
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 19 April 2024
Sec. Bone Research
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1352671
Osteoarthritis is the most prevalent age-related degenerative joint disease and a leading cause of pain and disability in aged people. Its etiology is multifaceted, involving factors such as biomechanics, pro-inflammatory mediators, genetics, and metabolism. Beyond its evident impact on joint functionality and the erosion of patients’ quality of life, OA exhibits symbiotic relationships with various systemic diseases, giving rise to various complications. This review reveals OA’s extensive impact, encompassing osteoporosis, sarcopenia, cardiovascular diseases, diabetes mellitus, neurological disorders, mental health, and even cancer. Shared inflammatory processes, genetic factors, and lifestyle elements link OA to these systemic conditions. Consequently, recognizing these connections and addressing them offers opportunities to enhance patient care and reduce the burden of associated diseases, emphasizing the need for a holistic approach to managing OA and its complications.
Osteoarthritis (OA) is a chronic degenerative joint disease, impacting the entire joint (1). It arises from an imbalance between the repair and degradation of joint tissues. This leads to structural changes in the hyaline articular cartilage, subchondral bone, ligaments, capsule, synovium, and periarticular muscles (2, 3), such as degeneration and loss of articular cartilage, subchondral bone sclerosis, subchondral bone cyst, weakened and frayed tendons/ligaments/muscles, capsular fibrosis, synovial hyperplasia and inflammation of the synovitis membrane, as well as meniscus degeneration and infrapatellar fat pad fibrosis and inflammation (4, 5) (Figure 1). These alterations ultimately lead to joint failure (6). Structural damage in OA, referred to as structural OA, includes cartilage loss, osteophyte formation, subchondral bone changes, and meniscal alteration, etc, all of which can be visualized through MRI. Joint pain may accompany these alterations, indicating symptomatic OA (7). Being the most common joint disease and a leading cause of disability among older adults, OA affects approximately 7% of the global population, exceeding 500 million people worldwide, with women being disproportionately affected (8, 9). The prevalence of OA is expected to rise further, given the trends of an aging population and increasing rates of obesity, making it more widespread than in previous decades (10–13). Traditionally regarded as a localized joint disorder, emerging evidences have shed light on OA as a condition that exacerbates various comorbidities, underscoring its profound health implications (14). The association between OA and the subsequent development of diverse common conditions highlights an overarching burden that demands attention from the broader medical community. Therefore, there is an urgent need to thoroughly investigate the relationship between OA and its comorbidities. This review aims to offer a comprehensive perspective on OA management, emphasizing the holistic approach necessary to address both OA and its comorbidities effectively. By highlighting the importance of comorbidities in symptomatic OA individuals, we strive to contribute to the development of improved strategies for patient care, ultimately reducing the burden of related diseases. Figure 2 visually represents the intricate interplay of OA with various systemic complications discussed in the subsequent sections of this paper.
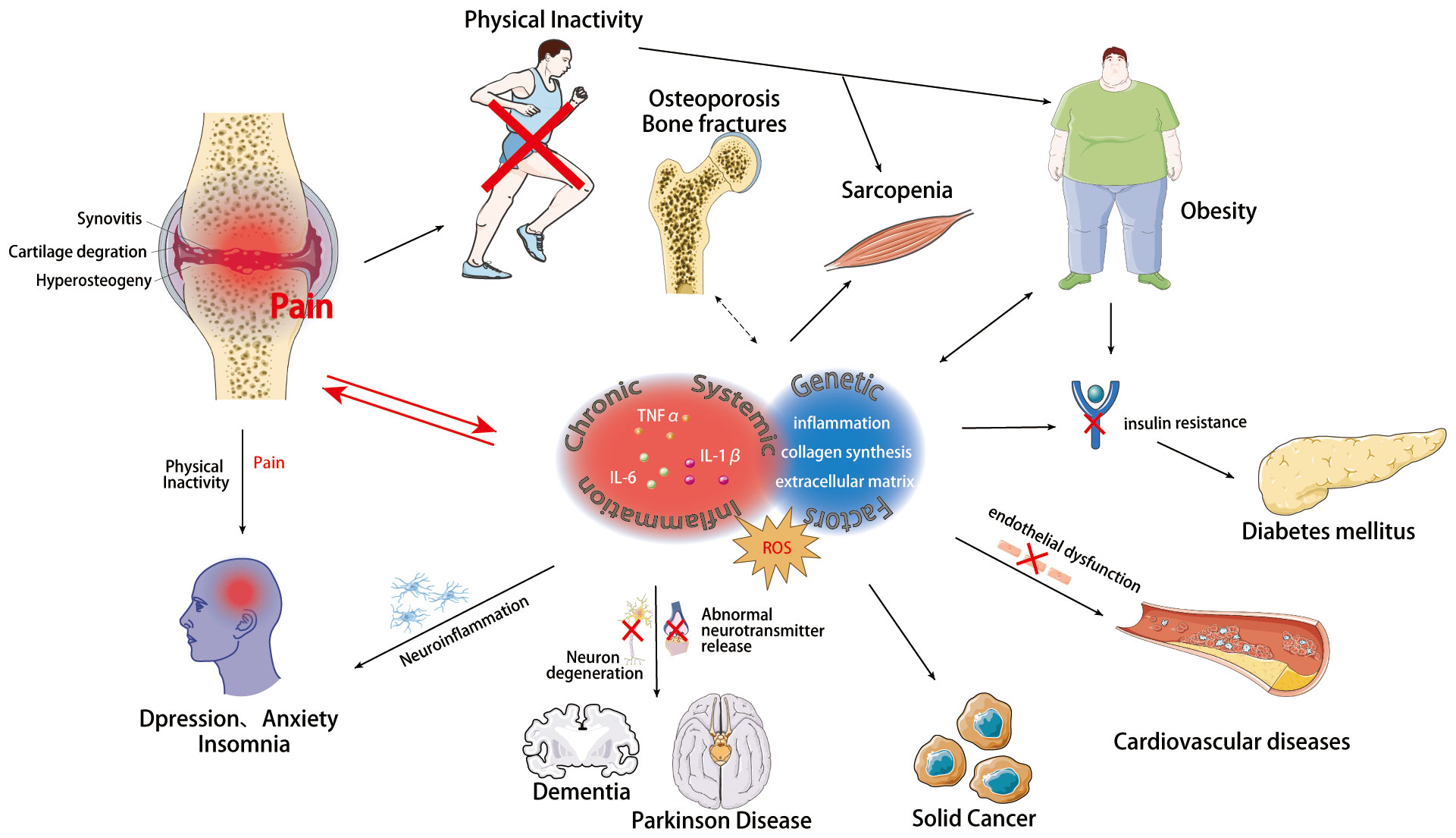
Figure 1 Chronic systemic inflammation, ROS, and genetic factors link osteoarthritis with multisystemic comorbidities mechanisms. Osteoarthritis, a chronic degenerative joint disease, involves structural changes in the hyaline articular cartilage, subchondral bone, ligaments, capsule, synovium, periarticular muscles, as well as meniscus degeneration and infrapatellar fat pad fibrosis and inflammation. There are intricate connections between OA and various systemic diseases, sharing common underlying mechanisms including Chronic Systemic Inflammation, ROS, and Genetic Influences. These mechanisms contribute to the pathogenesis of OA and serve as significant risk factors for multi-system comorbidities. Templates adapted to create this figure are freely available from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
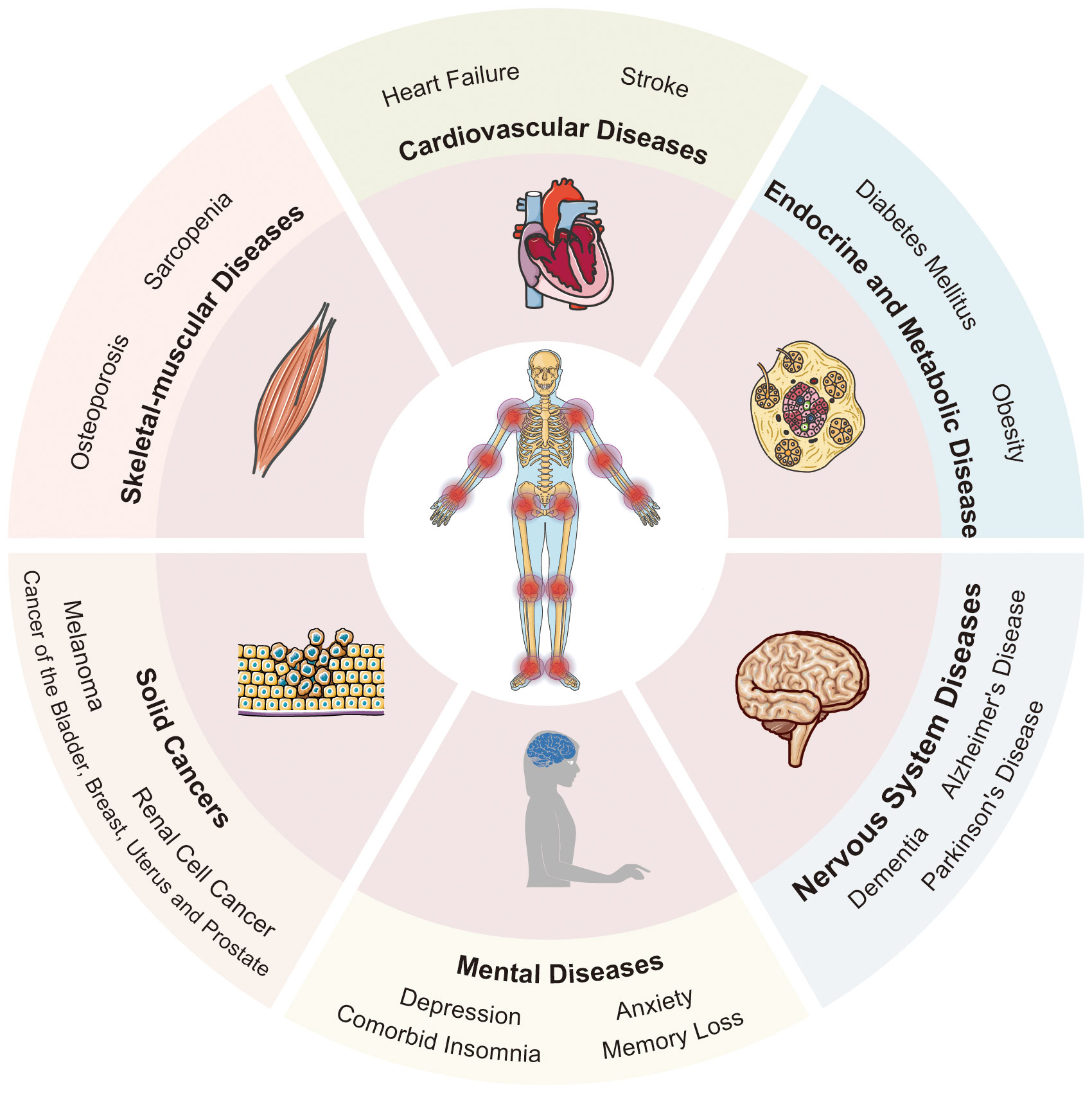
Figure 2 The multisystemic impact of osteoarthritis: a comprehensive overview. OA manifests symbiotic relationships with several systemic diseases, thereby giving rise to numerous comorbidities. These include skeletal-muscular diseases, cardiovascular diseases, endocrine and metabolic diseases, nervous system diseases, mental diseases, and solid cancers. Templates adapted to create this figure are freely available from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
