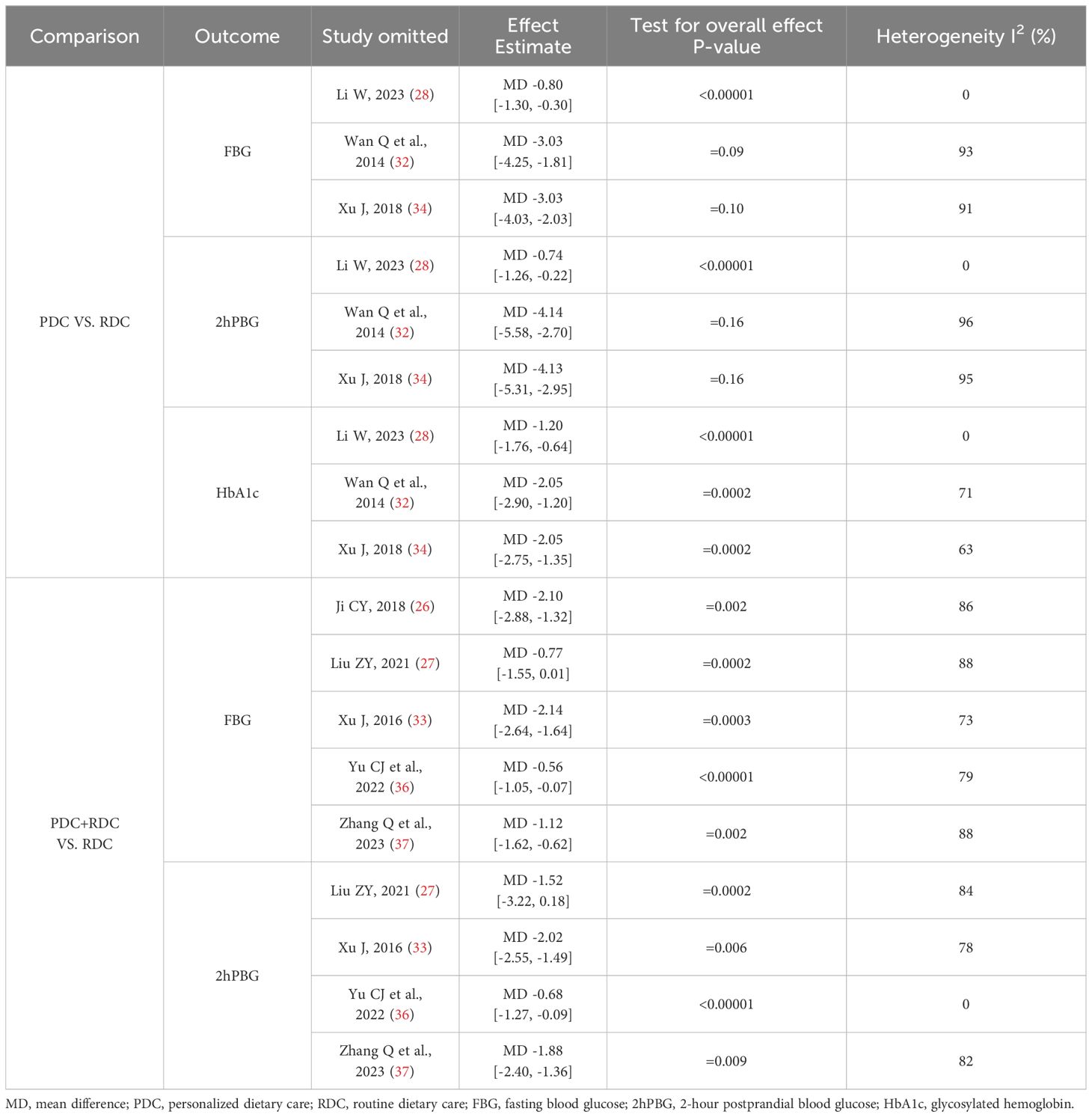
In the meta-analysis, we found that individual variables in the PDC and PDC+RDC groups had high heterogeneity, so we only conducted sensitivity analysis on these variables with high heterogeneity to explore the possible sources of heterogeneity. In the meta-analysis of PDC vs. RDC, we observed significant heterogeneity by omitting individual studies in variables such as FBG, 2hPBG, and HbA1c one by one. After excluding the study of Li W (28), the heterogeneity of research results related to FBG, 2hPBG, and HbA1c was significantly reduced (I2 = 0%). In the meta-analysis of PDC+RDC vs. RDC, by omitting individual trials in the FBG variables one by one, we did not observe significant changes in the results, and these heterogeneity did not affect the stability of the results. When omitting individual RCTs in the 2hPBG variables one by one, we found that after excluding the study by Yu CJ et al. (36), heterogeneity was significantly reduced (I2 = 0%). The specific details of sensitivity analysis are shown in Table 4.
