
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 10 May 2024
Sec. Endocrinology of Aging
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1374027
This article is part of the Research TopicEndocrinology of Obesity, Aging and StressView all 16 articles
Background: Aging has been implicated in many chronic inflammatory diseases, including periodontitis. Periodontitis is an inflammatory disease caused by long-term irritation of the periodontal tissues by the plaque biofilm on the surface of the teeth. However, only a few bibliometric analyses have systematically studied this field to date. This work sought to visualize research hot spots and trends in aging associated with periodontitis from 2002 to 2023 through bibliometric approaches.
Methods: Graphpad prism v8.0.2 was used to analyse and plot annual papers, national publication trends and national publication heat maps. In addition, CtieSpace (6.1.6R (64-bit) Advanced Edition) and VOSviewer (version 1.6.18) were used to analyse these data and visualize the scientific knowledge graph.
Results: The number of documents related to aging associated with periodontitis has steadily increased over 21 years. With six of the top ten institutions in terms of publications coming from the US, the US is a major driver of research in this area. journal of periodontology is the most published journal in the field. Tonetti MS is the most prolific authors and co-cited authors in the field. Journal of Periodontology and Journal of Clinical Periodontology are the most popular journals in the field with the largest literature. Periodontitis, Alzheimer’s disease, and peri-implantitis are current hot topics and trends in the field. Inflammation, biomarkers, oxidative stress cytokines are current research hotspots in this field.
Conclusion: Our research found that global publications regarding research on aging associated with periodontitis increased dramatically and were expected to continue increasing. Inflammation and aging, and the relationship between periodontitis and systemic diseases, are topics worthy of attention.
Periodontitis is an inflammatory disease resulting from infection with periodontal pathogenic microorganisms and dysregulation of the host immune system (1). More than 50% of adults worldwide are affected by different degrees of periodontitis (2, 3). Periodontitis is classified as mild, moderate or severe depending on the degree of inflammation of the disease (4). Severe periodontal tissue damage can lead to aesthetic complications that can be very disturbing to the patient, and new treatment techniques such as laser therapy and digital technology can improve patients’ quality of life (5, 6). Aging underlies the pathogenesis of a range of systemic diseases and has a critical effect on the development of chronic inflammatory diseases (7), such as atherosclerosis and autoimmune (8, 9). Numerous studies have shown a close connection between periodontitis and aging (10–12). During the development of periodontitis, the periodontal tissues are attacked by a large number of free radicals, which intensifies oxidative stress and causes oxidative damage to DNA, consequently accelerating telomere shortening. Shortened telomere length can exert cytotoxic effects, leading not only to disruption of epithelial connective tissue continuity, but also to interference with cell growth and differentiation processes. At the same time, persistent stimulation of bacterial-derived lipopolysaccharide affects cellular senescence of osteoblasts, driving alveolar bone resorption (13, 14). In conclusion, Aging may be one of the key risk factors that promote periodontitis progression.
With time changes as well as functional deterioration, the organism develops a number of chronic and age-related conditions. This process is defined as aging (15). Aging affects normal metabolism as well as promotes the progression of inflammatory responses, disrupting bone remodeling and leading to increased levels of bone resorption (16). In addition, aging impairs the organism’s immune system, mainly through damaging the physiological function of immune cells and weakening the immune effects of biomolecules. This causes a series of processes of immune senescence, immune activation, and inflammatory responses that ultimately result in older adults being more susceptible to autoimmune, and inflammatory diseases. Decreased immune responsiveness with chronic inflammation, which may accelerate the disease process (17–19). Periodontitis is a chronic inflammatory condition related to alterations in the oral microbiota (20). Notably, localized microecological dysregulation in the oral cavity is often caused by a high-inflammatory state in the host (21). Given that aging is related to a low-level “sterile” inflammatory state with no apparent infection, it is suggested that aging may influence the underlying process of periodontitis (22).
Bibliometrics allows for retrospective reviews to discover the relevance of data and make predictions for the future (23). Bibliometrics is a common method of measuring the academic impact of scientific and technical papers, and a means of demonstrating and encouraging emerging scholarship (24). Bibliometrics and visualization analysis can not only effectively integrate information and enhance understanding of research activities, but also analyze research hotspots and future trends in a certain field (25). In recent decades, Bibliometrics has been broadly used in the field of medicine, including obstetrics and gynecology, orthopedics, complementary medicine, and alternative medicine. It has promoted the development of medical research and clinical practice (26). However, the application of bibliometrics in dentistry is still limited at present, and there is a gap in the study of periodontitis related to aging. For this reason, this work intends to systematically sort out the aging research related to periodontitis, summarize the existing research results at home and abroad, assess their academic impact and characteristics, and provide new design ideas for further research.
Web of Science Core Collection(WoSCC)has better accuracy in labeling literature types than any other database and is considered the best choice for literature analysis, therefore we chose to search in this database. We searched WOS for all articles related to periodontitis and aging from January 1, 2002 to October 25, 2023 with the following search formula (Figure 1): (((TS=(aging)) OR TS=(Senescence)) OR TS=(Biological Aging)) OR TS=(Aging, Biological) AND (((TS=(periodontitis)) OR TS=(Periodontitides)) OR TS=(Pericementitis)) OR TS=(Pericementitides) (Supplementary Table S1). Literature selection inclusion criteria for this study were as follows: (1) the full text of publications related to periodontitis and aging; (2) the articles and reviews manuscript category were in English; and (3) the article was published between January 1, 2002, and October 25, 2023. The criteria for exclusion were as follows (1) the topic was not related to periodontitis and aging, and (2) the article was a conference abstract, news, or briefing paper. We exported the plain text version of the paper. Graphpad prism v8.0.2 was used to analyze and plot annual papers, national publication trends, and national publication heat maps. In addition, CtieSpace (6.1.6R (64-bit) Advanced Edition) and VOSviewer (version 1.6.18) were used to analyze these data and visualize the scientific knowledge graph. VOSviewer v.1.6.17, created by Waltman et al. in 2009, is a free JAVA-based software for analyzing large amounts of literature data and displaying it in a map format. In order to visualize the results of research in a particular field by mapping the literature co-citation network, Professor Chaomei Chen created the CiteSpace (6.1.6R) software, which envisions the use of an experimental framework for studying new concepts and evaluating existing technologies. This enables users to better understand areas of knowledge, research frontiers and trends, and to anticipate their future research progress.
The results showed that from January 1, 2002 to October 25, 2023, the WoSCC database contained a total of 3,240 publications on periodontitis and aging-related literature, including 2,339 (%) articles and 901 reviews (%). The literature covers 120 countries and regions, 3807 institutions and 19263 authors. From 2002 to 2004, the number of articles per year was less than 100, suggesting that the field was not noticed, and after 2005 the number of articles had a yearly increase, which increased rapidly after 2017 and reached the highest value in 2022. It indicates that the correlation between aging and the progression of periodontitis is receiving widespread attention after 2017 (Figure 2A).
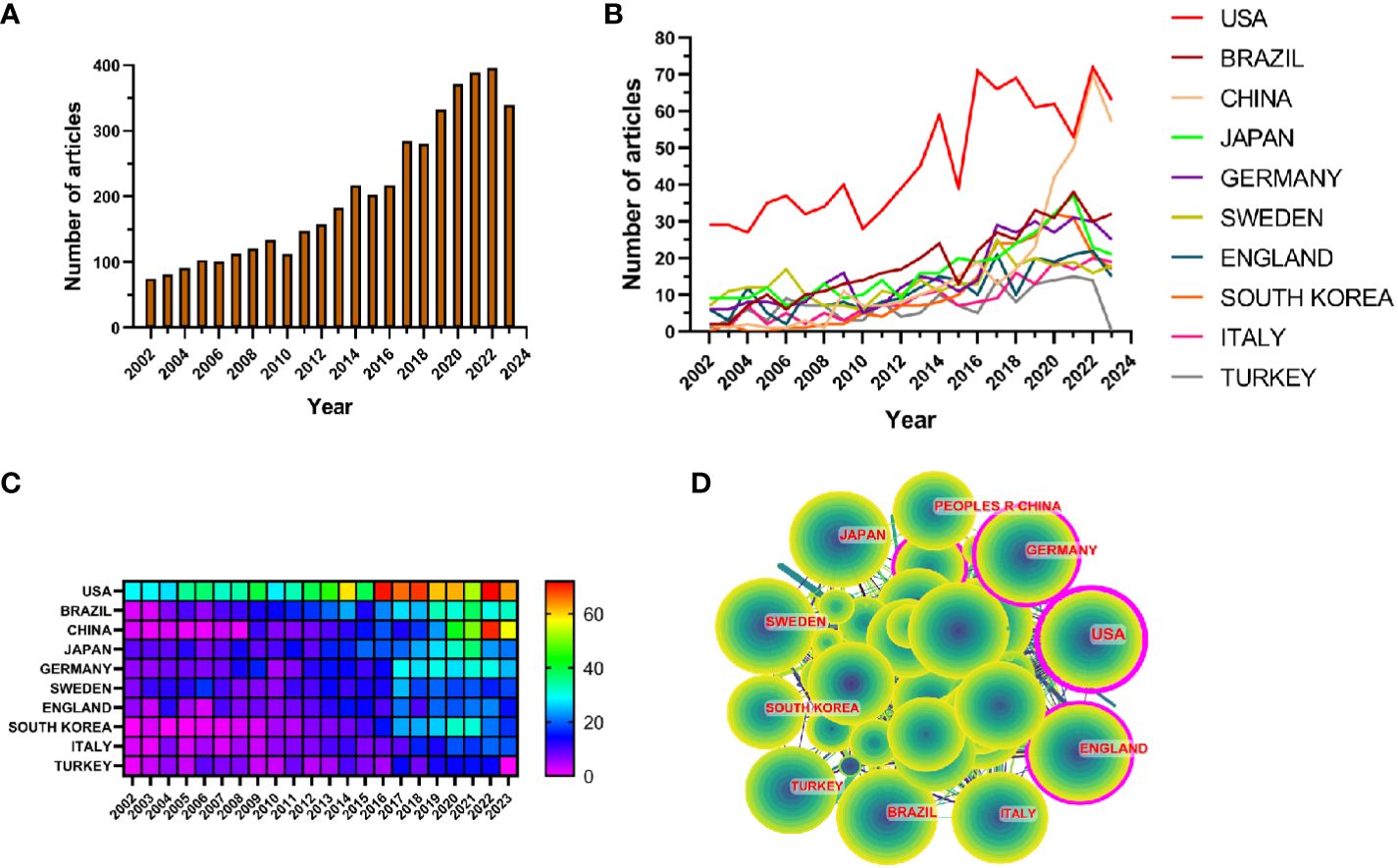
Figure 2 (A) Line graph of the volume of communications (B) Heat map of national issuances (C) Line graph of national issuances (D) Map of country cooperation network.
