- 1Department of Pediatrics, Rizhao Hospital of Traditional Chinese Medicine, Rizhao, China
- 2Department of Endocrinology & Diabetes Vascular Function Laboratory, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, China
With changes in lifestyle behaviors, including dietary structure and habits, the prevalence of Youth-onset Type 2 Diabetes Mellitus (YODM) has increased 2 to 3 times compared to 30 years ago. YODM patients experience complications earlier, progress faster, and exhibit more severe symptoms. However, limited and inconclusive direct evidence, coupled with poor patient compliance, poses challenges in the clinical management of YODM. Apart from the continuous decline in pancreatic β-cell function and quantity, tissue-specific insulin resistance (IR) is also a typical characteristic of YODM. The main mechanisms of IR in YODM involve different aspects such as obesity, dietary imbalance, abnormal substance metabolism, chronic inflammation, oxidative stress, and hormonal fluctuations during adolescence. For the comprehensive management of YODM, besides achieving good control of blood glucose levels, it may be necessary to apply the most appropriate methods considering the uniqueness of the patient population and the specifics of the disease. Early identification and detection of the disease are crucial. Precise screening of patients with well-functioning pancreatic insulin β-cells, primarily characterized by IR and obesity, represents the population most likely to achieve diabetes remission or reversal through lifestyle modifications, medications, or even surgical interventions. Additionally, considering potential emotional disorders or the impact of adolescent hormones in these patients, health education for patients and caregivers is essential to make them aware of the long-term benefits of well-controlled blood glucose. In conclusion, adopting comprehensive management measures to achieve diabetes remission or reversal is the ideal goal. Controlling high blood glucose, obesity, and other risk factors related to diabetes complications is the next priority to delay the occurrence and progression of complications. A comprehensive perspective on IR provides insights and references for identifying YODM and its management strategies.
1 Introduction
The International Diabetes Federation pointed out in its 2021 conference that globally, 537 million adults (20–79 years old) are affected by Diabetes Mellitus (DM), and it is projected to rise to 784 million by the year 2045. Changes in dietary structure (consumption of high-calorie foods) and lifestyle habits (such as staying up late and insufficient physical activity) contribute to a trend of younger onset Type 2 Diabetes Mellitus (T2DM). Research indicates that the prevalence of Youth-onset Type 2 Diabetes Mellitus (YODM), that occurs during adolescence (typically between the ages of 10 and 18), has increased 2 to 3 times compared to 30 years ago, with an estimated 41,600 new cases worldwide in 2021, particularly higher rates reported in China, India, and the United States (1–3).
While comprehensive management for adult DM patients is becoming increasingly standardized across various aspects such as diagnosis, monitoring, prevention, and treatment, the same cannot be said for YODM. This is primarily due to its unique characteristics, including the need for improved systemic management measures, rapid disease progression, poor compliance, and prominent psychological and emotional challenges. Data from “Healthy People 2020” reveals that only 27.1% of adolescents meet the recommended levels of physical activity, and just 31.7% get adequate sleep (4). Dietary imbalances are common among YODM, impacting obesity and psychosocial functionality. The Treatment Options for Type 2 Diabetes in Adolescents and Youth Study (TODAY) indicates that 20% and 6% of young individuals exhibit subclinical or clinical binge-eating behavior (5). These unfavorable lifestyle habits contribute to the rising incidence of YODM, and information about its pathophysiology is mostly derived from adult studies (6). Considering the unique nature of the affected population, YODM patients face a higher risk of depression and other mental health disorders, with an estimated depression prevalence exceeding 20% (7, 8). This not only affects blood glucose control but also directly contributes to lower patient compliance (9). In comparison to adult T2DM, YODM has an earlier onset, a faster decline in pancreatic β-cell function, and a higher likelihood of complications if not controlled promptly (10). Approximately 8 years after YODM diagnosis, 72% of T2DM patients experience at least one complication (11). As a result, YODM has become an increasingly serious global health issue for adolescents and young people, demanding more attention (12–14).
In addition to the continuous decline in pancreatic β-cell function and quantity, tissue-specific insulin resistance (IR) is also a typical characteristic of T2DM, including YODM, holding a significant position in its occurrence and development (15–17). Therefore, focusing on IR allows for the transfer of some T2DM management strategies to YODM. Simultaneously, expectations for the treatment goals of some YODM patients should be elevated, including diabetes remission, defined as having an HbA1c below 6.5% for at least one year without the use of glucose-lowering medications according to the standards recommended by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). And the most ideal state, crucial for long-term improvements in quality of life and survival (18). This is followed by controlling risk factors to delay the occurrence and progression of complications. However, the immediate priority lies in the early identification of adolescent IR or even YODM, enabling the prompt alteration of metabolic abnormalities. Thus, early detection and intensive management of YODM are paramount (19). We first summarize the clinical characteristics (specificities, risk factors) of YODM, focusing on IR, and then explores management strategies for YODM, aiming to provide reference points for the clinical understanding and management of YODM.
2 Comprehensive and accurate understanding of YODM is key to developing more effective comprehensive management strategies
2.1 More specific characteristics of YODM compared to adult T2DM
Both adult T2DM and YODM share common risk factors, pathological changes, and clinical manifestations as they both belong to the diabetes category. Common risk factors include obesity, family history, shared pathological changes such as reduced insulin sensitivity and insufficient insulin secretion, and common clinical manifestations like polydipsia, polyuria, increased appetite, and fatigue (20). However, due to the younger age of the YODM patient population, they exhibit unique clinical features, making management more challenging, disease progression faster, and the condition more severe (21).
2.1.1 Earlier onset of complications, faster disease progression, and more severe symptoms in YODM patients
Long-term follow-up reports from the TODAY study (average diabetes duration of 13.3 ± 1.8 years) show that YODM patients have higher rates of diabetic nephropathy (54.8%), neuropathy (32.4%), and retinopathy (13.7%) (22). The SEARCH study also indicates that compared to Type 1 Diabetes Mellitus (T1DM) patients, YODM patients have higher occurrence rates of diabetic nephropathy (19.9% vs. 5.8%), retinopathy (9.1% vs. 5.6%), and peripheral neuropathy (17.7% vs. 8.5%) (11). Although diabetic ketoacidosis is not a common initial presentation in YODM, its frequency can be as high as 3% to 11%, with high blood glucose and hyperosmolarity states being rare (23, 24).
2.1.2 Limited and challenging clinical management due to insufficient evidence and poor compliance in YODM patients
There are relatively few interventional clinical studies focused on YODM, with major studies including the TODAY study and SEARCH study. Ethical considerations, along with the transitional phases YODM patients undergo in physiological, psychological, and social aspects, make it challenging to conduct diagnostic and interventional clinical studies due to the significant emotional disturbances exhibited by patients. Despite partial references to the pathophysiology evidence from adult diabetes studies (6), the scarcity of such studies poses limitations. A study in Korea assessing the prevalence and characteristics of YODM using the National Health Insurance Service (NHIS) database highlighted non-adherence to treatment and low compliance as major obstacles in managing YODM patients (19). Adolescents (10 to 20 years old) are more likely to display medical non-adherence, and data indicates that the younger the age, the lower the blood glucose control rate in T2DM patients (25–27). YODM patients relying solely on lifestyle modifications and metformin for blood glucose control exhibit a high rate of poor blood glucose control, reaching 51.7% (28). Therefore, effective strategies and interventions are needed to manage YODM and reduce the risks of microvascular and macrovascular complications, addressing compliance issues more effectively than seen in adult patients.
2.2 Risk factors for the occurrence and development of YODM
Critical driving factors for the prevalence of YODM include both genetic factors inherited from parents and acquired risk factors related to oneself, such as obesity, unhealthy lifestyle habits, and psychological factors.
2.2.1 Genetic factors inherited from parents
For the majority of YODM cases (92%), genetic factors should be considered, particularly for patients with atypical presentations and/or a positive family history (23, 29). If one parent has T2DM, the estimated lifelong risk for their child developing T2DM is 40%, and if both parents have T2DM, the risk increases to as much as 70% (30, 31). Research also suggests that whether maternal diabetes is diagnosed during pregnancy or post-pregnancy, it is associated with poor blood glucose control and reduced glomerular filtration rate in YODM patients (32). Interestingly, a study in Denmark involving 2,448,753 individuals found that offspring of mothers with gestational hypertension (HR=1.37) or preeclampsia (HR=1.62) have a higher risk of developing T2DM (33), possibly due to intrauterine growth restriction caused by imbalanced nutrient intake leading to fetal adipose tissue and pancreatic β-cell dysfunction (34). The Progress in Genetic studies of youth-onset diabetes (ProDiGY) consortium conducted a genome-wide association study on YODM, identifying seven crucial loci in the genome, including rs7903146 in TCF7L2, rs72982988 near MC4R, rs200893788 in CDC123, rs2237892 in KCNQ1, rs937589119 in IGF2BP2, rs113748381 in SLC16A11, and rs2604566 in CPEB2, which may play a significant role in early detection of YODM in the future (35).
2.2.2 Acquired risk factors
The most crucial acquired risk factor for the development of YODM is obesity, primarily attributed to unhealthy dietary or exercise habits (36, 37). In recent years, with the widespread adoption of fast food culture and the increased consumption of ultra-processed foods, the proportion of adolescents consuming high-sugar and high-fat foods has significantly risen. Research indicates that high-sugar diets can lead to insulin resistance and fatty liver, thereby increasing the risk of DM (38). Moreover, high-fat diets not only cause weight gain but also affect insulin secretion and function (39). For instance, a study involving American adolescents found that those who consumed sugar-sweetened beverages daily had a significantly higher risk of obesity and T2DM compared to their peers who did not consume such beverages (40). Similarly, diets high in saturated and trans fats are associated with decreased insulin sensitivity and poor glycemic control (41). A prospective cohort study of adolescents also pointed out that a high-energy fast food diet is significantly associated with elevated fasting blood glucose levels and the occurrence of prediabetic symptoms (42).
