- 1Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Clinical Research Centre For Breast Disease In Hunan Province, Changsha, China
- 3Department of Breast Surgery, Xiangya Hospital, Central South University, Changsha, China
- 4Department of Breast Surgery, the First People's Hospital of Xiangtan City, Xiangtan, Hunan, China
Objective: Hormone receptor (HR)-low/HER2-negative breast cancers (BCs) are more likely to be basal-like BCs, with similar molecular features and gene expression profiles to HR-negative (estrogen receptor <1% or negative and progesterone receptor <1% or negative) BCs. Recently, with the clinical application of adjuvant intensive therapy for triple-negative breast cancer (TNBC), the prognosis of TNBC patients without pathological complete response (pCR) has significantly improved. Therefore, it is necessary to reanalyse the prognostic characteristics of clinically high-risk HR-low/HER2-negative BC.
Methods: According to the inclusion and exclusion standards, 288 patients with HR-low/HER2-negative BC and TNBC who received NAC and were followed up between 2015 and 2022 at three breast centres in Hunan Province, China, were enrolled. Inverse probability of treatment weighting (IPTW) was utilized to mitigate imbalances in baseline characteristics between the HR-low/HER2-negative BC group and TNBC group regarding event-free survival (EFS) and overall survival (OS). The primary clinical endpoints were pCR and EFS, while the secondary endpoints included OS, objective response rate (ORR), and clinical benefit rate (CBR).
Results: The pCR rate (27.1% vs. 28.0%, P = 1.000), ORR rate (76.9% vs. 78.3%, P = 0.827) and CBR rate (89.7% vs. 96.5%, P = 0.113) after NAC were similar between the HR-low/HER2-negative BC and the TNBC group. EFS in patients with non-pCR from the 2 groups was significantly inferior in comparison to patients with pCR (P = 0.001), and the 3-year EFS was 94.74% (95% CI = 85.21% to 100.00%) and 57.39% (95% CI =43.81% to 75.19%) in patients with pCR and non-pCR from the HR-low/HER2-negative BC group, respectively, and 89.70% (95% CI = 82.20% to 97.90%) and 69.73% (95% CI = 62.51% to 77.77%) in the TNBC patients with pCR and non-pCR, respectively.
Conclusions: In the real world, the therapeutic effects of NAC for HR-low/HER2-negative BCs and TNBCs were similar. EFS of patients with non-pCR in the HR-low/HER2-negative BC group was inferior to that of the TNBC group with non-pCR, suggesting that it is necessary to explore new adjuvant intensive therapy strategies for these patients.
Introduction
Breast cancer (BC) serves as the foremost contributor to cancer-related fatalities among women (1). Based on the immunohistochemistry (IHC) expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), BCs can be categorized into 4 distinct molecular subtypes as follows: HER2 overexpression, luminal A, luminal B, and triple-negative breast cancer (TNBC) (2).
Regarding clinical decisions and prognosis, the expression of hormone receptors (HR), which includes ER and PR, stands as one of the most critical biomarkers for breast cancer (BC) patients. The 2010 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines suggest that BCs with ER/PR expression of ≥1% should be regarded as HR positive (3). Moreover, the prognosis has remarkably benefited from endocrine therapy for the HR-positive BC patients (4). Recently, however, it has been reported that the efficacy of endocrine therapy in HR-low-expression BC remains uncertain (5–10). The 2020 ASCO/CAP guidelines recommend considering HR-low/HER2-negative BCs to be characterized by HER2-negative status and 1-10% ER/PR expression. They are resemble to basal-like BCs (11), with similar molecular features, gene expression profiles and incidence of BRCA 1/2 mutation to those of HR-negative (ER negative or <1% and PR negative or <1%) BCs (8, 12, 13).
It has been reported that the efficacy of neoadjuvant chemotherapy (NAC) and the survival of patients with HR-low/HER2-negative BCs and patients with TNBCs after NAC are similar (7). An analysis of neoadjuvant clinical trials (14) involving the randomized clinical trials of GeparQuinto (n=2491) (15, 16) and GeparSepto (n=1206) (17) from the German Breast Group (GBG) and Arbeitsgemeinschaft Gynäkologische Onkologie, Breast Group (AGO-B), found no statistically significant difference in the pathological complete response (pCR) rate between patients with HR-low/HER2-negative BC and those with TNBC (OR=1.47, 95% CI=0.89 to 2.42, P = 0.132). The same result was also observed in disease-free survival, distant disease-free survival, and overall survival (OS) between these 2 groups.
In recent years, the improvements of NAC and the proposal of an adjuvant intensive therapy strategy have significantly improved the prognosis of TNBC (18). The CREATE-X, SYSUCC-001 Randomized Clinical Trial provided the basis for the application of capecitabine in non-PCR patients with TNBC (19–21). However, adjuvant intensive therapies for HR-low/HER2-negative BCs have not been sufficiently studied. Therefore, it is necessary to reanalyse the prognostic characteristics of clinically high-risk HR-low/HER2-negative BCs, especially in non-pCR patients after NAC.
This real-world multicentre study intended to compare baseline characteristics, efficacy of NAC, and survival outcomes between patients with TNBCs and patients with HR-low/HER2-negative BCs.