Research related to periodontitis and aging has been conducted in 120 countries and regions. Figures 2B, C show the heat map and line graph of the annual publication volume of the top 10 countries during the past 20 years, and the top 5 countries with the highest publication volume in this field are the United States, Brazil, China, Japan, and Germany. The U.S. accounts for 23.02% of the total number of papers published, which is far more than other countries.
Research related to periodontitis and aging has been conducted in 120 countries and regions. Figures 2B, C show the heat map and line graph of the annual publication volume of the top ten countries during the past 20 years. The top five countries with the highest publication volumes are the United States, Brazil, China, Japan and Germany. The percentage of papers published in the United States is 23.02%, far exceeding that of other countries.
The number of citations for papers published in the United States is 41,954 (Supplementary Table S2), significantly surpassing all other countries/regions. Additionally, the citation/publication ratio (40.97%) places the United States as the second highest globally. Although China has the third highest number of publications, its citation/publication ratio is only 17.26%, the lowest among the ten countries, which suggests that the quality of China’s publications is not high. Despite a significant difference in the number of publications compared to the United States, the citation/publication ratio for the United Kingdom (56.03%) ranks first globally. This indicates the superior quality of materials published from the UK. The network of cooperation is shown in Figure 2D, with close cooperation between the US, the largest producer, and Korea and Japan. The US also has cooperative relationships with countries such as China, Germany and the UK. From the heat map of paper publications, it can be observed that since 2002, the United States has published a greater number of articles. However, the number of articles published by China has surged in recent years and caught up with the United States by 2021. The United States not only has a significantly larger number of publications compared to other countries, but its centrality value also reached 0.46, indicating a leadership role in the development of the field. The remaining countries are still in the process of development in this field.
3807 organizations systematically published articles related to periodontitis and aging. Among the top 10 institutions with the highest number of publications, six are from the United States, two from Switzerland, one from Finland and one from South Korea. Karolinska Instiv has published the most literature in this field (116 papers, 3,351 citations, 21.86 citations per paper). Univ Helsinki (105 papers, 3,315 citations, 57.56/paper) ranked second and Univ Washington (86 papers, 8,051 citations, 16.37/paper) ranked third (Figure 4A). It can be seen that institutions between countries prefer to cooperate with their own domestic institutions and there is a lack of international cooperation, so we call for the strengthening of cooperation between domestic and foreign institutions to break down academic barriers.
The top 10 journals with the highest number of publications are presented in Supplementary Table S3 and Figure 3A. The Journal of Periodontology (522 articles, 11.74%) is the most published journal in this field. It was followed by journal of clinical periodontology (461 articles, 10.36%),journal of periodontal research (166 articles, 3.73%)和clinical oral investigations (138 articles, 3.10%). Among the 10 most prolific journals, journal of dental research had the highest IF of 7.6. 50% of the journals are categorized as Q1 and the remaining 50% are categorized as Q2.
The impact factor of a journal is determined by the frequency with which it is cited and reflects the significant impact the journal has had on the scientific community. The most cited journal was J PERIODONTOL (3601), followed by J CLIN PERIODONTOL(3466)and J DENT RES (2711) (Supplementary Table S5 and Figure 4B). Among the top 10 most co-cited journals, LANCET was cited 1062 times and had the highest impact factor (168.9). And 60% of the co-cited journals were first quarter journals.
The thematic distribution of scholarly publications is shown by a double map overlay (Supplementary Figure S1). The colored tracks indicate citation links, with the citing journal on the left and the cited journal on the right. After analyzing the data, we have identified three primary citation paths, each color-coded for clarity. (1) Research published in molecular/biology/immunology journals is predominantly cited by studies in molecular/biology/genetics and health/nursing/medicine journals. (2) Research reports in medicine/health/clinical research receive the most citations from journals in molecular/biological/genetics, health/nursing/medicine, and dermatology/dentistry/surgery fields. (3) Research reports in the dentistry/dental/surgery field are cited by journals primarily in the medical/immunology field.
The top 10 authors with the highest number of publications related to periodontitis and aging are shown in Table S6 and Figure 3B. These authors have published a total of 276 papers, accounting for 6.21% of the total in the field. Kocher, Thomas (50 papers) published the most research papers, following papapanou, panos n. (31 papers) and holtfreter, birte (28 papers).
Figure 5A and Supplementary Table S1 respectively show the top 10 authors with the highest number of total citations and the highest number of total citations. 78 authors have a total of more than 100 citations, demonstrating the high reputation and impact of their research. The largest nodes are associated with the most co-cited authors, including TONETTI MS (698 citations), EKE PI (672 citations), and PAGE RC (534 citations).
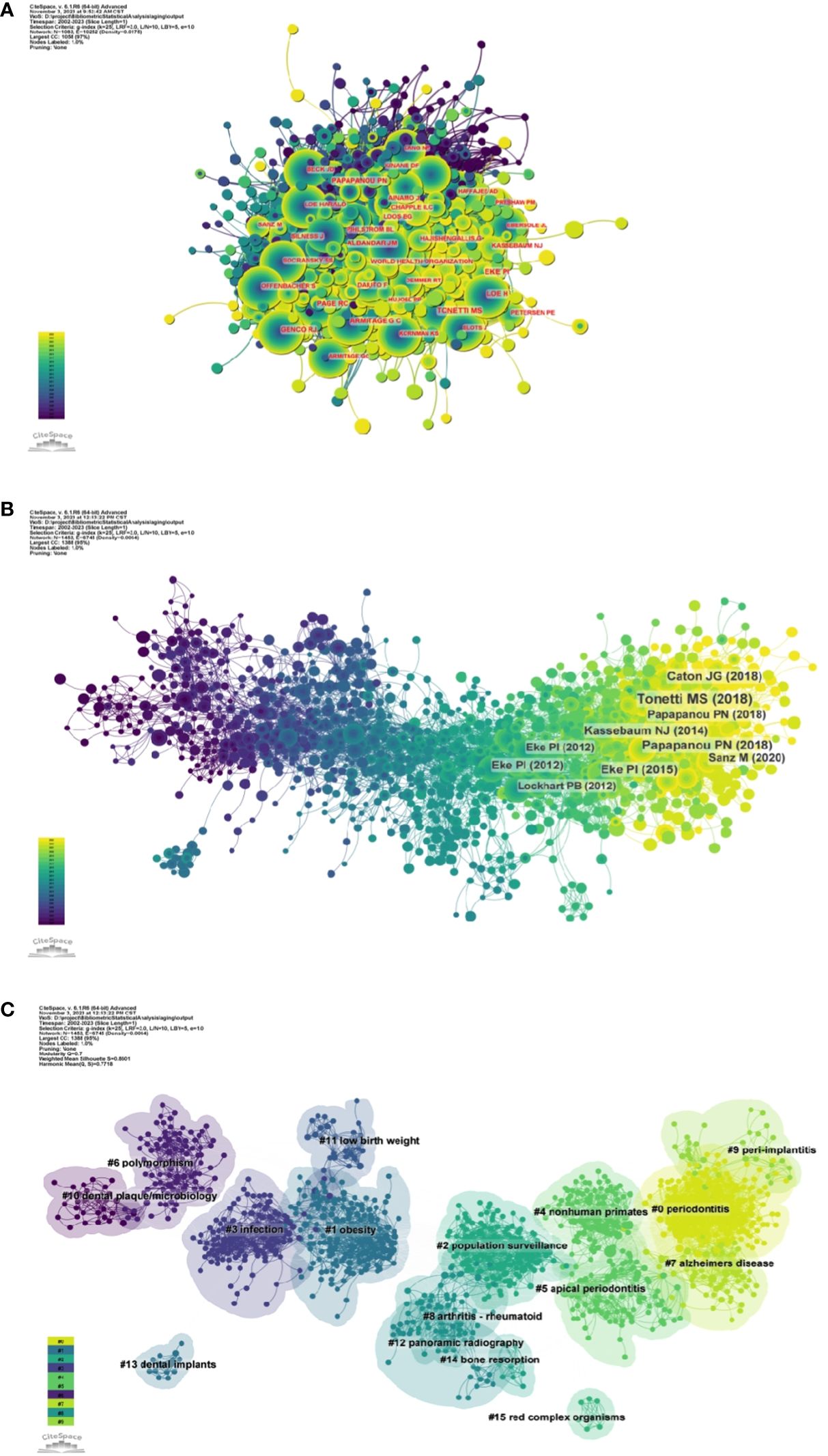
Figure 5 (A) Author co-citation network map. (B) Network diagram of co-cited literature. (C) Clustering map of co-cited literature.
