- 1Department of Respiratory and Critical Care Medicine, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, China
- 2Health Science Center, Ningbo University, Ningbo, China
Background: Obstructive sleep apnea (OSA) is an important but frequently overlooked risk factor for hypertension (HTN). The prevalence of hypertension is high in patients with OSA, but the differences in clinical symptoms and comorbidities between patients with OSA with hypertension and those with normal blood pressure have not been fully defined.
Methods: This study retrospectively analyzed OSA patients diagnosed for the first time in Lihuili Hospital Affiliated to Ningbo University from 2016 to 2020. Patients were divided into an OSA group with hypertension and an OSA group without hypertension. The sociodemographic information, clinical symptoms, comorbidities, and polysomnography results of the two groups were compared. The independent risk factors associated with hypertension in patients with OSA were explored.
Results: A total of 1108 patients with OSA initially diagnosed were included in the study, including 387 with hypertension and 721 without. Compared with OSA patients without hypertension, OSA patients with hypertension were older; had a higher body mass index (BMI) and Epworth sleepiness score (ESS); a higher incidence of nocturia; and a higher proportion of diabetes mellitus, coronary heart disease, and cerebrovascular disease. Multivariate analysis showed age (odds ratio [OR]:1.06, 95% confidence interval [CI]:1.04-1.08), BMI (OR:1.17, 95% CI:1.11-1.23), ESS score (OR:0.97, 95%CI: 0.94-1.00) and nocturia symptoms (OR:1.64, 95% CI:1.19-2.27) was independently associated with hypertension in OSA patients, and comorbid diabetes (OR: 3.86, 95% CI: 2.31-6.45), coronary heart disease (OR: 1.90, 95% CI:1.15-3.16), and ischemic stroke (OR: 3.69,95% CI:1.31-10.40) was independently associated with hypertension in OSA patients.
Conclusion: Compared to OSA patients with normal blood pressure, OSA patients with hypertension had more significant daytime sleepiness, more frequent nocturnal urination, and a higher risk of diabetes, coronary heart disease, and cerebrovascular disease.
1 Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder and is characterized by recurrent complete or partial collapse of the upper airway at night, leading sleep fragmentation and frequent nocturnal hypoxia. Approximately 936 million adults aged 30-69 suffer from OSA worldwide; China has the largest number of OSA patients in the world, with a prevalence of 23.6% in people aged 30-69 (1). As the population ages and the prevalence of obesity increases, the prevalence of OSA is also likely to increase rapidly. OSA can cause a series of pathophysiological results such as chronic intermittent hypoxia, oxidative stress and inflammatory response, increased negative pressure in the pleural cavity, and increased sympathetic nerve activity (2). Patients with OSA may present with a variety of symptoms, including habitual snoring, fatigue, poor sleep quality, excessive daytime sleepiness (EDS), memory loss, headaches, and psychological disturbances (3). Many studies have confirmed that untreated OSA can damage multiple systems and organs and cause a variety of complications, including cardiovascular diseases, ischemic stroke, metabolic-related diseases, and neuropsychiatric complications (4–7).
OSA and hypertension are closely associated. The prevalence of OSA in hypertensive patients is approximately 50%, the prevalence of OSA in patients with resistant hypertension is up to 71%, and the incidence of hypertensive crisis in OSA patients with hypertension is 15.70% (8). Approximately 30 to 50% of patients with OSA also have hypertension, which increases the risk of cardiovascular disease and cardiovascular death in these patients (9). Kario et al. found that OSA-related hypertension has the following features, which is usually characterized by increased diastolic blood pressure and nocturnal hypertension typically presents with nocturnal blood pressure may be non-descending (a small or absent nocturnal blood pressure drop) or ascending (nocturnal blood pressure higher than daytime blood pressure) (10). Cai et al. found that the incidence of occult hypertension in patients with OSA was higher than that in those without OSA, and the incidence of occult hypertension in subjects newly diagnosed with OSA was close to 30% (11). OSA induces intermittent hypoxia, which causes oxidative stress and inflammation, leading to vascular endothelial dysfunction, reduces the availability of nitric oxide and vascular endothelial relaxation ability, upregulates sympathetic nerve excitation, and causes vasoconstriction, increased vascular resistance, and vascular remodeling. Hypoxia leads to the activation of the renin-angiotensin-aldosterone system (RAAS), which in turn leads to hypertension (12–16).
To date, the clinical characteristics of patients with OSA and hypertension have not been fully clarified, and only individual studies have been conducted in Western countries. Moreover, OSA is a heterogeneous disease with some racial differences. Craniofacial structure differences have been proposed as one of the important reasons for the greater susceptibility of Asians to OSA (17). Besides, ethnic differences in body fat distribution, the impact of BMI on AHI and genetic susceptibility may be other factors of OSA characteristics in Asian populations (18, 19). This study examined the association of coexisting hypertension with OSA-related symptoms, other comorbidities, and polysomnography (PSG) outcomes in Chinese patients with OSA at first diagnosis.
2 Materials and methods
2.1 Study participants
This retrospective study used data from January 2016 to December 2020; 1257 patients who were admitted to the affiliated Lihuili Hospital of Ningbo University with suspected sleep disordered breathing underwent full-night PSG examination, and adult patients with newly diagnosed OSA were included in this study. The exclusion criteria were age<18 y, simple snoring, central sleep apnea, mixed sleep apnea, previous PSG and/or continuous positive airway pressure treatment, and incomplete clinical data. A total of 1108 patients met the inclusion criteria, including 387 with hypertension and 721 without hypertension. Hypertension in this study was defined according to Chinese Guidelines for the Prevention and Treatment of Hypertension: In the absence of antihypertensive drugs, room blood pressure was measured three times on different days, SBP≥140mmHg and/or DBP≥90 mmHg or the patient’s history of a definite diagnosis of hypertension. The grouping flow chart is presented in Figure 1. The study was approved by the Ethics Committee of The Affiliated Lihuili Hospital of Ningbo University (Approval Number: KY2021PJ241) and written informed consent was obtained from each participant.
