- 1School of Public Health and Preventive Medicine, Faculty of Medicine, Nursing & Health Sciences, Monash University, Melbourne, VIC, Australia
- 2Division of Sports Science and Physical Education, Tsinghua University, Beijing, China
- 3Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 4Department of Undergraduate, Taishan University, Taian, China
- 5Orthopedics Department, PLA Rocket Force Characteristic Medical Center, Beijing, China
- 6College of Public Health, Hebei University/Hebei Key Laboratory of Public Health Safety, Baoding, Hebei, China
- 7School of Pharmacy, Macau University of Science and Technology, Macao, Macao SAR, China
- 8Department of Earth Science, Kunming University of Science and Technology, Kunming, China
Background: Sufficient physical activity and sleep duration are essential for overall health. While one-third of the US population reports short sleep (<7 h), which is proven to link with negative health status. Current evidence on the relationship between physical activity, sedentary behavior, and serum insulin level in short sleep groups is limited.
Methods: The National Health and Nutrition Examination Survey (NHANES) was used to conduct this cross-sectional study of 8,494 adults (NHANES) 2007–2018. Serum insulin was quantitatively tested by human insulin immunoassay. Short sleep conditions were defined as ≤7 h per night. Physical activity conditions, including work activity, recreational activity, and sedentary behavior, were self-reported in NHANES by the Physical Activity Questionnaire using a 7-day recall method. The main analyses utilized weighted linear regression models due to the complex multistage sampling design of NHANES. Subgroup analysis and the influence of different lipid indices were explored in this study. In addition, a sensitivity analysis of participants without diabetes was conducted.
Results: In fully adjusted models, increased levels of work and recreational activity significantly reduced insulin levels, with β values 95% CI = -0.002 (-0.003, 0.001) and β values 95% CI = -0.008 (-0.012, -0.003), respectively. However, sedentary behavior was positively associated with insulin levels, with a β value 95% CI =0.022 (0.009, 0.034). The sensitivity analysis further confirmed the benefits of recreational activity in controlling insulin levels. Through sex stratification analysis, it seemed that physical activity was more obviously impacted in the male than female groups.
Conclusions: Overall, our analysis demonstrates that in short sleepers, an increased level of work and recreational activity is beneficial to control the insulin level, and more sedentary time is harmful. However, this association might be discrepant in different sexes and different levels of lipid indices.
Introduction
Sleep deprivation has been associated with the development and management of a number of chronic diseases, including depression, cognitive decline, type 2 diabetes, and coronary heart disease, and metabolic disorders such as insulin fluctuation (1–4). Sleep deprivation is also associated high rates of morbidity and mortality. It is concerning that nearly one-third of the U.S. population reports sleeping less than seven hours per night on average (5). Advancing occupational and social demands contribute to the continuous decline in sleep duration, and the knowledge for improving quality of life is still lacking among short sleepers.
Insulin, the body’s primary hormone, regulates glucose metabolism and peripheral tissue glucose uptake and utilization. Insulin levels rise rapidly after a meal, peaking 30 to 45 minutes after the meal’s onset (bolus/prandial), and then fall back to basal levels within an hour or so. In healthy individuals, basal insulin is secreted continuously at a low rate (concentrations of 5-15 μU/mL) (6). Maintaining normal blood glucose concentrations and keeping them within a narrow range (63-135 mg/dL [3.5-7.5 mmol/L]) is beneficial for people without diabetes (7). Insulin resistance (IR), characterized by the body’s reduced ability to respond to insulin, is a hallmark of type 2 diabetes mellitus (T2DM), where insulin release may be significantly decreased or absent (8, 9). Another possible scenario in T2DM is that insulin release may be significantly decreased or completely absent (6). Fasting hyperglycemia occurs as a result of inadequate basal insulin secretion to maintain normal fasting plasma glucose concentrations or as a result of the appearance of insulin resistance (10–12).
While numerous studies have highlighted the health risks of sedentary behavior (SB) and physical inactivity (PI) (13), a significant portion of the American and global population continues to engage in high levels of SB/PI and low levels of physical activity (PA), often coupled with inadequate sleep (14–16). Especially, current studies showed that the benefits of PA existed in short-sleep population (17, 18). Notably, diabetes or prediabetes is closely associated with high SB/PI levels. Data from the National Health and Nutrition Examination Survey (NHANES) 2003 - 2006 indicated a negative association between physical activity and prediabetes prevalence in middle-aged U.S. adults, independent of adiposity (19). Another study also pointed out that sedentary lifestyles are important drivers of the current global diabetes epidemic (20). Owing to the high levels of SB/PI having impacts on basal insulin levels, both of them can increase the risk of diabetes significantly. Long-term sedentary behavior will lead to the body secreting a large amount of insulin, and its excessive use and consumption will further lead to basal insulin deficiency or IR. One study used NHANES to support this view and found that SB might be an important modifiable determinant of concentrations of insulin, and simultaneously, a significant positive correlation was found between SB and PI (21). Christian K Roberts also showed that PI was an important reason for metabolic syndrome and insulin resistance (9).
PA demands fuel mobilization and oxidation, with insulin’s effects on fuel storage being suppressed during exercise. This is achieved by reducing insulin release during PA and initiating systemic and local fuel mobilization (22). The effects of insulin on fuel storage are diminished during PA, which is primarily accomplished by blocking the release of insulin during PA and triggering both systemic and local fuel mobilizing processes (22). After the PA process, it is necessary to increase the insulin sensitivity of exercise muscles to increase glycogen storage. Hence, there is plentiful evidence that PA can prevent the abnormal increase in insulin levels and the occurrence of IR (22, 23). Several studies have also confirmed that PA could reduce the occurrence of abnormal increases in triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total serum cholesterol (TC). Those are all higher risks of developing diabetes, heart disease, or other illnesses (24–26). Regarding the specific movement mode, moderate-to-vigorous PA has proven particularly effective. For the short-sleep population, current studies have reported that PA can induce positive effects on hemodynamic changes and cognitive health (27, 28). Moderate-to-vigorous PA, in particular, has proven effective in enhancing these health outcomes.
The 2008 Physical Activity Guidelines for Americans recommend engaging in at least 150 to 300 minutes of moderate-intensity aerobic activity each week (29). This level of activity has been shown to effectively improve ‘poor’ sleep quality (30–32). However, the relationship between PA, SB, and insulin levels in populations with short sleep duration has been less explored.
The present study aims to explore the relationship between physical activity, sedentary behavior, and insulin levels in individuals with short sleep durations. We hypothesize that increased daily physical activity and reduced sedentary behavior are associated with improved serum insulin levels in this population. This research seeks to provide insights into the clinical significance of these lifestyle factors for short sleepers, potentially informing interventions to improve their metabolic health (33).
Methods
Study population
The NHANES is a National Center for Health Statistics program that is part of the Centers for Disease Control and Prevention (CDC). A structured household interview evaluated participants, and a standardized physical examination in mobile examination centers (MEC) with room for blood draws and measurement. The NHANES was designed to represent the population of the United States, which has become a continuous program since 1999 using a stratified, multistage probability sampling design; the NHANES survey was conducted every two years. More information on the sampling procedure is available on the website (http://www.cdc.gov/nchs/nhanes/about_nhanes.htm#intro). The present study included participants aged 18 years and older in six NHANES cycles (2007-2008, 2009-2010, 2011-2012, 2013-2014, 2015-2016, 2017-2018). Sleep duration on a usual weekday or workday was self-reported by participants. Referring to previous literature (34, 35), a short sleep condition was defined as ≤7 h per night.
Outcome: insulin level
In the NHANES 2011-2012 data collection cycles, the University of Minnesota conducted insulin testing using the Roche Elecsys 2010 immunoassay. Between 2013 and 2018, insulin testing was performed by the University of Missouri-Columbia, utilizing a two-site immunoenzymetric assay with the Tosoh AIA System Analyzer. Within the AIA-PACK, insulin was bound to a monoclonal antibody immobilized on a solid permanent magnet phase and an enzyme-labeled monoclonal antibody. The magnetic beads were then incubated with the fluorogenic substrate 4-methylumbelliferyl phosphate to remove any unbound protease mabs. The quantity of enzyme-labeled specific antibodies that bind to the beads is proportional to the insulin concentration of the sample. The densities of unknown compounds were characterized using a constructed standard curve. More information about the quality control and test process can be found in a detailed description at http://www.cdc.gov/nchs/nhanes/.