Using a ten-year time slice with a time frame of 2013 to 2023, the co-cited reference network had 1453 nodes and 6745 links (Figure 5B). According to the top 10 most co-cited articles, the JOURNAL OF PERIODONTOLOGY (IF=6.7) entitled “Staging and grading of periodontitis: framework and proposal of a new classification and case definition” (12) was the most co-cited reference with Tonetti MS as the first author. We found that most of the 10 most co-cited articles were foundational literature related to periodontitis and aging.
We performed co-citation reference clustering and timeline analysis (Figures 5C, 6). We found that obesity (cluster1), infection (cluster3), polymorphism (cluster6), dental plaque/microbiology (cluster10), and low birth weight (cluster 11) were the population surveillance (cluster2), nonhuman primates (cluster4), apical periodontitis (cluster5), and arthritis-rheumatoid (cluster8), panoramic radiography (cluster12), dental implants (cluster13), and bone resorption (cluster14) are the hotspots of research in the mid-term, while periodontitis (cluster0), alzheimers Periodontitis (cluster0), alzheimers disease (cluster7), and peri-implantitis (cluster9) are the current hot topics and trends in this field.
By analyzing keywords, we can get a quick overview of a field and its direction. According to the co-occurrence of keywords in VOSwiever, the most popular keyword was PREVALENCE (747), followed by RISK (640), INFLAMMATION (600), and HEALTH (489) (Table 1 and Figures 7A, B). We constructed a network containing 217 keywords with at least 30 occurrences (Figure 8), yielding a total of 6 different clusters. Cluster 1 (red) had 76 keywords including inflammation, biomarkers, chronic periodontitis, oxidative stress, peophyromonas-gingivalis, subgingival microbiota, bone loss, cytokines. group 2 (green) has 67 keywords, including prevalence, epidemiology, tooth loss, oral health, global burden, risk-factors, nutrition. group 3 contains 32 keywords (blue), including therapy, dental implants. Includes therapy, dental implants, alveolar bone loss, diagnosis, radiography, apical periodontitis, surgery, management Group 4 contains 26 keywords (in yellow) and includes risk, diabetes, metabolic syndrome, blood pressure, insulin-resistance, c-reactive protein, markers, glycemic control. group 5 contains 11 keywords (purple), including obesity, risk factors, weight, overweight, body-mass-index. group 6 contains 5 keywords (sky blue), including cigarette-smoking, smokers, tobacco smoking, and young-adults. a volcano map to visualize the research hotspots over time (Figure 7C).
Through CiteSpace, we derived the 50 most reliable citation bursts related to the field of periodontitis and aging (Figure 8). One of them, “Staging and grading of periodontitis: framework and proposal of a new classification and case definition” by Maurizio S. Tonetti (13), the reference with the highest burst intensity (61.64). Forty-eight of the 50 references were published between 2002 and 2023, indicating that these papers have been cited frequently over the past 20 years and that research on periodontitis and aging will remain of interest in the future.
Among the 50 strongest burst keywords in the field, we focused on those that will still be mutating in 2023 (Figure 9), including “cohort study” (7.38 burst intensity), “global burden” (16.64 burst intensity), peri implant disease (24.76 burst intensity), oral microbiome (7.8 bursts), prevention (6.89 bursts), public health (6.82 bursts), classification (55.8 bursts), consensus report (26.23 bursts), 2017 world workshop (14.47 burst intensity), workshop (9.3 burst intensity), cellular senescence (6.95 burst intensity), cary (6.66 burst intensity), oral microbiota (6.55 burst intensity), and periapical lesion (6.33 burst intensity). (6.33 burst intensity).
In this work, we assessed research trends and hot topics in the field of aging and periodontitis over the last 20 years through bibliometric analysis. We searched 2,339 articles and 901 reviews published from January 1, 2002, to October 25, 2023, respectively. During that research phase, only a few papers were published in the field prior to 2004. The number of papers has increased every year since 2005, rapidly increasing after 2017 and peaking in 2022, indicating that the field is growing rapidly and maintaining strong research interest. Therefore, we may speculate that research related to aging and periodontitis remains a hot topic for the future.
In the field of periodontitis and aging research, papers published in the United States were cited 41,954 times, far exceeding all other countries/regions, and it also had the second highest citation/number of papers ratio (40.97%). A Although China has the third largest number of papers in this field in the world, its citation/publication ratio is only 17.26%, indicating that the quality of Chinese publications still needs improvement. It is worth noting that although the UK has a low number of publications, its citation/publication ratio (56.03%) is among the highest in the world, indicating the high quality of the material it publishes. The collaboration network indicates close cooperation between the United States, South Korea, and Japan, with the highest number of published papers. According to the heat map, it can be seen that in recent years, the U.S. has had a higher volume of publications since 2002, while China has seen a surge in publications in recent years and is gradually catching up with the U.S. by 2021. The United States not only has a much higher number of publications than other countries but also has a centrality score of 0.46, indicating its leading position in this field. The rest of the countries are far less than the United States, indicating that these countries are still in the process of development in this field.
In terms of institutions, 3807 institutions systematically published articles related to periodontitis and aging. Among the 10 institutions with the highest number of publications, six are from the United States, two from Switzerland, one from Finland, and the other from South Korea. Karolinska Instiv had the most publications in this area (116 papers, 3351 citations, 21.86 citations per paper). Univ Helsinki (105 papers, 3315 citations 57.56 citations/paper) ranked second and Univ Washington (86 papers, 8051 citations, 16.37 citations/paper) ranked third. It can be seen that institutions between countries prefer to cooperate with their own domestic institutions, and there is a lack of international cooperation, so we call for the strengthening of cooperation between domestic and foreign institutions to break down academic barriers. Among all the authors who have published literature related to periodontitis and aging, Table 2 and Figure 5B list the 10 authors who have published the most papers. The top 10 authors have collectively published 276 papers, accounting for 6.21% of all papers in this field. kocher, thomas (50 papers) published the most research papers, following by papapanou, panos n. (31 papers) and holtfreter, birte (28 papers). According to the top 10 most co-cited articles (Table 3), the JOURNAL OF PERIODONTOLOGY (IF=6.7) entitled “Staging and grading of periodontitis: framework and proposal of a new classification and case definition” (18) was the most co-cited reference, demonstrating the importance of this journal among periodontal journals, with Tonetti MS as the first author, demonstrating his centrality to the field. We found that most of the 10 most co-cited articles were foundational literature related to periodontitis and aging.
In bibliometric analyses, keyword bursts usually reflect hot topics in the research field (83), and keyword volcano maps can show the evolution of new research hotspots (84, 85). We found that obesity, infection, polymorphism, dental plaque/microbiology, and low birth weight were early research hotspots, population surveillance, nonhuman primates, apical periodontitis, arthritis-rheumatoid, panoramic radiography, dental implants and bone resorption are mid-term hotspots. While periodontitis, alzheimers disease and peri-implantitis are current hot topics and trends in the field. In periodontitis, a large accumulation of microorganisms leads to the recruitment of polymorphonuclear neutrophils to the lesion site within the periodontal pocket. As the first line of defense against pathogens, the recruitment of neutrophils is influenced by various factors, including immune cell cytokines and chemotactic factors (86, 87). Early keyword hotspots reflect the exploration of periodontitis and the mechanisms of aging, and current keywords in the field reflect the aging phenomenon in which the possible link between disease and illness in the aging process and how to prevent and treat it will become a hotspot.
The analysis of co-occurring keywords indicates a close relationship between periodontitis and aging, consistent with previous research findings in recent years (88–90). Aging may trigger a decline in both innate and adaptive immune functions in the body, as well as alterations in the functional efficacy of effector biomolecules, leading to immune aging, immune activation, and inflammatory processes. This may be one of the plausible mechanisms by which aging may lead to increased susceptibility to periodontitis (91). Notably, the in vivo microenvironment associated with aging may additionally increase the complexity of defects in innate immunity and adaptive function (92–94). In addition, the close relationship between aging and bone metabolism was analyzed by keywords. Many immune and non-immune cells in periodontal tissue interact with osteoblasts and osteoclasts, which can regulate the balance of physiological bone formation and bone resorption (95, 96). Therefore, bone metabolism is regulated by various factors, including aging. In particular, aging disrupts the balance between bone remodeling and metabolism, leading to increased bone resorption, changes in bone structure, and decreased fracture resistance (16).