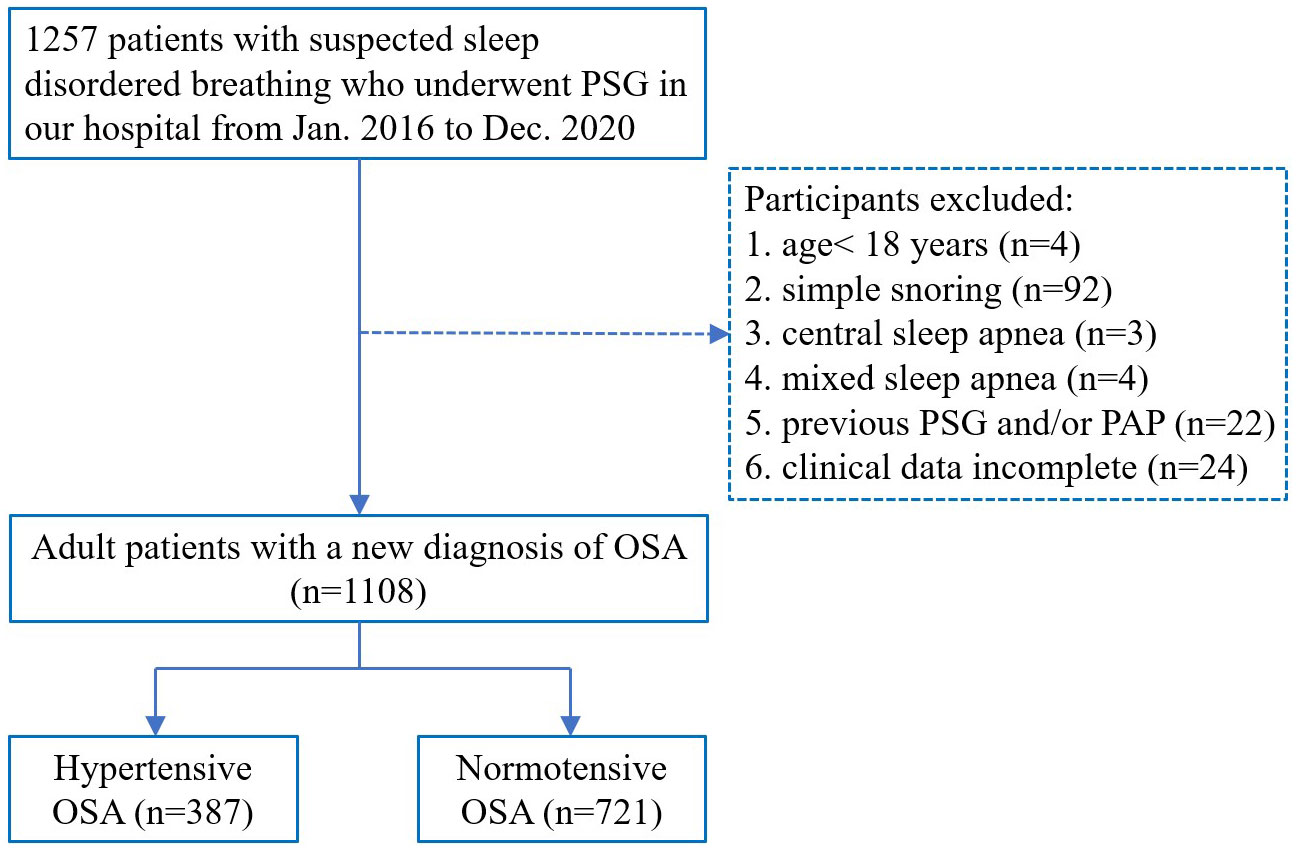
Figure 1 Flow chart of the patients’ inclusion process. PSG, polysomnography; OSA, obstructive sleep apnea; PAP, positive airway pressure.
2.2 Data collection
Age and sex of the participants were obtained from an inpatient system. Professional nurses measured the height, weight, and awake pulse oxygen saturation of the participants. Detailed medical histories of the participants, including comorbidities and other diseases, were collected and recorded by professional respiratory physicians. Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS). Body mass index (BMI) was defined as kg/m2.
2.3 Polysomnography
Participants underwent full-night PSG monitoring in the hospital’s sleep-monitoring room and were asked to avoid alcohol, tea, coffee, sedation, and hypnotic drugs that day. We had used a polysomnographic system (Alice 5, Respironics, Philips, Pittsburgh, Pennsylvania, USA) to test at least 7 hours of sleep. The monitoring items included electroencephalography, electrocardiography, electroophthalmography, mandibular electromyography, oronasal airflow, snoring, chest and abdominal movements, body position, and pulse oxygen saturation.
2.4 Data analysis
Continuous variables which conform to normal distribution are expressed by mean and standard deviation, those that do not conform to normal distribution are expressed by median (quartile), and categorical variable data are expressed by percentage. The t-test or analysis of variance (ANOVA) were used to compare continuous variables, and the chi-squared test was used to compare categorical variables. Multivariate analysis was performed using binary logistic regression. SPSS26.0 statistical analysis software was used for statistical analysis, and a P value <0.05 was considered statistically significant.
3 Results
3.1 Sociodemographic characteristics
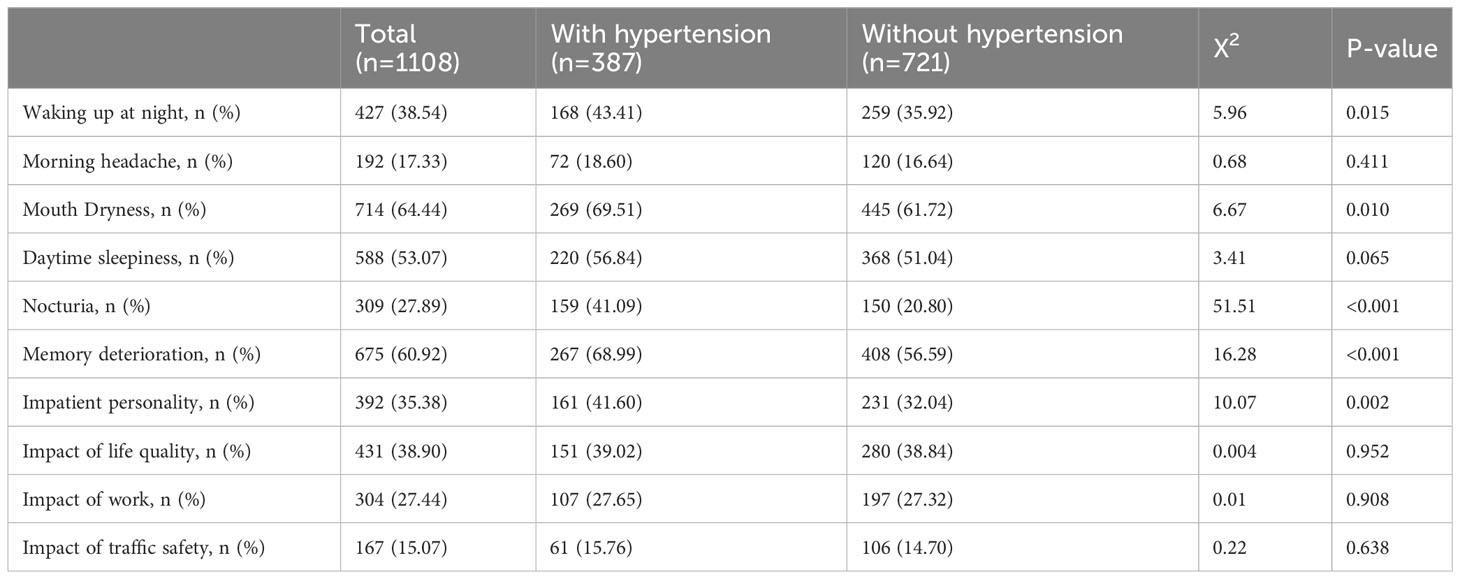
The sociodemographic characteristics of the 1108 patients with OSA are shown in Table 1. There were 387 patients with hypertension and 721 patients without hypertension, respectively. The average age of the total patients was 45.30 ± 11.58 y; the average age of patients with and without hypertension was 50.31 ± 10.92 and 42.6 ± 11.03 y, respectively. The mean age of patients with hypertension was significantly higher than that of patients without hypertension (P < 0.001). There was a significant increase in BMI (P < 0.001) and ESS scores (P =0.048) in patients with hypertension. The proportions of obese patients (P < 0.001) and smokers (P =0.003) with hypertension were significantly higher. No differences in sex or alcohol use were observed between OSA patients with and without hypertension.
