- 1Department of Nursing, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2The First Ward of the Neurosurgery Department, Affiliated Hospital of Zunyi Medical University, Zunyi, China
Purpose: The purpose of this study was to explore the factors influencing PRL levels in patients with prolactinoma and to investigate the correlations between anxiety, depression, sleep, self-efficacy, and PRL levels.
Methods: This retrospective study included 176 patients with prolactinoma who received outpatient treatment at the Affiliated Hospital of Zunyi Medical University from May 2017 to August 2022. The general information questionnaire, Hospital Anxiety and Depression Scale (HADS), Athens Insomnia Scale (AIS), and General Self-Efficacy Scale (GSES) were used for data collection. A generalized estimating equation (GEE) model was used to analyze the factors influencing PRL levels in patients with prolactinoma. GEE single-effect analysis was used to compare PRL levels at different time points between anxiety group and nonanxiety group, between insomnia group and normal group, and between low, medium, and high self-efficacy groups.
Results: The median baseline PRL level and the PRL levels at 1, 3, 6, and 12 months of follow-up were 268.50 ng/ml, 122.25 ng/ml, 21.20 ng/ml, 19.65 ng/ml, and 16.10 ng/ml, respectively. Among patients with prolactinoma, 59.10% had anxiety (HADS-A score = 7.35 ± 3.34) and 28.98% had depression (HADS-D score = 5.23 ± 3.87), 9.10% had sleep disorders (AIS score = 6.10 ± 4.31) and 54.55% had low self-efficacy (GSES score = 2.13 ± 0.83). Educational level, tumor size, number of visits, sleep quality, anxiety level, and self-efficacy level were found to be factors influencing PRL levels in patients with prolactinoma (P<0.05). Higher PRL levels were observed in the anxiety group compared to the non-anxiety group (P<0.001), in the insomnia group compared to the normal group (P<0.05), and in the low self-efficacy group compared to the medium and high self-efficacy groups (P<0.05).
Conclusion: PRL levels in patients with prolactinoma are related to education level, tumor size, number of visits, anxiety, self-efficacy, and sleep but not depression. PRL levels were higher in patients with anxiety, low self-efficacy, and sleep disorders.
1 Introduction
Prolactinoma is a common pituitary adenoma that occurs in individuals with hypothalamic-pituitary disease and is characterized by excessive secretion of prolactin (PRL) from anterior pituitary lactotroph cells. The incidence rate of prolactinoma is approximately 50 per 100,000 individuals, with an annual new case rate of 3 to 5 per 100,000 (1). The typical clinical manifestations of prolactinoma include menstrual disorders, galactorrhea, infertility, decreased libido, erectile dysfunction, hypogonadism and pituitary mass lesions (headache, partial hypopituitarism, visual field disturbances), all of which significantly impact the quality of life and mental health of affected patients (2). Currently, the clinical management of pituitary adenomas primarily consists of pharmacotherapy, surgery, and radiation therapy. The first-line pharmacological treatment for prolactinoma includes dopamine agonists (DAs), such as bromocriptine or cabergoline (3), which aim to suppress excessive PRL secretion and reduce tumor size using the minimum effective dose.
However, the relationships between PRL and anxiety and depression in prolactinoma patients remain to be explored. It has been reported that prolactinoma patients may experience dissatisfaction with their body image due to weight gain, and symptomatic patients have a higher rate of body dissatisfaction than asymptomatic patients (4). Prolactinoma patients often exhibit symptoms of depression, hostility, irritability, and anxiety (5, 6), and stress may trigger neuroendocrine changes involving dopamine or serotonin, thus affecting the release of PRL (7). The study demonstrated that compared to low anxiety behavior (LAB) rats, high anxiety behavior (HAB) rats exhibited increased basal and stress-induced PRL levels, possibly due to the association between inborn anxiety and HPA axis hyper-reactivity (8). High levels of PRL may hinder the formation of the homodimers required for PRL’s physiological functions. Persistent hyperprolactinemia reduces the ability of tuberoinfundibular neurons to make dopamine (9). Increased PRL levels result in higher levels of vasoinhibins, which could contribute to anxiety and depression behaviors (10, 11). However, studies by Sonino et al. have shown that even after restoring PRL levels to normal through treatment, patients may still experience persistent psychological issues, suggesting that the optimal endocrine balance may not be fully restored (12). However, the relationship between PRL levels in prolactinoma patients and sleep disturbances remains unclear. Some studies have indicated that there is no direct relationship between sleep disturbances and PRL levels in prolactinoma patients (13), but a retrospective study has reported a possible association between elevated PRL levels and excessive daytime sleepiness (14). In terms of self-efficacy, multiple studies have shown that patients with high levels of self-efficacy are more confident in coping with adversity or illness and are more likely to adopt a positive and healthy attitude toward them (15, 16). However, there is currently no research available on the relationship between PRL levels and self-efficacy in prolactinoma patients.
In summary, the correlations of PRL levels with anxiety, depression, sleep disturbances, and self-efficacy in prolactinoma patients have not been thoroughly investigated. Therefore, this study used a generalized estimating equation (GEE) to analyze the one-year follow-up data of prolactinoma patients and explore the factors influencing their PRL levels. Additionally, this study investigated the correlations between anxiety, depression, sleep disturbances, self-efficacy, and PRL levels in prolactinoma patients, thus providing evidence for targeted interventions to improve the quality of life and psychological status of prolactinoma patients.
2 Methods
2.1 Study subjects
This study included patients with prolactinoma who received outpatient treatment at the Affiliated Hospital of Zunyi Medical University from May 2017 to August 2022. The patients were divided into three groups: 128 patients with microadenomas, 38 patients with macroadenomas, and 10 patients with giant adenomas.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) aged older than 18 years, 2) had complete clinical data, and 3) had a confirmed diagnosis of prolactinoma. The exclusion criteria were as follows: 1) had complications in vital organs such as the heart, brain, or kidneys; 2) were unable to cooperate with assessments or had a history of cognitive impairment or psychiatric disorders; 3) were pregnant; and 4) had significant missing data on the core variables of interest in this study.
2.3 Research tools
A general information questionnaire was used, which included demographic information (sex, age at initial diagnosis of prolactinoma, education level, living arrangement, reproductive status, monthly family income, BMI, and number of hospital admissions) and disease-related information (recurrence of prolactinoma, tumor size, chronic diseases, occurrence of hyperprolactinemia during follow-up, Knosp grade, pretreatment prolactin levels, and prolactin levels at 1 month, 3 months, 6 months, and 1 year of follow-up). Prolactin levels at 1 month, 3 months, 6 months, and 1 year of follow-up were measured during routine examinations when patients visited the outpatient clinic. All data and information were collected retrospectively through the medical record system.