Based on results from keyword co-occurrence analysis, we also identified several terms related to systemic diseases, such as Alzheimer’s disease, atherosclerosis, and rheumatoid arthritis. These findings are consistent with recent research trends regarding the association between periodontitis and systemic diseases. These complex, multi-factorial diseases share common characteristics with periodontitis, including accelerated aging (97–99).
In recent years, Hajishengallis found that periodontitis can lead to dysbiosis, which is a serious risk to human health. In addition, localized treatment of periodontitis may serve as an additional indicator for the reduction of systemic inflammation and concomitant diseases (100, 101). There is substantial evidence suggesting that periodontitis may be influenced by systemic low-grade inflammation (102). Generally, periodontitis shows a bidirectional relationship with systemic inflammatory diseases. Aging is considered as a common factor that may explain the pathophysiological mechanisms underlying various inflammatory diseases (103–105). Therefore, aging may be a critical therapeutic target for inflammatory diseases.
Furthermore, citespace’s keyword exploration also helps to reveal future trends. We conclude that macrophage imbalance, oxidative stress may be current research hotspots. Meanwhile, pyroptosis and TLR9 may be potential research directions and targets.
Macrophages, as part of the innate immune system, possess high plasticity. When exposed to local stimuli, macrophages can exhibit various active states, which are classified into 2 major subsets, depending on the stimuli: classically activated (M1) and alternatively activated (M2) (106–108). Dysfunction of macrophages has been recognized as a key factor in periodontitis. Understanding the roles of different macrophage phenotypes contributes to the comprehension of potential mechanisms underlying periodontitis and aids in the development of new therapeutic strategies. Pro-inflammatory M1-like macrophages and reparative M2-like macrophages play an important role in inflammation and tissue homeostasis in periodontitis. Periodontal pathogens can induce the differentiation of macrophages into M1 type by stimulating CD14, Toll-like receptors (TLRs), and NOD-like receptors (NLRs) on the surface of macrophages with LPS. M2 macrophages, on the other hand, exert anti-inflammatory effects and play a role in wound healing and tissue repair. In the early stages of periodontitis, M1 macrophages dominate the infiltration of periodontal tissues, and the proportion of M1 is positively correlated with the progression of periodontal inflammation. There is evidence suggesting an increase in the abundance of M1 macrophages and a decrease in M2 counts in periodontal tissues from patients with periodontitis compared to healthy controls (109–112). During the progression of periodontitis, on one hand, periodontal pathogens may induce an excessive inflammatory response by modulating metabolic pathways to skew macrophage polarization towards M1, while on the other hand, sites of alveolar bone destruction accumulate large numbers of M1 macrophages, leading to the production of IL-1β and TNF-α, upregulation of RANKL expression, and increased bone resorption (113, 114). However, different studies have shown that no alterations in the M1/M2 ratio in periodontal tissues from patients with periodontitis (115, 116). Furthermore, the quantity of M1 macrophages notably decreases during the periodontitis stage. Therefore, in the pathogenesis of periodontitis, it is likely that the roles of M1 pro-inflammatory macrophages and M2 anti-inflammatory macrophages are not as straightforward as previously thought. They exist in a dynamic equilibrium, and disruption of this balance can lead to persistent inflammation (117, 118). During the natural healing process of periodontitis, M1 macrophages are capable of phagocytosing microorganisms and matrix debris. They exhibit a high antigen-presenting capacity during the early stages of healing. Consequently, the number of M1 macrophages increases in the early healing stages and then rapidly declines thereafter. M2 macrophages, on the other hand, peak in numbers during the later stages of healing (118–120). By producing IL-10 and reducing the expression of IL-6, M2 macrophages regulate the functions of Th17 and Treg cells, thereby influencing their anti-inflammatory and reparative properties (121, 122). In addition, the polarization of macrophages also undergoes changes with age. Research indicates that while there is no significant difference in the quantity of macrophages between young and old mice, the expression of M1-related markers, pro-inflammatory cytokines, and chemokines significantly increases in aged mice.[ (112, 123) Therefore, the aging immune system may play a crucial role in alveolar bone loss (124). Interventions targeting the aging immune system could emerge as a novel approach to managing periodontitis in elderly patients. Currently, the standard treatment for periodontitis typically involves mechanical removal of disrupted biofilms to control inflammation (125). However, this approach doesn’t always successfully halt the progression of the disease. Hence, exploring additional therapeutic targets contributes to the advancement of periodontal treatment. While therapeutic modulation of macrophage phenotypes has not yet been utilized in the treatment of periodontitis patients, inhibiting macrophage polarization to the M1 type or increasing the numbers and ratio of M2 macrophages may alleviate the progression of periodontitis. Therefore, the transition of macrophage phenotypes could potentially serve as a promising therapeutic target for periodontitis treatment, holding significant promise (Table 4).
Oxidative stress is an integral part of the pathogenesis of periodontitis and is the result of an imbalance between the oxidative and antioxidant systems in the body, an over-oxidized state caused by an overproduction of ROS and/or a lack of antioxidant defenses (126). A causal relationship between ROS-mediated oxidative stress and periodontal disease has been demonstrated (127, 128). Studies have shown that the serum levels of superoxide are significantly higher in periodontitis patients, especially in chronic periodontitis, than in healthy individuals. In addition, the levels of malondialdehyde (MDA), a biomarker of lipid peroxidation, and 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative DNA damage, were significantly elevated in saliva and gingival sulcus of patients with chronic periodontitis compared with healthy periodontal tissues (3, 129). Oxidative stress induced by the antimicrobial response during periodontitis may be an important cause of tissue damage. ROS play an important role in the pathomechanism of periodontitis.Gram-negative anaerobes colonizing dental plaque trigger the recruitment and activation of neutrophils, which subsequently produce a range of antimicrobial factors during phagocytosis of periodontal pathogens and release an excess of ROS through the NADPH oxidase pathway (130, 131). During phagocytosis, free radicals are the end-products of functional roles played by the mitochondria of polymorphonuclear neutrophils, mainly through lipid peroxidation (132–134). This leads to an oxidative imbalance that triggers a pro-inflammatory mechanism, which in turn promotes osteoclastogenesis, thus leading to alveolar bone resorption in patients with periodontitis (127, 135–139). In addition, reactive oxygen species affect Nuclear factor-erythroid 2-related factor 2 (Nrf2),Nrf2 down-regulation is associated with the progression of periodontitis, and finally, through direct damage to extracellular connective tissues (in addition to the bone itself), the production of reactive oxygen species is responsible for the loss of attachment that leads to periodontal destruction (140, 141).At the same time, reactive oxygen species production activates the NF-κB signaling pathway, which mediates the phosphorylation and degradation of NF-κB inhibitor a of NF-κB (IκBα) and the nuclear translocation of P65, a subunit of NF-κB, which promotes proinflammatory cytokines (such as interleukin-1, interleu-kin-6, and tumor necrosis factor-alpha), which can act directly or indirectly on periodontal tissues to cause bone resorption (142–144).In addition, increased reactive oxygen species disrupt the homeostatic relationship between bone formation and resorption via the RANKL/osteopro-tegerin axis, and thus oxidative stress-mediated inflammation may be one of the pathways leading to the development of periodontal disease. This means that conventional prophylaxis and treatment focusing on bacterial infection-mediated periodontal disease seems to be insufficient, especially when initial periodontal treatment fails to alleviate inflammation, and antioxidant scavenging of ROS to reduce overburdened inflammation is considered to be an effective way to stop the progression of periodontitis (Table 5).
Pyroptosis, a pro-inflammatory programmed cell death dependent on the Gasdermin family of proteins, plays an important role in the regulation of peridontal environmental homeostasis (145). Compared to apoptosis, pyroptosis occurs more rapidly as the cell swells until the membrane ruptures, releasing cellular contents and activating a strong inflammatory response (146, 147). Inflammatory mediators can cause collagen degradation and bone matrix resorption (148), causing destruction of periodontal hard and soft tissues. The initiation of cellular pyroptosis is dependent on the activation of intracellular inflammatory vesicles and their downstream cysteinyl aspartate-specific proteases1 (caspase-1), as well as the production of active fragments of the key pyroptosis protein Gasdermin. The invasion of periodontal pathogens induces an inflammatory response in the host, the molecular mechanism of which involves the activation of multiple inflammatory vesicles and triggers cellular pyroptosis, as well as the release of a large number of inflammatory factors, such as interleukin (IL)-1β, IL-18, and others, which mediate the destruction of periodontal tissues (149). The inflammatory response of periodontopathogenic bacteria in the periodontium is characterized by the release of a wide range of inflammatory factors. There is growing evidence that focal death plays a role in periodontitis pathology, such as Porphyromonas gingivalis, which can induce an inflammatory response through focal death. CASP4/GSDMD triggered by bacterial LPS leads to focal death of periodontal ligament stem cells in periodontitis patients (150). In the early stages of inflammation, gingival epithelial cell pyroptosis disrupts the epithelial barrier by interfering with intercellular junctions. When inflammation progresses, cell pyroptosis can further cause gingival fibroblast death, impaired migration of periodontal fibroblasts, impaired migration of osteoblasts, and active osteoclasts, which will be macroscopically manifested as loss of attachment and alveolar bone resorption. At the same time, the cellular contents released by pyroptosis, such as inflammatory factors and mtROS, can stimulate the production of MMP, causing collagen degradation in gingival and periodontal tissues. In summary elevated levels of focal death in periodontitis promote the secretion of active inflammatory factors (IL-1β, IL-18), which amplifies the inflammatory response and leads to an overactive immune response; this ultimately reduces bone formation, enhances bone resorption by up-regulating RANKL, exacerbates the destruction of periodontal tissues, and inhibits their regeneration. However, the regulation of cellular pyroptosis related to the osteoclastic mechanism needs to be further explored (Table 6).
