- 1Department of Urology II, The First Hospital of Jilin University, Changchun, China
- 2Department of Thoracic Surgery, The First Hospital of Jilin University, Changchun, China
- 3Key Laboratory of Pathobiology, Ministry of Education, Jilin University, Changchun, China
- 4Department of Prosthodontics, Hospital of Stomatology, Jilin University, Changchun, China
Objectives: The relationship between cathepsins and prostate cancer (PCa) has been reported. However, there is a lack of research on cathepsins and benign prostate diseases (BPDs). This study investigated the potential genetic link between cathepsins and BPDs through the utilization of Mendelian randomization (MR) analysis to determine if a causal relationship exists.
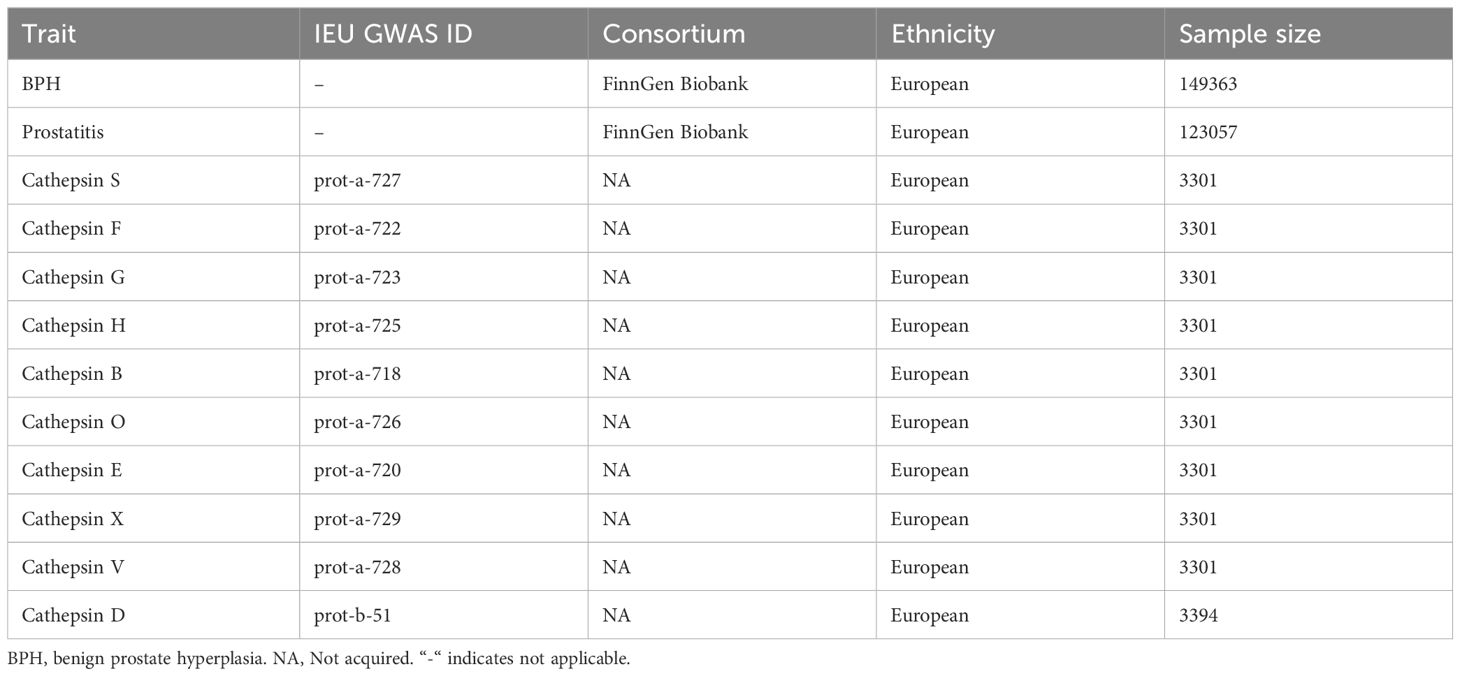
Methods: Publicly accessible summary statistics on BPDs were obtained from FinnGen Biobank. The data comprised 149,363 individuals, with 30,066 cases and 119,297 controls for BPH, and 123,057 individuals, with 3,760 cases and 119,297 controls for prostatitis. The IEU OpenGWAS provided the Genome-wide association data on ten cathepsins. To evaluate the causal relationship between BPDs and cathepsins, five distinct MR analyses were employed, with the primary method being the inverse variance weighted (IVW) approach. Additionally, sensitivity analyses were conducted to examine the horizontal pleiotropy and heterogeneity of the findings.
