
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 June 2024
Sec. Developmental Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1374682
 Yana Vanlaer1*
Yana Vanlaer1* Caro Minschart1
Caro Minschart1 Hannah Vrolijk2
Hannah Vrolijk2 Paul Van Crombrugge3
Paul Van Crombrugge3 Carolien Moyson1
Carolien Moyson1 Johan Verhaeghe4
Johan Verhaeghe4 Roland Devlieger4,5,6
Roland Devlieger4,5,6 Sofie Vandeginste7
Sofie Vandeginste7 Hilde Verlaenen7
Hilde Verlaenen7 Chris Vercammen8
Chris Vercammen8 Toon Maes8
Toon Maes8 Els Dufraimont9
Els Dufraimont9 Nele Roggen9
Nele Roggen9 Christophe De Block10
Christophe De Block10 Yves Jacquemyn11,12
Yves Jacquemyn11,12 Farah Mekahli13
Farah Mekahli13 Katrien De Clippel14
Katrien De Clippel14 Annick Van Den Bruel15
Annick Van Den Bruel15 Anne Loccufier16
Anne Loccufier16 Inge Van Pottelbergh17
Inge Van Pottelbergh17 Nele Myngheer18
Nele Myngheer18 Pascale Abrams19,20
Pascale Abrams19,20 Wouter Vinck20
Wouter Vinck20 Liesbeth Leuridan21
Liesbeth Leuridan21 Sabien Driessens21
Sabien Driessens21 Jaak Billen22
Jaak Billen22 Christophe Matthys23
Christophe Matthys23 Annick Bogaerts24,25
Annick Bogaerts24,25 Annouschka Laenen26
Annouschka Laenen26 Chantal Mathieu1
Chantal Mathieu1 Katrien Benhalima1
Katrien Benhalima1Aims: To determine the impact of breastfeeding on the risk of postpartum glucose intolerance in women with gestational diabetes.
Methods: Sub-analysis of two multi-centric prospective cohort studies (BEDIP-N and MELINDA) in 1008 women with gestational diabetes. Data were collected during pregnancy and at a mean of 12 weeks postpartum. Multivariate logistic regression was used to estimate the effect of breastfeeding on glucose intolerance, with adjustment for ethnicity, education, income, professional activity and BMI.
Results: Of all participants, 56.3% (567) breastfed exclusively, 10.1% (102) gave mixed milk feeding and 33.6% (339) did not breastfeed. Mean breastfeeding duration was 3.8 ± 2.4 and 3.7 ± 2.1 months in the breastfeeding and mixed milk feeding groups (p=0.496). The rate of glucose intolerance was lower in both the breastfeeding [22.3% (126)] and mixed milk feeding [25.5% (26)] groups compared to the no breastfeeding group [29.5% (100)], with an adjusted OR of 0.7 (95% CI 0.5–1.0) for glucose intolerance in the breastfeeding group compared to no breastfeeding group and an adjusted OR of 0.7 (95% CI 0.4–1.2) for the mixed milk feeding group compared to the no breastfeeding group. Postpartum, breastfeeding women had a lower BMI, less often postpartum weight retention, lower fasting triglycerides, less insulin resistance and a higher insulin secretion-sensitivity index-2 than the mixed milk feeding and no breastfeeding group. The mixed milk feeding group was more often from an non-White background, had a lower blood pressure and lower fasting triglycerides compared to the no breastfeeding group.
Conclusions: Breastfeeding (exclusive and mixed milk feeding) is associated with less glucose intolerance and a better metabolic profile in early postpartum in women with gestational diabetes.
Women with gestational diabetes mellitus (GDM), defined as “diabetes diagnosed during pregnancy, provided that overt diabetes has been excluded in early pregnancy” (1), are more likely to develop type 2 diabetes mellitus (T2DM) postpartum (2). Around 30–50% of all women with GDM develop T2DM within 10 years after their pregnancy (3–5). Previous studies have shown that T2DM can be prevented in this population, by adopting changes in lifestyle and/or by medication (6, 7). However, adherence to a healthy lifestyle is often low in early postpartum due to barriers such as lack of time, need for childcare and lack of social support (8). Besides lifestyle changes, lactation has also shown to reduce the risk to develop T2DM (9). The World Health Organization (WHO) and United Nations International Childrens Emergency Fund (UNICEF) recommend exclusive BF for the first six months after childbirth, since breastfeeding (BF) has known benefits for the child (10). A protective effect of BF, is particularly seen when women BF for a longer duration (at least six months) (11, 12). Two large cohort studies have demonstrated a protective effect of lactation on the evolution of GDM to T2DM (9, 13). BF is associated with lower fasting glucose levels and improved insulin sensitivity (14).
The evidence for the effect of BF on glucose intolerance in the early postpartum period in women with GDM remains incomplete (14). Limitations of previous research include small sample sizes, inclusion of only women with overweight or obesity and distinguished only between exclusive BF or exclusive formula feeding. Women who gave mixed milk feeding (MMF) were not included in previous studies (15–17). We investigated therefore the risk of glucose intolerance in early postpartum in a post-hoc analysis of two large existing cohorts of women with a recent history of GDM, comparing women with exclusive BF, women who gave MMF and women who did not breastfeed (NBF).
