- Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, China
Context: The coexistence of hypertension and elevated homocysteine (Hcy) levels has a mutually reinforcing impact on the susceptibility to cardio-cerebrovascular disease.
Objective: The aim was to assess the prevalence, clinical correlation, and demographic characteristics of hyperhomocysteinemia (HHcy) within the Chinese urban population with hypertension.
Methods: A cohort of 473 individuals with hypertension were selected from four communities in Shenzhen, China. Demographic attributes, clinical profiles, and lifestyle behaviors were gathered and compared between individuals with and without HHcy. A logistic regression model was employed to examine potential factors associated with the prevalence of HHcy. Correlation between Hcy levels and clinical characteristics was assessed through multiple linear regression analysis.
Results: The prevalence of HHcy in the population with hypertension was 31.3%. In comparison to individuals without HHcy, those with HHcy exhibited a higher proportion of males, a higher prevalence of smoking and alcohol consumption, and a higher proportion of cases with the homozygous (TT) genotype at the MTHFR C677T polymorphism. Moreover, individuals with HHcy had lower levels of folic acid (FA), and lower fruit and vitamin B12 intake. Furthermore, the risk factors for HHcy were male (B = 1.430, OR = 4.179) and MTHFR (TT) (B = 1.086, OR = 2.961). In addition, the multiple linear regression analysis revealed a significant association between Hcy levels and gender (B = -2.784, P = 0.004), MTHFR genotypes (B = 1.410, P = 0.005), and FA levels (B = -0.136, P = 0.030).
Conclusion: The high prevalence of HHcy among hypertensive patients in this Chinese urban population underscores the necessity for interventions targeting modifiable risk factors such as dietary choices and lifestyle practices.
Introduction
In the past three decades, there has been a persistent global rise in the prevalence of cardio-cerebrovascular disease. Specifically, cardiovascular disease (CVD) persists as the leading cause of mortality and morbidity on a global scale. Over the course of the last three decades, the burden of CVD has consistently escalated, with a notable 53.7% increase in the number of deaths (from 12.1 million in 1990 to 18.6 million in 2019) and a substantial 94.4% rise in years lived with disability (from 17.7 million in 1990 to 34.4 million in 2019) (1). Significantly, China experienced the highest number of CVD fatalities, with CVD ranking as the primary cause of death in both rural and urban regions, constituting 46.7% and 44.3% of total mortalities in 2019, respectively (2). Similarly, cerebrovascular disease ranks as the second most lethal ailment on a global scale, and acute cerebrovascular disease exhibits a higher incidence of disability compared to any other individual disease, thereby imposing substantial societal burdens (3). Hypertension has emerged as the primary risk factor for cardio-cerebrovascular disease (4). Due to the widespread adoption of unhealthy lifestyles resulting from rapid economic development and the accelerated aging process, hypertension has witnessed an increase in low- and middle-income countries, including China (5). According to recent survey data from China, the prevalence of hypertension among individuals aged 18 years from 2012 to 2015 reached a significant rate of 27.9% (6). Unfortunately, the existing measures for preventing and controlling hypertension are inadequate (7, 8). Consequently, determining the most effective approach to enhance hypertension management and alleviate the burden of cardio-cerebrovascular disease is a significant global concern.
Homocysteine (Hcy) is a sulfur-containing intermediary amino acid generated through the metabolic conversion of methionine to cysteine. The condition of having elevated plasma concentrations of Hcy above the established normal threshold of 15 μmol/l is referred to as hyperhomocysteinemia (HHcy) (9, 10). HHcy could potentially have detrimental effects on individuals with hypertension, as it collaboratively enhances the risk of cardio-cerebrovascular disease through a multiplicative effect (11, 12). At present, the specific mechanisms by which HHcy influences the susceptibility to hypertension and cardio-cerebrovascular disease remain uncertain. Several hypotheses have been proposed. HHcy has been suggested to potentially elevate the likelihood of developing hypertension and cardio-cerebrovascular disease through the subsequent mechanisms (13): (1) elevation of arterial blood pressure; (2) induction of endothelial dysfunction by augmenting oxidant stress and reducing nitric oxide release, thereby impairing vasodilation; (3) provoking oxidative damage to vascular endothelial cells and hindering the synthesis of nitric oxide, a potent vasodilator, by the endothelium; or (4) augmenting platelet adhesion to endothelial cells, thereby facilitating the proliferation of vascular smooth muscle cells. A nested case-control study conducted in China, involving a cohort of 39,165 Chinese participants who were followed for an average duration of 6.2 years, revealed that individuals diagnosed with hypertension and exhibiting elevated levels of Hcy faced a significantly heightened risk of stroke and stroke-related mortality (14). Compared to those with normal blood pressure and Hcy levels, the aforementioned group experienced an 11.7-fold increase in the likelihood of stroke and a 10.7-fold increase in the likelihood of stroke-related death (14). More recently, a retrospective cohort study conducted in China, involving a total of 1226 participants and a follow-up period of 17 years, presents compelling evidence indicating that individuals with hypertension and an Hcy level exceeding 15 μmol/L had a significantly higher risk of developing stroke and cardiovascular diseases compared to those with hypertension and an Hcy level below 10 μmol/L (15). The adjusted hazard ratios for stroke and cardiovascular diseases were found to be 2.12 and 2.24, respectively (15). Therefore, the identification of distinct risk factors associated with HHcy in hypertensive patients holds significant academic importance in mitigating the occurrence of cardio-cerebrovascular events.
In China, the prevalence of H-type hypertension, characterized by hypertension and HHcy, is observed to be higher compared to other populations (16). This can be attributed to inadequate dietary intake of folic acid (FA) and B vitamins, as well as a high mutation rate of the methylenetetrahydrofolate reductase (MTHFR) gene (the C677T single nucleotide polymorphism), leading to elevated levels of Hcy (17, 18). In the present study, we evaluated the prevalence, clinical association, and demographic attributes of HHcy among hypertensive individuals in the Chinese urban population, with the aim to identify specific risk factors of HHcy in hypertensive patients and provide intervention and management strategies for cardio-cerebrovascular disease.
Methods
Subjects
This study employed a cross-sectional observational design and involved the recruitment of 473 individuals diagnosed with hypertension from four communities in Shenzhen, China. Inclusion criteria for all participants encompassed: (1) being over 18 years of age; (2) belonging to the Han Chinese population; (3) adhering to the Chinese Guidelines for the Prevention and Treatment of Hypertension (2018 Revised Edition); (4) being local residents. Exclusion criteria for all participants encompassed: (1) the existence of a neurological diagnosis, a serious medical condition, or an unstable medical condition; (2) a diagnosis of major psychiatric disorders such as schizophrenia and major depressive disorder, severe somatic disease, or substance abuse. The study protocol received approval from the Clinical Research Ethics Committee of Shenzhen Second People’s Hospital (ID number: 20210824001) and was conducted in adherence to the principles outlined in the Declaration of Helsinki. Prior to participation, all participants provided written informed consent subsequent to receiving a comprehensive explanation of the study.