3.7 Publication bias
Nine studies were included in the meta-analysis of GET and CE, so we evaluated their publication bias. The funnel plot (Figure 5) shows an approximate symmetry between them. Egger’s test (Figure 6) also indicated that no significant publication bias (GET: P= 0.256; CE: P= 0.540).
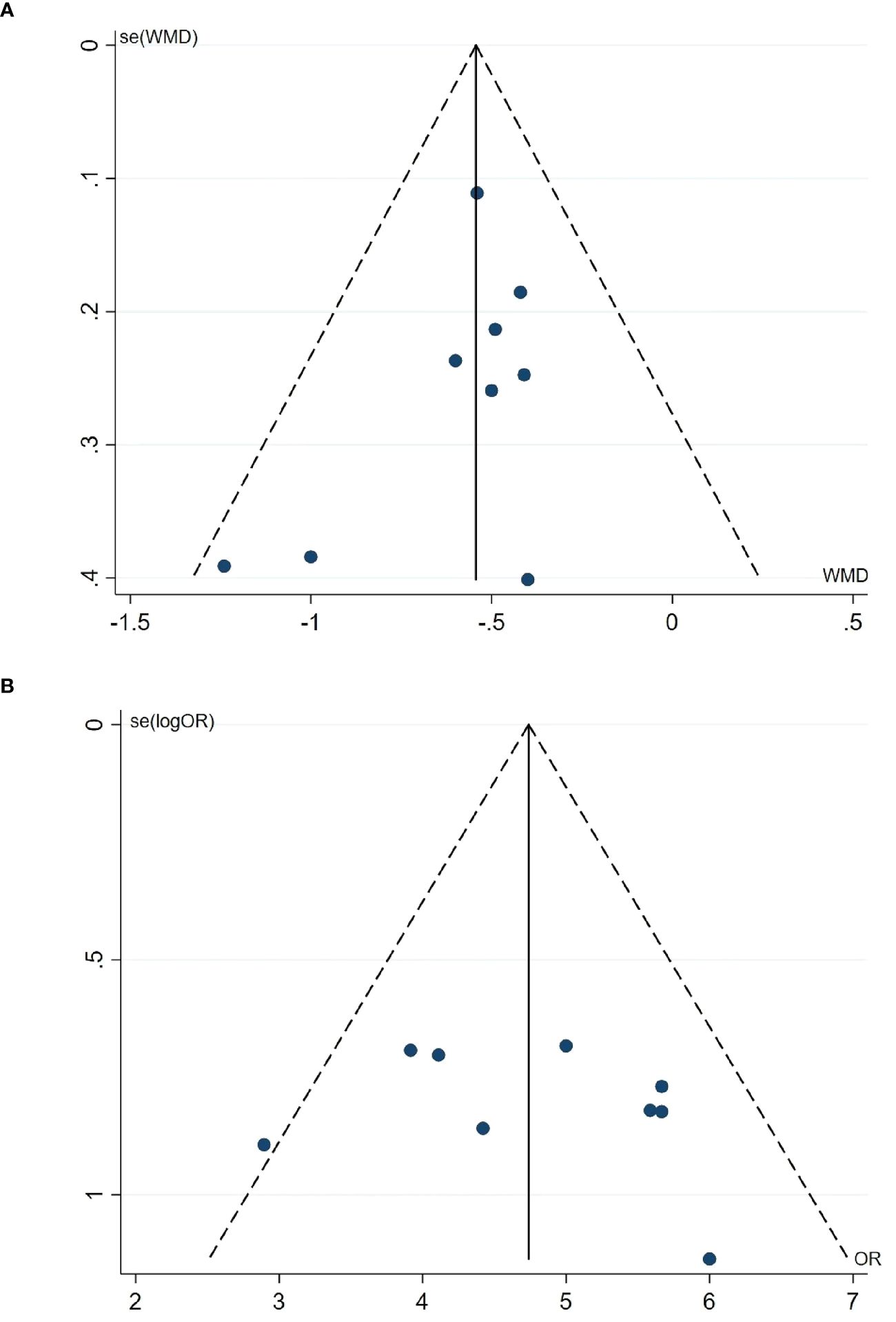
Figure 5 Funnel plot. (A) Funnel plot of the GET. (B) Funnel plot of the CE. GET, gastric emptying time; CE, clinical effect.
