
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 15 October 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1346476
Differentiated thyroid cancers (DTCs) constitute the primary histological subtype within thyroid cancer. Due to DTCs’ distinctive radioiodine (RAI) uptake mechanism, standard treatment involving surgery, with or without adjunctive therapy using RAI and levothyroxine inhibition, typically yields favorable prognoses for the majority of patients with DTCs. However, this favorable outcome does not extend to individuals with decreased RAI uptake, termed radioiodine-refractory thyroid cancers (RAI-RTCs). Recent research has revealed that the genetic mutations and gene rearrangements affecting sites such as RTKs, RAS, BRAF and TERTp lead to structural and functional abnormalities in encoded proteins. These abnormalities aberrantly activate signaling pathways like the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-hydroxykinase (PI3K) signaling pathways, resulting in thyroid cells dedifferentiation, sodium/iodide symporter (NIS) dysfunction, and consequent the RAI-refractory nature of DTCs. Targeted therapy tailored to mutations presents a promising avenue for the treatment of RAI-RTCs. Lenvatinib and sorafenib, multi-kinase inhibitors, represent the standard first-line systemic treatment options, while cabozantinib is the standard second-line treatment option, for this purpose. Furthermore, ongoing clinical trials are exploring selective kinase inhibitors, immune checkpoint inhibitors, and combination therapies. Notably, numerous clinical trials have demonstrated that selective kinase inhibitors like BRAF, MEK and mTOR inhibitors can restore RAI uptake in tumor cells. However, further validation through multicenter, large-sample, double-blinded randomized controlled trials are essential. Enhanced treatment strategies and innovative therapies are expected to benefit a broader spectrum of patients as these advancements progress.
Thyroid cancer is the most prevalent malignancy within endocrine system (1). According to data from GLOBOCAN 2020 by the International Agency for Research on Cancer, the year 2020 witnessed around 586,000 new cases of thyroid cancer globally, ranking it ninth among all cancers (2). The three encompassed pathological types are differentiated thyroid cancers (DTCs), medullary cancers and anaplastic cancers, with DTCs comprising about 90% of all thyroid cancers (3). The standard treatment for DTCs involves surgery often coupled with radioiodine (RAI) therapy and thyroid stimulating hormone (TSH) suppression. At the same time, more and more studies show that in addition to the above standard treatment for thyroid cancer, active surveillance is also an important strategy (4–6). Most patients with DTCs exhibit a favorable prognosis with low mortality (7). However, a subset of patients with DTCs shows resistance to RAI treatment, leading to disease progression post-treatment (8). These cases, constituting 5% to 15% of DTCs and 50% of metastatic DTCs (7, 9, 10), are termed as radioiodine-refractory thyroid cancers (RAI-RTCs) (11), displaying 5-year disease-specific survival rates of 60% to 70% (12) with a 10-year survival rate of 10% (13). Current treatment options for RAI-RTCs encompass targeted therapy utilizing tyrosine kinase inhibitors (TKIs), immunotherapy, cytotoxic chemotherapy and active surveillance (14). Among these, targeted therapy, particularly with sorafenib, lenvatinib and cabozantinib approved by the Food and Drug Administration (FDA) for RAI-RTCs treatment (15–18), emerges as a relatively established option. However, these agents offer only limited improvement in prognosis. With ongoing research into the pathogenesis of RAI-RTCs, patients now have access to new treatment avenues. This review aims to outline the pathogenesis of RAI-RTCs, novel therapies, particularly advancements in targeted tyrosine kinase inhibitor (TKI) therapies, and the status of clinical trials (completed and ongoing).
In 2006, Durante C et al. published a study of the long-term outcome of 444 patients with distant metastases of DTC, which showed that RAI uptake of the lesions in some DTC patients might gradually decrease or even disappear as the disease progressed, leading to limitations of RAI therapy (13). RAI-RTCs have gradually been recognized by researchers since then, but its definition has undergone evolution over time and is still controversial so far. It was not until September 2010, at the 14th International Thyroid Congress, that the definition of RAI-RTCs was initially proposed. RAI-RTCs were characterized by the absence of RAI uptake in one lesion or the lack of clinical evidence indicating additional benefits from RAI therapy (11). In 2015, the American Thyroid Association (ATA) broadened the scope of RAI-RTCs in the Guidelines for the Diagnosis and Treatment of Thyroid Nodules and Differentiated Thyroid Cancer in Adults, stating four manifestations: (i) the malignant/metastatic tissue does not ever concentrate RAI, (ii) the tumor tissue loses the ability to concentrate RAI after previous evidence of RAI-avid disease, (iii) RAI is concentrated in some lesions but not in others; and (iv) metastatic disease progresses despite significant concentration of RAI (3). But the precise definition of “ significant concentration of RAI “ was not specified. About one year later, the consensus for the management of advanced RAI-RTCs was issued by the Spanish Endocrine Society Thyroid Cancer Working Group and the Spanish Rare Cancer Working Group in 2016, recommending that lesions exhibiting high 18-F-deoxyglucose uptake on positron emission tomography/computed tomography (PET/CT) and total cumulative doses of RAI over 22.2GBq (600 mCi) could also be considered as diagnostic criteria for RAI-RTCs (19). Since many confounding factors in the likelihood appraisal and decision making about further RAI therapy, for example technique issues, standardization of radioactive iodine imaging and other limitations, were not given full consideration, the 2015 ATA guidelines were met with disagreement by extended nuclear medicine community. The European Association of Nuclear Medicine (EANM), the Society of Nuclear Medicine and Molecular Imaging (SNMMI), ATA and the European Thyroid Association (ETA) had an interactive meeting in Martinique thereafter in January 2018 with eight countries represented. A set of nine principles (Martinique Principles) were agreed on and published in 2019 (20). It was pointed out that characteristics used to classify patients as RAI refractory should be used to risk stratify patients and not necessarily as definitive criteria to mandate whether RAI therapy should be recommended. Five common clinical scenarios were summarized to be suggestive of the possibility of RAI-RTCs rather than absolute indicators, including: 1) no RAI uptake is present on a diagnostic RAI scan; 2) no RAI uptake is present on a RAI scan performed several days after RAI therapy; 3) RAI uptake is only present in some but not other tumor foci; 4) DTC metastasis(es) progress despite RAI uptake; 5) DTC metastasis(es) progress despite a cumulative RAI activity of 22.2GBq (600mCi). After Martinique meeting, ETA detailed the requirement for the assessment of RAI-RTCs. It was recommended that SPECT-CT be performed after high-activity RAI with preparation of high TSH and a diet with low iodine content; progression be defined as radiological progression, according to the response evaluation criteria in solid tumors (RECIST) 1.1) criteria, within a clinically relevant time frame, which is usually considered 6-12 months (21). In summary, no current definition, classification, criterion, or clinical scenario is an absolute indicator to label a patient as RAI refractory, but they convey the likelihood that a tumor will be refractory to additional RAI therapy. RAI-refractory criteria will continue to evolve, when confounding limitations and technical issues are addressed, techniques for radioactive iodine imaging are optimized and standardized, and the effectiveness of RAI therapy is enhanced by re-differentiation therapies (20).
The normal thyroid follicular cell membrane contains the sodium/iodide symporter (NIS), which actively transport two sodium ions and one iodide ion into the cytoplasm simultaneously (22). RAI is absorbed into thyroid tumor cells via NIS, releasing β rays that effectively destroy residual thyroid cancer cells within a range of 2.4 mm (23, 24), thereby playing an important role in the treatment of thyroid cancer. In general, lower NIS expression correlates with poorer differentiation of thyroid tumor cells, leading to less RAI uptake and ultimately the development of RAI-RTCs. Therefore, many thyroid-cancer-associated alterations are also playing a role in the development of RAI-RTCs. The pathogenesis process of RAI-RTCs is intricately associated with abnormal activation of the MAPK and/or PI3K signaling pathway, disruption of p53 functions, re-expression of telomerase reverse transcriptase (TERT), perturbation of the SWI-SNF chromatin remodeling complex and some rare genetic alterations (25–28). A brief description can be seen in Figure 1.
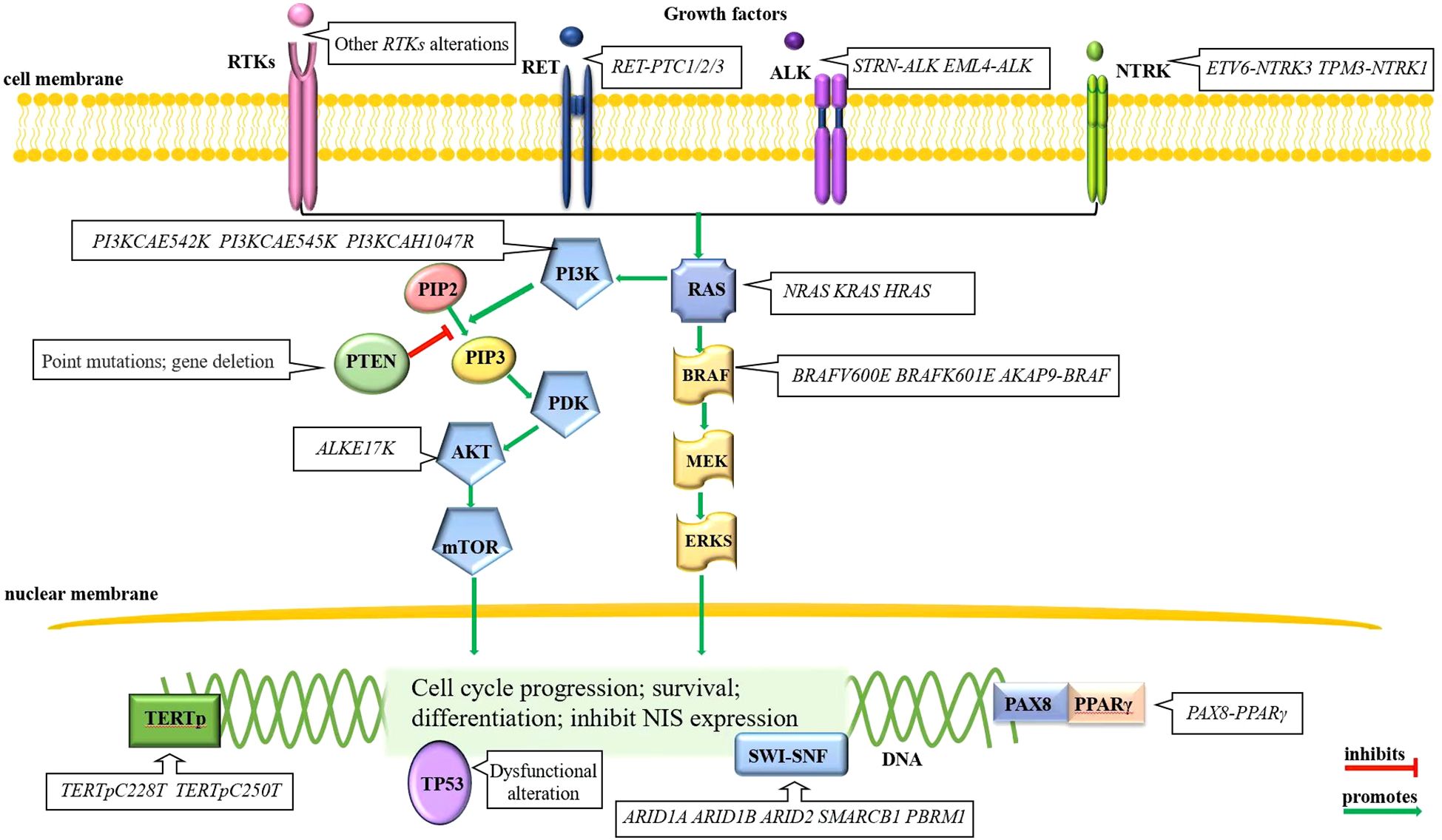
Figure 1. Molecular alterations of RAI-RTCs. Mutually exclusive BRAF and RAS alterations in the MAPK signaling pathway and gene alterations in RTKs, primarily RET, are often the initial events of thyroid cancer. Other mutational events, for example, the gene alterations in PI3K signaling pathway, disruption of p53 functions, re-express TERT, perturb the SWI-SNF chromatin remodeling complex, etc., drive disease progression (28).