For this review, an extensive search of the PubMed database and Google Scholar was conducted using the keywords “osteoarthritis” and “comorbidities”. Recent English-language original articles, meta-analyses, and systematic reviews focusing on prevalence or incidence and the risk ratio of OA comorbidity were included. Longitudinal studies were prioritized. Comorbidity was defined as any chronic condition coexisting with osteoarthritis, considering OA as the primary exposure, and outcomes were centered on the presence of comorbidities. We further screened for conditions closely linked to osteoarthritis and with a high prevalence, refining our search to systematically investigate the connections between OA and various health issues. This included exploring musculoskeletal, cardiovascular, endocrine-metabolic, and neurological diseases, such as osteoporosis, sarcopenia, cardiovascular diseases, obesity, diabetes mellitus, dementia, Parkinson’s disease, depression, insomnia, tumors, and more. Included studies were required to report relevant outcomes related to the prevalence, incidence, risk factors, pathophysiological mechanisms, clinical manifestations, or management strategies of comorbidities in individuals with osteoarthritis. Studies exclusively focusing on unrelated comorbidities, as well as those with insufficient data or inadequate sample sizes to draw meaningful conclusions, were excluded. Figures in this manuscript were made using Adobe Illustrator software.
OA is a chronic degenerative joint disease that primarily affects the hands, knees, hips, and spine. However, its impact extends beyond the joints, profoundly affecting other organ systems and exacerbating the morbidity and mortality associated with these conditions (15). Recent studies have shown that the presence and frequency of comorbidities such as CVDs, obesity, type 2 diabetes, and depression were associated with lower levels of physical activity in patients with OA (16–18). Kamps et al.’s (19) study in the Netherlands utilized a primary care database to explore the risk of general practitioner - diagnosed comorbidities following hip or knee OA diagnosis. After analyzing 58 prevalent comorbidities while adjusting for age and sex, they found individuals with knee and hip OA exhibited an elevated risk of being diagnosed with 30 and 26 different comorbidities, respectively. These findings confirm the strong association between OA and the subsequent development of various common conditions. Furthermore, these comorbidities further exacerbate the decline in patients’ quality of life and increase the burden on healthcare systems (20, 21). Therefore, understanding the relationship and underlying mechanisms between OA and its associated complications is crucial for effectively managing the disease and improving patient outcomes.
The onset of OA can be triggered by an array of factors, encompassing genetic predisposition, mechanical stress, and inflammatory processes (22). While a single pathogenic explanation for all OA cases remains elusive, genetic factors, sex disparities, age-related changes, and immunological processes collectively contribute to its development (22). Genetic investigations have identified multiple risk alleles distributed across the genome, underscoring the polygenic nature of OA (23). Epigenetic modifications, such as DNA methylation and histone affecting gene expression and tissue integrity (24), involving various factors like inflammatory mediators (IL-1β, IL-8), and stress factors associated with reactive oxygen species (SOD2, iNOS) (25, 26). Additionally, genetic alterations in key pathways like the TGF-β superfamily (27), Wnt/β-catenin (28), Notch (29), and Indian Hedgehog (Ihh) (30) have been implicated in osteoarthritis (OA) progression by disturbing the delicate balance between anabolic and catabolic processes in articular cartilage. These responses entail the upregulation of inflammatory mediators, leading to the degradation of the cartilage extracellular matrix (ECM) through increased expression of matrix metalloproteinases (MMPs) (31) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) (32). Mechanical factors, such as trauma, joint instability, and abnormal joint alignment, can also render individuals susceptible to the condition (33). OA emerges as a consequence of intricate interplays between local biomechanical factors and systemic susceptibility (34). Cartilage degradation and loss advance when the biomechanical stresses surpass the inherent resilience of the osteochondral structure (34). From a biomechanical perspective, injuries to cartilage and bone weaken their ability to withstand abnormal loads, leading to more severe structural damage (34, 35). Fractures and joint misalignments, such as varus and valgus deformities, result in incongruent joint lines and mechanical axis deviation, altering joint load distribution (36–38). Meniscal injuries lead to abnormal load transmission, increasing peak loads on joint cartilage and accelerating the progression of knee osteoarthritis, with meniscal protrusions also increasing local loading (39–41). Ligament laxity and muscle weakness result in joint instability, increasing shear forces on joint surfaces, leading to cartilage damage and the progression of osteoarthritis (42–44). Inflammation assumes a pivotal role in OA, with an increasing recognition of synovitis as a key feature. Inflammatory cytokines, notably IL-1β, are prominently involved in OA pathogenesis, with increased release observed from synovial fibroblasts in affected patients (45). This increase contributes to the development of an inflammatory pattern, including the elevation of TNF-α and IL-6 (46, 47). In general, the inflammatory condition not only exerts local effects but also leads to a pro-inflammatory state that can easily evolve into systemic inflammation. Disease progression involves the degradation of articular cartilage and subchondral bone, with a shift towards catabolic processes that favor tissue breakdown over repair mechanisms (48). Furthermore, the innate and adaptive immune systems have been implicated in OA, with immune cells, including lymphocytes, potentially contributing to the disease’s development (49, 50), although the exact mechanisms remain to be fully elucidated (Figure 1).
