- 1Endocrinology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
- 3Department of Pediatrics, Cystic Fibrosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Center for Preclinical Research, Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico, Milan, Italy
- 5Hepatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Introduction: One of the most common complications of cirrhosis is diabetes, which prevalence is strictly related to severity of hepatopathy. Actually, there are no data on the persistence of post-transplant glucose abnormalities and on a potential impact of diabetes on development of fibrosis in the transplanted liver. To this aim, we evaluated liver fibrosis in cirrhotic subjects before and after being transplanted.
Methods: The study included 111 individuals who had liver transplantation. The assessment was performed before and two years after surgery to investigate a potential impact of the persistence of diabetes on developing de novo fibrosis in the transplanted liver. The degree of fibrosis was assessed using the Fibrosis Index Based on 4 Factors (FIB-4) and the Aspartate to Platelet Ratio Index (APRI).
Results: At pre-transplant evaluation, 63 out of 111 (56.8%) subjects were diabetic. Diabetic subjects had higher FIB-4 (Geometric mean, 95% confidence interval: 9.74, 8.32-11.41 vs 5.93, 4.71-7.46, P<0.001) and APRI (2.04, 1.69-2.47 vs 1.18, 0.90-1.55, P<0.001) compared to non-diabetic subjects. Two years after transplantation, 39 out of 111 (35.1%) subjects remained with diabetes and continued to show significantly higher FIB-4 (3.14, 2.57-3.82 vs 1.87, 1.55-2.27, P<0.001) and APRI (0.52, 0.39-0.69 vs 0.26, 0.21-0.32, P<0.001) compared to subjects without diabetes.
Discussion: Thus, persistence of diabetes after surgery is a possible risk factor for an evolution to fibrosis in the transplanted liver, potentially leading to worsened long-term outcomes in this population.
1 Introduction
Actually, liver biopsy is considered the gold standard to assess liver fibrosis (1, 2). However, the widespread use of this procedure to determinate the degree of liver fibrosis in everyday practice is hardly feasible for several reasons. The procedure is costly and invasive, causing discomfort, pain and potential serious complications, as bleeding and, although rare, even death (3–6). Moreover, a considerable variability in sampling and in the histopatological interpretation has been reported, leading to possible underestimation of the stage of fibrosis (7, 8).
Transient elastography (FibroScan) has been proposed by the “European Association for the Study of the Liver” (EASL) and the “American Association for the Study of Liver Diseases” (AASLD) for the assessment of hepatic fibrosis in individuals with non-alcoholic fatty liver disease (NAFLD). Therefore, FibroScan is currently the most widely used and validated alternative to liver biopsy (9–12). Its value lies in its relatively inexpensive cost and portability, but this method can be considerably limited by obesity (11–13) and it is rarely available in the context of a diabetes outpatient visit.
Apart from FibroScan, over the past decade, other potential less-invasive techniques have been proposed for the evaluation of hepatic fibrosis, and their concordance with liver biopsy results has been demonstrated in different populations, especially in people with viral hepatitis and NAFLD. The most widely used are the “aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio” (13), the “age-platelet index (14), the aspartate aminotransferase to platelet ratio index” (APRI) (15) and the “Fibrosis Index Based on 4 Factors” (FIB-4) (16). In the “Edinburgh type 2 diabetes study”, Morling JR et al. demonstrated that the APRI and FIB-4 had the best positive agreement in detecting the presence of liver fibrosis in individuals with type 2 diabetes mellitus (17). Moreover, Ciardullo S et al. recently validated the use of non-invasive scores (in particular age-adjusted FIB-4) among a wide population of individuals with diabetes to characterize subjects at risk for fibrosis, making referrals to hepatologist more sustainable (18). Again, Ciardullo S et al. showed also that the screening for hepatopathy in a population of individuals with diabetes utilizing a combination of imaging-based techniques and serum-based indexes could reduce the need for hepatic biopsy (19). Finally, Kitajima T. et al. validated the FIB-4 for assessment of fibrosis in subjects who have undergone liver transplantation (20).
Although elastography overcomes the surrogate indexes for identify people at risk for fibrosis, serum markers have greater feasibility, being they are simpler, more reproducible and accessible with good reliability (9).
Type 2 diabetes mellitus is a very common condition in people with hepatopathy, and the relationship between these two conditions is bidirectional (21, 22): the contribution of cirrhosis to development of alterations in glucose metabolism has been widely demonstrated; conversely, diabetes can accelerate the progression to severe hepatopathy (23). Today, the real contribution of diabetes in developing and worsening liver disease is still debated (24), but a plenty of literature is available on the strong bond between diabetes, insulin resistance, plasma glucose and hepatic fibrosis in individuals with hepatopathy, and these evidences come primarily from HCV-infected subjects (25–31).