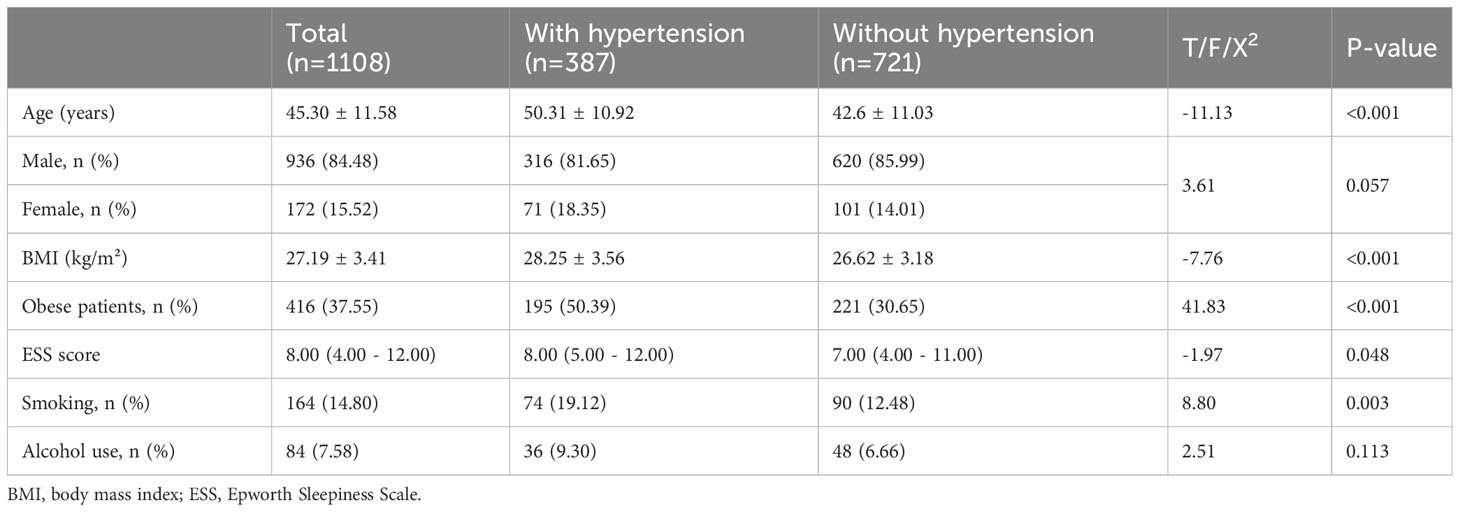
Table 1 Sociodemographic characteristics of total OSA patients and the patients with or without hypertension.
3.2 Clinical symptoms
Table 2 shows the clinical symptoms of total patients with OSA and those with or without hypertension. The proportions of patients waking up at night (P =0.015), mouth dryness (P=0.010), nocturia (P =0.010), memory deterioration(P<0.010), and impatient personality (P=0.010) were significantly increased in OSA patients with hypertension. There were no differences in the ratio of morning headache, daytime sleepiness, impact on quality of life, work, or traffic safety.
3.3 Clinical comorbidities
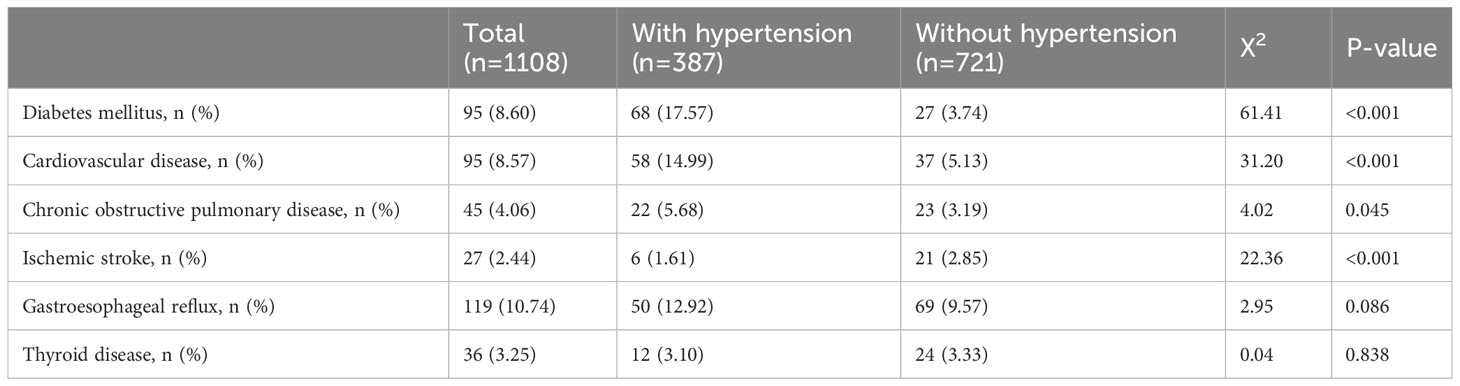
Other clinical comorbidities are shown in Table 3. The proportions for diabetes mellitus (P <0.010), coronary heart disease (P <0.010), chronic obstructive pulmonary disease (P =0.045), and ischemic stroke (P <0.010) were significantly higher in OSA patients with hypertension. There were no statistically significant differences in the proportions of gastroesophageal reflux and thyroid disease between OSA with and without hypertension.
