- 1Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Research Center for Antibiotic Stewardship and Antimicrobial Resistance, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
- 3Non-Communicable Diseases Research Center, Endocrine Population Sciences Institute, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 4Iranian Research Center for HIV/AIDS, Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences, Tehran, Iran
Objective: This systematic review and meta-analysis was conducted to compare the benefits of adrenalectomy and conservative treatment for comorbidities associated with mild autonomous cortisol secretion (MACS) in patients diagnosed with MACS.
Background: MACS is the most common benign hormone-secreting functional adrenal incidentaloma. Overproduction of cortisol is observed in MACS patients, resulting in a variety of long-term health issues, including arterial hypertension (HTN), diabetes mellitus (DM), dyslipidemia, obesity, and osteoporosis; however, the classic clinical manifestations of Cushing’s syndrome (CS) are not present.
Methods: A systematic search was conducted using MEDLINE, Embase, Web of Sciences, and Scopus databases on December, 2023. Two reviewers independently extracted data and assessed the quality of the included articles. A meta-analysis was performed to compare the beneficial effects of adrenalectomy versus conservative management for MACS-related comorbidities.
Results: Fifteen articles were included in this study, which evaluated 933 MACS patients (384 Adrenalectomy and 501 Conservative treatment, and 48 excluded due to incomplete follow-up duration). MACS diagnosis criteria were different among the included articles. All studies, however, stated that there must be no overt CS symptoms. Meta-analysis demonstrates the overall advantage of adrenalectomy over conservative treatment for MACS-related comorbidities (Cohen’s d = -0.49, 95% CI [-0.64, -0.34], p = 0.00). Subgroup analysis indicated that the systolic blood pressure (pooled effect size = -0.81, 95% CI [-1.19, -0.42], p = 0.03), diastolic blood pressure (pooled effect size = -0.63, 95% CI [-1.05, -0.21], p = 0.01), and BMD (pooled effect size = -0.40, 95% CI [-0.73, -0.07], p = 0.02) were significantly in favor of adrenalectomy group rather than conservative treatment but no significant differences between the two treatment groups in other MACS-related comorbidities were reported.
Conclusion: Despite the limited and diverse data, this study demonstrates the advantage of adrenalectomy over conservative treatment for MACS-related comorbidities.
1 Introduction
Mild Autonomous Cortisol Secretion (MACS) is initially identified as subclinical Cushing’s syndrome (SCS); however, the term “Subclinical or Preclinical” may not be appropriate for this condition as it implies a transition to Cushing’s syndrome, which is an uncommon occurrence (1–4). In this context, we employ the term “mild autonomous cortisol secretion” as recommended by the European Society of Endocrinology and European Network for the Study of Adrenal Tumors (ESE-ENSAT) (5). MACS is an adrenal incidentaloma, which refers to the incidental findings of an adrenal mass during diagnostic investigations conducted for reasons unrelated to suspected adrenal pathology (6, 7), characterized by increased cortisol production, which is independent to hypothalamic–pituitary–adrenal (HPA) axis, without clinical signs of overt Cushing’s syndrome (5, 8). Over the past decade, this issue has been debated, primarily because of an unclear definition and contentious treatment approaches (9, 10).
The prevalence of adrenal incidentaloma is between 0.4% to 7% And can be and up to 10% in patients > 80 years (7, 11–13), which can be hormone-secreting, nonfunctional, malignant, or benign (14). As hormone overproduction is one of the major clinical concerns in adrenal incidentaloma, MACS should be evaluated precisely since it is recognized as the most common benign hormone-secreting functional adrenal incidentaloma (15). The prevalence of MACS is estimated to be 79 cases per 100,000 people (16), accounting for 5 to 20% of adrenal incidentaloma masses (17, 18).
There is convincing evidence to suggest that MACS can cause physiological effects associated with excessive cortisol levels. These effects encompass a range of long-term health complications, such as arterial hypertension (HTN), diabetes mellitus (DM), dyslipidemia, obesity, and osteoporosis (19–21). Furthermore, there have been documented reports of elevated mortality linked to cardiovascular events in individuals diagnosed with MACS (22–25). The definite treatment for overt Cushing’s syndrome is adrenalectomy, but the optimal treatment for MACS is still controversial (26, 27). Several studies have documented a notable enhancement in comorbidities associated with MACS subsequent to adrenalectomy, while others have not observed any significant alterations in this regard (28–30).
This systematic review and meta-analysis were conducted to enhance comprehension of the available data regarding the positive impacts of adrenalectomy on cardiovascular risk factors and other comorbidities in patients with MACS. To reach this objective, we compared the effect of adrenalectomy with conservative treatment on comorbidities associated with MACS.
2 Methods
The research protocol for this study underwent examination and approval by the ethics committee at the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences (Ethical code: IR.TUMS.EMRI.REC.1402.013).
2.1 Data source and search strategy
The current review is designed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (31). A systematic search was conducted in several databases using a combination of keywords and MESH terms, including “Cushing Syndrome” or “adrenal incidentalomas’’ or “adrenal mass,” “subclinical Cushing’s syndrome’’ or “preclinical Cushing’s syndrome’’ or “autonomous cortisol secretion’’ or ‘‘Hypercortisolism’’ in combination with “Conservative Treatment”, and “Surgical Procedures, Operative” or “Adrenalectomy” or “General Surgery”. The Search strategy is provided in the Appendix 1. We searched MEDLINE, Embase, Web of Sciences, and Scopus databases in any language on December, 2023. There were no limitations placed on the search based on language and date. An experienced librarian and an endocrinologist with specialized knowledge in the field conducted the planning and implementation of the search strategy. Furthermore, the accuracy of the search strategy has been assessed by other authors.
2.2 Inclusion and exclusion criteria
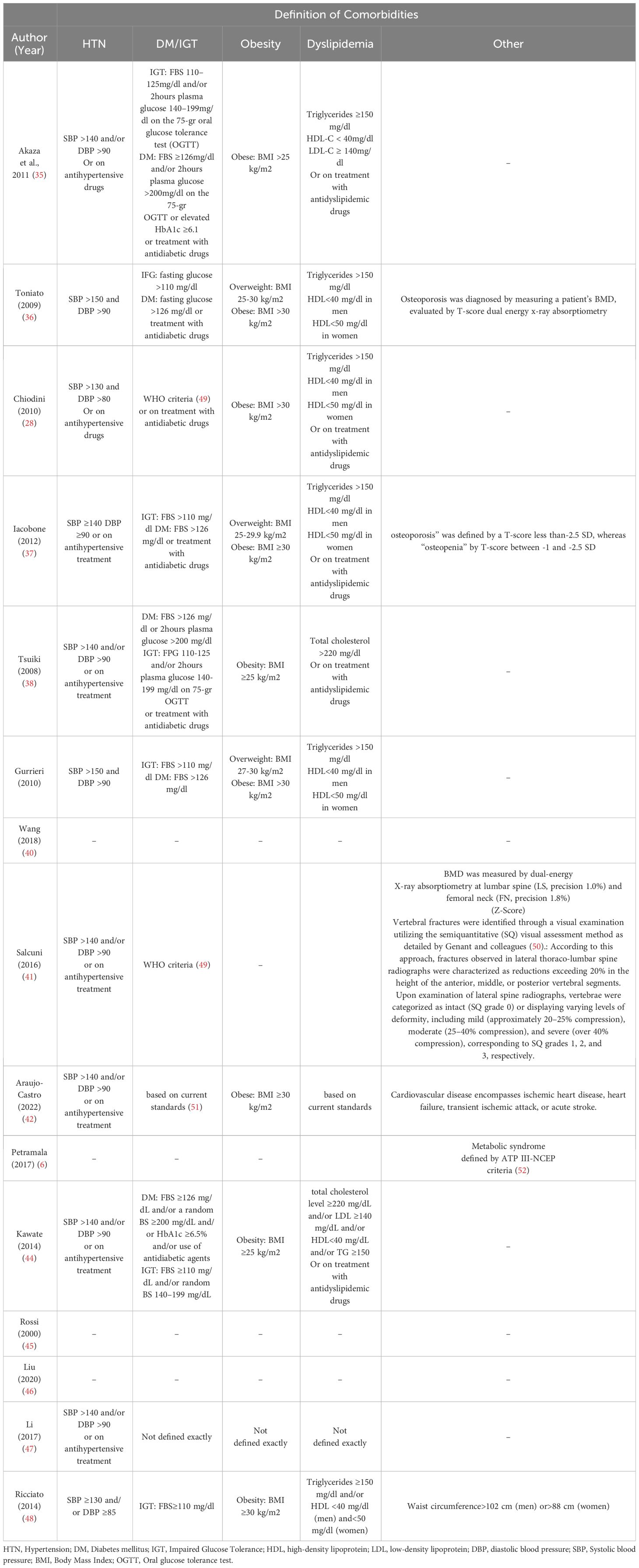
The selected studies were required to meet certain criteria to be considered for inclusion in this study. Specifically, they were required to provide data on both adrenalectomy and conservative treatment in patients diagnosed with MACS. Additionally, the studies were expected to examine at least one of the following outcomes: arterial hypertension, diabetes, dyslipidemia, obesity, osteoporosis, and vertebral fractures.
The excluded studies from the analysis were those that solely presented preoperative data or focused only on adrenalectomy or conservative therapy. Case reports and case series including fewer than ten patients were also excluded, as they were considered reviews and letters. Another exclusion criteria were those studies that encompass patients diagnosed with clinically evident Cushing’s syndrome and other adrenal disorders, including primary hyperaldosteronism, phaeochromocytoma, and non-functional adrenal tumors (NFA). Publications that did not have biochemically confirmed MACS and studies that did not distinguish between clinically evident CS and MACS were also excluded from our review.
