
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 17 May 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1369248
This article is part of the Research TopicAdipose tissue and adipokines: their roles in human reproductionView all 5 articles
 Konstantinos Nikolettos1*
Konstantinos Nikolettos1* Nikolaos Vlahos2
Nikolaos Vlahos2 Olga Pagonopoulou3
Olga Pagonopoulou3 Nikos Nikolettos1
Nikos Nikolettos1 Konstantinos Zikopoulos4
Konstantinos Zikopoulos4 Panagiotis Tsikouras1
Panagiotis Tsikouras1 Emmanouil Kontomanolis1
Emmanouil Kontomanolis1 Christos Damaskos5,6
Christos Damaskos5,6 Nikolaos Garmpis6,7
Nikolaos Garmpis6,7 Iason Psilopatis8
Iason Psilopatis8 Byron Asimakopoulos3
Byron Asimakopoulos3Background: Reproduction ability requires a certain amount of body fat that is necessary for ovulation, menstruation and pregnancy. Fat tissue represents an endocrine organ with high metabolic activity as it produces adipokines such as leptin and adiponectin. Our aim is to examine potential associations between women of reproductive age’s ovarian reserves and their levels of leptin and adiponectin.
Method: 74 women between 19 and 40 years of age consented to take part. Based on the patterns of their ovarian reserves, the women were divided into three main groups: women with adequate ovarian reserves (AOR - Group A, n=30), women with polycystic ovary syndrome (PCOS - Group B, n=31) and women with depleted ovarian reserves (DOR - Group C, n=13). Among these groups, several biochemical and demographic parameters were statistically compared.
Results: Compared to the other two groups, women with DOR had statistically higher age and follicle stimulation hormone (FSH) levels. For estradiol (E2) and thyroid-stimulating hormone (TSH), no statistically significant difference was seen between the groups. In addition, women with PCOS had higher body mass index (BMI), luteinizing hormone (LH), total testosterone (TT), 17 hydroxyprogesterone (17-OHP), dehydroepiandrosterone (DHEA), anti-Mullerian hormone (AMH), and antral follicle count (AFC) than the other two groups. In line with expectations, women with DOR also had lower levels of AMH and AFC than the other two groups. Women with PCOS had higher leptin levels than the other two groups, but there was no statistically significant difference. Women with PCOS had lower levels of adiponectin than the other groups, however the difference was not statistically significant.
Conclusion: The way we classified women in our study according to their ovarian reserves is completely consistent with what has been published internationally. The ovarian reserve in women of reproductive age is not strongly correlated with leptin and adiponectin levels. For safe conclusions, more research including a greater number of samples is required.
In 1974, Frisch and his team reported the ability to reproduce requires a certain amount of body fat that is necessary for ovulation, menstruation and pregnancy (1). Adipose tissue represents a dynamic organ with metabolic role and it contains adipocytes. It produces adipokines such as leptin and adiponectin (2).
Leptin plays crucial role in regulating female reproductive health through distinct molecular pathways. Leptin, primarily secreted by adipose tissue, acts on the hypothalamus to stimulate the release of gonadotropin-releasing hormone (GnRH), thereby influencing the menstrual cycle and fertility (3–5). Leptin concentrations are directly related to body fat content (6). Furthermore, leptin levels are very sensitive to caloric deprivation (7). Leptin is a 167 amino acid polypeptide that is mostly produced by fat cells but is also found in the placenta, mammary glands, and stomach (8). Moreover, leptin levels are at their lowest at 09:00 and reach their peak at 01:00 (9). Serum leptin levels are strongly lowered during fasting, even before weight decrease, despite the fact that they are positively correlated with adipose tissue (10). Despite the fact that leptin inhibits appetite, obese individuals have higher serum leptin levels and may be leptin resistant, which is comparable to the insulin resistance that is frequently observed in obesity (11). Serum leptin levels are also influenced by steroids, fat distribution, and gender (12). Additionally, subcutaneous and visceral adipose tissue express leptin differently (13).
The most widely released adipokine that is only expressed in adipose tissue is called adiponectin (2, 14). αdiponectin modulates reproductive function by affecting ovarian steroidogenesis and folliculogenesis, contributing to the regulation of ovulation and fertility (5). Three distinct forms of adiponectin have been identified: low molecular weight adiponectin, hexamer adiponectin, and high molecular weight adiponectin (15). Adipocyte mass is inversely correlated with circulating adiponectin levels, which rise with weight loss and fall with obesity (16). Men and women have different levels of adiponectin; women typically have higher levels of this hormone (17). Rats, chickens, pigs, cows, and other animals have also been shown to express adiponectin and its receptors in their ovarian granulosa cells (18–20).
These aforementioned adipokines serve as key links between energy metabolism and reproductive processes, highlighting the intricate interplay between adipose tissue and reproductive physiology. Suboptimal reproductive health, such as irregular menstrual cycles or polycystic ovarian syndrome (PCOS), can disrupt the balance of hormones involved in metabolism, including leptin and adiponectin. In conditions like PCOS, alterations in insulin sensitivity and ovarian function can lead to elevated leptin levels and decreased adiponectin levels, contributing to metabolic disturbances and further exacerbating reproductive dysfunction (3–5).