3.4 PSG results
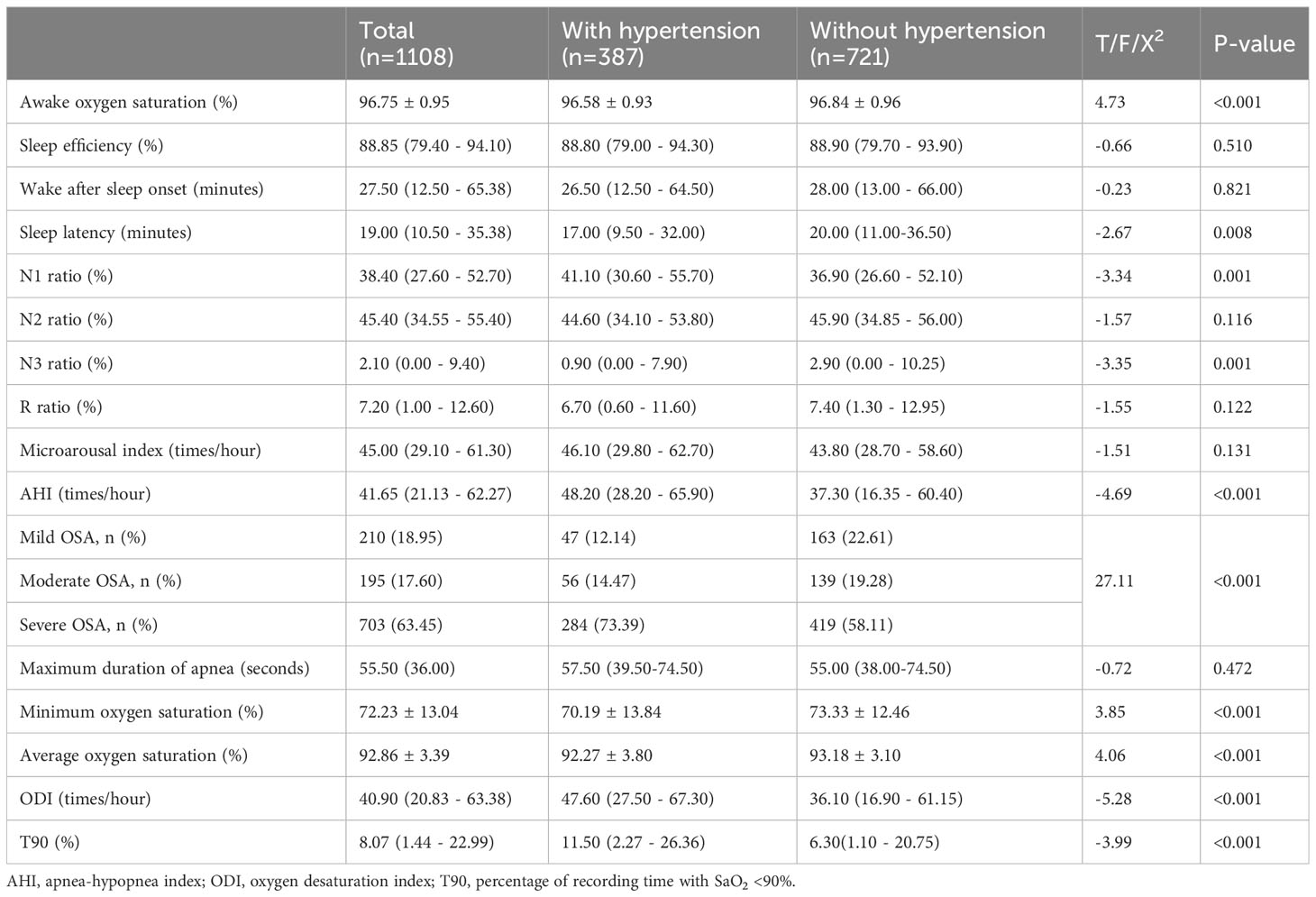
Table 4 shows the PSG results of all patients with OSA and those with or without hypertension. Awake oxygen saturation (P <0.010), minimum oxygen saturation (P <0.010), and average oxygen saturation (P <0.010) significantly decreased in OSA patients with hypertension. The sleep latency (P =0.008), N1 ratio (P=0.010), N3 ratio (P=0.010), AHI (P <0.010), oxygen desaturation index (P <0.010), and percentage of recording time with SaO2 <90% (T90) (P <0.010) differed significantly between hypertensive and normotensive OSA patients. The results of other PSG tests were not statistically significant.
3.5 Multi-variate analysis
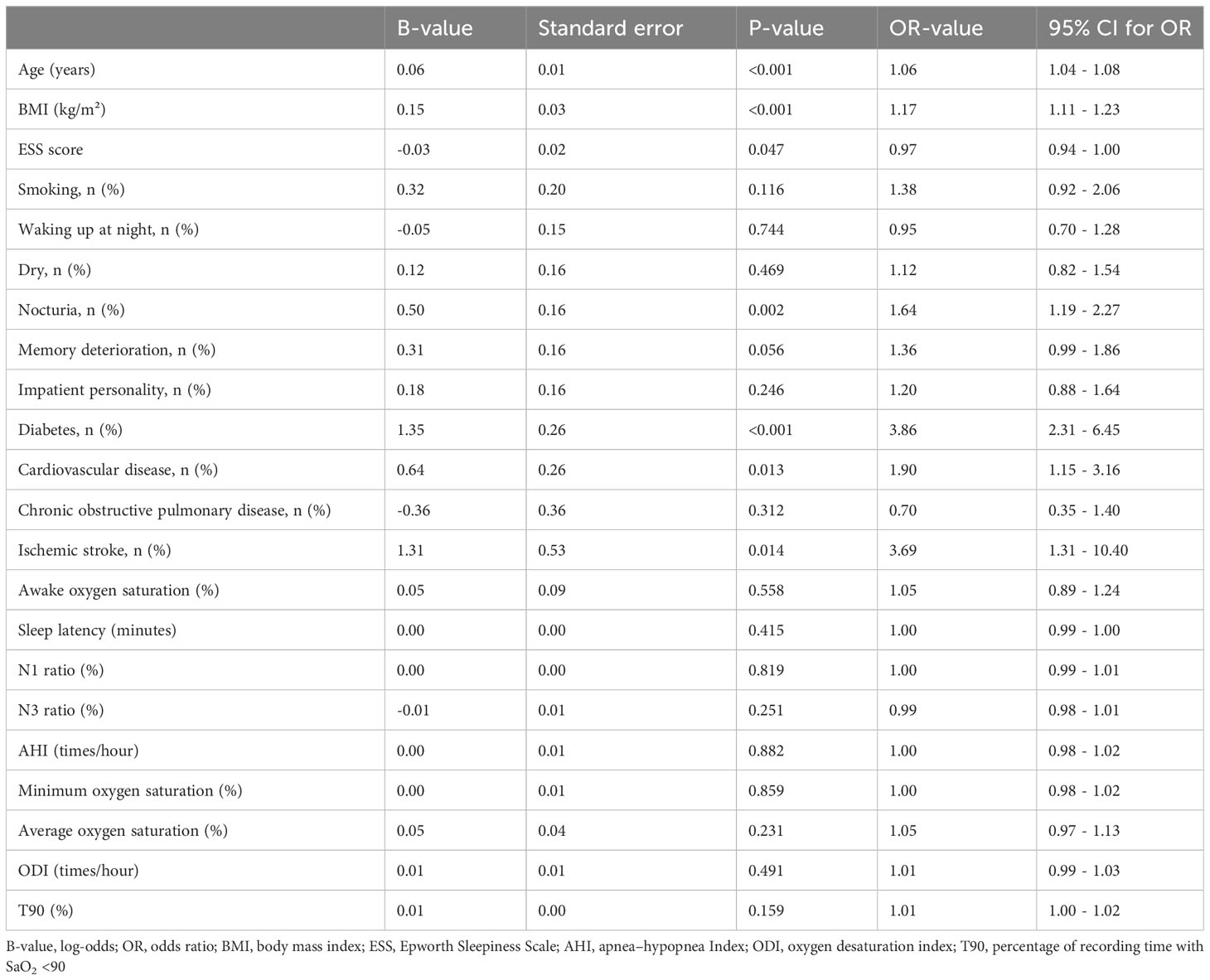
When controlling for sociodemographic features, clinical symptoms, comorbidities, and PSG results (Table 5), binary logistic regression analysis suggested that age, BMI, ESS score, nocturia, diabetes, cardiovascular disease, and ischemic stroke were independently associated with hypertension in OSA patients.
