
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 30 May 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1361683
This article is part of the Research TopicPapillary Thyroid Cancer: Prognostic Factors and Risk AssessmentView all 21 articles
Objectives: The objective of this study was to develop a predictive nomogram for intermediate-risk differentiated thyroid cancer (DTC) patients after fixed 3.7GBq (100mCi) radioiodine remnant ablation (RRA).
Methods: Data from 265 patients who underwent total thyroidectomy with central lymph node dissection (CND) and received RRA treatment at a single institution between January 2018 and March 2023 were analyzed. Patients with certain exclusion criteria were excluded. Univariate and multivariate logistic regression analyses were performed to identify risk factors for a non-excellent response (non-ER) to RRA. A nomogram was developed based on the risk factors, and its performance was validated using the Bootstrap method with 1,000 resamplings. A web-based dynamic calculator was developed for convenient application of the nomogram.
Results: The study included 265 patients with intermediate-risk DTC. Significant differences were found between the ER group and the non-ER group in terms of CLNM>5, Hashimoto’s thyroiditis, sTg level, TgAb level (P < 0.05). CLNM>5 and sTg level were identified as independent risk factors for non-ER in multivariate analysis. The nomogram showed high accuracy, with an area under the curve (AUC) of 0.833 (95% CI = 0.770–0.895). The nomogram’s predicted probabilities aligned closely with actual clinical outcomes.
Conclusions: This study developed a predictive nomogram for intermediate-risk DTC patients after fixed 3.7GBq (100mCi) RRA. The nomogram incorporates CLNM>5 and sTg levels as risk factors for a non-ER response to RRA. The nomogram and web-based calculator can assist in treatment decision-making and improve the precision of prognosis information. Further research and validation are needed.
Radioactive iodine therapy (RAIT) is a pivotal treatment for differentiated thyroid carcinoma (DTC) following total thyroidectomy, categorized into three modalities based on therapeutic objectives: 1) Radioiodine remnant ablation (RRA) aims to eliminate residual thyroid tissue post-surgery; 2) Radioiodine adjuvant therapy (RAT) targets undetected metastatic or residual lesions; 3) Radioiodine treatment (RT) addresses inoperable local or distant DTC metastases (1, 2). RRA is instrumental in post-thyroidectomy DTC management, enhancing serum thyroglobulin (Tg) level stratification, patient monitoring, the sensitivity of 131I whole-body scans for metastasis detection, and postoperative restaging (1, 3, 4). RAT extends beyond eliminating residual tissue to addressing latent lesions, thereby augmenting disease-free survival (DFS) rates (5, 6). Clinicians must grasp Tg level stratification in postoperative DTC patients and evaluate recurrence risk, especially in intermediate or low-risk cases, to optimize remnant ablation strategies.
The recommended 131I dose for RRA varies from 1.11 to 3.70 GBq (30 to 100 mCi) (1). Research shows comparable efficacy between 1.11 and 3.70 GBq doses in low or intermediate-risk DTC patients post-surgery, with lower doses minimizing short-term adverse effects. However, higher doses are suggested for patients with substantial thyroid remnants or as adjuvant treatment. Dose determination should integrate clinical and pathological characteristics, mortality and recurrence risks, and dynamic assessment, favoring tailored approaches over standardized low (1.11 GBq) or high (3.70 GBq) doses (7). Factors influencing RRA dose escalation include larger residual thyroid tissue, elevated thyroglobulin levels, and additional risk indicators. In scenarios where patients exhibit stimulated thyroglobulin (sTg) levels above 10ng/ml and are classified as high-risk for recurrence, RAT with doses exceeding 3.70 GBq is advisable (8, 9).
Studies associate factors like tumor size, elevated serum sTg levels, lymph node capsular invasion, N1a classification, and distant metastases with increased RRA failure risk (10). A serum sTg level below 2ng/mL is a vital predictor for positive response, with higher pre-ablation sTg levels indicating a potential initial treatment inadequacy (11). Hence, sTg levels serve as a predictive measure for RRA success. Notably, prior research involving patients with sTg levels above 10, classified as high-risk, and exhibiting structural lesions, employed varying 131I doses, potentially introducing biases. Therefore, a distinct risk predictive model to predict the response to fixed 3.7GBq (100mCi) RRA with intermediate-risk DTC is necessary.
The predominant staging systems for DTC are the TNM system and recurrence risk stratification. DTC typically demonstrates low disease-specific mortality. These staging systems primarily predict recurrence risk in patients rather than the efficacy of initial RRA. In contrast, nomograms, which have been developed for most cancer types, often outperform traditional staging methods (12–14). Consequently, many consider nomograms a potential alternative or new standard (15). This study aims to establish a risk nomogram utilizing clinicopathologic data from 265 intermediate-risk DTC patients who underwent a fixed dose of 3.7GBq (100mCi) RRA.
This retrospective study focused on patients who underwent thyroidectomy and RRA from January 2018 to March 2023 at the Affiliated Hospital of Guilin Medical University. Eligible participants had undergone thyroidectomy and central lymph node dissection (CND), with histopathologically confirmed DTC. They were categorized into the intermediate-risk group for recurrence and received an fixed RRA dose of 3.7GBq (100mCi). The intermediate risk group was defined in accordance with the ATA risk criteria (1). This group includes patients with the following characteristics: (1) microscopic invasion of the tumor into the perithyroidal soft tissues, (2) the presence of radioactive iodine (RAI)-avid metastatic foci in the neck as observed on the first post-treatment whole-body RAI scan, (3) aggressive histology (e.g., tall cell, hobnail variant, columnar cell carcinoma), (4) papillary thyroid cancer with vascular invasion, (5) clinical N1 or >5 pathologic N1 with all involved lymph nodes <3 cm in the largest dimension, and (6) multifocal papillary microcarcinoma with extrathyroidal extension (ETE) and BRAFV600E mutation (if known). The exclusion criteria included: sTg levels exceeding 10 ng/ml; serum thyroglobulin antibody (TgAb) positivity (>115 U/mL); evidence of cervical lymph node metastasis (CLNM) or distant metastasis (DM) post RRA whole-body scintigraphy (Rx-WBS); being under 18 years of age; and insufficient clinicopathologic data for analysis.