Results: The examination of IVW MR findings showed that cathepsin O had a beneficial effect on BPH (IVW OR=0.94, 95% CI 0.89–0.98, P=0.0055), while cathepsin X posed a threat to prostatitis (IVW OR=1.08, 95% CI 1.00–1.16, P=0.047). Through reverse MR analysis, it was revealed that prostatitis had an adverse impact on cathepsin V (IVW OR=0.89, 95% CI 0.80–0.99, P=0.035), while no favorable association was observed between BPH and cathepsins. The results obtained from MR-Egger, weighted median, simple mode, and weighted mode methods were consistent with the findings of the IVW approach. Based on sensitivity analyses, heterogeneity, and horizontal pleiotropy are unlikely to distort the results.
Conclusion: This study offers the initial evidence of a genetic causal link between cathepsins and BPDs. Our findings revealed that cathepsin O was beneficial in preventing BPH, whereas cathepsin X posed a potential threat to prostatitis. Additionally, prostatitis negatively affected cathepsin V level. These three cathepsins could be targets of diagnosis and treatment for BPDs, which need further research.
Introduction
Benign prostatic diseases (BPDs), mainly including benign prostatic hyperplasia (BPH) and prostatitis, are prevalent urological ailments affecting males globally (1, 2). Compression of the urethra caused by BPH results in clinical symptoms that impact the quality of life for individuals with BPH, including lower urinary tract symptoms (LUTS), episodes of acute urinary retention, and recurring urinary infections (3). Numerous investigations have been carried out regarding the development of BPH; nevertheless, the factors responsible for its occurrence and advancement are still poorly understood (4). Prostatitis is classified into types I–IV by the National Institutes of Health (NIH) in the United States. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) of Type III is categorized into inflammatory CPPS (IIIa) and noninflammatory CPPS (IIIb), making up approximately 90%–95% of cases related to prostatitis (5). Individuals diagnosed with CP/CPPS typically encounter discomfort in the pelvic region, such as pain in the pubic region or perineum, discomfort during sexual activity or ejaculation, painful urination, frequent urination at night and/or a sense of urgency, sexual difficulties, impotence, mental health disorders, and reduced sperm quality (6, 7). Exploring the etiology, diagnosis, and treatment of BPDs in public health has significant practical implications by identifying the causal association of risk factors that can be modified.
The group of lysosomal proteolytic enzymes known as cathepsins plays a crucial role in preserving cellular homeostasis, which are classified into multiple families, including serine proteases (cathepsins A and G), cysteine proteases (cathepsins B, C, F, H, K, L, O, S, V, W, and X), and aspartyl proteases (cathepsins D and E) (8, 9). They are associated with many cellular activities including protein and lipid metabolism, autophagy, antigen presentation, growth factor receptor recycling, cellular stress signaling, extracellular matrix degradation, and lysosome-mediated cell death (10). Various cathepsins have significant roles in various diseases due to their participation in these essential processes (11). The association between cathepsins and prostate cancer (PCa) has been reported, such as cathepsin B. Nalle et al. found that the inhibition of invasion and migration and the activation of apoptosis in PC3 and DU145 prostate cancer cell lines can be achieved by targeting cathepsin B (12). Moreover, the rise in serum levels of cathepsin B and the density of cathepsin B may serve as innovative indicators for the progression of the disease. However, they do not impact the survival of individuals diagnosed with PCa (13). Furthermore, the generation of sphingosine 1-phosphate through acid ceramidase facilitates the invasion of PCa by increasing the expression of cathepsin B (14). Despite several studies suggesting potential applications of cathepsins in PCa disease, there is a lack of studies exploring cathepsins in relation to BPDs. Based on this, we will use Mendelian randomization (MR) to do such a thing.
MR designs utilize genetic diversity as instrumental variables (IVs) to explore the causal association between potential factors and the specific disease, offering several advantages absent in conventional epidemiology (15). In addition, using MR designs can decrease the likelihood of reverse causality and minimize the impact of confounding variables (16). The objective of this study was to examine the causal connection between cathepsins and BPDs, potentially identifying novel avenues for diagnosing and treating BPDs.