4 Discussion
OSA is a complex and heterogeneous disease with multiple pathophysiological results such as chronic intermittent hypoxia, oxidative stress, inflammatory response, increased negative pressure in the chest, and increased sympathetic activity (2). Peppard et al. found a dose-effect relationship between baseline sleep-disordered breathing and hypertension after four years, independent of known confounding factors (20). This suggests that the risk of developing hypertension increases with AHI. Sleep in patients with OSA is highly fragmented, with a higher proportion of light sleep; the proportion of slow wave sleep (SWS) is significantly decreased, and SWS stage is accompanied by lower sympathetic nervous activity and higher parasympathetic nerve activity (3, 21). Experimental inhibition of human SWS can significantly reduce the drop in blood pressure at night, that is, the increase in blood pressure at night; a decrease in the proportion of SWS is considered a sign of elevated blood pressure (22). In patients with OSA, blood pressure is characterized by no decrease in blood pressure, nocturnal hypertension, and increased blood pressure variability, that is, non-dipping or rising patterns (23–27). Elevated nighttime BP and higher morning BP are associated with increased risk of cardiovascular events and worse cardiovascular outcomes (28, 29). In our study, SWS was statistically significant in the univariate analysis, but not in the multivariate analysis, which may be related to the imbalance of other factors, such as age, between the two groups. Through meta-analysis, Maurice et al. found that sleep time, sleep efficiency, and SWS decrease with increasing age (30).
The most commonly used quantitative method to assess the degree of daytime sleepiness is the subjective ESS. When the ESS score was greater than 10 was defined as EDS (31). EDS has become one of the most common and prominent symptoms in patients with OSA and is an important criterion for its diagnosis and treatment (32, 33). In this study, we found that the ESS score of OSA patients with hypertension was significantly higher than that of those without hypertension. Kapur et al. suggested that participants with sleep-disordered breathing who often felt excessively sleepy were more likely to develop high blood pressure than those who did not (34). Feng et al. found that OSA patients with higher ESS scores were more likely to have higher blood pressure than OSA patients with lower ESS scores, and even had a higher risk of being diagnosed with hypertension (35). Robinson et al. reported that EDS hypertensive patients with OSA experienced a decrease in blood pressure after receiving continuous positive airway pressure (CPAP) compared without EDS hypertensive patients (36). This suggests that daytime sleepiness may be related in some way to the pathogenesis of hypertension in sleep apnea. However, contrary results have also been reported in the literature. Martynowicz et al. found that in the moderate to severe OSA group, the total ESS score of hypertensive patients was significantly lower than that of patients with normal blood pressure (37). This may be because the ESS relies on patient self-reporting, and is susceptible to subjective factors.
Nocturia refers to the occurrence of two or more urinations at night (38). The main causes of nocturia include inflammation, benign prostatic hyperplasia, excessive bladder activity, neurogenic bladder, and OSA. Nocturia and nighttime hypertension are common in patients with obstructive sleep apnea (OSA) and share several physiopathological pathways (27). AHI is an independent predictor of nocturnal urination frequency in OSA patients (39). Nocturia became more common as the severity of OSA increased. OSA may lead to nocturia by several mechanisms: OSA can lead to airflow obstruction, intermittent hypoxia, sympathetic hyperactivity, and changes in thoracic pressure, resulting in atrial and ventricular secretion of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), stimulating water and sodium excretion, inhibiting RAAS and antidiuretic hormone (ADH) release (40–42). Consistent with our findings, Destors et al. showed that nocturia was strongly associated with OSA-associated hypertension after adjusting for confounders (43). Rahman et al. found that compared with patients with normal blood pressure, patients with hypertension had a 1.2-1.3 times increased risk of nocturia (44). Although the pathophysiological mechanism of the association between hypertension and nocturia has not been fully established, several hypotheses have been proposed. First, higher daytime blood pressure may reflect higher nighttime blood pressure, which promotes natriuresis (45). Hypertension can lead to nocturia through changes in glomerular filtration rate and tubular transport (46). Nocturnal polyuria due to RAAS activation, a corresponding increase in nocturnal sodium in the urine, and decreased bladder function due to chronic vascular insufficiency are mediated by hypertension and other atherosclerotic risk factors (47). Congestive heart failure due to hypertension leads to atrial extension, increased release of atrial natriuretic peptide (ANP) and increased nocturia due to renal hyperfiltration (48). Therefore, nocturia was more frequent in OSA patients with hypertension than in those without hypertension.
