
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 20 June 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1364106
Background: A rapid increase in the prevalence of diabetes is an urgent public health concern among older adults, especially in developing countries such as China. Despite several studies on lifestyle factors causing diabetes, sleep, a key contributor, is understudied. Our study investigates the association between night sleep duration and diabetes onset over a 7-year follow-up to fill information gaps.
Method: A population-based cohort study with 5437 respondents used 2011–2018 China Health and Retirement Longitudinal Study data. Using self-reported night sleep duration from the 2011 baseline survey, information on new-onset diabetes was collected in follow-up surveys. Baseline characteristics of participants with vs. without new-onset diabetes were compared using Chi-square and Mann-Whitney U tests. Multivariable Cox regression models estimated the independent relationship between night sleep and new-onset diabetes. The addictive Cox regression model approach and piece-wise regression described the nonlinear relationship between night sleep and new-onset diabetes. Subgroup analysis was also performed by age, gender, body measurement index, dyslipidemia, drinking status, smoking, hypertension, and afternoon napping duration.
Result: 549 respondents acquired diabetes during a median follow-up of 84 months. After controlling for confounders, night sleep duration was substantially linked with new-onset diabetes in the multivariable Cox regression model. The risk of diabetes is lower for respondents who sleep longer than 5 hours, except for those who sleep over 8 hours [5.1–6h Hazard ratios (HR) [95% confidence intervals (CI)] = 0.71 (0.55, 0.91); 6.1–7h HR = 0.69 (0.53, 0.89); 7.1–8h HR = 0.58 (0.45, 0.76)]. Nonlinear connections were delineated by significant inflection points at 3.5 and 7.5 hours, with a negative correlation observed only between these thresholds. With one hour more night sleep, the risk of diabetes drops 15%. BMI and dyslipidemia were identified as modifiers when only consider the stand linear effect of sleep duration on diabetes.
Conclusion: This study establishes a robust association between night sleep and new-onset diabetes in middle-aged and older Chinese individuals within the 3.5–7.5-hour range, offering a foundation for early glycemic management interventions in this demographic. The findings also underscore the pivotal role of moderate night sleep in preventing diabetes, marking a crucial juncture in community medical research.
The ever-expanding global public health concern of diabetes mellitus, coupled with its numerous comorbidities, has progressively captured the attention of the medical community (1). Over the antecedent decades, the adult populace grappling with diabetes has demonstrated a dramatic surge, particularly in countries with low to middle income such as China (2). According to the International Diabetes Federation, China carries the weighty mantle of possessing the highest diabetes-afflicted populace, exceeding 140 million sufferers as of 2021; a staggering figure anticipated to escalate beyond 174 million by the year 2045 (3). As known, type 2 diabetes is the predominant type of diabetes, accounting for approximately 90% of adult individuals with diabetes (4). Although genetic predisposition partially determines individual susceptibility to type 2 diabetes, the sedentary lifestyle and unhealthy diet serve as crucial driving factors of the current global epidemic (4, 5). Strong evidence suggests that a significant number of type 2 diabetes cases could be prevented by maintaining a healthy body weight, adhering to a healthy diet, engaging in at least 30 minutes of daily exercise, avoiding smoking, and practicing moderate alcohol (6, 7). Accordingly, identifying modifiable factors associated with diabetes and understanding their association can contribute to improving intervention strategies and alleviating the disease burden.
Adequate and high-quality night sleep is fundamentally indispensable for maintaining holistic physical and psychological health (8). With the inevitability of aging, however, comes an increased propensity for sleep irregularities, including an insufficiency or surplus of night sleep (9, 10). Sleep irregularity serves as a modifiable risk factor, which is considered as one of the most important causes of diabetes. Previous studies have indicated that new-onset diabetes is a prevalent affliction amongst middle-aged and older adults, with a close association with night sleep duration. The most comprehensive meta-analysis to date proposed a U-shaped relationship between night sleep duration and the incidence of Type 2 diabetes (11). Findings from a U.K. Biobank cohort analysis showed that individuals with unfavorable sleep and circadian patterns were at an elevated risk of developing Type 2 diabetes (12). In Liu’s Mendelian randomized study, the presence of insomnia traits or inadequate night sleep was causatively linked to elevated levels of HbA1c (13). When focusing specifically on the Chinese population, the association between nighttime sleep and diabetes remains valid, yet results are still inconsistent (10). For example, a prospective study from the Shanghai Men’s Health Study involving 34,825 participants found a slight increase in diabetes risk for those sleeping 8 hours or more compared to those sleeping 7 hours (HR = 1.2, 95% CI = 1.0–1.3) (14). Another study of 11,539 Chinese participants with three years follow-up observed higher diabetes risk in those sleeping over 9 hours, with no significant difference in those sleeping less than 6 hours compared to 7–8 hours (10). Though these studies unanimously indicate a nonlinear relationship between nighttime sleep and diabetes, inconsistencies in their results strongly reduce their practical relevance. Therefore, further study for the Chinese population using a representative cohort with a sufficient follow-up time is still required.
In this study, we utilized the latest 2018 follow-up data from China Health and Retirement Longitudinal Study (CHARLS) to assess the relationship between night sleep duration and the new-onset of diabetes among Chinese middle-aged and older individuals. Moreover, we employed non-linear methods to elucidate the association between night sleep duration and newly diagnosed diabetes. Our research aimed to lay the groundwork for devising early intervention strategies for glucose management in middle-aged and older populations, as an integral part of community healthcare practices.
The current cohort study embodies a secondary analysis of data originating from the CHARLS, an ongoing, nationally representative longitudinal investigation (15). The extensive details of the study design have been delineated in a separate publication (15). In summary, a total of 17,708 participants across 10,257 households were recruited between June 2011 and March 2012 from 150 distinct counties or districts, encompassing 450 villages spanning 28 provinces within China. The recruitment strategy was based on the application of a multistage stratified probability-proportional-to-size sampling technique. Household with a member 39 years of age or older was randomly selected, and at least one of member aged in 39 or older will be selected as study sample. If the selected person was between 39 and 45 years of age that person was designated for inclusion in a future refreshment sample and was not interviewed. If the chosen person was 45 years of age or older, both that person and his or her spouse were interviewed. Therefore, we only included those who were at least 45 years old at the first wave as our study sample. Participants undertook the completion of a standardized questionnaire aimed at capturing a diverse array of sociodemographic, lifestyle factors, and health-associated details. An impressive 80.5% response rate was achieved in the initial survey. Physical activity is acquired in a subsample of half the sample, using the modified International Physical Activity Questionnaire-short form. Subsequent follow-ups were planned biennially post the baseline survey. The CHARLS received the necessary ethical approval from the Institutional Review Board of Peking University and written informed consent was obtained from all participants. The present study adhered to the guidelines stipulated under the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (16).