In order to request further information or have a complete data, the corresponding authors were contacted via email. Once the desired data were obtained, they were used for analysis. Two researchers, working separately, evaluated the articles’ title, abstract, and full text from the initial search outcomes to select papers that aligned with the inclusion and exclusion criteria.
2.3 Data extraction
The data was extracted independently from included publications by two researchers using a standardized piloted web-based form and then were compared. Using full texts and debates, the third researcher made a decision concerning the conflicting and inconsistent data. A total of 13 publications were reviewed in the original language without transcription in English and two in Chinese.
2.4 Quality assessment
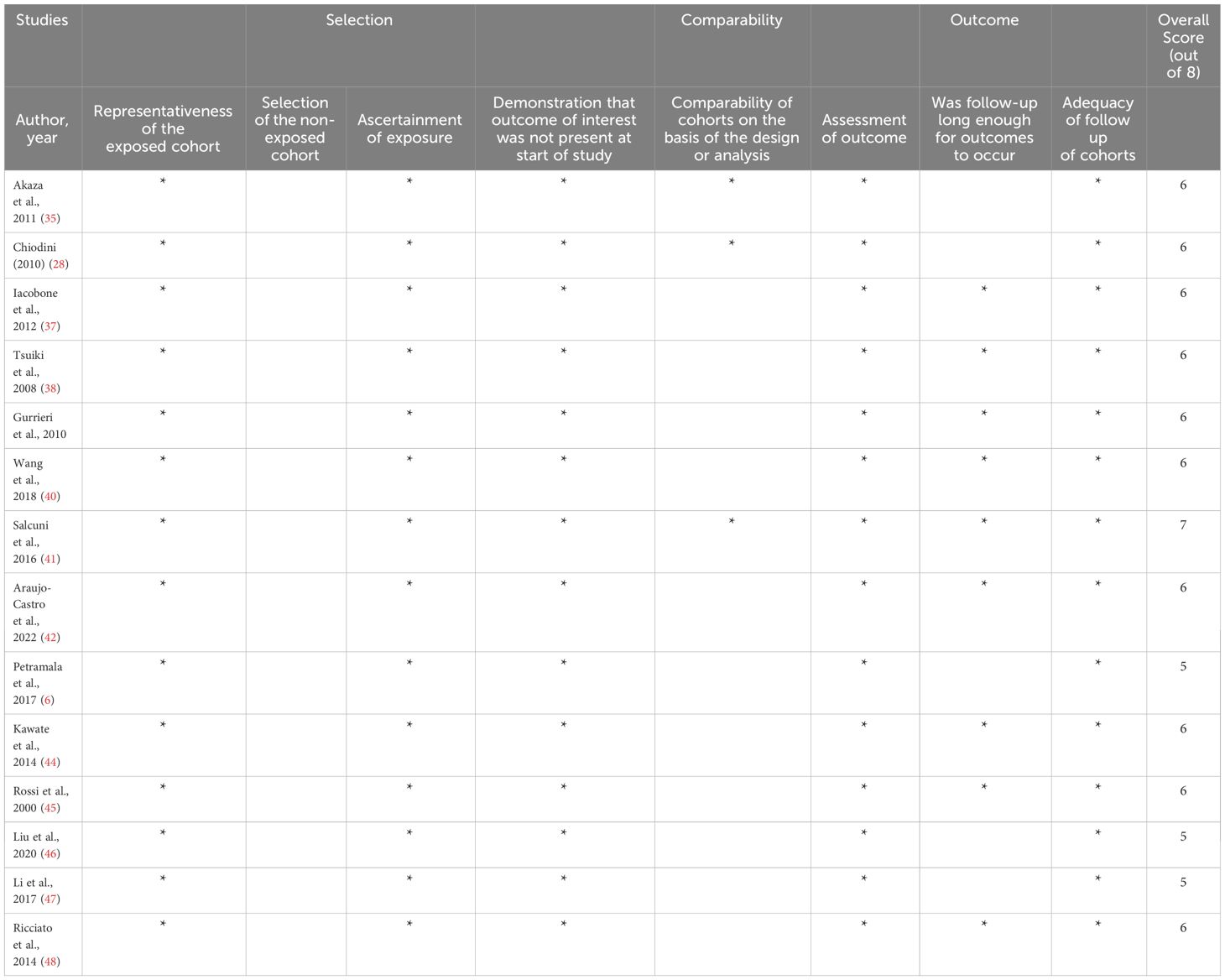
The risk of bias was evaluated using the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies. This tool evaluates how well the sample represented the population of interest, how the comparative group was chosen, how the outcome was assessed, and the length and sufficiency of follow-up (32). The Downs and Black checklist was used for a single study with a randomized controlled trial design (33). The methodological quality of the publications was individually appraised to determine their eligibility for inclusion in a meta-analysis.
2.5 Meta-analysis
Using the random-effects model, we conducted a meta-analysis to pool estimates from included studies. To account for heterogeneity between studies and within-study variability, a random-effects model was used instead of a fixed-effects model. The I2 statistic was used to calculate the percentage of total between-study variation owing to heterogeneity rather than chance ranging from 0 to 100%. Low, moderate, and high heterogeneity are represented by I2 values of 25, 50, and 75%, respectively. STATA version 17 was used for data analysis and statistical procedures. (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). For negative variables, the Cohen’s d value was used to calculate the effect size of the difference between the means of the two adrenalectomy and conservative treatment groups in terms of standard deviations. The Hedges’s g effect size was used for other variables including high-density lipoprotein (HDL) and bone mineral density (BMD). A significance threshold of P<0.05 was considered to determine statistical significance.