Diabetes is a common comorbidity of OSA, and increasing evidence supports a complex relationship between OSA and diabetes. Intermittent hypoxemia and sleep disruption can lead to activation of the sympathetic nervous system, increased oxidative stress, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and systemic inflammation. Sympathetic activation leads to increased levels of catecholamines such as norepinephrine and epinephrine, inducing insulin resistance, inhibiting pancreatic insulin secretion, and resulting in elevated blood sugar (49). Adrenaline can also promote hepatic glucose production and inhibit insulin secretion, and the uptake of glucose by tissues further increases blood glucose uptake (50). OSA can lead to a significant reduction or inhibition of SWS (21), and Herzog et al. found that selective suppression of SWS resulted in an increase in plasma glucose and a decrease in insulin sensitivity in the early morning (51). However, several studies have found that the relationship between OSA and type 2 diabetes may be bidirectional, and that diabetic neuropathy can affect central respiratory control and the upper respiratory tract nerve reflex and promote sleep-disordered breathing (52). Diabetes and hypertension have overlapping genetic, physiological, and environmental factors, and hypertension may be considered as an important cause of diabetes (53, 54). Diabetic complications are more common in patients with OSA and hypertension than in those without. Luo et al. found that non-falling blood pressure in hypertensive patients with OSA was associated with an approximately 1.5-fold increased risk of new-onset diabetes (55). This suggests that hypertension in OSA may induce or promote diabetes.
OSA is closely associated with cardiovascular and cerebrovascular diseases including transient ischemic attack, stroke, pulmonary hypertension, heart failure, coronary heart disease, atrial fibrillation, myocardial infarction, and sudden death (56). Intermittent hypoxia, systemic inflammation, sympathetic nerve activation, and endothelial dysfunction caused by OSA are important mechanisms that induce cardiovascular and cerebrovascular diseases (56–59). The strong association between OSA and coronary and cerebrovascular disease appears to be independent of shared risk factors, including obesity (60). Most studies have shown a causal relationship between OSA severity (as assessed using AHI) and the risk of cardiovascular events (61). It has also been found that the risk of stroke in patients with mild OSA is 2.44 times higher than that in normal people, while the risk of stroke in patients with moderate or severe OSA is 3.56 times higher than that in normal people (62). This indicates that the severer OSA is, the more likely it is to be complicated with cardiovascular and cerebrovascular diseases. Previous studies have found that the relationship between OSA and cardiovascular disease risk factors is bidirectional, suggesting that patients with cardiovascular disease may also develop OSA and that effective treatment of cardiovascular disease may improve OSA (63). Compared with patients without hypertension, hypertension also significantly increases the risk and severity of cardiovascular and cerebrovascular diseases (64). High blood pressure is an intermediary between OSA and cardiovascular disease, and its presence of high blood pressure also increases the risk of cardiovascular development in patients with OSA, ultimately increasing the risk of cardiovascular-related death (65). In addition, OSA has been shown to be an independent risk factor for hypertension, ischemic heart disease, and arrhythmia, which in turn constitute risk factors for ischemic stroke (66). Therefore, hypertensive OSA is associated with a significantly increased risk for cardiovascular and cerebrovascular diseases.
The advantage of this study is that the sample size was large, covering all genders and age stages, and the sample data were rich, including sociodemographic features, clinical characteristics, and PSG results. However, this study also had some limitations. First, ambulatory blood pressure monitoring was not performed in this study and data of blood pressure at night were unavailable. Second, there was no subgroup analysis of hypertension level.
5 Conclusion
In conclusion, our study found that patients with OSA and hypertension had more severe daytime sleepiness and more frequent nocturnal urination than OSA patients without hypertension. Simultaneously, there is a higher risk of combining with diabetes, coronary heart disease, and ischemic stroke. This indicates that the patients with OSA combined with hypertension had more symptoms and a heavier burden of cardiovascular, and cerebrovascular, and metabolic complications. More attention should be paid to these patients, and individualized treatment should be provided in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The Affiliated Lihuili Hospital of Ningbo University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MT: Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. XD: Resources, Writing – review & editing. JT: Resources, Writing – review & editing. QF: Resources, Writing – review & editing. CS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Science and Technology Program for Public Welfare of Ningbo, China (grant number 2021S175) and the Medical Science and Technology Program of Ningbo, China (grant number 2020Y03).
Acknowledgments
We would like to thank our sleep monitoring team and respiratory nurses for their support in this study. We also thank Rui Fan for his help in statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao B, Cao Z, Xie Y, Shi Y, Zhang Y, Liu S, et al. The relationship of tongue fat content and efficacy of uvulopalatopharyngoplasty in Chinese patients with obstructive sleep apnea. BMC Surg. (2023) 23:254. doi: 10.1186/s12893-023-02144-x
2. Gonzaga C, Bertolami A, Bertolami M, Amodeo C, Calhoun D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum hypertension. (2015) 29:705–12. doi: 10.1038/jhh.2015.15
3. Chen L, Bai C, Zheng Y, Wei L, Han C, Yuan N, et al. The association between sleep architecture, quality of life, and hypertension in patients with obstructive sleep apnea. Sleep breathing = Schlaf Atmung. (2023) 27:191–203. doi: 10.1007/s11325-022-02589-z
4. Sircu V, Colesnic SI, Covantsev S, Corlateanu O, Sukhotko A, Popovici C, et al. The burden of comorbidities in obstructive sleep apnea and the pathophysiologic mechanisms and effects of CPAP. Clocks sleep. (2023) 5:333–49. doi: 10.3390/clockssleep5020025