Over the past few decades, the number of obese adolescents has been steadily increasing in many countries (43). Estimates for childhood obesity from 2017 to March 2020 show prevalence rates of 20.7% for children aged 6 to 11 and 22.2% for children aged 12 to 19 (44). As a major risk factor for the development of T2DM, the rise in body mass index (BMI) in adolescents is associated with an increased risk of being diagnosed with T2DM at a young age (45). Meta-analysis results for 228,184 participants show that the prevalence of T2DM in obese individuals is 1.3%, which is 13 times higher than in normal-weight individuals, and the prevalence of prediabetes is three times higher in obese individuals compared to normal individuals (46). Furthermore, a multicenter cross-sectional study on 2,448 adolescents with T2DM showed prevalence rates of 10.4% for overweight and 79.4% for obesity (47). Adolescents are in a transitional phase during puberty, making many patients simultaneously experience emotional disturbances. Current research indicates that emotional disorders can lead to the onset of diabetes and exacerbate its severity. The estimated prevalence of depression in T2DM patients is 25%, and it is associated with poorer blood glucose control in these patients (9, 48). An analysis of data from the Taiwan National Health Insurance Research Database from 2001 to 2010 found that, compared to reactive depression patients responsive to antidepressants, patients with treatment-resistant depression were more likely to be diagnosed with T2DM in later life (HR 1.51) (49). Additionally, obesity, IR, and hypoglycemic abnormalities may result in relatively poor cognitive function in adolescents with both T2DM and obesity (50).
3 IR affects the occurrence and development of YODM
3.1 IR
In addition to the continuous decline in function and quantity of pancreatic β-cells, tissue-specific IR is also a typical characteristic of T2DM. When the main target tissues of insulin, such as muscles, fat, and the liver, fail to respond appropriately to insulin, insulin sensitivity decreases continuously, making glucose less likely to be cleared from the bloodstream. Tissues begin to exhibit IR, leading to increased insulin production by the pancreas or presenting as hyperinsulinemia (51). Research has confirmed that IR plays a crucial role in the occurrence and development of various metabolic disorders, including non-alcoholic fatty liver disease (NAFLD), metabolic syndrome, obesity, hypertension, and cardiovascular diseases (52, 53). Chronic hyperinsulinemia resulting from IR can also lead to a reduction in pancreatic β-cell mass (54).
Currently, the primary mechanisms of IR involve abnormalities in substance metabolism, chronic inflammation, oxidative stress, among other aspects (55). Firstly, excessive energy intake promotes an increase in free fatty acids (FFA) and lipid deposition, leading to IR (56). AMP-activated protein kinase (AMPK), as an AMP-dependent protein kinase, is a key molecule in regulating biological energy metabolism. Studies have shown that activated AMPK can enhance insulin sensitivity and promote glucose uptake (57). Protein tyrosine phosphatase 1B (PTP1B), a widely expressed prototype non-receptor tyrosine phosphatase, is a critical negative regulator in insulin signal transduction. Overexpression in adipose tissue can lead to dephosphorylation of insulin receptors and inhibit insulin signal transduction (58). GLUT4 is an insulin-dependent transmembrane carrier protein that facilitates the translocation of glucose on the cell membrane. IR leads to reduced glucose uptake and is associated with impaired GLUT4 translocation (59).
Certainly, there is ample evidence indicating that chronic inflammation is pervasive throughout the course of DM, and it also influences IR (60). Macrophages can be classified into two different subtypes based on their activation status: M1 polarization, which promotes inflammation and is associated with reduced insulin sensitivity (61), and M2 polarization, which inhibits inflammation and is associated with enhanced insulin sensitivity (62). Activation of the NF-κB and JNK pathways due to pro-inflammatory status increases the secretion of various inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), leading to the promotion of IR (63–65). Oxidative stress and inflammation often coexist and mutually influence each other in the host. Reactive oxygen species can stimulate the production of inflammatory factors, and, in turn, cellular inflammatory factors can promote the generation of oxygen free radicals (66). Oxidative stress reduces the translocation of IRS-1, decreases protein kinase B (PKB) phosphorylation, and lowers GLUT-4 expression (67, 68).
3.2 Key role of IR in the occurrence and development of YODM
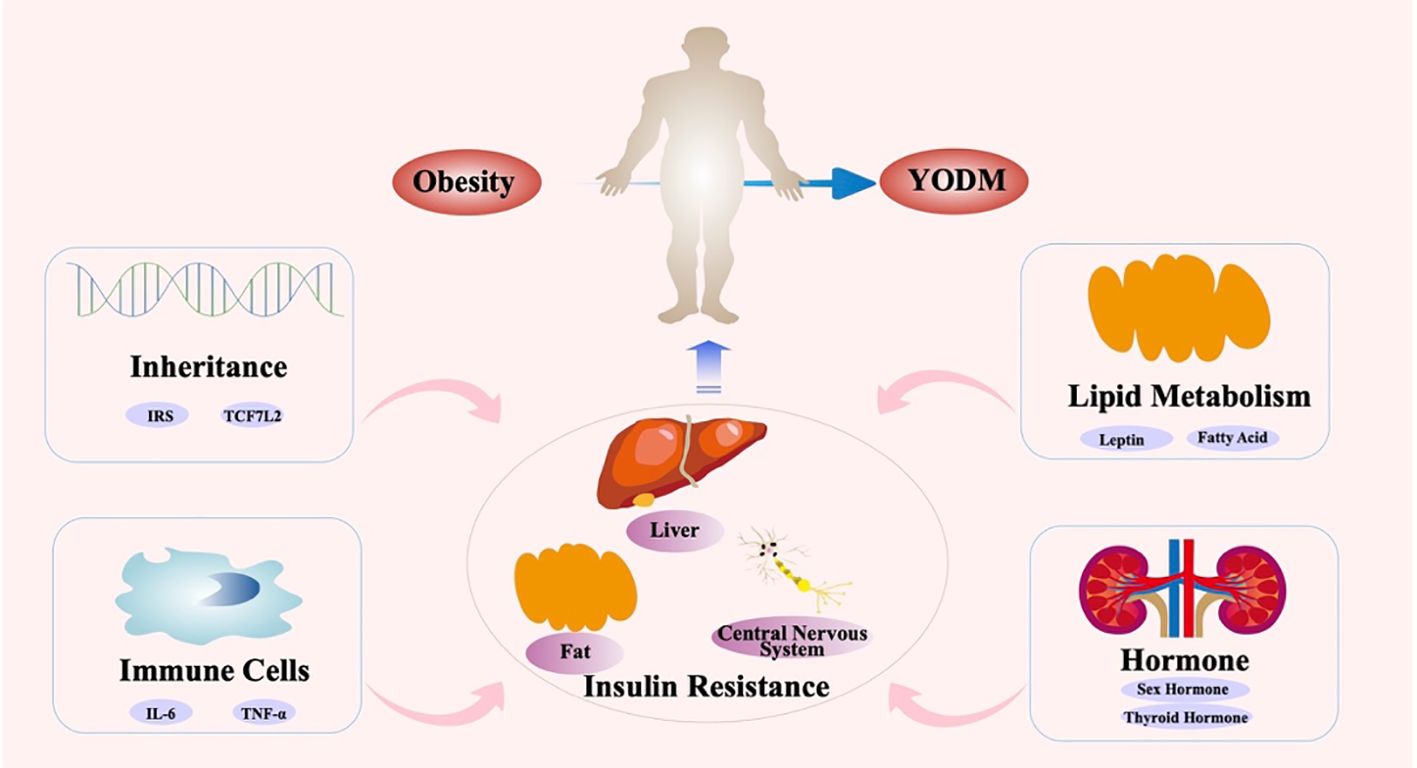
Existing clinical studies confirm that both insulin sensitivity and insulin secretion are impaired in YODM patients (69). Genetic-related β-cell dysfunction has been found in East Asian adolescents, and it is significantly associated with the development of T2DM even with mild decreases in insulin sensitivity (70). In contrast, IR is prevalent in the Indian population and may manifest as early as in neonates (71). The Restoring Insulin Secretion (RISE) study indicates that compared to adults with T2DM, YODM exhibits more severe IR, higher insulin secretion demand, and lower insulin clearance rates (15). A comparative study of YODM diagnosed within 1.5 years, matched with non-T2DM individuals in terms of BMI, showed a 75% decrease in the first-phase and a 55% decrease in the second-phase insulin secretion in YODM, accompanied by severe peripheral and hepatic IR (69). This may be attributed to hormonal changes affecting insulin sensitivity during puberty, and the more severe obesity issues in adolescent patients also affect peripheral tissue insulin sensitivity (72). IR may prompt β-cells to secrete more insulin, leading to earlier β-cell failure. Dysfunction, rather than death, is a common β-cell defect in T2D (73). Therefore, focusing on IR in the early stages of YODM and conducting intervention studies may improve β-cell function to some extent. A cross-sectional study, including 79 adolescents aged 10 to 18, assessed body composition indicators: body mass index (BMI), body fat percentage, waist circumference, and subcutaneous fat. IR was diagnosed in 29.1% of patients, and these body composition indicators were all correlated with IR (74) (Figure 1).
Considering the causes of obesity-induced DM, IR plays a crucial role (75, 76). A study conducted in Japan using intravenous glucose tolerance testing revealed IR in both obese non-diabetic adolescents and YODM individuals (77). Additionally, one of the major complications of obesity is IR, leading to disturbances in carbohydrate metabolism. Compared to normal-weight children, overweight and obese children have a higher incidence of IR (78). In the early stages of the disease, pancreatic β-cells can compensate for IR by increasing insulin secretion in the pathogenesis of glucose intolerance. Compensatory hyperinsulinemia induces increased appetite and weight gain. With the decline in pancreatic β-cell function and inadequate insulin secretion, the transition from IR to impaired glucose tolerance and then to T2DM occurs (79). Androutsos and colleagues applied principal component analysis to a health growth study cohort of over 2000 Greek children aged 9–13. They observed a positive correlation between a combination of high screen time, short sleep duration, and high sugary beverage consumption with HOMA-IR, while a combination of high to moderate-intensity physical activity and frequent eating showed a negative correlation with HOMA-IR (80). Dietary imbalance is prevalent in YODM and affects obesity and psychosocial function.