Materials and methods
Study design
To establish the bidirectional causal connection between BPDs and cathepsins, a Mendelian randomization (MR) study using two samples was conducted. To investigate the causal relationship between exposure and outcome, the research on MR utilizes instrumental variables (IVs) and examines Single Nucleotide Polymorphisms (SNPs). To acquire valid IVs, three essential assumptions must be met: i) relevance assumption: the IVs must be strongly linked to the exposure (cathepsins); ii) independence assumption: the IVs should not be correlated with any confounding factors; and iii) exclusion assumption: the IVs must solely impact the outcome (BPDs) through the exposure (cathepsins) (17, 18). Figure 1 shows the planned layout of bidirectional two-sample MR between cathepsins and BPDs.

Figure 1 The schematic of an MR study involving cathepsins and BPDs (BPH and prostatitis) using two samples. (A) In order for the MR study to be valid, three essential assumptions must be met: i) relevance assumption: the IVs must be linked to the exposure; ii) independence assumption: the IVs should not be correlated with any confounding factors; and iii) exclusion assumption: the IVs must solely impact the outcome through the exposure. (B) We explore the bidirectional two-sample MR between cathepsins and BPDs. MR, Mendelian randomization; BPDs, benign prostatic diseases; BPH, benign prostatic hyperplasia; IVs, instrumental variables.
GWAS statistics source
Summary statistics on BPDs obtained from FinnGen Biobank (website: https://www.finngen.fi/en) were utilized. The dataset included 149,363 (30,066 cases and 119,297 controls) and 123,057 (3,760 cases and 119,297 controls) separately for BPH and prostatitis. BPH cases are defined in the database based on the N40 criteria in the International Classification of Diseases 10 (ICD-10) Revision codes, as well as the codes 600 in ICD-8 and ICD-9. Prostatitis is a condition that causes inflammation in the prostate gland, which can be caused by infection or non-infectious factors. The objective of FinnGen Biobank, a collaboration between the public and private sectors, is to gather and examine genetic and medical information from 500,000 individuals involved in the Finnish biobank (19). The IEU OpenGWAS (https://gwas.mrcieu.ac.uk) provided the ten Genome-wide association studies (GWAS) of cathepsins (20, 21). The details of GWAS included in MR analyses are described in Table 1.
Selection of genetic instrumental variables
In order to obtain effective IVs, we selected SNPs that were highly correlated with exposure. A conventional threshold was considered, with significant genome-wide importance (P<5× 10-8), linkage disequilibrium (LD), and an r2 less than 0.001 within a 10,000 kb range. Consequently, the number of SNPs is insufficient to perform MR, thus the following criteria were adopted. We identified SNPs that showed genome-wide significance (P<5×10-6, and P<5×10-8 for BPH in reverse MR), LD, and an r2<0.001 threshold within a 10,000 kb range. We removed palindromic variants for incompatible alleles. The F-statistics were calculated utilizing the following equation:
R2 represents the proportion of variance in exposure that can be accounted for by the IVs, N is the sample size, and K is the number of IVs. SNPs were considered for inclusion if the F-statistic was more significant than or equal to 10, indicating a strong potential to predict exposure (22). The Steiger test posits that the correlation between SNPS and exposure should be greater than the outcome, otherwise reverse causality will occur, and SNPS that fail the test may be unrelated to exposure and excluded (23). MR-PRESSO is capable of detecting horizontal pleiotropy and correcting it by removing outliers. Furthermore, it is able to test the significance of causal inference both before and after the aforementioned correction (24). The chosen SNPs were the final instrumental variables (IVs) for the following MR study.
MR analyses and sensitivity analyses
For MR analyses, five MR methods were employed, namely MR Egger, weighted median, inverse-variance weighted (IVW), simple mode, and weighted mode. The IVW estimates were selected as the primary method, and four additional methods were used to improve their robustness in a broader range of scenarios (25). Cochran’s Q test of IVW and MR Egger (26) also identified heterogeneity. Additionally, the Pleiotropy Residual Sum and Outlier methods (MR-PRESSO) were employed to evaluate and rectify horizontal pleiotropy (24). Moreover, a Leave-one-out analysis was conducted to assess if a solitary SNP influenced or skewed the MR estimate. A funnel plot was employed to assess the likely directional pleiotropy. A statistical significance was determined when the significance level was below 0.05. Figure 2 displays the described workflow of MR and sensitivity analyses. The statistical analyses were conducted in R software 4.3.1 using the TwoSampleMR R package (version 0.5.7) and MR-PRESSO (version 1.0).