OA (OA) risk factors can be broadly divided into person-level and joint-level factors (51). Person-level factors include: (i) Age: increasing age is a prominent risk factor for OA, attributed to cumulative exposures and age-related changes in joint structures (52). (ii) Gender: women not only have a higher likelihood of developing OA but also tend to experience more severe cases (53). (iii) Obesity and metabolic syndrome: obesity, especially around the knee joint, is a recognized risk factor. Additionally, recent literature suggests an association between OA and metabolic syndrome (54). (iv) Genetic: genetic factors also play a role, with over 80 genes implicated in OA’s development, including those related to vitamin D receptors and bone health (55). (v) Diet: several dietary factors, including low levels of vitamins D, C, and K, are suspected contributors to OA development (56, 57). Joint-level factors encompass: (i) Injury: knee injuries, particularly anterior cruciate ligament (ACL) rupture, can lead to early-onset knee OA (13). The prevalence increases when associated with damage to cartilage, subchondral bone, and other structures (58, 59). Furthermore, prolonged joint damage can result in decreased joint function and disability, significantly impacting patients’ quality of life and social functioning. (ii) Abnormal loading of the joints: repetitive joint use is associated with OA development (51). Knee OA is linked to occupations requiring squatting and kneeling, hip OA is linked to prolonged lifting and standing (60), and hand OA is more frequent in people with occupations requiring increased manual dexterity (51).
Osteoporosis (OP) is a systemic disease characterized by low bone mass, microarchitectural deterioration and susceptible to fragility fractures (61). In a review of 40 studies, a prevalence of 21.7% of OP was reported among the elderly population worldwide (43), with expectations of further increases as the population continues to age. Both OA and OP were common and frequently-occurring diseases in the elderly, which seriously affect their quality of life (62). Does OA influence the occurrence and progression of OP or osteoporotic fractures? The verdict remains pending. A review of the literature suggests inversely relationship between OA and OP (63), or even perhaps a lack of direct correlation (64). However, when analyzed in individual bones, the bone mineral density (BMD) of the appendicular skeleton in OA-affected joints may decrease (65). An investigation conducted by M Güler-Yüksel revealed that progressive hand OA over a 2-year span is linked with accelerated metacarpal BMD loss (66). Furthermore, in a cohort encompassing 199 patients with hip and knee OA, the overall prevalence of OP at any site was found to be 23%, with an additional 43% of patients meeting osteopenic criteria according to World Health Organization standards (67). Interestingly, OP was more prevalent in the forearm (14%) compared to the lumbar spine (8.5%) and the proximal femur of the index side (8.2%) (67). Dongkeun Kim’s meta-analysis revealed intriguing insights, although the overall frequency of OP in OA participants remained similar, a notable exception surfaced in the lumbar spine, revealing a higher prevalence of OP in both men and women compared to matched controls (68). Therefore, it is conceivable that OA accompanies the onset of OP.
OA and OP are both bone disorders typically afflicting the elderly population, imposing significant burdens of morbidity and disability (69). Recent strides in the realm of osteoimmunology have shed light on a commonality: heightened bone loss occurs not only in OP but also in the early stages of OA (70). Moreover, inflammation has emerged as a shared risk factor for both OP and OA. Furthermore, an unfavorable body composition contributes to the genesis of these conditions (71, 72). However, the nature of this contribution differs: while a lower Body Mass Index escalates the risk of OP, obesity exacerbates OA development (73). This occurs through a dual mechanism: increased mechanical stress on weight-bearing joints and putative adverse effects stemming from specific adipose tissue-derived adipokines (74). Additionally, several factors influence the progression of both OA and OP, encompassing sex hormones, ethnicity, age, dietary components, genetic predispositions, and physical activity levels (75). Aging, in particular, serves as a predictive factor for radiographic OA, bone loss, OP, and susceptibility to fractures (76).
Sarcopenia, another age-related skeletal muscle disorder, entails the accelerated loss of muscle mass and function, which is associated with increased adverse outcomes, including falls, functional decline, frailty, and mortality (77). Previous research has suggested a complex, bidirectional relationship between muscle weakness and OA. According to Toda et al. (78), women in the early stages of knee OA often exhibit a decline in lower extremity lean body mass relative to their total body weight. Clinical evidence underscores the significance of quadriceps weakness in knee OA. Ikeda and colleagues (79) discovered that the quadriceps muscle cross-sectional area was, on average, 12% smaller in asymptomatic women with incident radiographic OA compared to age and body-mass-matched controls. Similarly, Quadriceps weakness has also been linked to the progression of knee OA, attributed to disuse atrophy due to the pain and disability common in knee OA (80).
A significant contributing factor is the pain and stiffness experienced in osteoarthritic joints, which often leads to reduced physical activity, even giving rise to sarcopenic obesity (81). Inflammatory cytokines play a pivotal role in OA. A direct correlation has been established between IL-6 plasma levels and sarcopenia (82). Inflammatory cytokines disrupt protein synthesis and degradation in muscles and articular cartilage, precipitating synovial inflammation, muscle loss, and cartilage damage. In a rat model of knee osteoarthritis (OA), heightened IL-1β expression was noted in the affected gastrocnemius muscle, coupled with a 10% reduction in cross-sectional area (83). Moreover, the inflammatory milieu of knee OA induces neural alterations, exacerbating muscle atrophy. Discovered by Cunha et al. (84)in a rat model, is the restructuring of the quadriceps neuromuscular junction associated with knee osteoarthritis, resulting in heightened expression of the atrophy protein MuRF-1 and accelerating muscle atrophy and strength decline. However, in other studies (85), no such association has been found. Perhaps, the multifactorial etiology of sarcopenia takes into account the possibility of an inflammatory role in its pathogenesis, while this connection has not yet been definitively demonstrated.
Cardiovascular diseases (CVDs), including coronary heart disease, hypertension, and stroke, stand as prominent contributors to global mortality, claiming over 17.6 million lives annually and projected to escalate to 23.6 million by 2030 (86). Notably, research has unveiled a significantly heightened prevalence of CVDs among individuals afflicted with OA, surpassing that observed in the general population. For instance, a comprehensive meta-analysis, encompassing data from 15 studies, further corroborated these findings, revealing a pooled prevalence of 38.4% for overall CVDs pathology in individuals with OA (87). A prospective longitudinal study shed light on the increased susceptibility of elderly men and adult women with OA to developing CVDs, particularly ischemic heart disease and congestive heart failure (88). Hypertension, a frequently encountered comorbidity in patients with OA, was found to increase the likelihood of developing hypertension by 13% in individuals with knee OA (89). Furthermore, a BMI-independent association between hypertension and radiographic knee OA was observed (90). Moreover, a Mendelian randomization study utilizing inverse variance weighted analysis demonstrated a significant impact of hip osteoarthritis(HOA) on the development of heart failure and stroke (91). Similarly, a recent study utilizing Mendelian Randomization in the UK Biobank unveiled a novel causal association between low systolic blood pressure (SBP) and the clinical diagnosis of OA (92).
Shared risk factors such as age, obesity, lack of physical activity, as well as inflammation and genetics, may contribute to the development of both OA and CVDs (93). Increasing evidence suggests that inflammation plays a role in the connection between OA and CVDs (94). Cytokines and inflammatory processes can contribute to the destruction of cartilage and endothelial cells, thereby promoting vascular inflammation and the development of atherosclerosis (95), which forms the basis of many CVDs. Furthermore, some evidence suggests that the association between OA and CVDs may be mediated by common genetic factors (96). Several genes have been identified to be associated with both conditions, including those involved in inflammation (97), lipid metabolism (98), and extracellular matrix (97). Additionally, it is worth noting that OA patients often require the use of nonsteroidal anti-inflammatory drugs(NSAIDs) and other medications, which may have adverse effects on the cardiovascular system (99). These findings emphasize the importance of comprehensive management of these conditions, including lifestyle modifications, pharmacological interventions, and regular monitoring of cardiovascular health in OA patients.
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (100). The global incidence of diabetes is predicted to reach 642 million adults by 2040 (101). Chronic hyperglycemia and insulin resistance have the potential to cause harm to chondrocytes and induce cellular apoptosis (102). Additionally, research indicates that OA may potentially induce the onset of DM. A more extensive population-based study reported a 30% prevalence of high blood glucose in OA patients, while non-OA individuals exhibited a 13% prevalence (103).These prior investigations predominantly focused on specific joint locations, such as the knee, hand, or unspecified OA-afflicted joints (104).Some longitudinal studies have assessed the risk of developing DM in OA patients. In a comprehensive 12-year follow-up study encompassing 19,089 OA patients and an equal-sized control cohort, OA emerged as a pivotal risk factor for DM onset, except for elderly males, even after meticulous adjustment for covariates such as obesity (105). Similarly, a large-scale, protracted tracking study averaging 5.16 years, substantiated the importance of knee and hip OA as significant predictive factors for DM events (106). Moreover, supplementary investigations propose a conceivable linkage between OA and DM, possibly associated with joint replacement surgery. In the most recent study, which employed baseline walking speed as a fundamental metric, the diabetes incidence rate was scrutinized among individuals with knee OA or those at risk of knee OA (107).This study underscored that during a 7-month follow-up period, the cumulative diabetes incidence rate surged to a remarkable 96%, accentuating that slowed walking speed potentially stands as a pivotal predictive indicator for diabetes events in knee OA patients and those at risk of knee OA.