Patients underwent RRA following surgery (total thyroidectomy with central lymph node dissection [CND]). Indications for total thyroidectomy included: (a) primary tumor size exceeding 4 cm; (b) bilateral/multiple lesions; (c) extrathyroidal extension; (d) clinical evidence of lymph node or distant metastasis. Ipsilateral CND was performed for all patients, while bilateral CND was conducted in cases of bilateral carcinoma or clinically suspicious nodal disease in the contralateral central compartment. Lateral neck dissection (LND), encompassing levels II–V, was reserved for clinically or pathologically confirmed metastatic lateral neck lymph nodes.
Prior to RRA, patients adhered to a low-iodine diet for 14 days, guided by our nutrition experts, avoiding iodine-containing medications. RRA was administered post levothyroxine (LT4) withdrawal, aligned with the American Joint Committee on Cancer (AJCC) TNM staging and recurrence risk stratification, at a dose of 3.7GBq (100mCi). Pre-RRA routine biochemical (serum thyrotropin [TSH], sTg, anti-Tg antibody [TgAb]) and imaging (ultrasonography, computed tomography) examinations were conducted. Post-RRA, LT4 was prescribed for TSH suppression therapy, and Rx-WBS was performed 2–3 days later.
Follow-up visits post-RRA included TSH-stimulated Tg level assessment and Dx-WBS at six months. Therapeutic responses were classified into four categories: Excellent Response (ER), Indeterminate Response (IDR), Biochemical Incomplete Response (BIR), and Structural Incomplete Response (SIR), with IDR, BIR, and SIR collectively termed as non-ER. ER was defined as negative imaging with suppressed Tg <0.2 ng/mL or TSH-stimulated Tg <1 ng/mL; BIR as negative imaging but suppressed Tg ≥1 ng/mL, stimulated Tg ≥10 ng/mL, or rising anti-Tg antibodies; SIR as any Tg level with structural or functional disease evidence, with or without anti-Tg antibodies; IDR as nonspecific imaging findings, faint thyroid bed uptake on RAI scanning, detectable non-stimulated Tg <1 ng/mL, stimulated Tg <10 ng/mL, or stable/declining anti-Tg antibodies without structural/functional disease. The therapeutic response assessment was conducted six months post-RAT, following the 2015 ATA guidelines (1). ER is defined as successful RRA.
Serum TSH concentrations, sTg levels, and TgAb levels were measured by cobas e 801 analytical unit for immunoassay tests.
Rx-WBS was obtained 2–3 days after RRA using dualhead γ-cameras (Siemens Symbia T16 SPECT/CT) equipped with medium-energy collimators, set to a peak energy of 364 keV with a window width of 20%. Both anterior and posterior planar images, from the vertex to the knee, were acquired and stored in 256×1024 matrices using a scan speed of 5 to 10 cm/min. Dx-WBS was performed at 24–48 hours after oral administration of 74 to 185 MBq of 131I using the same camera and protocol as for the Rx-WBS described above. A nuclear medicine physician with 14 years of experience visually analyzed WBS images. Rx-WBS findings were classified as hot uptake in the thyroid bed of the neck or not. Diagnostic WBS was used to evaluate the treatment response of RRA by classifying remaining fainted uptake lesion in the neck or not.
Patient clinical information was acquired through electronic medical record system, and cancer stages were determined using the eighth edition of the American Joint Committee on Cancer (AJCC) staging system. Patients were categorized into Excellent Response (ER), Biochemical Incomplete Response (BIR), Structural Incomplete Response (SIR), and Indeterminate Response (IDR) groups based on six-month Dx-WBS findings and sTg levels. This retrospective study’s design received approval from the institutional review board of the Affiliated Hospital of Guilin Medical University, under approval number 19–000896. The requirement for informed consent was waived.
Statistical analyses were performed using R version 3.6.3 and Python version 3.7. In instances where baseline variables had missing values, multiple imputation analysis was conducted. The fully conditional specification discriminant function was utilized for categorical missing data, and the fully conditional specification regression was employed for continuous missing data. Continuous data are presented as means ± standard deviations (SD) for normally distributed data and medians with interquartile ranges (IQR) for non-normally distributed data. Categorical variables are expressed as frequencies and percentages. For comparisons, the chi-square test or Fisher’s exact test was used for categorical variables, and analysis of variance for continuous variables with a normal distribution. Univariate and multivariate logistic regression analyses were conducted to identify risk factors for intermediate-risk DTC post-RRA. Variables in univariate analysis included gender, age, N staging, bilateral foci, multifocality, CLNM, AJCC staging, T staging, more than one operation before RRA, time from surgery to RRA exceeding one month, LND performance, primary tumor diameter, vascular invasion, thyroid capsule invasion, nodular goiter, TSH level, sTg level, TgAb level, 99mTcO4- thyroid imaging, Rx-WBS, and Dx-WBS. Multivariate analysis involved forward and backward selection procedures for parameters with P < 0.05 in log-rank tests, reporting odds ratios (ORs) with 95% confidence intervals (CIs). A nomogram was developed using the rms6.7.1 package in R version 3.6.3 (http://www.r-project.org/). The nomogram’s performance was assessed using the concordance index (C-index) and evaluated through calibration curves and clinical decision curves, employing the Bootstrap method with 1,000 resamplings. The workflow was shown in Figure 1.