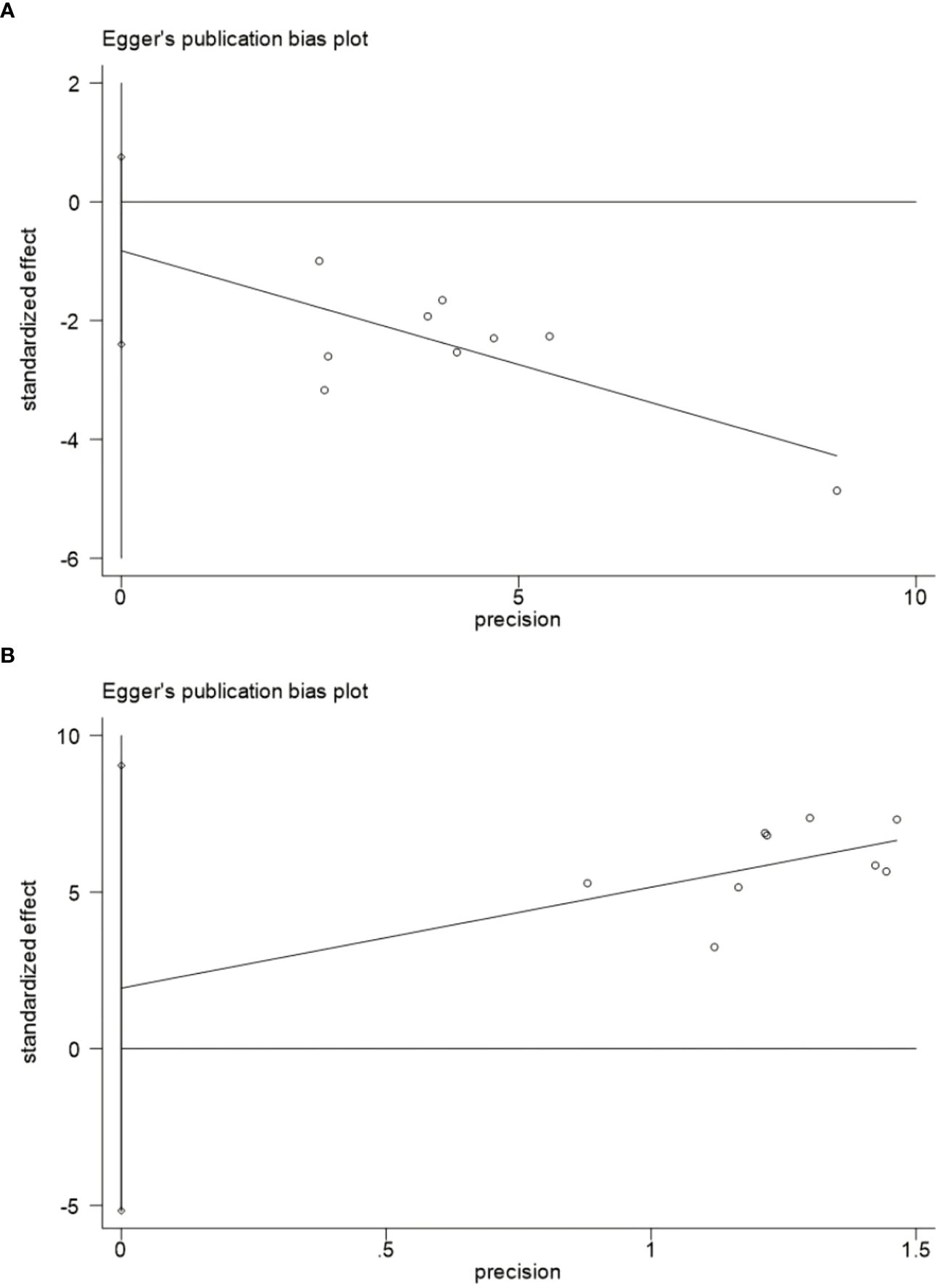
Figure 6 Egger’s publication bias plot.(A) Egger's publication bias plot for the GET. (B) Egger's publication bias plot for the CE. GET, gastric emptying time; CE, clinical effect.
3.8 Quality of evidence
We used the Cochrane Collaboration Network GRADE approach to evaluate the quality of the meta-analysis results, including five results in the PDC group (Figure 7A) and five outcomes in the PDC+RDC group (Figure 7B). Evidence based on GRADE ratings showed that the quality of evidence for most outcomes was low or moderate, mainly associated with the risk of bias, high heterogeneity, and small sample size.