Diabetes and OA exhibit a close molecular relationship. Elevated blood sugar levels, a hallmark of diabetes, play a pivotal role in triggering and activating various molecular pathways associated with the development of OA (108). This intricate process hinges on the expression of several byproducts of glucose metabolism, including advanced glycation end-products (AGEs), sorbitol, and diacylglycerol. The connection between diabetes and OA stems from the inflammatory cascade set in motion due to heightened production of pro-inflammatory cytokines. OA is often accompanied by a backdrop of chronic joint inflammation, initiating the release of various inflammatory mediators, including but not limited to tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) (109). These cytokines have the potential to directly or indirectly contribute to the development of insulin resistance, a pivotal characteristic of type 2 diabetes. The burden of pain and compromised joint function in OA may significantly curtail an individual’s physical activity levels, potentially resulting in weight gain and subsequent obesity, a well-established risk factor for DM (110). Numerous studies have substantiated the role of obesity and DM in promoting the onset and progression of OA. Obesity subjects joints to increased mechanical loading, thereby augmenting the pressure on joints and leading to damage and degeneration of joint cartilage, ultimately culminating in OA (111).It is plausible that obesity plays a crucial intermediary role, linking diabetes and OA, given their shared risk factors.
OA is not only intricately linked with DM, but evidence suggests that disrupted lipid metabolism may contribute to the pathogenesis of OA. A meta-analysis of cohort and cross-sectional investigations has exposed a notable heightened risk of OA in the presence of dyslipidemia, demonstrating a stark contrast with its absence (112). Additionally, a meta-analysis has identified a markedly elevated prevalence of dyslipidemia among OA sufferers, reaching up to 30%, significantly surpassing the 8.0% incidence found in the general population without OA. Furthermore, it demonstrated that the likelihood of dyslipidemia is significantly heightened in OA patients, with a risk ratio of 1.98 (113). Epidemiological evidence indicates a correlation between hypercholesterolemia and OA, implicating cholesterol as a systemic risk factor (114). Experimental studies using ApoE-knockout mice and diet-induced hypercholesterolemic rats have demonstrated that elevated cholesterol levels can induce OA-like pathologies, characterized by cartilage degradation, osteophyte development, and alterations in subchondral bone structure (115). Furthermore, increased circulating levels of cholesterol and triglycerides, coupled with dysfunctional high-density lipoprotein (HDL), have been associated with exacerbated joint pathology and cartilage loss in knee OA, mediated by synovial inflammation, ectopic bone formation, and an increased prevalence of bone marrow lesions, which are significant sources of pain in OA patients (116, 117).
Additionally, emerging research suggests a potential link between OA and other endocrine disorders, including hypothyroidism and hyperthyroidism. Studies have highlighted a correlation between these thyroid conditions and the onset of knee pain, as well as the development of knee OA (118). Notably, patients with hypothyroidism exhibit thinner femoral cartilage at all measured sites compared to healthy individuals, and are at a higher risk for OA (119). Intriguingly, meta-analytical reviews have revealed a more extensive connection, indicating that the presence of autoimmune thyroid diseases (AITD) not only elevates the risk of clinically significant thyroid diseases but also correlates with a higher prevalence of OA, rheumatoid arthritis (RA), and other musculoskeletal conditions (119, 120). Thyroid hormones play a crucial role in the development, growth, and maintenance of bone throughout life, but new research also suggests a potential significance of thyroid hormones in the articular cartilage lining of joint surfaces (121–123). Therefore, the relationship and mechanisms between thyroid hormones and osteoarthritis warrant further investigation.
The nervous system serves as the primary system for sensing and transmitting pain signals throughout the body. Recent research has indicated a higher prevalence of neuropathic pain (NP) in individuals with OA (124, 125), where OA may lead to stimulation or damage of nerve endings located within or surrounding the joints, resulting in persistent or intermittent pain (126). These pain-related manifestations could potentially prompt changes in neural excitability, alterations in neurotransmitter release, and reduced neural plasticity (127). These alterations, in turn, may pave the way for central sensitization, wherein pain is perceived or exacerbated even in the absence of external stimuli (128).
Furthermore, emerging evidence suggests that OA may exert an influence on degenerative diseases within the central nervous system (129). Dementia is a progressive neurodegenerative brain disorder, leading to cognitive impairment, disability, and death in the elderly population, standing out as one of the major causes of mortality in individuals afflicted with knee and hip OA (130). A recent nation-wide study conducted in Taiwan has unveiled a noteworthy association between OA and an increased risk of dementia (131). Individuals with OA showed a significantly elevated likelihood of being diagnosed with incident Alzheimer’s disease(AD) and related dementias following meticulous adjustments for various factors (18). Supporting these epidemiological findings, animal studies reported that OA may accelerate the progression and exacerbation of dementia by instigating peripheral inflammation and neuroinflammation (132). Parkinson’s disease (PD), the second most common neurodegenerative disorder, stems from dopaminergic neuron degeneration, leading to motor impairments and non-motor symptoms (133). Cohort studies in Danish (134) and Taiwanese (135) populations have linked OA with an increased risk of developing PD. A retrospective cohort study involving 260,224 UK patients revealed a significantly higher incidence of PD in those with OA, supported by adjusted Cox regression analyses showing a 1.82-fold increased risk (136).
The exact mechanisms responsible for the observed connection between OA and nervous system diseases remain partially elucidated. However, OA appears to contribute to cognitive decline and the development of dementia and PD through multiple pathways. The chronic pain associated with OA can significantly impact neurocognitive function (127), potentially leading to cognitive impairment. Moreover, both systemic and peripheral inflammation likely play a role in this relationship. Elevated levels of systemic markers such as Hs-CRP, IL-6, and other proinflammatory mediators have been implicated in the progression of both OA and cognitive impairment (137). Shared genes related to inflammation, cartilage homeostasis, and neuronal function further substantiate the intricate links between OA and neurodegenerative diseases (138). Concerning the connection between OA and PD, inflammation appears to be a significant risk factor contributing to the etiopathogenesis of both conditions (139, 140). Another shared risk factor for OA and PD is vitamin D deficiency, often a consequence of limited sun exposure and prevalent worldwide (141). Additionally, the association between OA and PD may involve various mediating factors, including physical inactivity (142) (such as failing to meet recommended physical activity levels) and depression (143).
OA exerts a potentially profound impact on mental health, a relationship elucidated by insightful epidemiological investigations (144). OA patients exhibit a prominent elevation in depression and anxiety prevalence, with a comprehensive meta-analysis of 49 studies divulging a pooled prevalence of 19.9% for depressive symptoms and 21.3% for anxiety symptoms among those with OA (145). Longitudinal data reinforces this, demonstrating that individuals with multi-site, hip, or knee OA exhibit greater odds of developing depressive symptoms compared to those without OA (113). Concomitantly, OA is linked to heightened likelihood of suicidal ideation underscoring its mental health implications (146). Moreover, individuals with OA also commonly report comorbid insomnia (147), as the pain and discomfort linked to the condition impede restful sleep, engendering fatigue and diminished quality of life (148). Moreover, OA patients were nearly three times more prone to frequent memory loss attributed to sleep and mood disturbances (149).
This symbiotic link is underscored by bidirectional interactions, where OA-related chronic pain, functional limitations, and distress contribute to the development and exacerbation of mental health conditions (150), while psychological factors, in turn, can further exacerbate the pain and inflammation (151), perpetuating a vicious cycle and influencing the perception and management of OA. In a recent systematic review, depressive symptoms were highlighted as a potential barrier to physical activity for people with OA (149). Furthermore, shared pathways involving neuroinflammation, oxidative stress, and genetic predispositions seem to underpin this connection (152).
