
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 01 July 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1376463
This article is part of the Research Topic Exploring the New Biomarkers and Clinical Indicators for Diabetes: Insights from Real-World Studies View all 29 articles
 Wei Xu1†
Wei Xu1† Yuntao Feng2†
Yuntao Feng2† Guzalnur Abdullah1†
Guzalnur Abdullah1† Ling Li1
Ling Li1 Ping Fang1
Ping Fang1 Sijing Tang1
Sijing Tang1 Huanhuan Yang1
Huanhuan Yang1 Dehong Kong1
Dehong Kong1 Hemin Huang1
Hemin Huang1 Yang Wang1
Yang Wang1 Ying Xue1*‡
Ying Xue1*‡Background and aims: The American Heart Association (AHA) recently introduced the Life’s Essential 8 (LE8) to improve cardiovascular health (CVH). However, the association between LE8 and the risk of prediabetes or diabetes is not yet fully understood. Consequently, this study aims to assess the association between CVH, as evaluated by LE8, and the risk of prediabetes and diabetes.
Methods and Results: This cross-sectional study encompassed 7,739 participants aged ≥20 years from the 2007-2018 National Health and Nutrition Examination Surveys (NHANES). The CVH of participants was evaluated using the LE8, combining four health behaviors and three health factors. Glucose metabolic status categories included normal glucose metabolism, prediabetes including isolated impaired fasting glucose, isolated impaired glucose tolerance, both IFG and IGT, and diabetes. The associations between CVH and prediabetes and diabetes were analyzed using logistic regression, linear regression, restricted cubic splines, and subgroup analyses. Among 7,739 participants, 1,949 had iIFG, 1,165 were diagnosed with iIGT, 799 were IFG+IGT, and 537 were diagnosed with diabetes. After multivariable adjustments, CVH scores were inversely associated with prediabetes and diabetes, with the most robust inverse association observed between IFG+IGT and CVH across all prediabetes subgroups. Of all CVH components not directly in the causal pathway, body mass index (BMI) had the most robust associations with prediabetes and diabetes. Subgroup analyses indicated that the negative correlation between CVH and prediabetes was stronger among those with university or higher education.
Conclusion: CVH, as defined by LE8, showed a significant negative association with prediabetes and diabetes.
• Cardiovascular health and prediabetes/diabetes association using Life’s Essential 8.
• Cross-sectional study of 7,739 NHANES participants.
• Inverse association between cardiovascular health scores and prediabetes/diabetes.
• Body mass index and education level as key modifiers.
The prevalence of diabetes mellitus has increased rapidly and dramatically worldwide in recent decades. In the United States, diabetes, characterized by elevated blood glucose levels, ranks among the top 10 leading causes of mortality (1). According to a current survey by the International Diabetes Federation (IDF), approximately 537 million adults worldwide have diabetes, and an additional 374 million adults have prediabetes (2), imposing a significant economic burden. In the United States, diabetes has reached epidemic proportions and affects over 10% of adults (3). It is well-established that a strong association exists between diabetes and cardiovascular disease (CVD). CVD stands as the primary cause of mortality in patients with diabetes, while diabetes serves as an independent risk factor for CVD (4). Clinical management of patients with diabetes centers on two overarching goals: enhancing glycemic control to mitigate diabetic complications (5), and modifying risk factors for complications, particularly those linked to CVD (6). Therefore, it is imperative to establish standardized metrics for assessing and continuously monitoring cardiovascular health (CVH) in patients with diabetes.
Diabetes is a progressive process, often preceded by a prediabetic stage, frequently under-diagnosed. There is no consensus on the potential link between prediabetes and the increased risk of CVD. While some studies have proposed a connection between prediabetes and an elevated risk of CVD (7–9), others have failed to establish a similar association (10, 11). In addition, prediabetes is primarily classified as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), and the relationship between these two types and CVD individually requires further exploration.
In 2010, the American Heart Association (AHA) introduced Life’s Simple 7 (LS7) to advocate for initiatives to reduce the risk of CVD. The LS7 evaluates seven modifiable and actionable metrics of CVH, including dietary intake, physical activity, smoking, body mass index (BMI), blood glucose, blood pressure (BP), and blood lipids (12). Over the past decade, the LS7 has been widely utilized and has made significant contributions to the advancement of CVH in the United States and globally. However, during this period, certain limitations of LS7 have come to light (13). In 2022, in response to these limitations, the AHA proposed Life’s Essential 8 (LE8), featuring key enhancements, including the addition of sleep quality indicators and refined scoring algorithms (13). A limited number of studies have found a negative correlation between LE8 and diabetes (14, 15); however, the association of LE8 with prediabetes remains uncertain.
Considering the well-established associations between diabetes and CVD, promoting CVH as a strategic approach for the prevention and management of diabetes could potentially alleviate the burden of diabetes. Previous studies have explored the relationship between LS7, LE8, and diabetes (14–18), yet no study has examined the associations between the newly launched LE8 and prediabetes. To address these research gaps, we conducted an in-depth analysis using data from the National Health and Nutrition Examination Surveys (NHANES) to comprehensively assess the connection between LE8 and various prediabetes subtypes, as well as diabetes, among US adults.
The data were from NHANES, which offers a comprehensive health and nutrition evaluation of noninstitutionalized civilians in the United States through a stratified, multi-stage, probabilistic cluster design that ensures national representation. The National Center for Health Statistics (NCHS) in the U.S. manages NHANES, involving in-person interviews, physical examinations, and laboratory tests. The NCHS Disclosure Review Board approved the survey methodologies. The NCHS Ethics Review Board granted ethical clearance, and participants submitted written informed consent to the NCHS Ethics Review Board. Protocol details are available at https://www.cdc.gov/nchs/ahcd/ahcd_confidentiality.htm. The Ethics Committee of Shanghai Tongji Hospital provided ethical approval for this study. Data from seven NHANES cycles (1999–2018) included a total of 101,316 individuals initially. Due to missing clinical data, unknown medical history, and incomplete LE8 data, the number of participants aged 20 years and older eligible for statistical analysis in the NHANES cycles (2007-2018) was 7,739 (Supplementary Figure 1).
The LE8 system assesses CVH by considering four health behavior scores: diet (as measured by the 2015 Healthy Diet Index from 24-hour recalls), physical activity, nicotine exposure, and sleep. It also incorporates four health factor scores: BMI, blood lipids, blood glucose, and blood pressure (13). Blood glucose was not included in the analysis as a LE8 metric, as prediabetes and diabetes were the outcomes of interest (19). Each indicator in the LE8 system is scored on a scale from 0 to 100, and the overall score is calculated as the mean of the scores. Detailed information is available in Supplementary Table 1. In accordance with the guidelines of the American Heart Association (AHA), LE8, including health behavior and health factors, was analyzed continuously and categorically by tertiles: high (80–100), moderate (50–79), and low (0–49) (13).