This study was a sub-analysis of the ‘Belgian Diabetes in Pregnancy study’ (BEDIP-N) (NCT02036619) and ‘Mobile-Based Lifestyle Intervention in Women with Glucose Intolerance after Gestational Diabetes Mellitus study’ (MELINDA) (NCT03559621). Both studies were published previously (18, 19). The BEDIP-N study was a large Belgian multi-centric prospective cohort study from 2014–2018 (18). This study enrolled 2006 pregnant women in early pregnancy to evaluate the diagnostic accuracy of different screening strategies for GDM based on the ‘International Association of Diabetes and Pregnancy Study Groups’ (IADPSG) criteria (20). All women without (pre)diabetes received universal screening for GDM between 24–28 weeks of pregnancy with a 75g 2-hour OGTT.
The Melinda study (performed between 2019–2023) was a multicenter randomized controlled trial (RCT), to investigate the efficacy and feasibility of a blended-care, telephone- and mobile-based lifestyle intervention to reach weight goals in 240 women with prediabetes after a recent history of GDM (diagnosed with the IADPSG criteria) (19, 20). In the Melinda study, baseline data were collected at 3 months postpartum of 1201 women with GDM who attended the postpartum OGTT (19). For this post-hoc analysis, only the baseline data were used.
In both studies, the American Diabetes Association (ADA)-recommended glycemic targets were used for treatment of GDM (21). If targets were not reached within two weeks after the start of lifestyle measures, treatment with insulin was started. Women with GDM received an invitation for a postpartum 75g OGTT 6–16 weeks after delivery. Glucose intolerance postpartum was defined as T2DM or prediabetes [defined as impaired fasting glucose (IFG; 100–125 mg/dL) and/or impaired glucose tolerance (IGT; 2-h glucose value on the OGTT between 140–199 mg/dL) or both] according to the ADA criteria (22).
Both studies received approval by the Institutional Review Boards of all participating centers and all investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. Participants gave written informed consent prior to any trial-related activity.
For both studies, baseline characteristics were collected for all eligible women through a clinical examination, self-administered questionnaires, collection of blood samples and extraction of data on medical history and pregnancy from the electronic medical records. At a mean of 12 weeks postpartum, a 75 g OGTT was performed with blood samples taken fasting and at 30, 60 and 120 min. Several self-administered questionnaires were completed by the participants. There was a self-designed questionnaire on general habits and socio-economic factors (18). The Food Frequency Questionnaire (FFQ) surveyed the frequency and portion size of consumption of foods and beverages (23). The International Physical Activity Questionnaire (IPAQ) measured physical activity such as job-related physical activity, transportation, house work and caring for family, recreation and time spent sitting (18, 24). The Center for Epidemiologic Studies-Depression (CES-D) questionnaire (widely used in pregnant and postpartum women) to assess symptoms of clinical depression over the past seven days (25). In both studies, the same self-designed questionnaire on BF and contraception was used, to collect information on the duration and frequency of BF. Women had to indicate what applied the most to them: [exclusive breastfeeding (< 45 ml formula feeding/day), half breastfeeding half formula feeding, or exclusive formula feeding (≥ 150ml formula feeding/day)] as well as on the type of contraception used (18). As the aim of this sub-analysis was to evaluate different degrees of intensity of BF, we only included women with a history of GDM, who received a postpartum OGTT and had data available on type of BF (exclusive, MMF or NBF) and on the duration of BF.
In line with normal routine, GDM was diagnosed between 24–28 weeks of pregnancy with a 75g 2-hour OGTT using the IADPSG criteria. Women who were diagnosed with GDM, were reevaluated at a mean of 12 weeks postpartum with a 2-h 75 g OGTT to screen for glucose intolerance. The 2-h 75 g OGTT consisted of measurements of glucose and insulin at fasting, 30 min, 60 min and 120 min. At the time of the OGTT, a fasting lipid profile (total cholesterol, triglycerides, HDL and LDL cholesterol) and HbA1c were also measured. Participants were instructed to fast for at least 10 h and not to smoke nor engage in any physical activity. They were also instructed to drink only water, but no coffee, cola or any drink containing sugar or caffeine. The analyses of glucose (fasting, 30 min, 60 min and 120 min in fluoride-containing tubes) were performed locally (and sent to the lab immediately after collection) so that there was no delay in diagnosing (pre)diabetes. The blood samples for the analyses of lipid profile, HbA1c and insulin, were analyzed centrally at the laboratory of Leuven University Hospital (UZ Leuven) to ensure uniformity. Plasma glucose was measured by an automated colorimetric-enzymatic method on a Hitachi/Roche-Modular P analyzer (Basel, Switzerland). Insulin was measured by the immunometric ECLIA (Roche Modular E170). HbA1c was measured by Tosoh Automated Glycohemoglobin Analyzer HLC-723G8. Lipid levels were measured by the immunoassay analyzer Cobas 8000 (Roche, Basel, Switzerland). Coefficients of variance are 1% for glucose, 6% for insulin, about 2% for lipids and 2% for HbA1c in the Lab of UZ Leuven (18, 19).