Demographic and clinical data collection
Each participant completed a comprehensive questionnaire capturing general information, sociodemographic characteristics, and lifestyle behaviors. Additionally, a physical examination was conducted, encompassing assessments of height, weight, body mass index (BMI), heart rate, and blood pressure measurements. Furthermore, fasting venous blood samples were collected from each subject between 8:00 and 9:00 AM. Subsequently, the blood samples were subjected to centrifugation at a speed of 2500 revolutions per minute for a duration of 20 minutes at a temperature of 4°C. The resulting serum was then collected and preserved at a temperature of -80°C until the time of analysis. Biochemical parameters pertaining to liver and kidney function, serum lipids, and glucose levels were quantified using an automated biochemical analyzer. Plasma Hcy, folic acid (FA), vitamin B12 and vitamin D levels, and methylenetetrahydrofolate reductase (MTHFR) C677T and reduced folate carrier (RFC) G80A genotypes were determined by the Shenzhen Tailored Medical Laboratory (Shenzhen, China). In terms of MTHFR C677T genotype and RFC G80A genotype, they were analyzed using a fluorescence PCR detection kit (PCR-fluorescence probe). Specifically, Fluorescence PCR detection was performed using a 4-μl whole genome DNA sample and a 10-μl PCR reaction system. The denaturation step was carried out at 95°C for 15 s, followed by annealing/extension at 60°C for 60 s, as recommended by the manufacturer. The reaction was subjected to 45 cycles. Following the completion of the PCR, the endpoint fluorescence in each well of the samples was quantified utilizing the ABI 7500 fluorescence quantitative PCR instrument (Applied Biosystems, Foster City, CA, USA). Subsequently, the genotyping outcomes were precisely ascertained employing the ABI 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
The data analysis was carried out utilizing SPSS (Version 17.0; SPSS, Inc, Chicago, IL, USA). The Kolmogorov-Smirnov one-sample test was utilized to identify normal distributions. Descriptive analyses were conducted, with normally distributed quantitative variables presented as means ± standard deviation (SD), and skewed quantitative variables presented as median and interquartile range (IQR; 25-75%). The Chi-square test was employed for categorical variables. To account for multiple testing, the Bonferroni correction was implemented. In order to investigate the risk factors associated with HHcy in individuals diagnosed with hypertension, the study employed univariate analyses to compare subjects with HHcy to those without HHcy. Variables with a significance level of P < 0.1 were further analyzed using logistic regression analyses. Additionally, Spearman correlation analysis and multiple linear regression analysis were conducted to examine the associations between Hcy levels and clinical factors. Statistical significance was determined at a P-value of less than 0.05 (two-tailed). Goodness-of-fit test based on the Chi-square was used to determine whether the genotype frequency distribution conformed to Hardy-Weinberg equilibrium (HWE). Sample size calculation was calculated using the G*Power 3.1 software, with power set at 0.99 to detect the medium-effect size (d = 0.50) and alpha = 0.01 with allocation ratio N2/N1 of 2. Considering a previous study involving 5935 hypertensive patients in China demonstrated a HHcy prevalence of 31.4% (13), the allocation ratio N2/N1 was set at 2. The required sample size was 436, with 145 subjects with HHcy and 291 subjects without HHcy. In the present study, a total of 473 individuals diagnosed with hypertension were enrolled, with 148 subjects with HHcy and 325 subjects without HHcy.
Results
Prevalence of HHcy in Chinese urban population with hypertension
HHcy was operationally defined as plasma Hcy levels exceeding 15 μmol/L. Consequently, a total of 473 hypertensive individuals were enrolled in this study, of which 148 (31.3%) fulfilled the diagnostic criteria for HHcy.
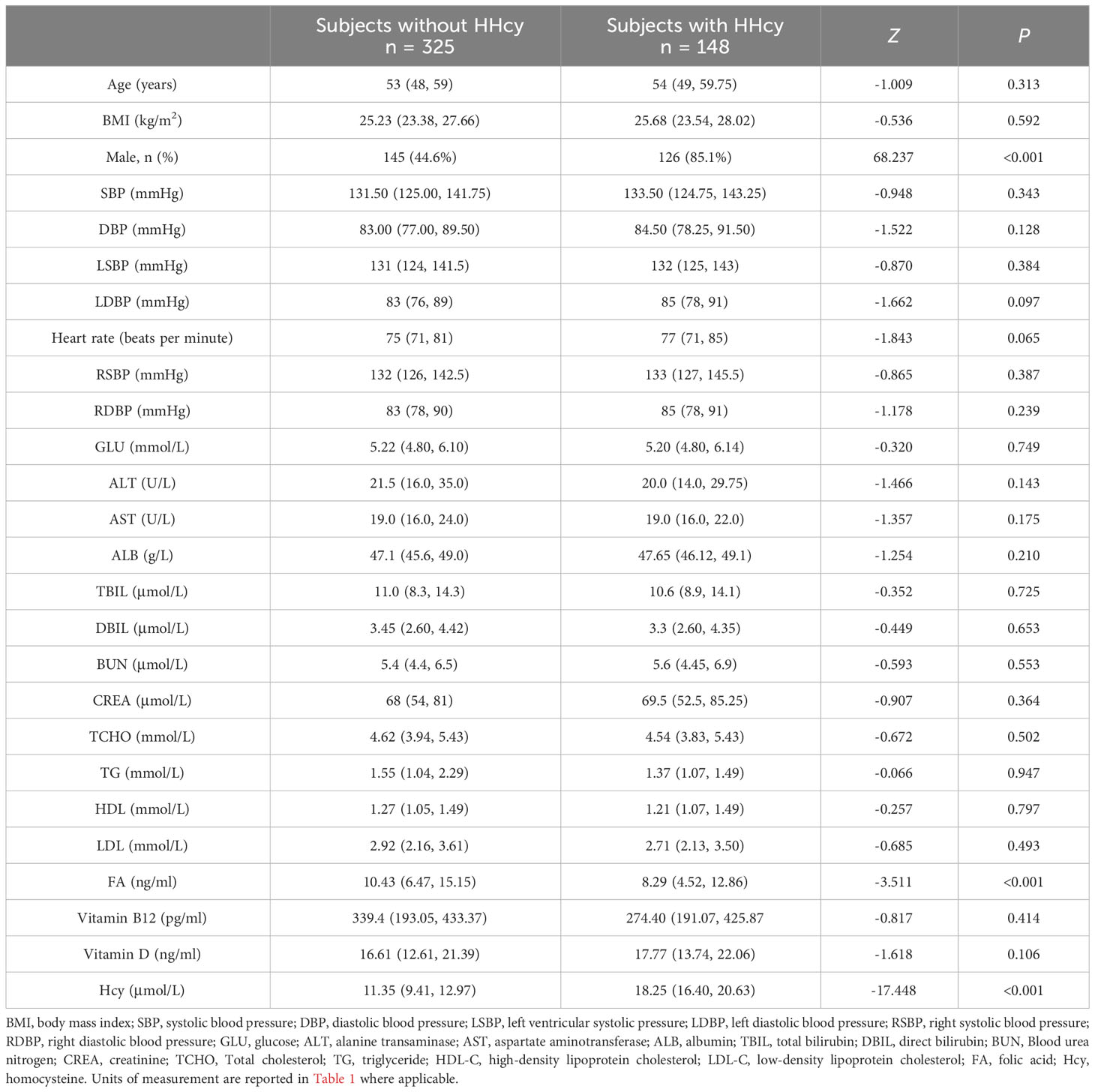
Differences in demographic characteristics, clinical profiles, distribution of MTHFR and RFC genotypes, and lifestyle behaviors of subjects with or without HHcy
According to the data presented in Table 1, there is a notable disparity in the gender distribution between individuals with and without HHcy. The proportion of males is significantly higher (χ2 = 68.237, P < 0.001, OR = 7.110; 95% confidence interval (CL): 4.30-11.76) among those with HHcy (85.1%) compared to those without HHcy (44.6%). Moreover, individuals with HHcy exhibit significantly lower levels of FA (Z = -3.511, P < 0.001) in comparison to individuals without HHcy.