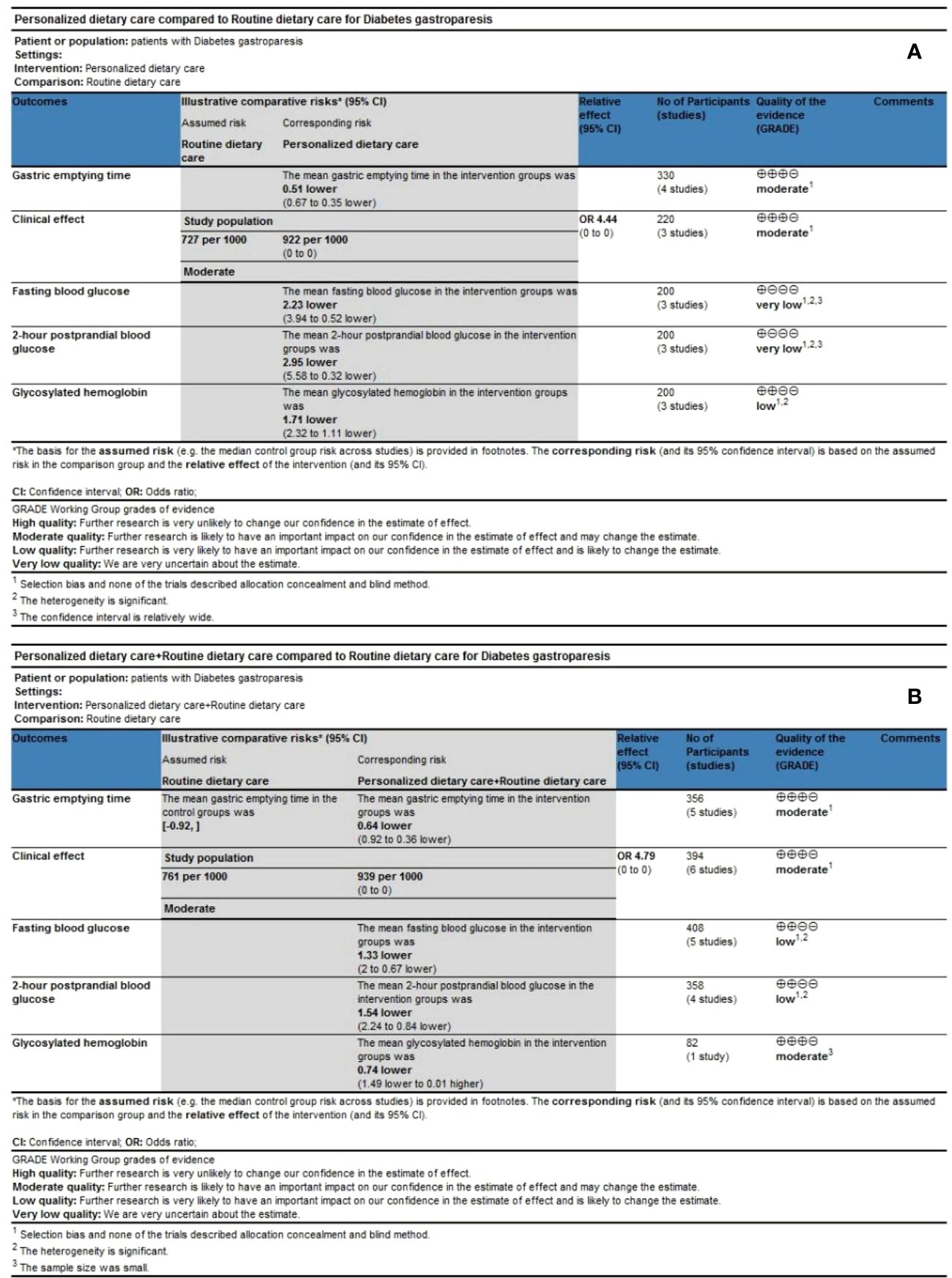
Figure 7 Quality of evidence. (A) GRADE evidence profiles for PDC versus RDC. (B) GRADE evidence profiles for PDC+RDC versus RDC. PDC, personalized dietary care; RDC, routine dietary care.