RAS mutations are early events in thyroid tumorigenesis, include three mutations (KRAS, HRAS, and NRAS), with the mutant in codon 61 of NRAS being the most common variant (29, 30). The RAS oncoprotein, as a common effector of the PI3K and MAPK signaling pathways, prevalently affects the latter signaling pathway (31). The mutated RAS protein is locked into guanosine triphosphate (GTP), persistently activating the PI3K pathway, which ultimately promotes tumor progression (32).
The RTKs genes encode cell-membrane-located kinases that are stimulated by insulin/insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF) (33), vascular endothelial growth factor (VEGF) and other cytokines (27) and then signal to downstream pathways including MAPK and PI3K signaling pathways. Mutations of RTKs genes can cause increased RTKs genes transcription, RTKs proteins mislocalization, aberrant RTKs fusion proteins, enhanced affinity for cytokines, cascade activation of the downstream MAPK and PI3K signaling pathways (34, 35), and thereby initiated a subset of thyroid cancers. RTKs genetic alterations are mainly gene rearrangement, which occur in proto-oncogene c-ret protein (RET), anaplastic lymphoma kinase (ALK), and neurotrophic tyrosine kinase receptor (NTRK). The RET rearrangement with coiled-coil domain-containing gene 6 (CCDC6), nuclear receptor coactivator 4 (NCOA4) and protein kinase cAMP-dependent type I regulatory subunit alpha (PRKAR1A) makes fusion gene RET-PTC1/2/3 (36), which is common in DTCs (37); the rearrangement of ALK and the striatin gene or EML4, results in the new fusion genes STRN-ALK (38) and EML4-ALK (39), respectively; the NTRK rearrangement with ETS variant gene 6 and α-tropomyosin (TPM3) forms the new fusion genes ETV6-NTRK3 (40) and TPM3-NTRK1 (41), respectively. Other rare genetic alterations, including gene copy number gain and missense mutations, occur in PDGFR, VEGFR, c-KIT, FGFR and FLT3 (31, 42–44).
It is worth noting that ALK fusion and NTRK fusion are rather rare in adult thyroid cancers (45, 46). Fusions involving the gene RET followed by NTRK and ALK, are the most prevalent rearrangements found in pediatric papillary thyroid carcinoma (PTC) (47) and have the highest association with invasive disease, particularly in cases of RET fusion (48). RET fusion genes are three times more frequently in pediatric than in adult patients. A total of 20 types of RET fusions have been identified, including CCDC6, NCOA4, RUFY2, AFAP1L2 and PRKAR1A, among others (48). Regarding NTRK fusion genes, the ETV6-NTRK3 fusion is most common, followed by TPR-NTRK1 and then other less frequent fusion patterns with both NTRK3 and NTRK1 (49). ALK fusion is rare in pediatric PTC, the predominant type identified is STRN-ALK (50).
BRAF mutations are early events in thyroid tumorigenesis (51), including BRAFV600E (52) and more rarely, BRAFK601E (52, 53). As the predominant form of mutation, the prevalence of BRAFV600E is lower in pediatric (especially ≤10 years old) PTC than in adult PTC (47). BRAF gene rearrangement (AKAP9-BRAF) is more common in radiation-related PTC but has also been reported in poorly differentiated thyroid cancers and anaplastic thyroid cancers (53, 54). It is noteworthy that BRAF mutations and RAS mutations occurs mutually exclusive.
Thyroid-cancer-associated genetic alterations in the PI3K signaling pathway mainly occur in three categories of genes: genes encoding phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA) α catalytic subunit, the serine-threonine protein kinase AKT, and phosphatase and tensin homolog phosphatase (PTEN). Genetic alterations of PIK3CA and AKT include point mutations and copy number gains, both are late events in thyroid tumorigenesis (55). Missense mutations of PIK3CA take place in exons 9 and 20 (E542K, E545K and H1047R) and are less frequent than copy number gains occurring at chromosome site 3q26.3 (56). These events increase PIK3CA protein expression, yet their tumorigenic role is not well defined. The AKT mutation is the E17K substitution, and this mutation can inhibit the apoptosis of thyroid cancer cells (55). It should be noted that unlike the first two oncogenes, PTEN, as a tumor suppressor gene (42), promotes NIS expression (57) and inhibits the PI3K signaling pathway (42). Genetic alterations of PTEN in thyroid cancer include point mutations, heterozygous deletion, whole gene deletion and epigenetic modification (58). The genetic alterations silence PTEN and activate the PI3K signaling pathway, resulting in enhanced tumor proliferation and invasion (32, 58).
The abnormal activation of the MAPK signaling pathway can promote the expression of tumor microenvironment (TME)-related genes (59–62) and impair the expression of genes that are required for normal thyroid function (63), which consequently leads to tumor enlargement and distant metastasis (34, 35). The physiological functions of PI3K signaling pathway activation facilitate cell metabolism, growth, proliferation and survival. Aberrant activation of PI3K signaling pathway can aid the uninhibited growth of cancer cells by increasing protein synthesis (64) and by interacting with unrestricted MAPK signaling pathway in thyroid tumor progression.
Beside of cell dedifferentiation and proliferation, impaired function or decreased expression of NIS is another major mechanism contributing to the RAI-refractory nature of thyroid cancer. The expression of NIS is regulated at both the transcription and posttranscription levels. The abnormal activation of MAPK and PI3K signaling pathway can inhibit NIS expression by promoting TGFβ-Smad signaling pathway and interfering the proximal promoter region of NIS for the former (65), and by inhibiting TSH-dependent NIS expression pathway for the latter (33, 66). The abnormal activation of both signaling pathways can also interfere with the correct localization of NIS protein to the cell membrane (67). A brief description can be seen in Figure 2.
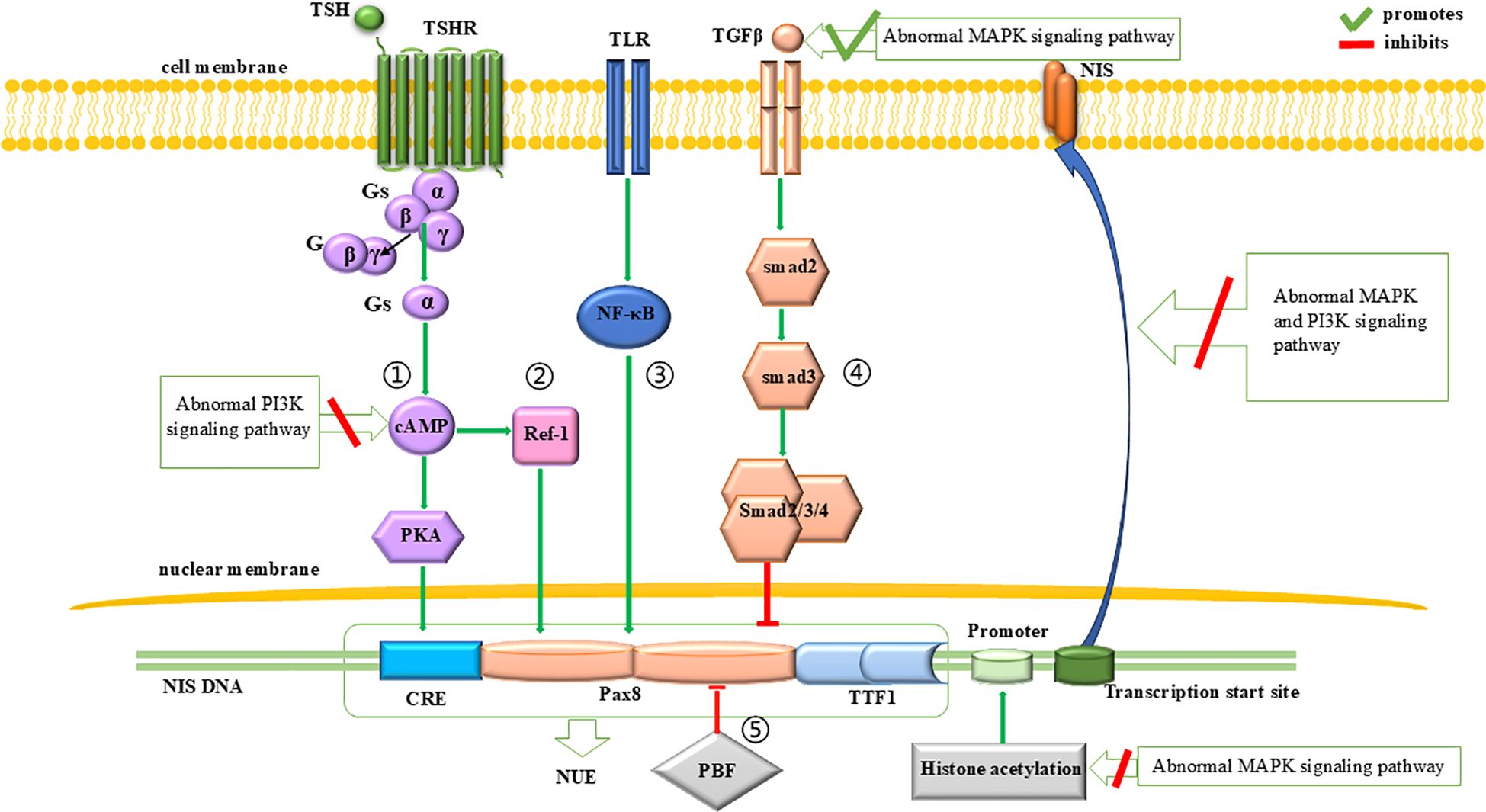
Figure 2. Regulation of NIS expression. The expression of NIS is influenced by the PI3K and MAPK signaling pathways. At the transcriptional level, abnormal MAPK signaling pathway initially reduces NIS expression by inhibiting histone acetylation at the promoter region. Subsequently, it downregulates NIS transcription by promoting the inhibitory effects of the TGFβ-Smad signaling pathway on NUE. Additionally, abnormal PI3K signaling pathway can inhibit the promotion effect of TSH-dependent NIS expression pathway on NUE, thereby leading to a downregulation of NIS expression. At the translational level, both abnormal PI3K and MAPK signaling pathways can impair the proper localization of NIS to the cell membrane.