Different indices of insulin sensitivity [the Matsuda index, a well-established measure of whole-body insulin sensitivity and the homeostasis model assessment of insulin resistance (HOMA-IR), a measure of largely hepatic insulin resistance] and β-cell function [HOMA-B, the insulinogenic index divided by HOMA-IR and the insulin secretion-sensitivity index-2 (ISSI-2), an OGTT-derived measure that is analogous to the disposition index obtained from the frequently sampled intravenous glucose tolerance test], were measured, as previously described (18, 19).
The following pregnancy outcome data were collected: parity and pre-pregnancy BMI was stratified into underweight (BMI < 18.5 kg/m²), normal weight (BMI 18.5–24.9 kg/m²), overweight (BMI 25–29.9 kg/m²), and obesity (BMI ≥ 30 kg/m²). Obesity was further subdivided into class I (BMI 30–34.9 kg/m²), class II (35–39.9 kg/m²) and class III (BMI ≥ 40 kg/m²). Early postpartum weight retention was defined as the difference in weight measured at the postpartum OGTT and the pre-pregnancy weight (self-reported weight up to 1 month before pregnancy or weight measured during first prenatal consultation). Other data collected include birth weight, length, macrosomia (>4 kg), birth weight ≥4.5 kg, Large-for-gestational age (LGA) defined as birth weight >90 percentile according to standardized Flemish birth charts adjusted for sex of the baby and parity (26), small-for-gestational age (SGA) defined as birth weight <10 percentile according to standardized Flemish birth charts adjusted for sex of the baby and parity (26), and admission on the neonatal intensive care unit (NICU) (18). In line with normal routine in each center, admission to the NICU was decided by the neonatologist. The difference in weight between first prenatal visit and the time of the OGTT was calculated as early weight gain. The total gestational weight gain (GWG) was calculated as the difference in weight between first prenatal visit and the delivery. Excessive total GWG and inadequate total GWG were defined according to the National Academy of Medicine (NAM) guidelines, previously known as Institute of Medicine (IOM) (27).
Descriptive statistics were presented as frequencies and percentages for categorical variables, and means with standard deviations or medians with interquartile range for continuous variables. Group comparisons were performed using the Mann-Whitney U test for continuous or ordinal variables, and Chi square test or Fisher exact test in case of low (<5) cell frequencies for categorical variables.
Logistic regression was used for estimating the effect of BF on glucose intolerance, with correction for the following confounders: ethnicity, education, income, professional activity and pre-pregnancy body mass index (BMI). Adjustment was performed for baseline characteristics for which group differences were observed. Results were presented as odds ratios with 95% confidence intervals. All tests were performed at a 5% two-sided significance level. Analyses were performed by statistician A. Laenen by using SAS software (version 9.4 of the SAS System for Windows, 2023).
Women without data on whether they gave breastfeeding (N=43) or without data on the duration of BF (N=339), or women indicating a different intensity of BF other than exclusive BF, MMF or NBF (N=33), were excluded [a total of 415 women (29.2%)]. Women excluded from this analyses had in general similar characteristics compared to women included in this study, except for lower rates of multiparity and a slightly higher BMI (Supplementary Table 3). In total, data from 1008 women were included in this sub-analysis (Figure 1, Supplementary Figures 1, 2). Of all participants from both cohorts, 56.3% (567) gave BF exclusively, 10.1% (102) gave MMF, and 33.6% (339) did NBF at a mean of 12 weeks postpartum (Figure 1). Mean breastfeeding duration was respectively 3.8 ± 2.4 and 3.7 ± 2.1 months in the BF and MMF groups (Table 1).
Compared to NBF women, the exclusive BF and MMF groups were significantly more often from an non-White background. In comparison with the NBF group, women who BF were significantly more often higher educated, had a significantly lower pre-pregnancy BMI, had significantly less often excessive GWG, but significantly more often inadequate low GWG. Furthermore, women who BF had also significantly less often excessive GWG compared to the MMF group (Table 2 and Supplementary Table 1).