Periodontitis is a common chronic disease, and its progression may be regulated by interactions between host immunity and periodontal pathogens (151, 152). TLRs are type I transmembrane glycoproteins that belong to the pattern recognition receptor (PRR) class, which recognizes highly conserved structures on the surface of a large number of microorganisms and activates intrinsic immune cells through intercellular signaling pathways, thereby triggering an acquired immune response (153, 154).In recent years, many studies have revealed the ability of TLRs to recognize periodontal pathogens and modulate host innate immune responses to periodontal bacteria, including plasma membrane-associated TLR2, TLR4, and more recently the intrinsic intracellular sensor TLR9 (74, 76, 78, 155). In vivo evidence shows that TLR9-deficient (TLR9/) mice are resistant to periodontitis. This provides the first conceptual evidence for the involvement of nucleic acid sensors in periodontitis (101). In addition, TLR9 has been shown to modulate inflammation triggered by TLR2 and TLR4, suggesting possible crosstalk between these sensors during periodontal inflammation via downstream signaling pathways. Recent data suggest that TLR9 is one of the most up-regulated PRRs expressed in chronic periodontitis (156). DNA of the periodontal pathogen Porphyromonas gingivalis promotes its virulence in periodontitis by expressing inflammatory cytokines via the TLR9 signaling pathway (80, 156). TLR9 is present in gingival tissues, and nucleic acids significantly upregulate TLR9 gene expression in patients with periodontitis (76). TLRs signal through two pathways: a MyD88-dependent pathway and a MyD88-independent pathway.TLR9 recognizes viral nucleic acids through a MyD88-dependent pathway. The TLR9 signaling pathway activates the transcription factor NF-κB through inhibition of the nuclear factor-κB (i -κB) kinase (IKK) complex, leading to enhanced NF-κB signaling (157, 158). TLR9 is also present in macrophages and can sense bacterial DNA. in addition the distribution of TLR9 haplotypes and TLR9 (T1486C) genotypes may be associated with chronic periodontitis (75, 159). To model chronic periodontitis, TLR9 knockout mice or wild-type mice were exposed to Porphyromonas gingivalis. The data showed that bone loss was increased in wild-type mice compared with controls (not infected with Porphyromonas gingivalis), and no bone loss was detected in TLR9 knockout mice compared with controls, suggesting that alveolar bone loss in patients with periodontitis may be regulated by TLR9 (74). TLR9 plays a key role in periodontitis, and aberrant expression of TLR9 can be observed in patients with periodontitis, thus TLR9 has the potential to serve as a diagnostic or prognostic biomarker for periodontitis. Understanding the mechanisms by which TLR9 promotes periodontal inflammation could provide important insights into how to control aberrant periodontal inflammation as well as identify therapeutic targets and disease biomarkers that are critical for local and systemic outcomes (Table 3).
This work demonstrates the dynamic evolutionary process and structural relationship between the fields related to periodontitis and aging by knowledge mapping and data visualization, and preliminarily analyzes the research frontiers in this field. This study will provide more theoretical basis for researchers in this field. In conclusion, we should place greater emphasis on research regarding the association between periodontal disease and aging. Additionally, it is important to strengthen communication and collaboration among research institutions to facilitate the development of this field.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
XL: Conceptualization, Data curation, Formal analysis,Investigation, Methodology, Software, Visualization, Writing –original draft, Writing – review & editing. HL: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81970938).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1374027/full#supplementary-material
Supplementary Table 1 | Search strategy.
Supplementary Table 2 | National communications scale.
Supplementary Table 3 | Institutional issuance scale.
Supplementary Table 4 | Scale of Journal Publications.
Supplementary Figure 1 | Double stacked diagram of journals.
Supplementary Table 5 | Table of Journal Commonly Cited.
Supplementary Table 6 | Table of authors’ publications and top 10 co-cited authors.
1. Ebersole JL, Kirakodu SS, Neumann E, Orraca L, Gonzalez Martinez J, Gonzalez OA. Oral microbiome and gingival tissue apoptosis and autophagy transcriptomics. Front Immunol. (2020) 11:585414. doi: 10.3389/fimmu.2020.585414
2. Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. (2020) 82:257–67. doi: 10.1111/prd.12323
3. Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol. (2019) 46:608–22. doi: 10.1111/jcpe.13112
4. Parameter on chronic periodontitis with advanced loss of periodontal support. J Periodontol. (2000) 71 Suppl 5S:856–8. doi: 10.1902/jop.2000.71.5-S.856
5. Valenti C, Pagano S, Bozza S, Ciurnella E, Lomurno G, Capobianco B, et al. Use of the er : YAG laser in conservative dentistry: evaluation of the microbial population in carious lesions. Materials (Basel). (2021) 14. doi: 10.3390/ma14092387
6. Ortensi L, Sigari G, La Rosa GRM, Ferri A, Grande F, Pedull E. Digital planning of composite customized veneers using Digital Smile Design: Evaluation of its accuracy and manufacturing. Clin Exp Dent Res. (2022) 8:537–43. doi: 10.1002/cre2.570
7. Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. (2023) 64:109–22. doi: 10.1007/s12016-021-08899-6
8. Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. (2021) 22:75–95. doi: 10.1038/s41580-020-00314-w
9. Ebersole JL, Graves CL, Gonzalez OA, Dawson D 3rd, Morford LA, Huja PE, et al. Aging, inflammation, immunity and periodontal disease. Periodontol 2000. (2016) 72:54–75. doi: 10.1111/prd.12135
10. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. (2012) 91:914–20. doi: 10.1177/0022034512457373
11. Bertl K, Tangl S, Rybaczek T, Berger B, Traindl-Prohazka M, Schuller-Götzburg P, et al. Prevalence and severity of periodontal disease in a historical Austrian population. J Periodontal Res. (2020) 55:931–45. doi: 10.1111/jre.12785
12. Mack F, Mojon P, Budtz-Jørgensen E, Kocher T, Splieth C, Schwahn C, et al. Caries and periodontal disease of the elderly in Pomerania, Germany: results of the Study of Health in Pomerania. Gerodontology. (2004) 21:27–36. doi: 10.1046/j.1741-2358.2003.00001.x
13. Baima G, Romandini M, Citterio F, Romano F, Aimetti M. Periodontitis and accelerated biological aging: A geroscience approach. J Dent Res. (2022) 101:125–32. doi: 10.1177/00220345211037977
14. Albuquerque-Souza E, Crump KE, Rattanaprukskul K, Li Y, Shelling B, Xia-Juan X, et al. TLR9 mediates periodontal aging by fostering senescence and inflammaging. J Dent Res. (2022) 101:1628–36. doi: 10.1177/00220345221110108
15. LL.ez-Ot0. C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
16. Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. (2012) 4:61–76. doi: 10.1177/1759720x11430858
17. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. (2014) 69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057
18. Huttner EA, MaChado DC, de Oliveira RB, Antunes AG, Hebling E. Effects of human aging on periodontal tissues. Spec Care Dentist. (2009) 29:149–55. doi: 10.1111/j.1754-4505.2009.00082.x
19. Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. (2010) 25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x
20. Boutin S, Hagenfeld D, Zimmermann H, El Sayed N, Höpker T, Greiser HK, et al. Clustering of subgingival microbiota reveals microbial disease ecotypes associated with clinical stages of periodontitis in a cross-sectional study. Front Microbiol. (2017) 8:340. doi: 10.3389/fmicb.2017.00340
21. Van Dyke TE. Shifting the paradigm from inhibitors of inflammation to resolvers of inflammation in periodontitis. J Periodontol. (2020) 91 Suppl 1:S19–s25. doi: 10.1002/jper.20-0088
22. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. (2000) 908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x
23. Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. (2015) 35:551–9. doi: 10.1002/phar.1586
24. Akmal M, Hasnain N, Rehan A, Iqbal U, Hashmi S, Fatima K, et al. Glioblastome multiforme: A bibliometric analysis. World Neurosurg. (2020) 136:270–82. doi: 10.1016/j.wneu.2020.01.027
25. Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: A bibliometric and visualized study. Front Oncol. (2020) 10:627891. doi: 10.3389/fonc.2020.627891
26. Wang S, Zhou H, Zheng L, Zhu W, Zhu L, Feng D, et al. Global trends in research of macrophages associated with acute lung injury over past 10 years: A bibliometric analysis. Front Immunol. (2021) 12:669539. doi: 10.3389/fimmu.2021.669539
27. Liu T, Chen D, Tang S, Zou Z, Yang F, Zhang Y, et al. P53 alleviates the progression of periodontitis by reducing M1-type macrophage differentiation. Inflammation. (2024). doi: 10.1007/s10753-024-01968-w
28. Chen MH, Wang YH, Sun BJ, Yu LM, Chen QQ, Han XX, et al. HIF-1, activator DMOG inhibits alveolar bone resorption in murine periodontitis by regulating macrophage polarization. Int Immunopharmacol. (2021) 99:107901. doi: 10.1016/j.intimp.2021.107901
29. Han Y, Huang Y, Gao P, Yang Q, Jia L, Zheng Y, et al. Leptin aggravates periodontitis by promoting M1 polarization via NLRP3. J Dent Res. (2022) 101:675–85. doi: 10.1177/00220345211059418
30. Yan X, Yuan Z, Bian Y, Jin L, Mao Z, Lei J, et al. Uncoupling protein-2 regulates M1 macrophage infiltration of gingiva with periodontitis. Cent Eur J Immunol. (2020) 45:9–21. doi: 10.5114/ceji.2020.94664
31. Hou L, Ye Y, Gou H, Tang H, Zhou Y, Xu X, et al. A20 inhibits periodontal bone resorption and NLRP3-mediated M1 macrophage polarization. Exp Cell Res. (2022) 418:113264. doi: 10.1016/j.yexcr.2022.113264
32. Wang Y, Zhang X, Wang J, Zhang Y, Ye Q, Wang Y, et al. Inflammatory Periodontal Ligament Stem Cells Drive M1 Macrophage Polarization via Exosomal miR-143-3p-Mediated Regulation of PI3K/AKT/NF-ed Signaling. Stem Cells. (2023) 41:184–99. doi: 10.1093/stmcls/sxac087
33. Cui Y, Hong S, Xia Y, Li X, He X, Hu X, et al. Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv Sci (Weinh). (2023) 10:e2302029. doi: 10.1002/advs.202302029
34. Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, et al. Exosomes from TNF-omes,2/a human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. (2021) 122:306–24. doi: 10.1016/j.actbio.2020.12.046
35. He XT, Li X, Zhang M, Tian BM, Sun LJ, Bi CS, et al. Role of molybdenum in material immunomodulation and periodontal wound healing: Targeting immunometabolism and mitochondrial function for macrophage modulation. Biomaterials. (2022) 283:121439. doi: 10.1016/j.biomaterials.2022.121439
36. Liu J, Wang H, Zhang L, Li X, Ding X, Ding G, et al. Periodontal ligament stem cells promote polarization of M2 macrophages. J Leukoc Biol. (2022) 111:1185–97. doi: 10.1002/jlb.1ma1220-853rr
37. Chen X, Wan Z, Yang L, Song S, Fu Z, Tang K, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnol. (2022) 20:110. doi: 10.1186/s12951-022-01314-y
38. Liao Y, Yan Q, Cheng T, Yao H, Zhao Y, Fu D, et al. Sulforaphene Inhibits Periodontitis through Regulating Macrophage Polarization via Upregulating Dendritic Cell Immunoreceptor. J Agric Food Chem. (2023) 71:15538–52. doi: 10.1021/acs.jafc.3c02619
39. Huang H, Pan W, Wang Y, Kim HS, Shao D, Huang B, et al. Nanoparticulate cell-free DNA scavenger for treating inflammatory bone loss in periodontitis. Nat Commun. (2022) 13:5925. doi: 10.1038/s41467-022-33492-6
40. Yang L, Tao W, Xie C, Chen Q, Zhao Y, Zhang L, et al. Interleukin-37 ameliorates periodontitis development by inhibiting NLRP3 inflammasome activation and modulating M1/M2 macrophage polarization. J Periodontal Res. (2024) 59:128–39. doi: 10.1111/jre.13196
41. Guo X, Huang Z, Ge Q, Yang L, Liang D, Huang Y, et al. Glipizide alleviates periodontitis pathogenicity via inhibition of angiogenesis, osteoclastogenesis and M1/M2 macrophage ratio in periodontal tissue. Inflammation. (2023) 46:1917–31. doi: 10.1007/s10753-023-01850-1
42. Wang Y, Li C, Wan Y, Qi M, Chen Q, Sun Y, et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small. (2021) 17:e2101505. doi: 10.1002/smll.202101505
43. Deng J, Golub LM, Lee HM, Bhatt HD, Johnson F, Xu TM, et al. A novel modified-curcumin 2.24 resolves inflammation by promoting M2 macrophage polarization. Sci Rep. (2023) 13:15513. doi: 10.1038/s41598-023-42848-x
44. Yuan G, Fu C, Yang ST, Yuh DY, Hajishengallis G, Yang S. RGS12 drives macrophage activation and osteoclastogenesis in periodontitis. J Dent Res. (2022) 101:448–57. doi: 10.1177/00220345211045303
45. Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, et al. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng Part A. (2021) 27:962–76. doi: 10.1089/ten.TEA.2020.0141
46. Li X, Zhou R, Han Y, Zeng J, Shi L, Mao Y, et al. Silibinin attenuates experimental periodontitis by downregulation of inflammation and oxidative stress. Oxid Med Cell Longev. (2023) 2023:5617800. doi: 10.1155/2023/5617800
47. Xiong Y, Zhao B, Zhang W, Jia L, Zhang Y, Xu X. Curcumin promotes osteogenic differentiation of periodontal ligament stem cells through the PI3K/AKT/Nrf2 signaling pathway. Iran J Basic Med Sci. (2020) 23:954–60. doi: 10.22038/ijbms.2020.44070.10351
48. Bhattarai G, Poudel SB, Kook SH, Lee JC. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. (2016) 29:398–408. doi: 10.1016/j.actbio.2015.10.031
49. Jia L, Xiong Y, Zhang W, Ma X, Xu X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp Cell Res. (2020) 386:111717. doi: 10.1016/j.yexcr.2019.111717
50. Wei Y, Fu J, Wu W, Ma P, Ren L, Yi Z, et al. Quercetin prevents oxidative stress-induced injury of periodontal ligament cells and alveolar bone loss in periodontitis. Drug Des Devel Ther. (2021) 15:3509–22. doi: 10.2147/dddt.S315249
51. Xin L, Zhou F, Zhang C, Zhong W, Xu S, Jing X, et al. Four-Octyl itaconate ameliorates periodontal destruction via Nrf2-dependent antioxidant system. Int J Oral Sci. (2022) 14:27. doi: 10.1038/s41368-022-00177-1
52. Liu X, Hou Y, Yang M, Xin X, Deng Y, Fu R, et al. N-acetyl-l-cysteine-derived carbonized polymer dots with ROS scavenging via keap1-nrf2 pathway regulate alveolar bone homeostasis in periodontitis. Adv Healthc Mater. (2023) 12:e2300890. doi: 10.1002/adhm.202300890
53. Li J, Li Y, Pan S, Zhang L, He L, Niu Y. Paeonol attenuates ligation-induced periodontitis in rats by inhibiting osteoclastogenesis via regulating Nrf2/NF-ngogenesi signaling pathway. Biochimie. (2019) 156:129–37. doi: 10.1016/j.biochi.2018.09.004
54. Zhu C, Zhao Y, Wu X, Qiang C, Liu J, Shi J, et al. The therapeutic role of baicalein in combating experimental periodontitis with diabetes via Nrf2 antioxidant signaling pathway. J Periodontal Res. (2020) 55:381–91. doi: 10.1111/jre.12722
55. Huang X, Liu Y, Shen H, Fu T, Guo Y, Qiu S. Chlorogenic acid attenuates inflammation in LPS-induced Human gingival fibroblasts via CysLT1R/Nrf2/NLRP3 signaling. Int Immunopharmacol. (2022) 107:108706. doi: 10.1016/j.intimp.2022.108706
56. Zhou J, Shi P, Ma R, Xie X, Zhao L, Wang J. Notopterol inhibits the NF-ib pathway and activates the PI3K/akt/nrf2 pathway in periodontal tissue. J Immunol. (2023) 211:1516–25. doi: 10.4049/jimmunol.2200727
57. Fan Q, Zhou XH, Wang TF, Zeng FJ, Liu X, Gu Y, et al. Effects of epigallocatechin-3-gallate on oxidative stress, inflammation, and bone loss in a rat periodontitis model. J Dent Sci. (2023) 18:1567–75. doi: 10.1016/j.jds.2023.02.019
58. Wang L, Pu W, Wang C, Lei L, Li H. Microtubule affinity regulating kinase 4 promoted activation of the NLRP3 inflammasome-mediated pyroptosis in periodontitis. J Oral Microbiol. (2022) 14:2015130. doi: 10.1080/20002297.2021.2015130
59. Yang S, Yin Y, Sun Y, Ai D, Xia X, Xu X, et al. AZGP1 aggravates macrophage M1 polarization and pyroptosis in periodontitis. J Dent Res. (2024), 220345241235616. doi: 10.1177/00220345241235616
60. Huang C, Zhang C, Yang P, Chao R, Yue Z, Li C, et al. Eldecalcitol inhibits LPS-induced NLRP3 inflammasome-dependent pyroptosis in human gingival fibroblasts by activating the nrf2/HO-1 signaling pathway. Drug Des Devel Ther. (2020) 14:4901–13. doi: 10.2147/dddt.S269223
61. Zhao D, Wu Y, Zhuang J, Xu C, Zhang F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1a in human periodontal ligament cells. Oncotarget. (2016) 7:68292–302. doi: 10.18632/oncotarget.11944
62. He D, Li X, Zhang F, Wang C, Liu Y, Bhawal UK, et al. Dec2 inhibits macrophage pyroptosis to promote periodontal homeostasis. J Periodontal Implant Sci. (2022) 52:28–38. doi: 10.5051/jpis.2101380069
63. Gao Y, Guo X, Zhou Y, Du J, Lu C, Zhang L, et al. Kynurenic acid inhibits macrophage pyroptosis by suppressing ROS production via activation of the NRF2 pathway. Mol Med Rep. (2023) 28. doi: 10.3892/mmr.2023.13098