NIS expression is regulated by two regions: the proximal promoter (68) and the NIS upstream enhancer (NUE) (69).
For the proximal promoter, the abnormal activation of the MAPK signaling pathway can downregulate the histone acetylation within this region, leading to the silencing of NIS expression (42, 70–73).
At the NUE, regulation of NIS transcription involves a cAMP-response element (CRE)-like sites (69, 74–76) and two paired box 8 (Pax8) binding sites (69). As for the CRE-like sites, their cAMP- and PKA-dependent phosphorylation activates NUE and promote NIS expression (76, 77). Since cAMP is produced by the action of extracellular thyroid stimulating hormone (TSH) (27), this pathway is also known as the TSH-dependent NIS expression pathway (Figure 2, pathway 1). This pathway can be inhibited by an abnormally activated PI3K signaling pathway (33, 66). As for the Pax8 binding sites, Pax8 binding to NUE can activate NUE and promote NIS expression (78, 79). This process is regulated by four signaling pathways: (1) A protein kinase A (PKA)-independent pathway, where cAMP promotes the binding of Pax8 to NUE through redox effector factor-1 (Ref-1) (75, 80–82) (Figure 2, pathway 2); (2) The Toll-like receptor (TLR)-NF-κB pathway, in which TLRs are stimulated by extracellular signals and promote the binding of Pax8 to NUE through NF-κB (83, 84) (Figure 2, pathway 3); (3) The TGFβ-Smad signaling pathway, in which Smad3 activation by TGFβ can inhibit the binding of Pax8 to NUE (59, 80) (Figure 2, pathway 4), and it is notable that the abnormal activation of the MAPK signaling pathway can promote TGFβ secretion and inhibit NIS expression (65); (4) the pituitary tumor-transforming gene-1 product (PTTG1)-binding factor (PBF) complex, which can interfere with the binding of Pax8 to NUE (85–87) (Figure 2, pathway 5).
Abnormal modification of the NIS protein or its incorrect localization to the cell membrane leads to NIS dysfunction. NIS protein modifications (including phosphorylation and glycosylation) and cell membrane localization (88, 89) are regulated not only by TSH (89–91) but also by the MAPK and PI3K signaling pathways. Previous studies have shown that abnormal activation of the MAPK and PI3K signaling pathways inhibit the correct localization of NIS protein to the cell membrane (67).
The p53 is a tumor suppressor encoded by TP53 that facilitates cell cycle control, DNA repair and apoptosis in response to various cellular stresses, thereby quenching the growth and proliferation of abnormal cells (92, 93). Genetic inactivation of p53 enable mutant tumor cells to circumvent these checkpoints (94). Indeed, p53 deficiency in association with activating mutations of oncogenes, such as RAS and BRAF, accounts for the high proliferation rate and increased aggressiveness of the more aggressive forms of thyroid cancer (95).
TERT is important for maintaining chromosomal integrity and genome stability (96). In most somatic cells, telomeres shorten with cell division, and when their length reaches a critical point, cells enter senescence or apoptosis (96).However, in thyroid cancer cells, an activating mutation in the TERT promoter (TERTp) prevents telomere shortening, allowing tumor cells to continue dividing and proliferating (97), thereby driving disease progression. The common TERTp mutations in thyroid cancer are two mutually exclusive mutations, C228T and C250T (98). These mutations form a consensus binding site for the E-twenty-six (ETS) transcription factor in the TERTp region, increasing its transcriptional activity (98). TERTp mutations, as a late event in tumor progression (55, 98), can also occur simultaneously with BRAF or RAS mutations in poorly differentiated and anaplastic thyroid cancers (99, 100), enhancing tumor aggressiveness (100–105). Therefore, they can be served as an early predictor of RAI-RTCs (106). Recently, some mouse model studies have shown that TERT reactivation can accelerate progression of BRAF-driven thyroid tumors via non-telomeric effects such as cytokine and PI3K signaling (107, 108). This association of TERT and PI3K signaling pathway may shed some light on the role of TERT in the pathogenesis of thyroid cancer as well as RAI-RTCs.
The function of SWI-SNF complexes is to reconfigure chromatin, thereby determining the expression of certain genetic programs. Dysfunction of SWI-SNF can induce stem cell-likeness (109). Genetic alterations disrupt genes that encode members of the SWI-SNF chromatin remodeling complex (for example, ARID1A, ARID1B, ARID2, SMARCB1 or PBRM1) occur in 6% of PDTCs and 36% of ATCs (55). Among those, thyroid cancers with BRAFV600E and SWI-SNF impairment were locked in a dedifferentiated transcriptional state that could not be reversed by MAPK signaling pathway inhibition (110), which would discourage the use of redifferentiation strategies.
Changes in the WNT/β-catenin signaling pathway (111), histone deacetylase (HDAC) isoforms (112), aberrant gene methylation (113, 114), peroxisome proliferator activated receptor gamma (PPAR-γ) rearrangement with PAX8 (115), and imbalance of ncRNAs (116) have also been observed in RAI-RTCs and take part in its occurrence and development.
The initiation of systemic therapy for RAI-RTCs should be done with caution (11). First of all, in some patients the condition can remain stable for many years and their life expectancy can be as long as several decades. In addition, drug-related adverse reactions may lead to a decreased quality of life. Finally, local treatment such as stereotactic radiotherapy and thermal ablation would be preferred for local advanced lesions (3), making immediate systemic therapy upon diagnosis of RAI-RTCs unnecessary. Some studies have suggested that targeted therapy be initiated when the tumor doubling time (VDT) is less than 6 months (117). Guidelines for the Diagnosis and Treatment of Thyroid Nodules and Differentiated Thyroid Cancer in Adults issued by ATA in 2015 recommended initiating TKI therapy for RAI-RTCs if the disease is metastatic, rapidly progressive, symptomatic, and/or imminently threatening disease not otherwise amenable to local control using other approaches (3). As TKIs are accompanied by side effects, ETA agree that treatment with TKIs should only be considered in patients with progressive RAI-RTC, with considerable tumor load and when, according to a multidisciplinary group of experts, refraining from treatment with MKIs would lead to considerable harm/clinical complications within the near future. Before starting MKIs, local treatments should be considered (21). European Society for Medical Oncology (ESMO) recommended initiating TKI therapy in patients with advanced/metastatic DTCs (118), and the specific evaluation criteria should be based on symptoms, tumor burden, the Eastern Cooperative Oncology Group performance status (ECOG PS), lesion characteristics (e.g. paratracheal location or other features likely to cause symptoms) and disease progression (119). In summary, decisions should be made after close monitoring and assessment of the disease. The best time to start TKI therapy for asymptomatic RAI-RTC patients was explored in the international, prospective, multicenter clinical study RIFTOS MKI (registration number NCT02303444), which recruited 647 asymptomatic RAI-RTCs patients (120). Unfortunately, the latest findings from RIFTOS MKI cannot answer the best time to initiate TKI therapy for asymptomatic RAI-RTC patients yet because of the slow accrual of events, with only 13 US patients receiving MKI treatment at study entry (14). Further research is needed to identify the best time to initiate systemic therapy for RAI-RTCs.
Most of systemic therapies for RAI-RTCs are targeted therapies for specific mutated proteins while immunotherapy and cytotoxic chemotherapy are also included. Targeted therapies mainly include MKIs and selective kinase inhibitors. Among the MKIs, sorafenib and lenvatinib are currently recommended as standard regimens. Selective kinase inhibitors that can increase the RAI uptake of tumor cells have become a research hotspot in recent years. Although the efficacy of immune checkpoint inhibitor (ICI) monotherapy is limited, there are many combinations of ICIs with targeted therapy or chemoradiotherapy in clinical trials. Cytotoxic chemotherapy is only used as a complementary treatment to the abovementioned options because of its limited clinical benefit (3).
In addition to VEGFR, PDGFR, FGFR, RET and c-KIT, which all belong to RTKs, the targets of MKIs also include BRAF and RAS. Representative drugs include sorafenib, lenvatinib, motesanib, pazopanib and sunitinib, among which sorafenib and lenvatinib have been approved by the US FDA for the clinical treatment of RAI-RTCs. The other drugs have been shown to be effective in preliminary clinical trials, yet have not been approved to include RAI-RTCs in their indications for their lack of multicenter large-sample trial data.
Sorafenib was originally developed by Bayer (Leverkusen, Germany), and its main targets are VEGFR 1-3, RET, RAF (including BRAF and C-Raf), PDGFR-β, c-KIT and FLT3 (121). In an earlier phase II single-arm clinical trial that included 31 patients with advanced RAI-RTCs, the median progression-free survival (PFS) after treatment was 18 months, and the median overall survival (OS) was 34.5 months (122). More reliable data were obtained in a multicenter, double-blinded, randomized controlled phase III clinical trial (DECISION; ClinicalTrials.gov number, NCT00984282) in 2012, with 417 patients included. The median PFS was significantly increased in the sorafenib group (n=207) compared with that in the placebo group (n=209) (10.8 months vs. 5.8 months) (123). Therefore, sorafenib was approved by the US FDA and the European Medicines Agency in November 2013 for the treatment of advanced RAI-RTCs with a recommended starting dose of 800 mg/day in divided doses. The common adverse effects are palm-plantar swelling, diarrhea, hair loss, rash and scaling, which primarily occur during the first 6 cycles of treatment and may gradually get tolerated as the course of treatment prolongs. However, these adverse events resulted in discontinuation of treatment in 66% of patients, dose reduction in 64% of patients, and permanent discontinuation in 18% of patients (124).
Lenvatinib targets VEGFR 1-3, FGFR 1-4, PDGFR-α, RET and c-KIT (125). Previous studies have suggested that its antitumor effect is mainly directed against the microvascular environment of VEGFR and FGFR rather than against tumor cell proliferation or a specific phase of the cell cycle (126, 127). The efficacy of lenvatinib has been demonstrated in a randomized, double-blinded, multicenter phase III clinical trial (SELECT; ClinicalTrials.gov number, NCT01321554). A total of 392 patients with advanced RAI-RTCs were recruited in this study, and the primary endpoint was PFS. The PFS of the lenvatinib group (n=261) and the placebo group (n=131) were 18.3 and 3.6 months, respectively. The treatment response rate of the Lenvatinib group was 64.8% and four patients had complete responses (CR), while that of the control group was only 1.5% and all responses were partial (128). The US FDA approved lenvatinib as the second drug, after sorafenib, for the treatment of advanced RAI-RTCs in 2015, with a recommended starting dose of 24 mg/day (129). The most common adverse reactions are hypertension, diarrhea, fatigue, nausea, loss of appetite and weight loss. Adverse reactions led to discontinuation in 82.4% of patients, dose reduction in 67.8% and permanent discontinuation in 14% (128). A study exploring the optimal dose of lenvatinib to treat RAI-RTCs has shown that the 24-week objective response rates (ORRs) of the lenvatinib were 57.3% in the 24 mg/day (n=75) group and 40.3% in the 18 mg/day (n=77) group, respectively, with the odds ratio (OR) being 0.5. As for the incidences of adverse reactions, treatment-emergent adverse events (TEAEs)≥3 in each group were 61.3% and 57.1%, respectively. Therefore, the starting dose of 24 mg/day is confirmed and recommended (130) (Table 1).