In the present study, we investigated whether the circulating levels of leptin and adiponectin correlate with the ovarian reserves.
Between February 2020 and November 2022, a prospective cross-sectional study was carried out in the Laboratory of Physiology, Faculty of Medicine, Democritus University of Thrace, in cooperation with the IVF clinic “Embryokosmogenesis,” in Alexandroupolis, Greece. The Democritus University of Thrace’s Research Ethics Committee (34792/223/13-2-2020) gave its approval to the study. Following a thorough explanation of the study’s objectives, all female participants signed a written consent form. Pregnancy, menopause, smoking, hypo- or hyperthyroidism, endometriosis, hysterectomy, oophorectomy, congenital adrenal hyperplasia, Cushing syndrome, and hypogonadotropic hypogonadism were the exclusion criteria.
Seventy-four women, ranging in age from 19 to 40 years, were enlisted. On these women ovarian reserve test was calculated by measuring anti-Mullerian hormone (AMH) and antral follicle count (AFC). Based on these results, these women were divided into three main groups: those with adequate ovarian reserves (AOR) (Group A - AOR, n=30), those with PCOS (Group B - PCOS, n=31), and those with depleted ovarian reserves (DOR) (Group C - DOR, n=13). PCOS was diagnosed using the Rotterdam criteria (21). In accordance with the Committee Opinion no 618 of the American College of Obstetricians and Gynecologists, diminished ovarian reserves were defined as <7 follicles in both ovaries (22). Figure 1 indicates the patient selection criteria and the flow chart of the current study.
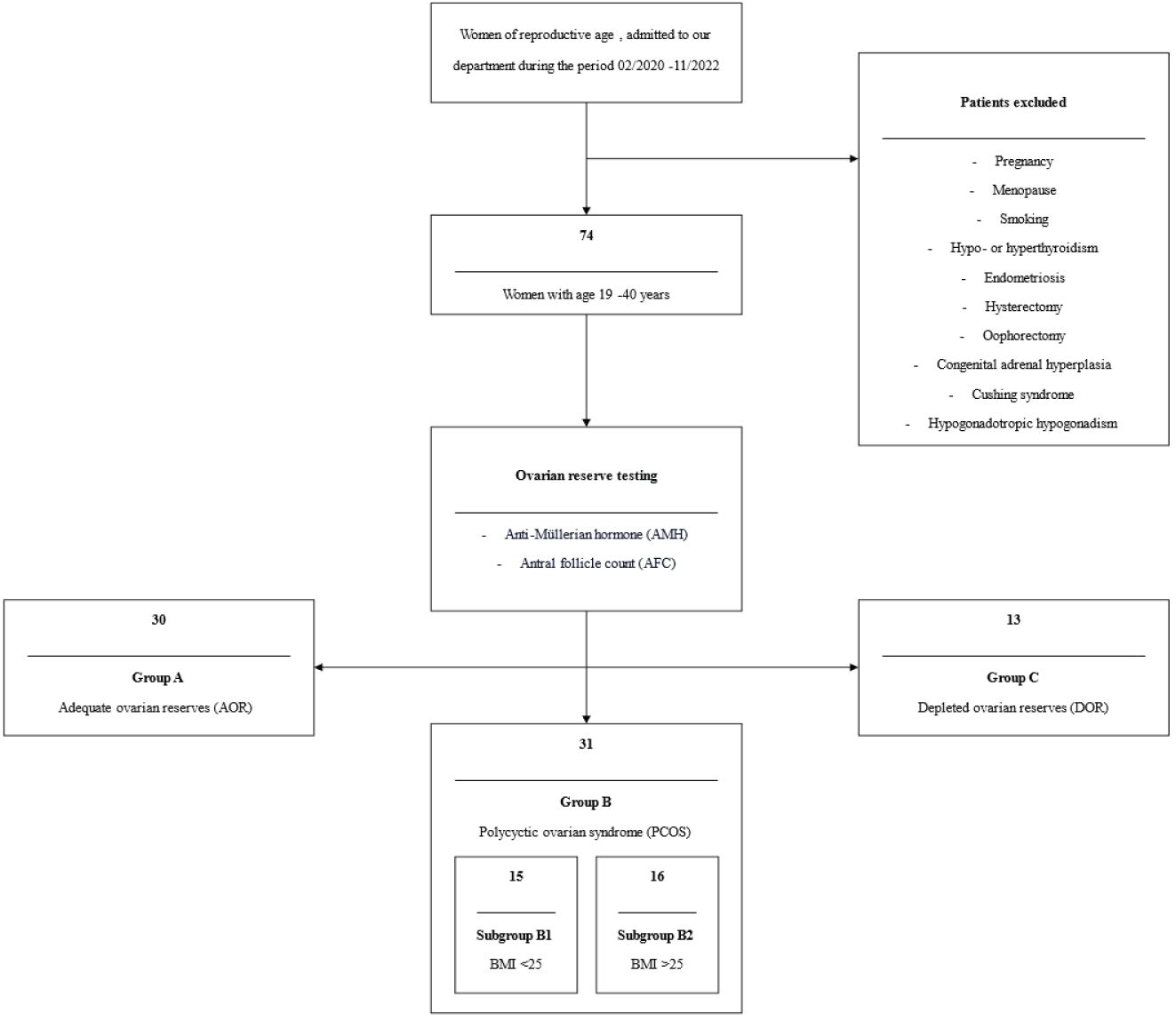
Figure 1 Patient’s selection criteria and the flow chart of the current study. AMH, Anti-Müllerian hormone; AFC, Antral follicle count; AOR, Adequate ovarian reserves; PCOS, Polycystic ovarian syndrome; BMI, Body mass index; DOR, Depleted ovarian reserves.