Exposure: PA status and SB
The independent variables of this study were physical activity conditions, including work and recreational activity, and sedentary behavior. The information on physical activity was self-reported in NHANES by the Physical Activity Questionnaire using a 7-day recall method. According to the questionnaire (36) provided by NHANES, work activity was defined as doing work and chores, such as cleaning the yard and trimming the sprays, which can be regarded as labor activities. Referring to previous literature (37–39), recreational activity was referred to as leisure time activities, including sports and exercise. The participants reported the duration and frequency of each type of physical activity at vigorous and moderate levels of intensity during a typical week. Subsequently, we summed the total number of minutes of physical activity at each intensity level to determine the remaining minutes of work and recreation. Sedentary behavior was measured as sitting time, defined as daily time spent “sitting at work, at home, getting to and from places, or with friends, including time spent sitting at a desk, traveling in a car or bus, reading, playing cards, watching television, or using a computer” and was assessed with the question “How much time do you usually spend sitting on a typical day?”
Covariates
Sociodemographic characteristics included age, sex race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other race), marital status (never married, married/living with partner, widowed/divorced) and education (< high school, High school or equivalent, and > High school), family income-to-poverty ratio (<1.3, [1.3, 3.5), ≥3.5). BMI was calculated as weight (kg) divided by height squared (m2) and classified into 3 groups (<25, [25, 30), ≥30 kg/m2). Lifestyle factors included smoking status (never, former, and current), alcohol use (never, moderate, high). The physiologic screening tests fast glucose (FG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured in blood samples. Participants were instructed to fast for at least 8.5 hours prior to blood sample collection. TG, TC, HDL-C, and LDL-C levels in serum samples were measured in mg/dL. Additionally, blood pressure, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), was manually recorded 3–4 times following the requirement of 5 minutes of quiet rest in a seated position and the determination of the maximum inflation level.
Statistical analysis
According to the complex survey design, all analyses in the current study were weighted by MEC exam weights and employed NHANES strata and population sampling units (40–42). Weighted means (standard error, SE) were utilized for continuous variables, and weighted proportions were utilized for categorical variables. The weighted linear regression model was used to determine the associations between insulin level and three types of physical activity: work activity, recreational activity, and sedentary behavior. For the crude model, no confounding factors were adjusted. For model 1, age, sex, and race/ethnicity were adjusted. For model 2, BMI categories, education, marital status, poverty status<1.3, SBP, DBP, total cholesterol, HDL-c, LDL-c, glucose, triglycerides, smoking status, and alcohol drinking status were additionally adjusted based on model 1. Statistical significance was set at P value < 0.05. All statistical analyses were performed using R (version 4.2.0, http://www.R-project.org, The R Foundation).
Results
Baseline participant characteristics
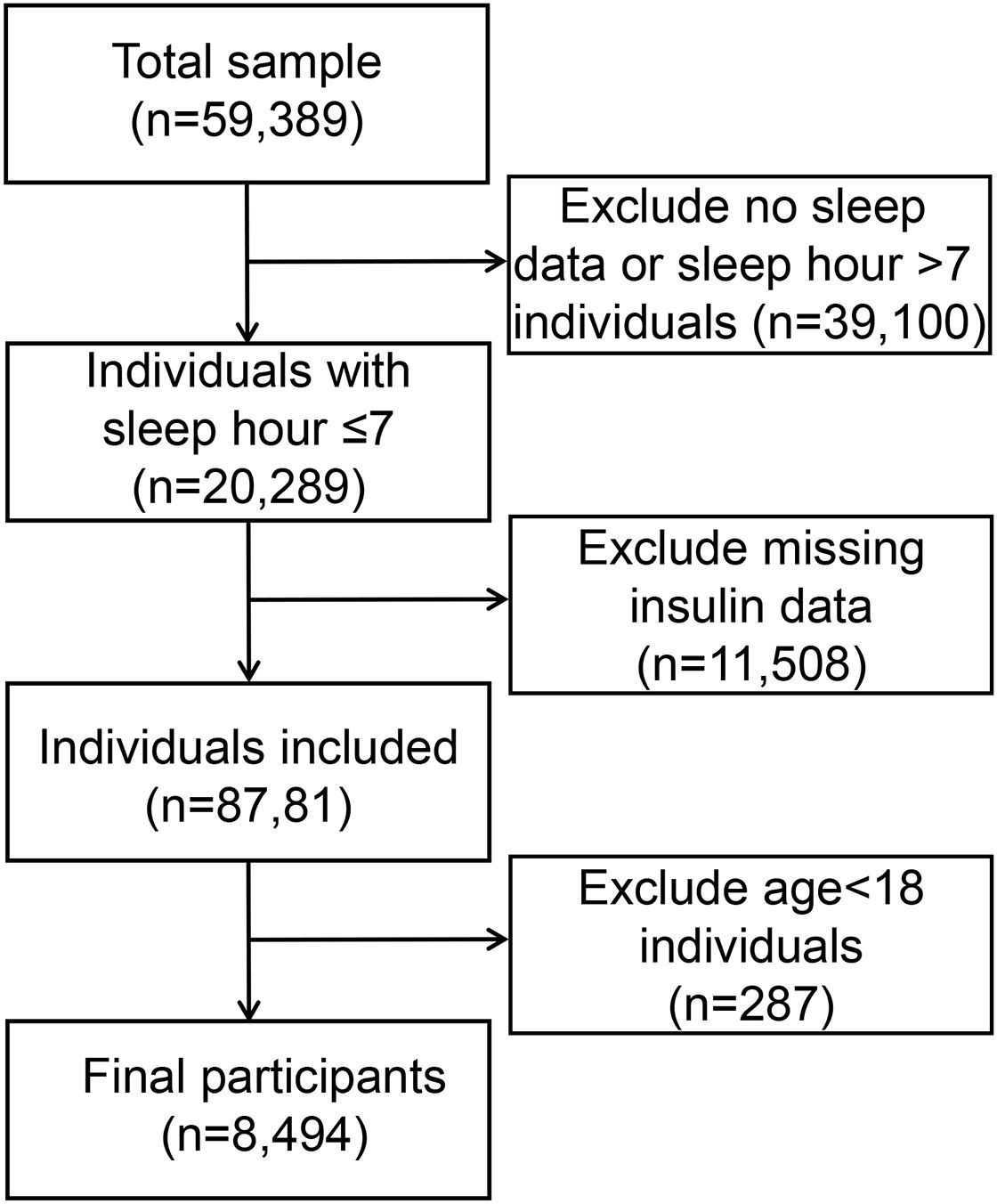
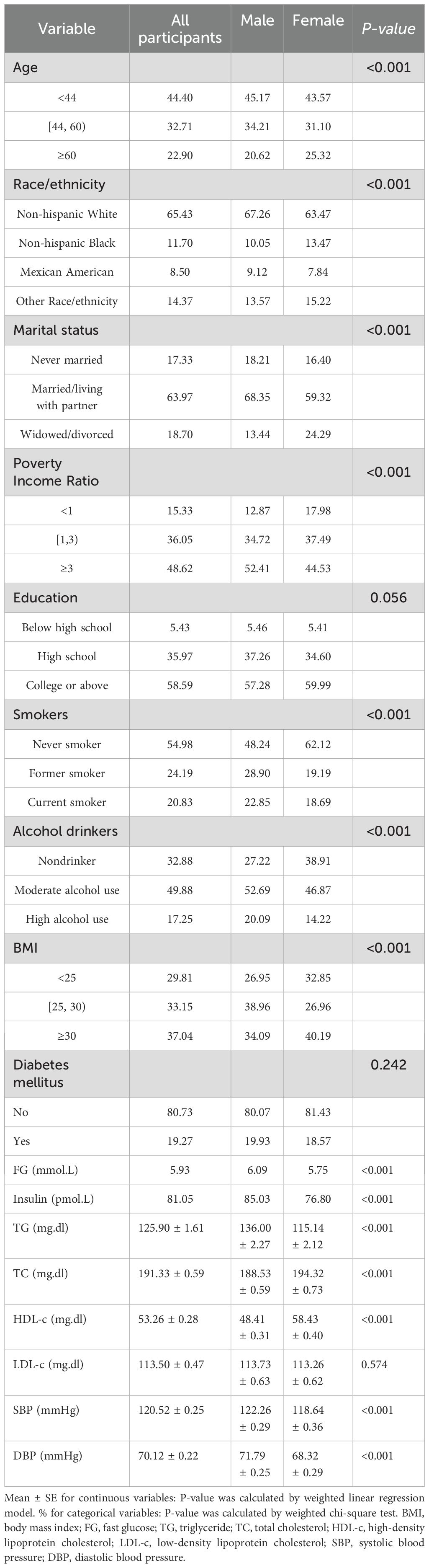
Out of the 59,389 participants (representing a weighted population of 55,989,817) in the 2007-2018 NH ANES cycles, 20,289 were identified as short sleepers (< 7 hours per night). Among these short sleepers, we included 8,494 participants aged ≥18 years with available insulin data. Figure 1 illustrates the detailed flow chart of the process of data filtering. The baseline clinical and demographic features are presented in Table 1, with the participants’ weighted characteristics sub-classified based on sex. The mean values of fasting glucose, insulin, total glucose, blood pressure, and diastolic blood pressure were significantly lower in females than in males (P < 0.001). However, females have a statistically significantly higher mean on HDL-C and TC than males (P < 0.001). Males were younger (P < 0.001) and had a higher proportion of smokers, alcohol consumption, poverty income ratio 3, married status or living with a partner, BMI between 25 and 30, non-Hispanic white and Mexican American (P < 0.001) compared to participants in female group, but a lower proportion of non-Hispanic black, unmarried or divorced status, poverty income ratio< 1 and BMI< 25 or ≥30 (There were no statistically significant differences in education, diabetes, or LDL-c (P > 0.05).