The analysis began with the inclusion of the data from 16,063 participants with complete baseline responses (W1, 2011) on night sleep duration. Next, individual meeting the relevant exclusion criteria were further excluded. Exclusion criteria comprised individuals 1) in the W1 baseline survey who satisfied any of the diagnostic criteria for diabetes (17) (namely, fasting plasma glucose levels ≥ 126 mg/dL [converted to mmol/L by multiplying by 0.0555], HbA1c ≥ 6.5%, current usage of modern antidiabetic medications, or self-reported history of diabetes), 2) with missing data for any of the diabetes diagnostic criteria, 3) with a self-reported history of cancer, and 4) aged 45 years or younger, 5) documented deaths before diabetes incidence or missing follow-up information, 6) with missing data in any control variables. The remaining pool of 5437 participants’ data was included for analysis: among them, 488 developed diabetes during follow-up. A detailed participant enrollment flowchart is illustrated in Figure 1, providing a comprehensive visual overview of the recruitment process.
Figure 2 depicts the proposed conceptual framework for the current study. The exposure variable is the duration of night sleep at baseline, and the outcome variable is new-onset diabetes throughout a 7-year period. The supposed casual effects are based on the temporal order of exposure and outcome variables. Potential confounders are considered because of their synergistic relationship with exposure and outcome variables, including age, gender, education level, marital status, living residence, smoking status, drinking status, BMI, hypertension, dyslipidemia, and afternoon snap sleep duration. Abbreviations: BMI, body mass index.
New-onset diabetes was characterized by an affirmative response to the question: “Have you received a diagnosis of diabetes or high blood sugar?” The person-time follow-up for each participant was computed from the baseline survey date to the date of diabetes diagnosis or the participant’s date of death.
We evaluated night sleep duration based on responses to the question: “In the past month, how many hours of actual sleep did you get on an average night? (Note, this may be less than the total number of hours you spent in bed.)” Based on the sleep patterns observed within the participant pool, responses were arranged into five groups: ≤5 hours, 5.1–6 hours, 6.1–7 hours, 7.1–8 hours, and >8 hours. Additionally, to structure a regression model with a smooth term and price-wise model, we employed continuous night sleep duration data.
The selection of covariates was informed by previous research and encompassed the following: age, gender, living residence, marital status, educational level, smoking and drinking status, body measurement index (BMI), hypertension, dyslipidemia, and afternoon snap duration (10, 18).
Trained interviewers collected sociodemographic and health-related information at baseline using a structured questionnaire. This encompassed details on age, sex, living residence (rural or urban), marital status (married or other [never married, separated, divorced, or widowed]), and educational level (formal education, primary school, middle or high school, or college or above). Health-related factors included self-reported smoking and drinking status, categorized into three levels: never, former, or current. A trained nurse undertook measurements of height, weight, and blood pressure.
BMI was computed as weight in kilograms divided by the square of height in meters, and classified into three levels: <18.5, 18.5–23.9, and ≥24 kg/m2. Hypertension was diagnosed if systolic blood pressure was ≥140 mm Hg, diastolic blood pressure was ≥90 mm Hg, if the participant was currently taking antihypertensive medications, or if they self-reported a history of hypertension. Dyslipidemia was defined as total cholesterol ≥240 mg/dL [converted to mmol/L by multiplying by 0.0259], triglycerides ≥150 mg/dL, low-density lipoprotein cholesterol ≥160 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, current use of lipid-lowering medications, or self-reported history of dyslipidemia.
The afternoon napping duration was based on responses to the question: “Over the past month, on average, how long have your post-lunch naps been?” Guided by earlier research (19, 20) and the participants’ reported nap habits, the responses were allocated into four distinct categories: 0, <30 min, 31–90 min, and >90 min.
Continuous variables adhering to a normal distribution are represented as means and standard deviations (SDs), while categorical variables are expressed as frequencies and percentages. Baseline traits of participants, stratified based on new-onset diabetes, were summarized and analyzed using the chi-squared test and Mann-Whitney U test for comparison.
Percentiles of missing values among covariates spanned from 0% to 11% (specifically, 8% and 473 observations for hypertension, 1% and 44 observations for dyslipidemia, 1 observation for drinking status, and 11% and 671 observations for BMI; the total observation count with all variables was 5437). In the primary analysis, instances of incomplete observations were omitted based on variables adjusted within the model.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to investigate the association between night sleep duration and new-onset diabetes by employing Cox proportional hazards models. The proportional hazards assumption was tested with Schoenfeld’s global test and found no violation. Two models were established to examine the association between night sleep duration and new-onset diabetes.
Model 1 was the crude model, with no adjustments for covariates.
Model 2 is the full model, which included adjustments for age, sex, education, marriage, living residence, smoking status, drinking status, BMI, hypertension, dyslipidemia, and daytime sleep duration.
To validate the potential non-linear association between night sleep duration and new-onset diabetes during 7-year follow-up, the additive Cox model was employed for curve-fitting method. Once non-linear trend was observed from the figure result of additive Cox model, a recursive algorithm (identifying the inflection point where the piecewise Cox has the largest maximum likelihood value using recursive method) was used to identifying inflection points.
Piecewise Cox model was constructed based on inflection points, and log-likelihood ratio test was used to test the difference between the piecewise model and one-line (non-segmented) model. To exploit whether this non-linear association remains stable in different stratification of given variable, we also conducted stratified analysis. Meanwhile, P-values for interaction were calculated by comparing models with or without interaction terms of night sleep duration and potential effect modifiers using likelihood ratio tests.
Three sensitivity analyses were performed to ensure the robustness of our results. In the first sensitivity analysis, we further considered sleep quality as a covariate. An evaluation question from Center for Epidemiologic Studies Short Depression Scale 10 (CESD-10) scale was used for evaluating the sleep quality: “Did you feel your sleep was restless during the past week?”. The following response options were provided: “Rarely or none of the time <1 day”, “some or a little of the time 1–2 days”, “occasionally or a moderate amount of 3” and “Most or all of the time 5–7 days”.
We further adjust this variable in our full model and serve it as the first sensitivity analysis., Given the different effects of moderate vs. heavy drinking on the diabetes risk in previous studies, we replaced the original drinking status with drinking among in the full model as the second sensitivity analysis. Drinking amount was categorized to non-drinker vs. moderate (≤1 drink/d for females, ≤2 drinks/d for males) vs. heavy (>1 drink/d for females, >2 drinks/d for males). In the third sensitivity analysis, missing values in covariates were assumed to be missing completely at random (MCAR), and multiple imputation method was used to generate 5 post-interpolation datasets which will be used for re-generating previous adjusted models.
Statistical significance was set at a two-sided P value of <0.05. All analyses were executed using R version 4.2.1 (R Foundation).