The Hospital Anxiety and Depression Scale (HADS) is a scale developed by Zigmond and Snaith in 1983 to assess anxiety and depression in pregnant patients in clinical or hospital settings (17, 18). The HADS consists of two parts: the Anxiety subscale (HADS-A) and the Depression subscale (HADS-D), each with 7 items. Each item is scored on a 4-point Likert scale, and the total score for each subscale ranges from 0 to 21. A cutoff score of 7 was used to determine the presence of anxiety or depression, with scores between 8 and 10 indicating mild anxiety or depression, scores between 11 and 14 indicating moderate anxiety or depression, and scores between 15 and 21 indicating severe anxiety or depression.
The Athens Insomnia Scale (AIS) was used to measure sleep quality in patients during the study period (19). The AIS is an internationally recognized self-report measure of sleep quality that consists of 8 items. Each item is scored on a 4-point Likert scale ranging from 0 to 3, with 0 indicating “no problem” and 3 indicating “severe problem”. The total score ranges from 0 to 24, with scores above 4 indicating the presence of sleep disorders. Higher scores indicate lower sleep quality and more severe sleep problems.
The General Self-Efficacy Scale (GSES), developed by Schwarzer et al., is widely used internationally (20). The Chinese version of the GSES has also demonstrated good applicability (21). We assessed the self-efficacy levels of prolactinoma patients using the Chinese version of the GSES. The Chinese version of the GSES consists of 10 items scored on a 4-point Likert scale. A higher total score indicates a stronger sense of self-efficacy. Based on the average self-efficacy scores, individuals can be classified into low-level self-efficacy (1.0-2.0), moderate-level self-efficacy (2.1-3.0), and high-level self-efficacy (3.1-4.0).
The HADS, AIS, and GSES were all collected retrospectively by the researchers from the outpatient medical record system.
2.4 Terminology definitions
BMI was calculated as body weight (kg)/height2 (m2). A BMI ≤18.5 indicates underweight, 18.6-23.9 indicates normal weight, ≥24 indicates overweight, and ≥28 indicates obesity (22). Pituitary prolactinomas are classified based on tumor size as microadenomas (≤1 cm), macroadenomas (1-4 cm), or giant adenomas (>4 cm)3. Knosp et al. (23) classified pituitary adenomas (PAs) into four grades based on the relative position of the tumor lateral to the internal carotid artery on MRI, using the mid-sagittal plane of the sella turcica as a reference and marking the inner, middle, and outer lines between the intracavernous segment of the internal carotid artery and the flow void. According to the Knosp classification, Grade 3 PAs exhibit tumor borders extending beyond the outer line of the internal carotid artery, while Grade 4 PAs encase the internal carotid artery, thus defining invasive PAs.
The detection method for PRL was as follows. Fasting blood samples were collected from patients during outpatient visits to monitor of PRL levels. PRL detection was performed using an Elecsys Prolactin II assay kit. The reference ranges were as follows: female, 4.79-23.30 ng/mL; male, 4.04-15.20 ng/mL. Patients with serum PRL levels above 23.30 ng/mL were diagnosed with hyperprolactinemia (according to the reference values provided by the PRL assay kit used in this study).
2.5 Statistical analysis
Normally distributed continuous variables are described using means and standard deviations (SDs), while nonnormally distributed continuous variables are described using medians (Ms) and interquartile ranges (IQRs). Categorical variables are described using frequencies and percentages. Since PRL exhibits a nonnormal distribution and is a repeated measurement variable, generalized estimating equation (GEE) models were used to analyze the factors influencing PRL levels in patients with pituitary prolactinoma. PRL was included as a continuous variable in the GEE model, with the Gaussian distribution selected for its distribution and the identity link function selected for its connection function. The independent variables were included in the GEE model as dummy variables to identify the factors affecting PRL levels. Furthermore, we compared the levels of PRL at different time points in the anxiety group versus the nonanxiety group, the sleep disorder group versus the nonsleep disorder group, and the low, medium, and high self-efficacy groups using separate effect analysis in the Generalized Estimating Equation (GEE) model. All the statistical analyses were performed using Stata 17 software, and the graphs were generated using GraphPad Prism 9.0. The significance level was set at two-sided (α=0.05), with P < 0.05 indicating statistical significance.
3 Results
3.1 Basic characteristics of patients
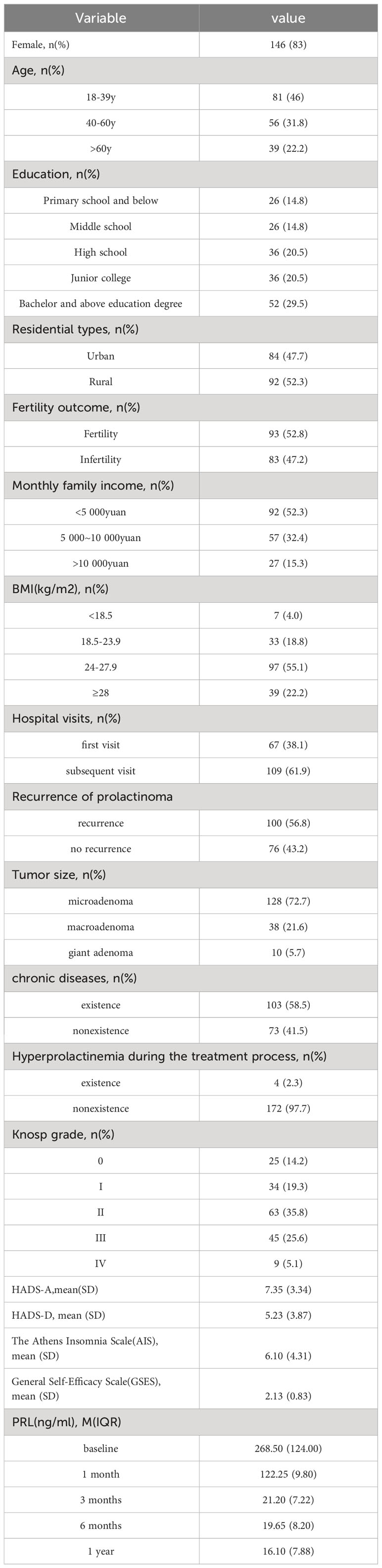
A total of 176 patients with prolactinoma, 30 males and 146 females, participated in this study. 2.2% of patients was over 60 years old. 29.5% of patients had a Bachelor and above education degree. 52.3% of the patients live in rural areas for a long time. 52.8% of the patients had reproductive capacity. 52.3% of the patients had a monthly family income of less than 5000 yuan. 77.3% of the patients had a BMI≥24. 61.9% of the patients had subsequent visits. 56.8% of patients experienced prolactinoma recurrence. The majority of patients (72.7%) had microadenomas, and 58.5% of patients had chronic diseases. A Knosp grade 3 or above was present in 30.7% of patients, and 4 patients reported hyperprolactinemia during the treatment process. The mean HADS-A score for prolactinoma patients was 7.35 ± 3.34, with 59.10% of patients experiencing anxiety. The mean HADS-D score was 5.23 ± 3.87, and 28.98% of patients had depression. The AIS score was 6.10 ± 4.31, with 59.10% of patients having sleep disorders. The GSES score was 2.13 ± 0.83, and 54.55% of patients had low self-efficacy levels. The PRL levels showed a marked decrease in the first month of treatment, followed by stabilization of the concentration. The detailed results are presented in Table 1.