Cabozantinib, an inhibitor of VEGFR, AXL, MET, and RET, is the second-line treatment option in advanced RAI-RTC patients. The latest results from COSMIC-311 (registration number NCT 03690388) indicated that cabozantinib benefits patients with RAI-RTC, regardless of prior lenvatinib or sorafenib treatments (131). COSMIC-311 is an international, randomized, double-blind trial in which patients with locally advanced or metastatic RAI-RTC that progressed during or following treatment with lenvatinib, sorafenib, or both were treated with either cabozantinib (n = 170) or placebo (n = 88). The median PFS with cabozantinib was 16.6, 5.8, and 7.6 months for those patients who received prior sorafenib only, prior lenvatinib only, or both, respectively, versus 3.2, 1.9, and 1.9 months with placebo; hazard ratios (HRs) 0.13, 0.28, and 0.27, respectively (132). Therefore, cabozantinib has been approved by FDA and Germany as a second-line treatment option in advanced RAI-RTC (133, 134).
Another MKI drug, motesanib, targets VEGFR 1-3, PDGFR, RET and c-KIT (135). In an open-label single-arm phase II clinical trial of 93 patients, 14% had a partial response (PR) and 35% had stable disease (SD) for more than 24 weeks (136). Vandetanib is shown efficacy in a randomized phase 2 trial in 145 patients with locally advanced or metastatic differentiated thyroid carcinoma (18). Patients who received vandetanib had longer PFS than did those who received placebo (HR 0·63, 60% CI 0·54-0·74; one-sided p=0·008): median PFS was 11·1 months (95% CI 7·7-14·0) for patients in the vandetanib group and 5·9 months (4·0-8·9) for patients in the placebo group. And vandetanib has been approved by the FDA for the treatment of advanced medullary thyroid carcinoma (137). A randomized double-blind phase III clinical trial of vandetanib efficacy in RAI-RTC patients is still ongoing (see Table 1 for details).
Other MKIs include pazopanib (138), sunitinib (139) and imatinib. Clinical trials, most of which are small sample, single-arm, open-label, single-center phase II studies, have shown that pazopanib (140, 141) and sunitinib (142, 143) have some efficacy in RAI-RTCs patients, which still needs further verification. As for imatinib, a small clinical trial investigating whether it can promote RAI reuptake in RAI-RTCs is ongoing (see Table 1 for details).
In summary, sorafenib and lenvatinib have been approved by the FDA for the treatment of RAI-RTCs with the dose reduction and treatment discontinuation due to adverse reactions worthy of attention yet. Cabozantinib can be used as a second-line treatment for RAI-RTC that has progressed after sorafenib and Lenvatinib. The other MKIs, such as pazopanib, motesanib and sunitinib, have shown certain clinical efficacy in RAI-RTCs but large sampled multicenter randomized controlled trials are still needed to confirm their efficacy. Off-label use of unapproved MKIs may also be considered if disease progression occurs despite treatment with sorafenib or lenvatinib (144).
There are many types of selective kinase inhibitors including RET inhibitors, BRAF inhibitors, MEK inhibitors, mTOR inhibitors, VEGFR-2 inhibitors and histone deacetylase inhibitors (HDACis). Their therapeutic effect in thyroid cancer, has been confirmed by a number of clinical studies. Notably, BRAF and MEK inhibitors have achieved a major breakthrough in the treatment of RAI-RTCs with the BRAFV600E mutation, which can cause RAI reuptake in some RAI-RTC lesions carrying BRAF/RAS mutations, allowing response to RAI treatment (145).
The representative drugs of this class are selpercatinib and pralsetinib. Selpercatinib is approved by US FDA for the treatment of thyroid cancer with RET gene mutations or fusions (146). In a phase I-II trial(NCT03157128), selpercatinib showed durable efficacy in 19 patients with previously-treated RET fusion-positive thyroid cancer, and the response rate was 79% (95% CI, 54–94) (147). Since RET mutation mainly occur in MTC (148), selpercatinib is mainly approved for the treatment of MTC. At present, several clinical trials of selpercatinib for RET-altered thyroid cancer are still ongoing, including the study of whether selpercatinib can increase the uptake of RAI in RET-altered RAI-RTC. The results of these studies are promising (see Table 2). Pralsetinib is another strong RET inhibitor. In the ARROW study (NCT03037385), the ORR of pralsetinib in 22 previously treated RET fusion positive thyroid cancer patients was 90.9% (95%CI: 70.8-98.9) (149). In December 2020, the FDA approved pralsetinib for use in patients with RET fusion positive RAT-RTC (150). More studies on pralsetinib for RAI-RTC are shown in Table 2.
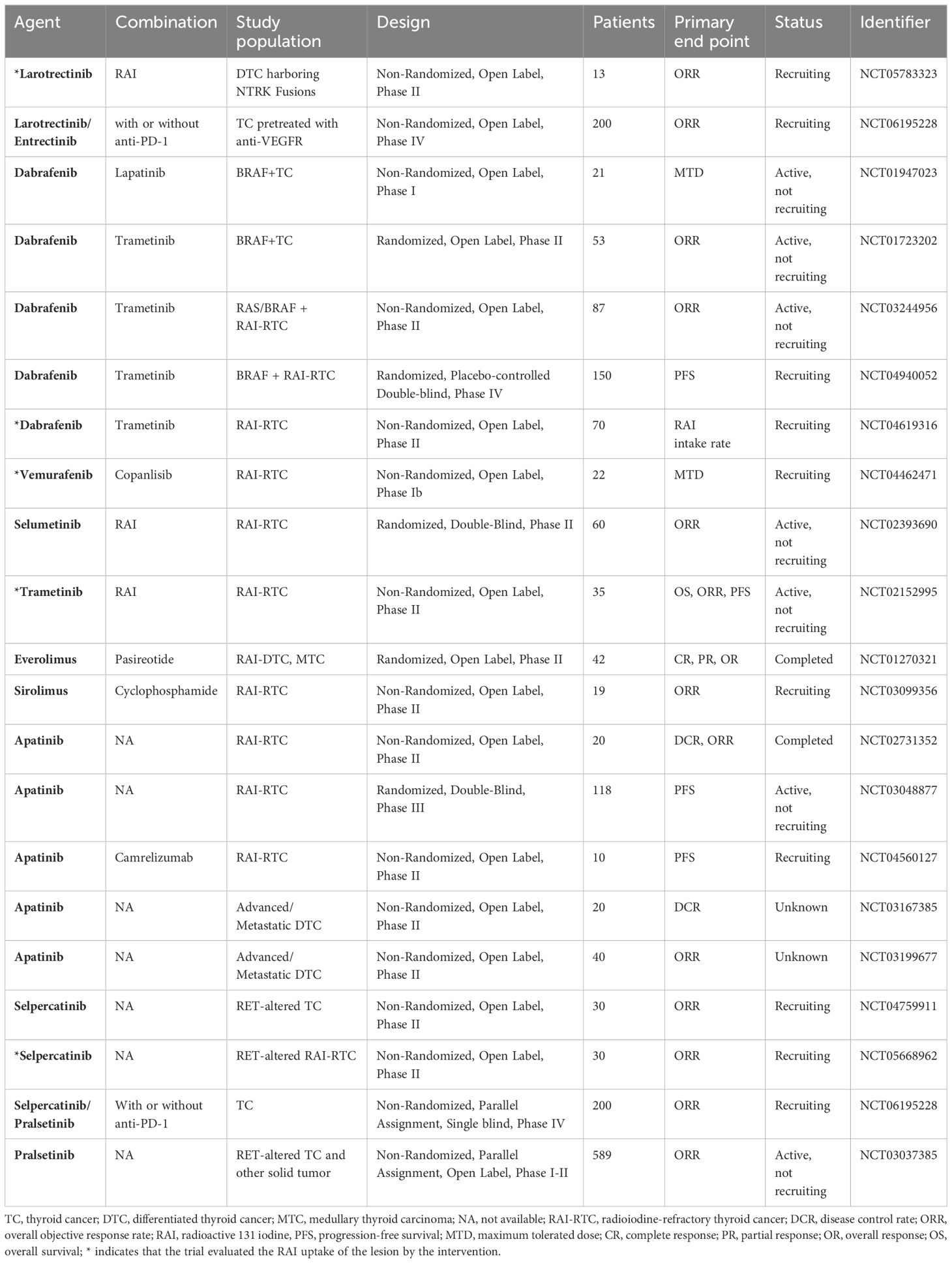
Table 2. Clinical trials of single-target inhibitors in the treatment of RAI-RTCS and other thyroid cancers.
Currently, two drugs have received approval for the treatment of solid tumors with NTRK fusions, larotrectinib and entrectinib.
The efficacy of larotrectinib, a highly selective TRK inhibitor targeting TRKA, TRKB, and TRKC, was examined in phase 1 and 2 clinical trials, in 17 different TRK fusion-positive cancer types, including thyroid cancer. The ORR response reached 75% (151). Based on these studies, larotrectinib received a tissue-agnostic drug approval in patients with TRK fusions. In a pooled analysis from three phase I/II larotrectinib clinical trials, there were 22 patients with TRK fusion-positive DTC treated with larotrectinib, ORR was 86% (152). See Table 2 for more information.
Entrectinib is another selective inhibitor targeting TRKA, TRKB, TRKC, ALK, and ROS1. Analysis of all phase 1/2 trials evaluating efficacy and safety of entrectinib showed that among 54 adult patients, the ORR was 57%. There were 13 patients with thyroid cancer, 10 patients with PTC, and 3 patients non-PTC, with 1 patient having CR and 6 patients PR, with median duration of response being 13.2 months (153). See Table 2 for more information.
That both the FDA and EMA approvals for larotrectinib and entrectinib are for patients with metastatic, unresectable solid tumors harboring NTRK fusions in tumor agnostic indication that have no satisfactory treatment options or have progressed on standard-of-care treatment (154).
Currently, the main BRAF inhibitors are dabrafenib and vemurafenib, while the MEK inhibitors are selumetinib and trametinib. Studies have shown that selective kinase inhibitors of the MAPK pathway can partially restore RAI uptake in RAI-RTC lesions.
For dabrafenib, a phase I clinical trial of 14 patients with BRAFV600E -positive RAI-RTCs, showed PR of 29% and SD of 50% (155), and a phase II clinical trial of 10 patients with BRAFV600E-positive RAI-RTC showed RAI reuptake, with PR of 20% and SD of 40% (156).
For vemurafenib, a phase II clinical trial of 51 patients with BRAFV600E-positive RAI-RTC showed some efficacy in restoring RAI reuptake (157). Another study suggested that four of 10 BRAFV600E-positive RAI-RTC patients showed RAI reuptake in lesions (158). The main adverse reactions are rash, weight loss, fatigue and hyperbilirubinemia (127). A clinical trial of the maximum tolerated dose of vemurafenib in combination with a PI3K inhibitor is currently ongoing (detailed in Table 2).