Currently there is no exhaustive evidence on the influence of diabetes on hepatic fibrosis progression after transplantation. For this reason, we calculated APRI and FIB-4 in people with cirrhosis referring to our Diabetology Unit, who have undergone liver transplantation, to assess the impact of diabetes on hepatic fibrosis progression before and after liver transplantation.
2 Research design and methods
2.1 Study design
We conducted an observational, prospective study aimed at assessing the relationship between diabetes and liver fibrosis in individuals with cirrhosis who underwent liver transplantation. The study complies with the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of the IRCCS Cà Granda – Ospedale Maggiore Policlinico Foundation (Prot. n. 516) and written informed consent was provided by each participant.
2.2 Patient population
From January 2014 to December 2018, 187 consecutive subjects with liver cirrhosis, who were candidates to liver Tx, were evaluated at our Endocrinology Unit. Of them, 111 individuals underwent transplantation (sex, according to SAGER guidelines – 32: 81 males/30 females), completed a 2-year follow-up and were included in this analysis.
2.3 Measurements
At enrolment, a complete medical history was collected for each patient. Before and two years after transplantation all individuals had an anthropometric assessment and clinical parameters were recorded. Furthermore, all patients had a fasting blood sample to evaluate glycaemic control and hepatic function. Both at enrolment and 24 months after surgery, subjects underwent a 75 g OGTT to diagnose diabetes according to the American Diabetes Association criteria (32).
Based on body mass index (BMI) values, they were classified as underweight (<18 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2) or obese (≥30 kg/m2).
2.4 Calculation of liver fibrosis indices
APRI was calculated as AST/(upper limit of the normal range) x 100/platelet counts (PLT) (109/L) (15),. FIB-4 was calculated as age (years) x AST (IU/L)/(PLT [109/L] x ALT [IU/L]1/2) (16),.
2.5 Statistical analysis
Categorical variables were summarized as frequencies and percentages, whereas continuous variables as median (25th-75th percentile). Differences in baseline characteristics between people without or with diabetes were compared using the Fisher’s exact test, the Chi-square test or the Wilcoxon rank sum test according to the type of variable and frequency count.
The prevalence of diabetes was calculated by dividing the number of diagnosed subjects by the total number of enrolled people, and 95% confidence intervals were calculated using the binomial distribution.
To account for the positive skewness in the data and to reduce the influence of outliers, data on liver fibrosis markers were summarized using the geometric mean and corresponding 95% confidence interval.
Linear mixed-effects models with random intercept were used to evaluate the effects of diabetes status (as time varying covariate) and timing of measurement (pre vs post transplantation) on the FIB-4 and APRI. The models included the indices of liver fibrosis as response variables, main effects for diabetes status and time, a diabetes-by-time interaction term and age at measurement as covariate. Response variables were included in the models as natural log transformed variables. Model-based means were then back-transformed, to geometric means in the original scale. Beta coefficients were exponentiated to represent ratios of geometric means. Metabolic risk factors including changes in weight and BMI as well as fasting glycaemia, HbA1c, serum creatinine, total cholesterol, LDL, HDL and triglycerides were compared between patients who had diabetes post transplantation and those who did not using the Wilcoxon rank sum test, with P values adjusted for multiple testing. Statistical significance was determined by P values < 0.05.
3 Results
The study included 111 individuals. Based on the results of the OGTT performed before transplantation, subjects were classified into glucose tolerance categories as follows: 63 as with diabetes, 23 with impaired glucose tolerance (IGT), four with impaired fasting glucose (IFG), two patients with both IFG and IGT, and 19 with normal glucose tolerance (NGT). The prevalence of diabetes among these subjects was 56.8% (95% CI: 47.0-66.1).