3.2 Factors influencing PRL levels in patients with prolactinoma
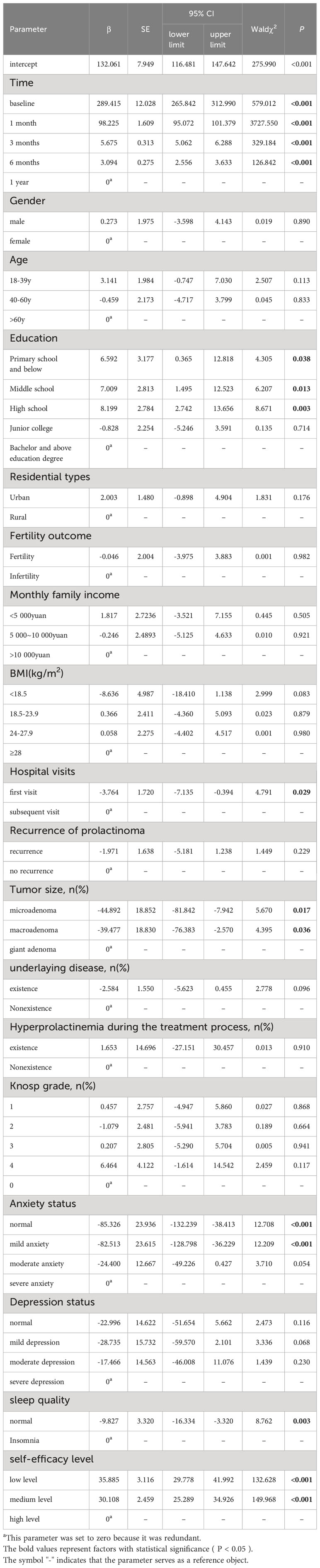
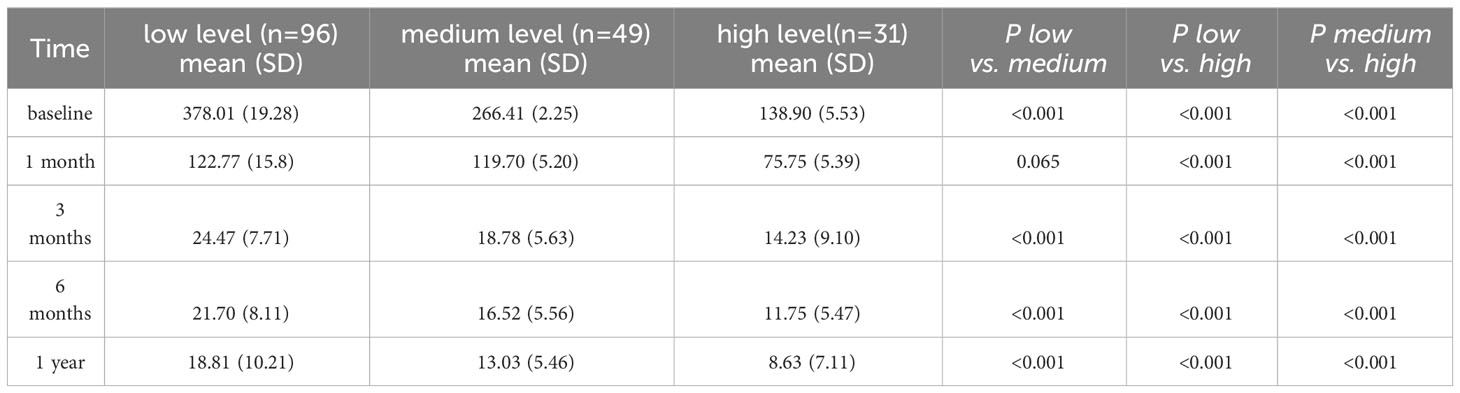
The GEE model showed that education level, tumor size, number of visits, sleep quality, anxiety level, and self-efficacy level were significantly related to PRL levels (P < 0.05, Table 2). Compared to patients with a Bachelor’s degree or higher education level, patients with a primary school or below (P = 0.038), middle school (P = 0.013), and high school education level (P = 0.003) had higher levels of PRL. Compared to patients with giant adenoma, patients with microadenoma (P = 0.017), macroadenoma (P = 0.036) had lower levels of PRL. Compared to patients who were visiting for the first time, those who had multiple visits for prolactinoma tended to have higher PRL levels (P = 0.029). Compared to patients with severe anxiety, those without anxiety (P < 0.001) and those with mild anxiety (P < 0.001) had lower PRL levels. The difference in PRL levels between patients with moderate anxiety (P = 0.054) and patients with severe anxiety was not statistically significant. Patients without sleep disorders had lower PRL levels than those with sleep disorders (P = 0.003). Patients with low self-efficacy (P < 0.001) and moderate self-efficacy (P < 0.001) had higher PRL levels than patients with high self-efficacy.