As shown in Table 2, the results of Goodness-of-fit test based on the Chi-square showed that the genotype frequency distribution of RFC G80A and MTHFR C677T conformed to HWE, and the difference was not statistically significant (P > 0.05), which demonstrated that the study objects were community representative. According to the data presented in Table 3, CT (OR = 1.611, 95% CI = 1.038-2.501, P = 0.034) and TT (OR = 3.054, 95% CI = 1.658-5.624, P < 0.001) genotypes of MTHFR C677T polymorphism increased the risk of HHcy compared to CC genotype. The RFC G80A variant was not associated with HHcy in any inheritance models tested (co-dominant, dominant and recessive).
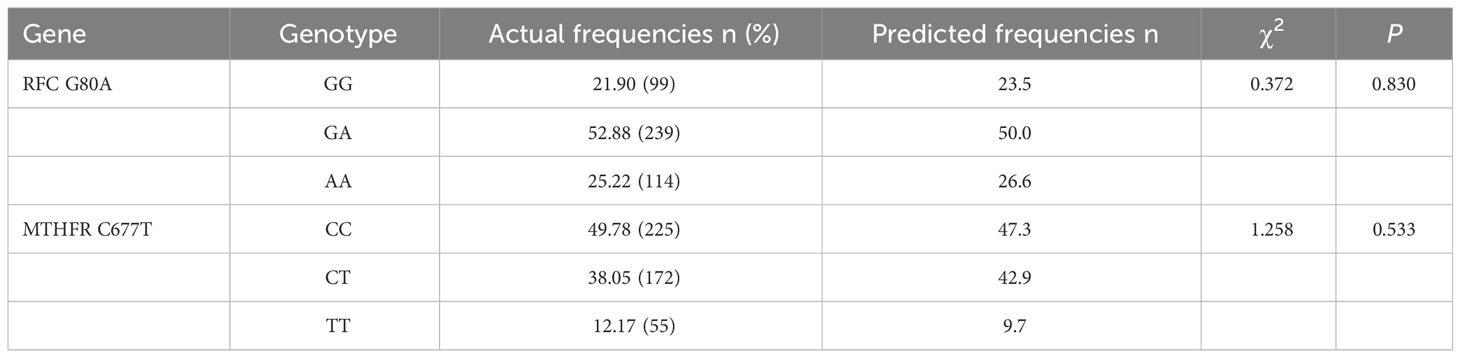
Table 2 Hardy-Weinberg equilibrium (HWE) results of RFC G80A and MTHFR C677T variants polymorphisms.
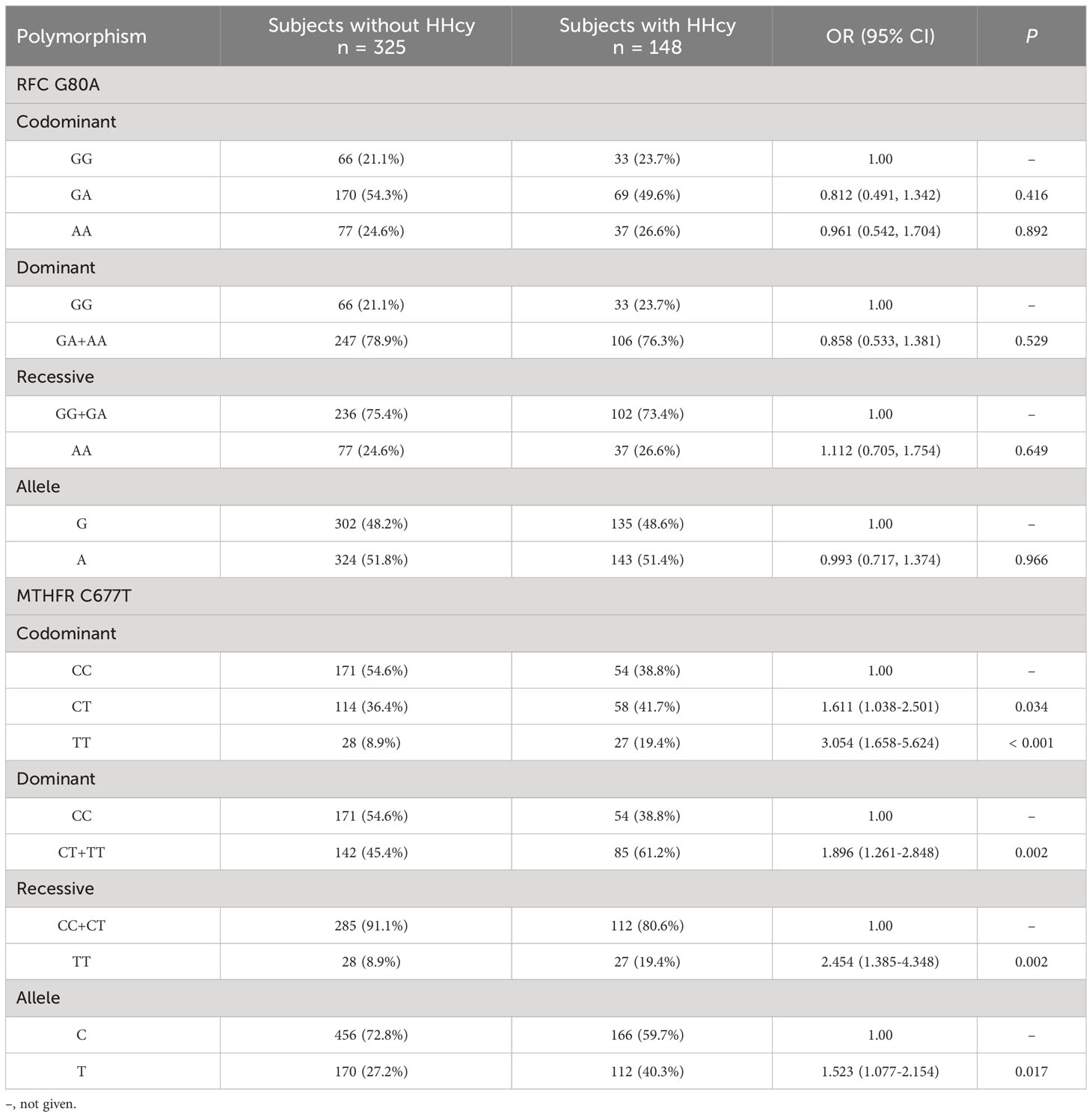
Table 3 Genotypic and allelic frequencies of RFC and MTHFR variants polymorphisms in subjects with or without HHcy.