The association between PA, SB and the level of insulin in short sleepers
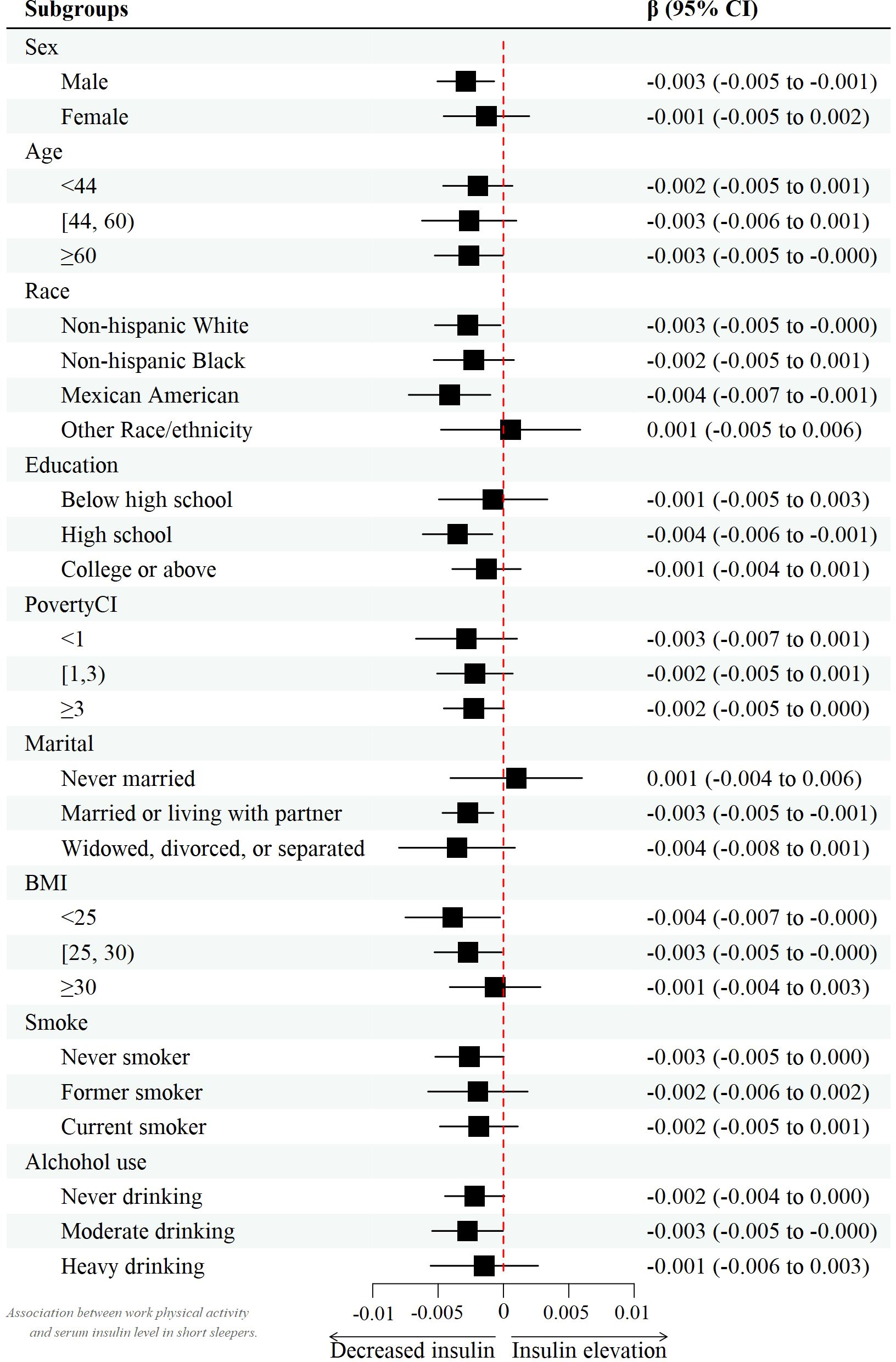
Data from the multivariate regression analysis are shown in Table 2. In the crude model, work activity and recreational activity were negatively associated with insulin [β value 95% CI = -0.002 (-0.002, -0.001), β value 95% CI = -0.012 (-0.017, -0.007), respectively], while sedentary behavior was positively correlated with insulin [β value 95% CI = 0.035 (0.022, 0.049)] in the total population. However, in the female work activity group, there seemed not to be a significant negative correlation with insulin (P > 0.05). Even after controlling for potential confounding factors, this relationship was found to persist in model 2 [β value 95% CI = -0.002 (-0.003, 0.001), β value 95% CI = -0.008 (-0.012, -0.003), β value 95% CI = 0.022 (0.009, 0.034)].
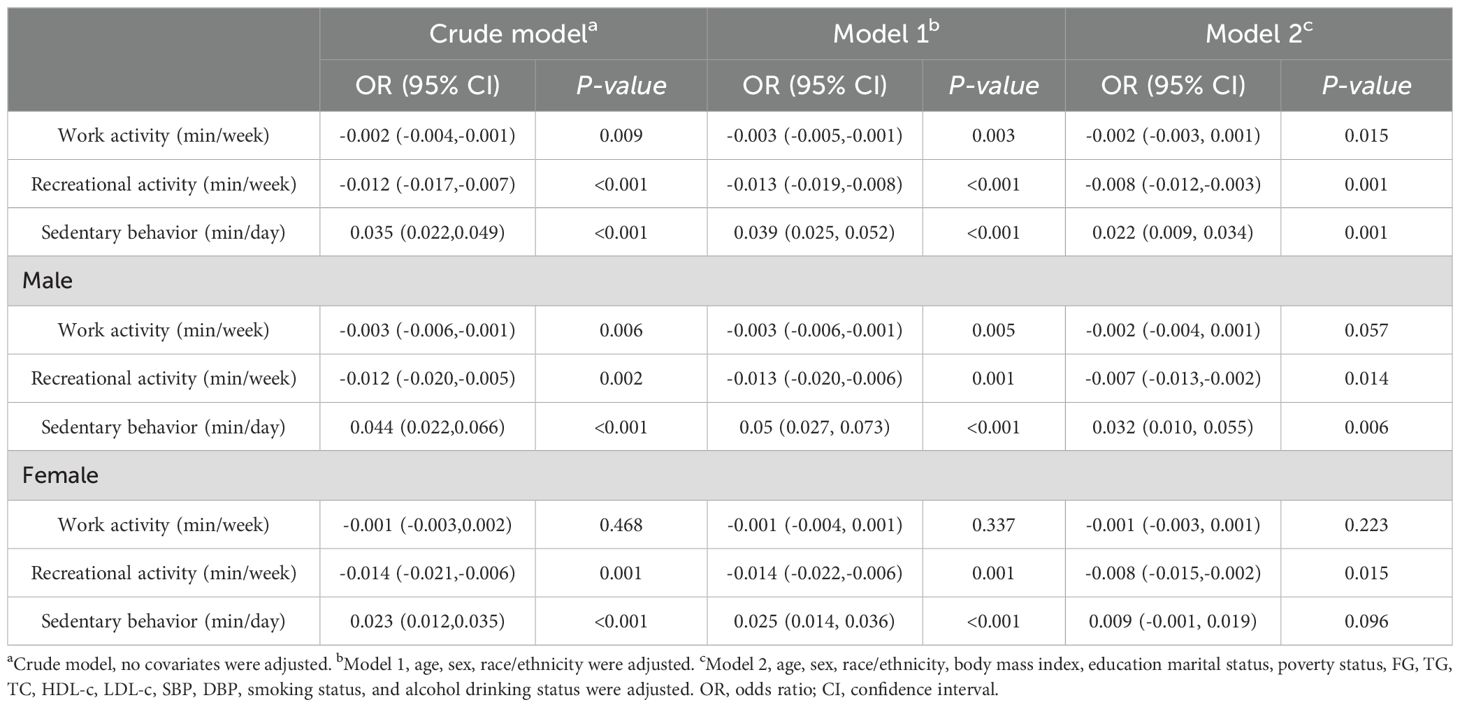
Table 2. Association between physical activity, sedentary behavior and serum insulin level in short sleepers.