Prediabetes and diabetes were defined according to the 2003 American Diabetes Association (ADA) criteria (20). Diabetes was defined by one of the following criteria: (1) fasting plasma glucose (FPG) ≥126 mg/dL (7mmol/L); (2) self-reported use of hypoglycemic medications; (3) 2 h plasma glucose (2hPG) ≥200 mg/dL (11.1mmol/L); (4) self-reported diabetes. After exclusion of diabetic subjects based on the above criteria, isolated impaired glucose tolerance (iIGT) was defined as FPG <100 mg/dL (5.6mmol/L) and 2hPG 140–199 mg/dL (7.8–11mmol/L); isolated impaired fasting glucose (iIFG) was defined as FPG 100–125 mg/dL (5.6–6.9mmol/L) and 2hPG <140 mg/dL(7.8mmol/L); both IFG and IGT (IFG+IGT) were FPG 100–125 mg/dL (5.6–11mmol/L)and 2hPG 140–199 mg/dL (7.8–11mmol/L); normal glucose metabolism (NGM) referred to FBG <100 mg/dL (5.6mmol/L) and 2hPG <140 mg/dL (7.8mmol/L).
Covariates included age, sex (male and female), race and ethnicity (non-Hispanic black, non-Hispanic white, Mexican American, or other races), poverty-to-income ratio (PIR) [low (<1.3), middle (1.3–3.5), and high (≥3.5)], marital status (divorced/separated/widowed, married/living with partner and never married), and education level (college graduate or above, high school or below, and college level). Insulin resistance (IR) was assessed using the homeostasis model of IR (HOMA-IR)=(fasting plasma insulin [μU/mL])×(fasting plasma glucose [mmol/L])÷22.5 (21).
NHANES employed design weighting to ensure the representativeness of the data. We performed weighted data analyses using relevant survey weights (MEC2yr) to generate nationally representative estimates. After applying the NHANES survey weights, our analyses provided estimates that represented approximately 59,055,054 individuals in the U.S. population. Categorical variables in the baseline information were expressed as weighted percentages, while continuous variables were presented as weighted means along with their respective confidence intervals (CIs). Group disparities were assessed by weighted variance tests and weighted chi-square tests. Additionally, we computed age-standardized prevalence estimates and 95% CIs for various score levels.
We utilized weighted multivariate logistic regression to examine the relationship between CVH with both diabetes and prediabetes. This analysis was stepwise adjusted for age, sex, marital status, education level, and race/ethnicity. We also employed restricted cubic spline regression to explore potential non-linear associations between the LE8 score and its sub-scale scores with diabetes and prediabetes. Furthermore, we conducted stratified analyses by age, sex, marital status, education level, and race/ethnicity to assess whether the association between LE8 and diabetes and prediabetes was influenced by these factors. The significance of the interaction was assessed by the interaction P-value between the LE8 score and the stratified factors.
All statistical analyses were performed using R software (Version 4.2.1, The R Foundation; http://www.R-project.org) and EmpowerStats software (Version 5.0, X&Y Solutions, Inc., Boston, MA; http://www.empowerstats.com). A significance level of p < 0.05 was considered statistically significant.
Tables 1 and 2 present the demographic characteristics and metabolic risk factors of the study population, categorized according to ADA criteria. The weighted average age of participants was 47.23 years, with 48.76% male, and a majority being non-Hispanic white (73.45%). Among the 7,739 participants, 1,949 had iIFG, 1,165 had iIGT, 799 had IFG+IGT, and 537 were diagnosed with diabetes. The mean CVH score for the entire study population was 66.94 ± 0.33. Stratified by glycemic metabolism status, the mean CVH scores for NGM, iIFG, iIGT, IFG+IGT, and diabetic subjects were 70.21 ± 0.39, 64.44 ± 0.53, 63.94 ± 0.85, 60.69 ± 0.67 and 59.28 ± 0.66, respectively.
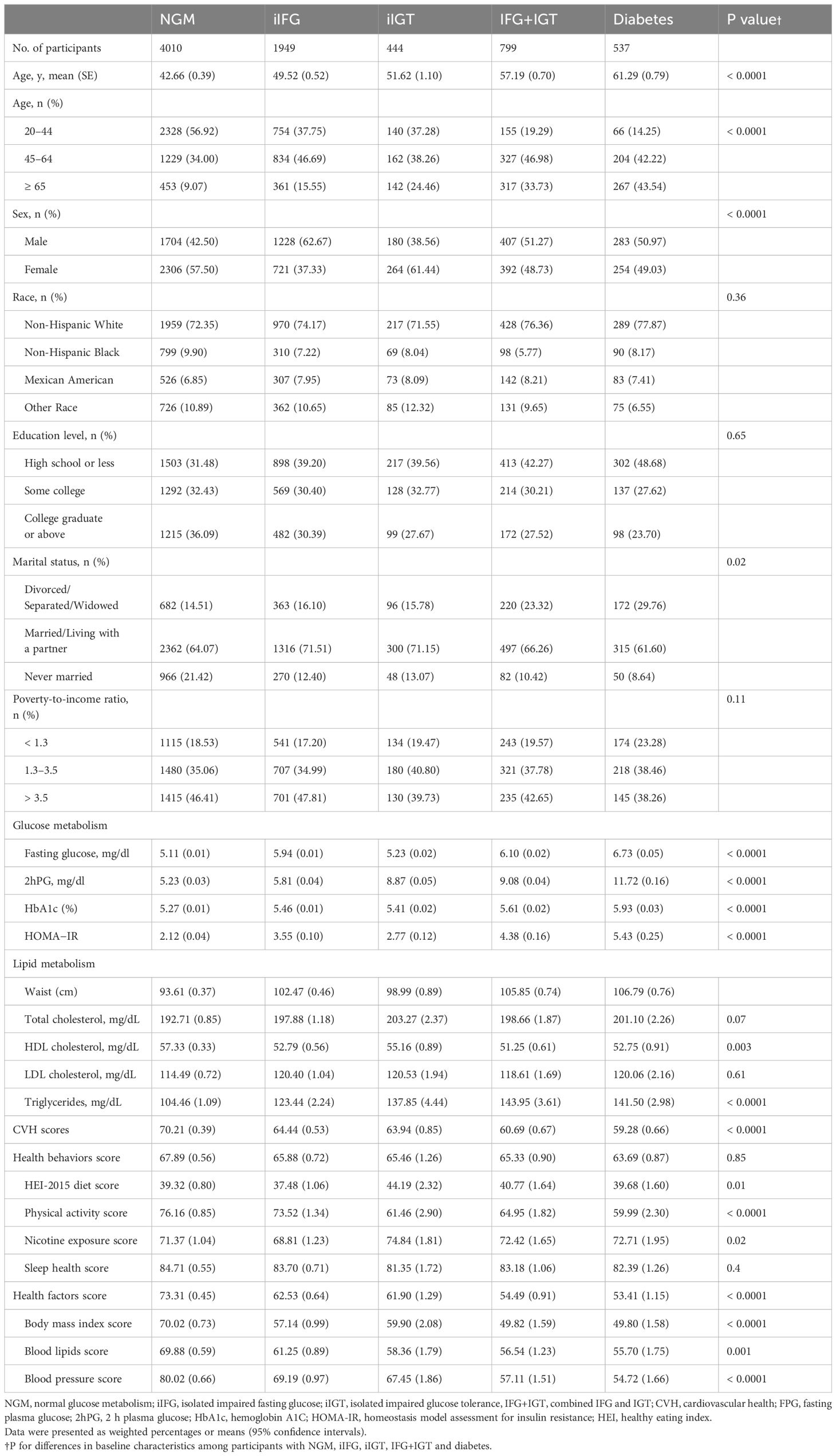
Table 2 Weighted baseline characteristics of participants with NGM, iIFG, iIGT, IFG+IGT and diabetes.