4 Discussion
It seems to be the first systematic review and meta-analysis to evaluate dietary therapy for DGP. This meta-analysis evaluated 15 RCTs of dietary interventions for DGP, including 1106 participants. Our analysis results showed that compared with RDC, PDC and PDC+RDC could shorten GET and promote gastric emptying better. Moreover, the CE of PDC and PDC+RDC group was better than that of RDC group. In addition, both PDC and PDC+RDC reduced FBG, 2hPBG and HbA1c levels in patients with DGP and improved blood glucose control. The stability of the overall results was also supported by sensitivity analysis and publication bias. However, in the three secondary outcomes of FBG, 2hPBG, and HbA1c, both the PDC group and the PDC+RDC group showed high heterogeneity. Sensitivity analysis seems to have identified the source of heterogeneity. In the PDC group, after excluding the study of Li W (28), the heterogeneity of FBG, 2hPBG, and HbA1c related research results was significantly reduced (I2 = 0%), which may indicate the risk of bias and methodological quality issues in this study. However, in the PDC+RDC group, it was found that after excluding the study by Yu CJ et al. (36), the heterogeneity of the 2hPBG variable was significantly reduced (I2 = 0%), which may be related to the risk of bias. Therefore, the results of the meta-analysis of FBG, 2hPBG, and HbA1c in the PDC group and the meta-analysis of 2hPBG in the PDC+RDC group should be treated with caution.
Although the quality of evidence is low to moderate, the findings suggest that personalized dietary interventions such as promoting a low-fiber diet, advocating the intake of liquid or semi-liquid foods, avoiding non-digestible foods such as fried and high-fat foods, following the principle of “a little each time but many times”, and adjusting the meal time and insulin injection time based on the patient’s postprandial blood glucose changes and gastric emptying. It can significantly shorten the GET of DGP patients, improve CE, reduce FBG, 2hPBG, and HbA1c levels.
DGP is an autonomic neuropathy caused by long-term poor blood sugar control (38). The independent risk factors for DGP include long-term hyperglycemia and high glycated hemoglobin (39, 40). There is a bidirectional relationship between blood glucose control and gastric emptying in DGP patients, that is, hyperglycemia-induced antral motility inhibition can delay gastric emptying, and delayed gastric emptying can also affect blood glucose control (41). Therefore, optimal control of blood glucose in patients with DGP may reduce the risk of future exacerbation of gastroparesis (1). The goal of blood glucose control should be controlled below 180 mg/dL to avoid inhibiting gastric myoelectric control and movement (38, 42).
Sympathetic and parasympathetic nervous system dysfunction, hyperglycemia and oxidative stress are all related to gastrointestinal motility dysfunction in diabetes (43, 44). Impaired gastric regulation in patient with diabetes is associated with decreased nitric oxide inhibitory innervation (45). Nitric oxide can affect pyloric dilation, and the loss of neuronal nitric oxide synthase may impair pyloric dilation and lead to pyloric spasm in patients with DGP (46, 47). This dysfunction (pyloric spasm) may be caused by vagus neuropathy and can hinder gastric emptying in DGP patients (48). Diet may have an impact on the regulation of the sympathetic nervous system, the release of nitric oxide, and vasodilation (49). A high-fat and carbohydrate diet can lead to activation of the sympathetic nervous system or regression of the parasympathetic nervous system, affecting the sympathetic nervous regulation of the heart (50). Research has shown that dysfunction of the cardiovascular vagus nerve can lead to delayed gastric emptying (51, 52).
Diet management is the first step in the treatment of gastroparesis patients (53, 54). Dietary interventions (including changes in dietary composition, size, and frequency of consumption, etc.) and stable blood sugar control are the fundamental management methods for DGP (7, 55). Dietary interventions can improve gastroparesis symptoms by regulating blood sugar control and promoting gastric emptying in DGP patients (56). Studies have shown that high-fat and high-fiber foods can delay gastric emptying, so patients with DGP should consume foods low-fat and low-fiber foods to compensate for the damage of gastric emptying (54, 57, 58). In addition, the diet of patients with DGP should be dominated by liquid or semi-liquid foods, as liquids are better tolerated than solid foods and are easier to empty (56, 59). Meanwhile, patients with DGP should eat small, frequent meals to maintain energy intake (54).
The PDC model of this review is different from the previous diet model of evaluating a single or several foods, because it systematically combines the power of multidisciplinary teams such as gastroenterologists, endocrinologists, nutritionists, psychologists, etc., which can more holistically and comprehensively evaluate the role of diet in DGP treatment. This review also has the following limitations. One is that the number of original studies related to dietary therapy for DGP is relatively small. Secondly, although the included studies have a consensus on PDC programs, there are also certain differences. However, these differences do not interfere with the positive effects of PDC in treating patients with DGP. Thirdly, the quality of evidence from the included RCTs was low to moderate. Fourthly, the included subjects are all from China. Considering the influence of dietary culture, religious customs and geographical environment, the results of this review should be cautiously applied to other countries and regions. Finally, due to significant differences in the treatment duration included in the study, this study did not discuss the length of intervention and the timepoint of evaluating intervention effectiveness.