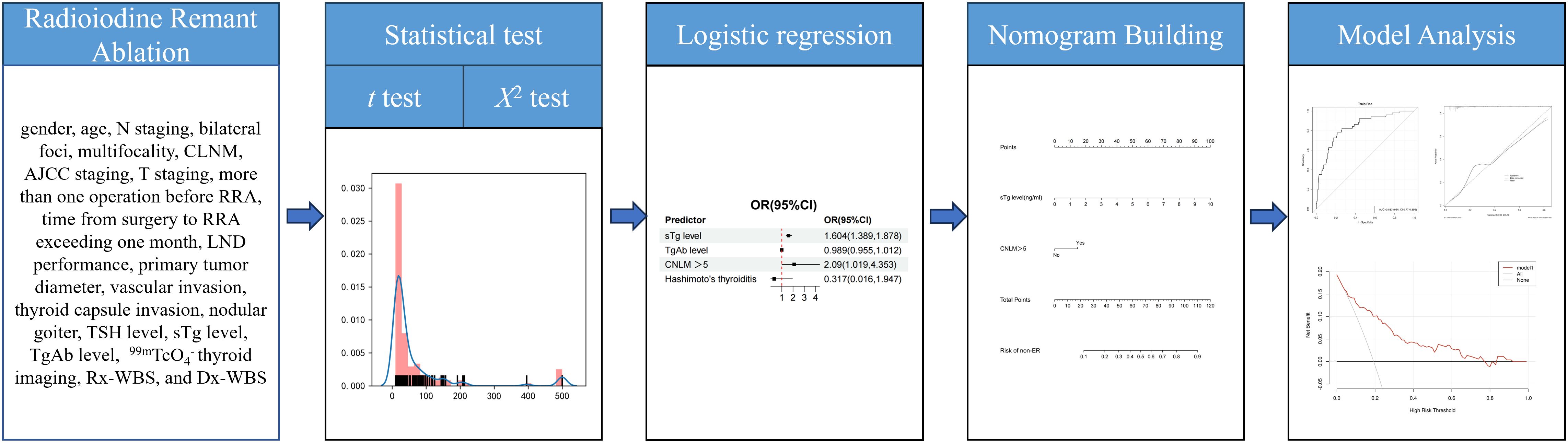
Figure 1 Workflow of this study. Univariate and multivariate logistic regression analyses were conducted to identify risk factors for intermediate-risk DTC post-RRA. A nomogram was developed from the result of multivariate logistic regression analyses. The nomogram’s performance was assessed using the concordance index (C-index) and evaluated through calibration curves and clinical decision curves, employing the Bootstrap method with 1,000 resamplings.
In this retrospective study, we analyzed 500 consecutive patients who underwent total thyroidectomy with CND and initial RRA treatment for intermediate-risk DTC at our institution between January 2018 and March 2023. From this cohort, we excluded 128 patients with sTg levels exceeding 10 ng/ml, 35 with serum thyroglobulin antibody (TgAb) levels above 115 U/mL, 52 with CLNM or DM identified Rx-WBS, 7 with CLNM or DM detected Dx-WBS at six months, 6 who were under 18 years old, and 7 lost to follow-up. Ultimately, 265 patients were included in the study. All patients were diagnosed with papillary thyroid carcinoma (PTC). The median age was 41 years (interquartile range: 34–50 years), with 28.7% (76/265) being male. The majority had T1 stage (83.4%), with more patients presenting with N1a disease (60.4%) than N1b; all were TNM stage M0, and 83.4% were AJCC stage I. Pre-RRA, 173 patients (65.3%) displayed hot uptake in the thyroid bed on 99mTcO4- thyroid imaging, reducing to 0.4% (1 patient) post-RRA. Pre-RRA, 259 patients (97.7%) showed hot uptake in the thyroid bed on Rx-WBS, decreasing to 3.4% (9 patients) post-RRA as per Dx-WBS.
Significant differences were noted between the Excellent Response (ER) group and the non-ER group in terms of CLNM>5, Hashimoto’s thyroiditis, sTg level, Tgoff level, and TgAb level (P < 0.05). However, no statistically significant differences were found in gender, age, N staging, bilateral foci, multifocality, CLNM, AJCC staging, T staging, more than one operation before RRA, time from surgery to RRA exceeding one month, LND performance, primary tumor diameter, vascular invasion, thyroid capsule invasion, nodular goiter, TSH level, 99mTcO4- thyroid imaging, Rx-WBS, and Dx-WBS between the two groups (P > 0.05). Detailed clinical characteristics of the patients are summarized in Table 1.