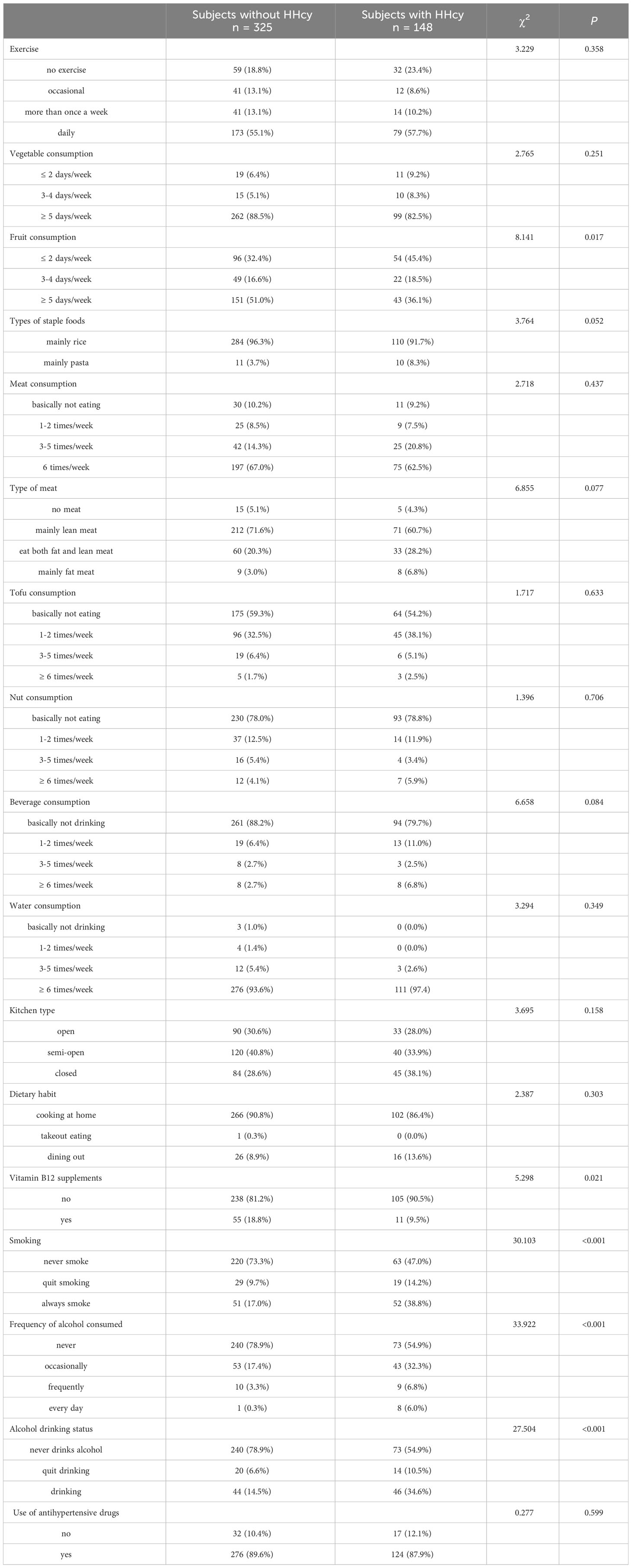
In terms of lifestyle behaviors, individuals with HHcy exhibited a greater prevalence of tobacco smoking (χ2 = 30.103, P < 0.001) and alcohol use (χ2 = 33.922, P < 0.001), as well as a lower intake of fruit (χ2 = 8.141, P = 0.017) and vitamin B12 (χ2 = 5.298, P = 0.021), in comparison to those without HHcy (Table 4).
Risk factors for HHcy in Chinese urban population with hypertension
A binary logistic regression model was applied to detect risk factors for HHcy (subjects without HHcy = 0, subjects with HHcy = 1), including gender (assignment: male = 1, female = 2), heart rate, FA, MTHFR genotypes (assignment: CC = 1, CT = 2, TT = 3), fruit consumption (assignment: ≤ 2 days/week = 1, 3-4 days/week = 2, ≥ 5 days/week = 3), types of staple foods (assignment: mainly rice = 1, mainly pasta = 2), type of meat (assignment: no meat = 1, mainly lean meat = 2, eat both fat and lean meat = 3, mainly fat meat = 4), beverage consumption (assignment: basically not drinking = 1, 1-2 times/week = 2, 3-5 times/week = 3, ≥ 6 times/week = 4), vitamin B12 supplements (assignment: no = 0, yes = 1), smoking (assignment: never smoke = 1, quit smoking = 2, always smoke = 3), frequency of alcohol consumed (assignment: never = 1, occasionally = 2, frequently = 3, every day = 4), and alcohol drinking status (assignment: never drinks alcohol = 1, quit drinking = 2, drinking = 3). As shown in Table 5, the risk factors for HHcy were as follows: male (B = 1.430, P < 0.001, OR = 4.179, 95%CL: 2.005-8.707) and MTHFR (TT) (B = 1.086, P = 0.006, OR = 2.961, 95%CL: 1.357-6.464).
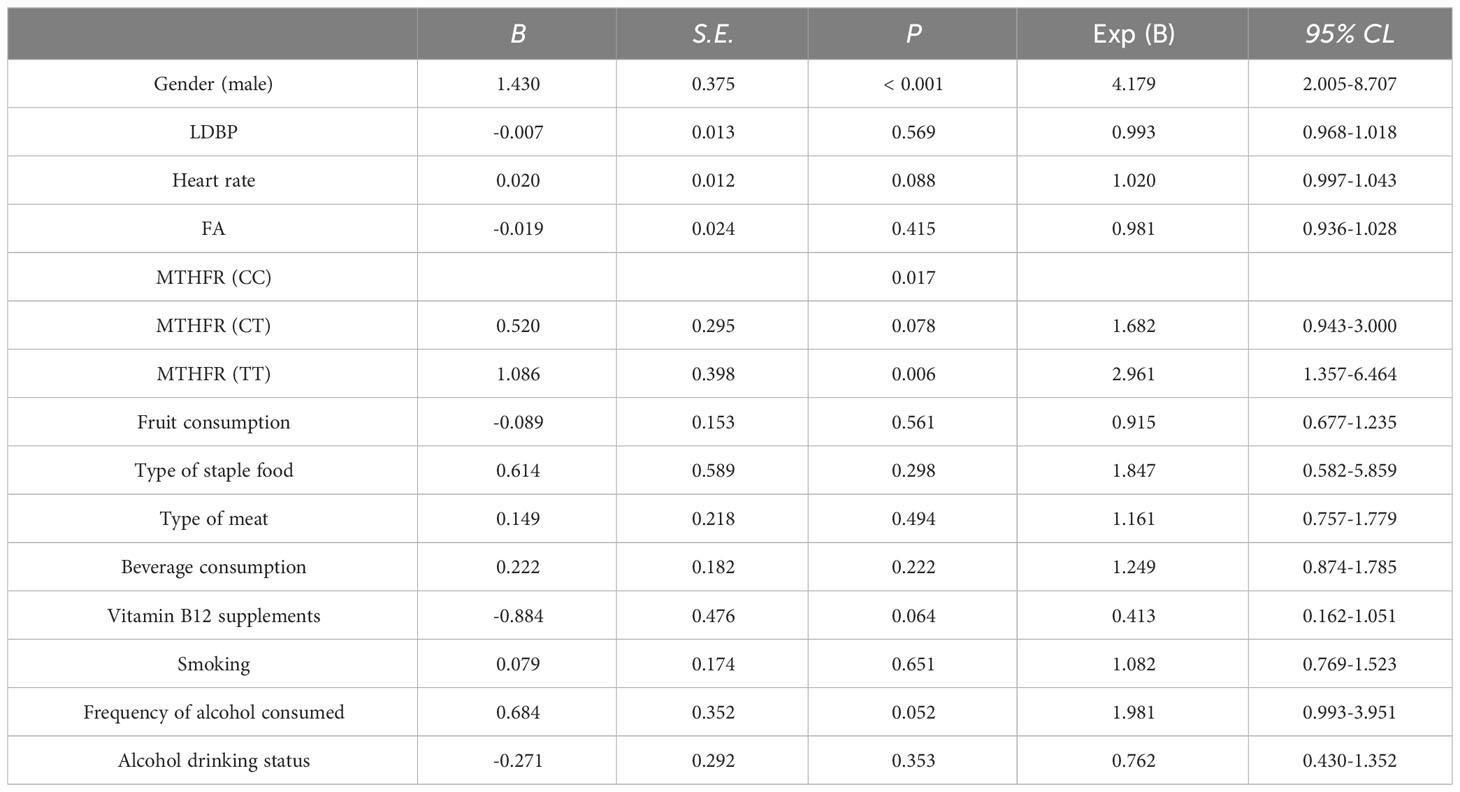
Table 5 Binary logistic regression analysis of potential risk factors related to HHcy in the hypertension population.