Women were first examined physically, with measurements taken for height, weight, and body mass index (BMI). On days 2–5 of the menstrual cycle, the same medical professional calculated the AFC using a Voluson 730P (General Electric Co.) ultrasound machine fitted with a vaginal transducer. Between day two and day five of the menstrual cycle, blood samples were drawn once for the purpose of measuring the following: follicle stimulation hormone (FSH), luteinizing hormone (LH), estradiol (E2), total testosterone (TT), 17 hydroxyprogesterone (17-OHP), dehydroepiandrosterone (DHEA), thyroid-stimulating hormone (TSH), AMH, leptin and adiponectin. After clotting at room temperature for ten minutes, blood samples were centrifuged at 1500 g for the purpose of extracting serum. Every day, serum samples were examined for FSH, LH, 17-OHP and E2. Serum samples were frozen at -80°C for the leptin, adiponectin, and AMH measurements within an hour of blood being drawn. ELISA was used to measure the serum levels of leptin and adiponectin using the following kits as per the manufacturer’s instructions: leptin (DLP00, R&D, USA) and adiponectin (DRP300, R&D, USA).
Finally, the biochemical and demographic traits of the three groups under investigation are presented in Table 1.
The statistical software Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA) was used to conduct the statistical analysis. Utilizing the Kolmogorov Smirnov and Shapiro-Wilks tests, the normality of the data was examined. Since some of the data did not fit into a normal distribution, group comparisons were made using non-parametric tests. The levels of adiponectin and leptin were tested for any significant correlation with the other parameters using Spearman rank R. A threshold of 0.05 was established for statistical significance.
Compared to the other two groups, the group with lower ovarian reserves (Group C) had statistically significantly higher age and FSH values (Kruskal-Wallis, p=0.0031 and p<0.001 respectively) (Figures 2A, B). In comparison to the other two groups, women with high ovarian reserves (Group B) had higher BMI, LH, TT, 17-OHP, DHEA and AMH (Kruskal-Wallis, p=0.0031, p<0.001, p<0.001, p=0.0134, p<0.001, p<0.001, respectively) (Figures 3A–F). In this group, AFC was also higher (Kruskal-Wallis, p<0.001) (Figure 4). As anticipated, women with low ovarian reserve had lower AMH and AFC compared to the other groups (p<0.001, p<0.001, respectively) (Figures 3F, 4). The Kruskal-Wallis analysis revealed no statistically significant difference inE2 or TSH between the groups (p=0.08 and p=0.479, respectively) (Figures 5A, B). Women with PCOS had higher leptin levels, but the difference was not statistically significant (Kruskal-Wallis, p=0.177) (Figure 6A). Additionally, although there was no statistically significant difference, women with PCOS had lower adiponectin levels (Kruskal-Wallis, p=0.77) (Figure 6B). The aforementioned results are described in Table 1.
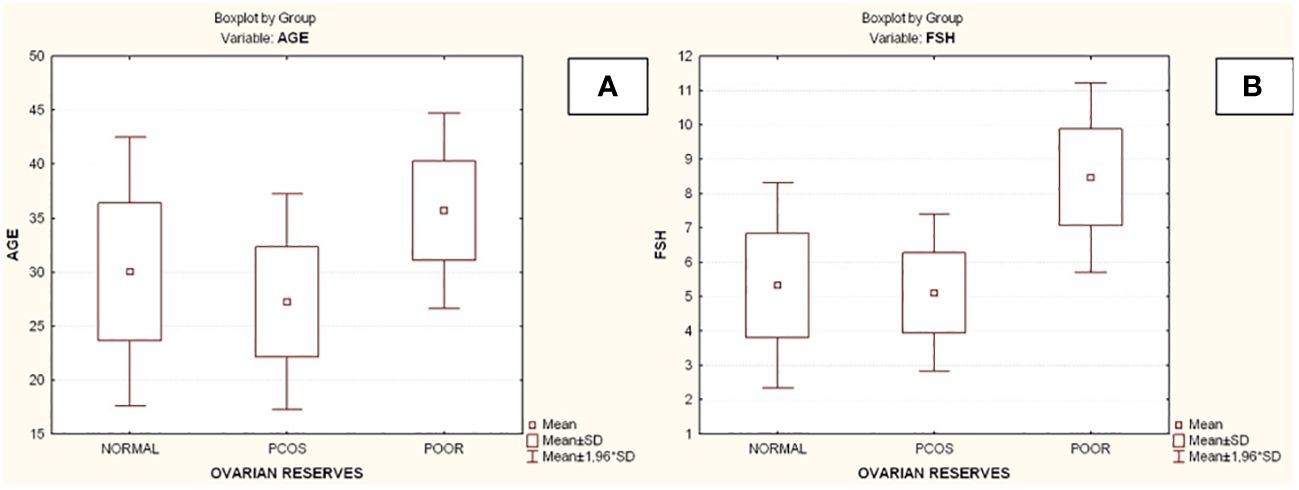
Figure 2 Comparison of parameters and ovarian reserves in all groups. (A) Age; (B) Follicle-stimulating hormone (FSH). PCOS, Polycystic ovarian syndrome; SD, Standard deviation; FSH, Follicle-stimulating hormone.