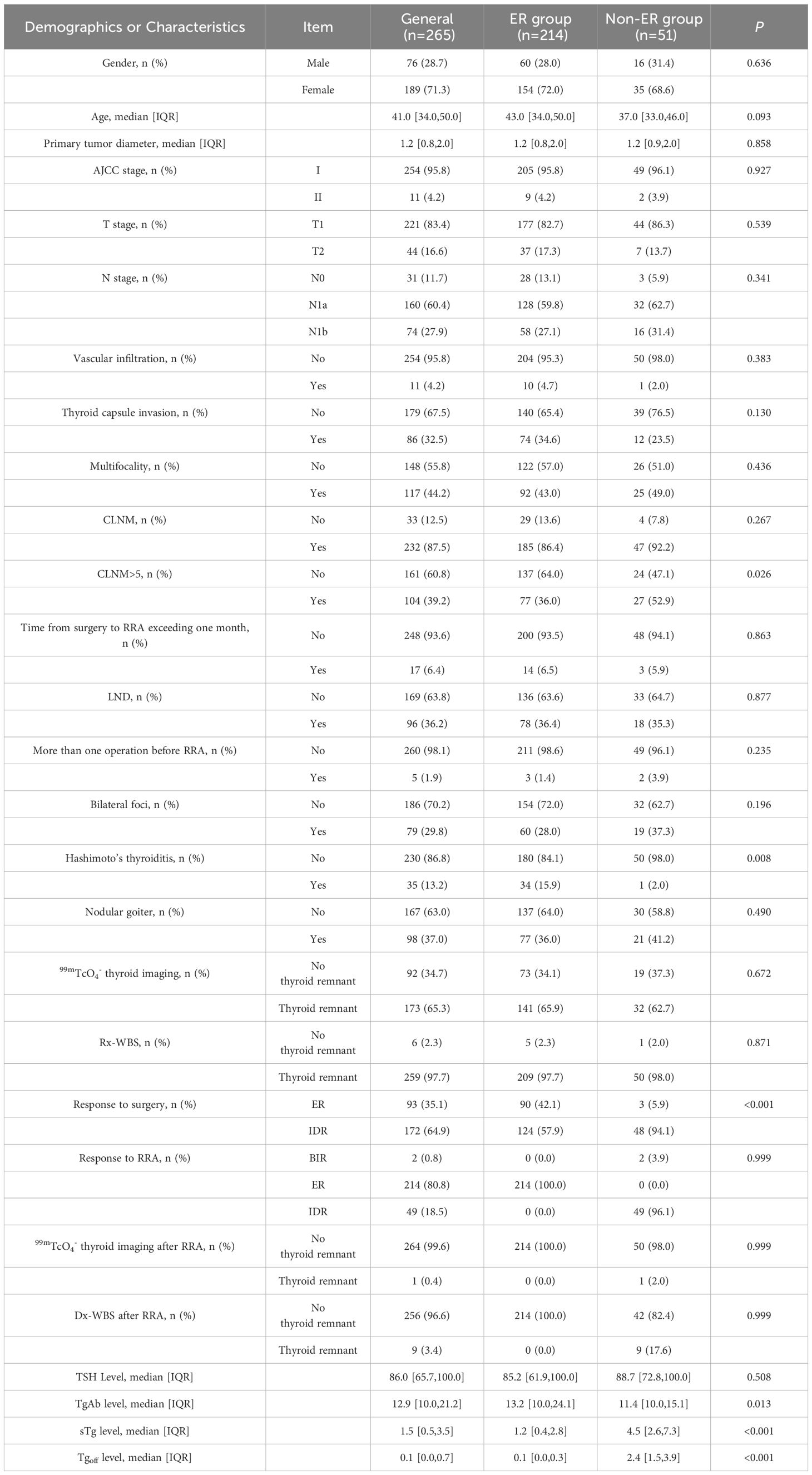
Table 1 Demographics and clinicopathologic characteristics of patients with intermediate-risk DTC after RRA.
In this study, 215 patients (81.1%) exhibited an Excellent Response (ER) upon follow-up, while 48 (18.1%) demonstrated an Indeterminate Response (IDR), and 2 (0.8%) had a Biochemical Incomplete Response (BIR). Univariate analyses identified CLNM>5, Hashimoto’s thyroiditis, sTg level, and TgAb level as significant risk factors for a non-ER outcome following RRA. Factors such as gender, age, N staging, bilateral foci, multifocality, CLNM, AJCC staging, T staging, more than one operation before RRA, surgery to RRA duration exceeding one month, LND performance, primary tumor diameter, vascular invasion, thyroid capsule invasion, nodular goiter, TSH level, 99mTcO4- thyroid imaging, Rx-WBS, and Dx-WBS were not significantly associated with non-ER outcomes. In multivariate analyses, CLNM>5 and sTg level emerged as independent risk factors for non-ER (Table 2). Logistic regression analysis was employed to estimate the likelihood of non-ER in patients with intermediate-risk DTC following RRA. In this model, specific point values were assigned to each predictor’s observed value (Figure 2), with the cumulative points for all variables representing an individual’s risk of non-ER post-RRA. The nomogram’s accuracy was rigorously validated using a bootstrap method with 1000 resamples, revealing an area under the curve (AUC) of 0.833 (95% CI = 0.770–0.895) (Figure 3A). The nomogram’s predicted probabilities were found to be in strong agreement with actual clinical outcomes (Figure 3B), and decision curve analysis demonstrated the model’s potential clinical applicability (Figure 3C).
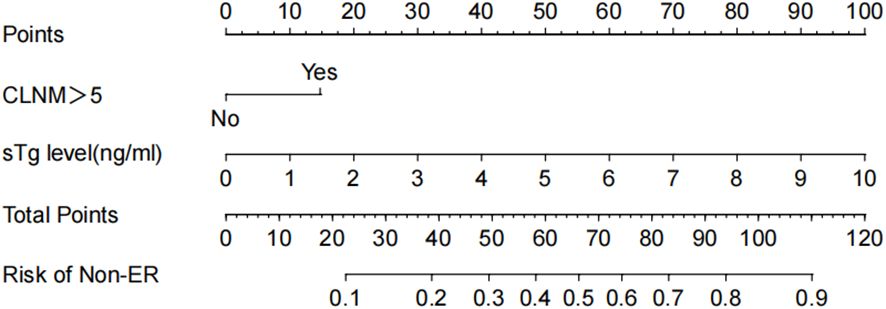
Figure 2 Nomogram for predicting non-ER risk. The value of each variable was scored on a point scale from 0 to 100, after which the scores for each variable were added together. That sum is located on the total points axis, which enables us to predict the probability of non-ER risk.