Methods
Study design
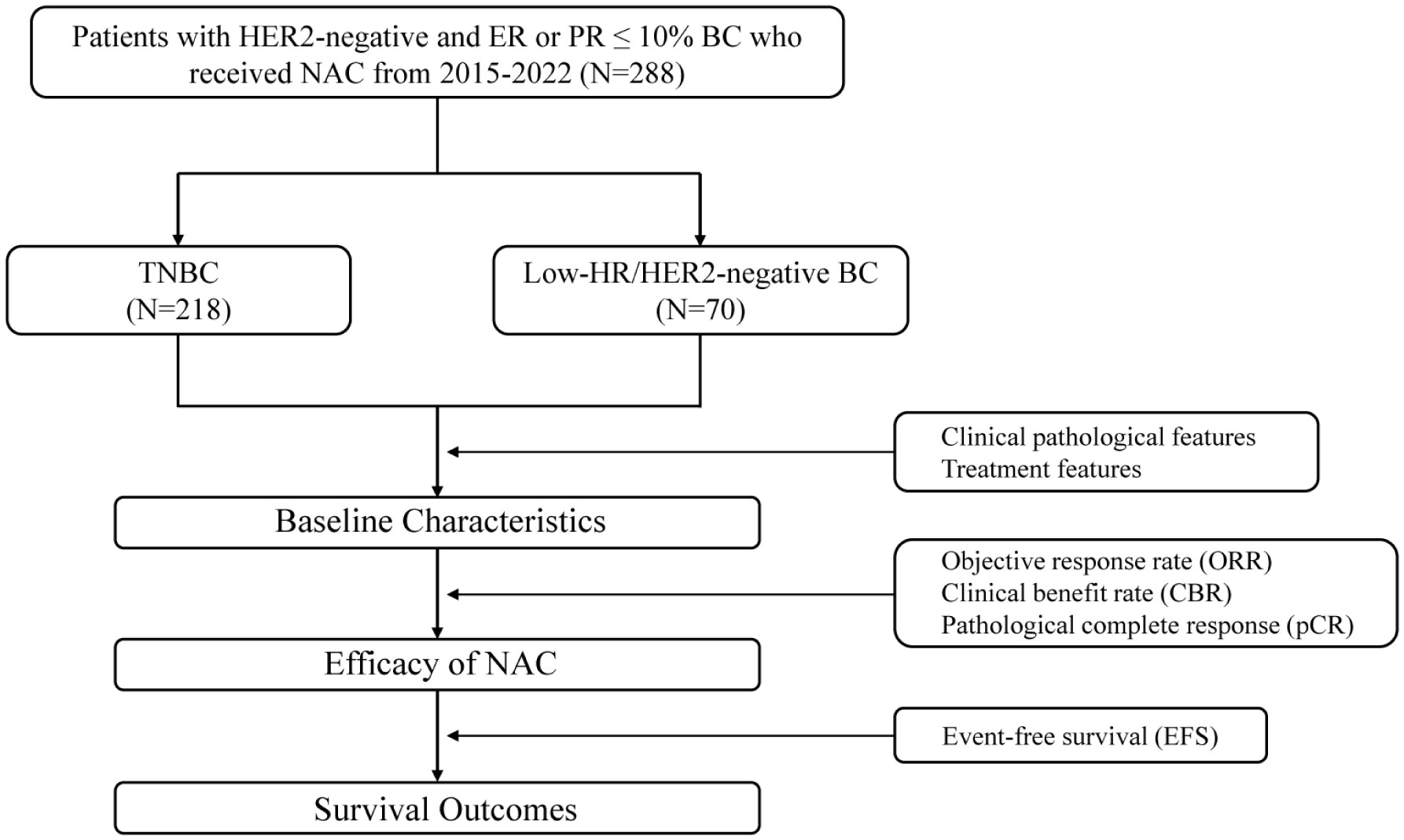
This was a retrospective multicentre real-world observational cohort study. This study included 288 BC patients from the Department of Breast Surgery of the Second Xiangya Hospital, Changsha, Hunan, China; Department of Breast Surgery of Xiangya Hospital, Changsha, Hunan, China; and the Department of Breast Surgery of the First People’s Hospital of Xiangtan City, Hunan, China, from 2015 to 2022. Patients were divided into 2 groups: HR-low/HER2-negative BC group (70 cases) and TNBC group (218 cases). We conducted follow-up assessments, collected clinical data, and compared the treatment efficacy and clinical outcomes of the 2 groups of patients (Figure 1).
Participants
Patients were included based on the following criteria: (1) female sex; (2) age of 20-80 years; (3) stage II or III; (4) histologically confirmed primary HER2-negative BC (IHC score of 0 or 1, or 2 with a negative result on fluorescence in situ hybridization) (20); (5) ER or PR ≤ 10% (the pathological results that contained a ER and PR percentage value IHC staining were adopted); and (6) received NAC. We strictly adhered to the following exclusion criteria: (1) unclear or missing ER or PR expression data; (2) advanced and metastatic BC; (3) inflammatory or bilateral BC; and (4) incomplete follow-up data. This study was conducted after approval at the ethics committee of the Second Xiangya Hospital of Central South University.
HR-low/HER2-negative BCs were defined as ER-low positive (1-10%) with PR-low positive (1-10%) or negative, PR-low positive with ER-low positive or negative, and HER2-negative status. ER, PR, and HER2 status were assessed by the pathology departments of the respective centres.
We extracted age, menopausal status (postmenopausal/premenopausal), ER expression percentage, PR expression percentage, clinical stage (T1-2/T3-4), pathological lymph node status (N0/N+), histological type (IDC/Other), tumour grade (II, III, unknown), NAC cycles (<6/≥6) and regimen (AC-T, TAC, Other), platinum-based NAC regimen (yes/no), breast surgery (breast-conserving surgery/mastectomy), axilla surgery (ALND/SLNB), therapeutic evaluation (Overall Response Rate/ORR, Clinical Benefit Rate/CBR, pCR), event-free survival (EFS) and OS from the database. Staging was evaluated according to the American Joint Committee on Cancer (AJCC) classification.
Clinical endpoints
The primary endpoints were the pCR rate and EFS. pCR was identified as the absence of invasive BC tumours in the breast and axillary lymph nodes surgical specimens after NAC (ypT0/is ypN0). EFS was described as the duration from the start of NAC until the first occurrence of any of the following events: disease progression without surgical treatment, local or distant recurrence, mortality from any cause, etc.