In multiple comparisons, significant differences were observed in CVH scores among NGM, prediabetes, and diabetes groups. The diabetes group exhibited significantly lower CVH scores compared to the NGM and prediabetes groups. Upon further comparison of the prediabetes subgroups, no significant difference was found in CVH scores between iIFG and iIGT, whereas the IFG+IGT group’s CVH score was significantly lower than those of the iIFG and iIGT groups (see Supplementary Figure 2). On average, patients with prediabetes or diabetes were older, less educated, and had poorer lipid profiles compared to those with NGM. Patients with IFG+IGT exhibited more pronounced metabolic deficits and more unfavorable CVD risk profiles, such as higher HOMA-IR, larger waist circumference, and altered lipid metabolism indices, compared to those with iIFG and iIGT (see Table 2).
When CVH was further subdivided into health behaviors (diet, physical activity, nicotine exposure, and sleep) and health factors (body mass index, blood lipids, and blood pressure), significant differences in scores were observed between the NGM, iIFG, iIGT, IFG+IGT, and diabetic groups (Table 2). The mean health behavior scores for the NGM, iIFG, iIGT, IFG+IGT, and diabetic subjects were 67.89 ± 0.56, 65.88 ± 0.72, 65.46 ± 1.26, 65.33 ± 0.90, and 63.69 ± 0.87, respectively; mean health factor scores were 73.31 ± 0.45, 62.53 ± 0.64, 61.90 ± 1.29, 54.49 ± 0.91, and 53.41 ± 1.15, respectively. Compared to patients with prediabetes (including iIFG, iIGT, IFG+IGT) or diabetes, those with NGM had higher health behaviors and health factors scores, except for nicotine exposure and sleep health scores, which were not statistically different.
The results of the logistic regression analysis of glucose metabolism status are summarized in Tables 3 and 4. This comprehensive evaluation revealed a robust inverse correlation between CVH scores and both prediabetes and diabetes. After adjusting for the variables of age, sex, ethnicity, marital status, PIR, and education, for every 10-point increase in CVH scores, the odds ratios (ORs) for each category were reduced as follows: iIFG (OR 0.77, 95% CI 0.72–0.82), iIGT (OR 0.76, 95% CI 0.69–0.84), IFG+IGT (OR 0.65, 95% CI 0.60–0.70), prediabetes (OR 0.74, 95% CI 0.70–0.78), and diabetes (OR 0.63, 95% CI 0.57–0.69). Notably, the inverse correlation between IFG+IGT and CVH was more pronounced than the negative association observed with iIFG or iIGT.
To further explore the relationship between CVH scores and prediabetes as well as diabetes, participants were stratified into low, medium, and high CVH groups based on CVH scores (refer to Table 3). Following multivariable adjustments, participants with moderate and high CVH had significantly lower odds of developing diabetes compared to those with low CVH, with ORs of 0.29 (95% CI 0.19–0.45) and 0.04 (95% CI 0.02–0.08), respectively. Participants in the moderate and high CVH groups also exhibited a significantly lower risk of iIFG, iIGT, IFG+IGT, and prediabetes compared to those in the low CVH group. Notably, the inverse association between IFG+IGT and CVH was more pronounced in the high CVH group compared to other prediabetes subgroups, with an OR of 0.13 (95% CI 0.09–0.21) per 10-point increase in CVH, second only to diabetes (OR 0.06, 95% CI 0.03, 0.12) (see Table 4). Logistics regression analysis of the association between CVH scores and prediabetes/diabetes showed that as the CVH scores went from low to high, the ORs for all types of glucose metabolism disorders, including iIFG, iIGT, IFG+IGT, and diabetes, then progressively decreased (p for trend <0.0001) (see Tables 3 and 4).
Furthermore, the age-adjusted prevalence of iIFG, iIGT, IFG+IGT, prediabetes, and diabetes showed varying decreases with increasing CVH levels, health behavior scores, and health factor scores, as illustrated in Figure 1. When FPG and 2hPG were used as continuous glycemic measures, both FPG and 2hPG exhibited an inverse association with CVH scores as presented in Figure 2 (β=-0.21, p < 0.0001 for both).
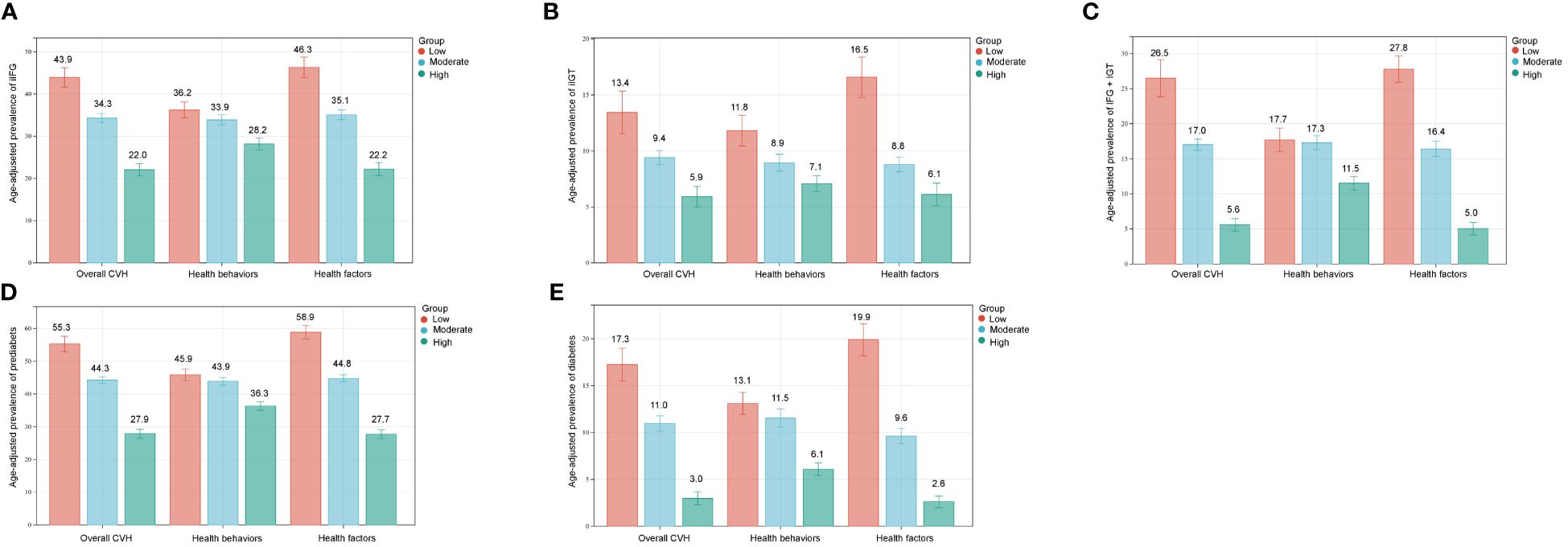
Figure 1 Age-adjusted prevalence of prediabetes and diabetes in different levels of CVH scores. (A) iIFG; (B) iIGT; (C) IFG+IGT; (D) prediabetes; (E) diabetes. Numbers at the top of the bars represent the weighted percentage. Bar whiskers represent the 95% confidence intervals. iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; IFG+IGT, combined IFG and IGT; CVH, cardiovascular health.