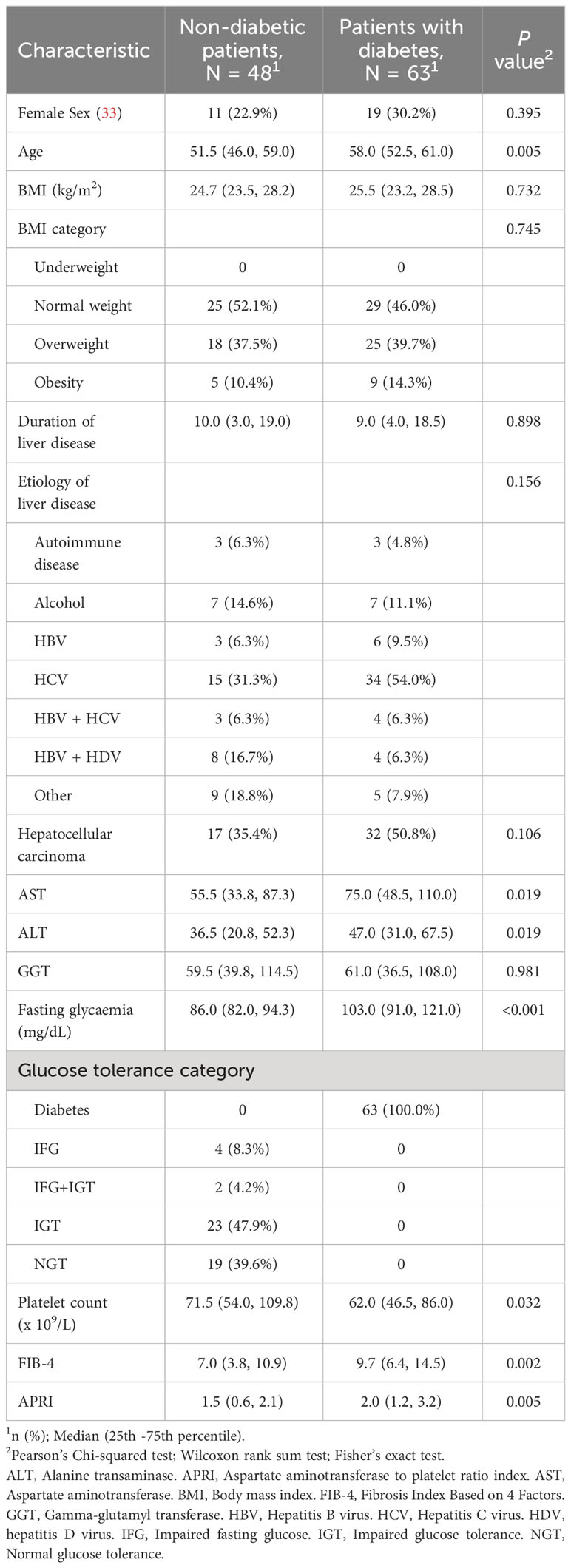
Table 1 shows a comparison of baseline characteristics between the 48 people without diabetes and the 63 ones with diabetes. subjects with diabetes were older (median age 58 vs 51.5), had higher levels of AST, ALT as well as higher values of FIB-4 and APRI. Platelet count was lower in people with diabetes as compared to individuals without diabetes. BMI was not significantly different between groups. Around half of the subjects were overweight or obese with no statistically significant differences between groups. In approximately 75% of the cases, viral hepatitis was identified as the primary cause of cirrhosis with no significant differences between the two subpopulations. Liver disease duration were comparable between groups.
At the two-year follow-up visit, 41 individuals had an OGTT indicative of diabetes resulting in a prevalence of 36.9% (95% CI: 28.0-46.6). Additionally, 12 subjects were classified as IGT, six as having IFG, 10 with both IFG and IGT and 42 as NGT. Out of the 48 who were non-diabetic prior to liver transplantation, three patients developed diabetes after transplantation.
Regarding to immunosuppressant therapy, subjects from both groups were placed on steroid therapy in the immediate post-surgery period and, after that, prednisone was gradually decreased (until suspended) and combined with calcineurin inhibitors (tacrolimus in most cases).
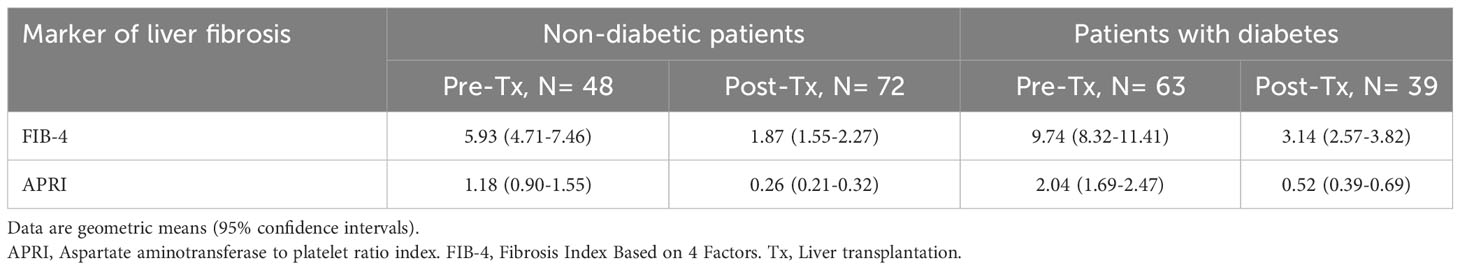
Table 2 summarizes the FIB-4 and the APRI values according to diabetes status and time from liver transplantation. Post-transplantation values were lower compared to those observed 2 years after liver transplantation. Having a diagnosis of diabetes is associated with higher FIB-4 and APRI values at both time points.