64. Peng Y, Wang H, Huang X, Liu H, Xiao J, Wang C, et al. Tet methylcytosine dioxygenase 1 modulates Porphyromonas gingivalis-triggered pyroptosis by regulating glycolysis in cementoblasts. Ann N Y Acad Sci. (2023) 1523:119–34. doi: 10.1111/nyas.14979
65. Lv X, Fan C, Jiang Z, Wang W, Qiu X, Ji Q. Isoliquiritigenin alleviates P. gingivalis-LPS/ATP-induced pyroptosis by inhibiting NF-ibi NLRP3/GSDMD signals in human gingival fibroblasts. Int Immunopharmacol. (2021) 101:108338. doi: 10.1016/j.intimp.2021.108338
66. Chu Y, Xu Y, Yang W, Chu K, Li S, Guo L. N-acetylcysteine protects human periodontal ligament fibroblasts from pyroptosis and osteogenic differentiation dysfunction through the SIRT1/NF-ontionetimp. signaling pathway. Arch Oral Biol. (2023) 148:105642. doi: 10.1016/j.archoralbio.2023.105642
67. Zhou X, Wang Q, Nie L, Zhang P, Zhao P, Yuan Q, et al. Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Arch Oral Biol. (2020) 116:104763. doi: 10.1016/j.archoralbio.2020.104763
68. Pang Y, Liu L, Wu S, Wang J, Liu L. Synoviolin alleviates GSDMD-mediated periodontitis by suppressing its stability. Immun Inflammation Dis. (2023) 11:e880. doi: 10.1002/iid3.880
69. Yumoto H, Chou HH, Takahashi Y, Davey M, Gibson FC 3rd, Genco CA. Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect Immun. (2005) 73:8050–9. doi: 10.1128/iai.73.12.8050-8059.2005
70. Sun Y, Shu R, Li CL, Zhang MZ. Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol. (2010) 81:1488–96. doi: 10.1902/jop.2010.100004
71. Wara-aswapati N, Chayasadom A, Surarit R, Pitiphat W, Boch JA, Nagasawa T, et al. Induction of toll-like receptor expression by Porphyromonas gingivalis. J Periodontol. (2013) 84:1010–8. doi: 10.1902/jop.2012.120362
72. Zhang Y, Li X. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: the role of toll-like receptors 2 and 4 and the MAPK pathway. J Periodontal Res. (2015) 50:141–51. doi: 10.1111/jre.12193
73. Kajita K, Honda T, Amanuma R, Domon H, Okui T, Ito H, et al. Quantitative messenger RNA expression of Toll-like receptors and interferon-alpha1 in gingivitis and periodontitis. Oral Microbiol Immunol. (2007) 22:398–402. doi: 10.1111/j.1399-302X.2007.00377.x
74. Kim PD, Xia-Juan X, Crump KE, Abe T, Hajishengallis G, Sahingur SE. Toll-like receptor 9-mediated inflammation triggers alveolar bone loss in experimental murine periodontitis. Infect Immun. (2015) 83:2992–3002. doi: 10.1128/iai.00424-15
75. Holla LI, Vokurka J, Hrdlickova B, Augustin P, Fassmann A. Association of Toll-like receptor 9 haplotypes with chronic periodontitis in Czech population. J Clin Periodontol. (2010) 37:152–9. doi: 10.1111/j.1600-051X.2009.01523.x
76. Sahingur SE, Xia XJ, Voth SC, Yeudall WA, Gunsolley JC. Increased nucleic Acid receptor expression in chronic periodontitis. J Periodontol. (2013) 84:e48–57. doi: 10.1902/jop.2013.120739
77. Chen YC, Liu CM, Jeng JH, Ku CC. Association of pocket epithelial cell proliferation in periodontitis with TLR9 expression and inflammatory response. J Formos Med Assoc. (2014) 113:549–56. doi: 10.1016/j.jfma.2012.07.043
78. Marchesan J, Jiao Y, Schaff RA, Hao J, Morelli T, Kinney JS, et al. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol Oral Microbiol. (2016) 31:243–58. doi: 10.1111/omi.12116
79. Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Chen YY, Singleton W, et al. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PloS One. (2016) 11:e0151967. doi: 10.1371/journal.pone.0151967
80. Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. (2002) 105:1158–61.
81. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. (2004) 5:190–8. doi: 10.1038/ni1028
82. González OA, Li M, Ebersole JL, Huang CB. HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves Toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. Clin Vaccine Immunol. (2010) 17:1417–27. doi: 10.1128/cvi.00009-10
83. Liu G, Jiang R, Jin Y. Sciatic nerve injury repair: a visualized analysis of research fronts and development trends. Neural Regener Res. (2014) 9:1716–22. doi: 10.4103/1673-5374.141810
84. Xiao F, Li C, Sun J, Zhang L. Knowledge domain and emerging trends in organic photovoltaic technology: A scientometric review based on citeSpace analysis. Front Chem. (2017) 5:67. doi: 10.3389/fchem.2017.00067
85. Ma L, Ma J, Teng M, Li Y. Visual analysis of colorectal cancer immunotherapy: A bibliometric analysis from 2012 to 2021. Front Immunol. (2022) 13:843106. doi: 10.3389/fimmu.2022.843106
86. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. (2008) 79:1585–91. doi: 10.1902/jop.2008.080183
87. Sczepanik FSC, Grossi ML, Casati M, Goldberg M, Glogauer M, Fine N, et al. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000. (2020) 84:45–68. doi: 10.1111/prd.12342
88. Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontol 2000. (2021) 87:143–56. doi: 10.1111/prd.12380
89. Tan J, Dai A, Pan L, Zhang L, Wang Z, Ke T, et al. Inflamm-aging-related cytokines of IL-17 and IFN-7 accelerate osteoclastogenesis and periodontal destruction. J Immunol Res. (2021) 2021:9919024. doi: 10.1155/2021/9919024
90. Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. (2016) 72:153–75. doi: 10.1111/prd.12129
91. Hajishengallis G. Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci. (2014) 56:30–7. doi: 10.1016/j.job.2013.09.001
92. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. (2004) 39:687–99. doi: 10.1016/j.exger.2004.01.009
93. LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. (1997) 160:115–26. doi: 10.1111/j.1600-065x.1997.tb01032.x
94. Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. (1993) 64:497–508. doi: 10.1902/jop.1993.64.6.497
95. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. (2011) 6:121–45. doi: 10.1146/annurev-pathol-011110-130203
96. Ogura N, Matsuda U, Tanaka F, Shibata Y, Takiguchi H, Abiko Y. In vitro senescence enhances IL-6 production in human gingival fibroblasts induced by lipopolysaccharide from Campylobacter rectus. Mech Ageing Dev. (1996) 87:47–59. doi: 10.1016/0047-6374(96)01701-0
97. Chen LP, Chiang CK, Chan CP, Hung KY, Huang CS. Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients? Am J Kidney Dis. (2006) 47:815–22. doi: 10.1053/j.ajkd.2006.01.018
98. Sanz M, Del Castillo AM, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases. Consensus report. Glob Heart. (2020) 15:1. doi: 10.5334/gh.400
99. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
100. D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. (2004) 83:156–60. doi: 10.1177/154405910408300214
101. Hajishengallis G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000. (2022) 89:9–18. doi: 10.1111/prd.12430
102. Pink C, Kocher T, Meisel P, Dörr M, Markus MR, Jablonowski L, et al. Longitudinal effects of systemic inflammation markers on periodontitis. J Clin Periodontol. (2015) 42:988–97. doi: 10.1111/jcpe.12473
103. Sharma P, Fenton A, Dias IHK, Heaton B, Brown CLR, Sidhu A, et al. Oxidative stress links periodontal inflammation and renal function. J Clin Periodontol. (2021) 48:357–67. doi: 10.1111/jcpe.13414
104. Sari A, Davutoglu V, Bozkurt E, Taner IL, Erciyas K. Effect of periodontal disease on oxidative stress markers in patients with atherosclerosis. Clin Oral Investig. (2022) 26:1713–24. doi: 10.1007/s00784-021-04144-8
105. Rodrigues LP, Teixeira VR, Alencar-Silva T, Simonassi-Paiva B, Pereira RW, Pogue R, et al. Hallmarks of aging and immunosenescence: Connecting the dots. Cytokine Growth Factor Rev. (2021) 59:9–21. doi: 10.1016/j.cytogfr.2021.01.006
106. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. (2014) 6:13. doi: 10.12703/p6-13
107. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. (2003) 3:23–35. doi: 10.1038/nri978
108. Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, et al. Induction of M2 macrophages prevents bone loss in murine periodontitis models. J Dent Res. (2019) 98:200–8. doi: 10.1177/0022034518805984
109. Almubarak A, Tanagala KKK, Papapanou PN, Lalla E, Momen-Heravi F. Disruption of monocyte and macrophage homeostasis in periodontitis. Front Immunol. (2020) 11:330. doi: 10.3389/fimmu.2020.00330
110. Sloniak MC, Lepique AP, Nakao LYS, Villar CC. Alterations in macrophage polarization play a key role in control and development of periodontal diseases. J Indian Soc Periodontol. (2023) 27:578–82. doi: 10.4103/jisp.jisp_75_23