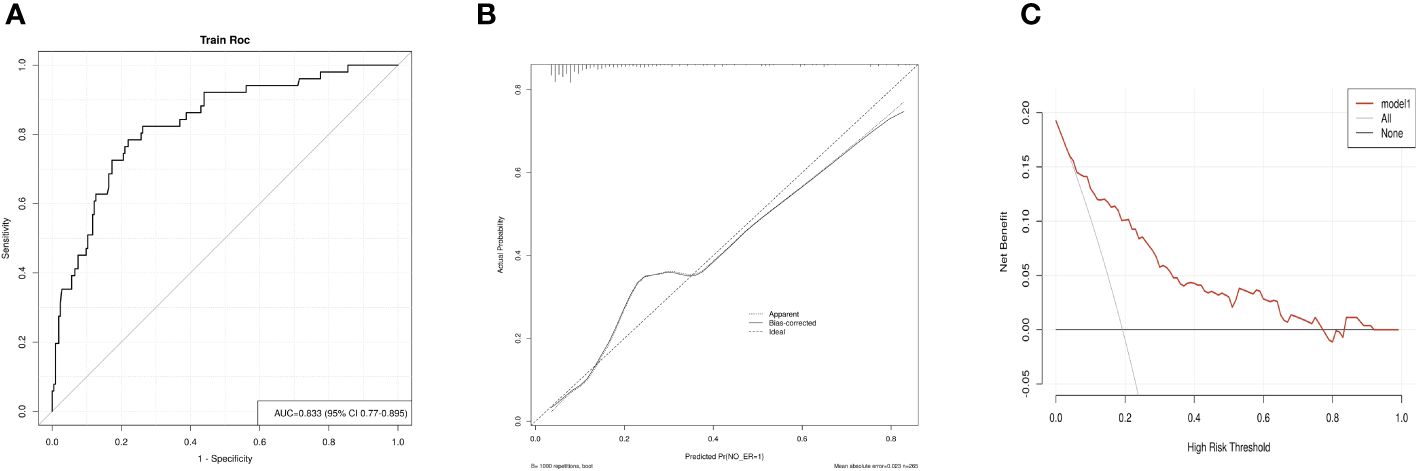
Figure 3 Evaluation of the nomogram model. (A) The Receiver Operating Characteristic (ROC) curve for the nomogram, derived using bootstrap resampling (1000 repetitions). (B) Calibration plot for the nomogram. The closer the performance nomogram’s solid line is to the dotted line of the ideal model, the higher the prediction accuracy of the nomogram. (C) Decision Curve Analysis (DCA) for the prediction model. The prediction model’s accuracy is represented by the red solid line, the gray line denotes the scenario where all patients exhibit non-complete response (non-ER), and the solid horizontal line implies that no patients experience non-ER. This graph illustrates the expected net benefit per patient in relation to the nomogram’s prediction of non-ER risk, with increased net benefit correlating to the extension of the model curve.
For convenient application of our nomogram, we developed dynamic calculators on the basis of a user-friendly website (https://www.evidencio.com/models/show/10176), which could be used directly by researchers and clinicians. By inputting certain clinical variables, we can easily obtain the corresponding individualized predicted survival probabilities through the output data generated by the website.
In this retrospective study, we found that sTg levels and CLNM>5 significantly contribute to the risk of non-ER in patients with intermediate-risk DTC following RRA. Utilizing these variables, we developed and validated a nomogram to estimate the risk of non-ER. This tool holds considerable potential importance for the primary prevention of non-ER in this patient population.
Contrary to previous research primarily centered on distinct factors influencing the prognosis of DTC post-surgery or radioactive iodine therapy, our study integrates clinicopathological characteristics and identifies that elevated sTg levels and CLNM>5 are associated with an increased risk of non-ER in patients with intermediate-risk DTC following RRA. Among these factors, elevated sTg levels were noted, corroborating findings from earlier studies (11, 16, 17). A retrospective analysis of 2,500 thyroid cancer patients established that a post-surgical thyroglobulin (ps-Tg) cutoff of ≤10.1 ng/mL predicts disease-free status with a negative predictive value of 95%. This threshold was consistently validated across all ATA risk categories. Additionally, the study revealed that a ps-Tg level of ≤10.1 ng/mL significantly reduces the likelihood of persistent or recurrent disease in patients classified as intermediate- and high-risk (18). In a prospective study of intermediate- to high-risk patients with sTg levels above 10 ng/mL, 28.4% displayed functional or structural disease following RAT. RAT, utilizing 5.55 GBq (150mCi) of Iodine-131, proved effective in detecting biochemical, functional, or structural disease, and elicited a substantial therapeutic response in a significant portion of these patients. Hence, these patients are deemed appropriate candidates for RAT (5). Therefore, in patients with a high risk of recurrence and sTg levels above 10 ng/ml, RAT has been shown to effectively enhance overall survival (OS) and disease-free survival (DFS). Consequently, it can be recommended as a standard treatment approach. Research on the risk factors of RRA in intermediate-risk DTC patients with low sTg levels (<10ng/ml) is relatively scarce. Previous studies examining the efficacy of total thyroidectomy and RRA in this demographic often included low-risk DTC patients, those with sTg levels over 10ng/ml, and patients with structural lesions in both Rx-WBS and Dx-WBS (19–22). Additionally, the variation in RRA dosages used in these studies contributes to biases in the research outcomes. This study specifically focuses on intermediate-risk DTC patients with sTg levels ≤10ng/ml and no structural lesions in either Rx-WBS or Dx-WBS. Furthermore, it standardizes the RRA dosage at 3.7GBq (100mCi) to enhance the reliability of the findings.