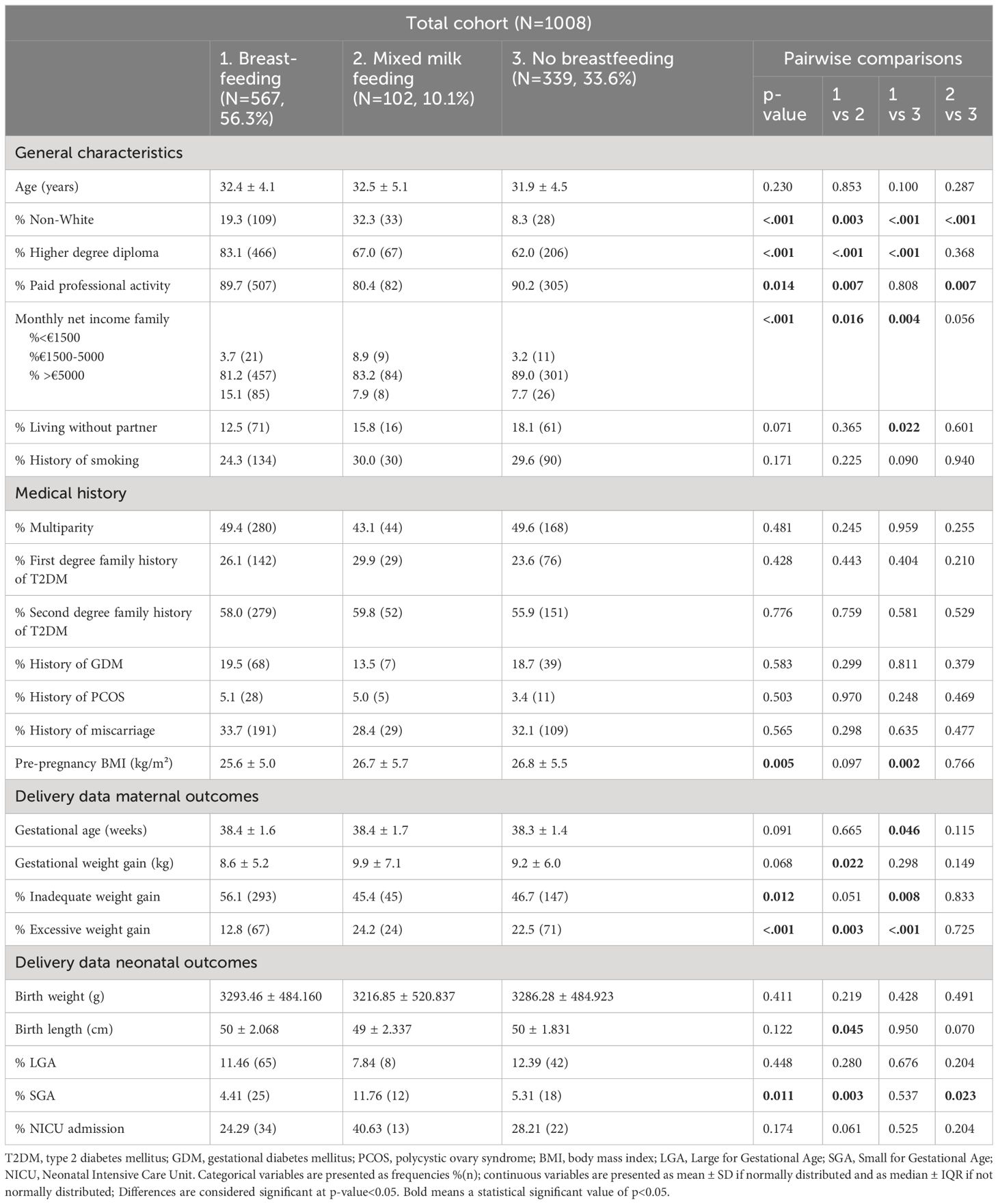
Table 2 Participant general characteristics, medical history and pregnancy outcomes according to breastfeeding behavior.
Compared to the MMF and NBF groups, women who BF exclusively had postpartum a significant lower BMI -and waist circumference, significantly less often postpartum weight retention, significantly lower fasting triglycerides, significantly less insulin resistance, a lower HOMA-B index [98.3 (72.1–140.6) vs. 115.5 (81.9–175.4), p=0.016; 98.3 (72.1–140.6) vs. 120.8 (91.0–172.0), p<0.001] but a higher ISSI-2 index [only significant higher compared to NBF group with respectively 2.0 (1.6–2.6) vs. 2.0 (1.5–2.7), p=0.960 for MMF; 2.0 (1.6–2.6) vs. 1.8 (1.4–2.4), p=0.003 for NBF] (Table 1). Compared to the NBF group, the BF and MMF groups had a significant lower systolic blood pressure (SBP), lower diastolic blood pressure (DBP) and lower fasting triglycerides at the postpartum OGTT (Table 1 and Supplementary Table 2).
The rate of glucose intolerance was significantly lower in both the BF (22.3%, p=0.019) and MMF (25.5%, p=0.011) groups compared to the NBF group (29.5%) (Table 1). Multivariate logistic regression was used to estimate the effect of BF on glucose intolerance in early postpartum, with adjustment for ethnicity, education, income, professional activity and pre-pregnancy BMI. The risk for glucose intolerance remained significantly lower in exclusively BF women compared to NBF women [adjusted OR of 0.7 (95% CI 0.5–1.0, p=0.0399)] (Table 3). The risk for glucose intolerance in the MMF group was no longer significantly lower compared to the NBF group after adjustment [adjusted OR of 0.7 (95% CI 0.4–1.2, p=0.2399)].
We show in a large cohort of more than 1000 women with a recent history of GDM that glucose intolerance was present in respectively 22.3% (BF), 25.5% (MMF) and 29.5% (NBF) of all participants, with a lower risk for glucose intolerance in the exclusively BF and MMF women compared to NBF women. However, the risk for glucose intolerance was no longer significantly lower in the MMF group after adjustment for confounders. Studies have previously demonstrated that breastfeeding is associated with improved insulin sensitivity and improved glucose tolerance (14). Breastfeeding is therefore an effective, low-cost intervention that can be easily applied after childbirth (15).
Our study provides novel data that also women who gave MMF had a lower rate of glucose intolerance compared to the NBF group, which suggests that even MMF might have a positive effect on the glucose levels. These data suggest therefore that when exclusive BF is not possible, MMF should also be stimulated in the first months of the postpartum period. However, after adjustment for confounders, the risk for glucose intolerance was no longer significantly lower in the MMF group compared to the NBF group. This might be due to the lower sample size in the MMF group and the lack of data on the long-term. Exclusive BF remains therefore preferable since the stronger association with a lower risk for glucose intolerance, as also demonstrated in our study. Research on the effects of MMF remain scarce. A previous study included only a small number of women who were undertaking mixed feeding; therefore a dose response relationship could not be established (17).