111. Yamamoto M, Fujihashi K, Hiroi T, McGhee JR, Van Dyke TE, Kiyono H. Molecular and cellular mechanisms for periodontal diseases: role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J Periodontal Res. (1997) 32:115–9. doi: 10.1111/j.1600-0765.1997.tb01391.x
112. Yang J, Zhu Y, Duan D, Wang P, Xin Y, Bai L, et al. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch Oral Biol. (2018) 96:234–42. doi: 10.1016/j.archoralbio.2017.03.006
113. Wang W, Zheng C, Yang J, Li B. Intersection between macrophages and periodontal pathogens in periodontitis. J Leukoc Biol. (2021) 110:577–83. doi: 10.1002/jlb.4mr0421-756r
114. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
115. Li W, Zhang Z, Wang ZM. Differential immune cell infiltrations between healthy periodontal and chronic periodontitis tissues. BMC Oral Health. (2020) 20:293. doi: 10.1186/s12903-020-01287-0
116. Zhou LN, Bi CS, Gao LN, An Y, Chen F, Chen FM. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. (2019) 25:265–73. doi: 10.1111/odi.12983
117. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
118. Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19061801
119. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
120. Chen B, Li S, Chang Y, Zhang J, Liu J, Dong Y, et al. Macrophages contribute to periodontal wound healing mainly in the tissue proliferation stage. J Periodontal Res. (2023) 58:122–30. doi: 10.1111/jre.13074
121. Ortega-Gga-Gr A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. (2013) 5:661–74. doi: 10.1002/emmm.201202382
122. Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front Immunol. (2021) 12:763334. doi: 10.3389/fimmu.2021.763334
123. Gibon E, Loi F, Córdova LA, Pajarinen J, Lin T, Lu L, et al. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regener Eng Transl Med. (2016) 2:98–104. doi: 10.1007/s40883-016-0016-5
124. Zhou F, Wang Z, Zhang G, Wu Y, Xiong Y. Immunosenescence and inflammaging: Conspiracies against alveolar bone turnover. Oral Dis. (2023). doi: 10.1111/odi.14642
125. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. (2005) 366:1809–20. doi: 10.1016/s0140-6736(05)67728-8
126. Novakovii N, Cakik S, Todorovii T, Raicevii BA, Dozi; I, Petrovii V, et al. Antioxidative status of saliva before and after non-surgical periodontal treatment. Srp Arh Celok Lek. (2013) 141:163–8. doi: 10.2298/sarh1304163n
127. Ling MR, Chapple IL, Matthews JB. Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy. J Clin Periodontol. (2016) 43:652–8. doi: 10.1111/jcpe.12575
128. Shin MS, Shin HS, Ahn YB, Kim HD. Association between periodontitis and salivary 8-hydroxydeoxyguanosine among Korean rural adults. Community Dent Oral Epidemiol. (2016) 44:381–9. doi: 10.1111/cdoe.12225
129. Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res. (2009) 44:43–51. doi: 10.1111/j.1600-0765.2007.01060.x
130. Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. (2007) 43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x
131. Ramesh A, Varghese SS, Doraiswamy JN, Malaiappan S. Herbs as an antioxidant arsenal for periodontal diseases. J Intercult Ethnopharmacol. (2016) 5:92–6. doi: 10.5455/jice.20160122065556
132. Baňasová L, Kamodyov4 N, Janodyov4 K, T.;odyo Ľ, Stanko P, Turnk J, et al. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin Oral Investig. (2015) 19:201–7. doi: 10.1007/s00784-014-1236-z
133. Su H, Gornitsky M, Velly AM, Yu H, Benarroch M, Schipper HM. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic Biol Med. (2009) 46:914–21. doi: 10.1016/j.freeradbiomed.2009.01.008
134. Konopka T, Kr;o K, Kopep W, Gerber H. Total antioxidant status and 8-hydroxy-2’-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp (Warsz). (2007) 55:417–22. doi: 10.1007/s00005-007-0047-1
135. Yin KJ, Huang JX, Wang P, Yang XK, Tao SS, Li HM, et al. No genetic causal association between periodontitis and arthritis: A bidirectional two-sample mendelian randomization analysis. Front Immunol. (2022) 13:808832. doi: 10.3389/fimmu.2022.808832
136. Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. (2005) 76:2066–74. doi: 10.1902/jop.2005.76.11-S.2066
137. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. (2007) 9 Suppl 1:S1. doi: 10.1186/ar2165
138. Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. (2012) 39:239–48. doi: 10.1111/j.1600-051X.2011.01810.x
139. Baltaccltac E, Yuva P, Ayda,c G, Alver A, Kahraman C, Karabulut E, et al. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: a new biomarker for periodontal disease? J Periodontol. (2014) 85:1432–41. doi: 10.1902/jop.2014.130654
140. Ikeda E, Ikeda Y, Wang Y, Fine N, Sheikh Z, Viniegra A, et al. Resveratrol derivative-rich melinjo seed extract induces healing in a murine model of established periodontitis. J Periodontol. (2018) 89:586–95. doi: 10.1002/jper.17-0352
141. Chiu AV, Saigh MA, McCulloch CA, Glogauer M. The role of nrF2 in the regulation of periodontal health and disease. J Dent Res. (2017) 96:975–83. doi: 10.1177/0022034517715007
142. Vo TTT, Chu PM, Tuan VP, Te JS, Lee IT. The promising role of antioxidant phytochemicals in the prevention and treatment of periodontal disease via the inhibition of oxidative stress pathways: updated insights. Antioxid (Basel). (2020) 9. doi: 10.3390/antiox9121211
143. Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, et al. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. (2017) 8:351. doi: 10.3389/fphys.2017.00351
144. Wei SJ, Zhang Q, Xiang YJ, Peng LY, Peng W, Ren Q, et al. Guizhi-Shaoyao-Zhimu decoction attenuates bone erosion in rats that have collagen-induced arthritis via modulating NF-ul signalling to suppress osteoclastogenesis. Pharm Biol. (2021) 59:262–74. doi: 10.1080/13880209.2021.1876100
145. Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. (2014) 73:263–9. doi: 10.1136/annrheumdis-2012-202726
146. Mei YM, Li L, Wang XQ, Zhang M, Zhu LF, Fu YW, et al. AGEs induces apoptosis and autophagy via reactive oxygen species in human periodontal ligament cells. J Cell Biochem. (2020) 121:3764–79. doi: 10.1002/jcb.29499
147. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. (2017) 42:245–54. doi: 10.1016/j.tibs.2016.10.004
148. Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18020440
149. Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, et al. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. (2009) 157:415–22. doi: 10.1111/j.1365-2249.2009.03972.x
150. Chen Q, Liu X, Wang D, Zheng J, Chen L, Xie Q, et al. Periodontal inflammation-triggered by periodontal ligament stem cell pyroptosis exacerbates periodontitis. Front Cell Dev Biol. (2021) 9:663037. doi: 10.3389/fcell.2021.663037
151. O’Brien-Simpson NM, Pathirana RD, Paolini RA, Chen YY, Veith PD, Tam V, et al. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J Immunol. (2005) 175:3980–9. doi: 10.4049/jimmunol.175.6.3980
152. Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. (2011) 63:108–13. doi: 10.1016/j.phrs.2010.10.005
153. Flórez-Álvarez L, Ruiz-Perez L, Taborda N, Hernandez JC. Toll-like receptors as a therapeutic target in cancer, infections and inflammatory diseases. Immunotherapy. (2020) 12:311–22. doi: 10.2217/imt-2019-0096
154. Kumar V. Toll-like receptors in adaptive immunity. Handb Exp Pharmacol. (2022) 276:95–131. doi: 10.1007/164_2021_543
155. Crump KE, Sahingur SE. Microbial nucleic acid sensing in oral and systemic diseases. J Dent Res. (2016) 95:17–25. doi: 10.1177/0022034515609062
156. Crump KE, Oakley JC, Xia-Juan X, Madu TC, Devaki S, Mooney EC, et al. Interplay of toll-like receptor 9, myeloid cells, and deubiquitinase A20 in periodontal inflammation. Infect Immun. (2017) 85. doi: 10.1128/iai.00814-16
157. O’Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. (2008) 29:12–20. doi: 10.1016/j.immuni.2008.06.004
158. Karapetyan L, Luke JJ, Davar D. Toll-like receptor 9 agonists in cancer. Onco Targets Ther. (2020) 13:10039–60. doi: 10.2147/ott.S247050
Keywords: periodontitis, bibliometric analysis, data visualization, inflammation, aging
Citation: Liu X and Li H (2024) Global trends in research on aging associated with periodontitis from 2002 to 2023: a bibliometric analysis. Front. Endocrinol. 15:1374027. doi: 10.3389/fendo.2024.1374027
Received: 21 January 2024; Accepted: 02 April 2024;
Published: 10 May 2024.
Edited by:
Kenju Shimomura, Fukushima Medical University, JapanReviewed by:
Stefano Pagano, University of Perugia, ItalyCopyright © 2024 Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjiao Li, c21tdWxoakAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.