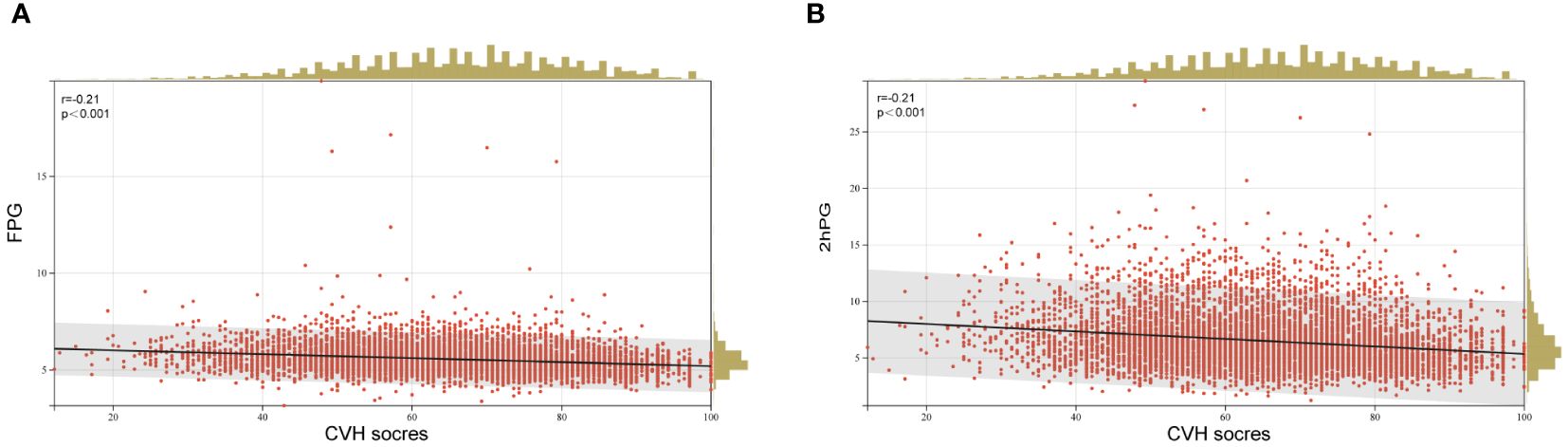
Figure 2 Linear regression analysis of CVH scores and (A) FPG and (B) 2hPG. FPG, fasting plasma glucose; 2hPG, 2 h plasma glucose; CVH, cardiovascular health.
We also focused on subgroups with abnormal glucose metabolism, including iIFG, iIGT, IFG+IGT, prediabetic, and diabetic subgroups (Figure 3). CVH scores showed a negative correlation with all of these subgroups. The negative associations between CVH scores and IFG+IGT, prediabetes, and diabetes did not significantly differ across age, gender, race, and PIR subgroups. Of note, our analysis revealed that, except for the iIGT subgroup, education level influenced the negative association between CVH scores and the risk of iIFG, IFG+IGT, prediabetes, or diabetes (p < 0.05 for the interaction). The inverse association between CVH scores and the risk of these four subgroups was more pronounced among participants with education levels of college graduation or above, with ORs of 0.69 (95% CI 0.62–0.77), 0.54 (95% CI 0.47–0.62), 0.64 (95% CI 0.58–0.71), and 0.52 (95% CI 0.44–0.61) for each 10-point increase in CVH scores, respectively.
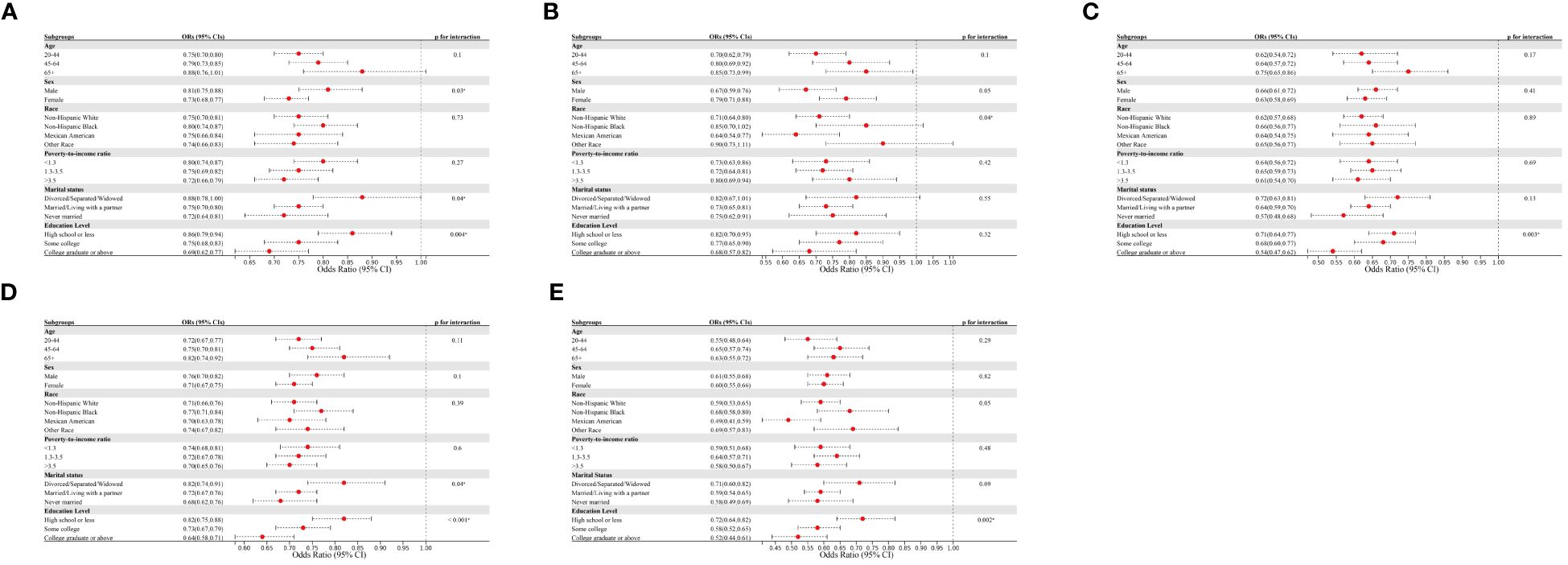
Figure 3 Subgroup analysis of the association of CVH scores and the presence of (A) iIFG; (B) iIGT; (C) IFG+IGT; (D) prediabetes; and (E) diabetes. ORs were calculated as per 10 scores increase in CVH scores. Each stratification was adjusted for age, sex, race/ethnicity, poverty-to-income ratio, education level, and marital status. iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; IFG+IGT, combined IFG and IGT; CVH, cardiovascular health; ORs, Odds ratios; CI: confidence interval.