In the TODAY study, 20% and 6% of young people exhibited subclinical and clinical binge eating (5). In the SEARCH study, 50.3% of youth and YODM had dietary imbalances (81). Changes in gastric redoxin secretion may be an early biomarker of impaired glucose regulation in obese children with IR (82). Relevant studies found that for each additional hour of sedentary time and screen use between ages 8–10 and 15–17, insulin sensitivity decreased by 8.2% and 6.4%, respectively, while fasting blood glucose increased by 0.03 mmol/L and 0.02 mmol/L. This emphasized the evidence of sedentary behavior and screen time as key driving factors for the high-risk development of YODM (83).
Simultaneously, numerous factors, including genetics, inflammation and immunity, lipid metabolism, and adolescent hormones, affect signaling pathways such as insulin receptors, mediating the occurrence of IR. Firstly, certain genetic variations may lead to IR during adolescence. Studies indicate that polymorphisms in specific genes may influence key molecules in insulin signal transduction pathways, resulting in the occurrence of IR. The development of IR in YODM usually involves complex regulation by various genetic mechanisms. Genes of the insulin receptor substrate (IRS) family are closely associated with IR. Proteins encoded by IRS genes are critical molecules in insulin signal transduction pathways, and their abnormal function may lead to IR. Polymorphisms in IRS genes have been found to be associated with the development of IR in YODM.
Polymorphisms in inflammatory factor genes may be associated with IR in YODM. Chronic inflammation associated with obesity can lead to IR and β-cell dysfunction (84). Abnormal expression of inflammatory factors may increase tissue inflammatory responses, thereby interfering with the normal function of insulin signal pathways, leading to IR. During adolescence, changes in hormone levels and cytokines may increase adipose tissue inflammatory responses, further exacerbating the degree of IR. Inflammatory responses lead to increased release of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). These cytokines may directly interfere with insulin signal transduction pathways, affecting the function of insulin receptors and the effectiveness of insulin. The pro-inflammatory cytokine TNF-α can induce IR by disrupting early insulin-stimulated tyrosine phosphorylation (85). The increase in inflammatory reactions may induce autoimmune reactions, activate immune cells, disrupt immune regulation, and cause immune stress, leading to increased release of inflammatory factors, thus interfering with the normal function of insulin signal pathways. Activation of autoimmune reactions may damage and destroy insulin-secreting cells. Activation of immune cells such as macrophages and lymphocytes may increase the release of inflammatory factors, affecting the normal function of insulin signal pathways. Imbalance in immune regulation may lead to an increase in inflammatory reactions and abnormal activation of immune cells. Chronic immune stress may lead to increased activation of immune cells and increased release of inflammatory factors. All these abnormal changes can affect the normal function of insulin signal pathways.
Patients with YODM often exhibit lipid metabolism disorders (86, 87), including hyperinsulinemia and lipid peroxidation. These factors lead to the release of more fatty acids from adipocytes, further worsening IR and insufficient insulin secretion. IR can also lead to abnormalities in lipid metabolism, where fatty acids are not efficiently utilized by muscle and adipose tissues, leading to the synthesis of triglycerides in the liver, resulting in a vicious cycle of fatty liver and IR. The main cause of lipid metabolism disorders may be attributed to poor lifestyle habits during adolescence. A high-sugar, high-fat, high-energy-density diet may lead to weight gain and fat accumulation. Additionally, poor dietary structure may lead to abnormal expression of key molecules in the insulin signal pathway, thereby disrupting normal insulin signal transduction. Lack of physical exercise leads to reduced energy consumption in muscles, lipid metabolism disorders, and decreased insulin sensitivity. Moderate physical exercise helps control weight, improve the function of insulin signal pathways, and thus alleviate the degree of IR. Mechanistically, adipose tissue secretes various factors, including leptin, adiponectin, etc., which may negatively impact insulin sensitivity. The IR in YODM is related to the total body fat mass, especially visceral fat. This is mediated by various fat factors, including leptin, resistin, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Additionally, non-esterified fatty acids contribute to IR by increasing hepatic glucose output and hindering glucose uptake in skeletal muscles (88, 89). Furthermore, an increase in visceral fat leads to decreased levels of adiponectin and reduced expression of adiponectin receptors on cell surfaces (90).
Adolescence is a critical period for growth and development, and changes in hormone levels during puberty have a significant impact on insulin sensitivity. During adolescence, changes in sex hormones may affect fat distribution and the growth and development of muscle tissue, influencing the function of insulin signal transduction pathways. Alternatively, hormonal changes during adolescence may affect IR through neuroendocrine regulatory mechanisms. Other factors, including changes in thyroid hormone levels and adrenal cortex hormone levels during adolescence, may also affect metabolic pathways and insulin signal transduction, thereby influencing the occurrence of IR.
4 Comprehensive management strategies for YODM from the perspective of IR
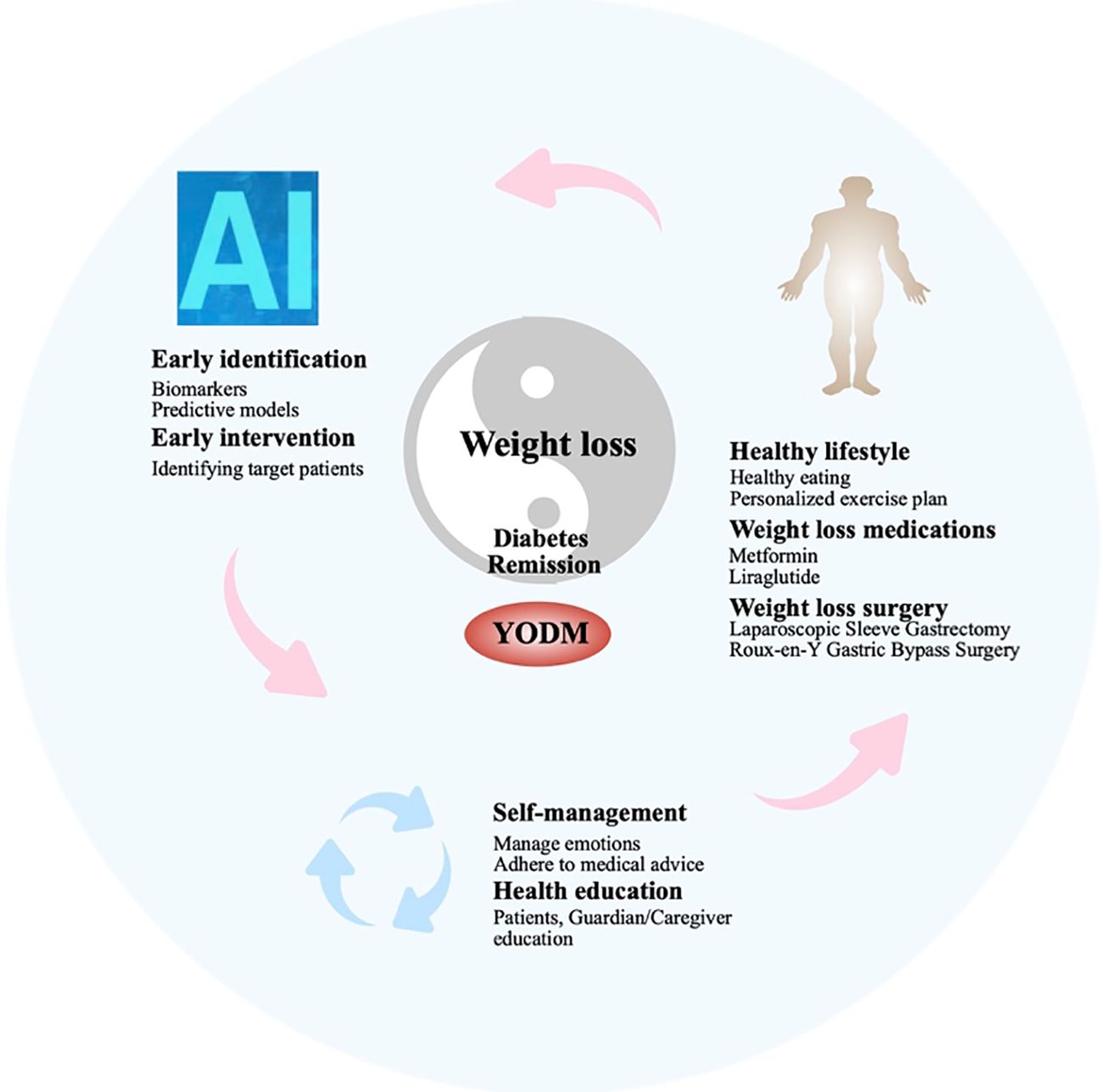
In 2016, the WHO’s “Global Diabetes Report” clearly stated that weight loss and restricted energy intake could lead to the remission of T2DM. In 2021, the ADA defined T2DM, recommending discontinuation of antidiabetic medications for at least 3 months, with a glycated hemoglobin (HbA1c) < 6.5% as the standard for T2DM remission. However, in certain situations, such as the presence of hemoglobin variants, diseases affecting red blood cell lifespan, or inaccuracies in HbA1c testing methods, HbA1c may not reflect true blood glucose levels. In such cases, FBG < 7.0 mmol/L or estimated HbA1c < 6.5% through continuous glucose monitoring (CGM) can be used as alternative criteria. There is clear clinical evidence supporting the significant reduction in weight and promotion of T2DM remission through enhanced lifestyle interventions or metabolic surgery in T2DM patients with a short course and obesity (91, 92). Additionally, short-term insulin therapy in newly diagnosed T2DM patients has also been shown to promote T2DM remission (93). While this evidence is focused on adults, it suggests that early intervention, including weight loss or medication targeting obesity, may be a crucial strategy for YODM to achieve diabetes remission (94). Therefore, early intervention strategies targeting IR, as a key factor in the occurrence and development of YODM, are crucial for achieving diabetes remission, with a focus on weight loss (95). Chronic hyperglycemia, or T2DM itself, is a significant cause of macrovascular and microvascular complications, including cardiovascular events, renal failure, and diabetes-related established microvascular and macrovascular damage leading to vision loss (96, 97). The remission of T2DM is associated with a lower risk of microvascular complications, especially in younger populations (under 45 years old) and those with fewer complications (none or less than 3) (98). Diabetes remission significantly helps improve patients’ quality of life and reduce the risk of complications. Therefore, the management strategy for YODM should shift some focus from controlling blood glucose and preventing complications to relieving diabetes. This should be the ideal treatment goal for such patients: early identification, early disease detection, precise screening of patients with well-functioning insulin beta cells, IR as the main pathological change, and obesity. This group is most likely to achieve diabetes remission or reversal through measures such as lifestyle changes, medication, or even surgery. Furthermore, considering that this population may have more emotional disorders or hormonal influences during adolescence, it is essential to provide health education to patients and caregivers, making them aware of the long-term benefits of maintaining good blood sugar control. In summary, the ideal goal is to adopt comprehensive measures to achieve diabetes remission or reversal, followed by controlling high blood sugar, obesity, and other risk factors associated with diabetes complications to delay the occurrence and progression of complications (Figure 2).