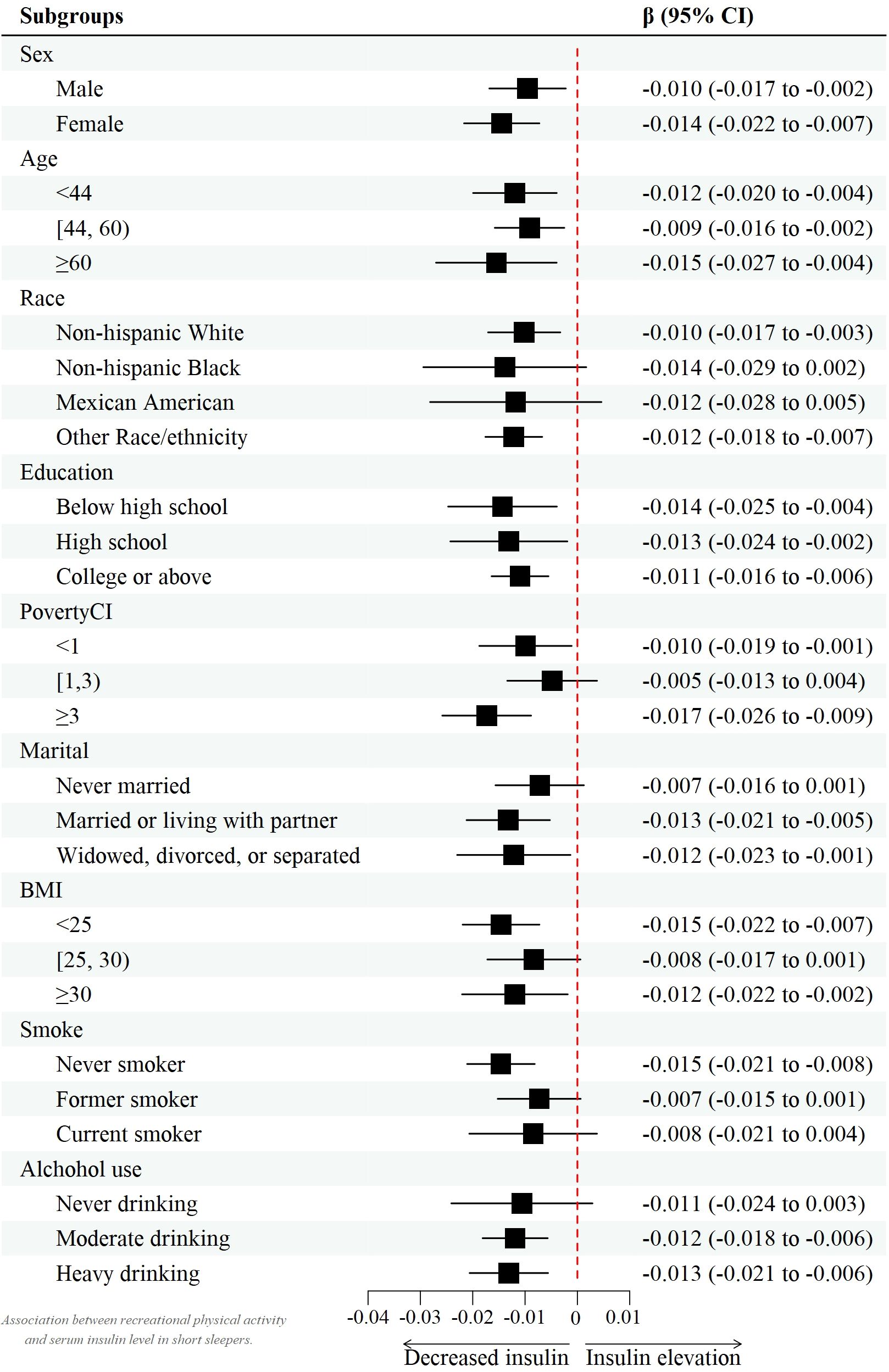
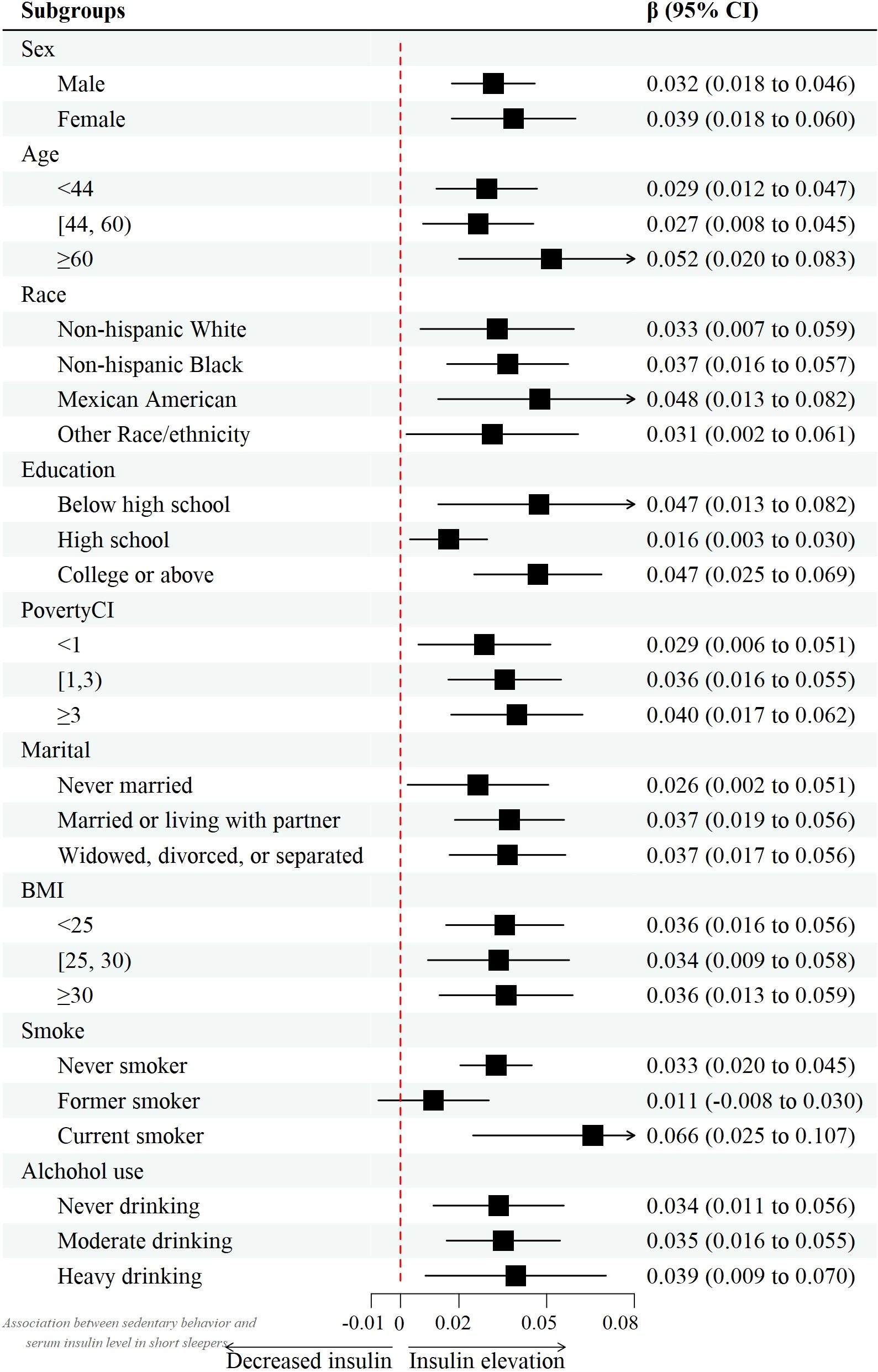
However, after controlling for confounders, this association only persisted in male leisure sport and sedentary behavior when grouped by sex. (model 2) [β value 95% CI = -0.007 (-0.013, -0.002), β value 95% CI =0.032 (0.010, 0.055), respectively] and female recreational activity [β value 95% CI =-0.008 (-0.015, -0.002)]. Figures 2–4 show the results of the subgroup analyses on the effect of work activity, recreational activity and sedentary behaviors on insulin levels, respectively.