5. Hirani R, Smiley A. A scoping review of sleep apnea: Where do we stand? Life (Basel Switzerland). (2023) 13:387. doi: 10.3390/life13020387
6. Lisik D, Pires GN, Zou D. Perspective: Systematic review and meta-analysis in obstructive sleep apnea - What is lacking? Sleep Med. (2023) 111:54–61. doi: 10.1016/j.sleep.2023.09.006
7. Myśliński W, Szwed M, Szwed J, Panasiuk L, Brożyna-Tkaczyk K, Borysowicz M, et al. Prevalence of target organ damage in hypertensive patients with coexisting obstructive sleep apnea. Ann Agric Environ Med AAEM. (2022) 29:294–9. doi: 10.26444/aaem/149469
8. Khamsai S, Chootrakool A, Limpawattana P, Chindaprasirt J, Sukeepaisarnjaroen W, Chotmongkol V, et al. Hypertensive crisis in patients with obstructive sleep apnea-induced hypertension. BMC Cardiovasc Disord. (2021) 21:310. doi: 10.1186/s12872-021-02119-x
9. Xia N, Wang H, Chen Y, Fan XJ, Nie XH. Association between sleep efficiency and hypertension in chinese obstructive sleep apnea patients. Nat Sci sleep. (2023) 15:79–88. doi: 10.2147/nss.S396893
10. Kario K, Hettrick DA, Prejbisz A, Januszewicz A. Obstructive sleep apnea-induced neurogenic nocturnal hypertension: A potential role of renal denervation? Hypertension (Dallas Tex 1979). (2021) 77:1047–60. doi: 10.1161/hypertensionaha.120.16378
11. Cai A, Wang L, Zhou Y. Hypertension and obstructive sleep apnea. Hypertension Res Off J Japanese Soc Hypertension. (2016) 39:391–5. doi: 10.1038/hr.2016.11
12. Zhang W, Si LY. Obstructive sleep apnea syndrome (OSAS) and hypertension: pathogenic mechanisms and possible therapeutic approaches. Upsala J Med Sci. (2012) 117:370–82. doi: 10.3109/03009734.2012.707253
13. Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, et al. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J Global Health. (2018) 8:10405. doi: 10.7189/jogh.08.010405
14. Bangash A, Wajid F, Poolacherla R, Mim FK, Rutkofsky IH. Obstructive sleep apnea and hypertension: A review of the relationship and pathogenic association. Cureus. (2020) 12:e8241. doi: 10.7759/cureus.8241
15. Pan M, Ou Q, Chen B, Hong Z, Liu H. Risk factors for obstructive sleep apnea-related hypertension in police officers in Southern China. J Thorac Dis. (2019) 11:4169–78. doi: 10.21037/jtd.2019.09.83
16. Ou YH, Tan A, Lee CH. Management of hypertension in obstructive sleep apnea. Am J Prev Cardiol. (2023) 13:100475. doi: 10.1016/j.ajpc.2023.100475
17. Genta PR, Marcondes BF, Danzi NJ, Lorenzi-Filho G. Ethnicity as a risk factor for obstructive sleep apnea: comparison of Japanese descendants and white males in São Paulo, Brazil. Braz J Med Biol Res = Rev Bras pesquisas medicas e biologicas. (2008) 41:728–33. doi: 10.1590/s0100-879x2008000800015
18. Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. (2016) 18:96–102. doi: 10.1016/j.sleep.2015.01.015
19. Sutherland K, Keenan BT, Bittencourt L, Chen NH, Gislason T, Leinwand S, et al. A global comparison of anatomic risk factors and their relationship to obstructive sleep apnea severity in clinical samples. J Clin sleep Med JCSM Off Publ Am Acad Sleep Med. (2019) 15:629–39. doi: 10.5664/jcsm.7730
20. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. New Engl J Med. (2000) 342:1378–84. doi: 10.1056/nejm200005113421901
21. Ren R, Covassin N, Zhang Y, Lei F, Yang L, Zhou J, et al. Interaction between slow wave sleep and obstructive sleep apnea in prevalent hypertension. Hypertension (Dallas Tex 1979). (2020) 75:516–23. doi: 10.1161/hypertensionaha.119.13720
22. Sayk F, Teckentrup C, Becker C, Heutling D, Wellhöner P, Lehnert H, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regulatory Integr Comp Physiol. (2010) 298:R191–197. doi: 10.1152/ajpregu.00368.2009
23. Saeed S, Romarheim A, Mancia G, Saxvig IW, Gulati S, Lehmann S, et al. Characteristics of hypertension and arterial stiffness in obstructive sleep apnea: A Scandinavian experience from a prospective study of 6408 normotensive and hypertensive patients. J Clin hypertension (Greenwich Conn). (2022) 24:385–94. doi: 10.1111/jch.14425
24. Hoshide S, Kario K, Chia YC, Siddique S, Buranakitjaroen P, Tsoi K, et al. Characteristics of hypertension in obstructive sleep apnea: An Asian experience. J Clin hypertension (Greenwich Conn). (2021) 23:489–95. doi: 10.1111/jch.14184
25. Altay S, Fırat S, Peker Y, The Turcosact C. A narrative review of the association of obstructive sleep apnea with hypertension: how to treat both when they coexist? J Clin Med. (2023) 12:4144. doi: 10.3390/jcm12124144
26. Gürün Kaya A, Gülbay B, Acıcan T. Clinical and polysomnographic features of hypertension in obstructive sleep apnea: A single-center cross-sectional study. Anatolian J Cardiol. (2020) 23:334–41. doi: 10.14744/AnatolJCardiol.2020.71429
27. Mansukhani MP, Covassin N, Somers VK. Apneic sleep, insufficient sleep, and hypertension. Hypertension (Dallas Tex 1979). (2019) 73:744–56. doi: 10.1161/hypertensionaha.118.11780
28. Sasaki N, Ozono R, Yamauchi R, Teramen K, Edahiro Y, Ishii K, et al. The relationship between morning hypertension and sleep quality in patients with obstructive sleep apnea syndrome. Clin Exp hypertension (New York NY 1993). (2013) 35:250–6. doi: 10.3109/10641963.2013.780069
29. Sapiña-Beltrán E, Torres G, Benitez I, Fortuna-Gutiérrez AM, Márquez PP, Masa JF, et al. Prevalence, characteristics, and association of obstructive sleep apnea with blood pressure control in patients with resistant hypertension. Ann Am Thorac Soc. (2019) 16:1414–21. doi: 10.1513/AnnalsATS.201901-053OC
30. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. (2004) 27:1255–73. doi: 10.1093/sleep/27.7.1255
31. Léger D, Stepnowsky C. The economic and societal burden of excessive daytime sleepiness in patients with obstructive sleep apnea. Sleep Med Rev. (2020) 51:101275. doi: 10.1016/j.smrv.2020.101275
32. Puretić H, Bosnar Puretić M, Pavliša G, Jakopović M. Revisiting the Epworth sleepiness scale : Is excessive daytime sleepiness still a valid screening tool for obstructive sleep apnea in a population at risk? Wiener klinische Wochenschrift. (2023). doi: 10.1007/s00508-023-02213-4
33. Zhang S, Meng Z, Zhang X, Huang M, Xu J. The rate of decrease in oxygen desaturation during severe obstructive sleep apnea syndrome is correlated with subjective excessive daytime sleepiness. Sleep breathing = Schlaf Atmung. (2021) 25:1285–91. doi: 10.1007/s11325-020-02223-w
34. Kapur VK, Resnick HE, Gottlieb DJ. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. (2008) 31:1127–32. doi: 10.5665/sleep/31.8.1127