4.1 YODM patients experience faster disease progression, emphasizing the importance of early identification and early intervention
Early diagnosis and intervention help control the progression of the disease. In the early stages of diabetes diagnosis, effective treatment measures can improve the patient’s condition and contribute to diabetes remission. Approximately 40% of YODM cases are asymptomatic at the time of diagnosis, as diabetes is detected through screening due to signs of obesity, other risk factors, or IR (99). Therefore, early identification is crucial and can be achieved through alternative indicators such as waist circumference, triglyceride-glucose (TyG) index, metabolites, and IR index.
A study analyzing data from 38,000 Brazilian adolescents aged 12 to 17 compared the associations between obesity, overweight, waist circumference, and IR. The results showed that waist circumference is more helpful in identifying adolescents with IR, especially in late adolescence (100). Another multicenter cross-sectional study analyzed 161 newly diagnosed T2DM children and adolescents, with 1,935 children with normal blood sugar as healthy controls. The results indicated that the increase in HOMA2-B and HOMA2-IR is associated with a higher risk of T2DM, suggesting that besides IR, impaired beta-cell function is closely related to Chinese YODM. Gender differences in susceptibility and higher complications require YODM screening and prevention strategies (101). The triglyceride-glucose (TyG) index [area under the curve (AUC) 0.839)] showed better performance in identifying T2DM patients than HOMA-IR (AUC 0.645), demonstrating a significant association between IR and T2DM (102). Respiratory metabolites, including limonene, nonane, and 2,7-dimethylundecane, are correlated with IR evaluated by the homeostasis model assessment (HOMA-IR). Given their simple and non-invasive breath-based testing, these substances have the potential to serve as effective measures for early detection of insulin resistance (103). Another study tracking 190 newly diagnosed T2DM patients for 5 years identified 12 different metabolites, with forty-two hexaenoic acid, oxygen tri-nucleotide, dihydrocholesterol, and trienoic acid being the most representative (104).
In recent years, the global prevalence of T2DM has been increasing annually, with a significant contributing factor being the rising rates of overweight or obesity due to lifestyle changes. Clinical research results indicate that whether through lifestyle, medication, or metabolic surgical interventions, it is possible to slow down the progression from prediabetes to diabetes or achieve the reversal of elevated blood sugar, leading to normal levels, as T2DM remission (105, 106). Current research indicates that only some patients can achieve diabetes remission. These conditions include the exclusion of specific types of diabetes, such as secondary diabetes or diabetes caused by certain genetic factors, as well as patients with a longer course, more severe complications, and poor pancreatic beta-cell function. For T2DM patients without autoimmune destruction of pancreatic islets, overweight or obesity (BMI ≥ 25 kg/m2), preserved beta-cell function, and a course of ≤ 5 years have a higher likelihood of remission (91, 107). However, among these conditions, early-stage obesity serves as a crucial signal for achieving diabetes remission.
The Japan Diabetes Data Management (JDDM74) analyzed data from over 4,000 patients over 30 years nationwide, with 3,454 patients achieving diabetes remission, significantly correlated with a moderate weight loss of 3.0–7.9% (108). Therefore, based on the current clinical characteristics achievable for diabetic patients, combined with ongoing interventional research, weight loss and subsequent visceral fat loss are crucial for relieving T2DM, with obesity being the central core for achieving remission. Obesity can lead to excessive growth of adipose tissue, especially abdominal fat. This adipose tissue releases various hormones and cytokines, including free fatty acids, leptin, tumor necrosis factor, etc. These substances can interfere with insulin signaling, leading to insulin resistance. In the obese population, adipose tissue releases an increasing amount of non-esterified fatty acids, glycerol, hormones, pro-inflammatory cytokines, and other factors involved in the development of insulin resistance (109).
4.2 Poor adherence in YODM patients: cultivating effective self-management and improving adherence are key to diabetes management
Unlike adults, adolescents are more heavily influenced by external factors, making it challenging for many YODM patients to effectively control their diet, engage in physical activity, and manage blood glucose in their daily lives. Adolescents, being in a sensitive period of growth transition, often experience unstable behavioral and emotional states, hindering stable blood sugar control. However, the remission of diabetes may have more significant benefits for adolescents. Medical goals for YODM include preventing further weight gain and/or achieving weight loss (encouraging obese patients to lose 7–10% of their weight), maintaining good blood sugar control, reducing disease-related stress, and managing adolescent psychosocial issues (110).
For diabetes patients, especially adolescents, considerations should extend beyond the treatment of diabetes itself to include other conditions caused by diabetes, including the emotional disorders often associated with adolescents. Diabetes distress encompasses complications, self-management demands, unresponsive provider reactions, poor interpersonal relationships, and negative emotional responses to diabetes itself. Depression and diabetes distress screening scores are closely related to YODM, similar to adolescents with T1DM (111). YODM exposed to various stressful life events often experience reduced quality of life, depressive symptoms, and lower compliance with prescription oral medications (112). In the Pediatric Diabetes Consortium, 22% of YODM cases were found to have comorbid depression, with only 9% receiving treatment in the past 12 months (113). Compared to non-diabetic peers, YODM is more likely to experience emotional or anxiety disorders before and after diagnosis (114). Therefore, patients must possess good self-management skills, adhere to dietary controls, medication regimens, and regularly monitor blood glucose levels. Patients should comply with treatment plans, including regular medication intake, follow-up appointments, and actively collaborate with doctors in adjusting and improving treatment plans.
4.3 IR and obesity: reciprocal causation, choosing appropriate weight loss methods is a crucial means of improving IR and achieving diabetes remission
Controlling body weight, especially for obese diabetes patients, can improve insulin sensitivity, contributing to the remission of diabetes. Therefore, weight loss and subsequent reduction in visceral fat are key to diabetes remission (115). Compared to adolescents, pre-adolescent YODM patients face a higher risk of related incidence, emphasizing the importance of intervention measures to prevent and treat obesity in early childhood (116). Patients need to adhere to a healthy lifestyle, including regular physical exercise, maintaining healthy dietary habits, and avoiding unhealthy habits such as smoking and excessive alcohol consumption. The Population-based Panasonic cohort study in Japan, analyzing the relationship between changes and diabetes remission in 1,903 patients over a 5-year period, showed that weight loss of ≥3.9 kg or ≥5.0% in patients with BMI ≥ 25 kg/m2 (obesity) may be effective in relieving new-onset type 2 diabetes, excluding patients with BMI < 25 kg/m2 (117). Lifestyle changes are an important component of YODM weight management. The T2DM youth weight management guidelines recommend that adolescents reach at least a 7–10% weight reduction from their final adult height, with weight indexes below the 85th percentile for those still growing (118) (Table 1).
While there is currently evidence supporting the effectiveness of certain treatments in adolescents with type 2 diabetes, we must also be mindful of the potential side effects associated with these treatments (119). These include the possibility of hypoglycemic reactions, gastrointestinal discomfort, drug allergies, and others. Additionally, weight-loss surgery may lead to postoperative malnutrition, gastric emptying disorders, abnormal insulin secretion, among other issues (120, 121). Considering the psychological sensitivity of patients, it is crucial to continuously monitor their psychological status when implementing treatment plans or making recommendations. Timely intervention for any emerging social and psychological barriers is also necessary.
4.3.1 Dietary therapy
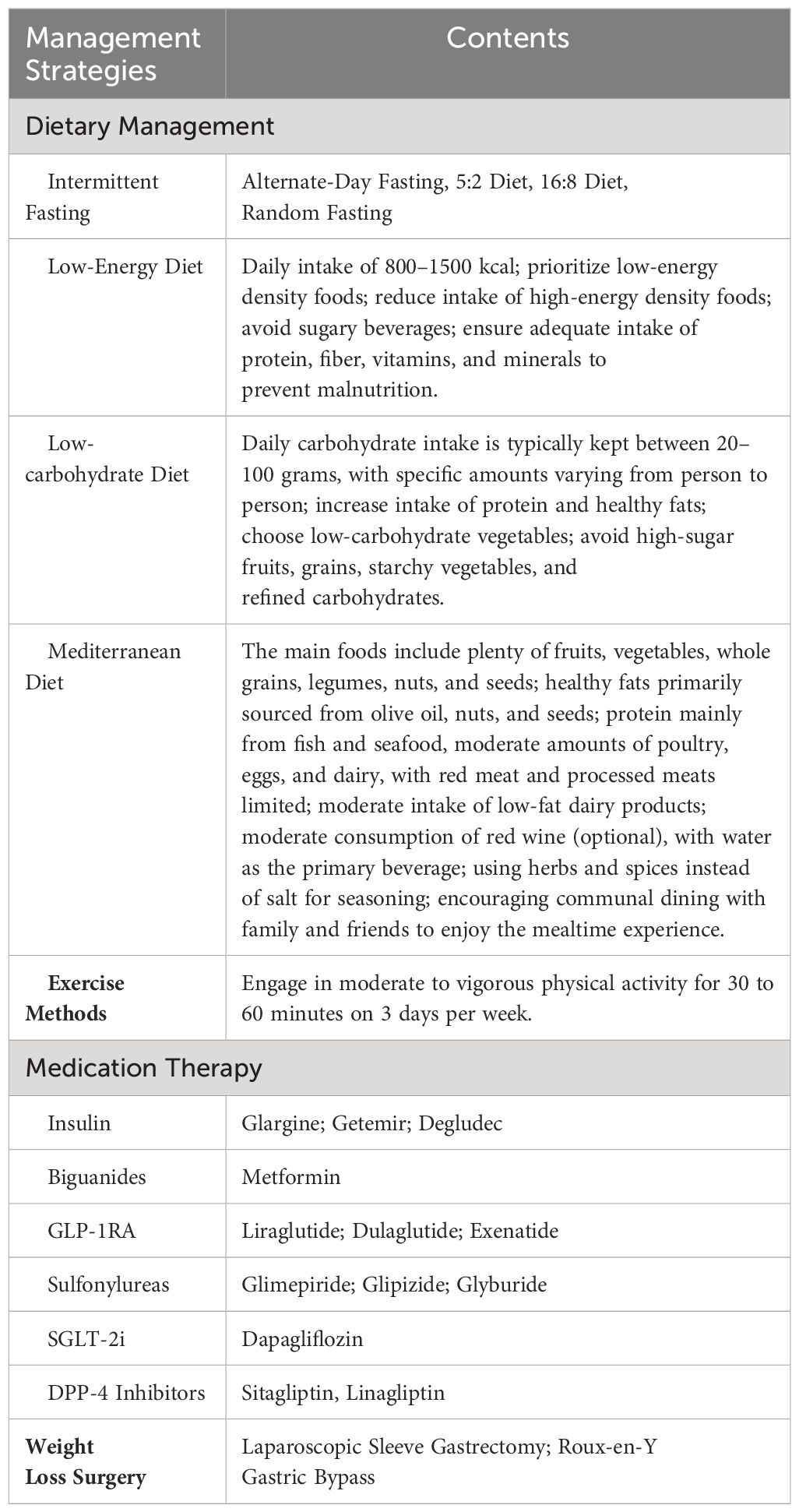
Dietary control can achieve standard weight and correct metabolic disorders. Dietary fiber can improve postprandial blood sugar and long-term diabetes control, with grain dietary fiber enhancing insulin sensitivity. As part of intensified lifestyle therapy, every T2DM patient should undergo comprehensive nutritional education (122). Reducing the consumption of sugary beverages, increasing the consumption of whole-grain bread and cereals, fruits, and vegetables is recommended (123). A randomized controlled clinical study in China confirmed that intermittent fasting intervention for 3 months could achieve diabetes remission for at least a year (124). A systematic review evaluating lifestyle changes (including three types of diets—low-energy diet, low-carbohydrate diet, Mediterranean diet, and exercise including aerobic and resistance sports, walking, and maintaining habitual physical activity) demonstrated effective diabetes remission, including weight loss and improved quality of life (125).