Overall, the evidence from this systematic review and meta-analysis suggests that patients with DGP benefit from dietary interventions (whether PDC alone or PDC+RDC), which could shorten their GET, reduce their FBG, 2hPBG, and HbA1c levels, enhance their gastric emptying ability, and stabilize their blood sugar control. The most important measures in the dietary interventions program include the adjustment of the dietary composition of patients with DGP (advocating a low-fiber and low-fat diet), adjusting the dietary form (focusing on semi-liquid or liquid foods), and adjusting the number of meals (following the principle of “a little each time but many times”). In addition, other measures in the dietary interventions program are also an indispensable part.
5 Conclusion
The research results evaluated in this system review support the therapeutic potential of diet in the treatment of DGP. Both PDC and PDC+RDC effective for the treatment of DGP, as can promote gastric emptying by shortening GET, and also reducing FBG, 2hPBG, and HbA1c levels in patients with DGP to stabilize blood sugar control. However, the number of included clinical trials are relatively few and the methodological quality is average, and well-designed, larger sample RCTs are needed to further corroborate our findings and determine whether diet has a place in DGP treatment guidelines.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
DL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. HW: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. YO: Conceptualization, Formal analysis, Software, Writing – original draft. LL: Conceptualization, Data curation, Formal analysis, Writing – original draft. QZ: Data curation, Formal analysis, Writing – original draft. JY: Data curation, Writing – original draft. DP: Conceptualization, Methodology, Project administration, Writing – review & editing. SP: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Project of National Natural Science Foundation of China (82104813), China Postdoctoral Science Foundation (2023MD734104), Sichuan Post-doctoral Innovative Talent Support Project (BX202205), Special Postdoctoral Project for Apricot Grove Scholars at Chengdu University of Traditional Chinese Medicine (No. BSH2023002), and the Science and Technology Development Fund of Hospital of Chengdu University of Traditional Chinese Medicine (23YY38)
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Camilleri M, Kuo B, Nguyen L, Vaughn VM, Petrey J, Greer K, et al. ACG clinical guideline: gastroparesis. Am J Gastroenterol. (2022) 117:1197–220. doi: 10.14309/ajg.0000000000001874
2. Magliano DJ, Boyko EJ, IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS. 10th ed. Brussels: International Diabetes Federation (2021).
3. Ud-Din M, Karout B, Torbé WM, Lunding J, Wegeberg AM, Drewes AM, et al. DIgestive COmplications in DIabetes - the DICODI population study. Scand J Gastroenterol. (2023) 58:3–6. doi: 10.1080/00365521.2022.2106149
4. Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: Quality of life and health care utilization. J Clin Gastroenterol. (2018) 52:20–4. doi: 10.1097/MCG.0000000000000728
5. Chen YJ, Tang W, Ionescu-Ittu R, Ayyagari R, Wu E, Huh SY, et al. Health-care resource use and costs associated with diabetic and idiopathic gastroparesis: A claims analysis of the first 3 years following the diagnosis of gastroparesis. Neurogastroenterol Motil. (2022) 34:e14366. doi: 10.1111/nmo.14366
6. Hyett B, Martinez FJ, Gill BM, Mehra S, Lembo A, Kelly CP, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. (2009) 137:445–52. doi: 10.1053/j.gastro.2009.04.055
7. Wang S, Wang R, Zhang Y, Zhang X, Cai B, Lu Y, et al. Therapies for diabetic gastroparesis: A protocol for a systematic review and network meta-analysis. Med (Baltimore). (2020) 99:e20461. doi: 10.1097/MD.0000000000020461
8. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: A systematic review and meta-analysis. Gastroenterology. (2019) 156:1650–60. doi: 10.1053/j.gastro.2019.01.249
9. Camilleri M, Atieh J. New developments in prokinetic therapy for gastric motility disorders. Front Pharmacol. (2021) 12:711500. doi: 10.3389/fphar.2021.711500
10. Heckert J, Sankineni A, Hughes WB, Harbison S, Parkman H. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. (2016) 61:168–75. doi: 10.1007/s10620-015-3837-z
11. Rajamanuri M, Mannava SM, Chhabra J, Karwarker GV, Chahal M, Maligireddy AR, et al. A systematic review of the therapeutic role of gastric pacemakers in adults with gastroparesis. Cureus. (2021) 13:e18152. doi: 10.7759/cureus.18152
12. Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. (2019) 68:2238–50. doi: 10.1136/gutjnl-2019–318712
13. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. (1997) 20:537–44. doi: 10.2337/diacare.20.4.537
14. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
15. Olausson EA, Störsrud S, Grundin H, Isaksson M, Attvall S, Simrén M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. (2014) 109:375–85. doi: 10.1038/ajg.2013.453
16. Pasricha PJ, Yates KP, Nguyen L, Clarke J, Abell TL, Farrugia G, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology. (2015) 149:1762–1774.e4. doi: 10.1053/j.gastro.2015.08.008
17. Camilleri M. Novel diet, drugs, and gastric interventions for gastroparesis. Clin Gastroenterol Hepatol. (2016) 14:1072–80. doi: 10.1016/j.cgh.2015.12.033
18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. (2013) 66:173–83. doi: 10.1016/j.jclinepi.2012.08.001
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. An FL. To study the diet nursing experience of type 2 diabetes patients with gastroparesis. New World Diabetes. (2021) 18:191.