For selumetinib, a clinical trial of 20 DTC patients with BRAF mutations or NRAS mutations revealed PR of 25% and SD of 15% after RAI treatment (159). To further evaluate whether selumetinib could improve RAI uptake in patients with DTC, AstraZeneca (Cambridge, UK) conducted a multicenter double-blinded phase III randomized controlled trial (NCT01843062) that included a total of 233 patients. The latest results from this study show that although the addition of selumetinib to adjuvant RAI failed to improve the CR rate for DTC patients (160). More information about this study is shown in Table 2. Trametinib is another MEK inhibitor. MERAIODE is a multicenter, prospective phase II trial in patients with RAI-RTC, with two independent cohorts: one for BRAFp.V600E patients and one for RAS mutated patients (NCT 03244956). In the cohort of RAS mutated patients, the treatment with trametinib is not highly effective for restoring/increasing RAI uptake (161). In the cohort of BRAFp.V600E patients, the designs were similar to the RAS mutated cohort except for treatment consisting of trametinib- dabrafenib, so we present its outcomes in the combined therapy (refer to 4.5). More studies on the efficacy of trametinib in the treatment of RAI-RTC are presented in the part 4.5 and Table 2.
Drugs that target this signaling pathway include the PI3K inhibitor (buparisib) and the mTOR inhibitors (everolimus, sirolimus and temsirolimus). The sample sizes of clinical trials for these drugs are small (19-43 cases in most trials), therefore the studies with larger sample size are needed to confirm their efficacy in RAI-RTC. Buparisib did not prolong PFS in RAI-RTC patients (162), thus its further study is limited. The efficacy of everolimus has been demonstrated in a study of 28 patients with RAI-RTCs (65% SD), with median PFS and OS being 9 and 18 months, respectively (163). A recent phase II trial of everolimus involving 33 patients with RAI-RTC achieved favorable results (median PFS 12.9 months, 2-year PFS 23.6%) (164).
The representative drug of this class is apatinib. Two clinical trials with 20 RAI-RTCs patients from mainland China showed that apatinib can reduce serum thyroglobulin levels and tumor volume in patients with RAI-RTCs (165) and obtain a certain efficacy (median PFS: 18.4 months, median OS: 51.6 months) (166). There are currently five ongoing studies evaluating the efficacy of apatinib in RAI-RTC and advanced DTC, one of which is a randomized double-blinded phase III clinical trial with a sample size of more than 100 cases (Table 2).
HDACis can inhibit tumor proliferation, promote tumor differentiation and induce tumor cell apoptosis as well as cell cycle arrest. Valproic acid is a representative drug. Basic studies have shown that HDACis can increase the RAI uptake rate of thyroid cancer cells (167). However, a phase II clinical trial of 13 patients showed that neither RAI uptake nor tumor response in RAI-RTC patients was increased by valproic acid (168). Currently, there is a lack of evidence supporting the efficacy of HDACis in RAI-RTC.
Immune checkpoint inhibitors (ICIs) are a new type of antitumor drug which can achieve antitumor goal by blocking the binding of immune checkpoints to their ligands, thereby enhancing the activity of T cells. ICIs mainly include CTLA-4 inhibitors and PD-1/PD-L1 inhibitors, which mainly used in the treatment of melanoma, Hodgkin’s lymphoma and non-small cell lung cancer (NSCLC). But the use of ICIs in thyroid cancer is questioned. On one hand, a large number of immune cells infiltrate DTC tissues, and this is closely related to tumor prognosis (169–173). Tumor patients with high expression of programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) often have an increased risk of tumor recurrence and shortened disease-free survival (DFS) (174–177). These mechanisms suggest that ICIs should be effective in treating thyroid cancer. On the other hand, the expression of PD-L1 in thyroid cancer fluctuates greatly (6.1% to 82.5%) (178), which creates uncertainty about the efficacy of ICIs to thyroid cancer.
Currently, the clinical application of ICIs for the treatment of malignant tumors includes CTLA-4 inhibitors (ipilimumab), PD-1 inhibitors (pembrolizumab and nivolumab), and PD-L1 inhibitors (durvalumab and atezolizumab). A single-arm study of 22 patients with PD-L1-positive DTC preliminarily explored the efficacy of pembrolizumab to thyroid cancer. The results were two cases of PR (9%) and a median PFS of 7 months (179), suggesting little efficacy of ICIs. Six studies of ICIs combined with targeted therapy or chemoradiotherapy are now in progress and most of them are nondouble-blinded phase II clinical trials with small sample sizes (refer to 4.5, see Table 3 for details).
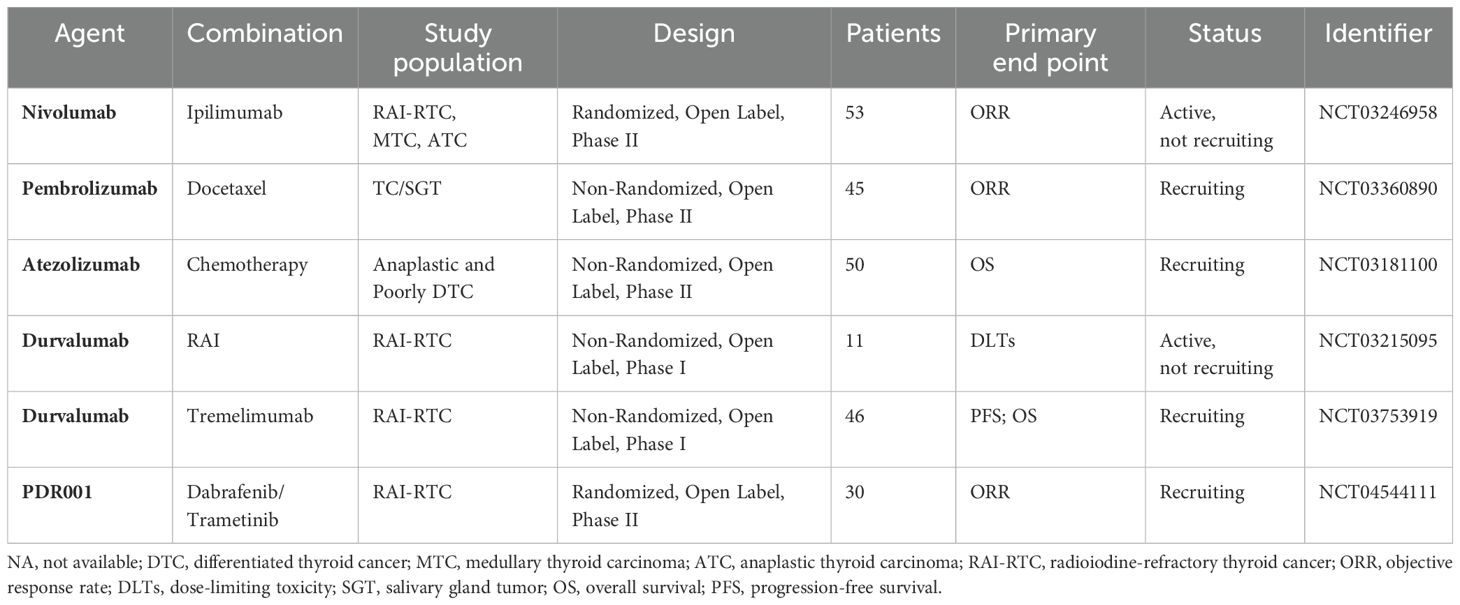
Table 3. Clinical trials of ICIs and their combination with other drugs in the treatment of thyroid cancer.
Chemotherapy is the use of the anticancer or cytotoxic properties of drugs to kill cancer cells. For most malignancies, cytotoxic chemotherapy is a well-documented treatment with good clinical outcomes. The US FDA approved in as early as 1974 doxorubicin for the treatment of DTC, but its clinical benefit is diminished by its toxicity and side effects (180). Therefore, ATA proposed in 2015 that systemic chemotherapy no longer be the standard treatment for RAI-RTCs and only be considered when other options, such as TKIs, are ineffective (3).
In addition to the monotherapies adopting the foregoing drugs, sequential therapy and combination therapy also showed certain effects.
The efficacy of sequential therapy was studied in a phase II clinical trial of vemurafenib. Fifty-one patients with BRAFV600E-positive RAI-RTC were divided into two groups in this trial according to whether they had received VEGFR-targeted TKI treatment before, and both groups were treated with vemurafenib. The antitumor effect was better in patients who had never received TKI therapy (PR, 38.5% vs. 27%; 6-month SD, 35% vs. 27%) (157).
The efficacy of combination therapies has been widely appraised. A retrospective study found that mTOR inhibitor sirolimus combined with chemotherapy drug cyclophosphamide reached a 1-year PFS rate comparable to standard treatment (0.45% vs. 0.30%) in the treatment of RAI-RTCs (181). There is currently a phase II clinical trial in progress that is using the two drugs combination in 19 patients with RAI-RTC (see Table 2 for details). Temsirolimus, another mTOR inhibitor, in combination with MKI sorafenib achieved PR of 22% and SD of 58% in 36 RAI-RTC patients (182). The combination therapy of somatostatin analog pasireotide and mTOR inhibitor everolimus in the treatment of RAI-RTC was also studied in a phase II clinical trial (NCT01270321), since it was observed that somatostatin receptor (SSTR) 2 was highly expressed in thyroid cancer and activation of the SSTR1-5 inhibited the signal transduction of the PI3K signaling pathway (183). It has been completed, but the results have not been published yet (see Table 2 for details). Another type of combination therapy is BRAF inhibitor dabrafenib combined with MEK inhibitor trametinib for BRAF-mutated RAI-ATC. In a randomized phase-II open-Label multicenter trial of 53 patients with BRAF-mutated RAI-RTC, this combination therapy was not superior in efficacy compared to dabrafenib monotherapy, within the ORR was 48% versus 42% (184). A new study shown that this combination therapy is effective in BRAF-mutated DTC patients for restoring RAI uptake with PR observed 6 months after RAI administration in 38% of the patients (185). There are also four ongoing clinical trials of dabrafenib in combination with other selective kinase inhibitors (see Table 2 for details). And two other studies are underway to investigate the response rate of MKI lenvatinib combined with ICIs in patients with RAI-RTC and the reuptake of RAI by lenvatinib on RAI-RTC lesions (see Table 2 for details).
The in-depth understanding of the molecular mechanisms involved in RAI-RTCs has promoted the development of therapies. Genetic mutations and gene rearrangements at sites such as RTKs, RAS, BRAF and TERTp lead to structural and functional abnormalities of the encoded proteins, which abnormally activate signaling pathways such as the MAPK and PI3K signaling pathways, thus the dedifferentiation of thyroid cells as well as NIS dysfunction, and consequently the RAI-refractory nature of DTCs. Targeted therapy for different mutations provides a new direction for the treatment of RAI-RTCs. The MKI drugs sorafenib, lenvatinib and cabozantinib have been approved by the US FDA for the treatment of RAI-RTCs. As for the other MKIs and selective kinase inhibitors, especially selective kinase inhibitors that restore RAI uptake in tumor cells (such as BRAF, MEK and mTOR inhibitors), encouraging results are shown in multiple clinical trials. However, more multicenter, large-sample, double-blinded randomized controlled trials are anticipated for further verification, and more patients will benefit from the improvement of treatments strategy and the innovation of therapies.