3.3 Comparison of PRL levels between the anxiety group and the nonanxiety group
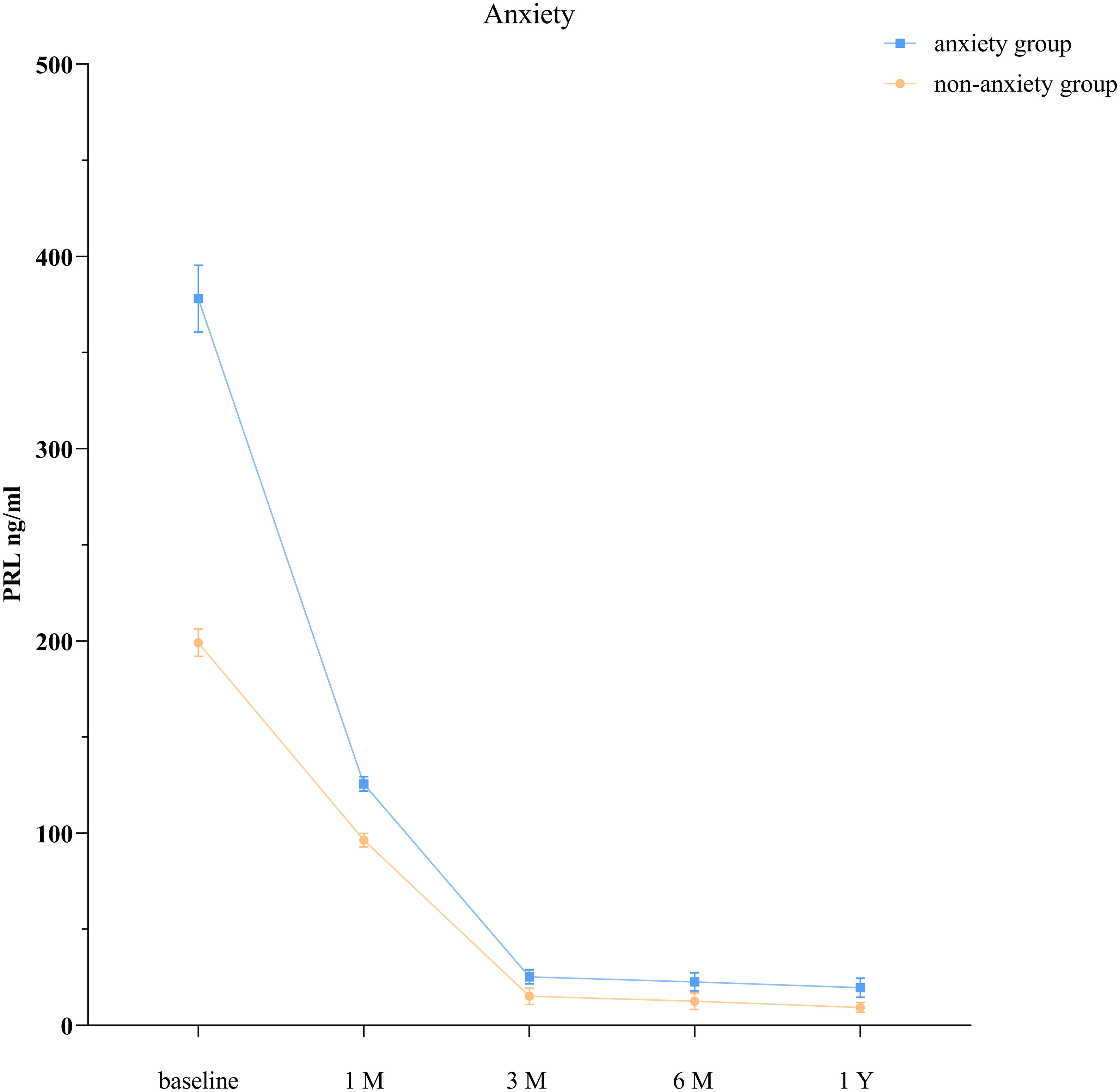
The GEE model showed that the anxiety group and the nonanxiety group had significantly different PRL levels at each time point (P < 0.001; Table 3, Figure 1).
3.4 Comparison of PRL levels between the normal group and sleep disorders group
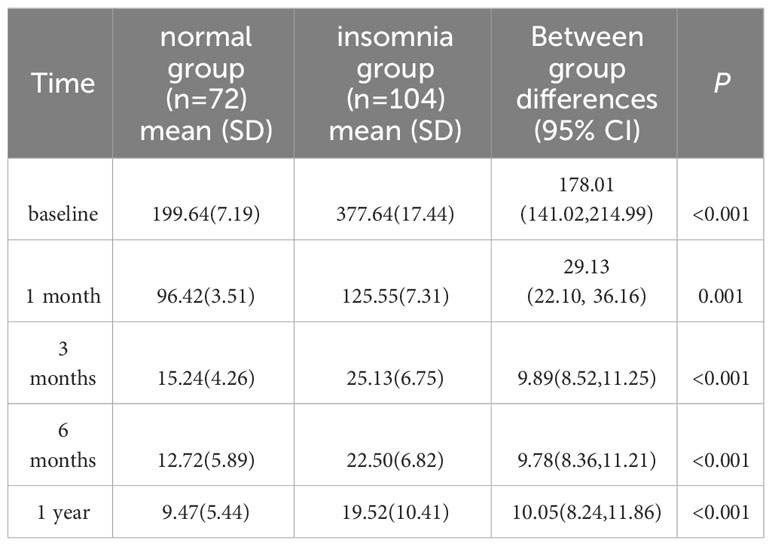
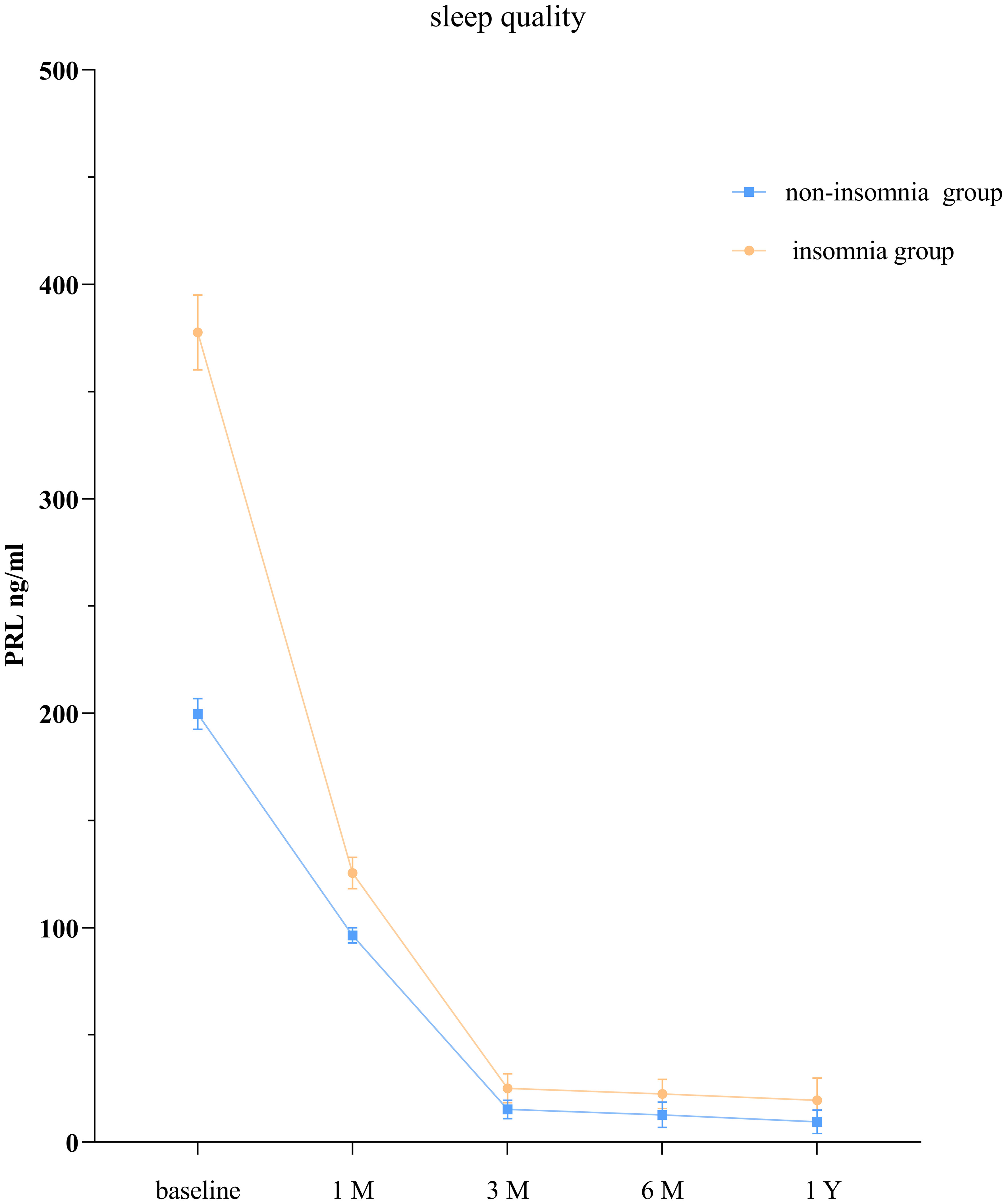
The GEE model showed that the group without sleep disorders and the group with sleep disorders had significantly different PRL levels at each time point (P < 0.05; Table 4, Figure 2).