The secondary endpoints were OS, ORR and CBR, which were evaluated based on response evaluation criteria in solid tumours (RECIST 1.1). OS was described as the duration from the start of NAC until mortality from any cause. The ORR was characterized as the proportion of patients with complete response and partial response, while the CBR was determined by those with complete response or partial response or stable disease (22, 23). Ultrasonography and/or magnetic resonance imaging (MRI) were used for disease assessments at baseline, once every 2 or 3 cycles of NAC and once until surgery.
Statistical analysis
Continuous variables were summarized using the mean, while categorical variables were presented using frequency. T tests were employed to assess the differences in age between the HR-low/HER2-negative BC and TNBC groups, and the chi-square test was applied to compare the clinicopathological data, ORR, CBR and pCR between the two groups. A logistic regression model was employed to explore the association between each variable and pCR. Cox regression was employed to model EFS in both groups. The original distribution of demographic and clinicopathological characteristics between the HR-low/HER2-negative BC and TNBC groups were evaluated by the standardized mean difference (SMD); a value >10% indicated an unbalanced distribution between the two groups. Inverse probability of treatment weighting (IPTW) was conducted to balance baseline characteristics between the HR-low/HER2-negative BC and TNBC groups (24) (Table 1). The weights were calculated as the inverse of propensity scores, defined as the predicted probability of subtypes on age, menopausal status (postmenopausal/premenopausal), clinical stage (T1-2/T3-4), pathological lymph node status (N0/N+), tumour grade (II, III, unknown), and histological type (IDC/Other), applying a nonparametric covariate balancing method to estimate propensity scores (25). Highly represented characteristic assignments were weighted less, and rarer characteristic assignments were weighted more. The SMD was utilized again to assess balance, and an SMD within 10% was considered acceptable, while 0% was considered ideal (24). EFS and OS were evaluated with Kaplan−Meier survival curves and the clog log transformation (26). All statistical tests were two-sided, and significance was defined as a P value < 0.05. All analyses were conducted using R 4.2.2.
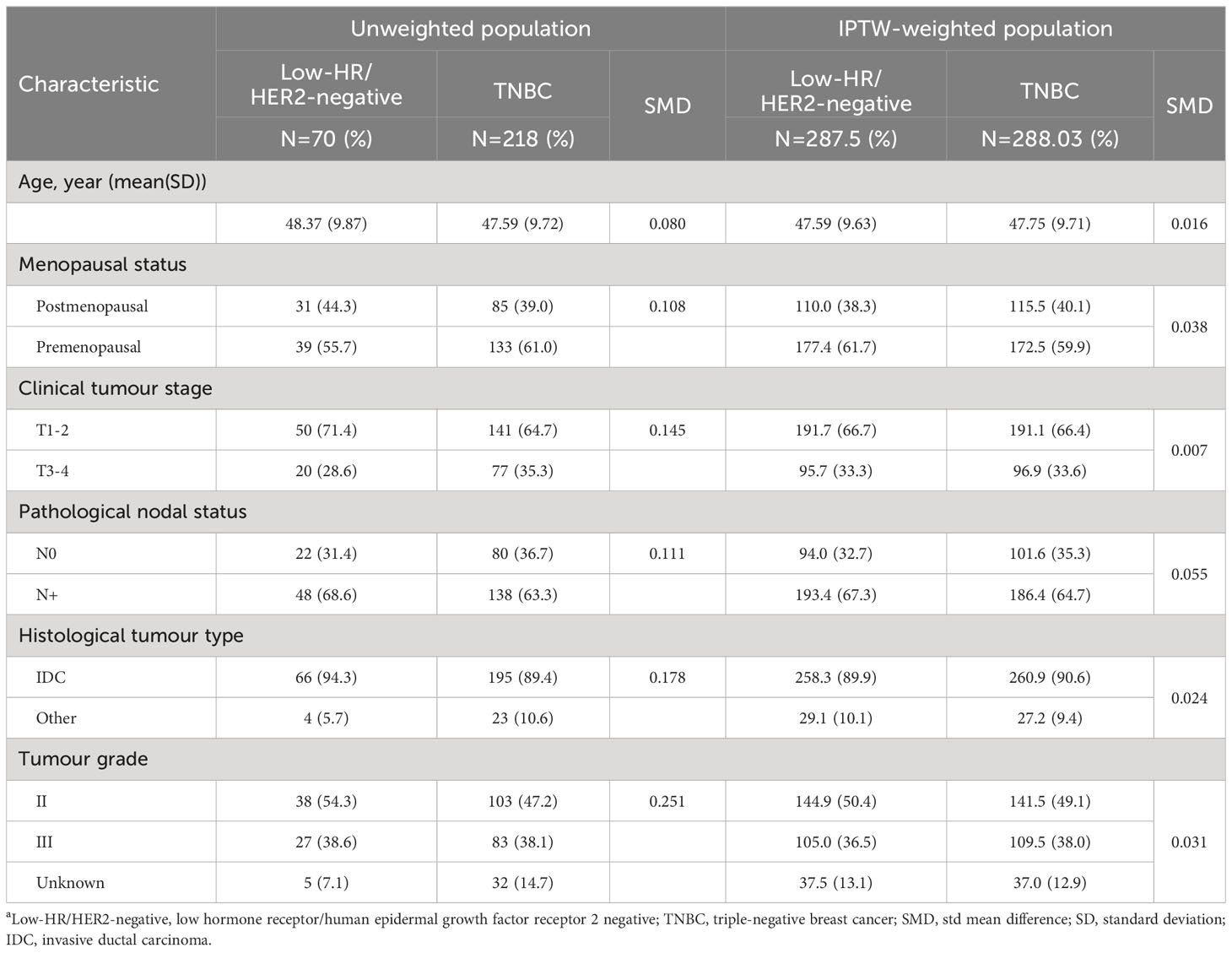
Table 1 Baseline patient characteristicsa.