Table 1 encapsulates the demographic and health characteristics of the analyzed participants, which have been systematically stratified according to whether they had been diagnosed with diabetes during the follow-up period. The mean age of the participants was found to be 58.80 years, with a standard deviation (SD) of 8.55.
The educational attainment was relatively low, with approximately half of the participants (47.99%) not having received formal education. Most participants were married or partnered (89.31%), lived in a rural area (67.94%), and were nonsmokers (61.38%) and nondrinkers (61.80%). A significant proportion of the cohort had a BMI within the average range (54.55%).
Participants reported an average night sleep duration of 6.34 hours (SD = 1.89), and 48.91% stated that they did not take afternoon snaps. In exploring the relationship between sleep patterns and the onset of diabetes, the study revealed that the mean night sleep duration was higher in the participants without new-onset diabetes (6.37 hours) compared to those with it (6.07 hours, P < 0.001). A trend was observed wherein participants with new-onset diabetes were more likely to be female, possess less formal education smoke and drink less, have a higher BMI, and have hypertension and dyslipidemia. Metabolic markers tested in the wave 1 also presented in Supplementary Table 1.
Compared with the group who do not have new-onset diabetes, participants who have new-onset diabetes during the 7 years follow-up had a higher levels of HbA1c (5.24 vs 5.09, P < 0.001), fasting glucose (105.02 vs 99.56 mg/dl, P < 0.001), total cholesterol (197.46 vs 192.34 mg/dl, P = 0.004), low-density lipoprotein (120.51 vs 117.10 mg/dl, P = 0.034), triacylglycerol (141.47 vs 119.32 mg/dl, P <0.001), a lower levels of high-density lipoprotein (49.06 vs 52.55 mg/dl, P < 0.001).
Table 2 presents the outcomes from the regression investigations. Model 1, when benchmarked against a night sleep duration of less than 5 hours, divulged HRs (and their 95% CIs) for new-onset diabetes as follows: 0.7 (0.54, 0.89) for 5.1–6 hours, 0.66 (0.51, 0.86) for 6.1–7 hours, 0.57 (0.44, 0.73) for 7.1–8 hours, and 0.7 (0.49, 0.99) for sleep duration exceeding 8 hours. These associations, even after adjusting for aspects such as afternoon snap duration, age, sex, education, marital status, geographical residence, lifestyle factors, BMI, hypertension, and dyslipidemia, remained significant in all models barring the group that sleeps more than 8-night hours (HR = 0.71, 95% CI: [0.50, 1.01]).
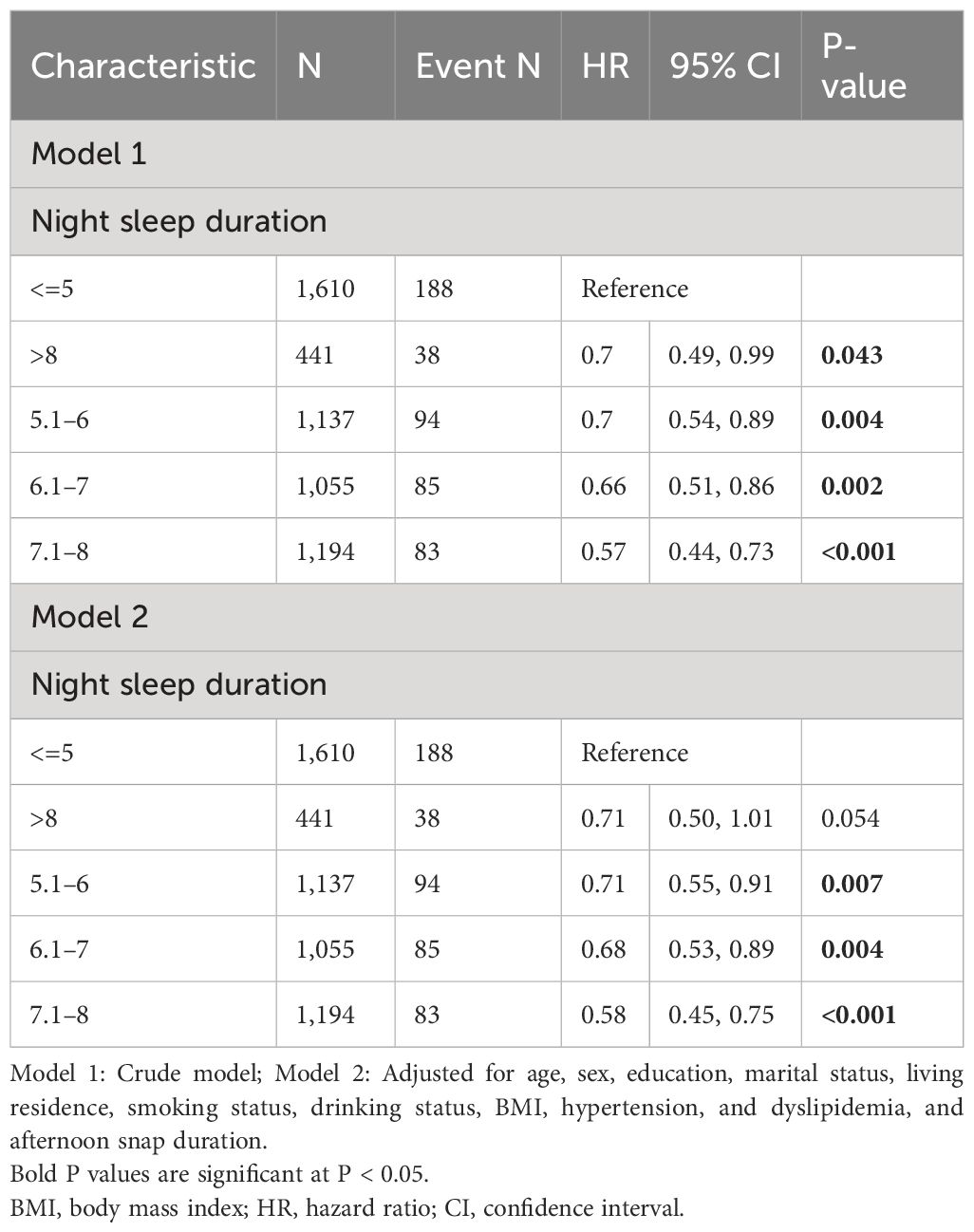
Table 2 Associations of night sleep duration (discontinuous) with new-onset diabetes in Chinese middle-aged and older adults.