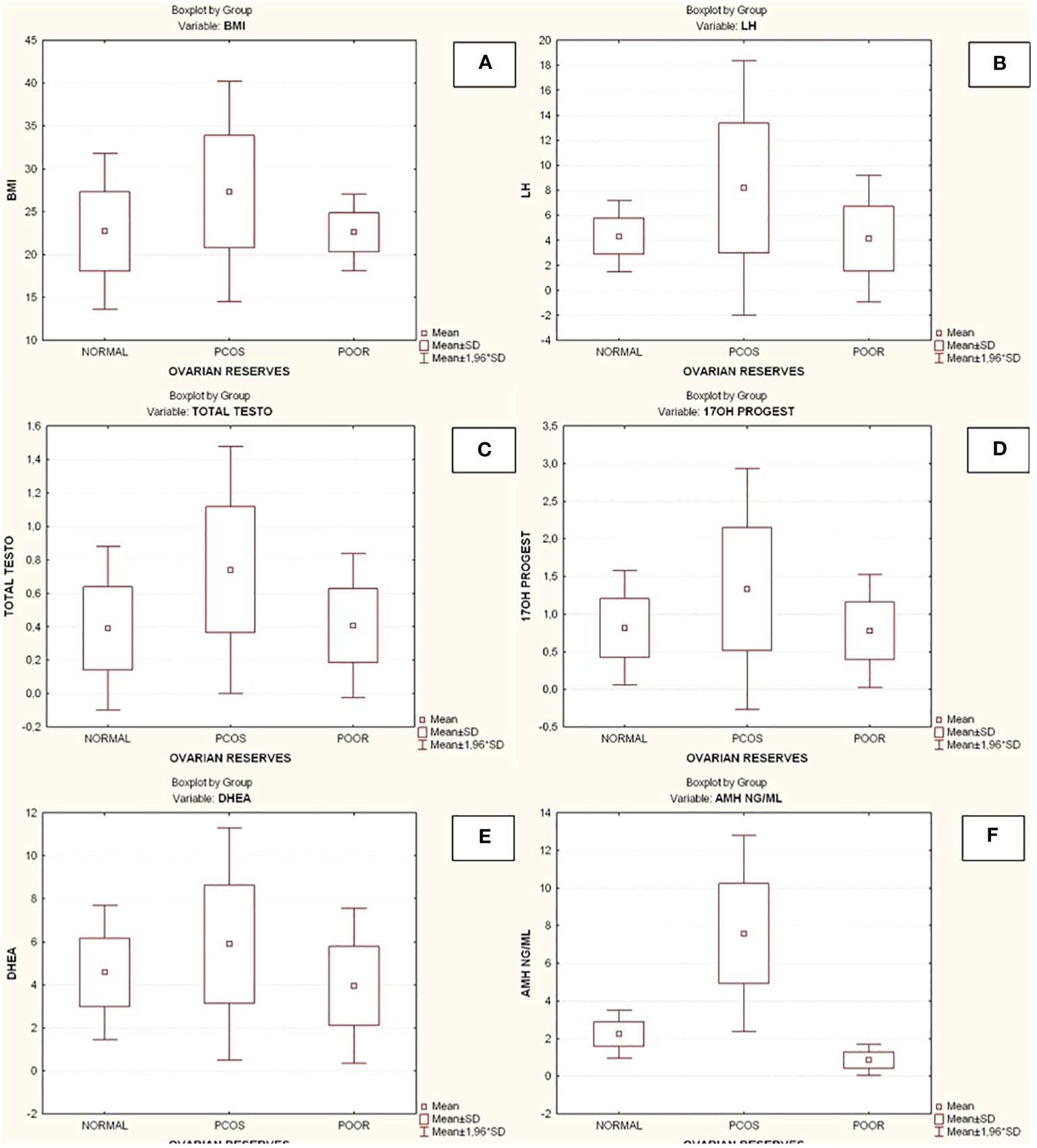
Figure 3 Comparison of parameters and ovarian reserves in all groups. (A) Body mass index (BMI); (B) Luteinizing hormone (LH); (C) Total testosterone (TT); (D) 17-hydroxy progesterone (17OHP); (E) Dehydroepiandrosterone (DHEA); (F) Anti-Mullerian hormone (AMH). BMI, Body mass index; PCOS, Polycystic ovarian syndrome; SD, Standard deviation; LH, Luteinizing hormone; TT, Total testosterone; 17OHP, 17-hydroxy progesterone; DHEA, Dehydroepiandrosterone; AMH, Anti-Mullerian hormone.