Our results also demonstrate that women who BF exclusively had lower glucose intolerance rates despite a lower HOMA-B index than the MMF and NBF groups (with also a lower non-significant HOMA-B in the MMF group compared to the NBF group). On the other hand, in our study the ISSI-2 index was higher in the BF group compared to the NBF group. ISSI-2 is in general considered to be a more accurate marker of β-cell function in women with GDM as it reflects an insulin resistance-adjusted insulin secretion (analogous to the disposition index obtained from the frequently sampled intravenous glucose tolerance test). Findings of a possible more impaired β-cell function (with lower HOMA-B) in women who exclusive BF, has also been reported in a prospective observational study in 106 mainly White women, between the 3rd and 5th month after the delivery (28). In this study, β-cell function was independently and negatively associated with BF and circulating prolactin concentrations. A recent meta-analysis confirmed that elevated prolactin was associated with lower beta-cell function and higher insulin sensitivity in the postpartum period. However, the direction of causality remains unclear (29). Lipolysis is also known to contribute to decreasing β-cell function (30). The pathophysiology of pregnancy and BF requires a certain β-cell plasticity, enabling adequate increase during pregnancy and immediate decrease in β-cell function postpartum. Reasons for not reaching this plasticity are mothers with obesity and high insulin resistance, but also lean mothers with impaired β-cell function because of genetic or aging reasons (28).
Compared to NBF women, BF and MMF women were more often from an non-White background. These results are consistent with results from previous studies indicating that non-White women had higher BF rates than women from a White origin (31). The BF group, compared to the NBF group, was more often higher educated, which is in line with previous studies that have shown that women with a Master’s degree (or higher) are more likely to start BF and also maintain BF for a longer period of time than mothers with primary studies (32–34). Pre-pregnancy BMI is also lower in our BF population compared to the NBF group. This can be explained by the fact that mothers with above-normal pre-pregnancy BMI are at increased risk of BF cessation (35). In addition, it has been demonstrated that women with underweight or obesity have significantly lower rates of BF initiation compared to women with normal pre-pregnancy BMI (36, 37). BF initiation in women with obesity may be lower due to a biological barrier, with a decreased hormonal response in lactation (38). A large population-based study from Florida, showed that women with overweight or obesity had a lower prolactin response to suckling. This can compromise the ability of women with overweight/obesity to produce milk and, over time, could lead to early cessation of lactation (36). In addition, also women with underweight were less likely to initiate breastfeeding (57, 58). Possible reasons might be maternal complications, insufficient milk supply, sucking problems and work resumption (39). It is therefore important to provide additional support to women with underweight or obesity to initiate and maintain breastfeeding.
Our results also showed lower fasting triglycerides in the MMF and BF group compared to the NBF group. This is in line with a study evaluating the lipid profile after 12 months postpartum among 79 predominantly Hispanic and socioeconomically disadvantaged women, indicating that each month increase in BF duration was associated with lower fasting glucose levels and lower triglycerides levels (17, 40). BF was also associated with less postpartum weight retention in our study, which has been confirmed in previous studies (41–43). BF may help mobilize fat stores built up during pregnancy, leading to weight loss, provided there is no compensatory increase in energy intake (44). Although BF may help some women lose weight, it cannot be generalized across all women who BF. This is due to the fact that women who BF often experience additional pressures, such as having to adapt to the needs of a newborn baby and recover from childbirth, difficulties in establishing feeding routines and baby sleep routines, which can affect the mother’s psychological health. A combination of these factors is likely to affect the ability to maintain a healthy lifestyle (45). More research is needed to assess the direct impact of BF on postpartum weight management on the long-term and to explore in-depth the reasons why not all women who BF lose weight (45–49).
Previous research has shown a decrease in both SBP and DBP during a BF session (50). Our study confirms these results, as there was also a decreased SBP and DBP in the MMF and exclusive BF groups compared to the NBF group. A study with over 400 Asian women, showed a lower SBP in BF women at one month postpartum compared with those using other feeding modalities (51). This suggests a direct effect of breastfeeding on maternal blood pressure. Animal studies suggest that oxytocin activates an “antistress” response that reduces cardiovascular stress reactivity, which may therefore lead to a lower BP (52). Studies in humans have shown that oxytocin, which is released when the baby starts suckling, has an anti-stress and blood pressure lowering effect (53–55).
A major strength of our study is that we used the data of two large multi-centric prospective cohort studies containing broad demographic, medical and obstetrical outcomes, as well as detailed postpartum characteristics. In addition, the same questionnaire was used to collected data on BF in both studies. The outcomes of this sub-analysis were adjusted for several confounding variables. Furthermore, we also included a large group of women with MMF, as the available data on the association with risk for glucose intolerance is very limited for this group. A first limitation of the study is that our population consisted mainly of White women. The results may therefore not to be applicable to other ethnic populations. Furthermore, this was a post-hoc analysis, with lack of longitudinal data, since data were only collected during pregnancy and at a mean of 12 weeks postpartum. A third limitation is that the questionnaire evaluating intensity and duration of breastfeeding was self-designed. Women had to indicate which of the three categories (BF, MMF or NBF) was most applicable to them. The degree of breastfeeding can however vary over time. We have no data on the exact dose of breastfeeding. In addition, for this analyses, we had to exclude women who did not have complete data on breastfeeding. However, the general characteristics of women not included in this analyses, were similar except for a lower rate of multiparity and slightly higher BMI compared to women included in the study.