Factors influencing Hcy levels in Chinese urban population with hypertension
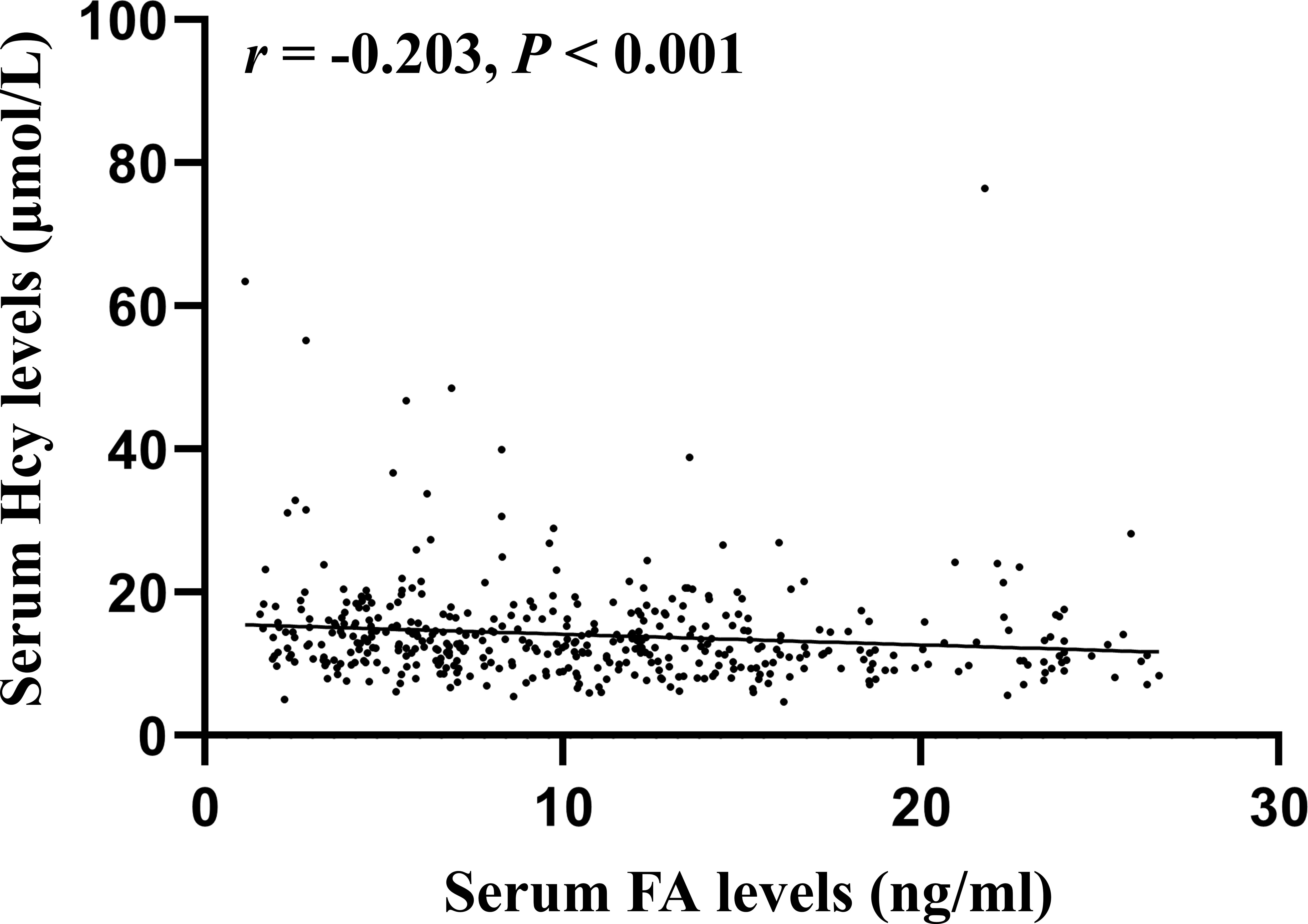
Spearman correlation analysis showed significant correlations between Hcy levels and the following parameters: gender (r = -0.462, P < 0.001), MTHFR genotypes (r = 0.231, P < 0.001), DBP (r = 0.098, P = 0.036), heart rate (r = 0.093, P = 0.047), ALB (r = 0.136, P = 0.025), FA (r = -0.203, P < 0.001), Vitamin D (r = 0.131, P = 0.010), fruit consumption (r = -0.179, P < 0.001), type of meat (r = 0.100, P = 0.044), vitamin B12 supplements (r = -0.123, P = 0.012), smoking (r = 0.304, P < 0.001), frequency of alcohol consumed (r = 0.276, P < 0.001), and alcohol drinking status (r = 0.270, P < 0.001). Figure 1 shows a scatter plot representing the correlation serum Hcy and FA levels. All variables except for DBP, ipulse, ALB, Vitamin D, type of meat, and vitamin B12 supplements passed Bonferroni correction (P < 0.05/30 = 0.0017). These significantly different variables from univariate analysis were included in multiple linear regression to detect correlates of Hcy levels (Table 6). Finally, the multiple linear regression revealed a significant association between Hcy levels and gender (B = -2.784, t = -2.931, P = 0.004), MTHFR genotypes (B = 1.410, t = 2.815, P = 0.005), and FA levels (B = -0.136, t = -2.192, P = 0.030).