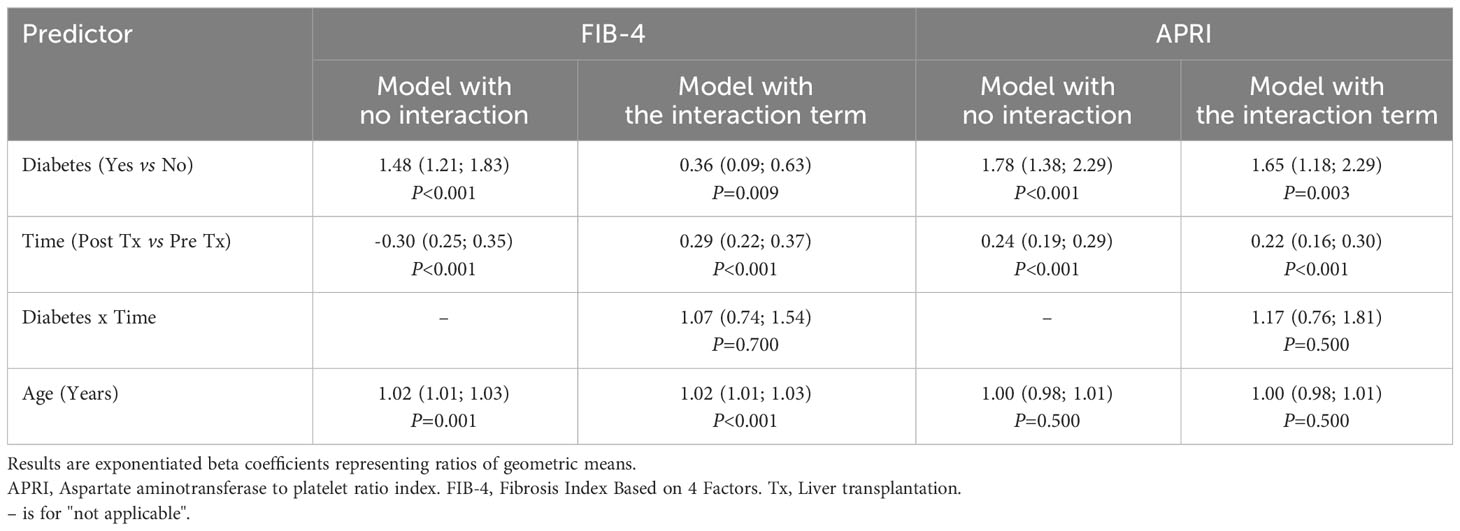
Table 3 shows the results of the regression models. Having diabetes was associated with a 48% (95% CI: 21-83) higher geometric mean for FIB-4 and 78% (38-129%) higher geometric mean for APRI as compared to not being diagnosed with diabetes. The geometric means observed after liver transplantation were 70% (95% 65-75) lower for FIB-4 and 66% (71–81) lower for APRI than those recorded before liver transplantation. The reduction was not significant different between non-diabetic and diabetic patients (P values for the interaction term: 0.70 for FIB-4 and 0.50 for APRI).
Figure 1 shows the model-based estimates of the geometric means of the two markers of liver fibrosis according to diabetes status and time from liver transplantation.