Sensitivity analysis in participants without DM
DM should always be identified as a major covariate. A diabetes-specific awareness evaluation was carried, and the relationship between regular exercise and insulin in short sleepers was observed among participants without have diabetes. As determined by multivariate linear regression, work activity and leisure activity were negatively associated with glycemia in attendees without hyperglycemia of both sexes.[β value 95% CI = -0.001 (−0.003, 0.000), β value 95% CI = -0.005 (-0.009, -0.001), respectively]. In spite of this, in all participants without DM only recreational activity was associated with lower insulin levels in those without diabetes mellitus after controlling for confounding variables.[Model 2, β value 95% CI =-0.005 (-0.008, -0.002)]. Males were also found to have this association when grouped by sex. [β value 95% CI =-0.006 (-0.010, -0.002)]. Moreover, sedentary behavior was positively correlated with insulin levels in this group [β value 95% CI =0.011 (0.001,0.021)]. However, according to the analytical results in females, there was no significant positive or negative association among all three groups (P > 0.05). Table 3 demonstrates the association between work activity, recreational activity, sedentary behavior, and serum insulin levels in short sleepers without DM.
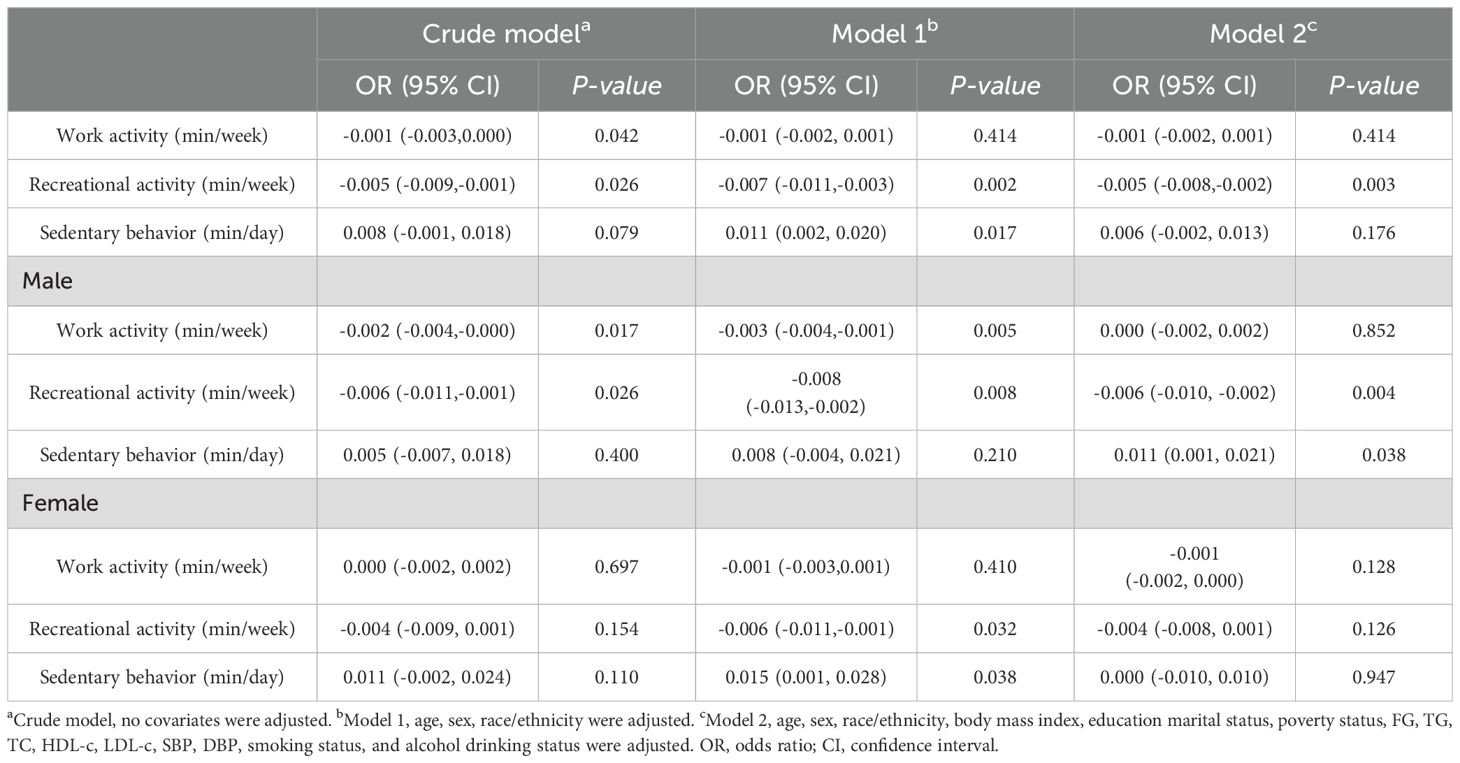
Table 3. Association between physical activity, sedentary behavior and serum insulin level in short sleepers without DM.
The Effect of PA, SB and lipid indices (LDL-C, HDL-C, FG, TC and TG) interaction on insulin levels in short sleepers
Shows in Table 4 that different quantile levels of physical activity and lipid indices (LDL-c, TC, TG and HDL-c) have different effects on insulin levels. First, only the recreational activity group showed significant differences in the lower HDL-c quantile, with β values. 95% CI =−0.010 (−0.017, −0.003) in the adjusted multivariate regression model. In addition to recreational activity, Insulin levels in the HDL-c tertile 2 were found to be linked to inactivity, according with study. [(β-value. 95% CI =0.019 (0.005,0.032)].
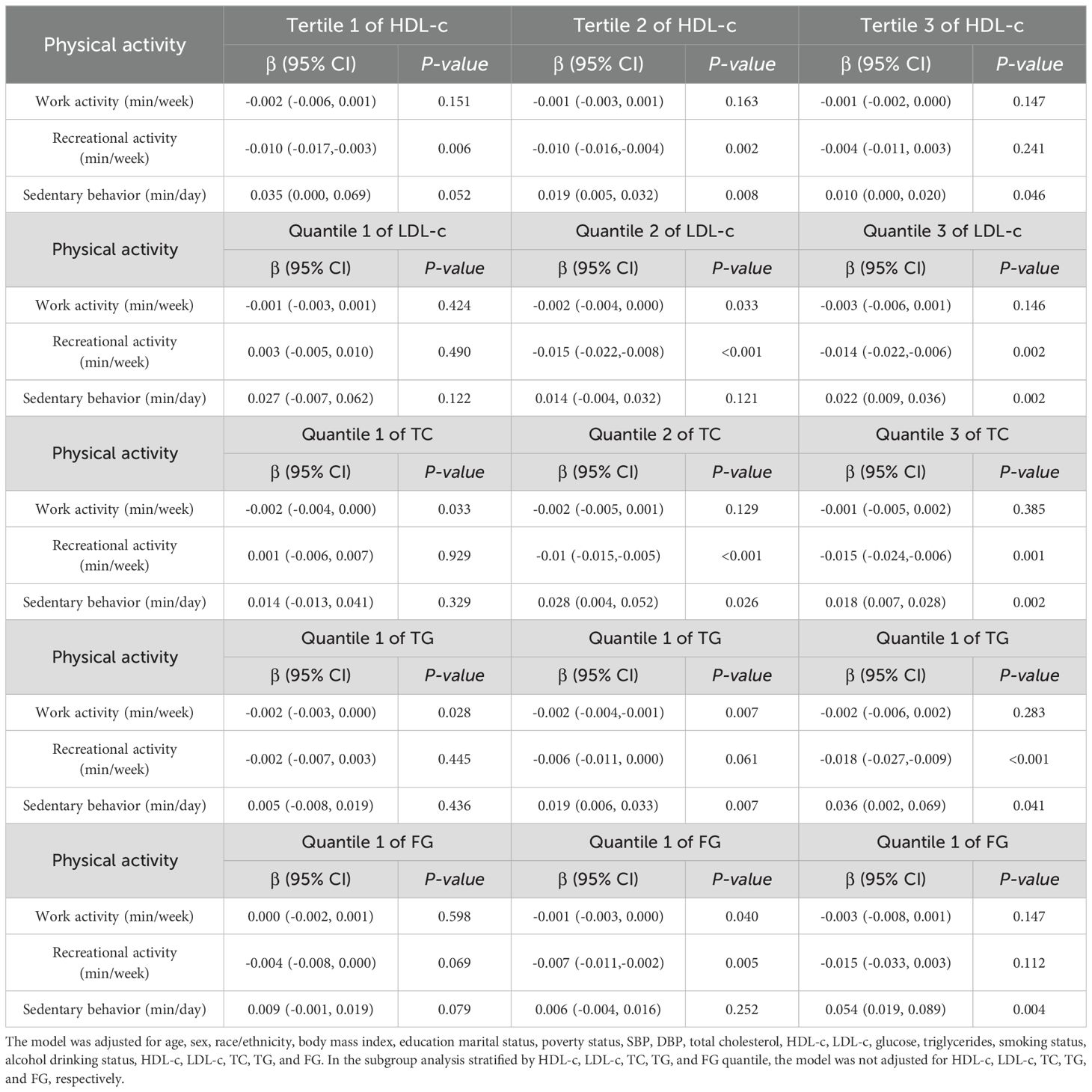
Table 4. The effect of PA and lipid indices (LDL-c, HDL-c, FG, TC and TG) interaction on the insulin levels in short sleepers.