In patients with intermediate-risk DTC, current evidence regarding the impact of RRA on disease recurrence remains inconclusive, indicating beneficial effects in some cases but showing no benefit in others (23). The 2015 ATA guidelines advise that, following total thyroidectomy in patients with low-risk thyroid cancer or intermediate-risk disease exhibiting lower risk features (such as low-volume central neck nodal metastases without additional gross residual disease or adverse features), a lower administered activity of approximately 30 mCi for RRA is generally favored over higher administered activities (1). A retrospective study evaluated the therapeutic efficacy and long-term clinical outcomes of varying doses of RRA in patients with intermediate-risk DTC. This study involved 204 intermediate-risk DTC patients, with 124 receiving a high dose (3.7 or 5.55 GBq) and 80 receiving a low dose (1.11 GBq) of radioactive iodine. The findings indicated no significant difference in treatment success rates between the high-dose (54.84%) and low-dose (45.00%) groups, regardless of whole-body scan results. According to the American Thyroid Association’s reclassification system, the post-treatment response rates were as follows: ER (high-dose 54.84%, low-dose 45.00%), IDR (high-dose 34.68%, low-dose 30.00%), BIR (high-dose 4.03%, low-dose 13.75%), and SIR (high-dose 6.45%, low-dose 11.25%). Additionally, long-term follow-up showed recurrence rates of 5.65% in the high-dose group and 8.75% in the low-dose group. Consequently, the study suggests that in high-iodine intake regions of Korea, low-dose radioactive iodine therapy may be less effective for treating intermediate-risk DTC patients, potentially necessitating additional treatment in the low-dose group (19). A retrospective study evaluated the efficacy of low-dose (1110 MBq) versus high-dose (2960–3700 MBq) RRA in patients with intermediate-to-high-risk DTC. This study found no significant difference in initial success rates between the low-dose (73.5%) and high-dose (70.6%) groups, though high-dose RRA may be more effective for high-risk patients (20). Another study concluded that low-dose 131I is as effective as high-dose in RRA of papillary thyroid carcinoma patients. In the intermediate-risk group, disease-free survival rates at 6 months, 1 year, and 2 years were marginally higher in the high-dose group compared to the low-dose group (24). Collectively, these studies indicate that the selection of RRA dosage for intermediate-risk DTC patients should be individualized, considering factors such as disease risk and pre-treatment thyroglobulin levels, instead of implementing a standardized dosage approach. The efficacy of low-dose RAI therapy, especially in specific patient subsets, underscores the importance of meticulous patient selection and monitoring. Consequently, we performed a retrospective analysis of risk factors in patients with intermediate-risk DTC who underwent 3.7 GBq (100 mCi) RRA therapy at our center. Our evaluation suggests that these patients may face an elevated risk of recurrence and higher sTg level. We also successfully developed a predictive nomogram for intermediate-risk DTC patients undergoing RRA dosage at 3.7GBq (100mCi). Furthermore, a web-based dynamics calculator was created for use by clinicians at our center and other institutions.
The 2015 ATA guidelines introduced revised risk stratifications compared to the 2009 version (1). Under this framework, high risk is characterized by lymph node metastases larger than 3 centimeters, while intermediate risk involves either palpable disease or more than five lymph nodes (CLNM > 5), each smaller than 3 centimeters. Yun et al. (25) determined that the optimal cutoff value for lymph node metastases (LNMs) impacting RAI treatment response is five. For patients with CLNM > 5, the most effective lymph node ratio (LNR) cutoff was identified as 0.30. Factors such as CLNM > 5, gender, lymph node dissection, and ATA risk classification were found to be independent predictors of RAI response. Correspondingly, another study indicated that patients with over five metastatic lymph nodes exhibit more aggressive clinicopathological features and poorer outcomes (26). Echoing our study’s results, these findings underscore the importance of both the quantity and characteristics of lymph node metastases in determining thyroid cancer prognosis. They further stress the need for precise assessment and monitoring of lymph node involvement to inform effective treatment plans and predict patient outcomes.
Recent research has advanced the development of nomograms, offering highly accurate prognostic information for thyroid cancer, surpassing traditional methods like the AJCC staging system (27–29). A study based on SEER data crafted a nomogram to predict the risk of DM and its prognostic value in female patients with DTC (30). Additionally, a nomogram has been created to forecast the cancer-specific survival (CSS) of patients with poorly differentiated thyroid carcinoma, aiding clinicians in formulating suitable treatment strategies (31). Further, specialized survival nomograms for patients with DTC and DM have been developed, enhancing prognostic understanding in this subgroup (32). Collectively, these studies represent significant advancements in predictive modeling for thyroid cancer, offering more precise and customized prognostic tools that could markedly influence patient management and treatment approaches. We aspire for our nomogram to be utilized in clinical practice, providing personalized predictions of RRA efficacy for intermediate-risk DTC patients, and facilitating individualized treatment options. We also eagerly anticipate collaborating with researchers from other centers to refine this clinical prediction model further.