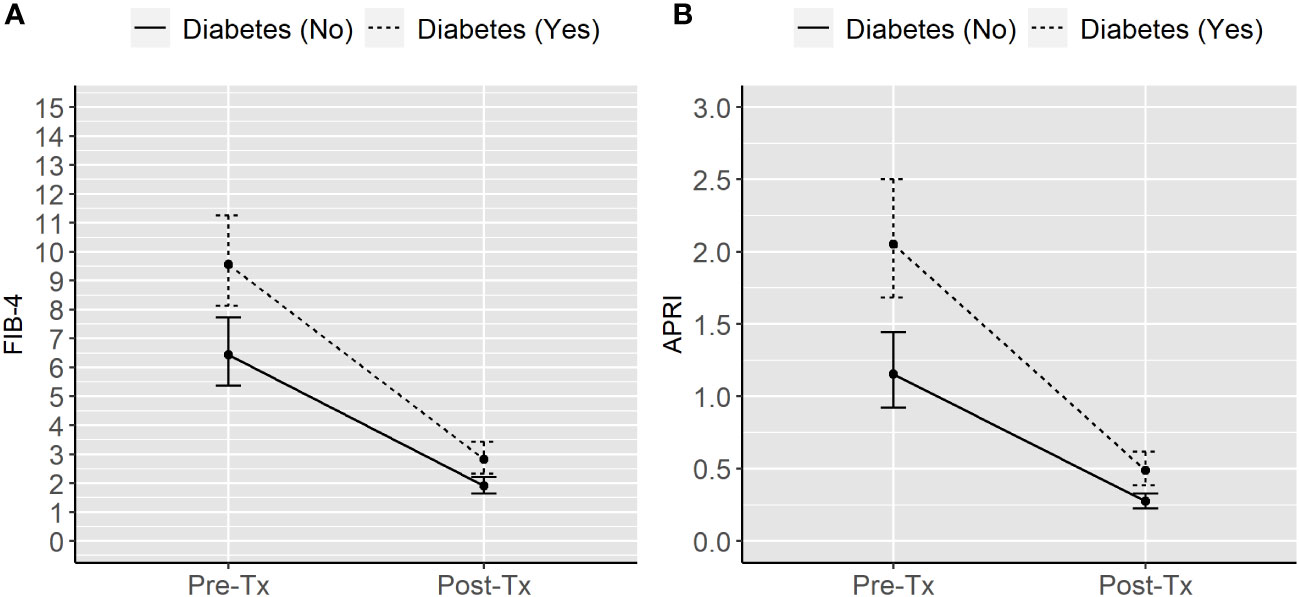
Figure 1 Model-based geometric means of FIB-4 (A) and APRI (B) in liver transplanted individuals according to diabetes status and time from transplantation. APRI, Aspartate aminotransferase to platelet ratio index. FIB-4, Fibrosis Index Based on 4 Factors. Tx, Liver transplantation.
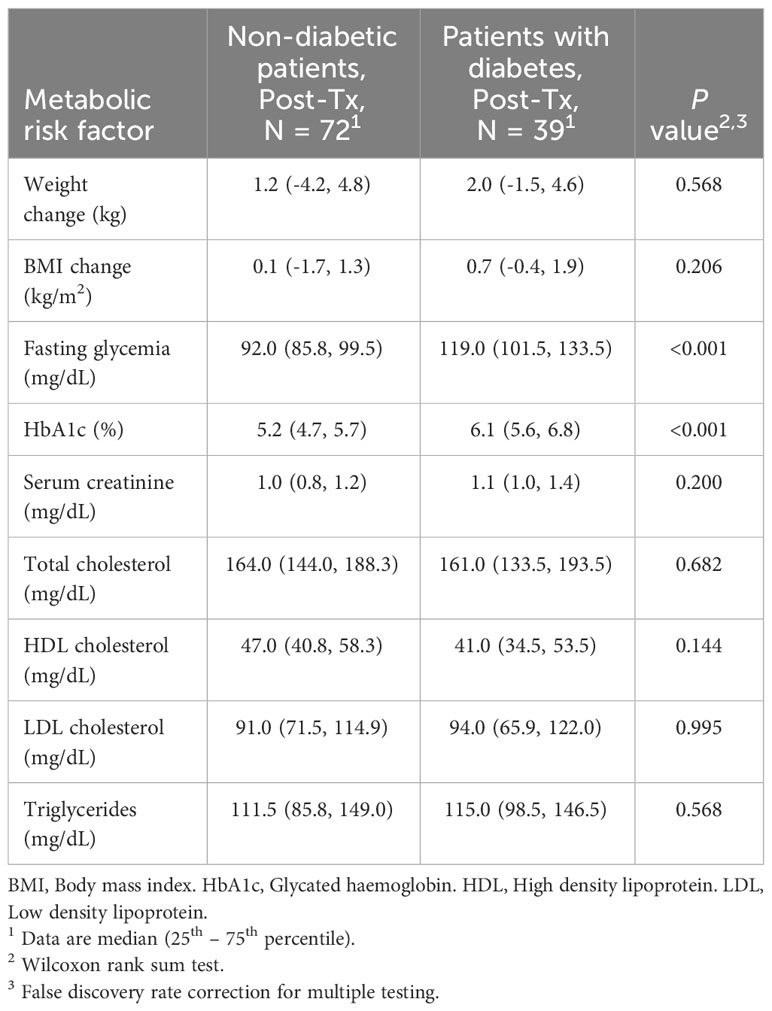
After liver transplantation, subjects with diabetes had higher fasting glycemia and HbA1c than non-diabetic individuals, while no significant differences were found for weight changes or other metabolic risk factors considered (Table 4).
4 Discussion
In our study, we recorded a high prevalence of diabetes in individuals with advanced hepatopathy who were candidates for liver transplantation and this condition was related to higher indices of liver fibrosis. Additionally, the study also found that after two years from liver transplantation the prevalence of diabetes remained elevated, with people with diabetes having a higher degree of liver fibrosis as compared to non-diabetic individuals.