To explore the potential non-linear association, we employed addictive Cox regression model. As shown in Figure 3, after adjusting for age, gender, education level, marriage, living residence, smoking status, drinking status, BMI, hypertension, dyslipidemia, and afternoon napping duration, we discovered a fluctuated association between night sleep duration and new-onset diabetes. With sequential usage of recursive algorithm, we identified two inflection points, which are 3.5 and 7.5 hours. Based on these two inflection points, we combined a Cox model with a three-piecewise Cox model to fit this nonlinear association (P for log-likelihood ratio <0.05). As Table 3 shown, we discovered that only when night sleep duration is within 3.5–7.5 hours, per hour increase of night sleep bring about 15% reduced risk of developing diabetes [HR (95% CI) = 0.85 (0.77, 0.93)]. When night sleep duration exceeds 7.5 or less than 3.5, this linear association became statistically insignificant, suggesting that this linear association may be restricted in 3.5–7.5 hours of night sleep.

Figure 3 Non-linear association of night sleep duration with new-onset diabetes in Chinese middle-aged and older adults (The association was adjusted for age, gender, education level, marital status, living residence, smoking status, drinking status, BMI, hypertension, dyslipidemia, and afternoon snap sleep duration. Blue dash line represents the 95% CI from the fit. The solid red line represents the smooth curve between night sleep duration and the risk of new-onset diabetes. BMI, body mass index).
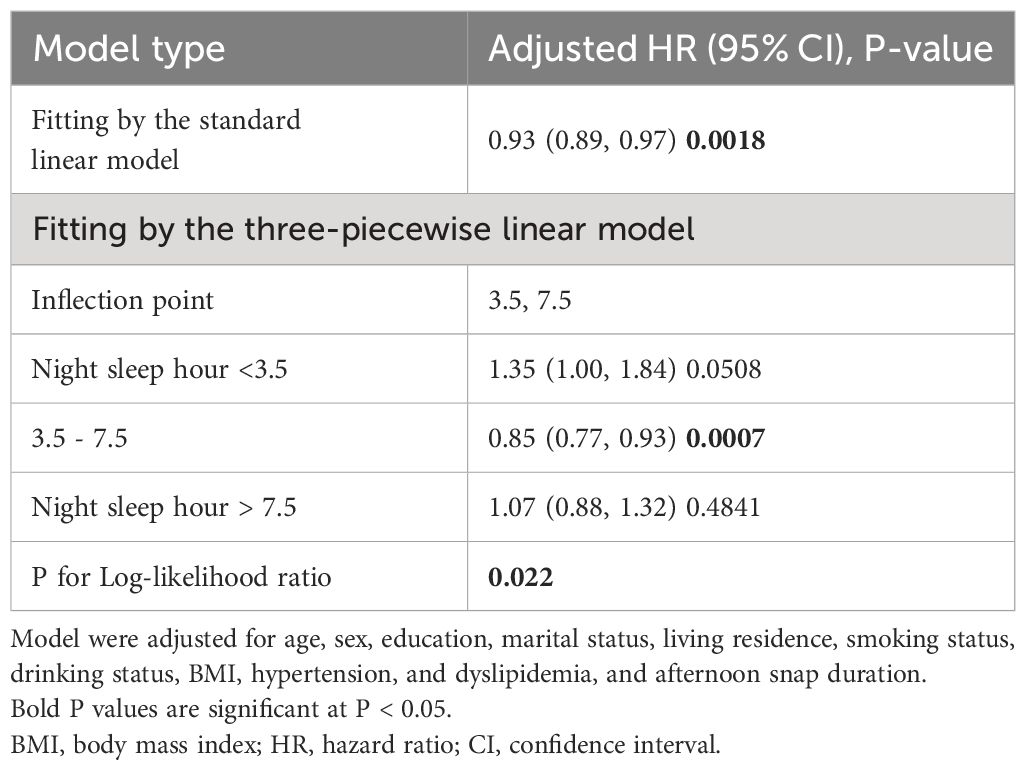
Table 3 Non-linear associations of night sleep duration with new-onset in Chinese middle-aged and older adults.