Logistic regression analysis revealed significant negative associations between both health factor scores and health behavior scores and the risk of prediabetes and diabetes (refer to Tables 3 and 4). The risk of iIFG, iIGT, IFG+IGT, prediabetes, and diabetes was significantly lower in the subgroups with moderate and high health factor scores compared with the subgroups with low health factor scores. However, the risk of iIFG, iIGT, IFG+IGT, prediabetes, and diabetes was not statistically different between the two subgroups with low health behavior scores versus moderate health behavior scores (see Tables 3 and 4). After adjusting for multiple confounding variables, individuals with high health behaviors scores had significantly lower odds of iIFG, iIGT, IFG+IGT, prediabetes, and diabetes, with ORs of 0.69 (95% CI 0.53–0.90), 0.62 (95% CI 0.42–0.92), 0.60 (95% CI 0.44–0.82), 0.66 (95% CI 0.53–0.82), and 0.49 (95% CI 0.33–0.72), respectively.
Additionally, the associations of each CVH component with prediabetes and diabetes were shown in Figure 4. Notably, among the health factors, BMI score exhibited the strongest association with prediabetes and diabetes, with ORs of 0.86 (95% CI 0.84–0.88) and 0.80 (95% CI 0.77–0.84) per 10-point increase, respectively. Following BMI score, BP score, and blood lipids score were secondary factors influencing the association of CVH with prediabetes and diabetes. In contrast, health behaviors demonstrated weaker associations with prediabetes and diabetes compared to health factors. Unexpectedly, nicotine exposure score displayed no significant association with either prediabetes (p=0.24) or diabetes (p=0.95). When categorizing prediabetes into the three subtypes, the associations between CVH components and iIFG, iIGT, and IFG+IGT followed a similar pattern of correlation as in prediabetes. In conclusion, both health behaviors and health factors were negatively associated with prediabetes and diabetes, with the negative associations being more pronounced for health factors, especially body mass index.
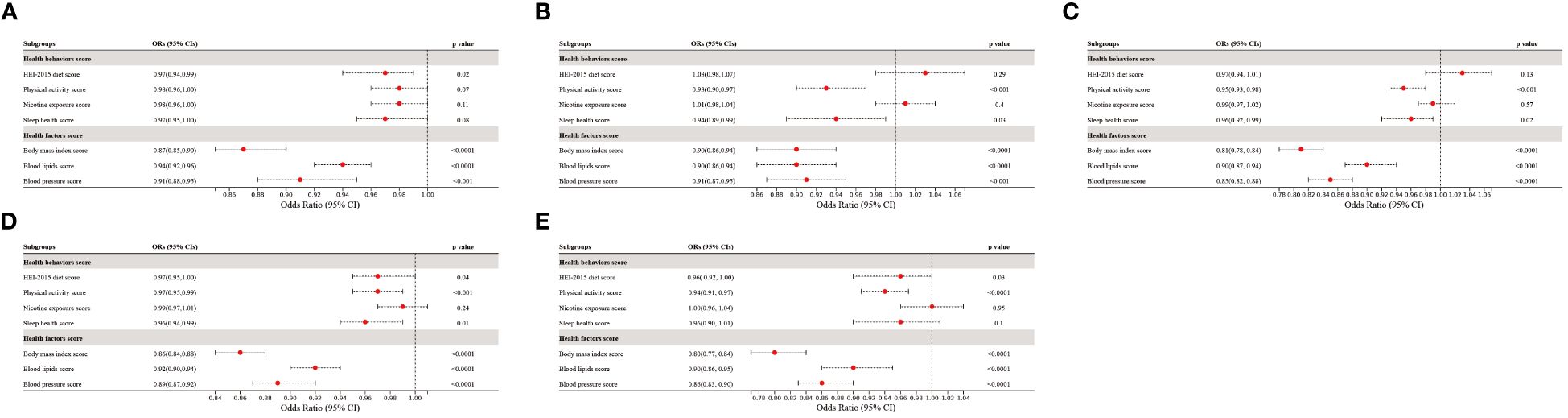
Figure 4 The association of CVH components with the presence of (A) iIFG; (B) iIGT; (C) IFG+IGT; (D) prediabetes; and (E) diabetes. ORs were adjusted for age, sex, race/ethnicity, poverty-to-income ratio, education levels, and marital status. iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; IFG+IGT, combined IFG and IGT; CVH, cardiovascular health; ORs, Odds ratios; CI: confidence interval.
As shown in Figure 5, restricted cubic spline (RCS) analysis with multivariate adjustment revealed that iIFG, iIGT, IFG+IGT, prediabetes, and diabetes were correlated with CVH scores (all p < 0.05). Among them, IFG+IGT, prediabetes, and diabetes had a significant non-linear dose-response relationship with CVH scores (p for nonlinear <0.05). In contrast, iIFG and iIGT had a linear dose-response association with CVH scores, with p-values of 0.09 and 0.84, respectively, for the non-linear test.