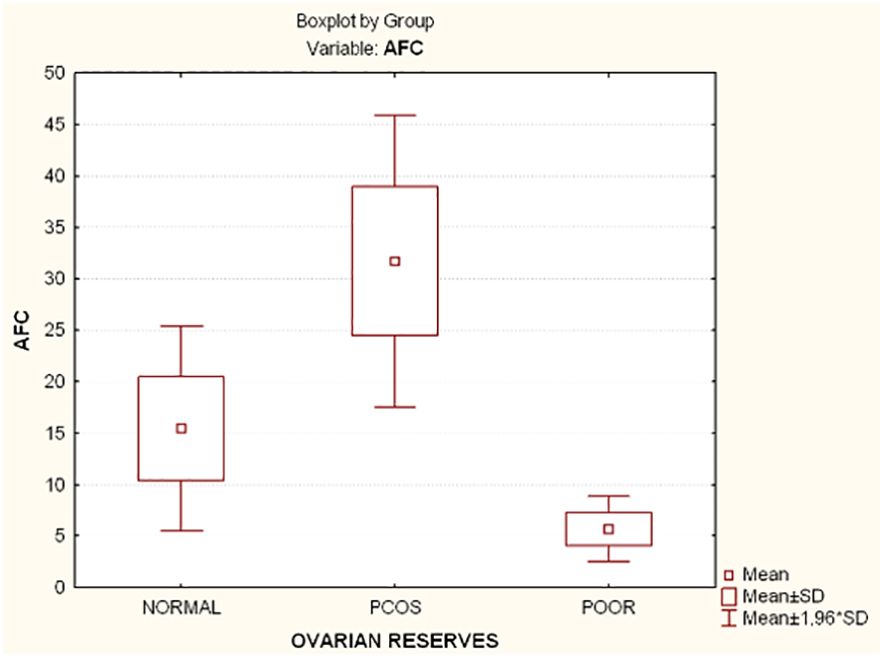
Figure 4 Comparison of antral follicle count (AFC) and ovarian reserves in all groups. AFC, Antral follicle count; PCOS, Polycystic ovarian syndrome; SD, Standard deviation.
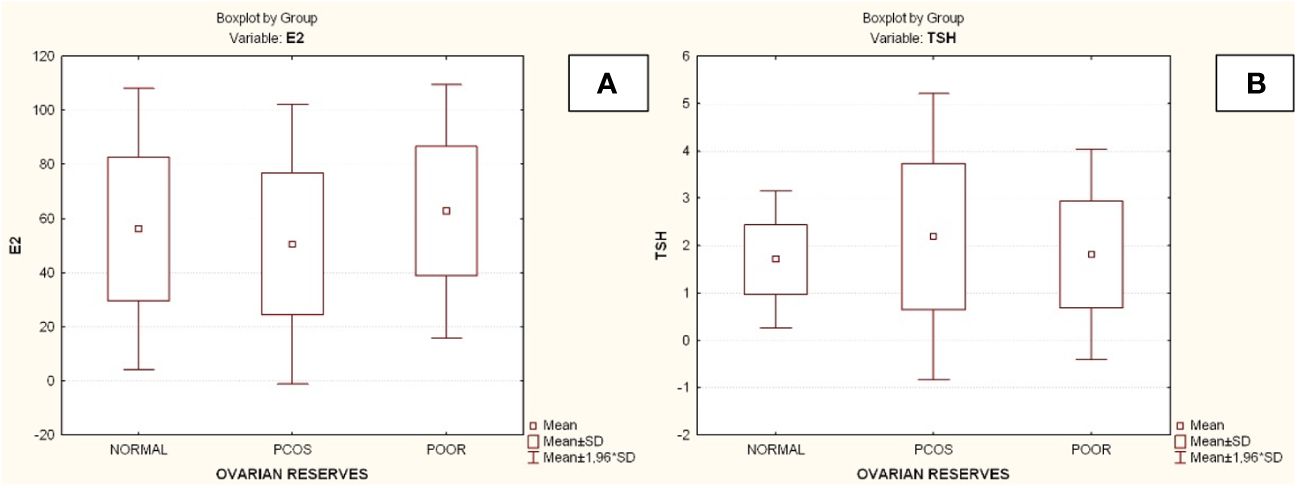
Figure 5 Comparison of parameters and ovarian reserves in all groups. (A) Estradiol (E2); (B) Thyroid-stimulating hormone (TSH). E2, Estradiol; PCOS, Polycystic ovarian syndrome; SD, Standard deviation; TSH, thyroid-stimulating hormone (TSH).