3.5 Comparison of PRL levels among low self-efficacy group, moderate self-efficacy group, and the high self-efficacy group
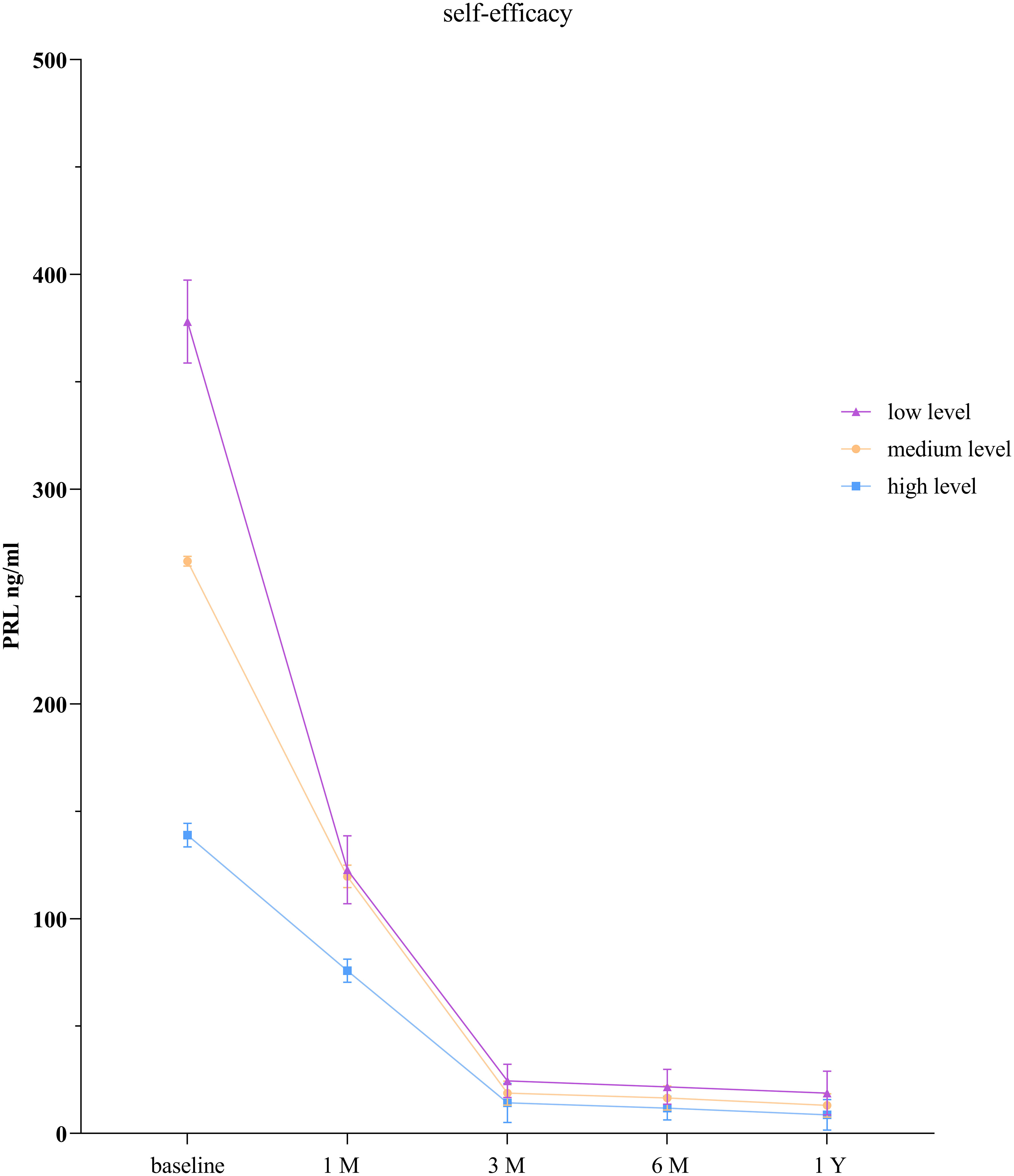
The GEE model showed that there were statistically significant differences in PRL levels between the low self-efficacy group and the high self-efficacy group as well as between the moderate self-efficacy group and the high self-efficacy group at each time point (P < 0.001). There was no statistically significant difference in PRL levels between the low self-efficacy group and the moderate self-efficacy group at the 1-month follow-up (P = 0.065), but significant differences were observed at all the other time points (P < 0.001; Table 5, Figure 3).