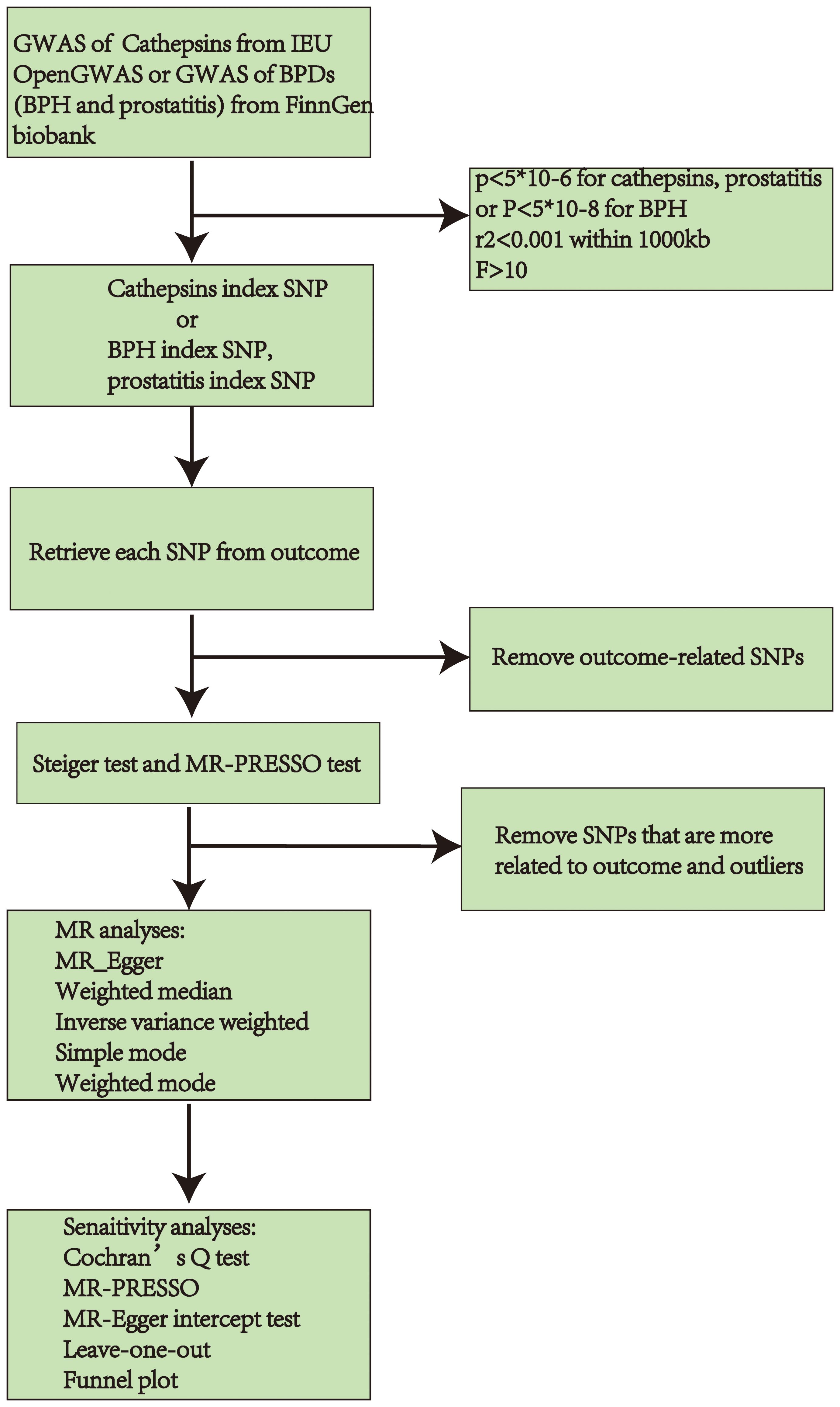
Figure 2 Workflow chart of MR discovering causality between cathepsins and BPDs (BPH and prostatitis). GWAS, Genome-Wide Association Study; BPDs, benign prostate diseases; BPH, benign prostate hyperplasia; SNP, Single Nucleotide Polymorphisms; MR, Mendelian randomization.