To identifying potential distinctive subgroups or potential modifiers on former identified association, we further performed interaction and stratification analysis in this section. Age, gender, BMI, smoking status, drinking status, hypertension, dyslipidemia, and afternoon napping were selected as potential modifiers. As shown in Figure 4, non-linear tendency only exists in some specified subgroups: age >=60, female, BMI >=24, having dyslipidemia, never drink and never smoke. Table 4 shown that BMI (BMI >=24: HR = 0.88,95%CI: 0.82–0.93; BMI<24: HR = 0.99, 95%CI: 0.92,1.06; P for interaction = 0.0215) and dyslipidemia (have dyslipidemia: HR = 0.88,95%CI: 0.82–0.94; have no dyslipidemia: HR = 0.97, 95%CI: 0.91,1.03; P for interaction = 0.0333) might exhibit interaction effects on the association between night sleep duration and new-onset diabetes.
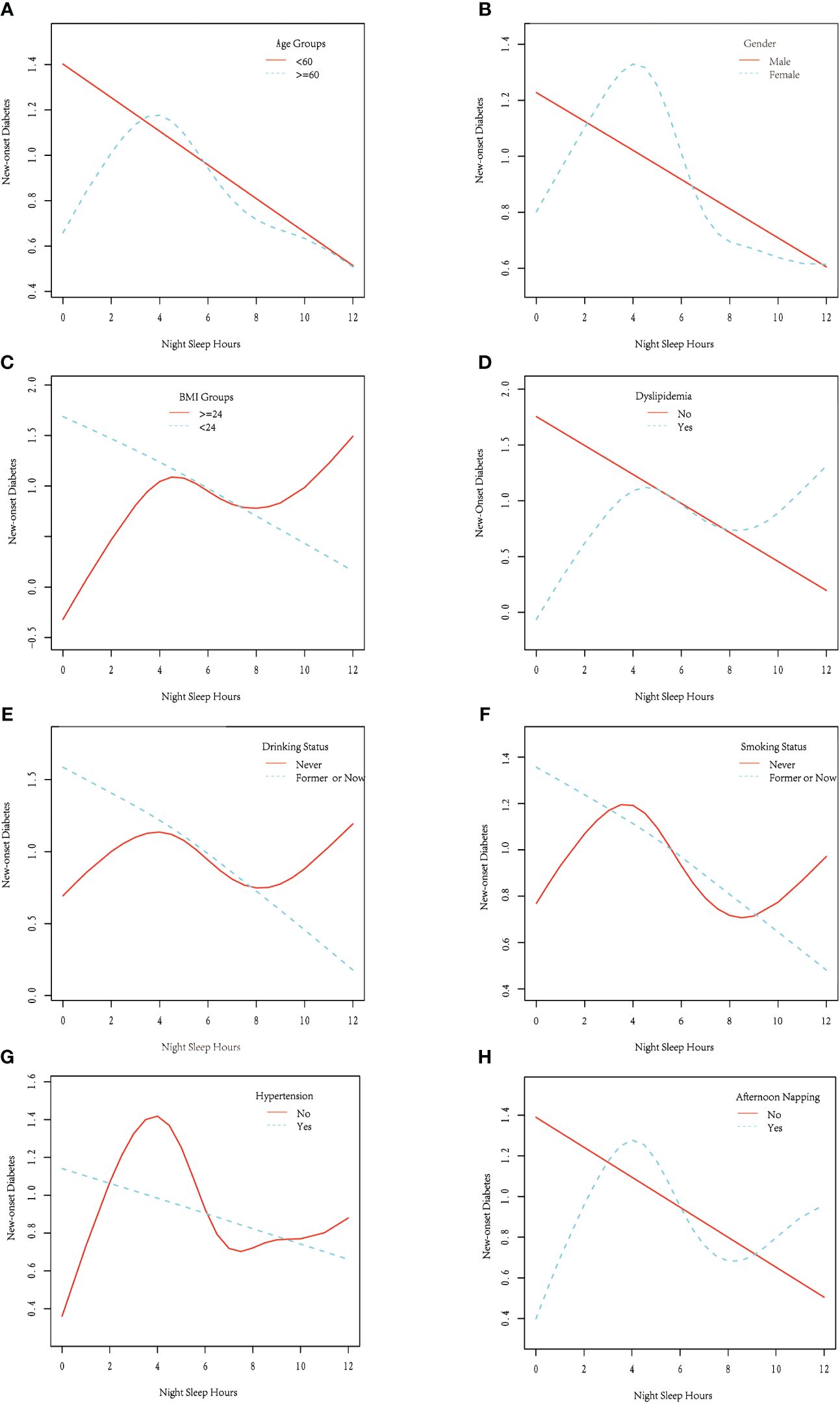
Figure 4 Stratified analysis of the non-linear association between night sleep duration and new-onset diabetes in Chinese middle-aged and older adults, stratified by (A) age, (B) gender, (C) BMI, (D) dyslipidemia, (E) drinking status, (F) smoking status, (G) hypertension and (H) afternoon napping (Each stratification was adjusted for age, sex, education, marital status, living residence, smoking status, drinking status, BMI, hypertension, and dyslipidemia, and afternoon snap duration, except the stratification factor itself). Blue dash line or solid red line represents the smooth curve fit between night sleep duration and the risk of new-onset diabetes for specific stratification. BMI, body mass index).
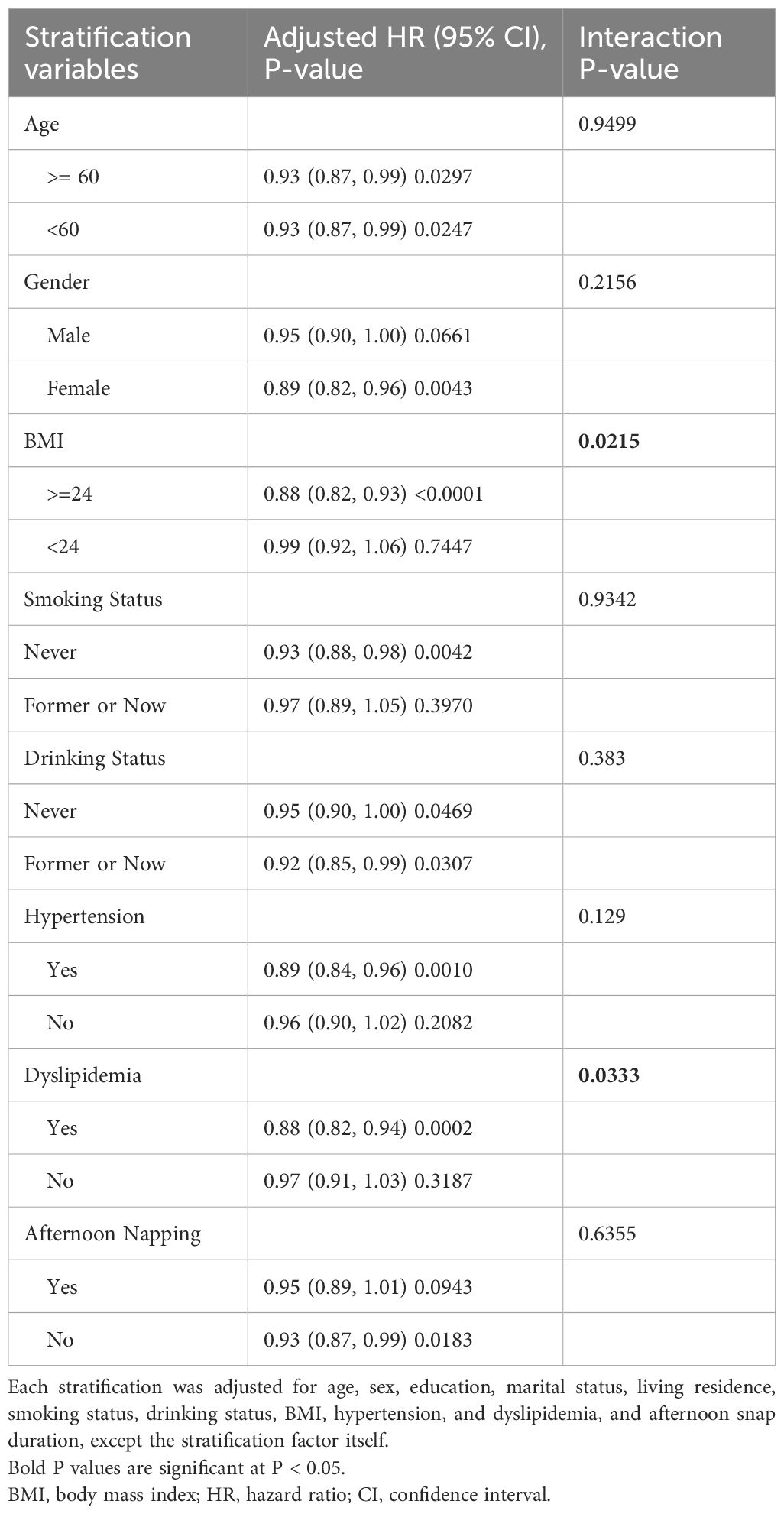
Table 4 Interaction and stratification analysis for the associations of night sleep duration (continuous) with new-onset diabetes in Chinese middle-aged and older adults.
Three sensitivity analyses as describe in Method were proposed to further confirm our findings. As shown in Supplementary Table 2, no obvious difference between the sensitivity analyses and our original results (Table 2), Curve-fitting with addictive Cox model also presented similar variation tendency (Supplementary Figures 1–3).