Results
Baseline patient characteristics
The HR-low/HER2-negative BC and TNBC groups were composed of 70 and 218 patients, respectively. The mean age at first diagnosis was 48.37 years in the HR-low/HER2-negative BC group and 47.59 years in the TNBC group. Females were more frequently premenopausal in both groups (HR-low/HER2-negative BC: 55.7%; TNBC: 61.0%). Most patients had T1 or T2 tumours (HR-low/HER2-negative BC: 71.4%; TNBC: 64.7%), positive nodal status (HR-low/HER2-negative BC: 68.6%; TNBC: 63.3%), and invasive ductal carcinoma (HR-low/HER2-negative BC: 94.3%; TNBC: 89.4%). Grade 2 and 3 disease accounted for 54.3% and 38.6% of the total number of patients in the HR-low/HER2-negative BC group, and in the TNBC group, 47.2% and 38.1% of patients had tumours classified as grade 2 and 3, respectively. After IPTW adjustment, the covariates between the HR-low/HER2-negative BC and TNBC groups were found to be homogenous (Table 1).
The median number of treatment cycles for all 288 eligible participants was 6. A total of 65.7% of patients in the HR-low/HER2-negative BC group and 65.1% of patients in the TNBC group received no less than 6 cycles of NAC. The majority of patients received anthracycline-taxane-based NAC (HR-low/HER2-negative BC: 94.3%; TNBC: 89.0%), and only a small percentage of patients were treated with platinum (HR-low/HER2-negative BC: 12.9%; TNBC: 17.9%). After neoadjuvant therapy, most patients underwent mastectomy (HR-low/HER2-negative BC: 90.0%; TNBC: 96.3%) or axillary lymph node dissection (ALND) (HR-low/HER2-negative BC: 97.1%; TNBC: 98.6%) (Table 2). In the TNBC group, 53.5% of patients with non-pCR received capecitabine as intensive therapy after definitive surgery and adjuvant therapy. In the HR-low/HER2-negative BC group, all patients received adjuvant endocrine therapy, mainly toremifene and tamoxifen.
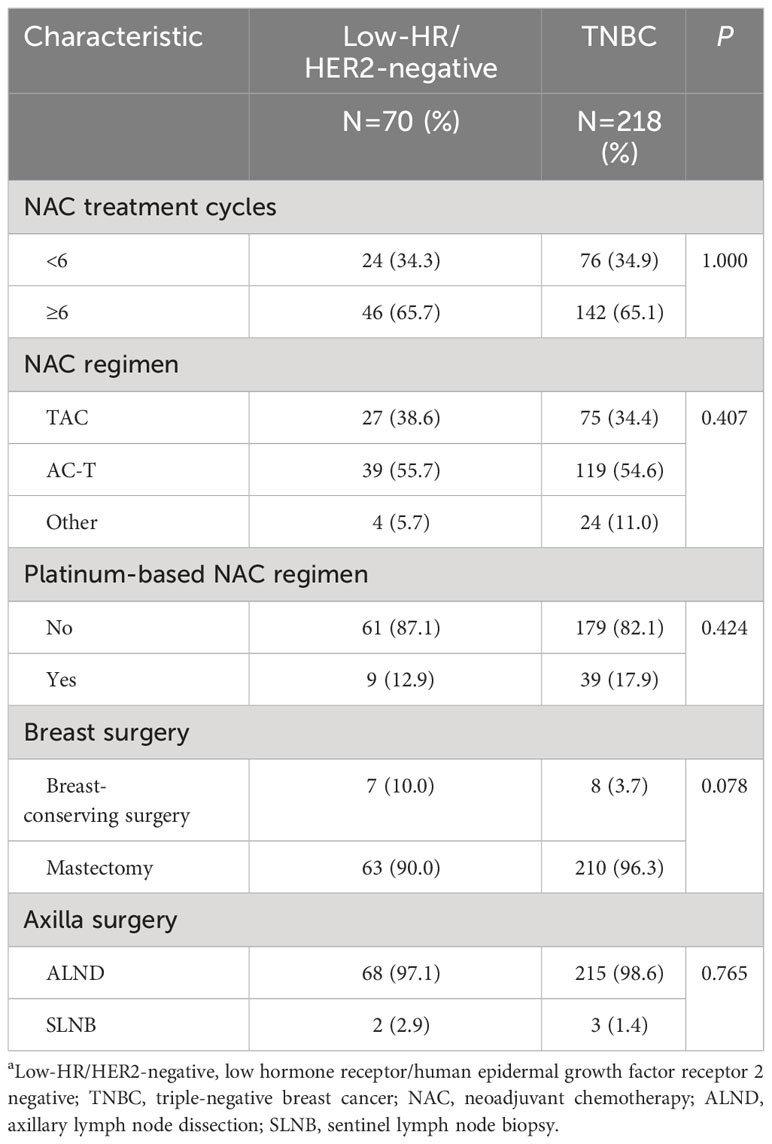
Table 2 Treatment characteristics of the patientsa.
Efficacy analysis
The ORR was 76.9% and 78.3% in the HR-low/HER2-negative BC group and the TNBC group, respectively. A total of 89.7% of patients with HR-low/HER2-negative BC and 96.5% of patients with TNBC met the CBR criteria. The pCR rate of the HR-low/HER2-negative BC group was similar to that of the TNBC group (27.1% vs. 28.0%). No significant difference was found in the ORR, CBR, or pCR rate between the 2 groups (ORR: P = 0.827; CBR: P = 0.113; pCR: P = 1.000) (Figure 2).