We depicted the forest plot, which illustrates the individual effect sizes and their corresponding confidence intervals for each study included in the analysis. We produced the Galbraith plot to demonstrate heterogeneity across studies. Furthermore, the funnel plots in Appendix shows a graphical representation of publication bias.
2.6 Publication bias assessment
Publication bias was evaluated through the utilization of funnel plots, while the extent of asymmetry was examined using Egger’s regression test (34). The analysis was performed in Stata version 17 using the “meta” package.
3 Results
3.1 Characteristics and overview of studies
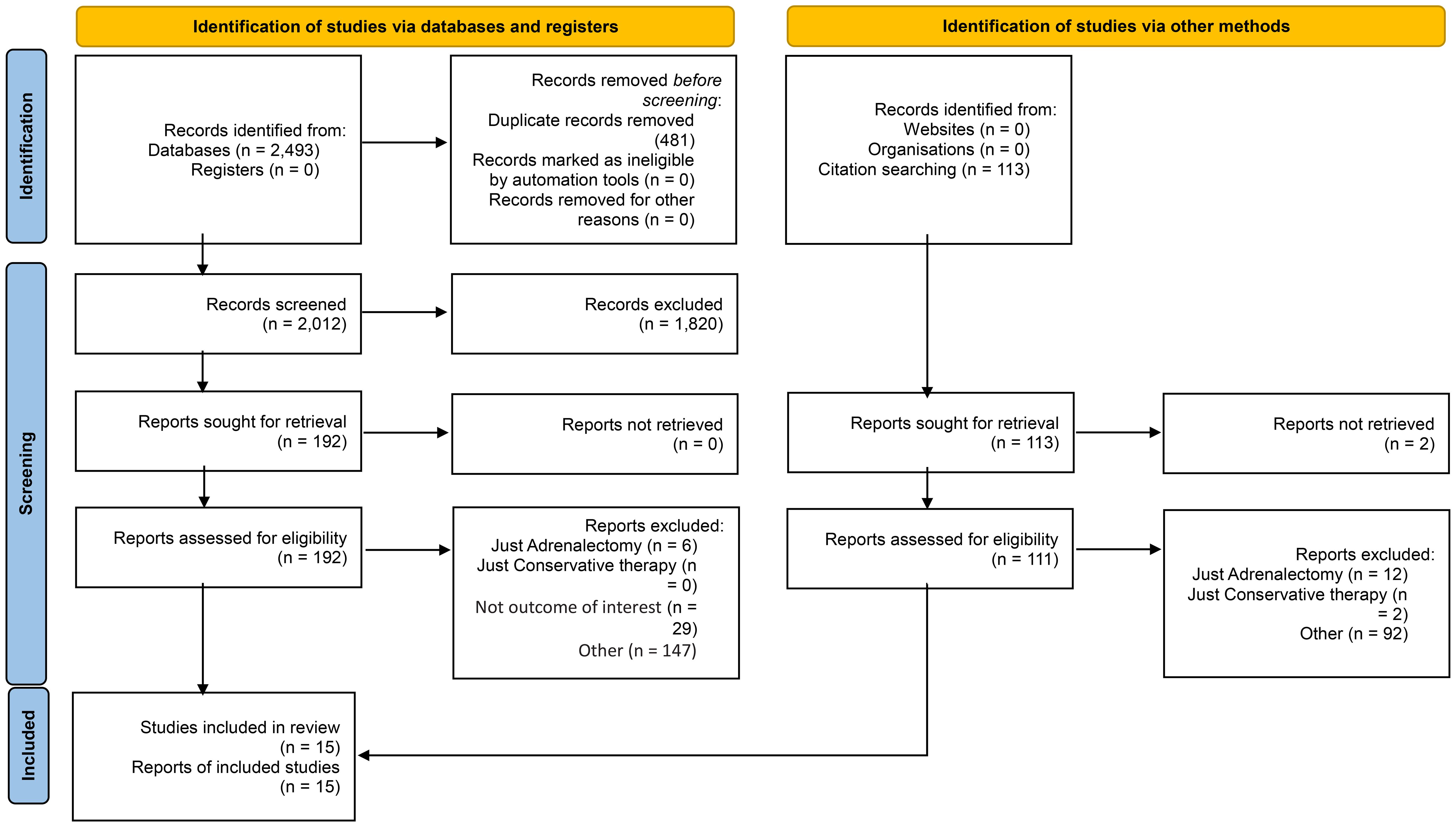
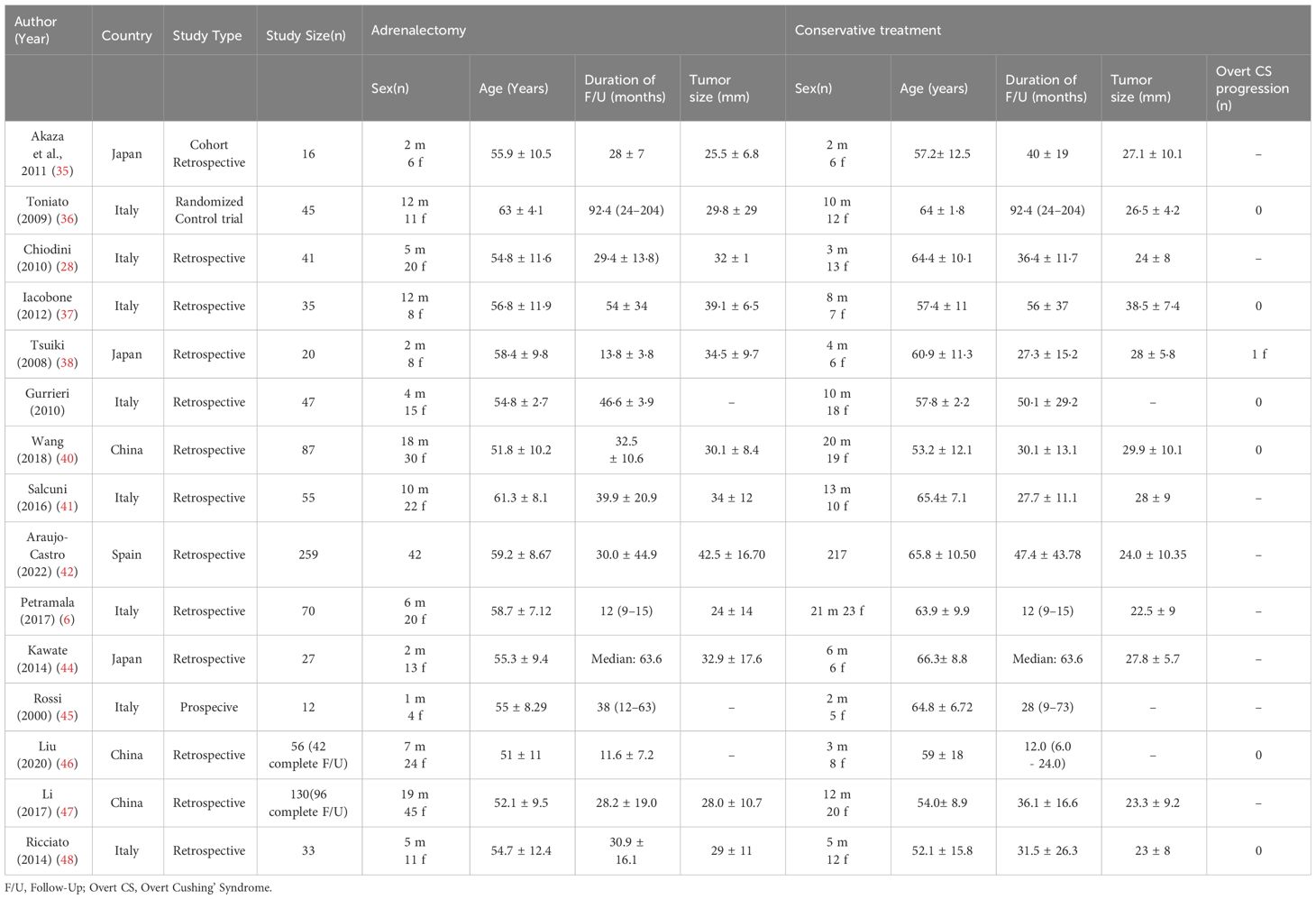
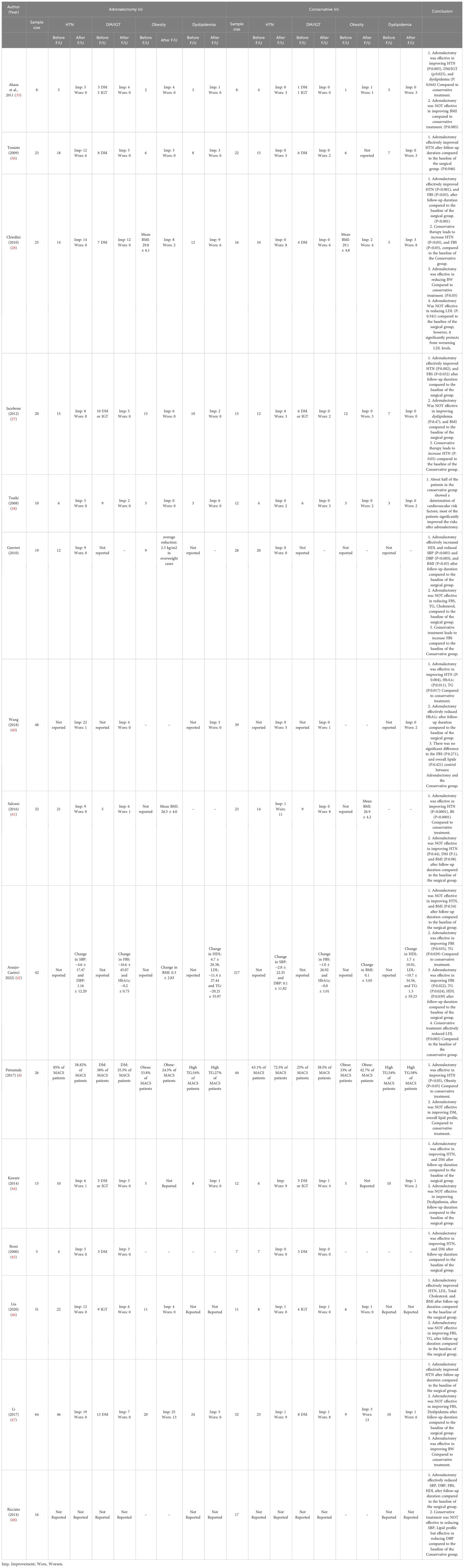
Our search yields a total of 2606 articles (2493 studies via databases and 113 via citation searching) that underwent title/abstract screening, eventually 15 references included in this study (28, 35–48), including 13 retrospective cohort and two prospective cohort studies. Six of the studies were from Asian centers (3 Japan and 3 China) and nine were from Europe centers (mostly from Italy). All of the included studies evaluated the comparative effects of conservative treatment and adrenalectomy for the MACS patients. Other studies which assess only one of these treatments were excluded. The characteristics of included articles are shown in Table 1. A total of 933 patients with MACS were included in this systematic review, among which 885 patients had completed the follow-up. A total of 384 patients underwent surgery (adrenalectomy) with a mean age of 56.1 years (Males: 30.7%, one study didn’t identify gender) (mean duration of follow-up: 34.8 months, one study only reported the median duration of follow-up) and 501 conservatively managed with the mean age of 60.4 years (Males: 40.4%, one study didn’t identify gender) (mean duration of follow up: 37.6 months, one study only reported median duration of follow-up). The average tumor size was 31.7 mm and 26.8 mm in the adrenalectomy and conservative groups, respectively. (Three studies didn’t report tumor size). The results show that 62.9% (number of patients: 345 of 548) of individuals with MACS experience hypertension, 29.2% (number of patients: 137 of 249) show impaired glucose metabolism (diabetes or impaired glucose tolerance), 41.4% (number of patients: 162 of 391) have dyslipidemia, and 38.2% (number of patients: 142 of 372) were affected by obesity. There were no reports of progression to overt Cushing’s syndrome, except for one study, which reported a female in a conservative therapy group developing symptoms of overt Cushing’s syndrome during follow-up.