4.3.2 Exercise therapy
Exercise plays a crucial role in the treatment of YODM, aiding in weight reduction, increasing insulin sensitivity, and enhancing peripheral tissue glucose uptake. The choice of exercise type and amount should be individualized based on gender, age, physique, physical strength, exercise habits, and preferences. Clear evidence indicates that regular physical activity benefits children and adolescents, including developing motor skills, promoting healthy weight and body composition, supporting bone and muscle development, and positively influencing insulin sensitivity (126, 127). Engaging in vigorous physical activity for 16 weeks twice a week significantly increased insulin sensitivity in overweight adolescents, independent of changes in total fat mass and lean tissue mass (128). The American Diabetes Association (ADA) and the International Society for Pediatric and Adolescent Diabetes (ISPAD) recommend encouraging adolescents with Youth-Onset Diabetes Mellitus (YODM) to engage in at least 60 minutes of moderate to vigorous physical activity daily, including muscle and bone strength training at least 3 days per week (129, 130). Exercise increases the expression of GLUT4 receptors, thereby enhancing peripheral glucose absorption. The benefits of improved insulin sensitivity persist for 16 hours after exercise in both healthy subjects and patients with Type 2 Diabetes Mellitus (T2DM) (131). In a cross-sectional study involving 164 YODM adolescents engaging in vigorous physical activity, lower HbA1c levels and better cardiovascular parameters were observed (132).
4.3.3 Pharmacological treatment
Despite significant progress in the treatment of adult T2DM over the past few decades, the efficacy in YODM has seen only slight changes. Besides insulin, metformin is the first-line medication approved by the U.S. Food and Drug Administration (FDA) for treating YODM (118, 133). Although emerging therapies have provided new perspectives for managing YODM, metformin remains a cost-effective and safe part of treatment plans for many YODM patients globally due to its beneficial metabolic effects (134). However, the reality reflects that in almost half of the cases, metformin has been found insufficient for treating YODM (135, 136), especially in adolescents with severe metabolic disturbances at the time of diagnosis (137). Given that postprandial insulin release is often blunted or impaired in T2DM patients, GLP-1 agonists improve blood glucose control by enhancing postprandial insulin release. Additionally, these drugs slow gastric emptying, prolong beta-cell lifespan by inhibiting apoptosis, increase peripheral glucose uptake in muscles, and reduce hepatic glucose output, thereby improving insulin sensitivity. An indirect mechanism for improving insulin sensitivity is weight loss. The weight-loss effects of these drugs are primarily attributed to delayed gastric emptying and direct effects on suppressing appetite in the hypothalamus (138, 139). Compared to liraglutide, semaglutide has shown promising weight-loss effects in adults with obesity and T2DM (140, 141). Until July 2019, the GLP-1 receptor agonist liraglutide was approved by the FDA for treating YODM and/or obesity in individuals aged 10 and above (142, 143). However, the approved dose for weight management (3 mg per day) is higher than the dose used for managing T2DM (1.8 mg per day). In a systematic review of 9 randomized controlled trials involving 286 obese, prediabetic, or YODM individuals aged 18 and under, GLP-1 agonist use was associated with an average weight loss of 2.7 kg. This effect was observed more in patients with higher BMI (144). A randomized controlled trial showed a favorable impact on YODM weight when GLP-1 agonists were used in conjunction with metformin. In a study of 134 children and adolescents (10–17 years) with T2DM, the liraglutide group experienced a weight loss of 2.3 kg at week 26, while the placebo group lost 0.99 kg (145). An investigation in Korea assessed the efficacy and side effects of once-weekly dulaglutide in treating YODM. The results indicated excellent glycemic control with once-weekly dulaglutide for YODM, with no significant side effects (146). However, other studies found additional side effects of GLP-1 agonists, including vomiting, diarrhea, abdominal pain, hypoglycemia, elevated transaminases, and pancreatitis (147). The use of sulfonylurea drugs in children effectively lowers HbA1c by 1.5–2% and has been approved in several countries. However, adverse reactions, including weight gain, accelerated β-cell loss, and hypoglycemia leading to seizures and negative cognitive consequences, have been observed (148). An evaluation of the efficacy and safety of several glucose-lowering medications provides additional options for YODM management. Compared to FDA-approved drugs, dapagliflozin, sitagliptin+metformin, and saxagliptin+metformin showed better efficacy, indicating the top ten treatment methods for YODM in individuals aged 10–17: saxagliptin+metformin, liraglutide+metformin, liraglutide, dapagliflozin, exenatide-2 mcg, sitagliptin+metformin, linagliptin-5 mg, linagliptin-1 mg, metformin, and exenatide-5/10 mcg (149).
Implementing short-term intensified insulin therapy in uncontrolled hyperglycemic patients in the early stages of the disease improves β-cell function, ensuring remission of T2DM for a considerable period (150, 151). Insulin therapy is typically associated with weight gain (152, 153). Remission after intensified insulin therapy has been shown to last for over 2 years, and the shorter the time interval between diagnosis and intensified insulin therapy, the greater the likelihood of remission (154). According to the International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines, if HbA1c levels do not reach the target of 6.5–7% after 4 months of metformin treatment for YODM, initiating basal insulin at a dose of 1.5 IU/kg/day is recommended. If adding basal insulin is insufficient, prandial insulin at a dose of 0.1 U/kg/meal may be necessary (148).
4.3.4 Bariatric surgical treatment
For patients who find it challenging to achieve weight loss after lifestyle interventions, or for those who prefer a more direct approach, bariatric surgery can be considered. The American Diabetes Association (ADA) recommends considering bariatric surgery for YODM patients with a BMI greater than 35 kg/m2, as surgery has a more favorable impact on blood glucose control (including HBG, HbA1c, HOMA-IR) compared to medical treatments (155). Metabolic and bariatric surgery (MBS) has been associated with excellent weight loss and T2DM remission in adolescents (156, 157). The two most common surgeries for adolescents are laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass. In the TEEN-LABS alliance study, among 242 adolescents undergoing MBS, the average weight decreased by 27% three years after surgical intervention. There was no significant difference in weight loss between patients undergoing Roux-en-Y gastric bypass (161 participants) or sleeve gastrectomy (67 participants) (28% and 26%, respectively). Furthermore, 95% (95% CI; 85 to 100) of patients with T2DM at the time of surgery achieved remission. Similarly, remission rates for other comorbidities were high (renal dysfunction at 86%, prediabetes at 76%, hypertension at 74%, dyslipidemia at 66%) (120). Laparoscopic sleeve gastrectomy (LSG) is gradually becoming a new benchmark for the treatment of morbid obesity and related complications in pediatric cases. A retrospective study involving 64 pediatric patients aged 5 to 14 provided further specific evidence of the beneficial metabolic effects of LSG surgery on morbidly obese children, effectively reducing complications related to Type 2 diabetes by lowering HbA1c levels after surgery (158).
5 Outlook and conclusion
Overall, based on the existing published research, there are still many areas that need improvement in screening, treatment, and other aspects of YODM. Given the continuously increasing number of YODM patients and the limited interventions available, there is a need to focus more on key aspects of the occurrence and development of YODM. This includes early identification and timely intervention, considering the unique characteristics of adolescents. For early identification, constructing predictive models that encompass various risk factors such as obesity, family history, unhealthy lifestyle habits, emotional disorders, along with data from metabolic and insulin resistance testing, may help identify patients early on. Specifically, in the future, leveraging new technologies such as artificial intelligence, big data, and others can provide more intelligent and precise support for early screening, diagnosis, and treatment of type 2 diabetes in adolescents. It is crucial not to wait until other chronic complications (such as peripheral neuropathy, kidney disease) or acute complications (such as ketoacidosis) emerge. Early intervention during this golden period is essential for achieving diabetes remission. In the future, precision medicine approaches such as genomics and metabolomics can be utilized to provide more personalized diagnosis and treatment for type 2 diabetes in adolescents, thereby improving treatment effectiveness and prognosis. Currently, the key interventions for diabetes patients consistently revolve around insulin resistance-mediated obesity, emphasizing the importance of maintaining normal weight. Good lifestyle habits are crucial in this regard and require supervision and monitoring from family and schools. Indeed, strengthening health education and intervention strategies across multiple levels such as family, school, and community can promote the adoption of healthy lifestyles among adolescents, thereby preventing and controlling the occurrence and progression of type 2 diabetes. Medications such as GLP-1 agonists can serve as effective supplementary interventions. For some patients, bariatric surgery may be more suitable and effective. Of course, in the future, precision medicine approaches such as genomics and metabolomics can be utilized to provide more personalized diagnosis and treatment for type 2 diabetes in adolescents, thereby improving treatment effectiveness and prognosis.