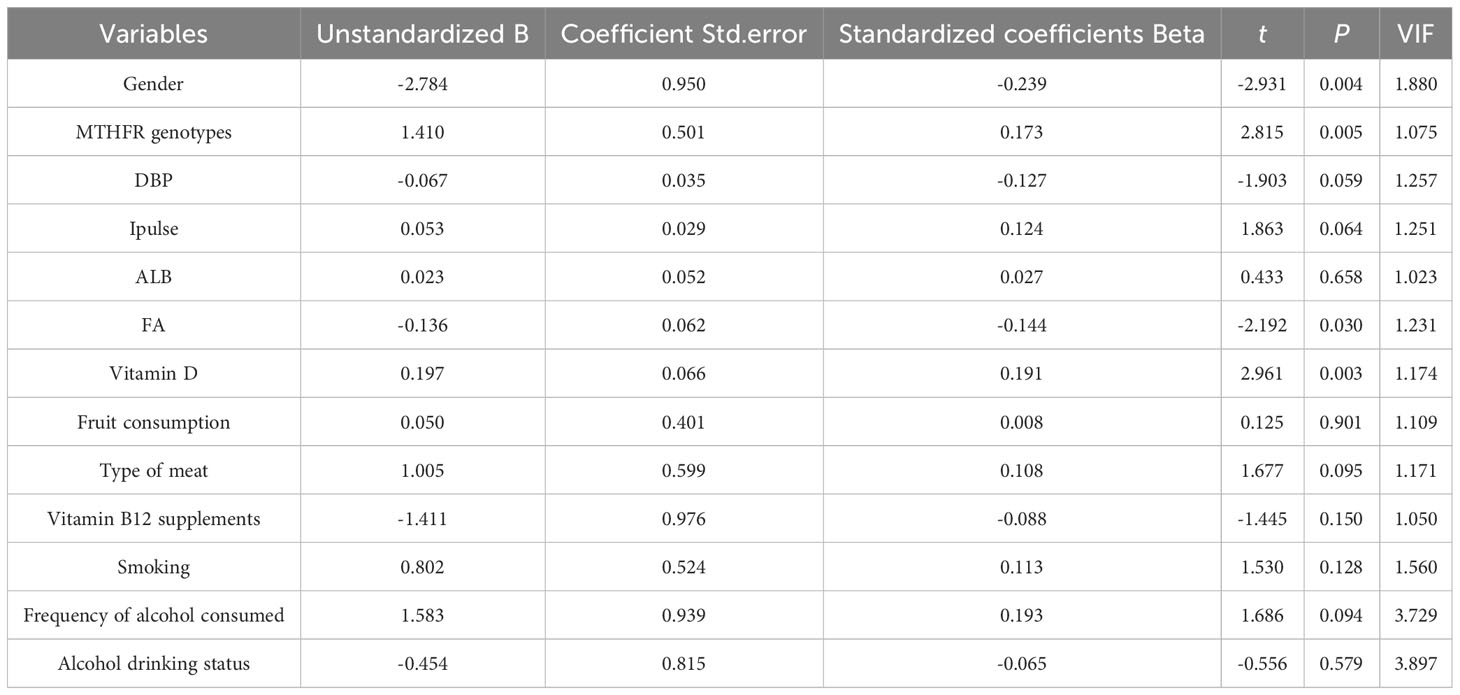
Table 6 Associated factors for Hcy levels in Chinese urban population with hypertension based on multiple linear regression model.
Discussion
The objective of this study was to assess the prevalence, clinical correlation, and demographic characteristics of HHcy in the Chinese urban population with hypertension, with a focus on identifying risk factors for HHcy in hypertensive patients. The study yielded three key findings. Firstly, the prevalence of HHcy in the Chinese urban population with hypertension was determined to be 31.3%. Secondly, logistic regression analysis identified male gender and the presence of the MTHFR (TT) genotype as significant risk factors for HHcy. Lastly, the multiple linear regression revealed a significant association between Hcy levels and gender, MTHFR genotypes, and FA levels.
Multiple studies have examined the occurrence of HHcy in individuals with hypertension. One such study revealed that 73.3% of newly diagnosed hypertensive patients residing in seven urban communities in Nanjing, China, exhibited HHcy (19). Similarly, another study found a comparable prevalence of HHcy. Specifically, among 105 patients diagnosed with primary hypertension in Vietnam, 74.3% (78 patients) displayed elevated plasma total Hcy levels ≥ 15 µmol/L (20). The current investigation aimed to ascertain the prevalence of HHcy among the urban Chinese population with hypertension, revealing a rate of 31.3%. This finding closely aligns with the outcomes of a prior study conducted within China (13). Notably, a separate investigation involving 5935 hypertensive patients in China demonstrated a HHcy prevalence of 31.4% (13). Discrepancies in HHcy prevalence across studies may be attributed to various contextual factors, including population demographics, geographical location, and patient age. A meta-analysis conducted in China involving a sample size of 60,754 individuals aged 3-97 years revealed a positive correlation between age and the prevalence of HHcy, with the highest occurrence observed among individuals aged 65 years and above (21). Consequently, it is reasonable to hypothesize that the dissimilarity in HHcy prevalence between the present study and the study conducted in Nanjing (31.3% vs. 73.3%) could potentially be attributed to the variance in age distribution among the respective subject groups (54.1 vs. 63.3).
Previous evidence has indicated that gender plays a significant role in determining the levels of Hcy in humans. A study conducted on healthy individuals revealed that males tend to have higher serum Hcy levels compared to females (22). Moreover, a meta-analysis encompassing 36 studies and involving 60,754 subjects from 19 provinces and municipalities in China found that the prevalence of HHcy was notably higher in men than in women (21). Furthermore, elevated levels of serum homocysteine have been observed in male patients with hypertension (23), primary chronic venous disease (24), and psoriasis (25). Potential factors contributing to the observed sex-specific variation encompass disparities in muscle mass, estrogen levels, lifestyles, and vitamin levels. Specifically, Notably, the connection between Hcy production and creatine-creatinine synthesis is widely acknowledged. Given that males typically possess greater muscle mass, they exhibit an augmented requirement for creatine synthesis, consequently leading to increased Hcy production (26). Moreover, Chinese men exhibit a higher prevalence of alcohol consumption and cigarette smoking compared to women, both of which exhibit positive correlations with Hcy concentrations (27). Furthermore, the presence of hormonal disparities between males and females may also play a role in the observed sex-related discrepancy. Research has indicated that the administration of long-term hormone replacement therapy leads to decreased overall concentrations of Hcy in women who have undergone menopause (28). In the present study, the proportion of males was notably greater among individuals with HHcy compared to those without HHcy. Through the utilization of logistic regression analysis, male gender was identified as a significant risk factor for HHcy, while multiple linear regression analysis demonstrated a significant association between Hcy levels and gender. These findings offer additional empirical evidence establishing a correlation between gender and Hcy levels.
The enzyme MTHFR, which is dependent on folate, plays a significant role in the conversion of the amino acid homocysteine to methionine (29). The activity of MTHFR has a detrimental impact on the levels of Hcy in the serum (25). The MTHFR rs1801133 polymorphism involves a substitution of C to T at position 677, resulting in the conversion of alanine to valine. This missense mutation leads to a reduction of approximately 70% and 35% in the normal activity of the MTHFR enzyme in carriers with the TT and CT genotypes, respectively (30). Additionally, the TT allele is associated with increased levels of homocysteine in the serum (31). In this study, CT and TT genotypes of MTHFR C677T polymorphism increased the risk of HHcy compared to CC genotype. Additionally, logistic regression analysis revealed that the presence of the MTHFR (TT) genotype was a significant risk factor for HHcy. Furthermore, multiple linear regression analysis demonstrated a significant association between homocysteine levels and the MTHFR genotype. Given that approximately 25% of the Chinese population possesses the MTHFR C677T TT genotype (32), the assessment of MTHFR genotype within the Chinese populace, particularly among individuals with hypertension, holds significant clinical implications in terms of cardio-cerebrovascular disease prevention and treatment.