Results
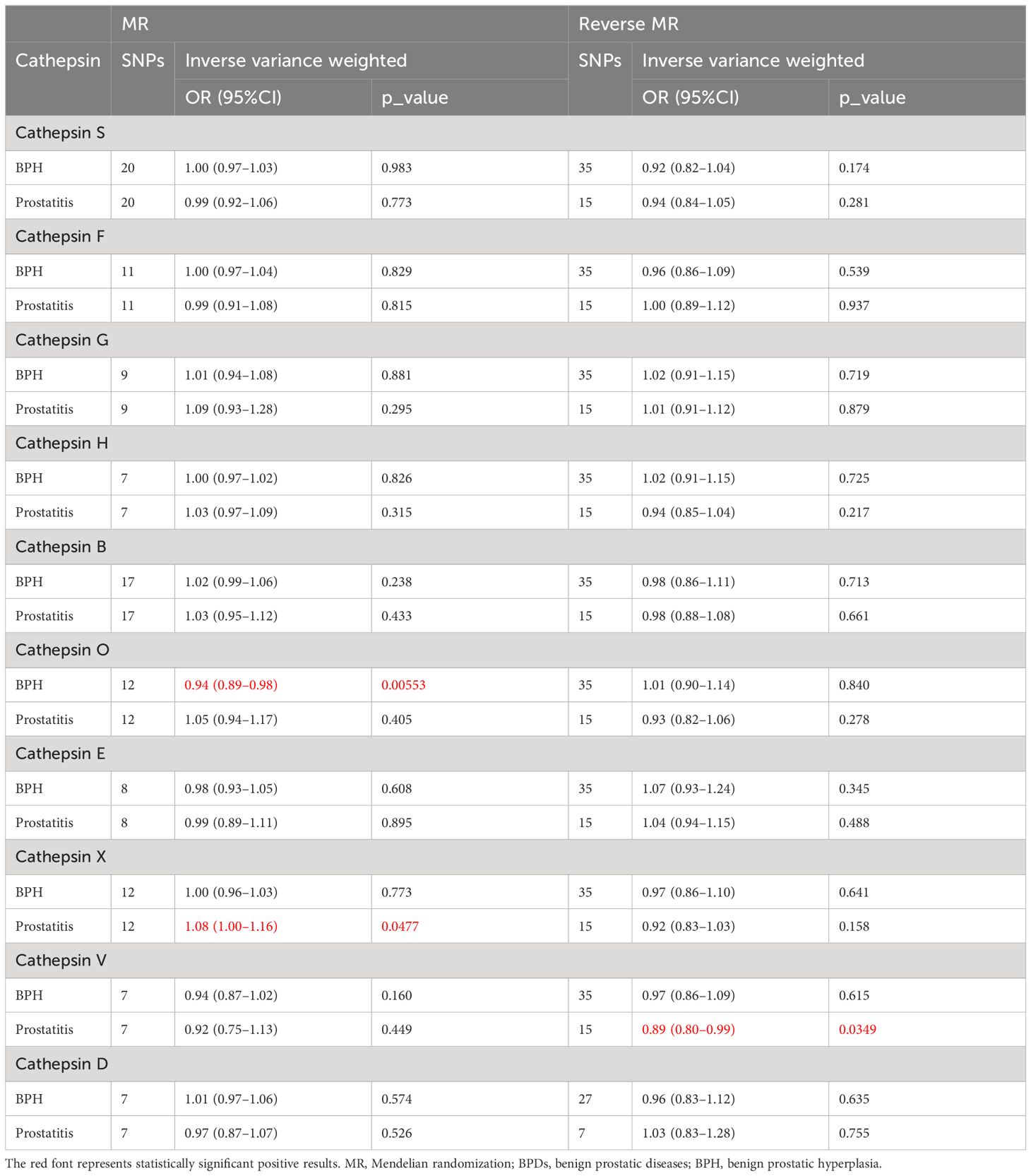
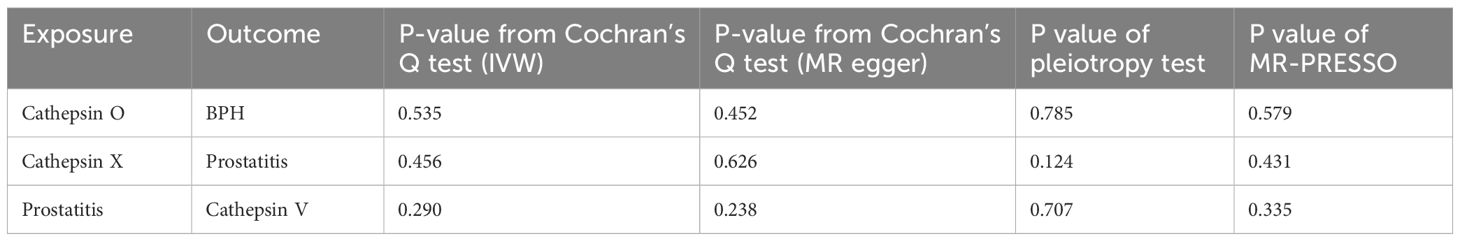
Table 2 displays the results of bidirectional two-sample MR between cathepsins and BPDs, and we found three statistically significant positive results. By utilizing the 12 SNPs associated with cathepsin O, we discovered that elevated levels of cathepsin O can decrease the risk of BPH (OR=0.94, 95%CI=0.89–0.98, p-value=0.00553) through the implementation of IVW techniques (Table 2; Supplementary Table 1; Supplementary Figure 1). Moreover, the absence of significant heterogeneity (P-value > 0.05) was confirmed by the MR-Egger and IVW heterogeneity tests. The absence of any impact was shown when using P-values obtained from MR-Pleiotropy Residual Sum and Outlier methods (MR-PRESSO) to evaluate horizontal pleiotropy (Table 3; Supplementary Table 2). In the Leave-one-out sensitivity analysis (Supplementary Figure 2A), there was no individual SNP that significantly undermined the overall impact of cathepsin O on BPH. The symmetrical funnel plot also suggests the absence of pleiotropy (Supplementary Figure 2B). Furthermore, by employing 12SNPs, we discovered a correlation between elevated cathepsin X levels and heightened susceptibility to prostatitis (OR=1.08, 95% CI=1.00–1.16, p-value=0.0477) through the implementation of IVW techniques (Table 2; Supplementary Table 3; Supplementary Figure 3). Comparable to the findings mentioned above, there was an absence of heterogeneity or horizontal pleiotropy in the outcomes (P-value > 0.05) (Table 3; Supplementary Table 4; Supplementary Figure 4).
In reverse MR, we found an association between genetically predicted prostatitis and cathepsin V levels by utilizing 15 prostatitis-related SNPs (Supplementary Table 5). Using IVW methods (Supplementary Figure 5), there is a decrease in the expression of cathepsin V in prostatitis cases (OR=0.89, 95% CI=0.80–0.99, p-value=0.0349). Similar to the analysis mentioned earlier, no signs of pleiotropy and heterogeneity were detected in the findings (P-value > 0.05) (Table 3; Supplementary Table 6; Supplementary Figure 6).