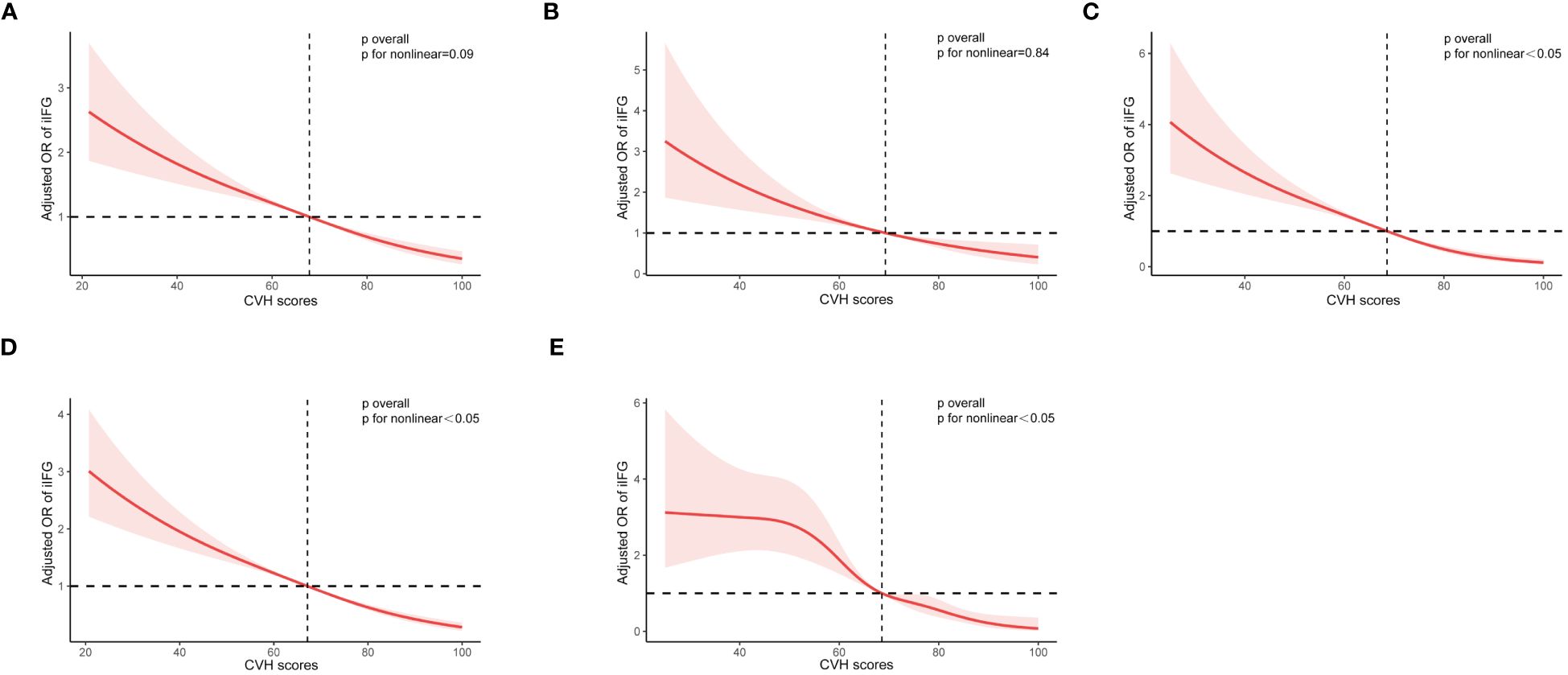
Figure 5 Restricted cubic curve of ORs for (A) iIFG; (B) iIGT; (C) IFG+IGT; (D) prediabetes; and (E) diabetes. ORs (red solid lines) and 95% confidence intervals (pink shaded areas) were adjusted for age, sex, race/ethnicity, poverty-to-income ratio, education levels, and marital status. iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; IFG+IGT, combined IFG and IGT; CVH, cardiovascular health; OR, Odds ratio.
This study is the first comprehensive, large-scale population-based analysis of the relationship between CVH, as defined by LE8, and prediabetes/diabetes. The findings indicate an inverse relationship between CVH scores, health behaviors, health factors, and risk of prediabetes and diabetes among U.S. adults. Among the non-causal CVH components, BMI exhibited the strongest association with prediabetes and diabetes. Notably, IFG+IGT demonstrated the strongest negative association with CVH among all prediabetes subtypes. Subgroup analyses showed that the negative association between CVH and prediabetes was stronger among individuals with a college or higher education level. These results remained robust after adjustments for various confounders, including age, sex, race/ethnicity, PIR, education level, and marital status.
Growing evidence suggests that LE8 is highly effective in assessing CVH for predicting cardiovascular disease events and cardiovascular-specific mortality in the general population and reducing premature deaths among diabetic patients (14, 22). We observed a notable decrease in CVH scores with the worsening of glucose metabolic status (from NGM to prediabetes and diabetes). This trend remained consistent when employing FBG and 2h-PG as continuous glucose measures. This negative correlation corroborates and validates previous findings in the literature. A prospective study by LU et al., including 193,846 participants aged over 40 years, demonstrated a J-shaped association between FBG, 2hPG, and cardiovascular disease events and mortality (23). In an international prospective cohort study of 18,990 participants from 21 countries, a 1 mmol/L increase in FPG or a 2.52 mmol/L increase in 2hPG was linked to an increased hazard ratio for cardiovascular events or death (1.17, 95% CI 1.13–1.22) (24). A meta-analysis pooling data from a large cohort revealed that among participants aged 55–64 years, each 1 mmol/L increase in FPG was linked to relative risks (RRs) of 1.18 (95% CI 1.08–1.29) for ischemic heart disease and 1.14 (95% CI 1.01–1.29) for stroke (25).
The LE8, as a novel assessment of CVH, remedies the shortcomings of the previous LS7 (13). The LS7, defining the original CVH component, may not adequately reflect current health behaviors and practices, particularly regarding dietary underassessment. Furthermore, the initial categorization of ideal, moderate, and poor CVH was not sufficiently sensitive to individual variations. Recent evidence has highlighted the significance of sleep in both assessing and promoting CVH. Considering the link between sleep, cardiovascular disease, and diabetes, the LS7 might not adequately capture health behaviors and physical characteristics due to the omission of sleep assessment (26, 27). Our findings underscore the inverse relationship between CVH and diabetes, consistent with prior studies based on LS7-defined CVH (16, 19, 28–30). For example, the Strong Heart Family Study (n = 1639) found that achieving a 2–3 or 4+ LS7 goal was associated with a reduced risk of diabetes, with ORs of 0.40 (95% CI 0.29–0.56) and 0.11 (95% CI 0.05–0.21), respectively (29). In the Coronary Artery Risk Development in Young Adults Study, Choi et al. observed that higher CVH scores in young adults were associated with a lower risk of diabetes and diabetic complications (30). This study was limited to black and white adults aged 20–30 years, restricting its applicability to other racial groups and to middle-aged or older populations.
Our study also revealed a significant negative correlation between CVH and prediabetes. To our knowledge, no studies have specifically addressed the correlation between CVH as assessed by LE8 and prediabetes. Prior research examining the association between LS7-based CVH and prediabetes has been limited. For instance, a study involving Japanese adults (n = 403,857) showed that an increase in non-ideal CVH metrics was associated with a higher risk of prediabetes or diabetes (31). The study utilized LS7 as an indicator for CVH assessment but omitted the impact of dietary components. Additionally, the findings of this study, based solely on Japanese participants, limit their broader applicability.