In conclusion, our results indicate that exclusive BF and with a lesser degree also MMF, is associated with a lower risk of glucose intolerance in early postpartum in women with GDM. In addition, our results show that both BF and MMF are associated with a better metabolic profile in early postpartum. These data suggest therefore that when exclusive BF is not possible, MMF should also be stimulated in the first months postpartum. It is therefore important to support both BF and MMF by educating women already prenatally about the benefits of breastfeeding.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by Ethics Committee Research UZ/KU Leuven. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YV: Writing – original draft, Writing – review & editing. CMi: Writing – review & editing. HV: Writing – review & editing. PVC: Writing – review & editing. CMo: Writing – review & editing. JV: Writing – review & editing. RD: Writing – review & editing. SV: Writing – review & editing. HV: Writing – review & editing. CV: Writing – review & editing. TM: Writing – review & editing. ED: Writing – review & editing. NR: Writing – review & editing. CDB: Writing – review & editing. YJ: Writing – review & editing. FM: Writing – review & editing. KDC: Writing – review & editing. AVDB: Writing – review & editing. ALo: Writing – review & editing. IVP: Writing – review & editing. NM: Writing – review & editing. PA: Writing – review & editing. WV: Writing – review & editing. LL: Writing – review & editing. SD: Writing – review & editing. JB: Writing – review & editing. ChrM: Writing – review & editing. AB: Writing – review & editing. AL: Formal analysis, Writing – review & editing. CM: Writing – review & editing. KB: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The author(s) declare that the BEDIP-N study received funding from Belgian National Lottery, the Fund of the Academic studies of UZ Leuven, and the Fund Yvonne and Jacques François-de Meurs of the King Boudewijn Foundation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The author(s) declare that the MELINDA study received funding from research fund of UZ Leuven and by an unrestricted grant of Novo Nordisk. The following companies provided limited research grants: Sanofi, AstraZeneca, Boehringer-Ingelheim and Lilly. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
KB and RD are the recipients of a ‘Fundamenteel Klinisch Navorserschap FWO Vlaanderen’. We thank the research assistants, paramedics and physicians of all participating centers for their support and we thank all women who participated in the studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1374682/full#supplementary-material
1. American Diabetes Association Professional Practice Committee. 1. Improving care and promoting health in populations: standards of care in diabetes—2024. Diabetes Care (2024) 47(Supplement_1):S11–S19. doi: 10.2337/dc24-S001
2. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
3. Benhalima K, Leuridan L, Calewaert P, Devlieger R, Verhaeghe J, Mathieu C. Glucose intolerance after a recent history of gestational diabetes. Int J Endocrinol. (2014) 2014:1–9. doi: 10.1155/2014/727652
4. Inoue H, Ishikawa K, Takeda K, Kobayashi A, Kurita K, Kumagai J, et al. Postpartum risk of diabetes and predictive factors for glucose intolerance in East Asian women with gestational diabetes. Diabetes Res Clin Pract [Internet]. (2018) 140:1–8. doi: 10.1016/j.diabres.2018.03.031
5. O’Shea E, Awang MH, Kgosidialwa O, Tuthill A. Abnormal glucose tolerance in women with prior gestational diabetes mellitus: a 4-year follow-up study. Irish J Med Sci (1971 -) [Internet]. (2023) 192:641–8. doi: 10.1007/s11845-022-03005-x
6. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New Engl J Med. (2001) 344:1343–50. doi: 10.1056/NEJM200105033441801
7. Shubrook JH, Chen W, Lim A. Evidence for the prevention of type 2 diabetes mellitus. J Am Osteopathic Assoc. (2018) 118. doi: 10.7556/jaoa.2018.158
8. Sharma M, Purewal TS, Fallows S, Kennedy L. The low-risk perception of developing type 2 diabetes among women with a previous history of gestational diabetes: a qualitative study. Pract Diabetes. (2019) 36:15. doi: 10.1002/pdi.2204
9. Much D, Beyerlein A, Roßbauer M, Hummel S, Ziegler AG. Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol Metab. (2014) 3:284–92. doi: 10.1016/j.molmet.2014.01.002
10. Bollipo S, Pagali D, Korrapolu HB, Rahman MA. The first golden hour of breastfeeding: where do we stand? Int J Contemp Pediatr. (2018) 6:27. doi: 10.18203/2349-3291.ijcp20184688
11. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387:475–90. doi: 10.1016/S0140-6736(15)01024-7
12. Gunderson EP, Hurston SR, Ning X, Lo JC, Crites Y, Walton D, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus. Ann Intern Med. (2015) 163:889–98. doi: 10.7326/M15-0807
13. Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, et al. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. (2012) 61:3167–71. doi: 10.2337/db12-0393
14. Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM. Diabetes Care. (2012) 35:50–6. doi: 10.2337/dc11-1409
15. Dijigow FB, Paganoti C de F, da Costa RA, Francisco RPV, Zugaib M. Influência da amamentação nos resultados do teste oral de tolerância à glicose pós-parto de mulheres com diabetes mellitusgestacional. Rev Bras Ginecologia e Obstetrícia. (2015) 37:565–70. doi: 10.1590/SO100-720320150005488
16. O’Reilly M, Avalos G, Dennedy MC, O’Sullivan EP, Dunne FP. Breast-feeding is associated with reduced postpartum maternal glucose intolerance after gestational diabetes. Ir Med J. (2012) 105.