Discussion
This study used MR to examine the reciprocal causal connections between cathepsins and BPDs. The results indicate that cathepsin O protected BPH, cathepsin X posed a risk for prostatitis, and prostatitis had a negative impact on cathepsin V. The findings provide valuable insights into the role of cathepsins in developing BPDs, potentially influencing the development of diagnostic and therapeutic strategies for cathepsins in patients with BPDs.
Previous research has indicated an association between cathepsins and PCa, which shows the possibility of clinical application value. Incorporating 474 males who had a prostate-specific antigen (PSA) concentration ranging from 2.0 to 10 ng/mL, a negative digital rectal examination, and an enlarged prostate (volume of at least 35 mL), a retrospective study revealed that combining thrombospondin 1, cathepsin D with %fPSA in a model could enhance the diagnosis of PCa and potentially decrease the need for unnecessary prostate biopsies. This model demonstrated improved specificity for PCa compared to using %fPSA alone (27). In PCa patients, a different protein expression test showed elevated levels of cathepsin S secreted by macrophages, indicating the progression of PCa. Another research by Jennica found that cathepsin H could regulate the migration of PCa cells (PC3) (28). Moreover, cathepsin E amplifies the effectiveness of doxorubicin against PCa cells in humans, which exhibit resistance to apoptosis induced by TRAIL. The up-regulation of cathepsin B promotes the invasion of PCa by producing sphingosine 1-phosphate by acid ceramidase (29). PCa is linked to the heightened presence of cathepsin B in plasma membrane/endosomal fractions, whereas BPH or normal prostate are not (30). Despite several studies suggesting potential applications of cathepsins in PCa disease, more studies need to explore cathepsins for BPDs. Importantly, this study is the initial MR to explore the correlation between cathepsins and BPDs, which warrants additional experimental and clinical investigation for validation.