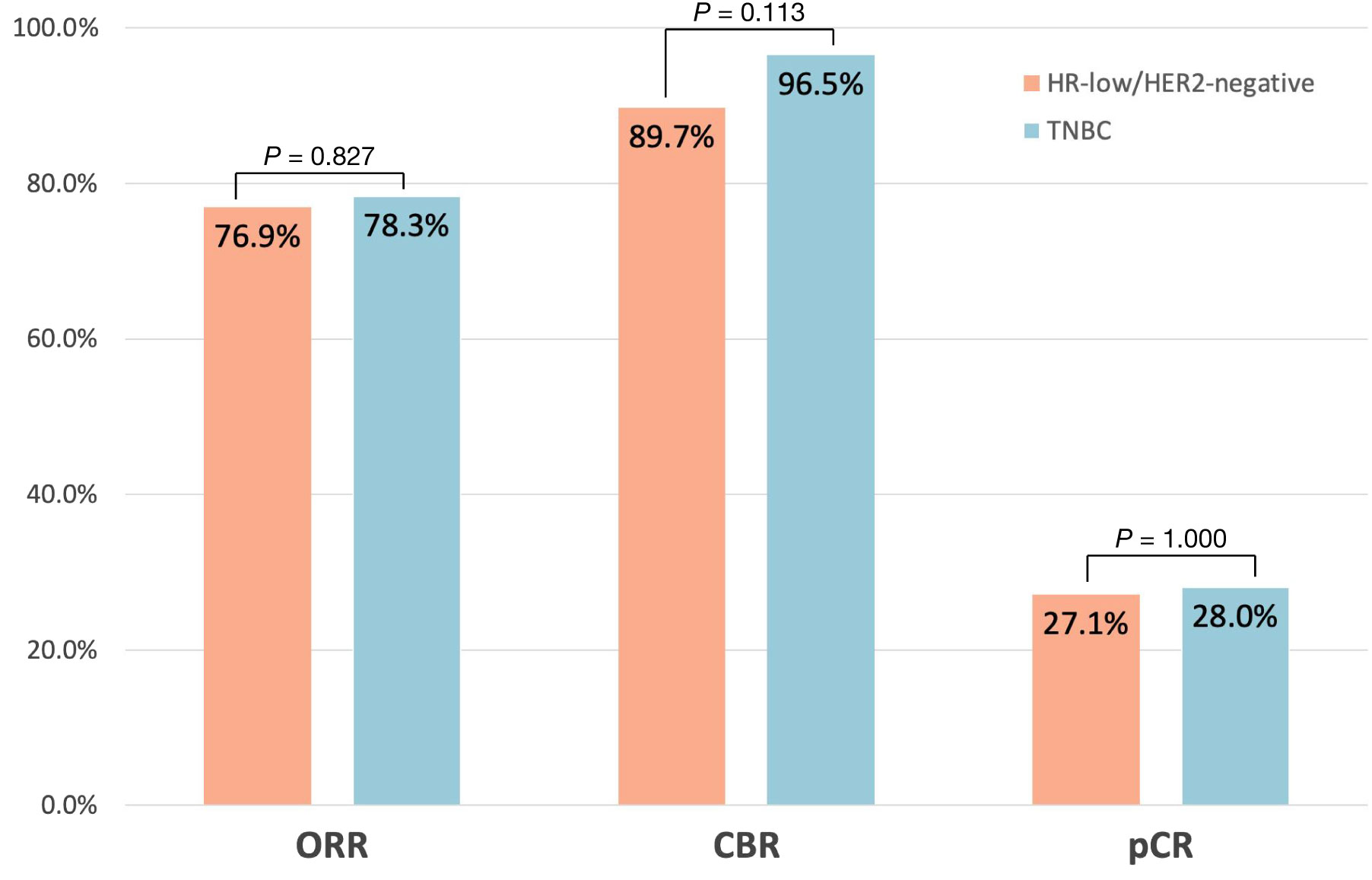
Figure 2 Pathological complete response (pCR: ypT0/is ypN0), objective response rate (ORR), and clinical benefit rate (CBR) across the HR-low/HER2-negative BC group and TNBC group.
According to the multivariate logistic regression analysis, NAC treatment cycles (P = 0.022) remained independently associated with pCR. The pCR rates in the HR-low/HER2-negative BC and TNBC groups were not significantly different according to either univariate or multivariate logistic analysis (Supplementary Table 1). According to the multivariate COX regression analysis, pathological nodal status (P = 0.001), histological tumour type (P = 0.009), and pCR (P = 0.013) were independently associated with EFS (Supplementary Table 2).
Comparing the EFS (P = 0.229) and OS (P = 0.579) between the 2 groups after IPTW, no significant difference was found (Figure 3A; Supplementary Figure 1). The 3-year EFS were 66.50% (95% CI = 54.83% to 80.66%) in the HR-low/HER2-negative BC group and 75.03% (95% CI = 69.15% to 81.41%) in the TNBC group.