Tumors, embodying uncontrolled cellular proliferation and invasive growth have conventionally stood apart from OA due to their divergent clinical manifestations and traditional spheres of study. Insights have been provided from extensive population-based investigations, illuminating the intriguing interplay between knee or hip osteoarthritis (KHOA) and cancer-related outcomes. Notably, two comprehensive studies, conducted in the United States and Sweden, revealed diminished cancer-related mortality among individuals afflicted with KHOA (153, 154). In contrast, a distinct meta-analysis presented a contrasting perspective, indicating an elevated risk of solid cancers ranging from melanoma to renal cell cancer, and cancers affecting the bladder, breast, uterus, and prostate in individuals with KHOA (155).A sole precedent study, delving into site-specific cancer incidence among OA patients, reported unexpectedly lower incidences of colorectal, stomach, and lung cancers, while noting an elevated incidence of prostate cancer (156). This variance may be plausibly attributed to prolonged use of NSAIDs (156), elucidating the multifaceted nuances of the intricate relationship between OA and cancer.
OA and tumorigenesis share complex molecular pathways. Chronic inflammation, a hallmark of OA, plays a crucial role in tumor progression, fostering an environment conducive to carcinogenesis (157). Pro-inflammatory cytokines and matrix metalloproteinases secreted by the synovium and the associated immune system can promote proliferation, viability, and migratory abilities of tumor cell (158). Genetic susceptibility has emerged as a key determinant in both OA and tumorigenesis (159). Shared genetic variants that influence inflammation, tissue remodeling, and cellular response have been implicated in both conditions. Oxidative stress, often observed in OA, similarly contributes to tumor development (160), prompting consideration of potential overlaps in therapeutic strategies targeting oxidative stress in both conditions.
OA stands as a chronic degenerative joint ailment characterized by articular cartilage injury, cartilage loss, and hyperosteogeny, impacting over 500 million individuals worldwide (161). This comprehensive review has explored the multifaceted landscape of OA and its intricate connections to various systemic diseases including skeletal-muscular diseases, CVDs, endocrine and metabolic diseases, nervous system diseases, mental diseases and tumors (Figure 1).
The significance of this review lies in its revelation of the extensive interconnections between OA and systemic diseases, as well as the recognition that OA frequently coexists with other systemic conditions, such as diabetes and obesity, in a bidirectional relationship. These findings underscore the need for a holistic approach to managing OA and its associated complications. It’s important for patients with OA and diverse complications to formulate the corresponding treatment strategy. Education about OA and treatment plans is crucial for patients. Regular cardiovascular checks are important, especially for those with obesity and diabetes. Effective diabetes management, emphasizing lifestyle changes and medication adherence, is vital. Additionally, addressing mental health issues is essential due to their high prevalence in OA patients. For those with neuropathic pain, specialized neurological care can improve their quality of life. Regular screenings for potential comorbidities, such as cancer, may be considered for long-standing OA or high-risk individuals. In addition, understanding the shared risk factors and pathogenesis, such as inflammation and genetics, provides an opportunity for more targeted and comprehensive interventions that can improve the quality of life for OA patients and potentially reduce the burden of associated systemic diseases.
Future prospects in this field are promising but require further research to elucidate the intricate mechanisms underlying the relationships between OA and systemic diseases. This necessitates exploring the potential therapeutic avenues for addressing both OA and its associated comorbidities. Furthermore, comprehensive patient care should encompass early screening, timely interventions, and a focus on improving overall health to attenuate the impact of OA on systemic health.
In conclusion, OA transcends its status as a joint disorder, extending its influence into multiple organ systems. Acknowledging these connections and addressing them through research and clinical practice promises to enhance the quality of life for the millions of individuals burdened by OA, as well as mitigate the broader systemic health consequences of the disease.
BL: Writing – original draft. ZY: Writing – review & editing. YL: Writing – review & editing. JZ: Writing – review & editing. CL: Writing – review & editing. NL: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OA, Osteoarthritis; OP, Osteoporosis; BMD, Bone Mineral Density; CVDs, Cardiovascular Diseases; NSAIDs, Anti-Nonsteroidal inflammatory drugs; DM, Diabetes Mellitus; HOA, Hip Osteoarthritis; KHOA, Knee or Hip Osteoarthritis; AD, Alzheimer’s Disease; PD, Parkinson’s Disease; TNF-α, Tumor Necrosis Factor-alpha; IL-1, Interleukin-1.
1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
2. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. (2012) 64:1697–707. doi: 10.1002/art.34453
3. Fontanella CG, Belluzzi E, Pozzuoli A, Scioni M, Olivotto E, Reale D, et al. Exploring anatomo-morphometric characteristics of infrapatellar, suprapatellar fat pad, and knee ligaments in osteoarthritis compared to post-traumatic lesions. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10061369
4. Ozeki N, Koga H, Sekiya I. Degenerative meniscus in knee osteoarthritis: from pathology to treatment. Life (Basel). (2022) 12. doi: 10.3390/life12040603
5. Zeng N, Yan ZP, Chen XY, Ni GX. Infrapatellar fat pad and knee osteoarthritis. Aging Dis. (2020) 11:1317–28. doi: 10.14336/AD.2019.1116
6. Martel-Pelletier J, Wildi LM, Pelletier JP. Future therapeutics for osteoarthritis. Bone. (2012) 51:297–311. doi: 10.1016/j.bone.2011.10.008
7. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
8. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. (2013) 105:185–99. doi: 10.1093/bmb/lds038
9. Yang G, Wang J, Liu Y, Lu H, He L, Ma C, et al. Burden of knee osteoarthritis in 204 countries and territories, 1990-2019: results from the global burden of disease study 2019. Arthritis Care Res (Hoboken). (2023). doi: 10.1002/acr.25158
10. Fan Z, Yan L, Liu H, Li X, Fan K, Liu Q, et al. The prevalence of hip osteoarthritis: a systematic review and meta-analysis. Arthritis Res Ther. (2023) 25:51. doi: 10.1186/s13075-023-03033-7
11. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. (2010) 26:355–69. doi: 10.1016/j.cger.2010.03.001
12. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. (2016) 59:134–8. doi: 10.1016/j.rehab.2016.01.006
13. Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train. (2017) 52:491–6. doi: 10.4085/1062-6050-51.5.08
14. King LK. Osteoarthritis and comorbidity: time for action. Osteoarthritis Cartilage. (2023) 31:423–4. doi: 10.1016/j.joca.2023.01.007
15. Veronese N, Cereda E, Maggi S, Luchini C, Solmi M, Smith T, et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum. (2016) 46:160–7. doi: 10.1016/j.semarthrit.2016.04.002
16. Dell’Isola A, Pihl K, Turkiewicz A, Hughes V, Zhang W, Bierma-Zeinstra S, et al. Risk of comorbidities following physician-diagnosed knee or hip osteoarthritis: A register-based cohort study. Arthritis Care Res (Hoboken). (2022) 74:1689–95. doi: 10.1002/acr.24717
17. Swain S, Sarmanova A, Coupland C, Doherty M, Zhang W. Comorbidities in osteoarthritis: A systematic review and meta-analysis of observational studies. Arthritis Care Res (Hoboken). (2020) 72:991–1000. doi: 10.1002/acr.24008
18. Innes KE, Sambamoorthi U. The association of osteoarthritis and related pain burden to incident Alzheimer’s disease and related dementias: A retrospective cohort study of U.S. Medicare beneficiaries. J Alzheimers Dis. (2020) 75:789–805. doi: 10.3233/JAD-191311
19. Kamps A, Runhaar J, Ridder MAJ, Wilde M, Lei der van J, Zhang W, et al. Comorbidity in incident osteoarthritis cases and matched controls using electronic health record data. Arthritis Res Ther. (2023) 25:114. doi: 10.1186/s13075-023-03086-8
20. Losina E, Silva GS, Smith KC, Collins JE, Hunter DJ, Shrestha S, et al. Quality-adjusted life-years lost due to physical inactivity in a US population with osteoarthritis. Arthritis Care Res (Hoboken). (2020) 72:1349–57. doi: 10.1002/acr.24035