The relationship between pre-transplant diabetes and a more advanced stage of fibrosis in subjects with hepatopathy has been previously demonstrated (34). However, the novelty of this study lies in the finding that subjects with diabetes continue to display elevated indicators of liver fibrosis two years after liver transplantation.
As mentioned before, diabetes is a condition frequently associated to liver cirrhosis. It is related to a worse outcome, due to increased mortality and more frequent complications of liver disease (22, 35, 36), although it’s not considered as a variable to assess the severity of liver disease in the most used staging and prognostic scores, as Child-Pugh and MELD.
Even after liver transplantation, the presence of glucose abnormalities is closely related to a worse prognosis, with higher risk of cardiovascular disease, liver rejection, infections and death (34, 37–39). Liver biopsy and FibroScan are actually the gold standard for assessing liver fibrosis, non-invasive methods as serum tests are gradually becoming more and more reproducible, available and accurate to detect liver fibrosis (9). In this context, FIB-4 and APRI have been demonstrated to be trustworthy as serum markers-based scores to assess liver fibrosis in subjects with hepatopathy from different aetiologies (15–17) and in liver-transplanted individuals (20, 40).
Activation of hepatic stellate cells has a crucial role in fibrosis development because of their extracellular matrix production during hepatic injury (41). Both genetic and environmental factors can impact on the pace of progression to cirrhosis. To date, the only established risk factor for developing new fibrosis after organ transplantation is the recurrence of the underlying hepatopathy such as viral hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis (42–45).
To our knowledge, there are no studies assessing the presence of other pre and post-transplantation risk factors for developing new fibrosis after liver transplantation. For this reason, we performed this simple and reproducible evaluation on a population of subjects with diabetes referring to our Diabetes Center, to evaluate if diabetes could worsen liver fibrosis before transplantation or could represent a further risk factor for developing new fibrosis after surgery.
The novelty of our research is the demonstration that diabetes could also represent a potential risk factor for developing new fibrosis, assessed with FIB-4, after surgery, although the underlying pathogenetic mechanisms are still to be completely clarified.
A limitation of this study is the possible presence of NAFLD in the transplanted organ, as a potential confounding factor in the assessment of liver fibrosis in the post-transplant evaluation. As well as the presence of NAFLD in the transplanted organ, several variables, as age of both donor and recipient, therapeutic schemes used for immunosuppression and concomitant viral infections, may negatively impact on a possible recurrence of fibrosis after surgery (46).
Moreover, data from literature report a prevalence of 20% of de novo NAFLD in liver transplanted individuals, mostly due to the significant weight gain and the developing of metabolic syndrome following surgery (47). Despite this we, couldn’t investigate the presence of insulin resistance, as fasting insulin levels being not available in this population for calculation of HOMA index.
Again, a recent meta-analysis, aimed to evaluate the accuracy of non-invasive indices and FibroScan in detecting de novo hepatic fibrosis after liver transplantation, demonstrated a better prediction of recurrent fibrosis by transient elastography, if compared to APRI and FIB-4 scores in liver-transplanted individuals (48, 49). APRI and FIB-4 have been also used as prognostic tools in people who had hepatic transplantation and their trend overtime has been related to several long-term outcomes, as death and liver rejection.
Heterogeneity of cut-offs used in the different studies is one of the most critical limits for these non-invasive biomarkers, which may affect their effective reliability in real-world practice (50).
For this, the gold standard for diagnosis and management of liver fibrosis remains liver biopsy.
Finally, we aim to confirm the evidences we found in this study on a wider population and in a longer follow up period.