In summary, early identification, early intervention, and effective evaluation centered around IR in YODM can be crucial for achieving diabetes remission. However, healthcare professionals and researchers may significantly help individuals with YODM by identifying and implementing better strategies for improving insulin resistance and intervening in obesity at an early stage.
Author contributions
QJ: Writing – original draft, Writing – review & editing. YZ: Writing – original draft. BZ: Conceptualization, Writing – review & editing. XA: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82305205), High Level Chinese Medical Hospital Promotion Project (HLCMHPP2023084), Youth Talent Support Project of the Chinese Association of Chinese Medicine (CACM-2023-QNRC2-A05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang J, Wu W, Dong G, Huang K, Fu J. Pediatric diabetes in China: Challenges and actions. Pediatr Diabetes. (2022) 23:545–50. doi: 10.1111/pedi.13344
2. Wu H, Patterson CC, Zhang X, Ghani RBA, Magliano DJ, Boyko EJ, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. (2022) 185:109785. doi: 10.1016/j.diabres.2022.109785
3. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. (2017) 376:1419–29. doi: 10.1056/NEJMoa1610187
4. U.S. Department of Health and Human Services. Healthy people. Washington, DC (2020). Available at: http://www.healthypeople.gov/2020.
5. Wilfley D, Berkowitz R, Goebel-Fabbri A, Hirst K, Ievers-Landis C, Lipman TH, et al. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care. (2011) 34:858–60. doi: 10.2337/dc10-1704
6. Type 2 diabetes in children and adolescents. Am Diabetes Assoc Diabetes Care. (2000) 23:381–9. doi: 10.2337/diacare.23.3.381
7. Gulley LD, Shomaker LB. Depression in youth-onset type 2 diabetes. Curr Diabetes Rep. (2020) 20:51. doi: 10.1007/s11892-020-01334-8
8. Wang J, Wu X, Lai W, Long E, Zhang X, Li W, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ Open. (2017) 7:e017173. doi: 10.1136/bmjopen-2017-017173
9. Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. (2015) 75:577–87. doi: 10.1007/s40265-015-0347-4
10. Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. (2011) 96:159–67. doi: 10.1210/jc.2010-1642
11. Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R Jr., Dolan L, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. Jama. (2017) 317:825–35. doi: 10.1001/jama.2017.0686
12. Gungor N, Hannon T, Libman I, Bacha F, Arslanian S. Type 2 diabetes mellitus in youth: the complete picture to date. Pediatr Clin North Am. (2005) 52:1579–609. doi: 10.1016/j.pcl.2005.07.009
13. Menke A, Casagrande S, Cowie CC. Prevalence of diabetes in adolescents aged 12 to 19 years in the United States, 2005–2014. Jama. (2016) 316:344–5. doi: 10.1001/jama.2016.8544
14. Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: systematic analysis of the Global Burden of Disease Study 2019. Bmj. (2022) 379:e072385. doi: 10.1136/bmj-2022-072385
15. Utzschneider KM, Tripputi MT, Kozedub A, Mather KJ, Nadeau KJ, Edelstein SL, et al. β-cells in youth with impaired glucose tolerance or early type 2 diabetes secrete more insulin and are more responsive than in adults. Pediatr Diabetes. (2020) 21:1421–9. doi: 10.1111/pedi.13113
16. Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. (2001) 86:4047–58. doi: 10.1210/jcem.86.9.7713
17. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. (1999) 104:787–94. doi: 10.1172/JCI7231
18. McCombie L, Leslie W, Taylor R, Kennon B, Sattar N, Lean MEJ. Beating type 2 diabetes into remission. Bmj. (2017) 358:j4030. doi: 10.1136/bmj.j4030
19. Yang YS, Han K, Sohn TS, Kim NH. Young-onset type 2 diabetes in South Korea: a review of the current status and unmet need. Korean J Intern Med. (2021) 36:1049–58. doi: 10.3904/kjim.2021.379
20. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. (2020) 16:321–31. doi: 10.1038/s41574-020-0334-z
21. La Grasta Sabolic L, Marusic S, Cigrovski Berkovic M. Challenges and pitfalls of youth-onset type 2 diabetes. World J Diabetes. (2024) 15:876–85. doi: 10.4239/wjd.v15.i5.876
22. Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. (2021) 385:416–26. doi: 10.1056/NEJMoa2100165
23. Klingensmith GJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Haro H, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. (2016) 17:266–73. doi: 10.1111/pedi.2016.17.issue-4
24. Chao LC, Vidmar AP, Georgia S. Spike in diabetic ketoacidosis rates in pediatric type 2 diabetes during the COVID-19 pandemic. Diabetes Care. (2021) 44:1451–3. doi: 10.2337/dc20-2733
25. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. (2004) 27:1218–24. doi: 10.2337/diacare.27.5.1218
26. Comellas M, Marrero Y, George F, Matthews L. Age and glycemic control among adults with type 2 diabetes in the United States: An assessment from the National Health and Nutrition Examination Survey (NHANES) 2013–2014. Diabetes Metab Syndr. (2019) 13:3069–73. doi: 10.1016/j.dsx.2019.11.004
27. Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. (2014) 2:935–43. doi: 10.1016/S2213-8587(14)70137-8
28. Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. (2007) 8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x
29. Sanderson EE, Shah M, Hooper AJ, Bell DA, Choong CS. Monogenic diabetes due to an INSR mutation in a child with severe insulin resistance. Endocrinol Diabetes Metab Case Rep. (2022) 2022:21-0114. doi: 10.1530/EDM-21-0114
30. Ali O. Genetics of type 2 diabetes. World J Diabetes. (2013) 4:114–23. doi: 10.4239/wjd.v4.i4.114
31. Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes. (2006) 55:460–5. doi: 10.2337/diabetes.55.02.06.db05-0823
32. Shah RD, Chernausek SD, El Ghormli L, Geffner ME, Keady J, Kelsey MM, et al. Maternal diabetes in youth-onset type 2 diabetes is associated with progressive dysglycemia and risk of complications. J Clin Endocrinol Metab. (2023) 108:1120–31. doi: 10.1210/clinem/dgac663
33. Yang L, Huang C, Zhao M, Lee PMY, Zhang C, Yu Y, et al. Maternal hypertensive disorders during pregnancy and the risk of offspring diabetes mellitus in childhood, adolescence, and early adulthood: a nationwide population-based cohort study. BMC Med. (2023) 21:59. doi: 10.1186/s12916-023-02762-5
34. Blasetti A, Quarta A, Guarino M, Cicolini I, Iannucci D, Giannini C, et al. Role of prenatal nutrition in the development of insulin resistance in children. Nutrients. (2022) 15:87. doi: 10.3390/nu15010087
35. Srinivasan S, Chen L, Todd J, Divers J, Gidding S, Chernausek S, et al. The first genome-wide association study for type 2 diabetes in youth: the progress in diabetes genetics in youth (ProDiGY) consortium. Diabetes. (2021) 70:996–1005. doi: 10.2337/db20-0443
36. Gao J, Lu Y, Gokulnath P, Vulugundam G, Li G, Li J, et al. Benefits of physical activity on cardiometabolic diseases in obese children and adolescents. J Transl Int Med. (2022) 10:236–45. doi: 10.2478/jtim-2022-0041
37. Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: A retrospective cohort study. J Endocr Soc. (2017) 1:524–37. doi: 10.1210/js.2017-00044
38. Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. (2016) 53:52–67. doi: 10.3109/10408363.2015.1084990
39. Barnard N, Levin S, Trapp C. Meat consumption as a risk factor for type 2 diabetes. Nutrients. (2014) 6:897–910. doi: 10.3390/nu6020897
40. Bleich SN, Vercammen KA. The negative impact of sugar-sweetened beverages on children’s health: an update of the literature. BMC Obes. (2018) 5:6. doi: 10.1186/s40608-017-0178-9
41. Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. (1998) 68:1157–73. doi: 10.1093/ajcn/68.6.1157
42. Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR Jr., Popkin BM. Regular consumption from fast food establishments relative to other restaurants is differentially associated with metabolic outcomes in young adults. J Nutr. (2009) 139:2113–8. doi: 10.3945/jn.109.109520
43. Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. Jama. (2021) 326:717–27. doi: 10.1001/jama.2021.11165
44. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. (2018) 141:e20181916. doi: 10.1542/peds.2017-3459
45. Karin A, Jon E, Martin A, Lena B, Martin L, Naveed S, et al. Body mass index in adolescence, risk of type 2 diabetes and associated complications: A nationwide cohort study of men. EClinicalMedicine. (2022) 46:101356. doi: 10.1016/j.eclinm.2022.101356
46. He QX, Zhao L, Tong JS, Liang XY, Li RN, Zhang P, et al. The impact of obesity epidemic on type 2 diabetes in children and adolescents: A systematic review and meta-analysis. Prim Care Diabetes. (2022) 16:736–44. doi: 10.1016/j.pcd.2022.09.006
47. Twig G, Zucker I, Afek A, Cukierman-Yaffe T, Bendor CD, Derazne E, et al. Adolescent obesity and early-onset type 2 diabetes. Diabetes Care. (2020) 43:1487–95. doi: 10.2337/dc19-1988
48. Hood KK, Beavers DP, Yi-Frazier J, Bell R, Dabelea D, McKeown RE, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. (2014) 55:498–504. doi: 10.1016/j.jadohealth.2014.03.011
49. Hsu JW, Chen LC, Huang KL, Tsai SJ, Bai YM, Su TP, et al. Risk of type 2 diabetes mellitus between adolescents with antidepressant-resistant and antidepressant-responsive depression: A cohort study of 15,651 adolescents. J Affect Disord. (2023) 328:210–4. doi: 10.1016/j.jad.2023.02.065
50. Snyder LL, Foland-Ross LC, Cato A, Reiss AL, Shah C, Hossain J, et al. Impact of dysglycemia and obesity on the brain in adolescents with and without type 2 diabetes: A pilot study. Pediatr Diabetes. (2022) 23:1674–86. doi: 10.1111/pedi.13420
51. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21:6275. doi: 10.3390/ijms21176275
52. Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). (2023) 14:1149239. doi: 10.3389/fendo.2023.1149239
53. James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. (2021) 22:751–71. doi: 10.1038/s41580-021-00390-6
54. Assunção SNF, Boa Sorte NCA, Alves CAD, Mendes PSA, Alves CRB, Silva LR. Glucose alteration and insulin resistance in asymptomatic obese children and adolescents. J Pediatr (Rio J). (2018) 94:268–72. doi: 10.1016/j.jped.2017.06.008
55. Xourafa G, Korbmacher M, Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. (2024) 20:27–49. doi: 10.1038/s41574-023-00898-1
56. Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. (2005) 280:35361–71. doi: 10.1074/jbc.M504611200
57. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. (2018) 27:299–313. doi: 10.1016/j.cmet.2017.10.009
58. Venable CL, Frevert EU, Kim YB, Fischer BM, Kamatkar S, Neel BG, et al. Overexpression of protein-tyrosine phosphatase-1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/Protein kinase B activation. J Biol Chem. (2000) 275:18318–26. doi: 10.1074/jbc.M908392199
59. Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. (2012) 13:383–96. doi: 10.1038/nrm3351
60. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. BioMed Pharmacother. (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
61. Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res. (2018) 191:29–44. doi: 10.1016/j.trsl.2017.10.004
62. Chen G, Ni Y, Nagata N, Xu L, Zhuge F, Nagashimada M, et al. Pirfenidone prevents and reverses hepatic insulin resistance and steatohepatitis by polarizing M2 macrophages. Lab Invest. (2019) 99:1335–48. doi: 10.1038/s41374-019-0255-4
63. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. (2012) 15:635–45. doi: 10.1016/j.cmet.2012.04.001
64. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. (2006) 116:1793–801. doi: 10.1172/JCI29069
65. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. (2007) 21:1443–55. doi: 10.1101/gad.1550907
66. Kwak HJ, Yang D, Hwang Y, Jun HS, Cheon HG. Baicalein protects rat insulinoma INS-1 cells from palmitate-induced lipotoxicity by inducing HO-1. PloS One. (2017) 12:e0176432. doi: 10.1371/journal.pone.0176432
67. Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. (2005) 7:1040–52. doi: 10.1089/ars.2005.7.1040
68. Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. BioMed J. (2017) 40:257–62. doi: 10.1016/j.bj.2017.06.007
69. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. (2005) 28:638–44. doi: 10.2337/diacare.28.3.638
70. Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-saharan africa. Lancet. (2010) 375:2254–66. doi: 10.1016/S0140-6736(10)60550-8
71. Yajnik CS. The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev. (2002) 3:217–24. doi: 10.1046/j.1467-789X.2002.00072.x
72. RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care. (2018) 41:1707–16. doi: 10.2337/dc18-0243
73. Cohrs CM, Panzer JK, Drotar DM, Enos SJ, Kipke N, Chen C, et al. Dysfunction of persisting β Cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep. (2020) 31:107469. doi: 10.1016/j.celrep.2020.03.033
74. Gobato AO, Vasques AC, Zambon MP, Barros Filho Ade A, Hessel G. Metabolic syndrome and insulin resistance in obese adolescents. Rev Paul Pediatr. (2014) 32:55–62. doi: 10.1590/S0103-05822014000100010
75. Huang RC, de Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA, et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. (2011) 34:1019–25. doi: 10.2337/dc10-1809
76. Peplies J, Börnhorst C, Günther K, Fraterman A, Russo P, Veidebaum T, et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int J Behav Nutr Phys Act. (2016) 13:97. doi: 10.1186/s12966-016-0424-4
77. Kobayashi K, Amemiya S, Higashida K, Ishihara T, Sawanobori E, Kobayashi K, et al. Pathogenic factors of glucose intolerance in obese Japanese adolescents with type 2 diabetes. Metabolism. (2000) 49:186–91. doi: 10.1016/S0026-0495(00)91221-6
78. van der Aa MP, Fazeli Farsani S, Knibbe CA, de Boer A, van der Vorst MM. Population-based studies on the epidemiology of insulin resistance in children. J Diabetes Res. (2015) 2015:362375. doi: 10.1155/2015/362375
79. Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. (2016) 9:235–9.
80. Androutsos O, Moschonis G, Mavrogianni C, Roma-Giannikou E, Chrousos GP, Kanaka-Gantenbein C, et al. Identification of lifestyle patterns, including sleep deprivation, associated with insulin resistance in children: the Healthy Growth Study. Eur J Clin Nutr. (2014) 68:344–9. doi: 10.1038/ejcn.2013.280
81. Nip ASY, Reboussin BA, Dabelea D, Bellatorre A, Mayer-Davis EJ, Kahkoska AR, et al. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for diabetes in youth study. Diabetes Care. (2019) 42:859–66. doi: 10.2337/dc18-2420
82. Corica D, Pepe G, Aversa T, Currò M, Curatola S, Li Pomi A, et al. Meal-related asprosin serum levels are affected by insulin resistance and impaired fasting glucose in children with obesity. Front Endocrinol (Lausanne). (2021) 12:805700. doi: 10.3389/fendo.2021.805700
83. Harnois-Leblanc S, Sylvestre MP, Van Hulst A, Barnett TA, Mathieu M, Mesidor M, et al. Estimating causal effects of physical activity and sedentary behaviours on the development of type 2 diabetes in at-risk children from childhood to late adolescence: an analysis of the QUALITY cohort. Lancet Child Adolesc Health. (2023) 7:37–46. doi: 10.1016/S2352-4642(22)00278-4
84. Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. (2020) 16:81–90. doi: 10.1038/s41574-019-0286-3
85. Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. (1993) 268:26055–8. doi: 10.1016/S0021-9258(19)74276-8
86. Sunil B, Ashraf AP. Dyslipidemia in pediatric type 2 diabetes mellitus. Curr Diabetes Rep. (2020) 20:53. doi: 10.1007/s11892-020-01336-6
87. Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. (2015) 16:678–89. doi: 10.1038/nrm4074
88. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. (2005) 365:1333–46. doi: 10.1016/S0140-6736(05)61032-X
89. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. (2002) 32 Suppl 3:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x
90. Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem. (2020) 126:67–73. doi: 10.1080/13813455.2018.1493606
91. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. (2018) 391:541–51. doi: 10.1016/S0140-6736(17)33102-1
92. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. (2013) 369:145–54. doi: 10.1056/NEJMoa1212914
93. Kramer CK, Zinman B, Choi H, Retnakaran R. Predictors of sustained drug-free diabetes remission over 48 weeks following short-term intensive insulin therapy in early type 2 diabetes. BMJ Open Diabetes Res Care. (2016) 4:e000270. doi: 10.1136/bmjdrc-2016-000270
94. Pyle L, Kelsey MM. Youth-onset type 2 diabetes: translating epidemiology into clinical trials. Diabetologia. (2021) 64:1709–16. doi: 10.1007/s00125-021-05480-w
95. Wang J, Lin H, Chiavaroli V, Jin B, Yuan J, Huang K, et al. High prevalence of cardiometabolic comorbidities among children and adolescents with severe obesity from a large metropolitan centre (Hangzhou, China). Front Endocrinol (Lausanne). (2022) 13:807380. doi: 10.3389/fendo.2022.807380
96. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
97. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. (2001) 44 Suppl 2:S14–21. doi: 10.1007/PL00002934
98. Hounkpatin H, Stuart B, Farmer A, Dambha-Miller H. Association of type 2 diabetes remission and risk of cardiovascular disease in pre-defined subgroups. Endocrinol Diabetes Metab. (2021) 4:e00280. doi: 10.1002/edm2.280
99. Jefferies C, Carter P, Reed PW, Cutfield W, Mouat F, Hofman PL, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. (2012) 13:294–300. doi: 10.1111/j.1399-5448.2012.00851.x
100. Deusdará R, de Moura Souza A, Szklo M. Association between obesity, overweight, elevated waist circumference, and insulin resistance markers among Brazilian adolescent students. Nutrients. (2022) 14:3487. doi: 10.3390/nu14173487
101. Xu ZR, Du HW, Cui LW, Zheng RX, Li GM, Wei HY, et al. Association of β-cell function and insulin resistance with pediatric type 2 diabetes among Chinese children. World J Diabetes. (2021) 12:1292–303. doi: 10.4239/wjd.v12.i8.1292
102. Yoon JS, Lee HJ, Jeong HR, Shim YS, Kang MJ, Hwang IT. Triglyceride glucose index is superior biomarker for predicting type 2 diabetes mellitus in children and adolescents. Endocr J. (2022) 69:559–65. doi: 10.1507/endocrj.EJ21-0560
103. Khan MS, Cuda S, Karere GM, Cox LA, Bishop AC. Breath biomarkers of insulin resistance in pre-diabetic Hispanic adolescents with obesity. Sci Rep. (2022) 12:339. doi: 10.1038/s41598-021-04072-3
104. Mora-Ortiz M, Alcala-Diaz JF, Rangel-Zuñiga OA, Arenas-de Larriva AP, Abollo-Jimenez F, Luque-Cordoba D, et al. Metabolomics analysis of type 2 diabetes remission identifies 12 metabolites with predictive capacity: a CORDIOPREV clinical trial study. BMC Med. (2022) 20:373. doi: 10.1186/s12916-022-02566-z
105. Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care. (2016) 39:808–15. doi: 10.2337/dc15-1942
106. Steven S, Taylor R. Restoring normoglycaemia by use of a very low calorie diet in long- and short-duration Type 2 diabetes. Diabetes Med. (2015) 32:1149–55. doi: 10.1111/dme.12722
107. Zou D, Zhang Z, Ji L. Consensus of chinese experts on the management of type 2 diabetes. Chin J Diabetes. (2021) 29:641–52.