3.2 Diagnostic criteria and definition of MACS
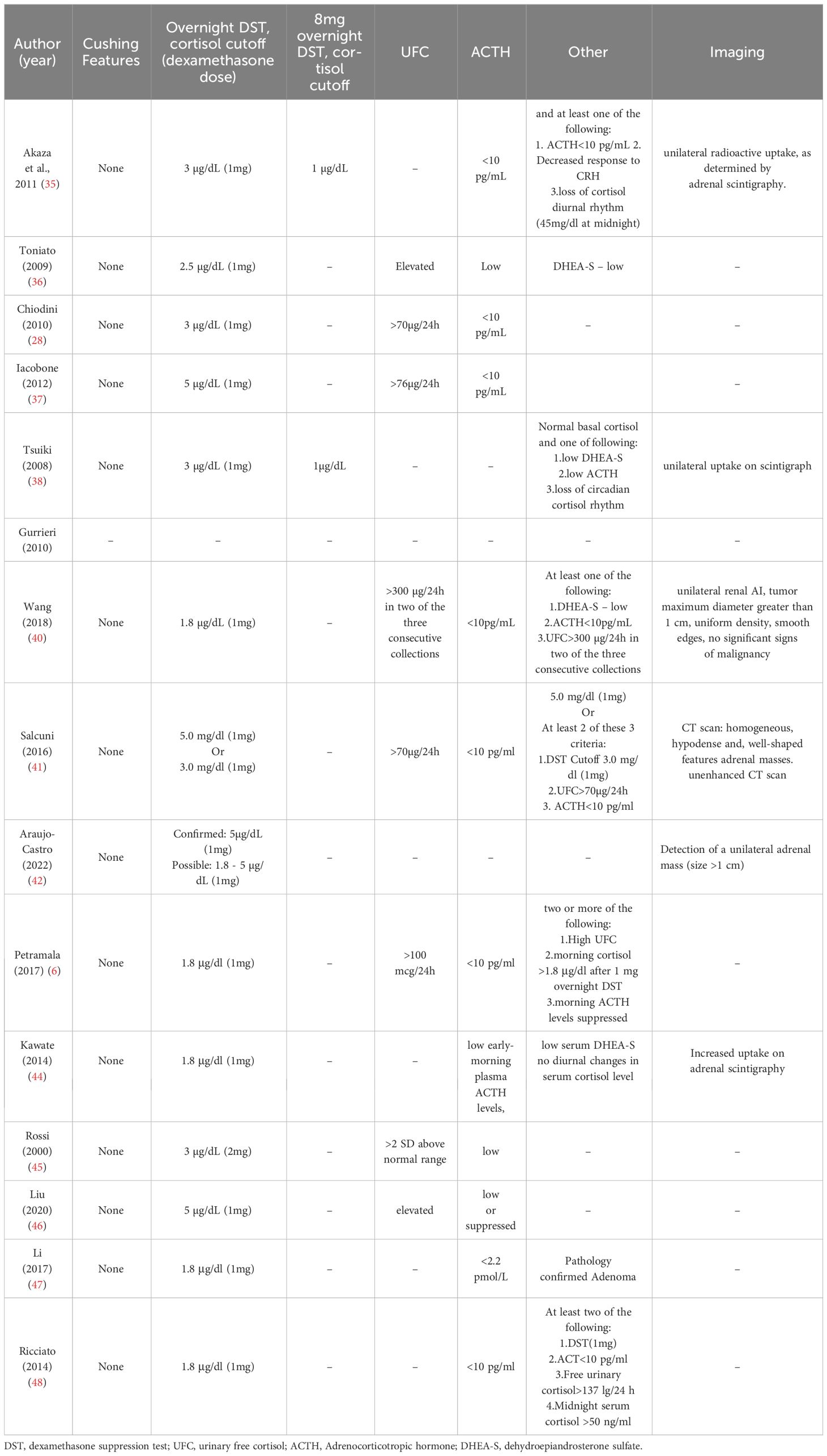
The diagnostic criteria for MACS varied between studies (see Table 2). One study yielded no information on diagnosis. However, all other studies agreed that there are no overt Cushing’s syndrome symptoms in patients with MACS. The cortisol cutoff after 1 mg-overnight-Dexamethasone Suppression Test (DST) varied; 5 studies used 3 μg/dl, 5 studies chose 1.8 μg/dl, and 1 study chose 2.5 μg/dl as a diagnostic criterion for cortisol level after 1mg-DST. The maximal cutoff was 5 μg/dl, which was used in three studies. Other criteria, such as low Adrenocorticotropic Hormone (ACTH) level, elevated urinary free cortisol (UFC), Low dehydroepiandrosterone sulfate (DHEA-S), 8 mg-overnight-DST, and imaging were evaluated in some of the included studies. The definition of comorbidities varied across studies, as were the “improvement” and “deterioration” of each specific comorbidity. (See Table 3)
3.3 Quality assessment
All included studies were observational (retrospective or prospective cohort) except one study with randomized control trial design. The sample size was not representative of most studies. Most studies reported a follow-up duration of more than 30 months, but it is still debatable whether this duration is sufficient to detect a significant change in outcome. Overall, the studies included exhibited a low to very low quality, and there was considerable heterogeneity in the data across the various studies. (See Table 4)
3.4 Publication bias
The funnel plot exhibited a symmetrical distribution of data points across the funnel, suggesting that the presence of publication bias was improbable. In addition, the Egger regression test showed that there was no significant difference in the degree of asymmetry of the funnel plot (p = 0.43) (Supplementary Figures 1–6).
3.5 Adrenalectomy versus conservative treatment outcome for MACS-related comorbidities
3.5.1 Meta analysis
Results of the subgroup analysis indicated no significant differences between the two treatment groups in various parameters, including body weight, body mass index (BMI), fasting blood sugar (FBS), glycated hemoglobin (HbA1C), total cholesterol, low-density lipoprotein (LDL) cholesterol, high –density lipoprotein (HDL) cholesterol, and triglyceride (TG). However, systolic blood pressure, diastolic blood pressure, and bone mineral density (BMD) (pooled effect size = -0.40, 95% CI [-0.73, -0.07], p = 0.02) were significantly in favor of adrenalectomy group rather than conservative treatment. The adrenalectomy group demonstrated a significant overall advantage over the conservative treatment group regarding negative variates of mild autonomous cortisol secretion following the procedure (Cohen’s d = -0.49, 95% CI [-0.64, -0.34], p = 0.00). However, substantial heterogeneity was observed (I^2 = 51.94%).
3.5.2 Blood pressure
Twelve studies evaluated HTN; in the adrenalectomy group, 300 patients with MACS were assessed for HTN, of whom 133 patients that underwent adrenalectomy showed improvement regarding HTN, and only 2 patients exhibited HTN deterioration. In contrast, 248 patients were assessed for HTN in the conservative treatment group; HTN improved in only 3 patients, and 177 patients experienced HTN worsening. Six studies were included in meta-analysis, revealing that the changes in systolic blood pressure (SBP) exhibited a pooled effect size of -0.81 (95% CI [-1.19, -0.42], p = 0.03), and diastolic blood pressure (DBP) demonstrated a pooled effect size of -0.63 (95% CI [-1.05, -0.21], p = 0.01). Heterogeneity analysis revealed moderate heterogeneity for both SBP and DBP among the included studies (SBP: I^2 = 58.46%, H^2 = 2.41, t^2 = 0.13, DBP: Heterogeneity: t2 = 0.18, I2 = 65.99%, H2 = 2.94). These findings support the notion that adrenalectomy is more advantageous than conservative treatment in terms of achieving a significant improvement in both SBP and DBP (Figure 1).
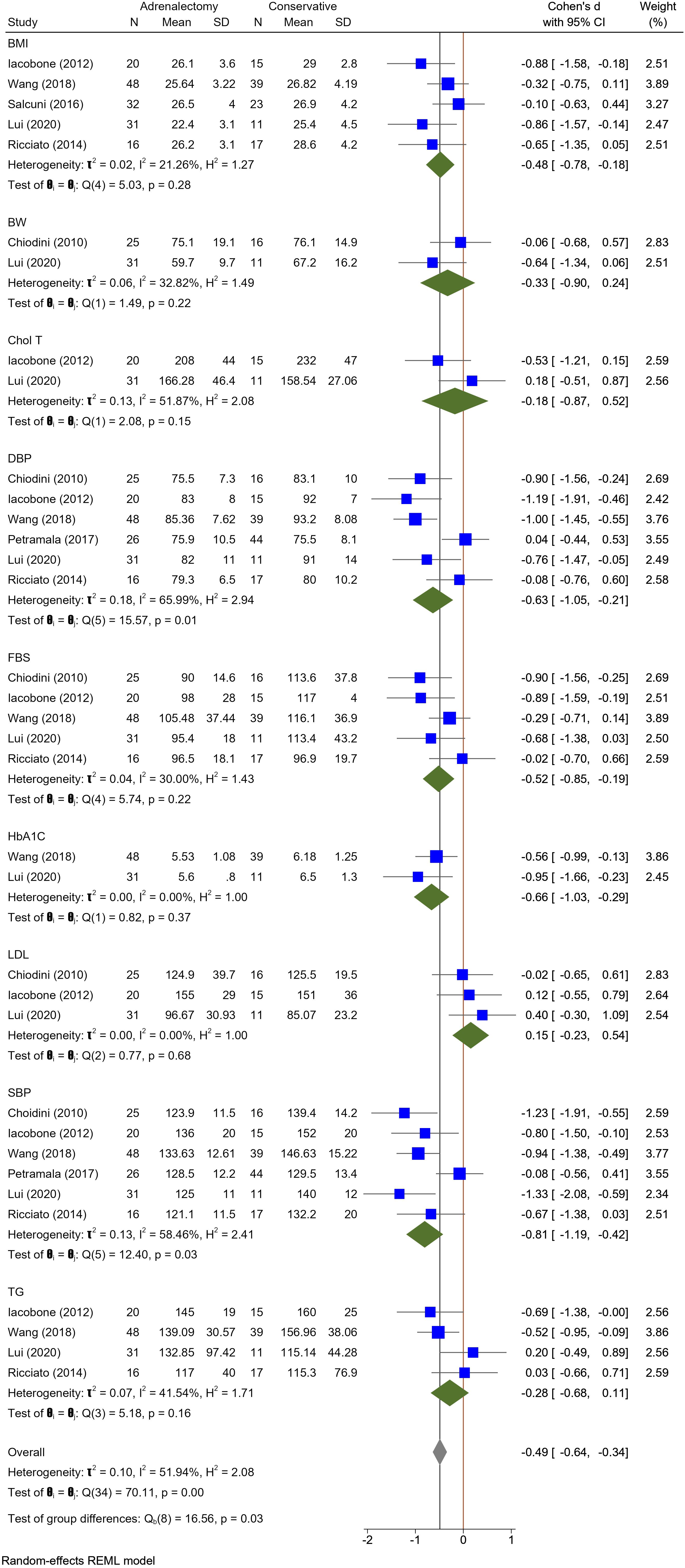
Figure 1 A comparison of the BMI, BW, Total Cholesterol, DBP, FBS, HbA1c, LDL, SBP, and triglyceride levels between the Adrenalectomy and conservative treatment groups. BMI, Body Mass Index; BW, Body Weight; Chol T, Total Cholesterol; DBP, Diastolic Blood Pressure; HbA1c, glycated hemoglobin; LDL, Low Density Lipoprotein; SBP, Systolic Blood Pressure; TG, triglyceride.