35. Feng J, He QY, Zhang XL, Chen BY. Epworth Sleepiness Scale may be an indicator for blood pressure profile and prevalence of coronary artery disease and cerebrovascular disease in patients with obstructive sleep apnea. Sleep breathing = Schlaf Atmung. (2012) 16:31–40. doi: 10.1007/s11325-011-0481-5
36. Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. (2006) 27:1229–35. doi: 10.1183/09031936.06.00062805
37. Martynowicz H, Skomro R, Gać P, Mazur G, Porębska I, Bryłka A, et al. The influence of hypertension on daytime sleepiness in obstructive sleep apnea. J Am Soc Hypertension JASH. (2017) 11:295–302. doi: 10.1016/j.jash.2017.03.004
38. van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourology urodynamics. (2002) 21:179–83. doi: 10.1002/nau.10053
39. Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J obstetrics gynecology. (2006) 194:1399–403. doi: 10.1016/j.ajog.2006.01.048
40. Arslan B, Gezmis CT, Çetin B, Gönültas S, Gökmen E, Gürkan O, et al. Is obstructive sleep apnea syndrome related to nocturia? Lower urinary tract symptoms. (2019) 11:139–42. doi: 10.1111/luts.12250
41. Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. (2010) 7:677–85. doi: 10.1038/nrcardio.2010.145
42. Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev. (2003) 7:403–11. doi: 10.1053/smrv.2002.0273
43. Destors M, Tamisier R, Sapene M, Grillet Y, Baguet JP, Richard P, et al. Nocturia is an independent predictive factor of prevalent hypertension in obstructive sleep apnea patients. Sleep Med. (2015) 16:652–8. doi: 10.1016/j.sleep.2014.10.019
44. Rahman SN, Cao DJ, Monaghan TF, Flores VX, Vaysblat M, Moy MW, et al. Phenotyping the association between nocturia and hypertension: A systematic review and meta-analysis. J Urol. (2021) 205:1577–83. doi: 10.1097/ju.0000000000001433
45. Promi T, Tologonova G, Roberts MC, Tena M, Dhuper S, Bamgbola O, et al. Nocturia and blood pressure elevation in adolescents. J Community Health. (2023). doi: 10.1007/s10900-023-01307-4
46. Chen J, Liu Z, Yang L, Zhou J, Ma K, Peng Z, et al. Relationship between nocturia and hypertension: findings from the NHANES 2005-2016. Front Cardiovasc Med. (2023) 10:1165092. doi: 10.3389/fcvm.2023.1165092
47. Mekki P, Monaghan TF, Lee L, Agudelo CW, Gong F, George CD, et al. Nocturia and electrocardiographic abnormalities among patients at an inner-city cardiology clinic. Neurourology urodynamics. (2021) 40:509–14. doi: 10.1002/nau.24590
48. Lombardo R, Tubaro A, Burkhard F. Nocturia: the complex role of the heart, kidneys, and bladder. Eur Urol Focus. (2020) 6:534–6. doi: 10.1016/j.euf.2019.07.007
49. Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. (2009) 179:235–40. doi: 10.1164/rccm.200809-1392OC
50. Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. (2000) 43:533–49. doi: 10.1007/s001250051341
51. Herzog N, Jauch-Chara K, Hyzy F, Richter A, Friedrich A, Benedict C, et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. (2013) 38:2075–82. doi: 10.1016/j.psyneuen.2013.03.018
52. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: A state of the art review. Chest. (2017) 152:1070–86. doi: 10.1016/j.chest.2017.05.009
53. Kamalumpundi V, Shams E, Tucker C, Cheng L, Peterson J, Thangavel S, et al. Mechanisms and pharmacotherapy of hypertension associated with type 2 diabetes. Biochem Pharmacol. (2022) 206:115304. doi: 10.1016/j.bcp.2022.115304
54. Tian X, Zuo Y, Chen S, Zhang Y, Zhang X, Xu Q, et al. Hypertension, arterial stiffness, and diabetes: A prospective cohort study. Hypertension (Dallas Tex 1979). (2022) 79:1487–96. doi: 10.1161/hypertensionaha.122.19256
55. Luo Q, Li N, Zhu Q, Yao X, Wang M, Heizhati M, et al. Non-dipping blood pressure pattern is associated with higher risk of new-onset diabetes in hypertensive patients with obstructive sleep apnea: UROSAH data. Front Endocrinol. (2023) 14:1083179. doi: 10.3389/fendo.2023.1083179
56. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. (2017) 69:841–58. doi: 10.1016/j.jacc.2016.11.069
57. Jones A, Vennelle M, Connell M, McKillop G, Newby DE, Douglas NJ, et al. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. (2013) 14:428–32. doi: 10.1016/j.sleep.2013.01.001
58. Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. (2009) 64:631–6. doi: 10.1136/thx.2008.105577
59. McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. (2007) 29:156–78. doi: 10.1183/09031936.00027406
60. Mone P, Kansakar U, Varzideh F, Boccalone E, Lombardi A, Pansini A, et al. Epidemiology of obstructive sleep apnea: What is the contribution of hypertension and arterial stiffness? J Clin hypertension (Greenwich Conn). (2022) 24:395–7. doi: 10.1111/jch.14426
61. Drager LF, Polotsky VY, O'Donnell CP, Cravo SL, Lorenzi-Filho G, MaChado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circulatory Physiol. (2015) 309:H1101–1111. doi: 10.1152/ajpheart.00094.2015
62. Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin sleep Med JCSM Off Publ Am Acad Sleep Med. (2010) 6:131–7. doi: 10.5664/jcsm.27760
63. Peker Y, Akdeniz B, Altay S, Balcan B, Başaran Ö, Baysal E, et al. Obstructive sleep apnea and cardiovascular disease: Where do we stand? Anatolian J Cardiol. (2023) 27:375–89. doi: 10.14744/AnatolJCardiol.2023.3307
64. Yen FS, Wei JC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J Trans Med. (2022) 20:9. doi: 10.1186/s12967-021-03217-2
65. Gourishetti SC, Taylor R, Isaiah A. Stratifying the risk of cardiovascular disease in obstructive sleep apnea using machine learning. Laryngoscope. (2022) 132:234–41. doi: 10.1002/lary.29852
66. Benbir G, Karadeniz D. A pilot study of the effects of non-invasive mechanical ventilation on the prognosis of ischemic cerebrovascular events in patients with obstructive sleep apnea syndrome. Neurological Sci Off J Ital Neurological Soc Ital Soc Clin Neurophysiol. (2012) 33:811–8. doi: 10.1007/s10072-011-0835-6
Keywords: obstructive sleep apnea, hypertension, daytime sleepiness, nocturia, comorbidity
Citation: Tao M, Dong X, Tu J, Fang Q and Shao C (2024) Symptom and comorbidity burden in hypertensive patients with obstructive sleep apnea. Front. Endocrinol. 15:1361466. doi: 10.3389/fendo.2024.1361466
Received: 05 January 2024; Accepted: 19 February 2024;
Published: 04 March 2024.
Edited by:
Pasquale Mone, University of Molise, ItalyCopyright © 2024 Tao, Dong, Tu, Fang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Shao, c2hhb2NodWFuMTk4NEBzaW5hLmNu
†ORCID: Chuan Shao, orcid.org/0000-0001-9073-219X
 MengShi Tao
MengShi Tao Xiaoqi Dong
Xiaoqi Dong Jinjing Tu
Jinjing Tu Qing Fang
Qing Fang Chuan Shao
Chuan Shao