108. Fujihara K, Khin L, Murai K, Yamazaki Y, Tsuruoka K, Yagyuda N, et al. Relationship between the magnitude of body mass index reductions and remission in patients with type 2 diabetes in real world settings: Analysis of nationwide patient registry in Japan (JDDM74). Diabetes Obes Metab. (2023) 25:3125–35. doi: 10.1111/dom.15206
109. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. (2006) 444:840–6. doi: 10.1038/nature05482
110. Urakami T, Kuwabara R, Habu M, Yoshida A, Okuno M, Suzuki J, et al. Pharmacologic treatment strategies in children with type 2 diabetes mellitus. Clin Pediatr Endocrinol. (2013) 22:1–8. doi: 10.1297/cpe.22.1
111. Hoffman RP, Damilano CP, Hong KMC, Glick BA, Kamboj MK. Glycemic control, depression, diabetes distress among adolescents with type 2 diabetes: effects of sex, race, insurance, and obesity. Acta Diabetol. (2022) 59:1083–9. doi: 10.1007/s00592-022-01902-2
112. Walders-Abramson N, Venditti EM, Ievers-Landis CE, Anderson B, El Ghormli L, Geffner M, et al. Relationships among stressful life events and physiological markers, treatment adherence, and psychosocial functioning among youth with type 2 diabetes. J Pediatr. (2014) 165:504–8.e1. doi: 10.1016/j.jpeds.2014.05.020
113. Silverstein J, Cheng P, Ruedy KJ, Kollman C, Beck RW, Klingensmith GJ, et al. Depressive symptoms in youth with type 1 or type 2 diabetes: results of the pediatric diabetes consortium screening assessment of depression in diabetes study. Diabetes Care. (2015) 38:2341–3. doi: 10.2337/dc15-0982
114. Sellers EAC, McLeod L, Prior HJ, Dragan R, Wicklow BA, Ruth C. Mental health comorbidity is common in children with type 2 diabetes. Pediatr Diabetes. (2022) 23:991–8. doi: 10.1111/pedi.13389
115. Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. (2013) 36:1047–55. doi: 10.2337/dc12-1805
116. Astudillo M, Tosur M, Castillo B, Rafaey A, Siller AF, Nieto J, et al. Type 2 diabetes in prepubertal children. Pediatr Diabetes. (2021) 22:946–50. doi: 10.1111/pedi.13254
117. Matsui T, Okada H, Hamaguchi M, Kurogi K, Murata H, Ito M, et al. The association between the reduction of body weight and new-onset type 2 diabetes remission in middle-aged Japanese men: Population-based Panasonic cohort study 8. Front Endocrinol (Lausanne). (2022) 13:1019390. doi: 10.3389/fendo.2022.1019390
118. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the american diabetes association. Diabetes Care. (2018) 41:2648–68. doi: 10.2337/dci18-0052
119. Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. (2023) 11:768–82. doi: 10.1016/S2213-8587(23)00225-5
120. Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. (2016) 374:113–23. doi: 10.1056/NEJMoa1506699
121. Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. Jama. (2019) 322:1155–66. doi: 10.1001/jama.2019.13772
122. Springer SC, Silverstein J, Copeland K, Moore KR, Prazar GE, Raymer T, et al. Management of type 2 diabetes mellitus in children and adolescents. Pediatrics. (2013) 131:e648–64. doi: 10.1542/peds.2012-3496
123. Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, Lichtenstein AH, et al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. (2006) 117:544–59. doi: 10.1542/peds.2005-2374
124. Yang X, Zhou J, Shao H, Huang B, Kang X, Wu R, et al. Effect of an intermittent calorie-restricted diet on type 2 diabetes remission: A randomized controlled trial. J Clin Endocrinol Metab. (2023) 108:1415–24. doi: 10.1210/clinem/dgac661
125. Zhang Y, Yang Y, Huang Q, Zhang Q, Li M, Wu Y. The effectiveness of lifestyle interventions for diabetes remission on patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Worldviews Evid Based Nurs. (2023) 20:64–78. doi: 10.1111/wvn.12608
126. Kim JY, Jeon JY. Role of exercise on insulin sensitivity and beta-cell function: is exercise sufficient for the prevention of youth-onset type 2 diabetes? Ann Pediatr Endocrinol Metab. (2020) 25:208–16. doi: 10.6065/apem.2040140.070
127. Hay J, Maximova K, Durksen A, Carson V, Rinaldi RL, Torrance B, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. (2012) 166:1022–9. doi: 10.1001/archpediatrics.2012.1028
128. Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Salem GJ, Crespo NC, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. (2006) 38:1208–15. doi: 10.1249/01.mss.0000227304.88406.0f
129. Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. (2022) 23:872–902. doi: 10.1111/pedi.13409
130. American Diabetes Association Professional Practice Committee. 14. Children and adolescents: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S208–s31. doi: 10.2337/dc22-S014
131. Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. (2000) 21:1–12. doi: 10.1055/s-2000-8847
132. Slaght JL, Wicklow BA, Dart AB, Sellers EAC, Gabbs M, Carino M, et al. Physical activity and cardiometabolic health in adolescents with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. (2021) 9:e002134. doi: 10.1136/bmjdrc-2021-002134
133. Gaylor AS, Condren ME, TODAY Study Group. Type 2 diabetes mellitus in the pediatric population. Pharmacotherapy. (2004) 24:871–8. doi: 10.1592/phco.24.9.871.36099
134. Alfaraidi H, Samaan MC. Metformin therapy in pediatric type 2 diabetes mellitus and its comorbidities: A review. Front Endocrinol (Lausanne). (2022) 13:1072879. doi: 10.3389/fendo.2022.1072879
135. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. (2013) 36:1749–57. doi: 10.2337/dc12-2393
136. Badaru A, Klingensmith GJ, Dabelea D, Mayer-Davis EJ, Dolan L, Lawrence JM, et al. Correlates of treatment patterns among youth with type 2 diabetes. Diabetes Care. (2014) 37:64–72. doi: 10.2337/dc13-1124
137. Bacha F, Cheng P, Gal RL, Kollman C, Tamborlane WV, Klingensmith GJ, et al. Initial presentation of type 2 diabetes in adolescents predicts durability of successful treatment with metformin monotherapy: insights from the pediatric diabetes consortium T2D registry. Horm Res Paediatr. (2018) 89:47–55. doi: 10.1159/000481687
138. Ard J, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. (2021) 38:2821–39. doi: 10.1007/s12325-021-01710-0
139. Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care. (2022) 45:1833–40. doi: 10.2337/dc21-2275
140. Iqbal J, Wu HX, Hu N, Zhou YH, Li L, Xiao F, et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. (2022) 23:e13435. doi: 10.1111/obr.13435
141. Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. (2022) 387:2245–57. doi: 10.1056/NEJMoa2208601
142. McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. (2014) 168:178–84. doi: 10.1001/jamapediatrics.2013.4200
143. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. (2020) 382:2117–28. doi: 10.1056/NEJMoa1916038
144. Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev. (2021) 22:e13177. doi: 10.1111/obr.13177
145. Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. (2019) 381:637–46. doi: 10.1056/NEJMoa1903822
146. Seo JY, Lee CG, Choi H, Lee HK, Lee SY, Kim HJ, et al. Effect of once weekly dulaglutide for juvenile type 2 Diabetes mellitus and obesity in Korea: a Pilot Study. Ann Pediatr Endocrinol Metab. (2023) 28:296-301. doi: 10.6065/apem.2244196.098
147. Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: A meta-analysis. J Pediatr. (2021) 236:137–47.e13. doi: 10.1016/j.jpeds.2021.05.009
148. Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. (2018) 19 Suppl 27:28–46. doi: 10.1111/pedi.2018.19.issue-S27
149. Wu S, He Y, Wu Y, Ji Y, Hou L, Liu X, et al. Comparative efficacy and safety of glucose-lowering drugs in children and adolescents with type 2 diabetes: A systematic review and network meta-analysis. Front Endocrinol (Lausanne). (2022) 13:897776. doi: 10.3389/fendo.2022.897776
150. Hu Y, Li L, Xu Y, Yu T, Tong G, Huang H, et al. Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care. (2011) 34:1848–53. doi: 10.2337/dc10-2105
151. Harrison LB, Adams-Huet B, Raskin P, Lingvay I. β-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. (2012) 35:1406–12. doi: 10.2337/dc11-2170
152. Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. (2007) 9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x
153. Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: What can be done? Diabetes Obes Metab. (2017) 19:1655–68. doi: 10.1111/dom.13009
154. Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2013) 1:28–34. doi: 10.1016/S2213-8587(13)70006-8
155. Zhu X, Zhou G, Gu X, Jiang X, Huang H, You S, et al. Comparison of bariatric surgery and medical therapy for obese adolescents with type 2 diabetes. Asian J Surg. (2022) 46:4337-43. doi: 10.1016/j.asjsur.2022.10.079
156. Pratt JSA, Browne A, Browne NT, Bruzoni M, Cohen M, Desai A, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. (2018) 14:882–901. doi: 10.1016/j.soard.2018.03.019
157. Armstrong SC, Bolling CF, Michalsky MP, Reichard KW. Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. (2019) 144:e20193223. doi: 10.1542/peds.2019-3223
Keywords: youth-onset diabetes mellitus, insulin resistance, obesity, mechanisms, management strategies, diabetes remission
Citation: Jia Q, Zhang Y, Zhang B and An X (2024) Reassessing type 2 diabetes in adolescents and its management strategies based on insulin resistance. Front. Endocrinol. 15:1377918. doi: 10.3389/fendo.2024.1377918
Received: 28 January 2024; Accepted: 05 June 2024;
Published: 19 June 2024.
Edited by:
Brenda Kohn, New York University, United StatesReviewed by:
Ershun Liang, Qilu Hospital of Shandong University, ChinaMeera Ladwa, Barts Health NHS Trust, United Kingdom
Copyright © 2024 Jia, Zhang, Zhang and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XueDong An, RG9jdG9yX2FueGRAMTYzLmNvbQ==; BaoFeng Zhang, Mjk4MjkzOTdAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 QianYou Jia
QianYou Jia YanMin Zhang1†
YanMin Zhang1† XueDong An
XueDong An