21. Parkinson L, Waters DL, Franck L. Systematic review of the impact of osteoarthritis on health outcomes for comorbid disease in older people. Osteoarthritis Cartilage. (2017) 25:1751–70. doi: 10.1016/j.joca.2017.07.008
22. Geyer M, Schonfeld C. Novel insights into the pathogenesis of osteoarthritis. Curr Rheumatol Rev. (2018) 14:98–107. doi: 10.2174/1573397113666170807122312
23. Aubourg G, Rice SJ, Bruce-Wootton P, Loughlin J. Genetics of osteoarthritis. Osteoarthritis Cartilage. (2022) 30:636–49. doi: 10.1016/j.joca.2021.03.002
24. Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. (2012) 20:339–49. doi: 10.1016/j.joca.2011.12.012
25. Shen J, Abu-Amer Y, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. (2017) 58:49–63. doi: 10.1080/03008207.2016.1208655
26. Hashimoto K, Oreffo RO, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. (2009) 60:3303–13. doi: 10.1002/art.24882
27. Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. (2001) 153:35–46. doi: 10.1083/jcb.153.1.35
28. Sassi N, Laadhar L, Allouche M, Achek A, Kallel-Sellami M, Makni S, et al. WNT signaling and chondrocytes: from cell fate determination to osteoarthritis physiopathology. J Recept Signal Transduct Res. (2014) 34:73–80. doi: 10.3109/10799893.2013.863919
29. Mirando AJ, Liu Z, Moore T, Lang A, Kohn A, Osinski AM, et al. RBP-Jkappa-dependent Notch signaling is required for murine articular cartilage and joint maintenance. Arthritis Rheum. (2013) 65:2623–33. doi: 10.1002/art.38076
30. Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. (2009) 15:1421–5. doi: 10.1038/nm.2055
31. Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, Hachem El K, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. (2011) 21:202–20. doi: 10.22203/eCM.v021a16
32. Kobayashi H, Hirata M, Saito T, Itoh S, Chung UI, Kawaguchi H. Transcriptional induction of ADAMTS5 protein by nuclear factor-kappaB (NF-kappaB) family member RelA/p65 in chondrocytes during osteoarthritis development. J Biol Chem. (2013) 288:28620–9. doi: 10.1074/jbc.M113.452169
33. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
34. Walker EA, Davis D, Mosher TJ. Rapidly progressive osteoarthritis: biomechanical considerations. Magn Reson Imaging Clin N Am. (2011) 19:283–94. doi: 10.1016/j.mric.2011.02.008
35. Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. (2003) 33:578–88. doi: 10.2519/jospt.2003.33.10.578
36. Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. (2005) 33:195–200. doi: 10.1097/00003677-200510000-00008
37. Egloff C, Hugle T, Valderrabano V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly. (2012) 142:w13583. doi: 10.4414/smw.2012.13583
38. Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthopedic Clinics North America. (1994) 25:367–77. doi: 10.1016/S0030-5898(20)31921-0
39. Heijink A, Gomoll AH, Madry H, Drobnic M, Filardo G, Espregueira-Mendes J, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. (2012) 20:423–35. doi: 10.1007/s00167-011-1818-0
40. Lee SJ, Aadalen KJ, Malaviya P, Lorenz EP, Hayden JK, Farr J, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. (2006) 34:1334–44. doi: 10.1177/0363546506286786
41. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. (2008) 90:1922–31. doi: 10.2106/JBJS.G.00748
42. Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. (2006) 34:612–20. doi: 10.1177/0363546505281813
43. Herzog W, Longino D. The role of muscles in joint degeneration and osteoarthritis. J Biomech. (2007) 40 Suppl 1:S54–63. doi: 10.1016/j.jbiomech.2007.03.001
44. Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. (2010) 89:541–8. doi: 10.1097/PHM.0b013e3181ddd5c3
45. Benito MJ, Veale DJ, FitzGerald O, Berg den van WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. (2005) 64:1263–7. doi: 10.1136/ard.2004.025270
46. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. (2010) 6:625–35. doi: 10.1038/nrrheum.2010.159
47. Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. (2013) 15:323. doi: 10.1007/s11926-013-0323-5
48. Grassel S, Zaucke F, Madry H. Osteoarthritis: novel molecular mechanisms increase our understanding of the disease pathology. J Clin Med. (2021) 10. doi: 10.3390/jcm10091938
49. Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. (2008) 20:565–72. doi: 10.1097/BOR.0b013e32830aba34
50. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis)! Osteoarthritis Cartilage. (2013) 21:16–21. doi: 10.1016/j.joca.2012.11.012
51. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. (2014) 28:5–15. doi: 10.1016/j.berh.2014.01.004
52. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. (2008) 34:515–29. doi: 10.1016/j.rdc.2008.05.007
53. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. (2005) 13:769–81. doi: 10.1016/j.joca.2005.04.014
54. Kluzek S, Newton JL, Arden NK. Is osteoarthritis a metabolic disorder? Br Med Bull. (2015) 115:111–21. doi: 10.1093/bmb/ldv028
55. Musumeci G, Aiello FC, Szychlinska MA, Rosa Di M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviors that influence disease onset and progression. Int J Mol Sci. (2015) 16:6093–112. doi: 10.3390/ijms16036093
56. Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. (2007) 56:129–36. doi: 10.1002/art.22292
57. McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. (2013) 309:155–62. doi: 10.1001/jama.2012.164487
58. Slauterbeck JR, Kousa P, Clifton BC, Naud S, Tourville TW, Johnson RJ, et al. Geographic mapping of meniscus and cartilage lesions associated with anterior cruciate ligament injuries. J Bone Joint Surg Am. (2009) 91:2094–103. doi: 10.2106/JBJS.H.00888
59. Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. (2009) 37:1434–43. doi: 10.1177/0363546509338827
60. Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure–a systematic overview of the evidence. J Rheumatol. (1997) 24:1599–607.
61. Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. (2017) 167:ITC17–32. doi: 10.7326/AITC201708010
62. Beaudart C, Biver E, Bruyere O, Cooper C, Al-Daghri N, Reginster JY, et al. Quality of life assessment in musculo-skeletal health. Aging Clin Exp Res. (2018) 30:413–8. doi: 10.1007/s40520-017-0794-8
63. Foss MV, Byers PD. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis. (1972) 31:259–64. doi: 10.1136/ard.31.4.259
64. Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. (1995) 38:907–16. doi: 10.1002/art.1780380706
65. Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. (2014) 32:101–9. doi: 10.1007/s00774-013-0531-0
66. Guler-Yuksel M, Bijsterbosch J, Allaart CF, Meulenbelt I, Kroon HM, Watt I, et al. Accelerated metacarpal bone mineral density loss is associated with radiographic progressive hand osteoarthritis. Ann Rheum Dis. (2011) 70:1625–30. doi: 10.1136/ard.2010.144147
67. Lingard EA, Mitchell SY, Francis RM, Rawlings D, Peaston R, Birrell FN, et al. The prevalence of osteoporosis in patients with severe hip and knee osteoarthritis awaiting joint arthroplasty. Age Ageing. (2010) 39:234–9. doi: 10.1093/ageing/afp222
68. Kim D, Pirshahid AA, Li Y, Varghese T, Pope JE. Prevalence of osteoporosis in osteoarthritis: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:1687–93. doi: 10.1007/s00198-022-06376-0
69. Hatta NNKNM, Hasan MKC. A review on osteoarthritis and osteoporosis: ongoing challenges for musculoskeletal care. Int J Care Scholars. (2019) 2:14–20. doi: 10.31436/ijcs.v2i2.127
70. Bultink IE, Lems WF. Osteoarthritis and osteoporosis: what is the overlap? Curr Rheumatol Rep. (2013) 15:328. doi: 10.1007/s11926-013-0328-0
71. Franco-Trepat E, Guillan-Fresco M, Alonso-Perez A, Jorge-Mora A, Francisco V, Gualillo O, et al. Visfatin connection: present and future in osteoarthritis and osteoporosis. J Clin Med. (2019) 8. doi: 10.3390/jcm8081178
72. Bultink IE, Vis M, Horst-Bruinsma der van IE, Lems WF. Inflammatory rheumatic disorders and bone. Curr Rheumatol Rep. (2012) 14:224–30. doi: 10.1007/s11926-012-0252-8
73. Geusens PP, van den Bergh JP. Osteoporosis and osteoarthritis: shared mechanisms and epidemiology. Curr Opin Rheumatol. (2016) 28:97–103. doi: 10.1097/BOR.0000000000000256
74. Conway R, McCarthy GM. Obesity and osteoarthritis: more than just mechanics. EMJ Rheumatol. (2015) 2:75–83. doi: 10.33590/emjrheumatol/10311763
75. Bai RJ, Li YS, Zhang FJ. Osteopontin, a bridge links osteoarthritis and osteoporosis. Front Endocrinol (Lausanne). (2022) 13:1012508. doi: 10.3389/fendo.2022.1012508
76. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. (2003) 81:646–56.
77. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
78. Toda Y, Segal N, Toda T, Kato A, Toda F. A decline in lower extremity lean body mass per body weight is characteristic of women with early phase osteoarthritis of the knee. J Rheumatol. (2000) 27:2449–54.