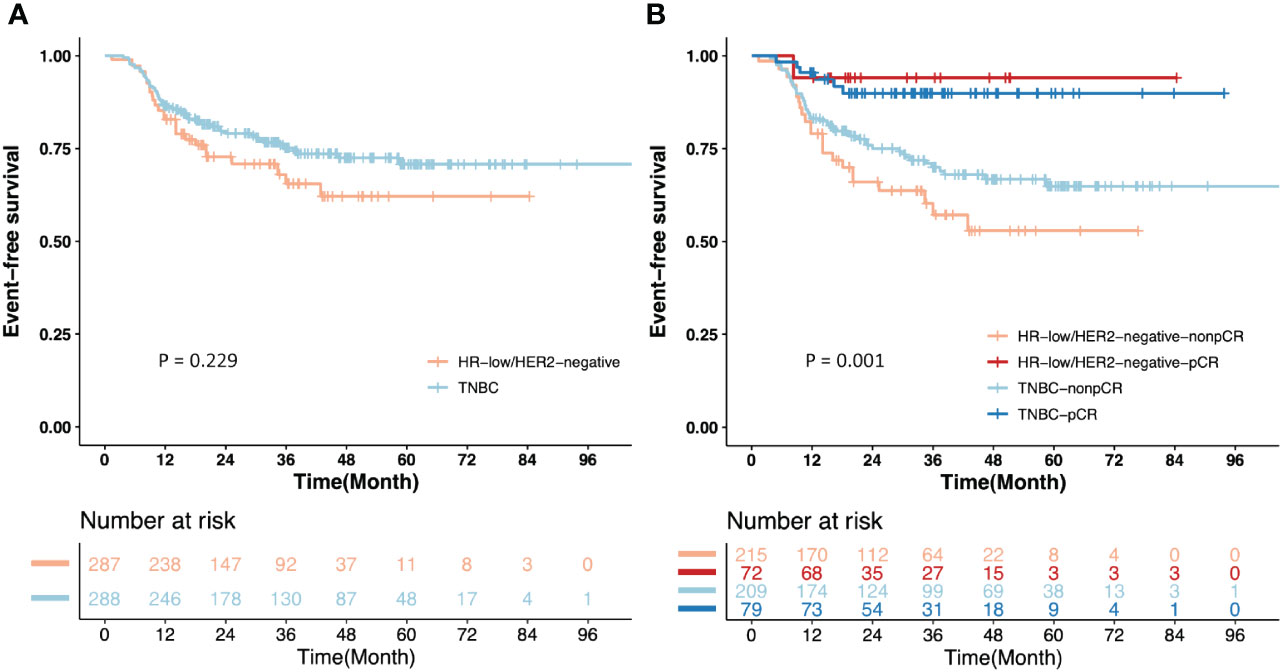
Figure 3 Kaplan-Meier estimates of event-free survival (EFS) after inverse probability of treatment weighting (IPTW). (A) EFS of the patients from the HR-low/HER2-negative BC and TNBC groups; (B) EFS of the patients with pCR or non-pCR from the HR-low/HER2-negative BC and TNBC groups.
Given that pCR is an important survival-relevant factor, additional survival analyses were performed among patients with pCR or non-pCR from the two groups separately. Non-pCR patients in the two groups showed a significantly higher risk of events than those with pCR (P = 0.001). The 3-year EFS of the patients with pCR and non-pCR were 94.74% (95% CI = 85.21% to 100.00%) and 57.39% (95% CI =43.81% to 75.19%), respectively, in the HR-low/HER2-negative BC group, and 89.70% (95% CI = 82.20% to 97.90%) and 69.73% (95% CI = 62.51% to 77.77%), respectively, in the TNBC group. For non-pCR patients, the 3-year EFS in the HR-low/HER2-negative BC group was lower than that in the TNBC group (57.39% vs. 69.73%). (Figure 3B).
Discussion
BC is a heterogeneous disease, and distinct subtypes of BC exhibit different treatment sensitivities. HR-low/HER2-negative BCs account for only 6% of all BCs (27). However, the similarity of the biological behaviour of HR-low/HER2-negative BCs and TNBCs has been described multiple times in recent years (6, 14, 28, 29).
Molecular profiling studies have reported that despite the majority of TNBCs being categorized as basal-like BCs, TNBCs contain other molecular subtypes, such as luminal BCs (30–32). It has also been reported that 1-10% of ER-positive tumours are heterogeneous and crossover with not only luminal A/B but also HER2-enriched and basal-like (14, 31–34), indicating a mixed distribution of molecular subtypes in HR-low/HER2-negative BC by IHC.
By comparing the data from our centres, we found that the proportion of TNBC tumours was approximately 3 times that of HR-low/HER2-negative BC tumours, which is consistent with the statistics from prior studies (9, 30). TNBCs generally exhibit a noticeably higher pCR rate than luminal tumours (35–38). However, we found that patients with HR-low/HER2-negative BCs (27.1%) and TNBCs (28.0%) had comparable pCR rates after NAC (P = 1.000). The low level of pCR rates in both groups are consistent with those in other real-world studies of the efficacy of NAC in TNBC (39–41), and may be related to patient compliance and differences in drug regimen selection between randomized controlled trials and real-world studies. Additionally, the grouping of HR-low/HER2-negative BCs or TNBCs had no impact on pCR in univariate (P = 0.524) and multivariate (P = 0.823) analyses of pCR.
In respect of EFS (P = 0.229) and OS (P = 0.579), no significant difference was found between the HR-low/HER2-negative BC group and the TNBC group (Figure 3A; Supplementary Figure 1). This outcome may be related to the relatively limited follow-up duration. However, EFS in patients with non-pCR from the two groups was significantly worse than those with pCR (P = 0.001). The HR-low/HER2-negative BC group had a lower 3-year EFS than the TNBC group (66.50% vs. 75.03%), and the patients with non-pCR from the HR-low/HER2-negative BC and TNBC groups showed an inferior 3-year EFS to that of the patients with pCR from the two groups (HR-low/HER2-negative BC with pCR and non-pCR: 94.74% vs. 57.39%; TNBC with pCR and non-pCR: 89.70% vs. 69.73%) (Figure 3B). No significant difference was observed in the 3-year EFS, but numerical differences did exist. We infer these may be attributed to the application of adjuvant intensive therapy, which remarkably improved the survival outcomes in non-pCR TNBC patients (20). Specifically, we propose that HR-low/HER2-negative BC patients, especially those with non-pCR after NAC, exhibit unmet treatment needs. This observation underscores their potential for intensified adjuvant therapy, such as combinations of capecitabine and endocrine therapy. We highlight the necessity for further prospective randomized controlled clinical studies to confirm these findings.