Presently, the utilization of FA and B vitamins is employed to attain desired Hcy levels, exhibiting evident efficacy in diminishing plasma Hcy concentrations (33). While not yet substantiated, the reduction of overall plasma Hcy levels via FA and/or vitamin B12 intake may potentially mitigate the susceptibility to vascular diseases among HHcy individuals (34). Another clinical trial investigation has documented that the addition of FA as a supplementary therapy, administered at a dosage of 5 mg per day, exhibits the potential to markedly decrease serum Hcy levels (35). This intervention may play a contributory role in the pathogenesis of vascular disorders, including Buerger’s disease (35). In the current investigation, individuals diagnosed with HHcy exhibited notably diminished levels of FA in comparison to those without HHcy. Regarding lifestyle habits, individuals with HHcy demonstrated a reduced intake of vitamin B12 in comparison to those without HHcy. Furthermore, inverse associations were observed between Hcy levels and both FA levels and vitamin B12 supplementation. Additionally, fruits and vegetables are recognized as abundant sources of FA and B vitamins, which contribute to elevated plasma levels of these vitamins and decreased Hcy levels (36). In the present study, it was consistently observed that individuals with HHcy exhibited lower levels of fruit intake compared to those without HHcy. These findings imply that augmenting FA levels and incorporating vitamin B12 supplements could potentially decrease Hcy levels in hypertensive patients, consequently reducing the incidence of cardio-cerebrovascular disease.
Evidence consistently supports the notion that smoking, alcohol consumption, and physical inactivity have a significant impact on elevating Hcy levels (37, 38). Consequently, these lifestyle factors were incorporated into the current study to ascertain the specific risk factors associated with HHcy in hypertensive individuals. Correspondingly, our findings revealed a heightened prevalence of tobacco smoking and alcohol use among subjects with HHcy, thereby reinforcing the recommendation for hypertensive patients with HHcy to adopt lifestyle modifications such as reducing alcohol intake and quitting smoking.
Several limitations to this study need to be acknowledged. First, this is a cross-sectional study design and cannot show a causal relationship between the presence of HHcy and a given risk factor. Second, this study is limited by its observational design and small subset size, which reduces the power of the statistical analysis. Third, generalizability was limited due to the fact that the study was performed in only one region of China. Fourth, an additional limitation of this study pertains to the absence of validation for the questionnaire tool and the omission of a reliability assessment. Fifth, given that diuretics decrease the absorption of FA, the absence of precise categorizations pertaining to the types of antihypertensive drugs is regarded as a limitation of this research.
In conclusion, our study reveals a notable occurrence of HHcy among the Chinese urban population with hypertension, those at greatest risk are male, genotype MTHFR 677TT, and a decreased FA level. Consequently, it is imperative to implement future strategies for managing and intervening in these factors to mitigate the development of H type hypertension and minimize associated cardio-cerebrovascular events within this population. For example, to mitigate the occurrence of HHcy and cardio-cerebrovascular disease, particularly among male individuals with the MTHFR C677T TT genotype, it is recommended to implement pharmacotherapy and/or lifestyle adjustments encompassing physical activity, nutrition, and dietary supplementation, such as FA. Notably, HHcy has been considered a specific disease in the Chinese Han population (39). The population with the TT genotype accounts for 25% of the Chinese Han population, whereas the population with the TT genotype in North America and Europe accounts for only approximately 12% of the combined population (40). Thus, the risk factors for HHcy identified in the Chinese population in this study, specifically the MTHFR677TT genotype, may not be applicable to other populations, such as North Americans and Europeans.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Committee of Shenzhen Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft. HF: Investigation, Methodology, Validation, Writing – original draft. LZ: Software, Supervision, Writing – original draft. YL: Formal analysis, Resources, Software, Writing – original draft. FC: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. LR: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shenzhen Team on the Fusion of Health Management and Prevention of Neurological Diseases (6099007), Science and Technology Innovation Commission of Shenzhen (ZDSYS20200811142600003) and Natural Science Foundation of Guangdong Province (2023A1515010169).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roth G, Mensah G, Fuster V. The global burden of cardiovascular diseases and risks: A compass for global action. J Am Coll Cardiol (2020) 76:2980–1. doi: 10.1016/j.jacc.2020.11.021
2. Wang H, Zhang H, Zou Z. Changing profiles of cardiovascular disease and risk factors in China: A secondary analysis for the global burden of disease study 2019. Chin Med J (2023) 136:2431–41. doi: 10.1097/CM9.0000000000002741
3. Katan M, Luft A. Global burden of stroke. Semin Neurol (2018) 38:208–11. doi: 10.1055/s-0038-1649503
4. Fan F, David Spence J, Huo Y. Beyond hypertension: Hypertension with hyperhomocysteinemia. Sci Bull (2023) 68:1975–7. doi: 10.1016/j.scib.2023.08.018
5. Zheng W, Wang X, Xue X, Li W, Fan L, Zhang S, et al. Characteristics of hypertension in the last 16 years in high prevalence region of China and the attribute ratios for cardiovascular mortality. BMC Public Health (2023) 23:114. doi: 10.1186/s12889-022-14974-0
6. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet (London England) (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
7. Lauder L, Mahfoud F, Azizi M, Bhatt D, Ewen S, Kario K, et al. Hypertension management in patients with cardiovascular comorbidities. Eur Heart J (2023) 44:2066–77. doi: 10.1093/eurheartj/ehac395
8. Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004-18: Findings from six rounds of a national survey. BMJ (Clinical Res ed.) (2023) 380:e071952. doi: 10.1136/bmj-2022-071952
9. Wang Y, Xia H, Song Z, Zhou X, Zhang H. Plasma homocysteine as a potential marker of early renal function decline in iga nephropathy. Front Med (2022) 9:812552. doi: 10.3389/fmed.2022.812552
10. Liu W, Li Q, Wang Q, Ma S, Yang X, Zhang J, et al. Association between body fat composition and hyperhomocysteinemia in the analysis of the baseline data of the northwest China natural population cohort: Ningxia project (cnc-nx). J Clin Hypertension (Greenwich Conn) (2023) 25:573–81. doi: 10.1111/jch.14666
11. Yan J, Zhou J, Huang J, Zhang H, Deng Z, Du Y. The outcomes of acute myocardial infarction patients comorbidity with hypertension and hyperhomocysteinemia. Sci Rep (2021) 11:22936. doi: 10.1038/s41598-021-02340-w
12. Ganguly P, Alam S. Role of homocysteine in the development of cardiovascular disease. Nutr J (2015) 14:6. doi: 10.1186/1475-2891-14-6