However, in the highest HDL-c tertile, only sedentary behavior was positively correlated with insulin levels [β value 95% CI =0.010 (0.000,0.020)]. Second, no significant positive or negative association was observed among all three groups (P > 0.05) with insulin levels in the lower LDL-c quantile. However, a significant negative association was observed for work activity [β value 95% CI =-0.002 (-0.004, 0.000)] and recreational activity [β value 95% CI =-0.015 (-0.022, -0.008)]. Similarly, we found a significant negative association between recreational activity and insulin levels [β value 95% CI =-0.014 (-0.022, -0.006)] and a positive association with sedentary behavior [β value 95% CI =0.022 (0.009, 0.036)]. Third, lower TC and TG tertile presented the same trend. Namely, only work activity was negatively correlated with insulin levels [β value 95% CI =−0.002 (−0.004,0.000), β value 95% CI =-0.002 (-0.003,0.000), respectively]. Whereas sedentary behavior was positively correlated with insulin levels [β value 95% CI =0.028 (0.004,0.052), β value 95% CI =0.019 (0.006,0.033), respectively] in this group, work activity was significantly negatively correlated with insulin levels in TG [β value 95% CI =-0.002 (-0.004, -0.001)] while TC was not. In contrast, recreational activity was significantly negatively correlated with insulin levels in TC [β value 95% CI =-0.01 (-0.015, -0.005)], while TG was not. Simultaneously, upper TC and TG quantiles also presented the same trend, namely, recreational activity was significantly negatively correlated with insulin levels [β value 95% CI =−0.015 (−0.024, -0.006), β value 95% CI =-0.018 (-0.027, -0.009), respectively], and a positive association [β value 95% CI =0.018 (0.007, 0.028), β value 95% CI = 0.036 (0.002, 0.069), respectively] was observed in sedentary behavior. Last but not least, similar to the lower LDL-c quantile, all three groups had no significant positive or negative association (P > 0.05) with insulin levels in the lower FG quantile. Nevertheless, work activity and recreational activity presented a significant negative association with insulin level [β value 95% CI = -0.001 (-0.003,0.000), β value 95% CI =-0.007 (-0.011, -0.002), respectively]. In addition, only sedentary behavior had significant differences in the sedentary behavior group, with β values 95% CI = 0.054 (0.019,0.089).
Discussion
To our knowledge, this is the first study to show the association between different forms of PA, SB and insulin levels in short sleepers, and we further analyzed this association based on characteristics of the large included population, conducted a sensitivity analysis in participants’ diabetes mellitus, and explored the effect of PA and different levels of lipid indices (LDL-C, HDL-C, FG, TC and TG). A previous study confirmed that PA can improve IR (43). Herein, we could regard sedentary behavior as an extensively low level of PA (44). Because of the findings in this study, our results showed that work and recreational activity were negatively correlated with insulin, while sedentary behavior was the opposite, and this tendency persisted in further stratified analysis. Consequently, even in short sleepers, it’s possible that higher PA densities might dramatically decrease insulin levels. However, the results also showed that recreational activity significantly reduced insulin levels more than work activity. Other studies have also confirmed this view. Namely, it is not enough to rely only on work-related physical activity to maintain fitness, and they have to increase recreational activity to ensure the effect of preventing a variety of chronic diseases (45, 46).
Although plentiful evidence showed that SB exerted great harm to the body, increasing the risk of multiple diseases and mortality (47, 48), the estimated prevalence of prolonged sitting per day generally remained high and stable, approximately 62% [95% CI, 58% to 66%] in US adults (49). Provided that plus the hazard risk caused by insufficient sleep, there is no doubt that the risk of multiple diseases will increase (50, 51). Our results also reflected this fact, while it was interesting that female SB seemed not to be significantly positively correlated with insulin levels in the adjusted model, which coincided with previous research results (52). Insulin sensitivity and SB may be influenced by estrogen and progesterone, which have the possibilities to govern neuro transmitters and maintain beta-cell competence, which could account for this finding (53, 54). This conclusion also applied to participants without diabetes mellitus in the sensitivity analysis after adjusting for confounding factors. Thus, it could be seen that males without diabetes should further reduce sedentary behavior to prevent the occurrence of IR.
In contrast, both recreational and work activities were significantly positively correlated with insulin levels in all participants. Unlike sedentary behavior, recreational activity was significantly associated with insulin levels in both males and females. However, work activity did not seem to have such a significant trend in all genders, even in this particular but enormous population that possessed insufficient sleep. Generally, leisure time is a major part of PA and the amount of activity generated by work is insufficient to maintain basic PA needs (45, 55, 56). Additionally, among females without diabetes, their recreational activity level was insignificantly correlated with insulin level. In addition to the reasons mentioned above, there are sex differences in substrate utilization during PA (57). As a result, more research is needed to determine the role of sexual hormones or sex discrepancies inside this route.
Among the explorations of the effect of PA and several lipid index interactions on insulin levels in short sleepers, we found that low or middle levels of HDL-C seemed to have a significant association with PA, while high levels demonstrated the opposite trend. LDL-C appeared to be the contrary result, with a high level positively correlated with SB A low level negatively correlated with recreational activity. Simultaneously, according to our results, it seemed that when TG was at the medium level, the relationship between PA or SB within lower degrees, and insulin was more significant. In contrast, with the increase in TG level, the greater the intensity of PA, the closer the negative relationship between PA and insulin was; obviously, TC presented similar results. The latest research has shown that insulin resistance affects the occurrence and development of dyslipidemia through several mechanisms, including TG, HDL-c, and LDL-c (58, 59).
Increased HTGL exercise is seen to be heavily associated to IR, which could lead to a faster HDL-C clearance and a reduction in HDL-C levels (52, 60). In addition, we can observe that when fast blood glucose (FG) is at a medium-high level, the intensity of PA shows a significant negative correlation with it. At the same time, SB would further significantly increase the body content of FG, which does not also increase the possibility of occurrence of IR but also a powerful independent risk marker for cardiovascular diseases (CVD) and contributes to the increased atherosclerotic risk in diabetes mellitus (DM) (61, 62). The latest study claimed that a high binding affinity was observed in patients with T2DM samples concerning healthy subjects against Methylglyoxal glycated fibrinogen antigen compared to native fibrinogen, which might explain this phenomenon to some extent (62). The above mentioned insulin resistance and dyslipidemia are all risk factors for CVD and DM. Based on our findings, the intensity and duration of PA can prevent these phenomena because the significant negative association between LDL-c, TG and PA is observed. This is consistent with recent research results, namely, moderate-intensity aerobic exercise can significantly improve insulin sensitivity, Each time about more than 30 minutes, three times a week for a minimum of eight weeks (54, 63).
Consequently, many chronic diseases have been linked to a lack of sleep, and we found that even short sleepers can benefit from the therapeutic benefits of PA, this conclusion is still suitable. In many cases, people lack a comfortable environment that can provide enough sleep duration and good sleep quality (64), or those short sleepers due to various reasons, such as anxiety and depression, can not only mitigate the severity of these emotional diseases (65) but also prevent the occurrence of CVD and DM by conducting regular moderate-intensity aerobic exercise. Additionally, PA usually has no other adverse effects and can cure multiple health concerns immediately, which is the most cost-effective means of maintaining health (66).