17. Shub A, Miranda M, Georgiou HM, McCarthy EA, Lappas M. The effect of breastfeeding on postpartum glucose tolerance and lipid profiles in women with gestational diabetes mellitus. Int Breastfeed J. (2019) 14:4. doi: 10.1186/s13006-019-0238-5
18. Benhalima K, Van Crombrugge P, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, et al. The Belgian Diabetes in Pregnancy Study (BEDIP-N), a multi-centric prospective cohort study on screening for diabetes in pregnancy and gestational diabetes: methodology and design. BMC Pregnancy Childbirth. (2014) 14:226. doi: 10.1186/1471-2393-14-226
19. Minschart C, Maes T, De Block C, Van Pottelbergh I, Myngheer N, Abrams P, et al. Mobile-based lifestyle intervention in women with glucose intolerance after gestational diabetes mellitus (MELINDA), A multicenter randomized controlled trial: methodology and design. J Clin Med. (2020) 9:2635. doi: 10.3390/jcm9082635
20. International Association of Diabetes and Pregnancy Study Groups. Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
21. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care. (2023) 46:S97–110. doi: 10.2337/dc23-S006
22. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33:S62–9. doi: 10.2337/dc10-S062
23. Matthys C, Meulemans A, Schueren B. Development and validation of general FFQ for use in clinical practice. Ann Nutr Metab. (2015) 67.
24. Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Activity. (2011) 8:19. doi: 10.1186/1479-5868-8-19
25. Dalfrà MG, Nicolucci A, Bisson T, Bonsembiante B, Lapolla A. Quality of life in pregnancy and post-partum: a study in diabetic patients. Qual Life Res. (2012) 21:291–8. doi: 10.1007/s11136-011-9940-5
26. Devlieger H, Martens G, Bekaert A, Eeckels R. Standaarden van geboortegewicht-voor-zwangerschapsduur voor de vlaamse boreling. Tijdschr Geneeskd. (2000) 56:1–14. doi: 10.47671/TVG.56.1.5000625
27. Rasmussen KM, Yaktine AL. Weight gain during pregnancy. Washington, D.C: National Academies Press (2009). Available at: https://pubmed.ncbi.nlm.nih.gov/20669500/.
28. Harreiter J, Vila G, Leitner K, Wattar L, Leutner M, Worda C, et al. Decreased beta-cell function in breastfeeding obese and non-obese women: A prospective observational study. Clin Nutr. (2019) 38:2790–8. doi: 10.1016/j.clnu.2018.11.035
29. Rassie K, Giri R, Joham AE, Mousa A, Teede H. Prolactin in relation to gestational diabetes and metabolic risk in pregnancy and postpartum: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13. doi: 10.3389/fendo.2022.1069625
30. DeFronzo RA. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. (2009) 58:773–95. doi: 10.2337/db09-9028
31. Griffiths LJ, Tate AR, Dezateux C. The contribution of parental and community ethnicity to breastfeeding practices: evidence from the Millennium Cohort Study. Int J Epidemiol. (2005) 34:1378–86. doi: 10.1093/ije/dyi162
32. Haas DM, Yang Z, Parker CB, Chung J, Parry S, Grobman WA, et al. Factors associated with duration of breastfeeding in women giving birth for the first time. BMC Pregnancy Childbirth. (2022) 22:722. doi: 10.1186/s12884-022-05038-7
33. Lechosa-Muñiz C, Paz-Zulueta M, Sota SM, de Adana Herrero MS, del Rio EC, Llorca J, et al. Factors associated with duration of breastfeeding in Spain: a cohort study. Int Breastfeed J. (2020) 15:79. doi: 10.1186/s13006-020-00324-6
34. Levinienė G, Tamulevičienė E, Kudzytė J, Petrauskienė A, Zaborskis A, Aželienė I, et al. Factors associated with breastfeeding duration. Medicina (Kaunas). (2013) 49:415–21. doi: 10.3390/medicina49090065
35. Martin H, Thevenet-Morrison K, Dozier A. Maternal pre-pregnancy body mass index, gestational weight gain and breastfeeding outcomes: a cross-sectional analysis. BMC Pregnancy Childbirth. (2020) 20:471. doi: 10.1186/s12884-020-03156-8
36. Thompson LA, Zhang S, Black E, Das R, Ryngaert M, Sullivan S, et al. The association of maternal pre-pregnancy body mass index with breastfeeding initiation. Matern Child Health J. (2013) 17:1842–51. doi: 10.1007/s10995-012-1204-7
37. Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr. (2014) 10:166–83. doi: 10.1111/j.1740-8709.2012.00439.x
38. Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. (2004) 113:e465–71. doi: 10.1542/peds.113.5.e465
39. Guelinckx I, Devlieger R, Bogaerts A, Pauwels S, Vansant G. The effect of pre-pregnancy BMI on intention, initiation and duration of breast-feeding. Public Health Nutr. (2012) 15:840–8. doi: 10.1017/S1368980011002667