24. Bao MM. Effect of personalized diet intervention on diabetic patients with gastroparesis. Chin Rural Med. (2016) 4:18–9. doi: 10.3969/j.issn.1006–5180.2016.04.008
25. Hu GF, Li JX. Application of diet nursing based on PDCA cycle in diabetic gastroparesis patients. J Contemp Clin Med. (2022) 35:94–5. doi: 10.3969/j.issn.2095–9559.2022.01.58
26. Ji CY. Effect and evaluation of dietary nursing intervention on gastric empting time in patients with diabetic gastroparesis. Health Literature. (2018) 11:260. doi: 10.3969/j.issn.1671–5217.2018.11.260
27. Liu ZY. Effects of dietary nursing intervention on blood glucose level and gastric emptying in patients with diabetic gastroparesis. Chin J Health Care. (2021) 17:89–90.
28. Li W. Observation on the application effect of diet nursing based on PDCA circulation in patients with diabetes gastroparesis. Peers. (2023) 10):82–4.
29. Pan ML, Ye CY, Pan YL, WANG YL. Effect of dietary nursing intervention on gastric emptying time in patients with diabetic gastroparesis. Chin Modern Med Doctor. (2014) 52:99–101.
30. Sun XL. Effect of diet nursing intervention on patients with diabetes gastroparesis. Chin Ethnic Folk Med. (2015) 19):137–137139. doi: 10.3969/j.issn.1007–8517.2015.19.zgmzmjyyzz201519087
31. Wang CX, Wang J. Effects of dietary nursing intervention on blood glucose attainment and gastric emptying in patients with diabetic gastroparesis. Chin Modern Med Doctor. (2017) 55:148–51.
32. Wan Q, Xie L. Effect of individualized diet on improving gastrointestinal symptoms in patients with diabetic gastroparesis. Chin J Clin Nurs. (2014) 5:391–3. doi: 10.3969/j.issn.1674–3768.2014.05.011
33. Xu J, Li XL. Effect of dietary nursing intervention on gastric emptying time in patients with diabetic gastroparesis. Modern Digest Interventional Diagnosis Treat. (2016) 21:438–40. doi: 10.3969/j.issn.1672–2159.2016.03.024
34. Xu J. Effect of individualized diet on improving gastrointestinal symptoms in patients with diabetic gastroparesis. New World Diabetes. (2018) 21:114–5. doi: 10.16658/j.cnki.1672–4062.2018.19.114
35. Xu LY, Jing Q, Zhou XH. Application of personalized diet intervention in diabetic gastroparesis patients. Chin J Rural Med. (2017) 24:80–1. doi: 10.3969/j.issn.1006–5180.2017.06.044
36. Yu CJ, Jiang S, Duan JT. Effect of individualized diet intervention on gastrointestinal function in type 2 diabetic patients with secondary gastroparesis. Modern Digest Interventional Diagnosis Treat. (2022) 5:583–7.
37. Zhang Q, Li MN, Yang Y. Individualized dietary intervention in type 2 diabetic secondary gastroparesis. Health Care Guide. (2023) 21:166–8.