This study had several limitations that must be acknowledged. First, since the statistical analysis was based solely on cross-sectional data, it is challenging to establish causality between physical activity (PA) and insulin levels. To strengthen these findings, larger population-based, high-quality randomized controlled trials or longitudinal cohort studies are needed to confirm these conclusions (67). Second, several potential confounding factors were not fully accounted for, such as underlying disorders, dietary habits, and medications, particularly hypoglycemic agents. In NHANES, dietary habits are assessed only once at baseline, without information on changes over time, which limits the accuracy of dietary data. Furthermore, the use of self-reported dietary information introduces recall bias. Third, most measurements of work activity, sedentary behavior, and recreational activity were based on self-reports, which may not be entirely accurate. Future research should consider incorporating more objective methods, such as accelerometers, to capture the volume and intensity of PA with greater precision.
Conclusions
This study explored the association between physical activity, sedentary behavior and insulin levels in short sleepers for the first time and demonstrated that work activity and recreational activity were negatively correlated with insulin. In contrast, sedentary behavior was the opposite in this population. The sensitivity analysis further confirmed that males without diabetes should especially focus on reducing sedentary behavior to prevent insulin resistance. Short sleepers with regular moderate-intensity aerial exercise can not only mitigate the severity but also prevent the occurrence of CVD and DM, making it an effective approach to maintaining health. Based on our results, further large-scale, high-quality randomized controlled trials are needed to be carried out to confirm the conclusion and further explore the association in the future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. PY: Data curation, Software, Writing – review & editing. QiZ: Formal analysis, Software, Writing – review & editing. XL: Formal analysis, Writing – review & editing. QuZ: Project administration, Supervision, Writing – review & editing. QC: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this publication are those of the authors.
Abbreviations
PA, Physical activity; IR, Insulin resistance; NHANES, National Health and Nutrition Examination Survey; SB, Sedentary behavior; PI, Physical inactivity; IR, Insulin resistance; DM, Diabetes mellitus; T2DM, Type 2 diabetes mellitus; TG, Triglycerides; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; TC, Total serum cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
References
1. Dutil C, Chaput JP. Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutr Diabetes. (2017) 7:e266. doi: 10.1038/nutd.2017.19
2. Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. (2020) 45:74–89. doi: 10.1038/s41386-019-0411-y
3. Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. (2016) 355:i5210. doi: 10.1136/bmj.i5210
4. You Y, Liu J, Li X, Wang P, Liu R, Ma X. Relationship between accelerometer-measured sleep duration and Stroop performance: a functional near-infrared spectroscopy study among young adults. PeerJ. (2024) 12:e17057. doi: 10.7717/peerj.17057
5. Mead MP, Irish LA. Application of health behaviour theory to sleep health improvement. J Sleep Res. (2020) 29:e12950. doi: 10.1111/jsr.12950
6. Niswender KD. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad Med. (2011) 123:17–26. doi: 10.3810/pgm.2011.07.2300
7. Thompson R, Christie D, Hindmarsh PC. The role for insulin analogues in diabetes care. Paediatrics and Child Health (2006) 16(2):117–22. doi: 10.1016/j.cupe.2005.12.011
8. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. (2001) 109 Suppl 2:S135–48. doi: 10.1055/s-2001-18576
9. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. (2013) 3:1-58. doi: 10.1002/cphy.c110062
10. Freeman JS. Insulin analog therapy: improving the match with physiologic insulin secretion. J Am Osteopath Assoc. (2009) 109:26–36. doi: 10.7556/jaoa.2009.109.1.26
11. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. (2006) 444:840–6. doi: 10.1038/nature05482
12. Zuo H, Shi Z, Yuan B, Dai Y, Hu G, Wu G, et al. Interaction between physical activity and sleep duration in relation to insulin resistance among non-diabetic Chinese adults. BMC Public Health. (2012) 12:247. doi: 10.1186/1471-2458-12-247
13. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee D-C, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. (2015) 117:207–19. doi: 10.1161/CIRCRESAHA.117.305205
14. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. (2019) 124:799–815. doi: 10.1161/CIRCRESAHA.118.312669
15. You Y, Chen Y, Fang W, Li X, Wang R, Liu J, et al. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front Immunol. (2022) 13:1080782. doi: 10.3389/fimmu.2022.1080782
16. You Y, Li W, Liu J, Li X, Fu Y, Ma X. Bibliometric review to explore emerging high-intensity interval training in health promotion: A new century picture. Front Public Health. (2021) 9:697633. doi: 10.3389/fpubh.2021.697633
17. You Y. Accelerometer-measured physical activity and sedentary behaviour are associated with C-reactive protein in US adults who get insufficient sleep: A threshold and isotemporal substitution effect analysis. J sports Sci. (2024) 42:527–36. doi: 10.1080/02640414.2024.2348906
18. You Y, Ablitip A, Chen Y, Ding H, Chen K, Cui Y, et al. Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: a cross-sectional study. BMC Public Health. (2024) 24:1920. doi: 10.1186/s12889-024-19432-7
19. Farni K, Shoham DA, Cao G, Luke AH, Layden J, Cooper RS, et al. Physical activity and pre-diabetes-an unacknowledged mid-life crisis: findings from NHANES 2003-2006. PeerJ. (2014) 2:e499. doi: 10.7717/peerj.499
20. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
21. Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism. (2010) 59:1268–75. doi: 10.1016/j.metabol.2009.11.020
22. Richter EA, Sylow L, Hargreaves M. Interactions between insulin and exercise. Biochem J. (2021) 478:3827–46. doi: 10.1042/BCJ20210185
23. Whillier S. Exercise and insulin resistance. Adv Exp Med Biol. (2020) 1228:137–50. doi: 10.1007/978-981-15-1792-1_9
24. Nagayama C, Burns SF, Thackray AE, Stensel DJ, Miyashita M. Postprandial metabolism and physical activity in Asians: A narrative review. Int J Sports Med. (2021) 42:953–66. doi: 10.1055/a-1493-2948
25. Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: A systematic review and meta-analysis. Sports Med. (2020) 50:295–330. doi: 10.1007/s40279-019-01183-w
26. Silva DR, Werneck AO, Collings PJ, Fernandes RA, Barbosa DS, Ronque ERV, et al. Physical activity maintenance and metabolic risk in adolescents. J Public Health (Oxf). (2018) 40:493–500. doi: 10.1093/pubmed/fdx077
27. You Y, Liu J, Yao Z, Zhang S, Chen K, Ma X. Neural mechanisms of long-term exercise intervention on cognitive performance among short-sleep young adults: A hemodynamic study. Sleep Med. (2023) 110:7-16. doi: 10.1016/j.sleep.2023.07.020
28. You Y, Liu J, Wang D, Fu Y, Liu R, Ma X. Cognitive performance in short sleep young adults with different physical activity levels: A cross-sectional fNIRS study. Brain Sci. (2023) 13:171. doi: 10.3390/brainsci13020171
29. Gollie JM, Cohen SD, Patel SS. Physical Activity and Exercise for Cardiorespiratory Health and Fitness in Chronic Kidney Disease. Rev Cardiol Med (2022) 23(8):273. doi: 10.31083/j.rcm23082
30. Štefan L, Sporiš G, Krističević T, Knjaz D. Associations between sleep quality and its domains and insufficient physical activity in a large sample of Croatian young adults: a cross-sectional study. BMJ Open. (2018) 8:e021902. doi: 10.1136/bmjopen-2018-021902
31. You Y, Chen Y, Zhang Q, Yan N, Ning Y, Cao Q. Muscle quality index is associated with trouble sleeping: a cross-sectional population based study. BMC Public Health. (2023) 23:489. doi: 10.1186/s12889-023-15411-6
32. You Y, Chen Y, Zhang Y, Zhang Q, Yu Y, Cao Q. Mitigation role of physical exercise participation in the relationship between blood cadmium and sleep disturbance: a cross-sectional study. BMC Public Health. (2023) 23:1465. doi: 10.1186/s12889-023-16358-4
33. Moscatelli F, De Maria A, Marinaccio LA, Monda V, Messina A, Monacis D, et al. Assessment of lifestyle, eating habits and the effect of nutritional education among undergraduate students in Southern Italy. Nutrients. (2023) 15:2894. doi: 10.3390/nu15132894
34. You Y, Chen Y, Chen X, Wei M, Yin J, Zhang Q, et al. Threshold effects of the relationship between physical exercise and cognitive function in the short-sleep elder population. Front Aging Neurosci. (2023) 15:1214748. doi: 10.3389/fnagi.2023.1214748
35. You Y, Wei M, Chen Y, Fu Y, Ablitip A, Liu J, et al. The association between recreational physical activity and depression in the short sleep population: a cross-sectional study. Front Neurosci. (2023) 17:1016619. doi: 10.3389/fnins.2023.1016619
36. National health and nutrition examination survey, data documentation, codebook, and frequencies(2024). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/ (Accessed October 01, 2024).