This study is subject to several limitations: (1) As a retrospective analysis, it may be affected by recall bias and incomplete data, necessitating further prospective studies for confirmation of results. (2) Being a single-center study with a relatively small sample size, its findings may lack generalizability, underscoring the need for multi-center studies to verify these results. (3) The study primarily focuses on certain clinical and pathological markers as predictors, which might neglect other vital factors affecting treatment outcomes. Notably, the BRAFV600E mutation, demonstrated by preclinical studies to significantly diminish sodium-iodide symporter expression and reduce RAI uptake, along with its correlation with ER rates and AXL expression in DTC patients, could influence results. Regrettably, only a limited subset of our cohort underwent testing for the BRAFV600E mutation. Future research models should incorporate genomic data to improve prognostic accuracy for responses to RRA across a more diverse patient population. (4) The outcome selected—response to RRA—may not accurately reflect the recurrence risk and overall survival of DTC patients. Despite this, the 2015 ATA guidelines indicate that DTC patients categorized as intermediate or high risk could significantly reduce their risk of recurrent or persistent disease by achieving an ER to RRA. Additionally, in a previous study with a protracted follow-up period, no patients transitioned from an ER outcome to a non-ER outcome six months post-treatment (33). These observations highlight the utility of RRA response as an effective and practical indicator for predicting clinical outcomes concerning both recurrence and specific mortality risks, particularly in DTC, which is typically slow-growing. However, a longer follow-up period for the cohorts in this study is necessary to verify the predictive capacity of this nomogram for DFS and overall survival in DTC patients after RRA, and to identify variations in prognostic factors across different outcomes. (5) Although a nomogram and an online calculator were developed, external and clinical validations are essential before their practical implementation.
This study reveals that in intermediate-risk DTC patients, the likelihood of an non-ER to RRA is significantly linked with the presence of CLNM>5 and higher sTg levels. A predictive model incorporating these factors has been established to assess the risk of Non-ER, accompanied by the development of a network-based dynamic calculator. This model has shown high accuracy in validation, aligning closely with actual clinical outcomes. This tool not only assists in treatment decision-making but also enhances the precision of prognosis information. Nevertheless, additional research and validation are required.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the ethics committee of the Affiliated Hospital of Guilin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
LL: Data curation, Methodology, Writing – original draft. QL: Data curation, Writing – review & editing. ZG: Formal analysis, Writing – review & editing. YL: Formal analysis, Supervision, Validation, Writing – review & editing. CL: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. JH: Data curation, Writing – review & editing. XM: Writing – review & editing. WF: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
2. Eric Pochin E. Radioiodine therapy of thyroid cancer. Semin Nucl Med. (1971) 1:503–15. doi: 10.1016/S0001-2998(71)81043-7
3. Hackshaw A, Harmer C, Mallick U, Haq M, Franklyn JA. 131I activity for remnant ablation in patients with differentiated thyroid cancer: A systematic review. J Clin Endocrinol Metab. (2007) 92:28–38. doi: 10.1210/jc.2006-1345
4. James DL, Ryan ÉJ, Davey MG, Quinn AJ, Heath DP, Garry SJ, et al. Radioiodine remnant ablation for differentiated thyroid cancer: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2021) 147:544–52. doi: 10.1001/jamaoto.2021.0288
5. Cheng L, Sa R, Luo Q, Fu H, Jin Y, Tang L, et al. Unexplained hyperthyroglobulinemia in differentiated thyroid cancer patients as an indication for radioiodine adjuvant therapy: A prospective multicenter study. J Nucl Med. (2021) 62:62–8. doi: 10.2967/jnumed.120.243642
6. Sun Y-Q, Sun D, Zhang X, Zhang Y-Q, Lin Y-S. Radioiodine adjuvant therapy in differentiated thyroid cancer: An update and reconsideration. Front Endocrinol (Lausanne). (2022) 13:994288. doi: 10.3389/fendo.2022.994288
7. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: A joint statement from the american thyroid association, the european association of nuclear medicine, the society of nuclear medicine and molecular imaging, and the european thyroid association. Thyroid. (2019) 29:461–70. doi: 10.1089/thy.2018.0597
8. Valerio L, Maino F, Castagna MG, Pacini F. Radioiodine therapy in the different stages of differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. (2023) 37:101703. doi: 10.1016/j.beem.2022.101703
9. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. (2007) 48:879–88. doi: 10.2967/jnumed.106.035535
10. Wang C, Diao H, Ren P, Wang X, Wang Y, Zhao W. Efficacy and affecting factors of 131I thyroid remnant ablation after surgical treatment of differentiated thyroid carcinoma. Front Oncol. (2018) 8:640. doi: 10.3389/fonc.2018.00640
11. Spaas M, Decallonne B, Laenen A, Billen J, Nuyts S. Prognostic value of stimulated thyroglobulin levels at the time of radioiodine administration in differentiated thyroid cancer. Eur Thyroid J. (2018) 7:211–7. doi: 10.1159/000489849
12. Kutikov A, Smaldone MC, Egleston BL, Manley BJ, Canter DJ, Simhan J, et al. Anatomic features of enhancing renal masses predict Malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol. (2011) 60:241–8. doi: 10.1016/j.eururo.2011.03.029
13. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
14. Hansen J, Auprich M, Ahyai SA, de la Taille A, van Poppel H, Marberger M, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. (2013) 63:201–9. doi: 10.1016/j.eururo.2012.07.030
15. Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. (2006) 24:3819–20. doi: 10.1200/JCO.2006.07.1290
16. Ha J, Kim MH, Jo K, Lim Y, Bae JS, Lee S, et al. Recombinant human TSH stimulated thyroglobulin levels at remnant ablation predict structural incomplete response to treatment in patients with differentiated thyroid cancer. Medicine. (2017) 96:e7512. doi: 10.1097/MD.0000000000007512
17. Park HJ, Min J-J, Bom H-S, Kim J, Song H-C, Kwon SY. Early stimulated thyroglobulin for response prediction after recombinant human thyrotropin-aided radioiodine therapy. Ann Nucl Med. (2017) 31:616–22. doi: 10.1007/s12149-017-1190-3
18. Tian T, Xu Y, Zhang X, Liu B. Prognostic implications of preablation stimulated Tg: A retrospective analysis of 2500 thyroid cancer patients. J Clin Endocrinol Metab. (2021) 106:e4688–97. doi: 10.1210/clinem/dgab445
19. Jeong JH, Kong EJ, Jeong SY, Lee S-W, Cho IH, Ah Chun K, et al. Clinical outcomes of low-dose and high-dose postoperative radioiodine therapy in patients with intermediate-risk differentiated thyroid cancer. Nucl Med Commun. (2017) 38:228–33. doi: 10.1097/MNM.0000000000000636
20. Iizuka Y, Katagiri T, Ogura K, Mizowaki T. Comparison between the different doses of radioactive iodine ablation prescribed in patients with intermediate-to-high-risk differentiated thyroid cancer. Ann Nucl Med. (2019) 33:495–501. doi: 10.1007/s12149-019-01357-6
21. Goksel S, Avci U. Factors affecting ablation success after I-131 radioactive iodine therapy in low and intermediate risk papillary thyroid cancer. Horm Metab Res. (2023) 55:677–83.