38. Krishnasamy S, Abell TL. Diabetic gastroparesis: principles and current trends in management. Diabetes Ther. (2018) 9:1–42. doi: 10.1007/s13300–018-0454–9
39. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. (2015) 44:9–19. doi: 10.1016/j.gtc.2014.11.002
40. Almogbel RA, Alhussan FA, Alnasser SA, Algeffari MA. Prevalence and risk factors of gastroparesis-related symptoms among patients with type 2 diabetes. Int J Health Sci. (2016) 10:397–404. doi: 10.12816/0048734
41. Halland M, Bharucha AE. Relationship between control of glycemia and gastric emptying disturbances in diabetes mellitus. Clin Gastroenterol Hepatol. (2016) 14:929–36. doi: 10.1016/j.cgh.2015.11.021
42. Kuznetsov KO, Mikheeva AJ, Ishmukhametova AA, Tolstykh TA, Gallyametdinova AR, Botirova ZU, et al. Diabetic gastroenteropathy: modern methods of diagnosis and treatment. Probl Endokrinol (Mosk). (2022) 68:67–78. doi: 10.14341/probl13082
43. Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. (2008) 20:8–18. doi: 10.1111/j.1365-2982.2007.01056.x
44. Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. (2007) 19:951–60. doi: 10.1111/j.1365-2982.2007.01023.x
45. Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. (2008) 20:635–42. doi: 10.1111/j.1365-2982.2008.01081.x
46. Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. (2014) 26:611–24. doi: 10.1111/nmo.12330
47. Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. (1986) 90:1919–25. doi: 10.1016/0016–5085(86)90262–3
48. Feldman M, Corbett DB, Ramsey EJ, Walsh JH, Richardson CT. Abnormal gastric function in longstanding, insulin-dependent diabetic patients. Gastroenterology. (1979) 77:12–7. doi: 10.1016/S0016-5085(79)80003-7
49. Martin VT, Vij B. Diet and headache: part 2. Headache. (2016) 56:1553–62. doi: 10.1111/head.12952
50. Kuwahara K, Okita Y, Kouda K, Nakamura H. Effects of modern eating patterns on the cardiac autonomic nervous system in young Japanese males. J Physiol Anthropol. (2011) 30:223–31. doi: 10.2114/jpa2.30.223
51. Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology. (2015) 149:330–9. doi: 10.1053/j.gastro.2015.05.007
52. Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. (2015) 13:466–76. doi: 10.1016/j.cgh.2014.06.034
53. Abell TL, Malinowski S, Minocha A. Nutrition aspects of gastroparesis and therapies for drug-refractory patients. Nutr Clin Pract. (2006) 21:23–33. doi: 10.1177/011542650602100123
54. Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. (2006) 18:263–83. doi: 10.1111/j.1365-2982.2006.00760.x
55. Farmer AD, Bruckner-Holt C, Schwartz S, Sadler E, Kadirkamanthan S. Diabetic gastroparesis: perspectives from a patient and health care providers. J Patient Cent Res Rev. (2019) 6:148–57. doi: 10.17294/2330–0698.1689
56. Asha MZ, Khalil SFH. Pharmacological Approaches to Diabetic Gastroparesis: A systematic review of randomised clinical trials. Sultan Qaboos Univ Med J. (2019) 19:e291–304. doi: 10.18295/SQUMJ.2019.19.04.004
57. Limketkai BN, LeBrett W, Lin L, Shah ND. Nutritional approaches for gastroparesis. Lancet Gastroenterol Hepatol. (2020) 5:1017–26. doi: 10.1016/S2468–1253(20)30078–9
58. Bharucha AE, Kudva YC, Prichard DO. Diabetic gastroparesis. Endocr Rev. (2019) 40:1318–52. doi: 10.1210/er.2018–00161
Keywords: diabetes, gastroparesis, diet, systematic review, meta-analysis
Citation: Lin D, Wang H, Ou Y, Li L, Zhang Q, Yan J, Peng D and Peng S (2024) The role of diet in diabetes gastroparesis treatment: a systematic review and meta-analysis. Front. Endocrinol. 15:1379398. doi: 10.3389/fendo.2024.1379398
Received: 23 April 2024; Accepted: 04 June 2024;
Published: 18 June 2024.
Edited by:
Parth Shah, ObvioHealth, United StatesReviewed by:
Meihua Ji, Capital Medical University, ChinaTao Yi, Hong Kong Baptist University, Hong Kong SAR, China
Copyright © 2024 Lin, Wang, Ou, Li, Zhang, Yan, Peng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dezhong Peng, MTU4MjgwNTU2OThAMTYzLmNvbQ==; Sihan Peng, cGVuZ3NoMzEwQDE2My5jb20=
†These authors share first authorship
 Dezhi Lin
Dezhi Lin Hui Wang
Hui Wang Yangxu Ou
Yangxu Ou Longlong Li
Longlong Li Qiang Zhang
Qiang Zhang Jiayin Yan
Jiayin Yan Dezhong Peng
Dezhong Peng Sihan Peng
Sihan Peng