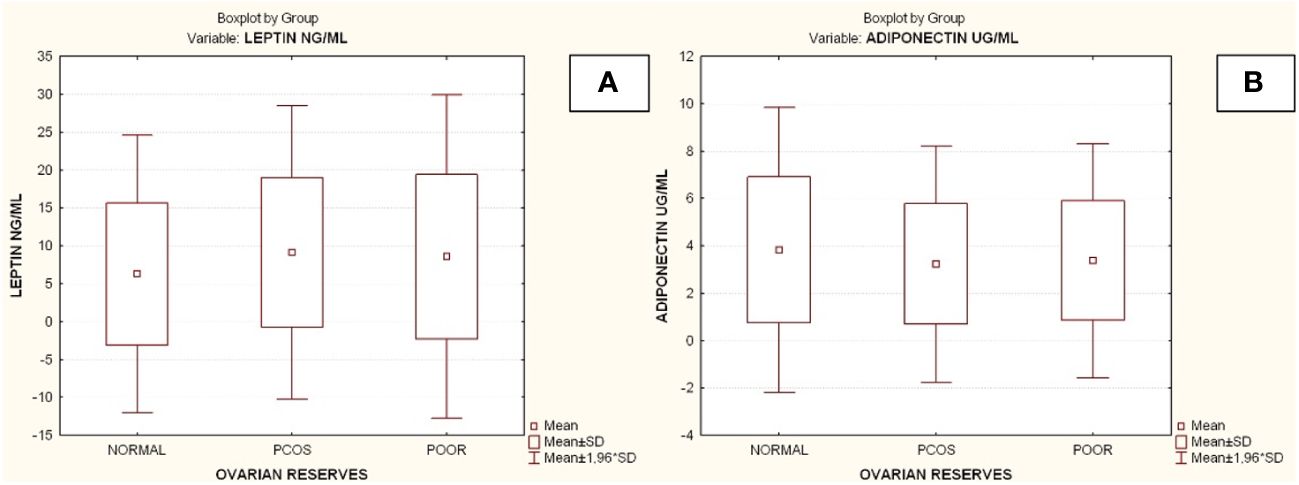
Figure 6 Comparison of parameters and ovarian reserves in all groups. (A) Leptin; (B) Adiponectin. PCOS. Polycystic ovarian syndrome; SD. Standard deviation. Diagrams Diagram 1. Patient’s selection criteria and the flow chart of the current study. AMH. Anti-Müllerian hormone; AFC. Antral follicle count; AOR. Adequate ovarian reserves; PCOS. Polycystic ovarian syndrome; BMI. Body mass index; DOR. Depleted ovarian reserves.
The correlation analyses of leptin and other parameters were performed in women of all groups. In group A and in group B there was not a correlation between leptin and the other parameters. In group C leptin levels were negative correlated with E2 (r=-0,57, p<0.05) and positive correlated with AMH (r =0.65, p<0.05) and adiponectin (r=0,59, p<0.05) (Table 2).
Also, the correlation analyses of adiponectin and other parameters were performed in women of all groups. In group A there was not a correlation between adiponectin and other parameters. In group B adiponectin levels were negative correlated with LH (r=-0.37, p<0.05) and 17-OHP (r =-0.4, p<0.05). Finally, in group C adiponectin levels were positive correlated with DHEA (r=0.62, p<0.05) and leptin (r=0.59, p<0.05) (Table 2).
Moreover, in group B, which included women with increased BMI compared to the other two groups, further statistical analysis was performed. Thus, group B was divided into two subgroups based on BMI: B1 (PCOS with BMI<25, n=15) and B2 (PCOS with BMI>25, n=16). In the comparison of subgroup B1 with B2, no statistically significant difference was found for the values of leptin (Mann – Whitney U Test, p=0.95) and adiponectin (Mann – Whitney U Test, p=0.54).
Furthermore, leptin was correlated with the parameters in subgroup B1, where there was a statistically significant positive correlation between leptin, FSH (r=0.52, p<0.05) and LH (r=0.58, p<0.05). In subgroup B2, no statistically significant correlation was found between leptin and the parameters (Table 3). In subgroup B1 there was a statistically significant negative correlation between adiponectin, LH (r=-0.53, p<0.05) and 17-OHP (r=-0.53, p<0.05). In subgroup B2, no statistically significant correlation was found between adiponectin and the parameters (Table 4).
In times of nutritional deficiency, adipose tissue serves as a vital source of energy in addition to offering protection against mechanical harm and heat. The amount and distribution of adipose tissue are influenced by a number of significant factors, including food consumption, physical activity, and psychological variables (23). Numerous studies have revealed that leptin, the first adipokine, is involved in reproductive functions since its discovery (24–27). These results piqued curiosity about studying other adipokines, like adiponectin.
This study’s primary goal was to look into any potential relationships between women of reproductive age’s ovarian reserves and their levels of leptin and adiponectin. Based on their ovarian reserves, the women in this study were divided into three groups. The ovarian reserves were assessed using AFC, AMH, and FSH. Given the sensitivity of female reproductive health to age-related changes, particularly after 35 years and the average age of participants in group C is notably higher than that of the other two groups (Figure 2A), there was significantly lower AFC and AMH values and higher FSH values than the other two groups. In contrary, Group B had significantly higher AFC and AMH values than the other two groups, as would be expected.
Likewise, it was anticipated that Group B would have higher LH and androgen values because the pathophysiology of this syndrome is associated with these hormones’ elevated levels. Generally speaking, great care was taken to select women and assign them to the various study groups in compliance with globally recognized scientific standards. The primary constraint on this study is the comparatively small group sizes.