This 7-year longitudinal cohort study examined the relationship between night sleep duration and diabetes risk, with a focus on middle-aged to older Chinese adults. After careful adjustment for potential confounders, we discovered that a night sleep duration of no more than 8 hours significantly lowered diabetes risk. Furthermore, 7.1–8 hours of nocturnal sleep was found to be associated with the lowest incidence of diabetes. Using the addictive Cox regression approach and a piece-wise model, we identified a temporal range of 3.5–7.5 hours where the negative linear connection between night sleep and new-onset diabetes is strong. Within this interval, each hour of prolonged night sleep duration was related with a 15% reduction in the chance of acquiring diabetes over the next seven years, when compared to not increasing sleep duration. 3.5 and 7.5 hours were discovered to be two critical threshold effect cutoffs for this limited time frame. When the sleep duration exceeds 7.5 hours or under 3.5 hours, no more extra benefit or risk will follow in regard of developing diabetes. As a result, we choose to refer to it as the “restricted linear association”. In the subsequent interaction and stratified analysis, we identified some potential modifiers of this restricted linear association. Restricted linear association was observed only in subgroup with age >=60, female, BMI >=24, having dyslipidemia, never drink and never smoke. In the interaction analysis, BMI (P for interaction = 0.0215) and dyslipidemia (P for interaction = 0.0333) both exhibited significant modification effects on the association between night sleep duration and new-onset diabetes when only considering their standard linear effect. Our results remain constant after three sensitivity analyses. Overall, we proposed a novel working model that has the potential to alter the conventional view of the non-linear relationship between night sleep length and diabetes risk.
The relationship between night sleep duration and the risk of diabetes remains an intriguing study topic in recent decades. Numerous studies have revealed a distinct non-linear association between night sleep duration and diabetes, with a widely supported U-shaped association being particularly notable (4, 10, 11, 14, 21–23). This working model suggests the existence of a narrow optimal sleep interval or optimal sleep duration for the lowest risk of diabetes, beyond or below which the risk of diabetes tends to rise. Many studies have substantially verified this characteristic U-shape association (4, 11, 21–23), and 7–8 hours of night sleep was selected as the optimal sleep duration and has been extensively promoted as a specific health recommendation (24). However, other studies using a Chinese sample with a perspective design report contradictory finding. A research of 34825 people from the Shanghai Men’s Health research found that prolonged sleep duration (>= 8 hours) was linked to an increased risk of diabetes [HR(95% CI) = 1.2(1.0–1.3), when compared to the group with 7 hours of sleep] (14). Another study of 11539 Chinese participants over 3 years found that those who slept more than 9 hours per night had a higher risk of newly diagnosed type 2 diabetes. However, there was no significant difference in risk between those who slept less than 6 hours per night and those who slept 7–8 hours per night (10). A study conducted by Li, using a parallel dataset to ours, validated a U-shaped nexus between sleep duration and diabetes incidence, though the generalizability was confined primarily to the older Chinese demographic aged over 65 years (11), despite the fact that not only the older but also the middle-aged bear the majority of the burden of diabetes (25). The variations in their findings could be attributed to differences in data sources and analytic methodology. Although these studies using a Chinese population have provided useful insights into diabetes prevention, evidence from a representative sample of middle-aged and older Chinese with adequate follow-up time remains scarce.
To the best of our knowledge, this is the first study to support the longitudinal relationship between night sleep duration and diabetes risk, employing a representative sample of middle-aged and older Chinese with a 7-year follow-up period. In this study, we developed a novel working model for the effect of night sleep duration on new-onset diabetes. Instead of the U-shaped link, we discovered a restricted time window in which each hour of longer night sleep duration was related with a 15% reduction in the chance of acquiring diabetes over the next 7 years. Outside of this interval, no substantial link between the two can be established since the risk of sleep deprivation and the benefit of increased sleep duration have both become saturated. This working model is especially relevant because it may not accurately reflect the diabetes risk associated with prolonged sleep and the supposed recommended sleep length for diabetes prevention. Intriguingly, Jin’s recent work utilizing UK biobank samples demonstrated that accelerometer-measured short but not long sleep duration is related with a greater risk of incident type 2 diabetes in a 7-year follow-up, which is consistent with our findings (26).
Potential reasons for the common U-shape relationship have been gathered. Multiple validated pathways strongly link insufficient night sleep duration at baseline to the onset of diabetes, implying biological roles for insulin resistance, leptin, ghrelin, and inflammatory cytokines, as well as behavioral roles for increased energy intake and decreased decision-making ability (27). However, the causes for the link between prolonged night sleep duration and diabetes are somewhat vague. Long sleep duration may indicate low socioeconomic level, poor health, insufficient physical exercise, and psychological illness (28, 29), or it may be related with poor sleep quality (30). Furthermore, because questionnaire-based surveys are subjective, they may overestimate sleep duration. However, a UK-biobank study that used accelerometer-measured sleep duration found results that were congruent with ours, indicating that there is no significant link between extended sleep duration and diabetes risk (26). Sizhi’s mendelian randomization (MR) research revealed that genetic predictors of lengthy sleep duration are not associated with an increased risk of diabetes (31), despite the fact that MR analysis can only assess exposure effects across a life span rather than individual life stages. Compared to the traditional U-shaped model, our analysis underscores the detrimental impact of short sleep duration on diabetes risk. Additionally, we demonstrate saturation effects for both the risk associated with decreased sleep duration and the protective influence of prolonged sleep. Our model aligns with the foundational mechanisms of the U-shaped curve but distinctly emphasizes the benefits of extended sleep as a preventive strategy against diabetes. In scenarios where sleep quality cannot be enhanced, longer sleep duration may serve as an effective compensatory mechanism. Our findings suggest that maintaining an extended sleep duration habitually does not increase diabetes susceptibility.
Some potential modifiers for the suggested nonlinear connection were identified in interaction and stratified analysis. Restricted linear connection only present in several specific subgroups, including those above the age of 60, female, with a BMI greater than 24, dyslipidemia, never drinking or smoking. There are two plausible explanations for this discovery. One is the smaller sample size will result in the removal of the nonlinear relationship in some subgroups, as detecting nonlinear relationships requires a large sample size. The other explanation is the subgroup factors may have a modifying effect on this nonlinear relationship. The restricted linear intervals appeared only in select subgroups, and their locations, lengths, and slopes varied, indicating that the stratified variables may have an interaction effect. When only the strand linear impact was investigated, BMI and dyslipidemia were identified as significant modifiers, with the overall beneficial benefit of night sleep time appearing to be more evident in people with greater BMI and dyslipidemia. These could be owing to a common mechanism between short sleep duration and obesity with diabetes (32). The high prevalence of obstructive sleep apnea (OSA) in the people with obesity also contributed to the modified effects of BMI (33) and dyslipidemia (34). These findings underscored the need for clinicians and policymakers to pay more attention to extending sleep time and overall sleep quality for persons with higher BMI and dyslipidemia.