In conclusion, individuals with diabetes need a closer follow-up in order to promptly recognize people to refer to a hepatology unit for elastography and, if the recurrence of new fibrosis is confirmed, to undertake the adequate therapeutic measures aimed at limiting its possible complications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato Etico Territoriale Lombardia 3. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. IC: Writing – original draft. GA: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. AG: Writing – original draft. SG: Writing – review & editing. MD: Writing – review & editing. EO: Supervision, Validation, Writing – review & editing. VR: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was (partially) supported by the Italian Ministry of Health (Ricerca Corrente 2023)”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bain VG, Bonacini M, Govindarajan S, Ma M, Sherman M, Gibas A, et al. A multicentre study of the usefulness of liver biopsy in hepatitis C. J ViralHepat. (2004) 11:375–82. doi: 10.1111/j.1365-2893.2004.00520.x
2. Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. (2002) 36:S152–60. doi: 10.1053/jhep.2002.36381
3. Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and wales: an audit by the British Society of Gastroenterology and the royal college of physicians of London. Gut. (1995) 36:437–41. doi: 10.1136/gut.36.3.437
4. Padia SA, Baker ME, Schaeffer CJ, Remer EM, Obuchowski NA, Winans C, et al. Safety and efficacy of sonographic-guided random real-time core needle biopsy of the liver. J Clin Ultrasound. (2009) 37:138–43. doi: 10.1002/jcu.20553
5. Myers RP, Fong A, Shaheen AAM. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. (2008) 28:705–12. doi: 10.1111/j.1478-3231.2008.01691.x
6. Piccinino F, Sagnelli E, Pasquale G, Giusti G, Battocchia A, Bernardi M, et al. Complications following percutaneous liver biopsy: a multicentre retrospective study on 68 276 biopsies. J Hepatol. (1986) 2:165–73. doi: 10.1016/s0168-8278(86)80075-7
7. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT. Sampling error and intra observer variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. (2002) 97:2614–18. doi: 10.1111/j.1572-0241.2002.06038.x
8. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. (2003) 38:1449–57. doi: 10.1016/j.hep.2003.09.022
9. Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. (2019) 13:361–74. doi: 10.1080/17474124.2019.1579641
10. Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY). (2017) 42:2037–53. doi: 10.1007/s00261-017-1211-7
11. Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and MR elastography for assessing liver fibrosis: part 1, principles and techniques. AJR. (2015) 205:22–32. doi: 10.2214/AJR.15.14552
12. Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology. (2015) 276:845–61. doi: 10.1148/radiol.2015150619
13. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. (1998) 93:44–8. doi: 10.1111/j.1572-0241.1998.044_c.x
14. Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. (1997) 4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x
15. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS. A simple non invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. doi: 10.1053/jhep.2003.50346
16. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J. Development of a simple non invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43:1317–25. doi: 10.1002/(ISSN)1527-3350
17. Morling JR, Fallowfield JA, Guha IN, Nee LD, Glancy S, Williamson RM, et al. Edinburgh type 2 diabetes study investigators. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the edinburgh type 2 diabetes study. J Hepatol. (2014) 60:384–91. doi: 10.1016/j.jhep.2013.10.017
18. Ciardullo S, Muraca E, Perra S, Bianconi E, Zerbini F, Oltolini A, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. (2020) 8:e000904. doi: 10.1136/bmjdrc-2019-000904
19. Ciardullo S, Sala I, Perseghin G. Screening strategies for nonalcoholic fatty liver disease in type 2 diabetes: Insights from NHANES 2005-2016. Diabetes Res Clin Pract. (2020) 167:108358. doi: 10.1016/j.diabres.2020.108358
20. Kitajima T, Kaido T, Hamaguchi Y, Yagi S, Taura K, Fujimoto Y, et al. Validation of the FIB-4 index for evaluation offibrosis inpatients with recurrent hepatitis C after living donor livertransplantation: A single center experience. Hepatol Res. (2016) 46:752–7. doi: 10.1111/hepr.12617
21. Grancini V, Trombetta M, Lunati ME, Boselli ML, Gatti S, Donato MF, et al. Central role of the β-cell in driving regression of diabetes after liver transplantation in cirrhotic patients. J Hepatol. (2019) 70:954–62. doi: 10.1016/j.jhep.2019.01.015
22. Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. (2009) 15:280–8. doi: 10.3748/wjg.15.280
23. Li X, Jiao Y, Xing Y, Gao P. Diabetes mellitus and risk of hepatic fibrosis/cirrhosis. BioMed Res Int. (2019) 2019:5308308. doi: 10.1155/2019/5308308
24. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. (2007) 30:734–43. doi: 10.2337/dc06-1539
25. Kita Y, Mizukoshi E, Takamura T, Sakurai M, Takata Y, Arai K. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metab - Clin Exp. (2007) 56:1682–8. doi: 10.1016/j.metabol.2007.07.011
26. Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. (2008) 103:1136–44. doi: 10.1111/j.1572-0241.2008.01813.x
27. Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology. (2003) 125:1695–704. doi: 10.1053/j.gastro.2003.08.032
28. Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: Implications for therapy. J Hepatol. (2003) 39:1042–8. doi: 10.1016/S0168-8278(03)00463-X
29. D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. (2005) 100:1509–15. doi: 10.1111/j.1572-0241.2005.41403.x
30. Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. (2005) 42:41–6. doi: 10.1016/j.jhep.2004.09.022
31. Cua IHY, Hui JM, Kench JG, George J. Genotype specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. (2008) 48:723–31. doi: 10.1002/hep.v48:3
32. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D. ADA Standards of Medical Care in Diabetes – 2023 Vol. 46. American Diabetes Association Diabetes Care (2023) p. S1–S284.
33. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. (2016) 1:2. doi: 10.1186/s41073-016-0007-6
34. Grancini V, Resi V, Palmieri E, Pugliese G, Orsi E. Management of diabetes mellitus in patients undergoing liver transplantation. Pharmacol Res. (2019) 141:556–73. doi: 10.1016/j.phrs.2019.01.042
35. Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. (2017) 37:950–62. doi: 10.1111/liv.13337
36. García-Compeán D, González-González JA, Lavalle-González FJ, González-Moreno EI, Villarreal-Pérez JZ, Maldonado-Garza HJ. Hepatogenous diabetes: is it a neglected condition in chronic liver disease? World J Gastroenterol. (2016) 22:2869–74. doi: 10.3748/wjg.v22.i10.2869
37. Lunati ME, Grancini V, Agnelli F, Gatti S, Masserini B, Zimbalatti D. Metabolic syndrome after liver transplantation: short-term prevalence and pre- and post-operative risk factors. Dig Liver Dis. (2013) 45:833–9. doi: 10.1016/j.dld.2013.03.009
38. Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. (2006) 82:603–11. doi: 10.1097/01.tp.0000235527.81917.fe
39. Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. (2006) 69:588–95. doi: 10.1038/sj.ki.5000116
40. Toniutto P, Fabris C, Bitetto D, Falleti E, Avellini C, Rossi E. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J Gastroenterol Hepatol. (2007) 22:1904–8. doi: 10.1111/j.1440-1746.2006.04628.x
41. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. (2011) 6:425–56. doi: 10.1146/annurev-pathol-011110-130246
42. Vasuri F, Malvi D, Gruppioni E, Grigioni WF, D’Errico-Grigioni A. Histopathological evaluation of recurrent hepatitis C after liver transplantation: a review. World J Gastroenterol. (2014) 20:2810–24. doi: 10.3748/wjg.v20.i11.2810
43. Neuberger J. Recurrent primary biliary cirrhosis. Liver Transpl. (2003) 9:539–46. doi: 10.1053/jlts.2003.50096
44. Jacob DA, Neumann UP, Bahra M, Klupp J, Puhl G, Neuhaus R. Long-term follow-up after recurrence of primary biliary cirrhosis after liver transplantation in 100 patients. Clin Transplant. (2006) 20:211–20. doi: 10.1111/j.1399-0012.2005.00471.x
45. Graziadei IW. Recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. (2002) 8:575–81. doi: 10.1053/jlts.2002.33952
46. Bosch W, Heckman MG, Pungpapong S, Diehl NN, Shalev JA, Hellinger WC. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation. (2012) 93:723–8. doi: 10.1097/TP.0b013e3182472876
47. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res 2020. (2020), 3920196. doi: 10.1155/2020/3920196
48. Azhie A, Sharma D, Sheth P, Qazi-Arisar FA, Zaya R, Naghibzadeh M, et al. A deep learning framework for personalised dynamic diagnosis of graft fibrosis after liver transplantation: a retrospective, single Canadian centre, longitudinal study. Lancet Digit Health. (2023) 5:e458–66. doi: 10.1016/S2589-7500(23)00068-7
49. Bhat M, Tazari M, Sebastiani G. Performance of transient elastography and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: A meta-analysis. PloS One. (2017) 12:e0185192. doi: 10.1371/journal.pone.0185192
Keywords: diabetes mellitus, liver transplantation, fibrosis, APRI score, FIB-4
Citation: Grancini V, Cogliati I, Alicandro G, Gaglio A, Gatti S, Donato MF, Orsi E and Resi V (2024) Assessment of hepatic fibrosis with non-invasive indices in subjects with diabetes before and after liver transplantation. Front. Endocrinol. 15:1359960. doi: 10.3389/fendo.2024.1359960
Received: 22 December 2023; Accepted: 21 February 2024;
Published: 05 March 2024.
Edited by:
Federico Bertuzzi, Niguarda Ca’ Granda Hospital, ItalyReviewed by:
Gianluca Perseghin, University of Milano-Bicocca, ItalyReynold Lopez-Soler, Loyola University Medical Center, United States
Copyright © 2024 Grancini, Cogliati, Alicandro, Gaglio, Gatti, Donato, Orsi and Resi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Grancini, dmFsZXJpYS5ncmFuY2luaUBwb2xpY2xpbmljby5taS5pdA==
 Valeria Grancini
Valeria Grancini Irene Cogliati
Irene Cogliati Gianfranco Alicandro
Gianfranco Alicandro Alessia Gaglio1
Alessia Gaglio1 Maria Francesca Donato
Maria Francesca Donato Veronica Resi
Veronica Resi