Despite the substantial evidence of the relationship between diabetes and CVH, prediabetes has garnered limited attention. Previous research has often omitted comparisons between the three distinct subtypes of prediabetes: iIFG, iIGT, and IFG+IGT. In our results, CVH scores were lower in the IFG+IGT group compared to the iIFG and iIGT groups, whereas the difference in CVH scores between the iIFG and iIGT groups was not statistically significant. With each 10-point increase in CVH scores, the odds ratios were lower in the IFG+IGT group (OR 0.65, 95% CI 0.60–0.70) compared to the iIFG group (OR 0.77, 95% CI 0.72–0.82) and iIGT group (OR 0.76, 95% CI 0.69–0.84). This association is likely related to IR. Our results revealed significantly higher HOMA-IR in the iIFG group(3.55 ± 0.10), iIGT group (2.77 ± 0.12), and IFG+IGT group (4.38 ± 0.16) than in the NGM group (2.12 ± 0.04), aligning with previous findings (32, 33). Prediabetes is heterogeneous regarding metabolic defects, hyperglycemia patterns, and cardiovascular risk. IGT is characterized by increased peripheral IR and compensated hyperinsulinemia, whereas IFG is associated with hepatic IR and excessive endogenous glucose production (34). Patients with IFG+IGT are thought to have more severe IR due to multiorgan (i.e., muscle + liver) defects (35). A cross-sectional study indicated that patients with IFG+IGT exhibited more pronounced metabolic defects, an increased likelihood of developing diabetes, and a higher CVD risk (36), which corroborates our findings. In Figure 1 of our study, we presented a prevalence plot illustrating the correlation between CVH and prediabetes/diabetes, offering a crucial foundation for motivating patients to enhance their CVH scores. The management of prediabetes has long played a crucial role in preventing diabetes and combating diabetes-related complications. Lifestyle modification is one of the important measures. Consequently, emphasizing patients with IFG+IGT and comprehensive CVH management in this cohort can contribute to reducing the global burden of diabetes. Attaining this objective will require concerted efforts by individuals, the healthcare sector, and society to actively improve the health of the population.
Among non-direct causal risk factors, BMI exhibited the strongest association with both prediabetes (OR 0.86, 95% CI 0.84–0.88) and diabetes (OR 0.80, 95% CI 0.77–0.84), aligning with previous findings regarding BMI’s significant impact on the risk of prediabetes and diabetes (16, 29, 31). Over the past three decades, an increase in BMI has been identified as a key contributor to the escalating prevalence of diabetes in the United States (28). Programs like the Diabetes Prevention Program (DPP) have illustrated that sustained weight loss plays a significant role in diminishing diabetes risk and enhancing cardiometabolic health (37). Adipose tissue, functioning as an active endocrine organ, secretes substantial quantities of cytokines and bioactive mediators, influencing insulin sensitivity, inflammation, coagulation, and ultimately, atherosclerosis. This phenomenon likely contributes to the strong association between BMI and the development of diabetes (38).
Notably, subgroup analyses revealed a more pronounced association between CVH scores and prediabetes/diabetes among participants with a university degree or higher education (interaction p < 0.01). McWilliams et al. reported that diabetic adults with lower education levels exhibited significantly poorer glycemic control (39). This disparity may stem from individuals with higher education levels having improved access to comprehensive diabetes medical care and an increased capacity to assimilate and comprehend new information related to diabetes management (40). Although the underlying mechanisms remain unclear, interventions should be tailored to the specific needs of the target community to improve CVH. Unexpectedly, the association between health behavior scores and prediabetes/diabetes was less pronounced than the association between health factor scores and prediabetes/diabetes. For example, smoking did not show a significant association with either prediabetes or diabetes. This could be attributed to individuals with prediabetes or diabetes refraining from making positive lifestyle changes, such as smoking cessation and increased physical activity, until after the diagnosis is confirmed.
The main strength of our study was that we explored, for the first time, the association between CVH, as defined by LE8, and prediabetes, including its three distinct subtypes. Additionally, we conducted extensive stratified analyses based on sociodemographic characteristics and risk factors for diabetes/prediabetes. However, this study had certain limitations. Firstly, it was challenging to establish a causal relationship between LE8 and prediabetes/diabetes owing to the cross-sectional design of the study. Secondly, individuals diagnosed with prediabetes or diabetes may have implemented necessary lifestyle changes, however, due to the cross-sectional design, we were unable to explore the causal relationship between lifestyle changes and risk of prediabetes/diabetes, which can be better explored in future large follow-up cohort studies. Thirdly, the diagnosis of diabetes partially relied on self-reporting, potentially leading to misclassification and estimation bias. Fourthly, the exclusion of participants with an unknown medical history or incomplete LE8 data might have led to a reduced study population and potential selection bias.
In conclusion, our study demonstrated a significant negative association between CVH, as defined by the LE8 score, and prediabetes/diabetes among US adults. Elevated CVH levels were associated not only with reduced risks of diabetes but also with lower risks of prediabetes. These findings offer robust evidence supporting the potential of the LE8 score in mitigating the burden of diabetes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
WX: Conceptualization, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YF: Writing – original draft, Writing – review & editing. GA: Data curation, Investigation, Writing – original draft, Writing – review & editing. LL: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. PF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. ST: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. HY: Conceptualization, Software, Writing – original draft. DK: Data curation, Investigation, Software, Writing – original draft. HH: Investigation, Software, Writing – original draft. YW: Data curation, Methodology, Software, Writing – original draft. YX: Data curation, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (82370867, 81974105), the Fundamental Research Funds for the Central Universities (22120220625), Clinical Research Project of Tongji Hospital of Tongji University (ITJ(QN)2301), Cultivation project for the National Natural Science Foundation of Shanghai Tongji Hospital (GJPY2202).
We appreciate the NCHS for their efforts in collecting the data for the NHANES.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1376463/full#supplementary-material
AHA, American Heart Association; LE8, Life’s Essential 8; CVH, Cardiovascular health; NHANES, National Health and Nutrition Examination Surveys; NGM, Normal glucose metabolism; iIFG, isolated impaired fasting glucose; iIGT, Isolated impaired glucose tolerance; IFG+IGT, Both IFG and IGT; BMI, body mass index; IDF, International Diabetes Federation; CVD, Cardiovascular disease; LS7, Life’s Simple 7; BP, Blood pressure; NCHS, National Center for Health Statistics; ADA, American Diabetes Association; FPG, Fasting plasma glucose; 2hPG, 2 h plasma glucose; PIR, Poverty-to-income ratio; IR, Insulin resistance; HOMA-IR, Homeostasis model of IR; CI, Confidence interval; DPP, Diabetes Prevention Program.
1. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162. doi: 10.1016/j.diabres.2020.108086
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183. doi: 10.1016/j.diabres.2021.109119
3. Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ-Br Med J. (2018) 362. doi: 10.1136/bmj.k1497
4. Seshasai SRK, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. (2011) 364:829–41. doi: 10.1056/NEJMoa1008862
6. Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence A scientific statement from the American heart association and the American diabetes association. CIRCULATION. (2015) 132:691–718. doi: 10.1161/CIR.0000000000000230
7. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ-Br Med J. (2016) 355. doi: 10.1136/bmj.i5953
8. Vistisen D, Witte DR, Brunner EJ, Kivimaki M, Tabak A, Jorgensen ME, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care. (2018) 41:899–906. doi: 10.2337/dc17-2530
9. Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, et al. Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. (2007) 153:552–8. doi: 10.1016/j.ahj.2007.01.003
10. Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population MESA (Multi-ethnic study of atherosclerosis). J Am Coll Cardiol. (2011) 58:140–6. doi: 10.1016/j.jacc.2011.03.025
11. Hubbard D, Colantonio LD, Tanner RM, Carson AP, Sakhuja S, Jaeger BC, et al. Prediabetes and risk for cardiovascular disease by hypertension status in black adults: the Jackson heart study. Diabetes Care. (2019) 42:2322–9. doi: 10.2337/dc19-1074
12. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction the American heart association’s strategic impact goal through 2020 and beyond. CIRCULATION. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
13. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. CIRCULATION. (2022) 146:E18–43. doi: 10.1161/CIR.0000000000001078
14. Sun Y, Yu Y, Zhang K, Yu B, Yu Y, Wang Y, et al. Association between Life’s Essential 8 score and risk of premature mortality in people with and without type 2 diabetes: A prospective cohort study. DIABETES-Metab Res Rev. (2023) 39. doi: 10.1002/dmrr.3636
15. Yu Y, Sun Y, Yu Y, Wang Y, Chen C, Tan X, et al. Life’s Essential 8 and risk of non-communicable chronic diseases: Outcome-wide analyses. Chin Med J (Engl). (2023). doi: 10.1097/CM9.0000000000002830
16. Joseph JJ, Bennett A, Tcheugui JBE, Effoe VS, Odei JB, Hidalgo B, et al. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. DIABETOLOGIA. (2019) 62:426–37. doi: 10.1007/s00125-018-4792-y
17. Aboagye-Mensah EB, Azap RA, Odei JB, Gray DM II, Nolan TS, Elgazzar R, et al. The association of the American heart associations lifes simple 7 with self-reported health, diabetes, and adiposity in African American males. DIABETES. (2020) 69. doi: 10.2337/db20-1423-P
18. Shen R, Guo X, Zou T, Ma L. Associations of cardiovascular health assessed by life’s essential 8 with diabetic retinopathy and mortality in type 2 diabetes. Prim Care Diabetes. (2023), 17:S1751991823001390. doi: 10.1016/j.pcd.2023.08.001
19. Climie RE, van Sloten TT, Perier M-C, Tafflet M, Fayosse A, Dugravot A, et al. Change in cardiovascular health and incident type 2 diabetes and impaired fasting glucose: the Whitehall II study. Diabetes Care. (2019) 42:1981–7. doi: 10.2337/dc19-0379
20. Amer Diabet Assoc. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2009) 32:S62–7. doi: 10.2337/dc09-S062
21. Effoe VS, Carnethon MR, Echouffo-Tcheugui JB, Chen H, Joseph JJ, Norwood AF, et al. The American heart association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson heart study. J Am Heart Assoc. (2017) 6. doi: 10.1161/JAHA.116.005008
22. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, et al. Association of the American Heart Association’s new “Life’s Essential 8” with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. (2023) 21. doi: 10.1186/s12916-023-02824-8
23. Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin A1c on risk of diabetes and complications in Chinese adults. Diabetes Care. (2019) 42:1539–48. doi: 10.2337/dc18-1390
24. Anand SS, Dagenais GR, Mohan V, Diaz R, Probstfield J, Freeman R, et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol. (2012) 19:755–64. doi: 10.1177/1741826711409327
25. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PloS One. (2013) 8. doi: 10.1371/journal.pone.0065174
26. Schipper SBJ, Van Veen MM, Elders PJM, van Straten A, van der Werf YD, Knutson KL, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. DIABETOLOGIA. (2021) 64:2367–77. doi: 10.1007/s00125-021-05541-0
27. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. (2020) 41:1182–+. doi: 10.1093/eurheartj/ehz849
28. Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, Bertoni AG, Shay CM, Ahmed HM, et al. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. DIABETOLOGIA. (2016) 59:1893–903. doi: 10.1007/s00125-016-4003-7
29. Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SAA, Mete M, et al. Life’s simple 7 and incidence of diabetes among American Indians: the strong heart family study. Diabetes Care. (2014) 37:2240–5. doi: 10.2337/dc13-2267
30. Choi Y, Jacobs DR, Bancks MP, Lewis CE, Cha E, Yan F, et al. Association of cardiovascular health score with early- and later-onset diabetes and with subsequent vascular complications of diabetes. J Am Heart Assoc. (2023) 12:e027558. doi: 10.1161/JAHA.122.027558
31. Okada A, Kaneko H, Matsuoka S, Itoh H, Suzuki Y, Fujiu K, et al. Association of cardiovascular health metrics with annual incidence of prediabetes or diabetes: Analysis of a nationwide real-world database. J Diabetes Investig. (2023) 14:452–62. doi: 10.1111/jdi.13958
32. Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose - The risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care. (2003) 26:868–74. doi: 10.2337/diacare.26.3.868
33. Martínez-Larrad MT, Corbatón-Anchuelo A, Fernández-Pérez C, Lazcano-Redondo Y, Escobar-Jiménez F, Serrano-Ríos M. Metabolic syndrome, glucose tolerance categories and the cardiovascular risk in Spanish population. Diabetes Res Clin Pract. (2016) 114:23–31. doi: 10.1016/j.diabres.2016.02.003
34. Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. (2018) 47:33–+. doi: 10.1016/j.ecl.2017.10.001
35. Malin SK. Exercise resistance across the prediabetes phenotypes: Impact on insulin sensitivity and substrate metabolism. Rev Endocr Metab Disord. (2016) 17:81–90. doi: 10.1007/s11154-016-9352-5
36. Pankow JS, Kwan DK, Duncan BB, Schmidt MI, Couper DJ, Golden S, et al. Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance - The atherosclerosis risk in communities study. Diabetes Care. (2007) 30:325–31. doi: 10.2337/dc06-1457
37. Delahanty LM, Pan Q, Jablonski KA, Aroda VR, Watson KE, Bray GA, et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the diabetes prevention program. Diabetes Care. (2014) 37:2738–45. doi: 10.2337/dc14-0018
38. Poirier P, Giles T, Bray G, Hong Y, Stern J, Pi-Sunyer F, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss - An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. CIRCULATION. (2006) 113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
39. McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U. S. Trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. (2009) 150:505–W90. doi: 10.7326/0003-4819-150-8-200904210-00005
Keywords: Life’s Essential 8, prediabetes, diabetes, NHANES, cardiovascular health
Citation: Xu W, Feng Y, Abdullah G, Li L, Fang P, Tang S, Yang H, Kong D, Huang H, Wang Y and Xue Y (2024) Association between the AHA life’s essential 8 and prediabetes/diabetes: a cross-sectional NHANES study. Front. Endocrinol. 15:1376463. doi: 10.3389/fendo.2024.1376463
Received: 25 January 2024; Accepted: 17 June 2024;
Published: 01 July 2024.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Nicolas Renna, Universidad Nacional de Cuyo, ArgentinaCopyright © 2024 Xu, Feng, Abdullah, Li, Fang, Tang, Yang, Kong, Huang, Wang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xue, ZG94eTA2MjJAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Ying Xue, orcid.org/0000-0002-6812-5665
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.