3.5.3 Glucose metabolism
In eleven studies, glucose metabolism including DM or Impaired Glucose Tolerance (IGT) was assessed. A total of 260 patients in the adrenalectomy group were evaluated for their glucose metabolism; 61 patients reported an improvement in fasting blood sugar (FBS) levels, while only one patient reported an increase. However, within the conservative treatment group of 209 patients, 3 cases improved, and 42 patients demonstrated FBS-level deterioration. Five Studies included in meta-analysis did not show differences in FBS levels of adrenalectomy group compared to the conservative treatment group with pooled effect size of -0.52 (95%CI [-0.85, -0.19], P = 0.22). Furthermore, the meta-analysis incorporating two studies revealed no substantial distinction between these groups concerning HbA1c levels. The pooled effect size was -0.66 (95% CI [-1.03, -0.29], p = 0.37), indicating a lack of statistically significant differences in HbA1c values between two compared groups (Figure 1). Heterogeneity analysis indicated low heterogeneity among the included studies for FBS (I^2 = 30.00%, H^2 = 1.43, t^2 = 0.04) and minimal heterogeneity among the included studies (I^2 = 0.00%, H^2 = 1.00, t^2 = 0.00), indicating a high degree of consistency in the findings across studies regarding HbA1c levels.
3.5.4 Lipid profile
In Ten included studies, Authors evaluate the lipid profile of patients with MACS. Improvement in dyslipidemia was seen in 34 patients in the adrenalectomy group (total patients: 214) and 5 patients in the conservative treatment group (total patients: 177). Dyslipidemia deteriorated in 6 MACS patients who had adrenalectomy and 28 patients who did not. The meta-analysis findings indicate an absence of statistically significant differences between the adrenalectomy group and the conservative group concerning various lipid profile parameters, including HDL (pooled effect size of 0.06 and 95% CI [-0.45, 0.57], P = 0.81), LDL (pooled effect size of 0.15 and 95% CI [-0.23, 0.54], P = 0.68), TG (pooled effect size of -0.28 and 95% CI [-0.68, 0.11], P = 0.16), and total cholesterol (pooled effect size of -0.18 and 95% CI [-0.87, 0.52], P = 0.15) (Figures 1, 2). Nevertheless, certain studies have demonstrated the favorable impact of adrenalectomy on lipid profiles. For instance, Akaza et al., 2011 (35) reported a significantly improvement in the overall lipid profile with adrenalectomy compared to conservative treatment. In the studies conducted by Wang et al. (42) Araujo-Castro et al., adrenalectomy exhibited a significant improvement in TG levels compared to conservative treatment. Furthermore, three studies documented a significant improvement in HDL levels following adrenalectomy compared to the levels observed before the procedure. Heterogeneity results also demonstrated minimal heterogeneity for LDL (t2 = 0.00, I2 = 0.00%, H2 = 1.00), moderate for cholesterol total, TG, and HDL (Chol: t2 = 0.13, I2 = 51.87%, H2 = 2.08; TG: t2 = 0.10, I2 = 51.94%, H2 = 2.08; HDL: t2 = 0.12, I2 = 57.42%, H2 = 2.35).
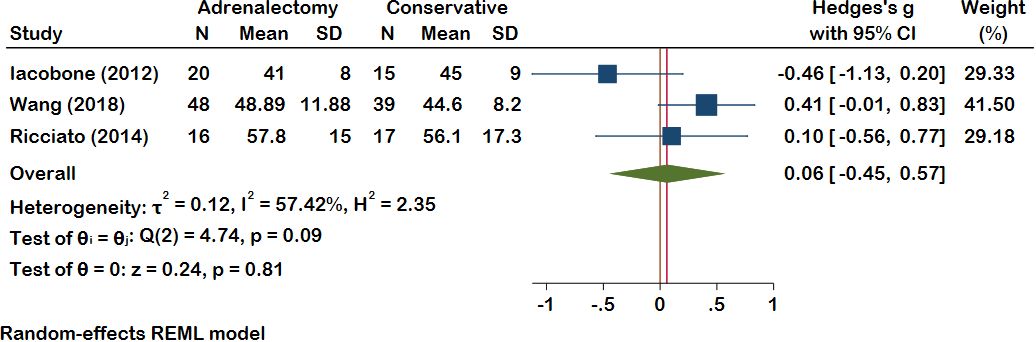
Figure 2 A comparison of the HDL levels between the Adrenalectomy and conservative treatment groups. HDL, High-Density Lipoprotein.
3.5.5 Weight
Among the included studies, five were considered in the meta-analysis for Body Mass Index (BMI), while two studies were included for Body Weight (BW). Nevertheless, the analysis revealed no significant difference between adrenalectomy and conservative treatment concerning BMI (pooled effect size of -0.48, 95% CI [-0.78, -0.18], p = 0.28) and BW (pooled effect size of -0.33, 95% CI [-0.90, 0.24], p = 0.22) (Figure 1). Heterogeneity analysis revealed a relatively low heterogeneity among the included studies (I^2 = 32.82%, H^2 = 1.49, t^2 = 0.06).
3.5.6 Bone
Within the meta-analysis, bone mineral density (BMD) exhibited a pooled effect size of -0.40 (95% CI [-0.73, -0.07], p = 0.02), decisively favoring the adrenalectomy over conservative treatment (Figure 3). Heterogeneity results also demonstrated minimal heterogeneity (I^2 = 00.00%, H^2 = 1.00, t^2 = 0.00). Only one study examined vertebral fractures (VFx), where 32 patients underwent adrenalectomy. Prior to the procedure, 15 of the 32 patients had fractures, but only three new Fx developed following surgery until the end of the follow-up. In another group with 23 conservatively treated patients, 15 of the 23 patients had VFx at the beginning of treatment, whereas, by the end of the follow-up period, 12 patients experienced new VFx. It has been shown that adrenalectomy effectively reduced VFx compared to conservative treatment.
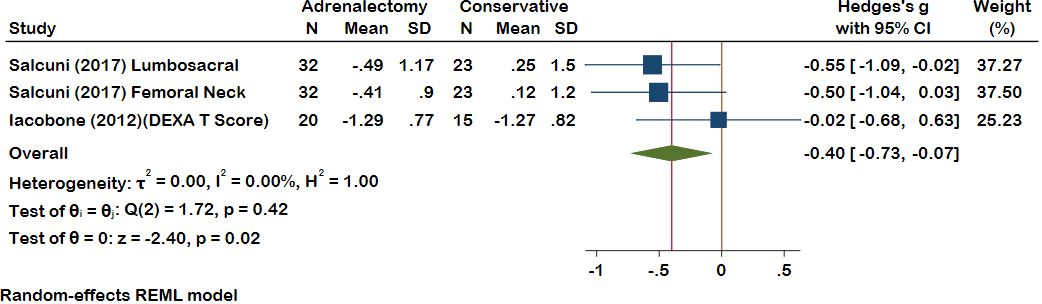
Figure 3 A comparison of the BMD levels between the Adrenalectomy and conservative treatment groups. BMD, Bone Mineral Density.
3.5.7 Mental status
One study evaluated mental health using the Short Form 36 Mental Health Component Summary (SF-36 MCS) test and found that adrenalectomy significantly improved mental health (p = 0.003), whereas conservative treatment did not yield similar outcomes.
Table 5 presents a comprehensive summary of all comorbidities along with the results from each individual study.
4 Discussion
Since the majority of patients with MACS are of an age range (in this study, the mean age for males was 56.1 years, and the mean age for females was 60.4 years) when HTN, diabetes, and obesity are highly prevalent (53–57), it is difficult to determine whether these metabolic complications are influenced by excess cortisol only or are affected by age either. However, some researches indicate that prolonged exposure to mild glucocorticoid excess resulting from adrenal incidentalomas is closely linked to a notable rise in cardiometabolic risk (22, 58). Both in-vivo and in-vitro evidence highlight how glucocorticoids (GCs) excess contribute to the pathophysiology of diabetes, osteoporosis, and dyslipidemia. Elevated cortisol levels affect blood glucose (on both liver and skeletal muscles), insulin sensitivity, and pancreatic function, linking MACS to a heightened risk of type 2 diabetes (59–67). Additionally, GCs excess adversely affects bone health by influencing osteoblast and osteocyte activity, leading to increased osteoclast activity and a potential risk of osteoporosis (68–71). In adipose tissue, GCs play a dual role in promoting both lipolysis and lipogenesis, contributing to dyslipidemia and adipose tissue changes as well as visceral obesity in conditions like MACS (60, 72–74). Additionally, it has been demonstrated that cortisol-mediated activation of the mineralocorticoid receptor may induce vascular changes even in mild and subclinical hypercortisolism (22). The outcomes might be associated with the severity and duration of hypersecretion and the sensitivity of each patient to cortisol (20). This systematic review and meta-analysis revealed that a notable proportion of patients with MACS experience various comorbidities. The findings of our study indicate that 62.9% of MACS patients have hypertension, 29.2% exhibit impaired glucose metabolism (DM or IGT), 41.4% suffer from dyslipidemia, and 38.2% are affected by obesity. Our findings are similar to the ENSAT NAPACA-Outcome Study conducted by Deutschbein et al., involving 3640 evaluated patients (7% with Autonomous Cortisol Secretion (ACS), 36% with possible Autonomous Cortisol Secretion (PACS), and 57% with NFA), the prevalence of cardiovascular risk factors among patients with PACS and ACS included higher rates of hypertension (72% and 73%, respectively), dyslipidemia (42% and 49%), and diabetes (22% and 25%). These rates were significantly higher compared to patients with NFA (25). [The 2016 ESE-ENSAT guideline introduced two categories for this condition at that time, defining patients as possible autonomous cortisol secretion (PACS) or autonomous cortisol secretion (ACS) based on post-1mg-DST cortisol levels (75)].