In our study leptin levels were increased in women of Group B compared to the other two groups without a statistical significance. However, leptin is affected by many different factors such as the distribution of body fat, fasting and the time of collecting the blood samples. In addition, we investigated the association of leptin with all parameters with each group. In group A and group B no significant statistical correlation of leptin with the other measured parameters was found. But in Group C a negative correlation was found between leptin and E2 and a positive correlation between leptin, AMH and adiponectin. The literature review did not provide any explanation for these findings, so they remain open to further investigation in the future.
Aiming to more thoroughly investigation we further sub-divided group B into two subgroups, based on BMI. In subgroup B1, a positive correlation of leptin with FSH and LH was found. LH is elevated in women with PCOS (28). Even today it is unclear what the role of leptin is in women with PCOS, although most researchers have found elevated leptin levels as well as leptin resistance in PCOS (both obese and lean subjects) (9, 29, 30). In subgroup B2, no statistically significant correlation was found between leptin and the remaining parameters.
Regarding adiponectin, the comparison of its values between the three groups (A, B and C) showed that group B had lower values compared to the other groups, but the difference was not statistically significant. Circulating adiponectin levels decrease with obesity and increase with weight loss (16). In our study group B had a statistically significant higher BMI than the other two groups.
In addition, we investigated the correlation of adiponectin with all parameters in each group. No statistically significant was found in group A. However, in group B, adiponectin was negatively correlated with LH and 17-OHP. As we know LH and 17-OHP are increased in PCOS (31). Also, most publications reported that adiponectin levels are decreased in women with PCOS (32–36). Therefore, we expected a correlation like this. In the subgroup B1 the same statistical result was found as we reported a negative correlation of adiponectin with LH and 17-OHP. No statistically significant correlation was found between adiponectin and parameters in subgroup B2. Finally, in the correlation analysis between adiponectin and Group C parameters, adiponectin was positively correlated with DHEA and leptin.
The main limitation of this study is the low sample number. Although there was no significant difference in leptin and adiponectin levels within the groups, due to the low sample number, it is difficult to generally conclude that leptin and adiponectin levels do not correlate with ovarian reserve. Furthermore, several factors determine plasma leptin and adiponectin levels which need to be dissected out in carefully controlled study design. As far as we know, there is no corresponding finding in the international literature, so further assays are needed.
In our study, leptin levels were elevated in women with PCOS who had an increased BMI, but there was no statistically significant difference compared to the other two groups. Leptin levels are affected by many different factors. Another possible explanation that there was no statistically significant difference between leptin and the three groups is that the number of samples was limited. Moreover, women with PCOS had lower adiponectin levels compared to the other two groups, but the difference was not statistically significant. The majority of studies found low adiponectin levels in women with PCOS regardless of BMI level although some studies have reported the exact opposite. Other factors that affect adiponectin levels are the body composition and the measured form.
In conclusion, we did not find compelling evidence linking circulating levels of leptin and adiponectin to ovarian reserves when we examined three groups of women of reproductive age (women with adequate ovarian reserves, women with high ovarian reserves, and women with diminished ovarian reserves). In our view, more research involving a greater number of participants is required to draw firm findings.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Research Ethics Committee of the Democritus University of Thrace (34792/223/13-2-2020). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KN: Writing – review & editing, Writing – original draft. NV: Writing – review & editing, Supervision. OP: Writing – review & editing. NN: Writing – review & editing, Supervision. KZ: Writing – review & editing. PT: Writing – review & editing. EK: Writing – review & editing. CD: Writing – review & editing. NG: Writing – review & editing. IP: Writing – review & editing, Data curation. BA: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. (1974) 185:949–51. doi: 10.1126/science.185.4155.949
2. Singh A, Choubey M, Bora P, Krishna A. Adiponectin and chemerin: contrary adipokines in regulating reproduction and Metabolic Disorders. Reprod Sci. (2018) 25:1462–73. doi: 10.1177/1933719118770547
3. Zhao X, Xiong Y, Shen Y. Leptin plays a role in the multiplication of and inflammation in ovarian granulosa cells in polycystic ovary syndrome through the JAK1/STAT3 pathway. Clinics (Sao Paulo). (2023) 78:100265. doi: 10.1016/j.clinsp.2023.100265
4. Nikolettos K, Nikolettos N, Vlahos N, Pagonopoulou O, Asimakopoulos B. Role of leptin, adiponectin, and kisspeptin in polycystic ovarian syndrome pathogenesis. Minerva Obstet Gynecol. (2023) 75:460–7. doi: 10.23736/S2724-606X.22.05139-9