40. Niu Z, Naya CH, Reynaga L, Toledo-Corral CM, Johnson M, Yang T, et al. Association of breastfeeding duration with 12-month postpartum blood lipids in a predominately lower-income hispanic pregnancy cohort in los angeles. Int J Environ Res Public Health. (2022) 19:3008. doi: 10.3390/ijerph19053008
41. Baker JL, Gamborg M, Heitmann BL, Lissner L, Sørensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. (2008) 88:1543–51. doi: 10.3945/ajcn.2008.26379
42. He X, Zhu M, Hu C, Tao X, Li Y, Wang Q, et al. Breast-feeding and postpartum weight retention: a systematic review and meta-analysis. Public Health Nutr. (2015) 18:3308–16. doi: 10.1017/S1368980015000828
43. Tahir MJ, Haapala JL, Foster LP, Duncan KM, Teague AM, Kharbanda EO, et al. Association of full breastfeeding duration with postpartum weight retention in a cohort of predominantly breastfeeding women. Nutrients. (2019) 11:938. doi: 10.3390/nu11040938
44. van Raaij J, Schonk C, Vermaat-Miedema S, Peek M, Hautvast J. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: reappraisal of the extra energy requirements of lactation. Am J Clin Nutr. (1991) 53:612–9. doi: 10.1093/ajcn/53.3.612
45. Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change—a systematic review and critical evaluation. Int J Obes. (2014) 38:577–90. doi: 10.1038/ijo.2013.132
46. Hollis JL, Crozier SR, Inskip HM, Cooper C, Godfrey KM, Harvey NC, et al. Modifiable risk factors of maternal postpartum weight retention: an analysis of their combined impact and potential opportunities for prevention. Int J Obes. (2017) 41:1091–8. doi: 10.1038/ijo.2017.78
47. Brandhagen M, Lissner L, Brantsaeter AL, Meltzer HM, Häggkvist AP, Haugen M, et al. Breast-feeding in relation to weight retention up to 36 months postpartum in the Norwegian Mother and Child Cohort Study: modification by socio-economic status? Public Health Nutr. (2014) 17:1514–23. doi: 10.1017/S1368980013001869
48. Martin JE, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. Predictors of post-partum weight retention in a prospective longitudinal study. Matern Child Nutr. (2014) 10:496–509. doi: 10.1111/j.1740-8709.2012.00437.x
49. Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes. (2003) 27:117–27. doi: 10.1038/sj.ijo.0802156
50. Jonas W, Nissen E, Ransjö-Arvidson AB, Wiklund I, Henriksson P, Uvnäs-Moberg K. Short- and long-term decrease of blood pressure in women during breastfeeding. Breastfeeding Med. (2008) 3:103–9. doi: 10.1089/bfm.2007.0031
51. Kashiwakura I, Ebina S. Influence of breastfeeding on maternal blood pressure at one month postpartum. Int J Womens Health. (2012) 333:334-7. doi: 10.2147/IJWH
52. Groer MW, Jevitt CM, Sahebzamani F, Beckstead JW, Keefe DL. Breastfeeding status and maternal cardiovascular variables across the postpartum. J Womens Health (Larchmt). (2013) 22:453–9. doi: 10.1089/jwh.2012.3981
53. Grewen KM, Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol Psychol. (2011) 87:340–9. doi: 10.1016/j.biopsycho.2011.04.003
54. Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, et al. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. (2001) 86:4798–804. doi: 10.1210/jcem.86.10.7919
Keywords: breastfeeding, mixed milk feeding, postpartum glucose intolerance, gestational diabetes mellitus, impaired beta-cell function, insulin resistance
Citation: Vanlaer Y, Minschart C, Vrolijk H, Van Crombrugge P, Moyson C, Verhaeghe J, Devlieger R, Vandeginste S, Verlaenen H, Vercammen C, Maes T, Dufraimont E, Roggen N, De Block C, Jacquemyn Y, Mekahli F, De Clippel K, Van Den Bruel A, Loccufier A, Van Pottelbergh I, Myngheer N, Abrams P, Vinck W, Leuridan L, Driessens S, Billen J, Matthys C, Bogaerts A, Laenen A, Mathieu C and Benhalima K (2024) Impact of breastfeeding on risk of glucose intolerance in early postpartum after gestational diabetes. Front. Endocrinol. 15:1374682. doi: 10.3389/fendo.2024.1374682
Received: 22 January 2024; Accepted: 23 May 2024;
Published: 12 June 2024.
Edited by:
Wei Ge, University of Macau, ChinaReviewed by:
Jami Josefson, Ann & Robert H. Lurie Children’s Hospital of Chicago, United StatesCopyright © 2024 Vanlaer, Minschart, Vrolijk, Van Crombrugge, Moyson, Verhaeghe, Devlieger, Vandeginste, Verlaenen, Vercammen, Maes, Dufraimont, Roggen, De Block, Jacquemyn, Mekahli, De Clippel, Van Den Bruel, Loccufier, Van Pottelbergh, Myngheer, Abrams, Vinck, Leuridan, Driessens, Billen, Matthys, Bogaerts, Laenen, Mathieu and Benhalima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yana Vanlaer, eWFuYS52YW5sYWVyQGt1bGV1dmVuLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.