79. Ikeda S, Tsumura H, Torisu T. Age-related quadriceps-dominant muscle atrophy and incident radiographic knee osteoarthritis. J Orthop Sci. (2005) 10:121–6. doi: 10.1007/s00776-004-0876-2
80. Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. (1997) 127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001
81. Lee S, Kim TN, Kim SH. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. (2012) 64:3947–54. doi: 10.1002/art.37696
82. Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. (2003) 284:E481–7. doi: 10.1152/ajpendo.00319.2002
83. Silva JMS, Alabarse PVG, Teixeira VON, Freitas EC, Oliveira FH, Chakr R, et al. Muscle wasting in osteoarthritis model induced by anterior cruciate ligament transection. PloS One. (2018) 13:e0196682. doi: 10.1371/journal.pone.0196682
84. Cunha JE, Barbosa GM, Castro P, Luiz BLF, Silva ACA, Russo TL, et al. Knee osteoarthritis induces atrophy and neuromuscular junction remodeling in the quadriceps and tibialis anterior muscles of rats. Sci Rep. (2019) 9:6366. doi: 10.1038/s41598-019-42546-7
85. Santos ML, Gomes WF, Pereira DS, Oliveira DM, Dias JM, Ferrioli E, et al. Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA). Arch Gerontol Geriatr. (2011) 52:322–6. doi: 10.1016/j.archger.2010.05.009
86. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
87. Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur J Prev Cardiol. (2016) 23:938–46. doi: 10.1177/2047487315610663
88. Rahman MM, Kopec JA, Anis AH, Cibere J, Goldsmith CH. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis Care Res (Hoboken). (2013) 65:1951–8. doi: 10.1002/acr.22092
89. Veronese N, Stubbs B, Solmi M, Smith TO, Noale M, Schofield P, et al. Knee osteoarthritis and risk of hypertension: A longitudinal cohort study. Rejuvenation Res. (2018) 21:15–21. doi: 10.1089/rej.2017.1917
90. Lo K, Au M, Ni J, Wen C. Association between hypertension and osteoarthritis: A systematic review and meta-analysis of observational studies. J Orthop Translat. (2022) 32:12–20. doi: 10.1016/j.jot.2021.05.003
91. Wang Z, Kang C, Xu P, Zhang S, Song JH, Wang D, et al. Osteoarthritis and cardiovascular disease: A Mendelian randomization study. Front Cardiovasc Med. (2022) 9:1025063. doi: 10.3389/fcvm.2022.1025063
92. Funck-Brentano T, Nethander M, Moverare-Skrtic S, Richette P, Ohlsson C, et al. Causal factors for knee, hip, and hand osteoarthritis: A Mendelian randomization study in the UK Biobank. Arthritis Rheumatol. (2019) 71:1634–41. doi: 10.1002/art.40928
93. Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest. (2015) 45:405–14. doi: 10.1111/eci.12413
94. Findlay DM. Vascular pathology and osteoarthritis. Rheumatol (Oxford). (2007) 46:1763–8. doi: 10.1093/rheumatology/kem191
95. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatol (Oxford). (2005) 44:7–16. doi: 10.1093/rheumatology/keh344
96. Kielbowski K, Herian M, Bakinowska E, Banach B, Sroczynski T, Pawlik A. The role of genetics and epigenetic regulation in the pathogenesis of osteoarthritis. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241411655
97. Zhang S, Feng Y, Yin X, Su Q, Xi Y, Cheng T, et al. Genetic evidence suggesting the predicted causality between osteoarthritis and cardiovascular diseases. Rheumatol Autoimmun. (2023) 3:230–9. doi: 10.1002/rai2.12097
98. Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog Lipid Res. (2011) 50:133–40. doi: 10.1016/j.plipres.2010.11.001
99. Atiquzzaman M, Karim ME, Kopec J, Wong H, Anis AH. Role of nonsteroidal antiinflammatory drugs in the association between osteoarthritis and cardiovascular diseases: A longitudinal study. Arthritis Rheumatol. (2019) 71:1835–43. doi: 10.1002/art.41027
100. American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2011) 34 Suppl 1:S62–9. doi: 102337/dc11-S062
101. Ogurtsova K, Fernandes Rocha JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. (2017) 128:40–50. doi: 10.1016/j.diabres.2017.03.024
102. Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. (2015) 23:1955–65. doi: 10.1016/j.joca.2015.05.016
103. Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. (2009) 121:9–20. doi: 10.3810/pgm.2009.11.2073
104. Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vila LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol. (2013) 19:1–6. doi: 10.1097/RHU.0b013e31827cd578
105. Rahman MM, Cibere J, Anis AH, Goldsmith CH, Kopec JA. Risk of type 2 diabetes among osteoarthritis patients in a prospective longitudinal study. Int J Rheumatol. (2014) 2014:620920. doi: 10.1155/2014/620920
106. Rogers-Soeder TS, Lane NE, Walimbe M, Schwartz AV, Tolstykh I, Felson DT, et al. Association of diabetes mellitus and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis. Arthritis Care Res (Hoboken). (2020) 72:98–106. doi: 10.1002/acr.23809
107. Alenazi AM, Alshehri Mm Alqahtani B, Alanazi AD, Khunti K, Vennu V. 1462-P: baseline gait speed can predict diabetes incidence in individuals with or at risk of knee osteoarthritis: A longitudinal study using data from the osteoarthritis initiative. Diabetes. (2020) 69. doi: 10.2337/db20-1462-P
108. Aiello FC, Trovato FM, Szychlinska MA, Imbesi R, Castrogiovanni P, Loreto C, et al. Molecular links between diabetes and osteoarthritis: the role of physical activity. Curr Diabetes Rev. (2017) 13:50–8. doi: 10.2174/1573399812666151123104352
109. Jhuma KA, Giasuddin AS, Hossain MS. Status of serum pro-inflammatory cytokines (IL-1, IL-6, TNF-alpha) and anti-inflammatory cytokines (IL-4, IL-10, IL-13) in newly diagnosed Bangladeshi patients with type 2 diabetes mellitus. Mymensingh Med J. (2023) 32:1149–55.
110. Piva SR, Susko AM, Khoja SS, Josbeno DA, Fitzgerald GK, Toledo FG. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin Geriatr Med. (2015) 31:67–87, viii. doi: 10.1016/j.cger.2014.08.019
111. Chen L, Zheng JJY, Li G, Yuan J, Ebert JR, Li H, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: Impact of mechanical loading. J Orthop Translat. (2020) 24:66–75. doi: 10.1016/j.jot.2020.05.001
112. Xiong J, Long J, Chen X, Li Y, Song H. Dyslipidemia might be associated with an increased risk of osteoarthritis. BioMed Res Int. (2020) 2020:3105248. doi: 10.1155/2020/3105248
113. Veronese N, Stubbs B, Solmi M, Smith TO, Noale M, Cooper C, et al. Association between lower limb osteoarthritis and incidence of depressive symptoms: data from the osteoarthritis initiative. Age Ageing. (2017) 46:470–6. doi: 10.1093/ageing/afw216
114. Cho BW, Kim DS, Kwon HM, Yang IH, Lee WS, Park KK. Cross-sectional association between hypercholesterolemia and knee pain in the elderly with radiographic knee osteoarthritis: data from the Korean national health and nutritional examination survey. J Clin Med. (2021) 10. doi: 10.3390/jcm10050933
115. Farnaghi S, Prasadam I, Cai G, Friis T, Du Z, Crawford R, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J. (2017) 31:356–67. doi: 10.1096/fj.201600600r
116. de Munter W, Blom AB, Helsen MM, Walgreen B, Kraan der van PM, Joosten LA, et al. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res Ther. (2013) 15:R178. doi: 10.1186/ar4367
117. Davies-Tuck ML, Hanna F, Davis SR, Bell RJ, Davison SL, Wluka AE, et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - a prospective cohort study. Arthritis Res Ther. (2009) 11:R181. doi: 10.1186/ar2873
118. Chen S, Sun X, Zhou G, Jin J, Li Z. Association between sensitivity to thyroid hormone indices and the risk of osteoarthritis: an NHANES study. Eur J Med Res. (2022) 27:114. doi: 10.1186/s40001-022-00749-1
119. Devrimsel G, Beyazal MS, Turkyilmaz AK, Sahin SB. Ultrasonographic evaluation of the femoral cartilage thickness in patients with hypothyroidism. J Phys Ther Sci. (2016) 28:2249–52. doi: 10.1589/jpts.28.2249
120. Botello A, Herran M, Salcedo V, Rodriguez Y, Anaya JM, Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: A systematic review and meta-analysis. Clin Endocrinol (Oxf). (2020) 93:375–89. doi: 10.1111/cen.14304