22. Zhang X, Liu J-R, Mu Z-Z, Cheng X-Q, Lin Y-S. Response to surgery assessments for sparing radioiodine remnant ablation in intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metab. (2023) 108:1330–7. doi: 10.1210/clinem/dgac745
23. van Velsen EFS, Verburg FA. Adjuvant radioiodine for intermediate-risk papillary thyroid cancer-to treat or not to treat. J Clin Endocrinol Metab. (2023) 108:e1149–50. doi: 10.1210/clinem/dgad171
24. Yasmin T, Adnan S, Younis MN, Fatima A, Shahid A. Comparing high and low-dose radio-iodine therapy in thyroid remnant ablation among intermediate and low-risk papillary thyroid carcinoma patients-single centre experience. Dose Response. (2021) 19:15593258211062775. doi: 10.1177/15593258211062775
25. Yun C, Xiao J, Cao J, Shao C, Wang L, Zhang W, et al. Lymph node metastases >5 and metastatic lymph node ratio >0.30 of differentiated thyroid cancer predict response to radioactive iodine. Cancer Med. (2021) 10:7610–9. doi: 10.1002/cam4.4288
26. Zhan L, Feng H, Yu X, Li L, Song J, Tu Y, et al. Clinical and prognosis value of the number of metastatic lymph nodes in patients with papillary thyroid carcinoma. BMC Surg. (2022) 22:235. doi: 10.1186/s12893-022-01635-7
27. Wang X, Zheng X, Zhu J, Li Z, Wei T. A nomogram and risk classification system for predicting cancer-specific survival in tall cell variant of papillary thyroid cancer: a SEER-based study. J Endocrinol Invest. (2023) 46:893–901. doi: 10.1007/s40618-022-01949-6
28. Cui H, Wang R, Zhao X, Wang S, Shi X, Sang J. Development and validation of a nomogram for predicting the early death of anaplastic thyroid cancer: a SEER population-based study. J Cancer Res Clin Oncol. (2023) 149:16001–13. doi: 10.1007/s00432-023-05302-z
29. Tang J, Zhanghuang C, Yao Z, Li L, Xie Y, Tang H, et al. Development and validation of a nomogram to predict cancer-specific survival in middle-aged patients with papillary thyroid cancer: A SEER database study. Heliyon. (2023) 9:e13665. doi: 10.1016/j.heliyon.2023.e13665
30. Wang W, Shen C, Yang Z. Nomogram individually predicts the risk for distant metastasis and prognosis value in female differentiated thyroid cancer patients: A SEER-based study. Front Oncol. (2022) 12:800639. doi: 10.3389/fonc.2022.800639
31. Jin S, Liu H, Yang J, Zhou J, Peng D, Liu X, et al. Development and validation of a nomogram model for cancer-specific survival of patients with poorly differentiated thyroid carcinoma: A SEER database analysis. Front Endocrinol (Lausanne). (2022) 13:882279. doi: 10.3389/fendo.2022.882279
32. Ma Q, Chen Z, Fang Y, Wei X, Wang N, Zhou X, et al. Development and validation of survival nomograms for patients with differentiated thyroid cancer with distant metastases: a SEER Program-based study. J Endocrinol Invest. (2023). doi: 10.1007/s40618-023-02129-wp
Keywords: intermediate-risk, differentiated thyroid cancer, radioiodine remnant ablation, predictive nomogram, thyroglobulin, cervical lymph node metastasis
Citation: Lu L, Li Q, Ge Z, Lu Y, Lin C, Lv J, Huang J, Mu X and Fu W (2024) Development of a predictive nomogram for intermediate-risk differentiated thyroid cancer patients after fixed 3.7GBq (100mCi) radioiodine remnant ablation. Front. Endocrinol. 15:1361683. doi: 10.3389/fendo.2024.1361683
Received: 26 December 2023; Accepted: 16 May 2024;
Published: 30 May 2024.
Edited by:
Yuebang Yin, Erasmus Medical Center, NetherlandsReviewed by:
Qianqian Lu, Peking University, ChinaCopyright © 2024 Lu, Li, Ge, Lu, Lin, Lv, Huang, Mu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyu Mu, muxingyu1992@hotmail.com; Wei Fu, fuwei19700513@163.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.