There are multiple advantages to the current research. Initially, it was the primary study to investigate the impact of cathepsins on BPDs by utilizing extensive GWAS data from Finngen Biobank and the IEU OpenGWAS. Furthermore, since the chosen IVs were situated on a distinct chromosome, it is plausible that any potential gene-gene interaction would have minimal impact on the projected outcome. Furthermore, we employed various reliable techniques to acquire the MR effects, including MR-PRESSO and the Steiger test. Additionally, we assessed the existence of horizontal pleiotropy. By employing the two-step MR analysis, we successfully pinpointed the association between cathepsins and BPDs.
There are certain constraints in the current investigation. Initially, while three cathepsins were found to be associated with BPDs in our study, the effect was marginal and, probably, of limited clinical relevance. At present, there is a paucity of studies investigating the mechanism between cathepsins and BPDs. Previous studies have demonstrated that cathepsins are involved in all aspects of cellular activity and are strongly linked to the development of various diseases. Further research may yet reveal cathepsins to be a new diagnostic and therapeutic target for BPDs. Nevertheless, this hypothesis requires further experimental verification. Furthermore, the GWAS data may give rise to potential nonlinear relationships or stratification effects. Additionally, the findings might have restricted applicability to individuals of non-European origin because only participants with European heritage were included exclusively. This implies that the causal relationship postulated in this paper is applicable solely to European populations. The emergence of future GWAS data from other ethnic groups will be useful to further explore this causal inference. A comprehensive assessment of mixed populations will be more useful to explore the causal relationship between Cathepsins and BPDs at the genetic level. Lastly, given the complexity of genetic and environmental factors influencing BPDs and levels of cathepsins, we did not include possible confounding factors in our analysis. It is very difficult to identify confounding factors that affect both, and future studies should further identify confounding factors that can affect both to further improve the accuracy of results.
Conclusion
To summarize, our findings indicate that cathepsin O played a beneficial role in BPH, cathepsin X posed a potential risk for prostatitis, and prostatitis had an adverse impact on the expression of cathepsin V. These cathepsins may become new targets for future BPDs diagnosis and treatment, but further validation is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HC: Writing – original draft, Supervision, Software, Formal analysis, Conceptualization. BL: Writing – review & editing, Software, Formal analysis, Data curation. KG: Writing – original draft, Software, Formal analysis, Data curation. HW: Writing – review & editing, Software, Formal analysis, Data curation. YW: Writing – review & editing, Software, Data curation. HZ: Writing – original draft, Software, Data curation. CS: Writing – original draft, Supervision. PW: Writing – review & editing, Supervision. HD: Writing – review & editing, Supervision. HZ: Writing – original draft, Investigation, Conceptualization. SW: Writing – review & editing, Investigation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by grants from the Jilin Provincial Department of Finance (No: JLSWSRCZX2020-058), Jilin Provincial Department of Science and Technology (No: 20210401154), WU JIEPING Medical Foundation (No: 320.6750.2020-06-37), WU JIEPING Medical Foundation (No: 320.6750.2020-06-36), and Jilin Provincial Department of Science and Technology (No: 20230204011YY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1348310/full#supplementary-material
References
1. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. (2022) 3:e754–e76.
2. Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. (2008) 31 Suppl 1:S85–90. doi: 10.1016/j.ijantimicag.2007.08.028
3. Kim EH, Larson JA, Andriole GL. Management of benign prostatic hyperplasia. Annu Rev Med. (2016) 67:137–51. doi: 10.1146/annurev-med-063014-123902
4. Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. (2021) 127:389–99. doi: 10.1111/bju.15229
5. Krieger JN, Nyberg L Jr., Nickel JC. NIH consensus definition and classification of prostatitis. Jama. (1999) 282:236–7. doi: 10.1001/jama.282.3.236
6. Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. (2016) 19:132–8. doi: 10.1038/pcan.2016.8
7. Wagenlehner FM, Naber KG, Bschleipfer T, Brähler E, Weidner W. Prostatitis and male pelvic pain syndrome: diagnosis and treatment. Dtsch Arztebl Int. (2009) 106:175–83. doi: 10.3238/arztebl.2009.0175
8. Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. (2010) 120:3421–31. doi: 10.1172/JCI42918
9. Patel S, Homaei A, El-Seedi HR, Akhtar N. Cathepsins: Proteases that are vital for survival but can also be fatal. BioMed Pharmacother. (2018) 105:526–32. doi: 10.1016/j.biopha.2018.05.148
10. Conus S, Simon HU. Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol. (2008) 76:1374–82. doi: 10.1016/j.bcp.2008.07.041
11. Yadati T, Houben T, Bitorina A, Shiri-Sverdlov R. The ins and outs of cathepsins: physiological function and role in disease management. Cells. (2020) 9. doi: 10.3390/cells9071679
12. Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. (2010) 17:599–613. doi: 10.1038/cgt.2010.16
13. Miyake H, Hara I, Eto H. Serum level of cathepsin B and its density in men with prostate cancer as novel markers of disease progression. Anticancer Res. (2004) 24:2573–7.
14. Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, et al. Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int J Cancer. (2012) 131:2034–43. doi: 10.1002/ijc.27480
15. Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. (2022) 12. doi: 10.1101/cshperspect.a041302
16. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
17. Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. (2015) 44:496–511. doi: 10.1093/ije/dyv071
18. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. (2018) 33:947–52. doi: 10.1007/s10654-018-0424-6
19. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18.
20. Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature. (2018) 558:73–9. doi: 10.1038/s41586-018-0175-2
21. Folkersen L, Fauman E, Sabater-Lleal M, Strawbridge RJ, Frånberg M, Sennblad B, et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PloS Genet. (2017) 13:e1006706. doi: 10.1371/journal.pgen.1006706
22. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
23. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
24. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
25. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z
26. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
27. Steuber T, Tennstedt P, Macagno A, Athanasiou A, Wittig A, Huber R, et al. Thrombospondin 1 and cathepsin D improve prostate cancer diagnosis by avoiding potentially unnecessary prostate biopsies. BJU Int. (2019) 123:826–33. doi: 10.1111/bju.14540
28. Jevnikar Z, Rojnik M, Jamnik P, Doljak B, Fonovic UP, Kos J. Cathepsin H mediates the processing of talin and regulates migration of prostate cancer cells. J Biol Chem. (2013) 288:2201–9. doi: 10.1074/jbc.M112.436394
29. Yasukochi A, Kawakubo T, Nakamura S, Yamamoto K. Cathepsin E enhances anticancer activity of doxorubicin on human prostate cancer cells showing resistance to TRAIL-mediated apoptosis. Biol Chem. (2010) 391:947–58. doi: 10.1515/bc.2010.087
Keywords: cathepsins, benign prostate diseases, Mendelian randomization, bi-directional causal effects, benign prostatic hyperplasia (BPH)
Citation: Cao H, Liu B, Gong K, Wu H, Wang Y, Zhang H, Shi C, Wang P, Du H, Zhou H and Wang S (2024) Association between cathepsins and benign prostate diseases: a bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 15:1348310. doi: 10.3389/fendo.2024.1348310
Received: 05 December 2023; Accepted: 20 May 2024;
Published: 05 June 2024.
Edited by:
Vittoria Rago, University of Calabria, ItalyReviewed by:
Mohammeed Aufy, University of Vienna, AustriaYong Zhang, The Second Affiliated Hospital of Xi’an Jiaotong University, China
Copyright © 2024 Cao, Liu, Gong, Wu, Wang, Zhang, Shi, Wang, Du, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honglan Zhou, aGx6aG91QGpsdS5lZHUuY24=; Song Wang, d19zQGpsdS5lZHUuY24=
 Hongliang Cao
Hongliang Cao Bin Liu
Bin Liu Kejian Gong
Kejian Gong Hao Wu
Hao Wu Yishu Wang
Yishu Wang Haiyang Zhang4
Haiyang Zhang4 Chengdong Shi
Chengdong Shi Honglan Zhou
Honglan Zhou Song Wang
Song Wang