37. You Y, Chen Y, Liu R, Zhang Y, Wang M, Yang Z, et al. Inverted U-shaped relationship between sleep duration and phenotypic age in US adults: a population-based study. Sci Rep. (2024) 14:6247. doi: 10.1038/s41598-024-56316-7
38. You Y, Chen Y, Wei M, Tang M, Lu Y, Zhang Q, et al. Mediation role of recreational physical activity in the relationship between the dietary intake of live microbes and the systemic immune-inflammation index: A real-world cross-sectional study. Nutrients. (2024) 16:777. doi: 10.3390/nu16060777
39. You Y, Mo L, Tong J, Chen X, You Y. The role of education attainment on 24-hour movement behavior in emerging adults: evidence from a population-based study. Front Public Health. (2024) 12:1197150. doi: 10.3389/fpubh.2024.1197150
40. You Y, Wang R, Li J, Cao F, Zhang Y, Ma X. The role of dietary intake of live microbes in the association between leisure-time physical activity and depressive symptoms: a population-based study. Appl physiology nutrition Metab = Physiologie appliquee Nutr metabolisme. (2024) 49:1014–24. doi: 10.1139/apnm-2023-0550
41. You Y, Li J, Zhang Y, Li X, Li X, Ma X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: Insights from population-based and mice studies. CNS Neurosci Ther. (2024) 30:e14783. doi: 10.1111/cns.14783
42. You Y, Chen Y, Wang X, Wei M, Zhang Q, Cao Q. Accelerometer-measured physical activity patterns are associated with phenotypic age: Isotemporal substitution effects. Heliyon. (2023) 9:e19158. doi: 10.1016/j.heliyon.2023.e19158
43. Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, et al. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 62:98-103. doi: 10.1016/j.rehab.2018.11.001
44. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, et al. Level and correlates of physical activity and sedentary behavior in patients with type 2 diabetes: A cross-sectional analysis of the Italian Diabetes and Exercise Study_2. PloS One. (2017) 12:e0173337. doi: 10.1371/journal.pone.0173337
45. Abd TT, Kobylivker A, Perry A, Miller Iii J, Sperling L. Work-related physical activity among cardiovascular specialists. Clin Cardiol. (2012) 35:78–82. doi: 10.1002/clc.21954
46. Matsushita M, Harada K, Arao T. Socioeconomic position and work, travel, and recreation-related physical activity in Japanese adults: a cross-sectional study. BMC Public Health. (2015) 15:916. doi: 10.1186/s12889-015-2226-z
47. Patterson R, McNamara E, Tainio M, de Sá TH, Smith AD, Sharp SJ, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. (2018) 33:811–29. doi: 10.1007/s10654-018-0380-1
48. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. (2012) 55:2895–905. doi: 10.1007/s00125-012-2677-z
49. Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. (2019) 321:1587–97. doi: 10.1001/jama.2019.3636
50. Tambalis KD, Panagiotakos DB, Psarra G, Sidossis LS. Insufficient sleep duration is associated with dietary habits, screen time, and obesity in children. J Clin Sleep Med. (2018) 14:1689–96. doi: 10.5664/jcsm.7374
51. Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int J Obes (Lond). (2009) 33 Suppl 1:S82–6. doi: 10.1038/ijo.2009.23
52. Lin Y, Fan R, Hao Z, Li J, Yang X, Zhang Y, et al. The association between physical activity and insulin level under different levels of lipid indices and serum uric acid. Front Physiol. (2022) 13:809669. doi: 10.3389/fphys.2022.809669
53. Bertoli A, De Pirro R, Fusco A, Greco AV, Magnatta R, Lauro R. Differences in insulin receptors between men and menstruating women and influence of sex hormones on insulin binding during the menstrual cycle. J Clin Endocrinol Metab. (1980) 50:246–50. doi: 10.1210/jcem-50-2-246
54. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. (2016) 2:e000143. doi: 10.1136/bmjsem-2016-000143
55. Ma L, Hagquist C, Kleppang AL. Leisure time physical activity and depressive symptoms among adolescents in Sweden. BMC Public Health. (2020) 20:997. doi: 10.1186/s12889-020-09022-8
56. Wong PM, Manuck SB, DiNardo MM, Korytkowski M, Muldoon MF. Shorter sleep duration is associated with decreased insulin sensitivity in healthy white men. Sleep. (2015) 38:223–31. doi: 10.5665/sleep.4402%JSleep
57. Ruby BC, Robergs RA. Gender differences in substrate utilisation during exercise. Sports Med. (1994) 17:393–410. doi: 10.2165/00007256-199417060-00005
58. Bjornstad P, Eckel RH. Pathogenesis of lipid disorders in insulin resistance: a brief review. Curr Diabetes Rep. (2018) 18:127. doi: 10.1007/s11892-018-1101-6
59. Nôga DA, Meth E, Pacheco AP, Tan X, Cedernaes J, van Egmond LT, et al. Habitual short sleep duration, diet, and development of type 2 diabetes in adults. JAMA network Open. (2024) 7:e241147–e241147. doi: 10.1001/jamanetworkopen.2024.1147%JJAMANetworkOpen
60. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. (2012) 32:2104–12. doi: 10.1161/ATVBAHA.111.241463
61. Dunn EJ, Ariëns RAS. Fibrinogen and fibrin clot structure in diabetes. Herz. (2004) 29:470–9. doi: 10.1007/s00059-004-2607-z
62. Rehman S, Song J, Faisal M, Alatar AA, Akhter F, Ahmad S, et al. The neoepitopes on methylglyoxal- (MG-) glycated fibrinogen generate autoimmune response: its role in diabetes, atherosclerosis, and diabetic atherosclerosis subjects. Oxid Med Cell Longev. (2021) 2021:6621568. doi: 10.1155/2021/6621568
63. McGarrah RW, Slentz CA, Kraus WE. The effect of vigorous- versus moderate-intensity aerobic exercise on insulin action. Curr Cardiol Rep. (2016) 18:117. doi: 10.1007/s11886-016-0797-7
64. Antunes BM, Campos EZ, Parmezzani SS, Santos RV, Franchini E, Lira FS. Sleep quality and duration are associated with performance in maximal incremental test. Physiol Behav. (2017) 177:252–6. doi: 10.1016/j.physbeh.2017.05.014
65. Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. (2019) 107:525–39. doi: 10.1016/j.neubiorev.2019.09.040
66. Gebreslassie M, Sampaio F, Nystrand C, Ssegonja R, Feldman I. Economic evaluations of public health interventions for physical activity and healthy diet: A systematic review. Prev Med. (2020) 136:106100. doi: 10.1016/j.ypmed.2020.106100
Keywords: physical activity, sedentary behavior, serum insulin, NHANES, short sleepers
Citation: Chen Y, You Y, Wei M, Yang P, Zhang Q, Li X, Zuo Q and Cao Q (2024) Exploration of physical activity, sedentary behavior and insulin level among short sleepers. Front. Endocrinol. 15:1371682. doi: 10.3389/fendo.2024.1371682
Received: 16 January 2024; Accepted: 20 September 2024;
Published: 14 October 2024.
Edited by:
Xun Lei, Chongqing Medical University, ChinaReviewed by:
Giovanni Messina, University of Foggia, ItalyRakhmat Ari Wibowo, Gadjah Mada University, Indonesia
Copyright © 2024 Chen, You, Wei, Yang, Zhang, Li, Zuo and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Zuo, enVvcXVuMjAwNkAxMjYuY29t; Qiang Cao, MjkxODI5Mjg2MUBxcS5jb20=
†These authors have contributed equally to this work
 Yuquan Chen
Yuquan Chen Yanwei You
Yanwei You Mengxian Wei2†
Mengxian Wei2† Ping Yang
Ping Yang Qi Zhang
Qi Zhang Xingzhong Li
Xingzhong Li Qiang Cao
Qiang Cao