Based on the available and diverse published data, our analysis revealed a significant advantage of the adrenalectomy group compared to the conservative treatment group in general; However, it is crucial to consider that observational studies, by their design, cannot conclusively prove causality (76). Our meta-analysis revealed that SBP, DBP, and osteoporosis management of adrenalectomy were significantly better than conservative therapy. Additionally, in some included studies, the benefits of adrenalectomy in the control of dyslipidemia and obesity were reported but they were not significantly different in our subgroup analysis (28, 35, 39, 40, 42, 43, 46–48). It’s important to take into account that weight loss after abdominal surgery may influence parameters such as lipid profile and blood glucose levels (77, 78). thus, we cannot definitively attribute these outcomes solely to hormonal effects following adrenalectomy. Previous reviews revealed the same benefits of adrenalectomy in terms of cardiovascular risk factors (20, 79, 80). During the course of conservative treatment for MACS, it is possible for patients to experience a deterioration in their comorbidities, even when they are being appropriately monitored and receiving suitable medical therapy. However, reports almost indicated no worsening of hypertension and diabetes after surgical treatment. In our review, only two patients for hypertension and one patient for DM exhibited deterioration after surgical intervention.
The occurrence of adrenal insufficiency following adrenalectomy, even in cases of unilateral adrenalectomy (81–84), may lead to the hesitation in proceeding with the surgical procedure. Research findings indicate a significant decrease in post-operative stimulated cortisol levels in nearly 28% of the patients (85, 86). However, given that none of the included studies reported any incidence of adrenal insufficiency during the follow-up, this systematic review abstained from evaluating this particular aspect. moreover, Consideration must be given to surgical complications. Hemorrhage from the adrenal and renal veins or the adrenal cortex, injuries to the vena cava, puncture of the diaphragm, and laceration of the spleen represent the primary intraoperative complications. Postoperatively, prevalent complications include retroperitoneal hematoma, incisional hernia, pancreatic fistula, hyponatremia, and intestinal damage (87). Additionally, mortality has been documented in a limited number of adrenalectomy cases (88). BMI is one of the factors that contribute to increased complications after laparoscopic adrenalectomy (89, 90), and as we showed in this study, patients with MACS are at risk of obesity; therefore, the increase in the risk of laparoscopic adrenalectomy complications for these patients must be considered. Furthermore, patients with MACS are at older ages, which contributes to an increase in the risk of laparoscopic adrenalectomy complications (91, 92).
There is a lack of uniform and homogeneous standards for diagnosing and defining MACS in the included studies; as shown in Table 2, these variations in MACS definition could cause bias. All studies confirmed that there must not be any symptoms of overt Cushing’s syndrome, and, with the exception of one, all studies employed 1mg-DST, which previously has been shown to be the most sensitive screening test for an aberrant hypothalamic-pituitary-adrenal axis (93). However, the cortisol cut-off following DST differs across the included studies. The most recent guideline for managing adrenal incidentalomas, jointly developed by the European Society of Endocrinology and the European Network for the Study of Adrenal Tumors (ESE, ENSAT), recommends considering a 1-mg overnight dexamethasone suppression test with a cutoff value of (>1.8 µg/dL) to identify MACS (94). Previously, The National Institutes of Health (NIH), American Association of Clinical Endocrinologists (AACE), and American Association of Endocrine Surgeons (AAES) recommend >5.0 μg/dL, Endocrine Society (ES) and French Society of Endocrinology (FSE) recommend >1.8 μg/dL as a cut-off for DST (95).
It is unlikely that MACS can be considered an early stage of Cushing’s syndrome, as the progression of overt Cushing’s syndrome is rare among individuals with MACS. In our systematic review, only one female patient with MACS progressed to overt Cushing’s syndrome. Additional research has also demonstrated that the progression from MACS to Overt Cushing’s syndrome is infrequent (96–98). The ESE-ENSAT guideline also recommends against considering patients with MACS as being at high risk for the development of overt Cushing’s syndrome (94).
Previous systematic reviews which evaluated the effect of adrenalectomy on cardiovascular risk factors in patients with MACS showed the benefits of surgical treatment over conservative treatment, particularly regarding HTN, DM, and obesity (20, 79, 80). Furthermore, based on the ESE-ENSAT guideline, The recommendation for surgical intervention (Adrenalectomy) should be determined for all MACS patients as the standard care that aligns with and supports our findings (94).
5 Limitations
This systematic review and meta-analysis exhibit certain limitations. The definition and diagnostic criteria of MACS varied among the included studies, reflecting the absence of a universally accepted definition at that time. In our efforts to assess the improvement or worsening of comorbidities such as hypertension, diabetes mellitus, osteoporosis, dyslipidemia, and obesity, it is important to acknowledge that variations in the diagnostic criteria and definitions of the improvement or deterioration of each comorbidity among studies may impact our research outcomes. Another limitation of this study is the lack of clarity in the included studies regarding the specific details of conservative treatment. Consequently, we are unable to assess the pharmacological effects. Variations in conventional treatment, such as the type of medications used or dosages administered, could introduce discrepancies in the outcomes of conservative treatments across different studies, which indeed affect the accuracy. Furthermore, information regarding postoperative corticosteroid supplementation, such as the dosage, the quantity of patients who received corticosteroids, and the duration of drug administration, was not specified in the included studies. Nevertheless, it should be acknowledged as an important factor that may influence the outcomes of this study. The duration of follow-up is an additional important limitation that could notably impact the decision about adrenalectomy. It is not well recognized whether the durations of follow-ups were sufficient to determine the efficacy of each treatment. Also, the incomplete data in some studies made us to exclude them from our meta-analysis.
While our meta-analysis provides valuable insights, cautious interpretation is advised. The heterogeneity observed between studies, particularly in the sub-groups of BW, DBP, SBP, cholesterol, and TG (I2 > 30%), poses a significant limitation in the interpretability of these findings. This heterogeneity may arise from methodological differences, participant demographics, variations in the type of conservative treatment received by control groups across different studies, and other unaccounted factors. While this meta-analysis represents the only study conducted on this topic, future original trials with similar aims may either reinforce or weaken the findings of our study.
6 Conclusion
In this study, we showed that the presence of MACS is linked to an elevated likelihood of experiencing various Comorbidities. Additionally, this study demonstrated the advantage of adrenalectomy over conservative treatment for MACS-related comorbidities. However, heterogeneous data were used in current research. Additionally, the patients undergoing adrenalectomy experienced an overall improvement in cardiovascular risk factors compared to their initial baseline characteristics. However, in patients receiving conservative care, cardiovascular risk factors may deteriorate. Further and more accurate research, particularly in terms of follow-up duration and sample size, is required to make a precise decision.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
MMK: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. SM: Methodology, Writing – review & editing. HH: Formal analysis, Methodology, Software, Writing – original draft. MPS: Conceptualization, Resources, Writing – review & editing. MAK: Writing – original draft, Writing – review & editing. RA: Methodology, Writing – review & editing. SS: Formal analysis, Methodology, Writing – review & editing. MM-T: Resources, Supervision, Writing – review & editing. BL: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1374711/full#supplementary-material
Abbreviations
MACS, mild autonomous cortisol secretion; SCSm, subclinical Cushing’s syndrome; CS, Cushing’s syndrome; HTN, hypertension; DM, diabetes mellitus; BMI, Body Mass Index.
References
1. Beierwaltes WH, Sturman MF, Ryo U, Ice RD. Imaging functional nodules of the adrenal glands with 131-I-19-iodocholesterol. J Nucl Med. (1974) 15:246–51.
2. Charbonnel B, Chatal JF, Ozanne P. Does the corticoadrenal adenoma with “pre-Cushing’s syndrome” exist? J Nucl Med. (1981) 22:1059–61.
3. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. (2003) 149:273–85. doi: 10.1530/eje.0.1490273
4. Young WF Jr. Management approaches to adrenal incidentalomas. A view Rochester Minnesota. Endocrinol Metab Clin North Am. (2000) 29:159–85. doi: 10.1016/S0889-8529(05)70122-5
5. Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. (2023) 189:G1–G42. doi: 10.1093/ejendo/lvad066
6. Petramala L, Cavallaro G, Galassi M, Marinelli C, Tonnarini G, Concistrè A, et al. Clinical benefits of unilateral adrenalectomy in patients with subclinical hypercortisolism due to adrenal incidentaloma: results from a single center. High Blood Pressure Cardiovasc Prev. (2017) 24:69–75. doi: 10.1007/s40292-017-0182-7
7. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. (1995) 16:460–84. doi: 10.1210/edrv-16-4-460
8. Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. (2021) 9:847–75. doi: 10.1016/S2213-8587(21)00235-7
9. Goddard GM, Ravikumar A, Levine AC. Adrenal mild hypercortisolism. Endocrinol Metab Clin North Am. (2015) 44:371–9. doi: 10.1016/j.ecl.2015.02.009
10. Starker LF, Kunstman JW, Carling T. Subclinical Cushing syndrome: a review. Surg Clin North Am. (2014) 94:657–68. doi: 10.1016/j.suc.2014.02.008
11. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. (2006) 29:298–302. doi: 10.1007/BF03344099
12. Davenport C, Liew A, Doherty B, Win HHN, Misran H, Hanna S, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. (2011) 40:80–3. doi: 10.1007/s12020-011-9445-6
13. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. (2006) 29:298–302. doi: 10.1007/BF03344099
14. Bancos I, Prete A. Approach to the patient with adrenal incidentaloma. J Clin Endocrinol Metab. (2021) 106:3331–53. doi: 10.1210/clinem/dgab512
15. Comlekci A, Yener S, Ertilav S, Secil M, Akinci B, Demir T, et al. Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single center experience. Endocrine. (2010) 37:40–6. doi: 10.1007/s12020-009-9260-5
16. Reincke M. Subclinical cushing’s syndrome. Endocrinol Metab Clinics North America. (2000) 29:43–56. doi: 10.1016/S0889-8529(05)70115-8
17. Thompson GB, Young WF Jr. Adrenal incidentaloma. Curr Opin Oncol. (2003) 15:84–90. doi: 10.1097/00001622-200301000-00013
18. Terzolo M, Bovio S, Pia A, Osella G, Borretta G, Angeli A, et al. Subclinical cushing’s syndrome. Arq Bras Endocrinol Metabol. (2007) 51:1272–9. doi: 10.1590/S0004-27302007000800013
19. Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, et al. Patients with subclinical Cushing’s syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. (2002) 87:4872–8. doi: 10.1210/jc.2001-011766
20. Khan U. Nonfunctioning and subclinical cortisol secreting adrenal incidentalomas and their association with metabolic syndrome: A systematic review. Indian J Endocrinol Metab. (2019) 23:332–46. doi: 10.4103/ijem.IJEM_52_19
21. Erbil Y, Ademoğlu E, Ozbey N, Barbaros U, Yanik BT, Salmaslioğlu A, et al. Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg. (2006) 30:1665–71. doi: 10.1007/s00268-005-0681-x
22. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. (2014) 2:396–405. doi: 10.1016/S2213-8587(13)70211-0
23. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. (2014) 99:4462–70. doi: 10.1210/jc.2014-3007
24. Morelli V, Arosio M, Chiodini I. Cardiovascular mortality in patients with subclinical Cushing. Paris, France: Annales d'Endocrinologie (2018). doi: 10.1016/j.ando.2018.03.005
25. Deutschbein T, Reimondo G, Dalmazi GD, Bancos I, Falhammar H, Tsagarakis S, et al. OR25–05 increased overall mortality and cardiovascular morbidity in patients with adrenal incidentalomas and autonomous cortisol secretion: results of the ENS@T NAPACA-outcome study. J Endocrine Soc. (2020) 4:OR25-05. doi: 10.1210/jendso/bvaa046.1640
26. Ritzel K, Beuschlein F, Mickisch A, Osswald A, Schneider HJ, Schopohl J, et al. Outcome of bilateral adrenalectomy in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. (2013) 98:3939–48. doi: 10.1210/jc.2013-1470
27. Vella A, Thompson GB, Grant CS, van Heerden JA, Farley DR, Young WF Jr. Laparoscopic adrenalectomy for adrenocorticotropin-dependent cushing’s syndrome. J Clin Endocrinol Metab. (2001) 86:1596–9. doi: 10.1210/jcem.86.4.7399
28. Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, et al. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab. (2010) 95:2736–45. doi: 10.1210/jc.2009-2387
29. Chiodini I, Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Viti R, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. (2009) 94:3207–14. doi: 10.1210/jc.2009-0468
30. Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract. (2009) 15:450–3. doi: 10.4158/EP.15.5.450
31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. (2009) 339:b2535. doi: 10.1136/bmj.b2535
32. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. (2000) 59:5–8. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
33. Report COU. Downs and black checklist for clinical trial quality assessment (2013). Available at: https://www.ncbi.nlm.nih.gov/books/NBK361373/.
34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
35. Akaza I, Yoshimoto T, Iwashima F, Nakayama C, Doi M, Izumiyama H, et al. Clinical outcome of subclinical Cushing’s syndrome after surgical and conservative treatment. Hypertens Res. (2011) 34:1111–5. doi: 10.1038/hr.2011.90
36. Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg. (2009) 249:388–91. doi: 10.1097/SLA.0b013e31819a47d2
37. Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Mondi I, et al. Adrenalectomy may improve cardiovascular and metabolic impairment and ameliorate quality of life in patients with adrenal incidentalomas and subclinical Cushing’s syndrome. Surgery. (2012) 152:991–7. doi: 10.1016/j.surg.2012.08.054
38. Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing’s syndrome. Endocr J. (2008) 55:737–45. doi: 10.1507/endocrj.K07E-177
39. Guerrieri M, Campagnacci R, Patrizi A, Romiti C, Arnaldi G, Boscaro M. Primary adrenal hypercortisolism: minimally invasive surgical treatment or medical therapy? A retrospective study with long-term follow-up evaluation. Surg Endosc. (2010) 24:2542–6. doi: 10.1007/s00464-010-1000-7
40. Wang D, Ji ZG, Li HZ, Zhang YS. Adrenalectomy was recommended for patients with subclinical Cushing’s syndrome due to adrenal incidentaloma. Cancer biomark. (2018) 21:367–72. doi: 10.3233/CBM-170531
41. Salcuni AS, Morelli V, Eller Vainicher C, Palmieri S, Cairoli E, Spada A, et al. Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur J Endocrinol. (2016) 174:261–9. doi: 10.1530/EJE-15-0977
42. Araujo-Castro M, Mínguez Ojeda C, Sánchez Ramírez MN, Gómez Dos Santos V, Pascual-Corrrales E, Fernández-Argüeso M. Adrenalectomy improves blood pressure control in nonfunctioning adrenal incidentalomas and glycemic and lipid control in patients with autonomous cortisol secretion. Endocrine. (2022) 78:142–50. doi: 10.1007/s12020-022-03120-w
43. Petramala L, Cavallaro G, Galassi M, Marinelli C, Tonnarini G, Concistrè A, et al. Clinical benefits of unilateral adrenalectomy in patients with subclinical hypercortisolism due to adrenal incidentaloma: results from a single center. High Blood Pressure Cardiovasc Prev. (2017) 24. doi: 10.1007/s40292-017-0182-7
44. Kawate H, Kohno M, Matsuda Y, Akehi Y, Tanabe M, Horiuchi T, et al. Long-term study of subclinical Cushing’s syndrome shows high prevalence of extra-adrenal Malignancy in patients with functioning bilateral adrenal tumors. Endocr J. (2014) 61:1205–12. doi: 10.1507/endocrj.EJ14-0155
45. Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, et al. Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. (2000) 85:1440–8. doi: 10.1210/jcem.85.4.6515
46. Liu MS, Zhang WJ, Zhu KY, Feng WH, Huang H, Zhu DL, et al. [Clinical features and outcomes of surgical versus conservative management in patients with subclinical Cushing’s syndrome]. Zhonghua Yi Xue Za Zhi. (2020) 100:2834–40. doi: 10.3760/cma.j.cn112137-20200213-00274
47. Li LL, Zhao L, Dou JT, Yang GQ, Gu WJ, Lü ZH, et al. [Surgery versus conservative management for subclinical Cushing’s syndrome in adrenal incidentalomas]. Zhonghua Yi Xue Za Zhi. (2017) 97:3152–7. doi: 10.3760/cma.j.issn.0376-2491.2017.40.007
48. Ricciato MP, Di Donna V, Perotti G, Pontecorvi A, Bellantone R, Corsello SM. The role of adrenal scintigraphy in the diagnosis of subclinical Cushing’s syndrome and the prediction of post-surgical hypoadrenalism. World J Surg. (2014) 38:1328–35. doi: 10.1007/s00268-014-2482-6
49. Federation. WHOID. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation (2006). Available at: https://apps.who.int/iris/handle/10665/43588.
50. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. (1993) 8:1137–48. doi: 10.1002/jbmr.5650080915
51. Organization WHHEARTS D: diagnosis and management of type 2 diabetes (2020). Available at: https://apps.who.int/iris/handle/10665/331710.
52. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). Jama. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
53. Babatsikou F, Zavitsanou A. Epidemiology of hypertension in the elderly. Health Sci J. (2010) 4:24.
54. Chinnakali P, Mohan B, Upadhyay RP, Singh AK, Srivastava R, Yadav K. Hypertension in the elderly: prevalence and health seeking behavior. N Am J Med Sci. (2012) 4:558–62. doi: 10.4103/1947-2714.103314
55. Tuomilehto J, Nissinen A, Kivelä SL, Pekkanen J, Kaarsalo E, Wolf E, et al. Prevalence of diabetes mellitus in elderly men aged 65 to 84 years in eastern and western Finland. Diabetologia. (1986) 29:611–5. doi: 10.1007/BF00869258
56. Jain A, Paranjape S. Prevalence of type 2 diabetes mellitus in elderly in a primary care facility: An ideal facility. Indian J Endocrinol Metab. (2013) 17:S318. doi: 10.4103/2230-8210.119647
57. Li Y, Zhao L, Yu D, Ding G. The prevalence and risk factors of dyslipidemia in different diabetic progression stages among middle-aged and elderly populations in China. PloS One. (2018) 13:e0205709. doi: 10.1371/journal.pone.0205709
58. Kalafatakis K, Russell GM, Ferguson SG, Grabski M, Harmer CJ, Munafò MR, et al. Glucocorticoid ultradian rhythmicity differentially regulates mood and resting state networks in the human brain: A randomized controlled clinical trial. Psychoneuroendocrinology. (2021) 124:105096. doi: 10.1016/j.psyneuen.2020.105096
59. Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int J Mol Sci. (2021) 22:5–8. doi: 10.3390/ijms22020623
60. Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. (2007) 275:43–61. doi: 10.1016/j.mce.2007.05.015
61. Rooney DP, Neely RD, Cullen C, Ennis CN, Sheridan B, Atkinson AB, et al. The effect of cortisol on glucose/glucose-6-phosphate cycle activity and insulin action. J Clin Endocrinol Metab. (1993) 77:1180–3. doi: 10.1210/jcem.77.5.8077310
62. Pagano G, Cavallo-Perin P, Cassader M, Bruno A, Ozzello A, Masciola P, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest. (1983) 72:1814–20. doi: 10.1172/JCI111141
63. Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. (2000) 49:1700–8. doi: 10.2337/diabetes.49.10.1700
64. Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism. (1998) 47:3–6. doi: 10.1016/S0026-0495(98)90184-6
65. Gremlich S, Roduit R, Thorens B. Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic beta cells. Comparison effects Fatty Acids J Biol Chem. (1997) 272:3216–22. doi: 10.1074/jbc.272.6.3216
66. Borboni P, Porzio O, Magnaterra R, Fusco A, Sesti G, Lauro R, et al. Quantitative analysis of pancreatic glucokinase gene expression in cultured beta cells by competitive polymerase chain reaction. Mol Cell Endocrinol. (1996) 117:175–81. doi: 10.1016/0303-7207(95)03745-4
67. Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol. (2009) 46:85–95. doi: 10.1007/s00592-009-0112-9
68. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential Mech their deleterious effects Bone J Clin Invest. (1998) 102:274–82. doi: 10.1172/JCI2799
69. O’brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. (2004) 145:1835–41. doi: 10.1210/en.2003-0990
70. Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int. (2010) 21:1457–69. doi: 10.1007/s00198-010-1194-5
71. Seibel MJ, Cooper MS, Zhou H. Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. (2013) 1:59–70. doi: 10.1016/S2213-8587(13)70045-7
72. Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fueling fat redistribution in the metabolic syndrome. J Endocrinol. (2008) 197:189–204. doi: 10.1677/JOE-08-0054
73. Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. (2009) 23:1161–70. doi: 10.1210/me.2008-0464
74. Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, et al. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing’s syndrome. FASEB J. (2008) 22:1672–83. doi: 10.1096/fj.07-094144
75. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. (2016) 175:G1–g34. doi: 10.1530/EJE-16-0467
76. Berry AJ. Observational studies identify associations, not causality. Anesth Analgesia. (2005) 101:1238. doi: 10.1213/01.ANE.0000173754.86959.9D
77. Matory WE Jr., O’Sullivan J, Fudem G, Dunn R. Abdominal surgery in patients with severe morbid obesity. Plast Reconst Surg. (1994) 94:976–87. doi: 10.1097/00006534-199412000-00011
78. Wagner M, Probst P, Haselbeck-Köbler M, Brandenburg JM, Kalkum E, Störzinger D, et al. The problem of appetite loss after major abdominal surgery: A systematic review. Ann Surg. (2022) 276:256. doi: 10.1097/SLA.0000000000005379
79. Bancos I, Alahdab F, Crowley RK, Chortis V, Delivanis DA, Erickson D, et al. THERAPY OF ENDOCRINE DISEASE: Improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. (2016) 175:R283–r95. doi: 10.1530/EJE-16-0465
80. Iacobone M, Citton M, Scarpa M, Viel G, Boscaro M, Nitti D. Systematic review of surgical treatment of subclinical Cushing’s syndrome. Br J Surg. (2015) 102:318–30. doi: 10.1002/bjs.9742
81. Heinrich DA, Adolf C, Holler F, Lechner B, Schneider H, Riester A, et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab. (2019) 104:5658–64. doi: 10.1210/jc.2019-00996
82. Kahramangil B, Montorfano L, Gutierrez D, Erten O, Zhou K, Li D, et al. Biochemical assessment of adrenal insufficiency after adrenalectomy for non-cortisol secreting tumors: clinical correlation and recommendations. Surg Endoscopy. (2022) 36:7638–46. doi: 10.1007/s00464-022-09232-8
83. Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J Clin Endocrinol Metab. (2014) 99:2637–45. doi: 10.1210/jc.2014-1401
84. Mitchell J, Barbosa G, Tsinberg M, Milas M, Siperstein A, Berber E. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surg endoscopy. (2009) 23:248–54. doi: 10.1007/s00464-008-0189-1
85. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. (2017) 2:7. doi: 10.1172/jci.insight.93136
86. Heinrich DA, Adolf C, Holler F, Lechner B, Schneider H, Riester A, et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab. (2019) 104:5658–64. doi: 10.1210/jc.2019-00996
87. Conzo G, Tartaglia E, Gambardella C, Esposito D, Sciascia V, Mauriello C, et al. Minimally invasive approach for adrenal lesions: systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. Int J Surg. (2016) 28:S118–S23. doi: 10.1016/j.ijsu.2015.12.042
88. Murphy MM, Witkowski ER, Ng SC, McDade TP, Hill JS, Larkin AC, et al. Trends in adrenalectomy: a recent national review. Surg endoscopy. (2010) 24:2518–26. doi: 10.1007/s00464-010-0996-z
89. Dancea HC, Obradovic V, Sartorius J, Woll N, Blansfield JA. Increased complication rate in obese patients undergoing laparoscopic adrenalectomy. JSLS: J Soc Laparoendoscopic Surgeons. (2012) 16:45. doi: 10.4293/108680812X13291597715862
90. Thompson LH, Nordenström E, Almquist M, Jacobsson H, Bergenfelz A. Risk factors for complications after adrenalectomy: results from a comprehensive national database. Langenbeck’s Arch Surg. (2017) 402:315–22. doi: 10.1007/s00423-016-1535-8
91. Bergamini C, Martellucci J, Tozzi F, Valeri A. Complications in laparoscopic adrenalectomy: the value of experience. Surg endoscopy. (2011) 25:3845–51. doi: 10.1007/s00464-011-1804-0
92. Green RL, Gao TP, Hamilton AE, Kuo LE. Older age impacts outcomes after adrenalectomy. Surgery. (2023) 174:819–27. doi: 10.1016/j.surg.2023.06.007
93. Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. (2012) 26:405–19. doi: 10.1016/j.beem.2011.12.006
94. Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. (2023) 189:G1–g42. doi: 10.1093/ejendo/lvad066
95. Yanase T, Oki Y, Katabami T, Otsuki M, Kageyama K, Tanaka T, et al. New diagnostic criteria of adrenal subclinical Cushing’s syndrome: opinion from the Japan Endocrine Society. Endocrine J. (2018) 65:383–93. doi: 10.1507/endocrj.EJ17-0456
96. De Leo M, Cozzolino A, Colao A, Pivonello R. Subclinical cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. (2012) 26:497–505. doi: 10.1016/j.beem.2012.02.001
97. Terzolo M, Pia A, Reimondo G. Subclinical Cushing’s syndrome: definition and management. Clin Endocrinol. (2012) 76:12–8. doi: 10.1111/j.1365-2265.2011.04253.x
Keywords: mild autonomous cortisol secretion, subclinical Cushing’s syndrome, Cushing’s syndrome, adrenalectomy, conservative, systematic review, meta-analysis
Citation: Khadembashiri MM, Mohseni S, Harandi H, Pejman Sani M, Khadembashiri MA, Atlasi R, SeyedAlinaghi S, Mohajeri- Tehrani M and Larijani B (2024) Comparison of adrenalectomy with conservative treatment on mild autonomous cortisol secretion: a systematic review and meta-analysis. Front. Endocrinol. 15:1374711. doi: 10.3389/fendo.2024.1374711
Received: 22 January 2024; Accepted: 29 April 2024;
Published: 13 May 2024.
Edited by:
Henrik Falhammar, Karolinska Institutet (KI), SwedenReviewed by:
Ivana Kraljevic, University of Zagreb, CroatiaAnna Angelousi, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Khadembashiri, Mohseni, Harandi, Pejman Sani, Khadembashiri, Atlasi, SeyedAlinaghi, Mohajeri- Tehrani and Larijani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahnaz Pejman Sani, bXBzYW5pQHNpbmEudHVtcy5hYy5pcg==; Rasha Atlasi, cmFzaGFhdGxhc2lAZ21haWwuY29t
†These authors have contributed equally to this work
 Mohamad Mehdi Khadembashiri
Mohamad Mehdi Khadembashiri Shahrzad Mohseni1†
Shahrzad Mohseni1† Hamid Harandi
Hamid Harandi Mahnaz Pejman Sani
Mahnaz Pejman Sani Mohamad Amin Khadembashiri
Mohamad Amin Khadembashiri SeyedAhmad SeyedAlinaghi
SeyedAhmad SeyedAlinaghi Bagher Larijani
Bagher Larijani