5. Athar F, Karmani M, Templeman NM. Metabolic hormones are integral regulators of female reproductive health and function. Biosci Rep. (2024) 44:BSR20231916. doi: 10.1042/BSR20231916
6. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. (1996) 334:292–5. doi: 10.1056/NEJM199602013340503
7. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. (2003) 111:1409–21. doi: 10.1172/JCI17490
8. Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. (1998) 83:1810–3. doi: 10.1210/jcem.83.5.4952
9. Laughlin GA, Yen SS. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab. (1997) 82:318–21. doi: 10.1210/jcem.82.1.3840
10. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. (1996) 382:250–2. doi: 10.1038/382250a0
11. Münzberg H, Myers MG JrMolecular and anatomical determinants of central leptin resistance. Nat Neurosci. (2005) 8:566–70. doi: 10.1038/nn1454
12. Iuorno MJ, Islam LZ, Veldhuis PP, Boyd DG, Farhy LS, Johnson ML, et al. Leptin secretory burst mass correlates with body mass index and insulin in normal women but not in women with polycystic ovary syndrome. Metabolism. (2007) 56:1561–5. doi: 10.1016/j.metabol.2007.06.027
13. Van Harmelen V, Reynisdottir S, Eriksson P, Thörne A, Hoffstedt J, Lönnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. (1998) 47:913–7. doi: 10.2337/diabetes.47.6.913
14. Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obes (Lond). (2008) 32:223–31. doi: 10.1038/sj.ijo.0803719
15. Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. (2010) 94:1949–57. doi: 10.1016/j.fertnstert.2010.05.010
16. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. (2003) 88:2838–43. doi: 10.1210/jc.2002-021721
17. Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol. (2005) 153:409–17. doi: 10.1530/eje.1.01978
18. Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. (2006) 147:5178–86. doi: 10.1210/en.2006-0679
19. Choubey M, Ranjan A, Bora PS, Baltazar F, Martin LJ, Krishna A. Role of adiponectin as a modulator of testicular function during aging in mice. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:413–27. doi: 10.1016/j.bbadis.2018.11.019
20. Choubey M, Ranjan A, Bora PS, Krishna A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie. (2020) 168:41–52. doi: 10.1016/j.biochi.2019.10.014
21. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
22. Committee opinion no. 618: Ovarian reserve testing. Obstet Gynecol. (2015) 125:268–73. doi: 10.1097/01.AOG.0000459864.68372.ec
23. Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. (2006) 27:762–78. doi: 10.1210/er.2006-0033
24. Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. (2011) 301:E567–584. doi: 10.1152/ajpendo.00315.2011
25. Casabiell X, Piñeiro V, Vega F, de la Cruz LF, Diéguez C, Casanueva FF. Leptin, reproduction and sex steroids. Pituitary. (2001) 4:93–9. doi: 10.1023/a:1012999130035
26. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. (2006) 393:7–20. doi: 10.1042/BJ20051578
27. Duggal PS, van der Hoek KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, et al. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. (2000) 141:1971–6. doi: 10.1210/endo.141.6.7509
28. Li Y, Chen C, Ma Y, Xiao J, Luo G, Li Y, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. (2019) 228:167–75. doi: 10.1016/j.lfs.2019.04.046
29. Brzechffa PR, Jakimiuk AJ, Agarwal SK, Weitsman SR, Buyalos RP, Magoffin DA. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (1996) 81:4166–9. doi: 10.1210/jcem.81.11.8923878
30. Hahn S, Haselhorst U, Quadbeck B, Tan S, Kimmig R, Mann K, et al. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. Eur J Endocrinol. (2006) 154:287–94. doi: 10.1530/eje.1.02078
31. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. (2006) 147:5817–25. doi: 10.1210/en.2006-0787
32. Carmina E, Chu MC, Moran C, Tortoriello D, Vardhana P, Tena G, et al. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril. (2008) 89:642–8. doi: 10.1016/j.fertnstert.2007.03.085
33. Aroda V, Ciaraldi TP, Chang SA, Dahan MH, Chang RJ, Henry RR. Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil Steril. (2008) 89:1200–8. doi: 10.1016/j.fertnstert.2007.04.046
34. Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchón R, Luque-Ramírez M, et al. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Hum Reprod. (2006) 21:2257–65. doi: 10.1093/humrep/del146
35. Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrøm V, Flyvbjerg A, et al. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin ghrelin leptin body composition hirsute PCOS patients controls. Eur J Endocrinol. (2006) 155:337–45. doi: 10.1530/eje.1.02207
Keywords: leptin, adiponectin, ovarian reserves, polycystic, reproductive age, ovarian, syndrome
Citation: Nikolettos K, Vlahos N, Pagonopoulou O, Nikolettos N, Zikopoulos K, Tsikouras P, Kontomanolis E, Damaskos C, Garmpis N, Psilopatis I and Asimakopoulos B (2024) The association between leptin, adiponectin levels and the ovarian reserve in women of reproductive age. Front. Endocrinol. 15:1369248. doi: 10.3389/fendo.2024.1369248
Received: 11 January 2024; Accepted: 02 May 2024;
Published: 17 May 2024.
Edited by:
Mayank Choubey, NYU Grossman Long Island School of Medicine, United StatesReviewed by:
Nisha Thomas, University of Oklahoma, United StatesCopyright © 2024 Nikolettos, Vlahos, Pagonopoulou, Nikolettos, Zikopoulos, Tsikouras, Kontomanolis, Damaskos, Garmpis, Psilopatis and Asimakopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Nikolettos, ay5uaWtvbGV0dG9zQHlhaG9vLmdy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.