121. Bos SD, Bovee JV, Duijnisveld BJ, Raine EV, Dalen van WJ, Ramos YF, et al. Increased type II deiodinase protein in OA-affected cartilage and allelic imbalance of OA risk polymorphism rs225014 at DIO2 in human OA joint tissues. Ann Rheum Dis. (2012) 71:1254–8. doi: 10.1136/annrheumdis-2011-200981
122. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. (2010) 362:906–16. doi: 10.1056/NEJMoa0905633
123. Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, Wijk der van HJ, et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. (2008) 17:1867–75. doi: 10.1093/hmg/ddn082
124. Magni N, Collier J, McNair P, Rice DA. Neuropathic pain in hand osteoarthritis: A cross-sectional study. J Clin Med. (2021) 10. doi: 10.3390/jcm10194439
125. French HP, Smart KM, Doyle F. Prevalence of neuropathic pain in knee or hip osteoarthritis: A systematic review and meta-analysis. Semin Arthritis Rheum. (2017) 47:1–8. doi: 10.1016/j.semarthrit.2017.02.008
126. Dimitroulas T, Duarte RV, Behura A, Kitas GD, Raphael JH. Neuropathic pain in osteoarthritis: a review of pathophysiological mechanisms and implications for treatment. Semin Arthritis Rheum. (2014) 44:145–54. doi: 10.1016/j.semarthrit.2014.05.011
127. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. (2011) 13:211. doi: 10.1186/ar3306
128. Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. (2010) 149:573–81. doi: 10.1016/j.pain.2010.04.003
129. Weber A, Mak SH, Berenbaum F, Sellam J, Zheng YP, Han Y, et al. Association between osteoarthritis and increased risk of dementia: A systemic review and meta-analysis. Med (Baltimore). (2019) 98:e14355. doi: 10.1097/MD.0000000000014355
130. Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. (2011) 342:d1165. doi: 10.1136/bmj.d1165
131. Huang SW, Wang WT, Chou LC, Liao CD, Liou TH, Lin HW. Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep. (2015) 5:10145. doi: 10.1038/srep10145
132. Kyrkanides S, Tallents RH, Miller JN, Olschowka ME, Johnson R, Yang M, et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J Neuroinflamm. (2011) 8:112. doi: 10.1186/1742-2094-8-112
133. Kalia LV, Lang AE. Parkinson’s disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
134. Rugbjerg K, Friis S, Jorgensen TL, Ritz B, Korbo L, Olsen JH. Risk for Parkinson’s disease among patients with osteoarthritis: a Danish cohort study. Mov Disord. (2010) 25:2355–60. doi: 10.1002/mds.23274
135. Feng SH, Chuang HJ, Yeh KC, Pan SL. Association of osteoarthritis with increased risk of Parkinson’s disease: A population-based, longitudinal follow-up study. Arthritis Care Res (Hoboken). (2022) 74:1842–8. doi: 10.1002/acr.24708
136. Jacob L, Smith L, Koyanagi A, Schnitzler A, Shin Il J, Kostev K. Association between osteoarthritis and the incidence of Parkinson’s disease in the United Kingdom. Clin Park Relat Disord. (2021) 5:100120. doi: 10.1016/j.prdoa.2021.100120
137. Al-Khazraji BK, Appleton CT, Beier F, Birmingham TB, Shoemaker JK. Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: a narrative review. Osteoarthritis Cartilage. (2018) 26:462–70. doi: 10.1016/j.joca.2018.01.011
138. Heng H, Liu J, Hu M, Li D, Su W, Li J. WDR43 is a potential diagnostic biomarker and therapeutic target for osteoarthritis complicated with Parkinson’s disease. Front Cell Neurosci. (2022) 16:1013745. doi: 10.3389/fncel.2022.1013745
139. Pajares M, A IR, Manda G, Bosca L, Cuadrado A. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells. (2020) 9. doi: 10.3390/cells9071687
140. Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. (2017) 29:79–85. doi: 10.1097/BOR.0000000000000353
141. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. (2008) 87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S
142. Fang X, Han D, Cheng Q, Zhang P, Zhao C, Min J, et al. Association of levels of physical activity with risk of Parkinson disease: A systematic review and meta-analysis. JAMA Netw Open. (2018) 1:e182421. doi: 10.1001/jamanetworkopen.2018.2421
143. Wang J, Song Y, Chen Z, Leng SX. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev. (2018) 2018:1972714. doi: 10.1155/2018/1972714
144. Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil. (2009) 90:1866–73. doi: 10.1016/j.apmr.2009.06.012
145. Stubbs B, Aluko Myint Smith PK TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. (2016) 45:228–35. doi: 10.1093/ageing/afw001
146. Kye SY, Park K. Suicidal ideation and suicidal attempts among adults with chronic diseases: A cross-sectional study. Compr Psychiatry. (2017) 73:160–7. doi: 10.1016/j.comppsych.2016.12.001
147. Jacob L, Aluko Y, Myint PK, Smith TO. Association between sleep disorders and osteoarthritis: A case-control study of 351,932 adults in the UK. J Sleep Res. (2021) 30:e13367. doi: 10.1111/jsr.13367
148. De Baets L, Runge N, Labie C, Mairesse O, Malfliet A, Verschueren S, et al. The interplay between symptoms of insomnia and pain in people with osteoarthritis: A narrative review of the current evidence. Sleep Med Rev. (2023) 70:101793. doi: 10.1016/j.smrv.2023.101793
149. Innes KE, Sambamoorthi U. The association of perceived memory loss with osteoarthritis and related joint pain in a large Appalachian population. Pain Med. (2018) 19:1340–56. doi: 10.1093/pm/pnx107
150. Iijima H, Aoyama T, Fukutani N, Isho T, Yamamoto Y, Hiraoka M, et al. Psychological health is associated with knee pain and physical function in patients with knee osteoarthritis: an exploratory cross-sectional study. BMC Psychol. (2018) 6:19. doi: 10.1186/s40359-018-0234-3
151. Phyomaung PP, Dubowitz J, Cicuttini FM, Fernando S, Wluka AE, Raaijmaakers P, et al. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskelet Disord. (2014) 15:10. doi: 10.1186/1471-2474-15-10
152. Shimura Y, Kurosawa H, Tsuchiya M, Sawa M, Kaneko H, Liu L, et al. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol. (2017) 36:2781–7. doi: 10.1007/s10067-017-3826-z
153. Turkiewicz A, Kiadaliri AA, Englund M. Cause-specific mortality in osteoarthritis of peripheral joints. Osteoarthritis Cartilage. (2019) 27:848–54. doi: 10.1016/j.joca.2019.02.793
154. Mendy A, Park J, Vieira ER. Osteoarthritis and risk of mortality in the USA: a population-based cohort study. Int J Epidemiol. (2018) 47:1821–9. doi: 10.1093/ije/dyy187
155. Ward MM, Alehashemi S. Risks of solid cancers in elderly persons with osteoarthritis or ankylosing spondylitis. Rheumatol (Oxford). (2020) 59:3817–25. doi: 10.1093/rheumatology/keaa166
156. Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of Malignancy among patients with rheumatic conditions. Int J Cancer. (2000) 88:497–502. doi: 10.1002/1097-0215(20001101)88:3<497::AID-IJC27>3.0.CO;2-J
157. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
158. Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. (2016) 7:75571–84. doi: 10.18632/oncotarget.12289
159. Zhu S, Qiu H, Bennett S, Kuek V, Rosen V, Xu H, et al. Chondromodulin-1 in health, osteoarthritis, cancer, and heart disease. Cell Mol Life Sci. (2019) 76:4493–502. doi: 10.1007/s00018-019-03225-y
160. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. (2020) 38:167–97. doi: 10.1016/j.ccell.2020.06.001
Keywords: osteoarthritis, osteoporosis, sarcopenia, cardiovascular diseases, diabetes mellitus, dementia, Parkinson’s disease, mental disease
Citation: Li B, Yang Z, Li Y, Zhang J, Li C and Lv N (2024) Exploration beyond osteoarthritis: the association and mechanism of its related comorbidities. Front. Endocrinol. 15:1352671. doi: 10.3389/fendo.2024.1352671
Received: 08 December 2023; Accepted: 12 March 2024;
Published: 19 April 2024.
Edited by:
Kok Yong Chin, National University of Malaysia, MalaysiaReviewed by:
Thomas Wilson, Henry Ford Health System, United StatesCopyright © 2024 Li, Yang, Li, Zhang, Li and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naishan Lv, bHZuYWlzaGFuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.