4 Discussion
Prolactinomas account for approximately 50% of all pituitary tumors requiring treatment (24). The 2022 edition of the ICCE/AME consensus states that DAs are the first-line treatment for most prolactinomas (3). These medications can normalize PRL levels in nearly 90% of patients with idiopathic hyperprolactinemia or prolactin microadenomas and in 75-80% of patients with macroprolactinomas (25). Most patients with macroadenomas also experience tumor shrinkage in the early stages of treatment (26), although the exact mechanisms of action are not yet fully understood (27, 28). In this study, we found that PRL levels rapidly decreased in patients with prolactinoma after the 1st month of drug therapy and gradually stabilized at the 3-month, 6-month, and 1-year. PRL levels in patients with prolactinoma may be related to sleep quality, anxiety level, and self-efficacy level. However, due to the limitations of retrospective studies, the causal relationship between psychological factors and prolactin levels could not be clearly established. The rapid decrease in PRL levels is attributed to DAs treatment as prolactinoma cells typically exhibit a high expression of D2-receptor. Long-term DAs therapy leads to significant tumor shrinkage in most case (29).
Our research has shown that prolactin levels in prolactinoma patients are associated with anxiety but not with depression. One possible explanation is that patients with high prolactin levels require continuous administration of DAs to control prolactin levels, and the emotions and behaviors of patients with hyperprolactinemia receiving Das treatment may change (30). Previous study suggested that the occurrence of depression in prolactinoma patients is related to the secondary decrease in estrogen levels caused by hyperprolactinemia (31). However, this was a case series of low quality. Several studies have indicated that patients with hyperprolactinemia are more likely to experience depression, anxiety, and hostility (5, 6). These symptoms are generally alleviated after hyperprolactinemia is corrected, suggesting that prolactin plays a direct role in psychological disorders. Therefore, healthcare professionals should promptly assess the anxiety and depression status of prolactinoma patients receiving long-term DAs treatment and provide psychological counseling. Furthermore, interdisciplinary collaboration is encouraged for the follow-up and symptom management of prolactinoma patients.
Our study revealed that compared to patients without sleep disorders, those with insomnia seemed to have lower PRL levels. A retrospective study reported that 42.3% of hospitalized patients admitted for mental illness and sleep disorders had elevated PRL levels and suggested an association between elevated PRL levels and excessive daytime sleepiness (14), which may be caused by nocturnal insomnia (32). Our study may help explain the relationship between prolactin levels and sleep in prolactinoma patients.
Prolactin can act on different brain regions, including those involved in neural growth; development; protection; sleep; learning; and memory, among others (33). Due to pituitary dysfunction; excessive hormone secretion; and psychological, behavioral, or environmental factors, patients with sellar region tumors may experience various forms of sleep disorders (34). However, due to limitations in the target population and study design, the relationship between prolactin levels in prolactinoma patients and sleep disorders is still controversial. Several studies have indicated that sleep disorders in prolactinoma patients are not directly related to prolactin levels, and they suggest that obesity and higher BMI are associated with sleep disorders in prolactinoma patients (13). Future prospective studies should use objective measures of sleep quality, control for confounding factors, and measure PRL levels at different time points to further explore the relationship between sleep and PRL levels in prolactinoma patients.
Patients with low self-efficacy tend to have higher PRL levels than do those with high self-efficacy. This may be because individuals with high self-efficacy are more likely to develop positive beliefs, increase positive emotions, and actively engage in health-promoting behaviors, leading to better control of PRL levels. Several studies have shown that individuals with higher levels of self-efficacy are more confident in facing adversity or illness and are more likely to adopt a positive attitude toward them (15, 16). Self-efficacy is beneficial for patients to establish a healthy and positive mindset and can improve their confidence in disease treatment (35). Studies have also indicated that an increase in self-efficacy can positively influence the prognosis of patients and improve sleep quality (36). Therefore, interventions aimed at improving sleep self-efficacy should be explored to enhance patients’ sleep quality.
PRL levels in prolactinoma patients are associated with education level. Currently, there is no direct research linking education level to PRL levels, but education level may influence patients’ perception of the disease, choice of treatment plans, and treatment adherence. Prolactinoma patients require the use of DAs to suppress PRL synthesis and secretion, control PRL levels, and prevent tumor recurrence or further growth (3, 37). Patients with higher education levels tend to have better medication compliance, resulting in more effective and stable control of PRL levels.
This study revealed that prolactin (PRL) levels in patients with prolactinoma are correlated with tumor size. Consistent with previous reports (1, 38–41), Osorio et al. (41) demonstrated a significant correlation between preoperative and postoperative tumor volume and PRL levels. For every 1 cubic centimeter increase in preoperative tumor volume, the serum PRL levels increased by 101.31 μg/L. Burke et al. (39) showed a strong correlation between prolactinoma volume and serum PRL levels, while nonfunctioning adenomas did not exhibit a significant correlation. Tumor size has been a subject of debate regarding its relationship with serum PRL levels (42). March et al. (43) reported two patients with galactorrhea and hyperprolactinemia who showed an increase in tumor size without a significant increase in PRL levels. However, the lack of MRI visualization for tumor assessment in these patients may have led to potentially inaccurate detection results. Several researchers have also proposed that the secretory capacity of prolactin is related to the number of intracellular secretory granules, which may not be correlated with tumor volume (44). Therefore, the prolactin levels in some larger prolactinomas may not be high.
Compared to patients who were visiting for the first time, those who had multiple visits for prolactinoma tended to have higher PRL levels. On the one hand, this could be due to tumors developing resistance to DAs, resulting in uncontrolled tumor growth and continued abnormal PRL secretion (45). On the other hand, prolactinomas that have not achieved remission after multiple visits may have a unique molecular composition, leading to higher prolactin levels during tumor growth (41). However, further molecular studies are needed in the future to explore the relationship between the molecular composition of prolactinomas and PRL levels.