13. Wang C, Chen Z, Zhang T, Liu J, Chen S, Liu S, et al. Elevated plasma homocysteine level is associated with ischemic stroke in chinese hypertensive patients. Eur J Internal Med (2014) 25:538–44. doi: 10.1016/j.ejim.2014.04.011
14. Li J, Jiang S, Zhang Y, Tang G, Wang Y, Mao G, et al. H-type hypertension and risk of stroke in chinese adults: A prospective, nested case-control study. J Trans Internal Med (2015) 3:171–8. doi: 10.1515/jtim-2015-0027
15. Feng Y, Kang K, Xue Q, Chen Y, Wang W, Cao J. Value of plasma homocysteine to predict stroke, cardiovascular diseases, and new-onset hypertension: A retrospective cohort study. Medicine (2020) 99:e21541. doi: 10.1097/MD.0000000000021541
16. Zhang P, Zhang Y. Association of homocysteine with acute stroke and its subtypes in the chinese population. Neuropsychiatr Dis Treat (2023) 19:1435–42. doi: 10.2147/NDT.S409591
17. Kataria N, Yadav P, Kumar R, Kumar N, Singh M, Kant R, et al. Effect of vitamin b6, b9, and b12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus (2021) 13:e14958. doi: 10.7759/cureus.14958
18. Peng X, Zhou Y, Wu X, Wang X, Bai H, Li Y, et al. Association of methylenetetrahydrofolate reductase (mthfr) variant c677t and risk of carotid atherosclerosis: A cross-sectional analysis of 730 chinese han adults in chongqing. BMC Cardiovasc Disord (2020) 20:222. doi: 10.1186/s12872-020-01505-1
19. Wang W, Ji P, Wang Y, Guo H, Bian R, Xu J, et al. Prevalence of hyperhomocysteinemia and its associated factors in patients with primary hypertension in chinese urban communities: A cross-sectional study from nanjing. Clin Exp Hypertension (New York NY: 1993) (2018) 40:495–500. doi: 10.1080/10641963.2017.1403621
20. Tran S, Ngo T, Nguyen P, Truong A, Truong G, Tran K, et al. Hyperhomocysteinemia in patients with newly diagnosed primary hypertension in can tho city, Vietnam. Healthc (Basel Switzerland) (2023) 11:234. doi: 10.3390/healthcare11020234
21. Yang B, Fan S, Zhi X, Wang Y, Wang Y, Zheng Q, et al. Prevalence of hyperhomocysteinemia in China: A systematic review and meta-analysis. Nutrients (2014) 7:74–90. doi: 10.3390/nu7010074
22. Moon H, Whang D, Ko Y, Joo S, Yun Y, Hur M, et al. Reference interval and determinants of the serum homocysteine level in a korean population. J Clin Lab Anal (2011) 25:317–23. doi: 10.1002/jcla.20476
23. Pang H, Han B, Fu Q, Hao L, Zong Z. Association between homocysteine and conventional predisposing factors on risk of stroke in patients with hypertension. Sci Rep (2018) 8:3900. doi: 10.1038/s41598-018-22260-6
24. Smith R, Quigley F, Tosenovsky P, Velu R, Bradshaw B, Buettner P, et al. Serum homocysteine is associated with the severity of primary chronic venous disease. Phlebology (2016) 31:409–15. doi: 10.1177/0268355515592076
25. Zhang Q, Lin J, Zhang Z, Han L, Huang Q, Zhu J, et al. Mthfr polymorphism and folic acid supplementation influence serum homocysteine levels in psoriatic patients treated with methotrexate. J Clin Med (2022) 11:4580. doi: 10.3390/jcm11154580
26. Rauh M, Verwied S, Knerr I, Dörr H, Sönnichsen A, Koletzko B. Homocysteine concentrations in a german cohort of 500 individuals: Reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids (2001) 20:409–18. doi: 10.1007/s007260170037
27. de Bree A, Verschuren W, Blom H, Kromhout D. Lifestyle factors and plasma homocysteine concentrations in a general population sample. Am J Epidemiol (2001) 154:150–4. doi: 10.1093/aje/154.2.150
28. Madsen J, Kristensen S, Klitgaard N, Bladbjerg E, Abrahamsen B, Stilgren L, et al. Effect of long-term hormone replacement therapy on plasma homocysteine in postmenopausal women: A randomized controlled study. Am J Obstet Gynecol (2002) 187:33–9. doi: 10.1067/mob.2002.123030
29. Zhao L, Li T, Dang M, Li Y, Fan H, Hao Q, et al. Association of methylenetetrahydrofolate reductase (mthfr) rs1801133 (677c>t) gene polymorphism with ischemic stroke risk in different populations: An updated meta-analysis. Front Genet (2022) 13:1021423. doi: 10.3389/fgene.2022.1021423
30. Kong Y, Zheng J, Li L, Lu L, Wang J. Association of mthfr polymorphisms with h-type hypertension: A systemic review and network meta-analysis of diagnostic test accuracy. Int J Hypertension (2022) 2022:2861444. doi: 10.1155/2022/2861444
31. Chen L, Wu C, Dong Z, Cao S, Ren N, Yan X. Methylenetetrahydrofolate reductase polymorphisms and elevated plasma homocysteine levels in small vessel disease. Brain Behav (2023) 13:e2960. doi: 10.1002/brb3.2960
32. Wu D, Wu Y, Deng J. Changes in homocysteine levels affect serum lipid response to atorvastatin in patients with acute coronary syndrome: A retrospective observational study. Clin Appl Thromb/Hemost (2020) 26:1076029620920369. doi: 10.1177/1076029620920369
33. Zhou Y, Tang J, Wu M, Lu J, Wei X, Qin Y, et al. Effect of folic acid supplementation on cardiovascular outcomes: A systematic review and meta-analysis. PloS One (2011) 6:e25142. doi: 10.1371/journal.pone.0025142
34. Boushey C, Beresford S, Omenn G, Motulsky A. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA (1995) 274:1049–57. doi: 10.1001/jama.274.13.1049
35. Modaghegh M, Ravari H, Haghighi M, Rajabnejad A. Effect of folic acid therapy on homocysteine level in patients with atherosclerosis or buerger’s disease and in healthy individuals: A clinical trial. Electronic Physician (2016) 8:3138–43. doi: 10.19082/3138
36. Khan M, Saeedullah A, Andrews S, Iqbal K, Qadir S, Shahzad B, et al. Adolescent afghan refugees display a high prevalence of hyperhomocysteinemia and associated micronutrients deficiencies indicating an enhanced risk of cardiovascular disease in later life. Nutrients (2022) 14:1751. doi: 10.3390/nu14091751
37. Kowluru R, Mohammad G, Sahajpal N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vision (London England) (2020) 7:4. doi: 10.1186/s40662-019-0167-9
38. Losso J. The potential of dietary bioactive compounds against sars-cov-2 and covid-19-induced endothelial dysfunction. Mol (Basel Switzerland) (2022) 27:1623. doi: 10.3390/molecules27051623
39. Hou Y, Yun L, Zhang L, Lin J, Xu R. A risk factor-based predictive model for new-onset hypertension during pregnancy in chinese han women. BMC Cardiovasc Disord (2020) 20:155. doi: 10.1186/s12872-020-01428-x
Keywords: hyperhomocysteinemia, hypertension, urban, prevalence, clinical correlates
Citation: Xu Y, Feng H, Zhang L, Li Y, Chi F and Ren L (2024) Prevalence and clinical correlates of hyperhomocysteinemia in Chinese urban population with hypertension. Front. Endocrinol. 15:1369997. doi: 10.3389/fendo.2024.1369997
Received: 13 January 2024; Accepted: 05 February 2024;
Published: 20 February 2024.
Edited by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroReviewed by:
Tatsuo Shimosawa, International University of Health and Welfare (IUHW), JapanAzadeh Anna Nikouee, Loyola University Chicago, United States
Maryam Pirhoushiaran, Tehran University of Medical Sciences, Iran
Svetlana Stoica, Institute of Cardiovascular and Heart Diseases of Timișoara, Romania
Palani Selvam Mohanraj, All India Institute Of Medical Sciences Gorakhpur, India
Copyright © 2024 Xu, Feng, Zhang, Li, Chi and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijie Ren, MTM2MzE2MDU5NjZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yayun Xu
Yayun Xu Haixing Feng†
Haixing Feng† Lijie Ren
Lijie Ren