Our findings reveal a restricted linear association between night sleep duration and diabetes risk within the 3.5 to 7.5-hour range, where each additional hour of sleep correlates with a 15% reduction in the likelihood of developing diabetes. This restricted time window suggests that within this specific range, the benefits of extended sleep duration on reducing diabetes risk are significant. However, beyond this range, the protective effects of sleep become saturated, and no further significant association is observed. This phenomenon implies that both the detrimental effects of insufficient sleep and the benefits of prolonged sleep reach a plateau within this window.
The explanation for this restricted time window could be attributed to the body’s physiological and metabolic adaptations. Within the 3.5 to 7.5-hour sleep duration, the body optimally balances its restorative processes, including hormonal regulation, glucose metabolism, and inflammatory responses. Shorter sleep durations may lead to sleep deprivation, resulting in metabolic dysregulation and increased diabetes risk. On the other hand, longer sleep durations might not provide additional benefits due to a saturation effect, where the body’s capacity to benefit from extended sleep is maximized.
This study highlights the importance of maintaining an optimal sleep duration within the identified restricted time window to minimize diabetes risk. Our findings differ from the U-shaped relationship reported in other studies, suggesting a more nuanced understanding of the sleep-diabetes nexus in the Chinese population.
The strengths of the present study include its prospective design, long follow-up duration, and precise demonstration of the non-linear association. However, this study had several limitations. Our study, for instance, did not incorporate potential confounding determinants like economic status, physical activity levels, and depressive symptomatology in the relationship matrix of sleep duration and diabetes incidence. Given our employment of the modified International Physical Activity Questionnaire-short form and the ensuing sizable missing data proportion pertaining to physical activity, the integration of such variables could have inadvertently distorted energy expenditure computations, leading to a significantly constrained sample scope. Additionally, the intricate interplay between excessive sleep, insomnia, and depression is noteworthy. While both sleep extremes can catalyze depressive states, depression can reciprocally truncate sleep spans (35–37). Thus, the sleep-diabetes nexus might be confounded or partially mediated by underlying depressive dynamics. Another potential pitfall lies in the self-reported nature of diabetes diagnosis, potentially introducing recall biases. Yet, a multitude of studies have consistently asserted the high congruence between self-reported diabetes, corroborative medical histories, or physician-validated diabetes diagnoses, boasting a specificity surpassing 90% (38–41). Besides, the lack of dietary data for CHARLS is another limitation which should be noticed. Additionally, only baseline data were used to predict future diabetes risk in this study. Future studies should account for the dynamic nature of nighttime sleep duration and time-varying confounders, employing updated longitudinal data and advanced modelling techniques to enhance causality assessments. Conclusively, it is imperative to interpret our findings with a geographical and demographic lens, principally catering to middle-aged to older populations within China and analogous middle-income nations.
In conclusion, a time interval was identified within which night sleep duration is linearly associated with diabetes risk, but outside of which no significant link between the two can be established because both the risk of sleep deprivation and the benefit of increased sleep duration have become saturated. Furthermore, the protective benefits of increased sleep duration on diabetes were reconfirmed and found to be modified by BMI and dyslipidemia.
This study has established a precise temporal correlation between night sleep duration and the risk of new-onset diabetes among middle-aged and older adults in a Chinese cohort, underlining a restricted linear association within a defined interval of 3.5 to 7.5 hours of nightly sleep. Beyond this range, the relationship attenuates, signaling a saturation point where both the detrimental effects of sleep deprivation and the advantageous impacts of extended sleep no longer influence diabetes risk. This phenomenon suggests a potential threshold effect, where sleep durations outside this range do not contribute additional protective benefits against diabetes.
Furthermore, our analysis reconfirmed the protective role of increased sleep duration on diabetes risk and highlighted its modification by BMI and dyslipidemia. Specifically, individuals with higher BMI and dyslipidemia exhibited a more pronounced protective effect from extended sleep duration. These findings underscore the need for tailored sleep recommendations that consider individual metabolic profiles, particularly for those at higher risk due to obesity and lipid abnormalities.
Our study contributes to the growing body of evidence on the importance of adequate sleep for metabolic health and emphasizes the necessity for diabetes and pre-diabetes interventions that promote optimal sleep duration as part of diabetes prevention strategies. Future research should explore the underlying mechanisms of this restricted linear association and investigate the potential benefits of sleep interventions in high-risk populations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Peking University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MC: Conceptualization, Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. BL: Conceptualization, Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing. GF: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. General program of Guangdong Natural Science Foundation (2022A1515012192), Medical research fund project of Guangdong Province (A2021500, A2022535), Philosophy and Social Sciences Planning Foundation Discipline Co-construction Project of Guangdong Province of 2021 (GD22XXW10), 2021 Guangdong Provincial Science and Technology Special Fund (“Major Tasks +Task List”) (210728156901595). Undergraduate teaching quality and teaching reform project of Guangdong Province in 2022 (Yue Gao Jiao Han[2023]4-581), Clinical Teaching Base Teaching Reform Research Project of Guangdong Province in 2021(2021JD062). Guangdong Province 2022 Annual Education Science Planning Project (Higher Education Special) (2022GXJK192), 2021 Guangdong Provincial Quality Engineering Project for University Student Practical Training Base: ‘Heart’ Practical Training Base for University Students (Yue Gao Jiao Han [2021] No. 19-18).
We thank the China Health and Retirement Longitudinal Study team for providing data and training in using the datasets. We thank the students who participated in the survey for their cooperation. We thank all volunteers and staff involved in this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1364106/full#supplementary-material
CHARLS, China Health and Retirement Longitudinal Study; HRs, Hazard Ratios; CIs, Confidence Intervals; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SD, Standard Deviation.
1. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. (2016) 387:1513–30. doi: 10.1016/S0140-6736(16)00618-8
3. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
4. Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. (2017) 32:246–56. doi: 10.1016/j.sleep.2016.08.006
5. Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clinics. (2022) 17:173–91. doi: 10.1016/j.jsmc.2022.03.003
6. Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. (2007) 176:1299–304. doi: 10.1503/cmaj.060792
7. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. (2015) 38:529–37. doi: 10.2337/dc14-2073
8. Liu J, Richmond RC, Bowden J, Barry C, Dashti HS, Daghlas I, et al. Assessing the causal role of sleep traits on glycated hemoglobin: A mendelian randomization study. Diabetes Care. (2022) 45:772–81. doi: 10.2337/dc21-0089
9. Li ZH, Zhang PD, Chen Q, Gao X, Chung VCH, Shen D, et al. Association of sleep and circadian patterns and genetic risk with incident type 2 diabetes: a large prospective population-based cohort study. Eur J Endocrinol. (2021) 185:765–74. doi: 10.1530/EJE-21-0314
10. Liu H, Chen G, Wen J, Wang A, Mu Y, Dou J, et al. Association between sleep duration and incidence of type 2 diabetes in China: the REACTION study. Chin Med J. (2022) 135:1242–8. doi: 10.1097/CM9.0000000000001835
11. Lin L, Lu C, Chen W, Guo VY. Daytime napping and nighttime sleep duration with incident diabetes mellitus: A cohort study in chinese older adults. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph18095012
12. Fang W, Li Z, Wu L, Cao Z, Liang Y, Yang H, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. (2013) 14:950–4. doi: 10.1016/j.sleep.2013.04.015
13. Lam KB, Jiang CQ, Thomas GN, Arora T, Zhang WS, Taheri S, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. (2010) 33:402–7. doi: 10.1093/sleep/33.3.402
14. Dai F, Cai H, Li H, Yang G, Ji BT, Zheng W, et al. Association of sleep duration and incidence of diabetes modified by tea consumption: a report from the Shanghai men's health study. Sleep Med. (2017) 38:135–41. doi: 10.1016/j.sleep.2017.07.024
15. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
17. (2) Classification and diagnosis of diabetes. Diabetes Care. (2015) 38:S8–s16. doi: 10.2337/dc15-S005
18. Yin X, Liu Q, Wei J, Meng X, Jia C. Association of daytime napping with prediabetes and diabetes in a Chinese population: Results from the baseline survey of the China Health and Retirement Longitudinal Study. J Diabetes. (2018) 10:302–9. doi: 10.1111/1753-0407.12605
19. Li J, Cacchione PZ, Hodgson N, Riegel B, Keenan BT, Scharf MT, et al. Afternoon napping and cognition in chinese older adults: findings from the China health and retirement longitudinal study baseline assessment. J Am Geriatrics Soc. (2017) 65:373–80. doi: 10.1111/jgs.14368
20. Li J, Chang YP, Riegel B, Keenan BT, Varrasse M, Pack AI, et al. Intermediate, but not extended, afternoon naps may preserve cognition in chinese older adults. journals gerontology Ser A Biol Sci Med Sci. (2018) 73:360–6. doi: 10.1093/gerona/glx069
21. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. (2010) 33:414–20. doi: 10.2337/dc09-1124
22. Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev. (2018) 39:25–36. doi: 10.1016/j.smrv.2017.06.011
23. Jang JH, Kim W, Moon JS, Roh E, Kang JG, Lee SJ, et al. Association between sleep duration and incident diabetes mellitus in healthy subjects: A 14-year longitudinal cohort study. . J Clin Med. (2023) 12. doi: 10.3390/jcm12082899
24. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. (2015) 1:233–43. doi: 10.1016/j.sleh.2015.10.004
25. Magliano DJ, Boyko EJ, committee IDFDAtes. IDF Diabetes Atlas Vol. 2021. Brussels: International Diabetes Federation (2021).
26. Jin X, Chen Y, Feng H, Zhou M, Chan JWY, Liu Y, et al. Association of accelerometer-measured sleep duration and different intensities of physical activity with incident type 2 diabetes in a population-based cohort study. J Sport Health Sci. (2024) 13(2):222–32. doi: 10.1016/j.jshs.2023.03.001
27. Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms. Curr Diabetes Rep. (2016) 16:106. doi: 10.1007/s11892-016-0805-8
28. Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. (2009) 169:1052–63. doi: 10.1093/aje/kwp023
29. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. (2006) 29:881–9. doi: 10.1093/sleep/29.7.881
30. Mokhlesi B, Temple KA, Tjaden AH, Edelstein SL, Utzschneider KM, Nadeau KJ, et al. Association of self-reported sleep and circadian measures with glycemia in adults with prediabetes or recently diagnosed untreated type 2 diabetes. Diabetes Care. (2019) 42:1326–32. doi: 10.2337/dc19-0298
31. Ai S, Zhang J, Zhao G, Wang N, Li G, So HC, et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK Biobank. Eur Heart J. (2021) 42:3349–57. doi: 10.1093/eurheartj/ehab170
32. Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. (2021) 252:125–41. doi: 10.1530/JOE-21-0155
33. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. Jama. (2004) 291:2013–6. doi: 10.1001/jama.291.16.2013
34. Barros D, García-Río F. Obstructive sleep apnea and dyslipidemia: from animal models to clinical evidence. Sleep. (2019) 42. doi: 10.1093/sleep/zsy236
35. Ji S, Wang J, Wang W, Liao F. Longer depressive duration reduces sleep duration more: A longitudinal study in the middle-aged and elderly Chinese. J Affect Disord. (2022) 317:185–92. doi: 10.1016/j.jad.2022.08.051
36. Ouyang P, Sun W. Depression and sleep duration: findings from middle-aged and elderly people in China. Public Health. (2019) 166:148–54. doi: 10.1016/j.puhe.2018.10.007
37. Dong L, Xie Y, Zou X. Association between sleep duration and depression in US adults: A cross-sectional study. J Affect Disord. (2022) 296:183–8. doi: 10.1016/j.jad.2021.09.075
38. Koller KR, Wilson AS, Asay ED, Metzger JS, Neal DE. Agreement between self-report and medical record prevalence of 16 chronic conditions in the alaska EARTH study. J primary Care Community Health. (2014) 5:160–5. doi: 10.1177/2150131913517902
39. Steinkirchner AB, Zimmermann ME, Donhauser FJ, Dietl A, Brandl C, Koller M, et al. Self-report of chronic diseases in old-aged individuals: extent of agreement with general practitioner medical records in the German AugUR study. J Epidemiol Community Health. (2022) 76:931–8. doi: 10.1136/jech-2022-219096
40. Sidorkiewicz S, Malmartel A, Prevost L, Partouche H, Pinot J, Grangé-Cabane A, et al. Patient-physician agreement in reporting and prioritizing existing chronic conditions. Ann Family Med. (2019) 17:396–402. doi: 10.1370/afm.2444
41. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. (2004) 57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005
Keywords: older adults, night sleep duration, diabetes mellitus, non-linear association, Chinese population, CHARLS
Citation: Chen M, Li B, Fan G and Zhou Y (2024) Restricted linear association between night sleep duration and diabetes risk in middle-aged and older adults: a 7-year follow-up analysis from the China health and retirement longitudinal study. Front. Endocrinol. 15:1364106. doi: 10.3389/fendo.2024.1364106
Received: 02 January 2024; Accepted: 17 May 2024;
Published: 20 June 2024.
Edited by:
Junhong Zhou, Harvard Medical School, United StatesReviewed by:
Fan Chen, University of Massachusetts Lowell, United StatesCopyright © 2024 Chen, Li, Fan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanhua Fan, ZmFuZ2hAc3R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.