This study has several limitations. First, the sample size was relatively small, but the population consisted of patients with prolactinoma and still held a certain representativeness. Second, this was a single-center retrospective study, and future research could include multicenter prospective studies to further investigate the relationships and mechanisms between PRL levels and both prolactinoma and sleep disorders as well as the severity of anxiety. Thirdly, our study lacks a correlation analysis between depression scores and the dosage/exposure of DAs. Due to the constraints of retrospective research and patient medication compliance, it was challenging to accurately collect specific information on the types and doses of DAs taken by the patients. Future prospective studies are needed to analyze the relationship between the dosage/types/duration of DAs medication and depression in prolactinoma patients. Finally, due to limited data, subgroup analysis was not conducted for patients receiving bromocriptine, cabergoline, or combination therapy. Future research should further explore and analyze the impact of DAs on patients’ sleep and psychological well-being.
5 Conclusion
In this retrospective study, we analyzed the relationship between prolactin (PRL) levels in patients with prolactinoma and anxiety, self-efficacy, and sleep. Our results showed that PRL levels in prolactinoma patients were related to anxiety, self-efficacy, and sleep but not depression. PRLs were higher in anxious patients than in nonanxious patients. PRL levels were also higher in patients with low self-efficacy than in those with high self-efficacy. Compared with patients without sleep disorders, insomnia patients had higher PRL levels. Future research should further investigate the mechanisms through which psychological and sleep factors affect PRL levels in prolactinoma patients and explore the relationships among psychological factors, sleep quality, and self-efficacy in prolactinoma patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because this study employs a retrospective research design, where participants are not directly involved physically, and only previously collected data pertaining to them is used for analysis. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because the study is focused on the utilization of informational data.
Author contributions
XM: Investigation, Writing – original draft. ZF: Conceptualization, Writing – original draft, Formal analysis. XL: Methodology, Supervision, Writing – review & editing. JW: Methodology, Writing – review & editing. LY: Supervision, Writing – review & editing. SZ: Data curation, Writing – review & editing. YF: Data curation, Writing – review & editing. SH: Conceptualization, Supervision, Writing – review & editing. SX: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Zunyi City science and technology plan project: Zuncity Kehe HZ character (2023) No. 293; Zuncity Kehe HZ character (2023) No. 289.
Acknowledgments
The authors would like to express their gratitude to the staff who participated in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chanson P, Maiter D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab. (2019) 33:101290. doi: 10.1016/j.beem.2019.101290
2. Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: A review. JAMA. (2023) 329:1386–98. doi: 10.1001/jama.2023.5444
3. Cozzi R, Ambrosio MR, Attanasio R, Battista C, Bozzao A, Caputo M, et al. Italian Association of Clinical Endocrinologists (AME) and International Chapter of Clinical Endocrinology (ICCE). Position statement for clinical practice: prolactin-secreting tumors. Eur J endocrinol. (2022) 186:P1–p33. doi: 10.1530/eje-21-0977
4. Pereira HS, Naliato EC, Moraes AB, Gadelha MR, Vieira Neto L, Almeida RM, et al. Body self-image disturbances in women with prolactinoma. Rev Bras psiquiatria (Sao Paulo Brazil 1999). (2020) 42:33–9. doi: 10.1590/1516-4446-2018-0325
5. Fava M, Fava GA, Kellner R, Buckman MT, Lisansky J, Serafini E, et al. Psychosomatic aspects of hyperprolactinemia. Psychother psychosomatics. (1983) 40:257–62. doi: 10.1159/000287773
6. Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary Sep. (2003) 6:81–7. doi: 10.1023/B:PITU.0000004798.27230.ed
7. Sonino N, Guidi J, Fava GA. Psychological aspects of endocrine disease. J R Coll Physicians Edinburgh. (2015) 45:55–9. doi: 10.4997/jrcpe.2015.113
8. Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J neuroendocrinol. (1999) 11:405–7. doi: 10.1046/j.1365-2826.1999.00342.x
9. Mohankumar PS, Mohankumar SM, Quadri SK, Voogt JL. Chronic hyperprolactinemia and changes in dopamine neurons. Brain Res bulletin. (1997) 42:435–41. doi: 10.1016/s0361-9230(96)00369-3
10. Zamorano M, Ledesma-Colunga MG, Adán N, Vera-Massieu C, Lemini M, Méndez I, et al. Prolactin-derived vasoinhibins increase anxiety-and depression-related behaviors. Psychoneuroendocrinology. (2014) 44:123–32. doi: 10.1016/j.psyneuen.2014.03.006
11. Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. (2002) 64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049
12. Sonino N, Fava GA. Improving the concept of recovery in endocrine disease by consideration of psychosocial issues. J Clin Endocrinol Metab Aug. (2012) 97:2614–6. doi: 10.1210/jc.2012-1710
13. Barbosa FR, Silva CM, Lima GA, Warszawski L, Domingues RC, Dominic M, et al. Prevalence of obstructive sleep apnea in patients with prolactinoma before and after treatment with dopamine agonists. Pituitary Oct. (2014) 17:441–9. doi: 10.1007/s11102-013-0524-y
14. Mogavero MP, Cosentino FII, Lanuzza B, Tripodi M, Lanza G, Aricò D, et al. Increased serum prolactin and excessive daytime sleepiness: an attempt of proof-of-concept study. Brain Sci. (2021) 11:1574. doi: 10.3390/brainsci11121574
15. Selzler AM, Habash R, Robson L, Lenton E, Goldstein R, Brooks D. Self-efficacy and health-related quality of life in chronic obstructive pulmonary disease: A meta-analysis. Patient Educ counseling. (2020) 103:682–92. doi: 10.1016/j.pec.2019.12.003
16. Merluzzi TV, Pustejovsky JE, Philip EJ, Sohl SJ, Berendsen M, Salsman JM. Interventions to enhance self-efficacy in cancer patients: A meta-analysis of randomized controlled trials. Psycho-oncology. (2019) 28:1781–90. doi: 10.1002/pon.5148
17. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
18. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. J psychosomatic Res. (2010) 69:371–8. doi: 10.1016/j.jpsychores.2010.04.006
19. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J psychosomatic Res. (2000) 48:555–60. doi: 10.1016/s0022-3999(00)00095-7
20. Schwarzer R. Optimistic self-beliefs: Assessment of general perceived self-efficacy in thirteen cultures. World Psychol. (1997) 3:177–90.
21. Zhang JX, Schwarzer R. Measuring optimistic self-beliefs: A Chinese adaptation of the General Self-Efficacy Scale. PSYCHOLOGIA. (1995) 38:174–81.
22. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis childhood. (1995) 73:25–9. doi: 10.1136/adc.73.1.25
23. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. (1993) 33:610–7; discussion 617-8. doi: 10.1227/00006123-199310000-00008
24. Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. (2009) 23:543–54. doi: 10.1016/j.beem.2009.05.008
25. Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. (1999) 84:2518–22. doi: 10.1210/jcem.84.7.5810
26. Colao A, Di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. (2000) 85:2247–52. doi: 10.1210/jcem.85.6.6657
27. Tang C, Sun R, Wen G, Zhong C, Yang J, Zhu J, et al. Bromocriptine and cabergoline induce cell death in prolactinoma cells via the ERK/EGR1 and AKT/mTOR pathway respectively. Cell Death disease. (2019) 10:335. doi: 10.1038/s41419-019-1526-0
28. Zhang SL, Tang HB, Hu JT, Zang ZL, Ding X, Li S, et al. PGAM5-CypD pathway is involved in bromocriptine-induced RIP3/MLKL-dependent necroptosis of prolactinoma cells. Biomed pharmacothe = Biomed pharmacotherapie Mar. (2019) 111:638–48. doi: 10.1016/j.biopha.2018.12.128
29. LaPierre MP, Godbersen S, Torres Esteban M, Schad AN, Treier M, Ghoshdastider U, et al. MicroRNA-7a2 regulates prolactin in developing lactotrophs and prolactinoma cells. Endocrinology. (2021) 162:bqaa220. doi: 10.1210/endocr/bqaa220
30. Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan Iii TB, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur J endocrinol. (2019) 180:31–40. doi: 10.1530/eje-18-0682
31. Liao WT, Bai YM. Major depressive disorder induced by prolactinoma–a case report. Gen Hosp Psychiatry. (2014) 36:125.e1–2. doi: 10.1016/j.genhosppsych.2013.01.010
32. Gandhi KD, Mansukhani MP, Silber MH, Kolla BP. Excessive daytime sleepiness: A clinical review. Mayo Clinic Proc. (2021) 96:1288–301. doi: 10.1016/j.mayocp.2020.08.033
33. Cabrera-Reyes EA, Limón-Morales O, Rivero-Segura NA, Camacho-Arroyo I, Cerbón M. Prolactin function and putative expression in the brain. Endocrine Aug. (2017) 57:199–213. doi: 10.1007/s12020-017-1346-x
34. van Schaik J, Pillen S, van Litsenburg RRL, Vandenbussche NLE, de Bont JM, Schouten-van Meeteren AYN, et al. The importance of specialized sleep investigations in children with a suprasellar tumor. Pituitary. (2020) 23:613–21. doi: 10.1007/s11102-020-01065-9
35. Thornton CP, Li M, Yeh CH, Ruble K. Self-efficacy in symptom management for adolescents and young adults with cancer: a systematic review. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer. (2021) 29:2851–62. doi: 10.1007/s00520-020-05960-6
36. Ghose SM, Dzierzewski JM, Dautovich ND. Sleep and self-efficacy: The role of domain specificity in predicting sleep health. Sleep Health. (2023) 9:190–5. doi: 10.1016/j.sleh.2022.09.008
37. Landeiro JA, Fonseca EO, Monnerat AL, Taboada GF, Cabral GA, Antunes F. Nonfunctioning giant pituitary adenomas: Invasiveness and recurrence. Surg Neurol Int. (2015) 6:179. doi: 10.4103/2152-7806.170536
38. Glezer A, Bronstein MD. Prolactinomas. Endocrinol Metab Clinics North A. (2015) 44:71–8. doi: 10.1016/j.ecl.2014.11.003
39. Burke WT, Penn DL, Castlen JP, Donoho DA, Repetti CS, Iuliano S, et al. Prolactinomas and nonfunctioning adenomas: preoperative diagnosis of tumor type using serum prolactin and tumor size. J neurosurg. (2019) 133:321–8. doi: 10.3171/2019.3.Jns19121
40. Wright K, Lee M, Escobar N, Pacione D, Young M, Fatterpekar G, et al. Tumor volume improves preoperative differentiation of prolactinomas and nonfunctioning pituitary adenomas. Endocrine. (2021) 74:138–45. doi: 10.1007/s12020-021-02744-8
41. Osorio RC, Pereira MP, Oh T, Joshi RS, Haddad AF, Pereira KM, et al. Correlation between tumor volume and serum prolactin and its effect on surgical outcomes in a cohort of 219 prolactinoma patients. J neurosurg. (2022) 138:1669–79. doi: 10.3171/2022.8.Jns221890
42. Alkabbani AG, Mon SY, Hatipoglu B, Kennedy L, Faiman C, Weil RJ, et al. Is a stable or decreasing prolactin level in a patient with prolactinoma a surrogate marker for lack of tumor growth? Pituitary. (2014) 17:97–102. doi: 10.1007/s11102-013-0473-5
43. March CM, Kletzky OA, Davajan V, Teal J, Weiss M, Apuzzo ML, et al. Longitudinal evaluation of patients with untreated prolactin-secreting pituitary adenomas. Am J obstetrics gynecol. (1981) 139:835–44. doi: 10.1016/0002-9378(81)90553-6
44. Melting C, Yijun S, Wei L, Ring X, Renzhi W. Analysis of curative effect of surgical therapy for male prolactinoma. Zhonghua yi xue za zhi. (2016) 96:1477–80. doi: 10.3760/cma.j.issn.0376-2491.2016.19.003
Keywords: prolactinoma, PRL levels, generalized estimating equations, anxiety, depression, sleep disorders, self-efficacy
Citation: Miao X, Fu Z, Luo X, Wang J, Yuan L, Zhao S, Feng Y, Huang S and Xiao S (2024) A study on the correlations of PRL levels with anxiety, depression, sleep, and self-efficacy in patients with prolactinoma. Front. Endocrinol. 15:1369729. doi: 10.3389/fendo.2024.1369729
Received: 12 January 2024; Accepted: 29 February 2024;
Published: 19 March 2024.
Edited by:
Luiz Augusto Casulari, University of Brasilia, BrazilReviewed by:
Atanaska Petrova Elenkova, Medical University - Sofia, BulgariaSylvère Störmann, LMU Munich University Hospital, Germany
Copyright © 2024 Miao, Fu, Luo, Wang, Yuan, Zhao, Feng, Huang and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunwu Xiao, eHN3bG92ZTE5NzZAMTI2LmNvbQ==; Shiming Huang, MTg4NDg0ODg1NjNAMTYzLmNvbQ==
†These authors share first authorship
 Xiaoju Miao
Xiaoju Miao Zhongmin Fu1,2†
Zhongmin Fu1,2†