In addition to the pCR rate, the ORR and CBR are also commonly used therapeutic sensitivity parameters. To our knowledge, this is the first study to estimate ORR and CBR for patients with HR-low/HER2-negative BCs and TNBCs after NAC; likewise, no statistically significant difference was detected between the two subgroups (ORR: P = 0.827; CBR: P = 0.113; pCR: P = 1.000). Taken together, the absence of differences in patients’ baseline characteristics and NAC response between the 2 phenotypes highlights the fact that HR-low/HER2-negative BC appears to be biologically similar to TNBC. Current findings underscore the substantial unmet treatment needs of HR-low/HER2-negative BC patients, particularly those who fail to achieve a pCR following neoadjuvant chemotherapy. The potential efficacy of intensified adjuvant therapies, notably the combination of capecitabine and endocrine therapy, has emerged as a crucial area for addressing these needs. Such an approach could potentially improve outcomes for these patients, underlining the need for a more tailored therapeutic strategy based on HR and HER2 status. To validate these preliminary findings and fully understand the implications for treatment protocols, further prospective randomized controlled clinical studies are warranted.
This study had several limitations. First, this was a multicentre retrospective study, which may have been biased by the completeness of data collection and the accuracy of follow-up information. For example, there may have been slight differences in the data recording standards of different centres, and some prognostic follow-up information, which is derived from patient recollection, may have contained errors. These potentially compromised the accuracy and statistical power of the results. Second, the sample size was relatively small, and the follow-up time was relatively limited, to some extent, possibly posing challenges related to limited statistical ability and the capability to identify significant differences. However, HR-low/HER2-negative BC is indeed a rare subtype, which inherently limits the availability of a larger cohort for analysis. Despite these limitations, our preliminary findings contributed to a deeper understanding of HR-low/HER2-negative BC, and future studies are needed to validate and expand our findings through more rigorous data verification processes, larger cohorts, and extended follow-up periods.
Conclusions
In summary, the real-world therapeutic effects of NAC for HR-low/HER2-negative BCs and TNBCs were similar. EFS of patients with non-pCR in the HR-low/HER2-negative BC group was inferior to that of the TNBC group with non-pCR, suggesting that it is necessary to explore new adjuvant intensive therapy strategies for HR-low/HER2-negative BC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WY: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – review & editing. JP: Data curation, Formal analysis, Investigation, Software, Writing – original draft. YH: Data curation, Methodology, Supervision, Visualization, Writing – review & editing. QC: Data curation, Resources, Validation, Writing – review & editing. FX: Data curation, Resources, Writing – review & editing. DZ: Data curation, Resources, Writing – review & editing. JY: Data curation, Resources, Writing – review & editing. QZ: Data curation, Resources, Writing – review & editing. LY: Data curation, Resources, Writing – review & editing. LL: Data curation, Resources, Writing – review & editing. QL: Data curation, Resources, Writing – review & editing. LL: Data curation, Resources, Writing – review & editing. ML: Data curation, Resources, Writing – review & editing. XL: Data curation, Resources, Writing – review and editing. SW: Conceptualization, Data curation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Innovation Platform and Talent Plan of Hunan Province (Grant No.2023SK4019), the Science and Technology Innovation Program of Hunan Province (Grant No. 2021SK2026), the Clinical Medical Boot Technology Innovation Project of Hunan Province (Grant No. 2021SK53504), the Health and Family Planning Commission of Hunan Province (Grant No. 2022JJ70143), and the Clinical Research Special Fund of Wu Jieping Medical Foundation (Grant No. 320.6750.2022-19-29).
Acknowledgments
We are indebted to all the authors and our colleagues for the fruitful suggestions and discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1347762/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier estimates of overall survival (OS) of the patients from the HR-low/HER2-negative BC and TNBC groups after inverse probability of treatment weighting (IPTW).
References
1. Smolarz B, Nowak AZ, Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (Review of literature). Cancers (Basel). (2022) 14(10):2569. doi: 10.3390/cancers14102569
2. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol Sep. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
3. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol Jun 1. (2010) 28:2784–95. doi: 10.1200/JCO.2009.25.6529
4. Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol. (2016) 2:1477–86. doi: 10.1001/jamaoncol.2016.1897
5. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. (2015) 26:1533–46. doi: 10.1093/annonc/mdv221
6. Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, et al. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol. (2014) 25:1004–11. doi: 10.1093/annonc/mdu053
7. Yoder R, Kimler BF, Staley JM, Schwensen K, Wang YY, Finke K, et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer. (2022) 8:80. doi: 10.1038/s41523-022-00448-4
8. Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. (2012) 30:729–34. doi: 10.1200/jco.2011.36.2574
9. Raghav KP, Hernandez-Aya LF, Lei X, Chavez-Macgregor M, Meric-Bernstam F, Buchholz TA, et al. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. (2012) 118:1498–506. doi: 10.1002/cncr.26431
10. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. (2020) 38:1346–66. doi: 10.1200/jco.19.02309
11. Landmann A, Farrugia DJ, Zhu L, Diego EJ, Johnson RR, Soran A, et al. Low estrogen receptor (ER)-positive breast cancer and neoadjuvant systemic chemotherapy: is response similar to typical ER-positive or ER-negative disease? Am J Clin Pathol. (2018) 150:34–42. doi: 10.1093/ajcp/aqy028
12. Ohara AM, Naoi Y, Shimazu K, Kagara N, Shimoda M, Tanei T, et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat. (2019) 173:533–43. doi: 10.1007/s10549-018-5020-7
13. Sanford RA, Song J, Gutierrez-Barrera AM, Profato J, Woodson A, Litton JK, et al. High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer. (2015) 121:3422–7. doi: 10.1002/cncr.29572
14. Villegas SL, Nekljudova V, Pfarr N, Engel J, Untch M, Schrodi S, et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors - An analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer. (2021) 148:159–70. doi: 10.1016/j.ejca.2021.02.020
15. Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. (2012) 13:135–44. doi: 10.1016/s1470-2045(11)70397-7
16. von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. (2012) 366:299–309. doi: 10.1056/NEJMoa1111065
17. Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. (2016) 17:345–56. doi: 10.1016/s1470-2045(15)00542-2
18. Montemurro F, Nuzzolese I, Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother. (2020) 21:1071–82. doi: 10.1080/14656566.2020.1746273
19. Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. (2021) 325:50–8. doi: 10.1001/jama.2020.23370
20. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. (2017) 376:2147–59. doi: 10.1056/NEJMoa1612645
21. Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, et al. Adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide for early breast cancer: the randomized clinical FinXX trial. JAMA Oncol. (2017) 3:793–800. doi: 10.1001/jamaoncol.2016.6120
22. Chi P, Qin LX, Nguyen B, Kelly CM, D'Angelo SP, Dickson MA, et al. Phase II trial of imatinib plus binimetinib in patients with treatment-naive advanced gastrointestinal stromal tumor. J Clin Oncol. (2022) 40:997–1008. doi: 10.1200/JCO.21.02029
23. Chen IM, Johansen JS, Theile S, Hjaltelin JX, Novitski SI, Brunak S, et al. Randomized phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol. (2022) 40:3180–9. doi: 10.1200/jco.21.02511
24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34:3661–79. doi: 10.1002/sim.6607
25. Fong C, Hazlett C, Imai K. Covariate balancing propensity score for a continuous treatment: Application to the efficacy of political advertisements. Ann Appl Statistics. (2018) 12(1):156–77. doi: 10.1214/17-aoas1101
26. Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. (2007) 26:4505–19. doi: 10.1002/sim.2864
27. Allred DC, Carlson RW, Berry DA, Burstein HJ, Edge SB, Goldstein LJ, et al. NCCN task force report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw. (2009) 7 Suppl 6:S1–S21; quiz S22-3. doi: 10.6004/jnccn.2009.0079
28. Fujii T, Kogawa T, Dong W, Sahin AA, Moulder S, Litton JK, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. (2017) 28:2420–8. doi: 10.1093/annonc/mdx397
29. Zhang T, Ho B, Chan PMY, Tan EY. Hormonal therapy confers clinically relevant benefit in women with low estrogen receptor-positive breast tumor. Ann Oncol. (2018) 29:9–9. doi: 10.1093/annonc/mdy427.004
30. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. (2010) 7:683–92. doi: 10.1038/nrclinonc.2010.154
31. Benefield HC, Allott EH, Reeder-Hayes KE, Perou CM, Carey LA, Geradts J, et al. Borderline estrogen receptor-positive breast cancers in black and white women. J Natl Cancer Inst Jul 1. (2020) 112:728–36. doi: 10.1093/jnci/djz206
32. Cheang MC, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. (2015) 20:474–82. doi: 10.1634/theoncologist.2014-0372
33. Prabhu JS, Korlimarla A, Desai K, Alexander A, Raghavan R, Ce A, et al. A majority of low (1-10%) ER positive breast cancers behave like hormone receptor negative tumors. J Cancer. (2014) 5:156–65. doi: 10.7150/jca.7668
34. Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. (2013) 20:87–93. doi: 10.1245/s10434-012-2588-8
35. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. (2005) 11:5678–85. doi: 10.1158/1078-0432.Ccr-04-2421
36. Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. (2010) 116:1431–9. doi: 10.1002/cncr.24876
37. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. (2007) 13:2329–34. doi: 10.1158/1078-0432.Ccr-06-1109
38. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. (2023) 41:1809–15. doi: 10.1200/jco.22.02572
39. Walbaum B, Acevedo F, Medina L, Bravo ML, Merino T, Camus M, et al. Pathological complete response to neoadjuvant chemotherapy, but not the addition of carboplatin, is associated with improved survival in Chilean triple negative breast cancer patients: a report of real world data. Ecancermedicalscience. (2021) 15:1178. doi: 10.3332/ecancer.2021.1178
40. Li Y, Chen H, He J, Fan Z, Zhang H. The outcome of neoadjuvant chemotherapy and the current trend of surgical treatment in young women with breast cancer: A multicenter real-world study (CSBrS-012). Front Public Health. (2023) 11:1100421. doi: 10.3389/fpubh.2023.1100421
Keywords: breast cancer, estrogen receptor, progesterone receptor, triple-negative breast cancer, HR-low/HER2-negative breast cancer, neoadjuvant chemotherapy
Citation: Peng J, Hong Y, Chen Q, Xu F, Zhang D, Yao J, Zou Q, Yuan L, Li L, Long Q, Liao L, Liu M, Liu X, Wang S and Yi W (2024) Comparison of neoadjuvant chemotherapy response and prognosis between HR-low/HER2-negative BC and TNBC: an exploratory real-world multicentre cohort study. Front. Endocrinol. 15:1347762. doi: 10.3389/fendo.2024.1347762
Received: 01 December 2023; Accepted: 26 February 2024;
Published: 19 March 2024.
Edited by:
Umar Mehraj, Duke University, United StatesReviewed by:
Basharat Ahmad Bhat, University of Kashmir, IndiaMiguel J. Gil Gil, Catalan Institute of Oncology, Spain
Copyright © 2024 Peng, Hong, Chen, Xu, Zhang, Yao, Zou, Yuan, Li, Long, Liao, Liu, Liu, Wang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjun Yi, eWl3ZW5qdW5AY3N1LmVkdS5jbg==; Shouman Wang, d2FuZ3Nob3VtYW5AMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jing Peng1,2†
Jing Peng1,2† Qitong Chen
Qitong Chen Danhua Zhang
Danhua Zhang Lun Li
Lun Li Qian Long
Qian Long Wenjun Yi
Wenjun Yi