PC: Writing – original draft. YY: Writing – original draft. HT: Writing – review & editing. JL: Validation, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Grant 1.3.5 project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital Sichuan University (Grant No. 2020HXFH034) and the Gangbao Project of the Health Commission of Sichuan Province (Grant No. Chuanganyan 2021-102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. (2017) 24:332–6. doi: 10.1097/MED.0000000000000359
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 26:1–133. doi: 10.1089/thy.2015.0020
4. Koot A, Soares P, Robenshtok E, Locati LD, de la Fouchardiere C, Luster M, et al. Position paper from the Endocrine Task Force of the European Organisation for Research and Treatment of Cancer (EORTC) on the management and shared decision making in patients with low-risk micro papillary thyroid carcinoma. Eur J Cancer. (2023) 179:98–112. doi: 10.1016/j.ejca.2022.11.005
5. Campopiano MC, Ghirri A, Prete A, Lorusso L, Puleo L, Cappagli V, et al. Active surveillance in differentiated thyroid cancer: a strategy applicable to all treatment categories response. Front Endocrinol. (2023) 14:1133958. doi: 10.3389/fendo.2023.1133958
6. Ahmadi S, Alexander EK. Active surveillance for low-risk differentiated thyroid cancer. Endocr Pract. (2023) 29:148–53. doi: 10.1016/j.eprac.2022.10.005
7. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. (2013) 381:1058–69. doi: 10.1016/s0140-6736(13)60109-9
8. Huang J, Harris EJ, Lorch JH. Treatment of aggressive thyroid cancer. Surg Pathol Clin. (2019) 12:943–50. doi: 10.1016/j.path.2019.08.004
9. Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, et al. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. (2002) 132:984–90. doi: 10.1067/msy.2002.128690
10. Worden F. Treatment strategies for radioactive iodine-refractory differentiated thyroid cancer. Ther Adv Med Oncol. (2014) 6:267–79. doi: 10.1177/1758834014548188
11. Brose MS, Smit J, Capdevila J, Elisei R, Nutting C, Pitoia F, et al. Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev Anticancer Ther. (2012) 12:1137–47. doi: 10.1586/era.12.96
12. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. (2012) 22:884–9. doi: 10.1089/thy.2011.0535
13. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
14. Gianoukakis AG, Choe JH, Bowles DW, Brose MS, Wirth LJ, Owonikoko T, et al. Real-world practice patterns and outcomes for RAI-refractory differentiated thyroid cancer. Eur Thyroid J. (2024) 13. doi: 10.1530/etj-23-0039
15. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN guidelines insights: thyroid carcinoma, version 2. 2018. J Natl Compr Canc Netw. (2018) 16:1429–40. doi: 10.6004/jnccn.2018.0089
16. Traynor K. Cabozantinib approved for advanced medullary thyroid cancer. Am J Health Syst Pharm. (2013) 70:88. doi: 10.2146/news130005
17. Thornton K, Kim G, Maher VE, Chattopadhyay S, Tang S, Moon YJ, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. (2012) 18:3722–30. doi: 10.1158/1078-0432.Ccr-12-0411
18. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. (2012) 13:897–905. doi: 10.1016/S1470-2045(12)70335-2
19. Capdevila J, Galofré JC, Grande E, Zafón Llopis C, Ramón YCAT, Navarro González E, et al. Consensus on the management of advanced radioactive iodine-refractory differentiated thyroid cancer on behalf of the Spanish Society of Endocrinology Thyroid Cancer Working Group (GTSEEN) and Spanish Rare Cancer Working Group (GETHI). Clin Transl Oncol. (2017) 19:279–87. doi: 10.1007/s12094-016-1554-5
20. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: A joint statement from the american thyroid association, the european association of nuclear medicine, the society of nuclear medicine and molecular imaging, and the european thyroid association. Thyroid. (2019) 29:461–70. doi: 10.1089/thy.2018.0597
21. Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, et al. European thyroid association guidelines for the treatment and follow-Up of advanced radioiodine-Refractory thyroid cancer. Eur Thyroid J. (2019) 8:227–45. doi: 10.1159/000502229
22. Riesco-Eizaguirre G, Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol. (2006) 155:495–512. doi: 10.1530/eje.1.02257
23. Wyszomirska A. Iodine-131 for therapy of thyroid diseases. Physical and biological basis. Nucl Med Rev Cent East Eur. (2012) 15:120–3.
24. Howell RW, Rao DV, Sastry KS. Macroscopic dosimetry for radioimmunotherapy: nonuniform activity distributions in solid tumors. Med Phys. (1989) 16:66–74. doi: 10.1118/1.596404
25. Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. (2006) 13:257–69. doi: 10.1677/erc.1.01119
26. Romei C, Ciampi R, Faviana P, Agate L, Molinaro E, Bottici V, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. (2008) 15:511–20. doi: 10.1677/erc-07-0130
27. Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul Korea). (2019) 34:215–25. doi: 10.3803/EnM.2019.34.3.215
28. Landa I, Cabanillas ME. Genomic alterations in thyroid cancer: biological and clinical insights. Nat Rev Endocrinol. (2024) 20:93–110. doi: 10.1038/s41574-023-00920-6
29. Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci. (2019) 16:450–60. doi: 10.7150/ijms.29935
30. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. (2018) 24:3059–68. doi: 10.1158/1078-0432.Ccr-18-0373
31. Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. (2008) 93:3106–16. doi: 10.1210/jc.2008-0273
32. Tirrò E, Martorana F, Romano C, Vitale SR, Motta G, Di Gregorio S, et al. Molecular alterations in thyroid cancer: from bench to clinical practice. Genes. (2019) 10. doi: 10.3390/genes10090709
33. Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. (2001) 22:631–56. doi: 10.1210/edrv.22.5.0444
34. Bunone G, Vigneri P, Mariani L, Butó S, Collini P, Pilotti S, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. (1999) 155:1967–76. doi: 10.1016/s0002-9440(10)65515-0
35. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. (2001) 86:656–8. doi: 10.1210/jcem.86.2.7226
36. Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol. (2006) 155:645–53. doi: 10.1530/eje.1.02289
37. Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. (2016) 12:192–202. doi: 10.1038/nrendo.2016.11
38. Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U.S.A. (2014) 111:4233–8. doi: 10.1073/pnas.1321937111
39. McFadden DG, Dias-Santagata D, Sadow PM, Lynch KD, Lubitz C, Donovan SE, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. (2014) 99:E2457–62. doi: 10.1210/jc.2014-2611
40. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. (2014) 120:799–807. doi: 10.1002/cncr.28484
41. Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol. (2010) 321:44–9. doi: 10.1016/j.mce.2009.10.009
42. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. (2013) 13:184–99. doi: 10.1038/nrc3431
43. St Bernard R, Zheng L, Liu W, Winer D, Asa SL, Ezzat S. Fibroblast growth factor receptors as molecular targets in thyroid carcinoma. Endocrinology. (2005) 146:1145–53. doi: 10.1210/en.2004-1134
44. Gerber TS, SChad A, Hartmann N, Springer E, Zechner U, Musholt TJ. Targeted next-generation sequencing of cancer genes in poorly differentiated thyroid cancer. Endocr Connect. (2018) 7:47–55. doi: 10.1530/ec-17-0290
45. Chu YH, Sadow PM. Kinase fusion-related thyroid carcinomas: towards predictive models for advanced actionable diagnostics. Endocr Pathol. (2022) 33:421–35. doi: 10.1007/s12022-022-09739-9
46. Vuong HG, Le HT, Le TTB, Le T, Hassell L, Kakudo K. Clinicopathological significance of major fusion oncogenes in papillary thyroid carcinoma: An individual patient data meta-analysis. Pathol Res Pract. (2022) 240:154180. doi: 10.1016/j.prp.2022.154180
47. de Sousa MSA, Nunes IN, Christiano YP, Sisdelli L, Cerutti JM. Genetic alterations landscape in paediatric thyroid tumours and/or differentiated thyroid cancer: Systematic review. Rev Endocr Metab Disord. (2024) 25:35–51. doi: 10.1007/s11154-023-09840-2
48. Bulanova Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, et al. RET fusion genes in pediatric and adult thyroid carcinomas: cohort characteristics and prognosis. Endocr Relat Cancer. (2023) 30. doi: 10.1530/erc-23-0117
49. Ricarte-Filho JC, Halada S, O’Neill A, Casado-Medrano V, Laetsch TW, Franco AT, et al. The clinical aspect of NTRK-fusions in pediatric papillary thyroid cancer. Cancer Genet. (2022) 262-263:57–63. doi: 10.1016/j.cancergen.2022.01.002
50. Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, et al. BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. (2020) 30:1771–80. doi: 10.1089/thy.2019.0802
51. Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. (2003) 88:5399–404. doi: 10.1210/jc.2003-030838
52. Torregrossa L, Viola D, Sensi E, Giordano M, Piaggi P, Romei C, et al. Papillary thyroid carcinoma with rare exon 15 BRAF mutation has indolent behavior: A single-institution experience. J Clin Endocrinol Metab. (2016) 101:4413–20. doi: 10.1210/jc.2016-1775
53. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
54. Ciampi R, Knauf JA, Kerler R, Gandhi M, Zhu Z, Nikiforova MN, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. (2005) 115:94–101. doi: 10.1172/jci23237
55. Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. (2011) 71:4403–11. doi: 10.1158/0008-5472.Can-10-4041
56. Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM, et al. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. (2005) 90:4688–93. doi: 10.1210/jc.2004-2281
57. Haghpanah V, Fallah P, Tavakoli R, Naderi M, Samimi H, Soleimani M, et al. Antisense-miR-21 enhances differentiation/apoptosis and reduces cancer stemness state on anaplastic thyroid cancer. Tumour Biol. (2016) 37:1299–308. doi: 10.1007/s13277-015-3923-z
58. Hou P, Ji M, Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. (2008) 113:2440–7. doi: 10.1002/cncr.23869
59. Riesco-Eizaguirre G, Rodriguez I, de la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased Malignancy in thyroid cancer. Cancer Res. (2009) 69:8317–25. doi: 10.1158/0008-5472.CAN-09-1248
60. Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. (2006) 91:3667–70. doi: 10.1210/jc.2005-2836
61. Nucera C, Porrello A, Antonello ZA, Mekel M, Nehs MA, Giordano TJ, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci U.S.A. (2010) 107:10649–54. doi: 10.1073/pnas.1004934107
62. Hu S, Liu D, Tufano RP, Carson KA, Rosenbaum E, Cohen Y, et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. (2006) 119:2322–9. doi: 10.1002/ijc.22110
63. Mitsutake N, Knauf JA, Mitsutake S, Mesa C Jr., Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. (2005) 65:2465–73. doi: 10.1158/0008-5472.Can-04-3314
64. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. (2020) 20:74–88. doi: 10.1038/s41568-019-0216-7
65. Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, et al. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene. (2011) 30:3153–62. doi: 10.1038/onc.2011.44
66. García B, Santisteban P. PI3K is involved in the IGF-I inhibition of TSH-induced sodium/iodide symporter gene expression. Mol Endocrinol. (2002) 16:342–52. doi: 10.1210/mend.16.2.0774
67. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. (2011) 7:569–80. doi: 10.1038/nrendo.2011.142
68. Behr M, Schmitt TL, Espinoza CR, Loos U. Cloning of a functional promoter of the human sodium/iodide-symporter gene. Biochem J. (1998) 331:359–63. doi: 10.1042/bj3310359
69. Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol. (1999) 19:2051–60. doi: 10.1128/mcb.19.3.2051
70. Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. (2010) 95:820–8. doi: 10.1210/jc.2009-1888
71. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. (2007) 92:2840–3. doi: 10.1210/jc.2006-2707
72. Di Cristofaro J, Silvy M, Lanteaume A, Marcy M, Carayon P, De Micco C. Expression of tpo mRNA in thyroid tumors: quantitative PCR analysis and correlation with alterations of ret, Braf, ras and pax8 genes. Endocr Relat Cancer. (2006) 13:485–95. doi: 10.1677/erc.1.01164
73. Zhang Z, Liu D, Murugan AK, Liu Z, Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. (2014) 21:161–73. doi: 10.1530/erc-13-0399
74. Riedel C, Dohán O, de la Vieja A, Ginter CS, Carrasco N. Journey of the iodide transporter NIS: from its molecular identification to its clinical role in cancer. Trends Biochem Sci. (2001) 26:490–6. doi: 10.1016/s0968-0004(01)01904-1
75. Taki K, Kogai T, Kanamoto Y, Hershman JM, Brent GA. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3’,5’-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol Endocrinol. (2002) 16:2266–82. doi: 10.1210/me.2002-0109
76. Chun JT, Di Dato V, D’Andrea B, Zannini M, Di Lauro R. The CRE-like element inside the 5’-upstream region of the rat sodium/iodide symporter gene interacts with diverse classes of b-Zip molecules that regulate transcriptional activities through strong synergy with Pax-8. Mol Endocrinol. (2004) 18:2817–29. doi: 10.1210/me.2004-0020
77. Fenton MS, Marion KM, Hershman JM. Identification of cyclic adenosine 3’,5’-monophosphate response element modulator as an activator of the human sodium/iodide symporter upstream enhancer. Endocrinology. (2008) 149:2592–606. doi: 10.1210/en.2007-1390
78. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. (1994) 97:418–28. doi: 10.1016/0002-9343(94)90321-2
79. Su D, Delaplane S, Luo M, Rempel DL, Vu B, Kelley MR, et al. Interactions of apurinic/apyrimidinic endonuclease with a redox inhibitor: evidence for an alternate conformation of the enzyme. Biochemistry. (2011) 50:82–92. doi: 10.1021/bi101248s
80. Costamagna E, García B, Santisteban P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-beta repression of the sodium/iodide symporter gene. J Biol Chem. (2004) 279:3439–46. doi: 10.1074/jbc.M307138200
81. Kambe F, Nomura Y, Okamoto T, Seo H. Redox regulation of thyroid-transcription factors, Pax-8 and TTF-1, is involved in their increased DNA-binding activities by thyrotropin in rat thyroid FRTL-5 cells. Mol Endocrinol. (1996) 10:801–12. doi: 10.1210/mend.10.7.8813721
82. Tell G, Pellizzari L, Cimarosti D, Pucillo C, Damante G. Ref-1 controls pax-8 DNA-binding activity. Biochem Biophys Res Commun. (1998) 252:178–83. doi: 10.1006/bbrc.1998.9548
83. Nicola JP, Vélez ML, Lucero AM, Fozzatti L, Pellizas CG, Masini-Repiso AM. Functional toll-like receptor 4 conferring lipopolysaccharide responsiveness is expressed in thyroid cells. Endocrinology. (2009) 150:500–8. doi: 10.1210/en.2008-0345
84. Nicola JP, Nazar M, Mascanfroni ID, Pellizas CG, Masini-Repiso AM. NF-kappaB p65 subunit mediates lipopolysaccharide-induced Na(+)/I(-) symporter gene expression by involving functional interaction with the paired domain transcription factor Pax8. Mol Endocrinol. (2010) 24:1846–62. doi: 10.1210/me.2010-0102
85. Boelaert K, Smith VE, Stratford AL, Kogai T, Tannahill LA, Watkinson JC, et al. PTTG and PBF repress the human sodium iodide symporter. Oncogene. (2007) 26:4344–56. doi: 10.1038/sj.onc.1210221
86. Sáez C, Martínez-Brocca MA, Castilla C, Soto A, Navarro E, Tortolero M, et al. Prognostic significance of human pituitary tumor-transforming gene immunohistochemical expression in differentiated thyroid cancer. J Clin Endocrinol Metab. (2006) 91:1404–9. doi: 10.1210/jc.2005-2532
87. Stratford AL, Boelaert K, Tannahill LA, Kim DS, Warfield A, Eggo MC, et al. Pituitary tumor transforming gene binding factor: a novel transforming gene in thyroid tumorigenesis. J Clin Endocrinol Metab. (2005) 90:4341–9. doi: 10.1210/jc.2005-0523
88. Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, et al. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. (2005) 67:868–76. doi: 10.1124/mol.104.007583
89. Dohán O, de la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. (2003) 24:48–77. doi: 10.1210/er.2001-0029
90. Levy O, Dai G, Riedel C, Ginter CS, Paul EM, Lebowitz AN, et al. Characterization of the thyroid Na+/I- symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci U.S.A. (1997) 94:5568–73. doi: 10.1073/pnas.94.11.5568
91. Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci U.S.A. (2002) 99:15462–7. doi: 10.1073/pnas.242328999
92. Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. (2002) 2:103–12. doi: 10.1016/s1535-6108(02)00102-2
93. Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. (2019) 26:199–212. doi: 10.1038/s41418-018-0246-9
94. Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. (2013) 502:333–9. doi: 10.1038/nature12634
95. Manzella L, Stella S, Pennisi MS, Tirrò E, Massimino M, Romano C, et al. New insights in thyroid cancer and p53 family proteins. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18061325
96. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. (2005) 6:611–22. doi: 10.1038/nrg1656
97. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
98. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. (2016) 23:R143–55. doi: 10.1530/erc-15-0533
99. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. (2014) 99:E754–65. doi: 10.1210/jc.2013-3734
100. Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. (2014) 99:E1130–6. doi: 10.1210/jc.2013-4048
101. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. (2014) 32:2718–26. doi: 10.1200/jco.2014.55.5094
102. Ngeow J, Eng C. TERT and BRAF in thyroid cancer: teaming up for trouble. J Clin Oncol. (2014) 32:2683–4. doi: 10.1200/jco.2014.56.5614
103. Melo M, da Rocha AG, Vinagre J, Sobrinho-Simões M, Soares P. Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: added value in patient prognosis? J Clin Oncol. (2015) 33:667–8. doi: 10.1200/jco.2014.59.4614
104. Dettmer MS, Schmitt A, Steinert H, Capper D, Moch H, Komminoth P, et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. (2015) 22:419–29. doi: 10.1530/erc-15-0057
105. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAF(V600E) and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: A meta-analysis. Thyroid. (2017) 27:651–60. doi: 10.1089/thy.2016.0350
106. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
107. Yu P, Qu N, Zhu R, Hu J, Han P, Wu J, et al. TERT accelerates BRAF mutant-induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci Adv. (2023) 9:eadg7125. doi: 10.1126/sciadv.adg7125
108. Landa I, Thornton CEM, Xu B, Haase J, Krishnamoorthy GP, Hao J, et al. Telomerase upregulation induces progression of mouse brafV600E-driven thyroid cancers and triggers nontelomeric effects. Mol Cancer Res. (2023) 21:1163–75. doi: 10.1158/1541-7786.Mcr-23-0144
109. Sun X, Chuang JC, Kanchwala M, Wu L, Celen C, Li L, et al. Suppression of the SWI/SNF component arid1a promotes mammalian regeneration. Cell Stem Cell. (2016) 18:456–66. doi: 10.1016/j.stem.2016.03.001
110. Saqcena M, Leandro-Garcia LJ, Maag JLV, Tchekmedyian V, Krishnamoorthy GP, Tamarapu PP, et al. SWI/SNF complex mutations promote thyroid tumor progression and insensitivity to redifferentiation therapies. Cancer Discovery. (2021) 11:1158–75. doi: 10.1158/2159-8290.Cd-20-0735
111. Sastre-Perona A, Santisteban P. Role of the wnt pathway in thyroid cancer. Front Endocrinol. (2012) 3:31. doi: 10.3389/fendo.2012.00031
112. Giaginis C, Alexandrou P, Delladetsima I, Giannopoulou I, Patsouris E, Theocharis S. Clinical significance of histone deacetylase (HDAC)-1, HDAC-2, HDAC-4, and HDAC-6 expression in human Malignant and benign thyroid lesions. Tumour Biol. (2014) 35:61–71. doi: 10.1007/s13277-013-1007-5
113. Barros-Filho MC, Dos Reis MB, Beltrami CM, de Mello JBH, Marchi FA, Kuasne H, et al. DNA methylation-based method to differentiate Malignant from benign thyroid lesions. Thyroid. (2019) 29:1244–54. doi: 10.1089/thy.2018.0458
114. Liu T, Wang J, Xiu Y, Wu Y, Xu D, Methylation Age Drift Is Associated with Poor Outcomes DNA. De-differentiation in papillary and follicular thyroid carcinomas. Cancers. (2021) 13. doi: 10.3390/cancers13194827
115. Raman P, Koenig RJ. Pax-8-PPAR-γ fusion protein in thyroid carcinoma. Nat Rev Endocrinol. (2014) 10:616–23. doi: 10.1038/nrendo.2014.115
116. Cao J, Zhang M, Zhang L, Lou J, Zhou F, Fang M. Non-coding RNA in thyroid cancer - Functions and mechanisms. Cancer Lett. (2021) 496:117–26. doi: 10.1016/j.canlet.2020.08.021
117. Sacks W, Braunstein GD. Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr Pract. (2014) 20:263–75. doi: 10.4158/ep13305.Ra
118. Filetti S, Durante C, Hartl DM, Leboulleux S, Locati LD, Newbold K, et al. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol. (2022) 33:674–84. doi: 10.1016/j.annonc.2022.04.009
119. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
120. Brose MS, Smit J, Lin CC, Pitoia F, Fellous M, DeSanctis Y, et al. Timing of multikinase inhibitor initiation in differentiated thyroid cancer. Endocr Relat Cancer. (2017) 24:237–42. doi: 10.1530/erc-17-0016
121. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. (2004) 64:7099–109. doi: 10.1158/0008-5472.Can-04-1443
122. Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. (2012) 167:643–50. doi: 10.1530/EJE-12-0405
123. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. (2014) 384:319–28. doi: 10.1016/s0140-6736(14)60421-9
124. Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer. (2015) 22:877–87. doi: 10.1530/ERC-15-0252
125. Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. (2013) 340:97–103. doi: 10.1016/j.canlet.2013.07.007
126. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. (2014) 6. doi: 10.1186/2045-824x-6-18
127. Yeung KT, Cohen EE. Lenvatinib in advanced, radioactive iodine-refractory, differentiated thyroid carcinoma. Clin Cancer Res. (2015) 21:5420–6. doi: 10.1158/1078-0432.Ccr-15-0923
128. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
129. Nair A, Lemery SJ, Yang J, Marathe A, Zhao L, Zhao H, et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin Cancer Res. (2015) 21:5205–8. doi: 10.1158/1078-0432.Ccr-15-1377
130. Brose MS, Panaseykin Y, Konda B, de la Fouchardiere C, Hughes BGM, Gianoukakis AG, et al. A Randomized Study of Lenvatinib 18 mg vs 24 mg in Patients With Radioiodine-Refractory Differentiated Thyroid Cancer. J Clin Endocrinol Metab. (2022) 107:776–87. doi: 10.1210/clinem/dgab731
131. Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2021) 22:1126–38. doi: 10.1016/s1470-2045(21)00332-6
132. Capdevila J, Krajewska J, Hernando J, Robinson B, Sherman SI, Jarzab B, et al. Increased progression-free survival with cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer irrespective of prior vascular endothelial growth factor receptor-targeted therapy and tumor histology: A subgroup analysis of the COSMIC-311 study. Thyroid. (2024) 34:347–59. doi: 10.1089/thy.2023.0463
133. Brandenburg T, Machlah YM, Führer-Sakel D. Systemic therapies for advanced thyroid cancer - an update. Dtsch Med Wochenschr. (2023) 148:1412–8. doi: 10.1055/a-1951-2902
134. Duke ES, Barone AK, Chatterjee S, Mishra-Kalyani PS, Shen YL, Isikwei E, et al. FDA approval summary: cabozantinib for differentiated thyroid cancer. Clin Cancer Res. (2022) 28:4173–7. doi: 10.1158/1078-0432.Ccr-22-0873
135. Polverino A, Coxon A, Starnes C, Diaz Z, DeMelfi T, Wang L, et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. (2006) 66:8715–21. doi: 10.1158/0008-5472.Can-05-4665
136. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. (2008) 359:31–42. doi: 10.1056/NEJMoa075853
137. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. (2013) 31:3639–46. doi: 10.1200/jco.2012.48.4659
138. Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. (2007) 6:2012–21. doi: 10.1158/1535-7163.Mct-07-0193
139. Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. (2007) 25:884–96. doi: 10.1200/jco.2006.06.3602
140. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. (2010) 11:962–72. doi: 10.1016/s1470-2045(10)70203-5
141. Bible KC, Menefee ME, Lin CJ, Millward MJ, Maples WJ, Goh BC, et al. An international phase 2 study of pazopanib in progressive and metastatic thyroglobulin antibody negative radioactive iodine refractory differentiated thyroid cancer. Thyroid. (2020) 30:1254–62. doi: 10.1089/thy.2019.0269
142. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. (2010) 16:5260–8. doi: 10.1158/1078-0432.Ccr-10-0994
143. Bikas A, Kundra P, Desale S, Mete M, O’Keefe K, Clark BG, et al. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur J Endocrinol. (2016) 174:373–80. doi: 10.1530/eje-15-0930
144. Gosain R, Alexander JS, Gill A, Perez C. Radioactive iodine-refractory differentiated thyroid cancer in the elderly. Curr Oncol Rep. (2018) 20:82. doi: 10.1007/s11912-018-0736-4
145. Jaber T, Waguespack SG, Cabanillas ME, Elbanan M, Vu T, Dadu R, et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. (2018) 103:3698–705. doi: 10.1210/jc.2018-00612
146. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. (2021) 27:2130–5. doi: 10.1158/1078-0432.Ccr-20-3558
147. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. (2020) 383:825–35. doi: 10.1056/NEJMoa2005651
148. Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. (1994) 367:375–6. doi: 10.1038/367375a0
149. Subbiah V, Hu MI, Mansfield AS, Taylor MH, Schuler M, Zhu VW, et al. Pralsetinib in patients with advanced/metastatic rearranged during transfection (RET)-altered thyroid cancer: updated efficacy and safety data from the ARROW study. Thyroid. (2024) 34:26–40. doi: 10.1089/thy.2023.0363
150. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. (2021) 9:491–501. doi: 10.1016/s2213-8587(21)00120-0
151. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. (2018) 378:731–9. doi: 10.1056/NEJMoa1714448
152. Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. (2022) 186:631–43. doi: 10.1530/eje-21-1259
153. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. (2020) 21:271–82. doi: 10.1016/s1470-2045(19)30691-6
154. Cortas C, Charalambous H. Tyrosine kinase inhibitors for radioactive iodine refractory differentiated thyroid cancer. Life (Basel). (2023) 14. doi: 10.3390/life14010022
155. Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. (2015) 25:71–7. doi: 10.1089/thy.2014.0123
156. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. (2015) 21:1028–35. doi: 10.1158/1078-0432.Ccr-14-2915
157. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. (2016) 17:1272–82. doi: 10.1016/S1470-2045(16)30166-8
158. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab. (2019) 104:1417–28. doi: 10.1210/jc.2018-01478
159. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. (2013) 368:623–32. doi: 10.1056/NEJMoa1209288
160. Ho AL, Dedecjus M, Wirth LJ, Tuttle RM, Inabnet WB 3rd, Tennvall J, et al. Selumetinib plus adjuvant radioactive iodine in patients with high-risk differentiated thyroid cancer: A phase III, randomized, placebo-controlled trial (ASTRA). J Clin Oncol. (2022) 40:1870–8. doi: 10.1200/jco.21.00714
161. Leboulleux S, Benisvy D, Taieb D, Attard M, Bournaud C, Terroir-Cassou-Mounat M, et al. MERAIODE: A phase II redifferentiation trial with trametinib and (131)I in metastatic radioactive iodine refractory RAS mutated differentiated thyroid cancer. Thyroid. (2023) 33:1124–9. doi: 10.1089/thy.2023.0240
162. Borson-Chazot F, Dantony E, Illouz F, Lopez J, Niccoli P, Wassermann J, et al. Effect of buparlisib, a pan-class I PI3K inhibitor, in refractory follicular and poorly differentiated thyroid cancer. Thyroid. (2018) 28:1174–9. doi: 10.1089/thy.2017.0663
163. Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, et al. Everolimus in patients with advanced follicular-derived thyroid cancer: results of a phase II clinical trial. J Clin Endocrinol Metab. (2017) 102:698–707. doi: 10.1210/jc.2016-2525
164. Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, et al. Genomic correlates of response to everolimus in aggressive radioiodine-refractory thyroid cancer: A phase II study. Clin Cancer Res. (2018) 24:1546–53. doi: 10.1158/1078-0432.Ccr-17-2297
165. Lin Y, Wang C, Gao W, Cui R, Liang J. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget. (2017) 8:42252–61. doi: 10.18632/oncotarget.15036
166. Lin YS, Zhang X, Wang C, Liu YQ, Guan WM, Liang J. Long-term results of a phase II trial of apatinib for progressive radioiodine refractory differentiated thyroid cancer. J Clin Endocrinol Metab. (2021) 106:e3027–36. doi: 10.1210/clinem/dgab196
167. Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(-) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. (2001) 86:3430–5. doi: 10.1210/jcem.86.7.7621
168. Nilubol N, Merkel R, Yang L, Patel D, Reynolds JC, Sadowski SM, et al. A phase II trial of valproic acid in patients with advanced, radioiodine-resistant thyroid cancers of follicular cell origin. Clin Endocrinol (Oxf). (2017) 86:128–33. doi: 10.1111/cen.13154
169. French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. (2010) 95:2325–33. doi: 10.1210/jc.2009-2564
170. Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. (2010) 107:57–117. doi: 10.1016/s0065-230x(10)07003-x
171. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. (2009) 182:4499–506. doi: 10.4049/jimmunol.0802740
172. Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, et al. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab. (2012) 97:1474–82. doi: 10.1210/jc.2011-1838
173. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. (2008) 15:1069–74. doi: 10.1677/erc-08-0036
174. Chowdhury S, Veyhl J, Jessa F, Polyakova O, Alenzi A, MacMillan C, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. (2016) 7:32318–28. doi: 10.18632/oncotarget.8698
175. Rosenbaum MW, Gigliotti BJ, Pai SI, Parangi S, Wachtel H, Mino-Kenudson M, et al. PD-L1 and IDO1 are expressed in poorly differentiated thyroid carcinoma. Endocr Pathol. (2018) 29:59–67. doi: 10.1007/s12022-018-9514-y
176. Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. (2017) 102:1943–50. doi: 10.1210/jc.2016-3756
177. Wan B, Deng P, Dai W, Wang P, Dong Z, Yang C, et al. Association between programmed cell death ligand 1 expression and thyroid cancer: A meta-analysis. Med (Baltimore). (2021) 100:e25315. doi: 10.1097/md.0000000000025315
178. Zhang GQ, Wei WJ, Song HJ, Sun ZK, Shen CT, Zhang XY, et al. Programmed cell death-ligand 1 overexpression in thyroid cancer. Endocr Pract. (2019) 25:279–86. doi: 10.4158/ep-2018-0342
179. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. (2019) 19:196. doi: 10.1186/s12885-019-5380-3
180. Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol (R Coll Radiol). (2010) 22:464–8. doi: 10.1016/j.clon.2010.03.014
181. Manohar PM, Beesley LJ, Taylor JM, Hesseltine E, Haymart MR, Esfandiari NH, et al. Retrospective study of sirolimus and cyclophosphamide in patients with advanced differentiated thyroid cancers. J Thyroid Disord Ther. (2015) 4. doi: 10.4172/2167-7948.1000188
182. Sherman EJ, Dunn LA, Ho AL, Baxi SS, Ghossein RA, Fury MG, et al. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer. (2017) 123:4114–21. doi: 10.1002/cncr.30861
183. Atkinson H, England JA, Rafferty A, Jesudason V, Bedford K, Karsai L, et al. Somatostatin receptor expression in thyroid disease. Int J Exp Pathol. (2013) 94:226–9. doi: 10.1111/iep.12024
184. Busaidy NL, Konda B, Wei L, Wirth LJ, Devine C, Daniels GA, et al. Dabrafenib versus dabrafenib + Trametinib in BRAF-mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial. Thyroid. (2022) 32:1184–92. doi: 10.1089/thy.2022.0115
185. Leboulleux S, Do Cao C, Zerdoud S, Attard M, Bournaud C, Lacroix L, et al. A phase II redifferentiation trial with dabrafenib-trametinib and 131I in metastatic radioactive iodine refractory BRAF p. V600E-Mutated Differentiated Thyroid Cancer. Clin Cancer Res. (2023) 29:2401–9. doi: 10.1158/1078-0432.CCR-23-0046
Keywords: radioiodine-refractory thyroid cancers, sodium/iodide symporter, mitogen-activated protein kinase, phosphatidylinositol-3-hydroxykinase, TERTp, tyrosine kinase inhibitors, systemic treatments
Citation: Chen P, Yao Y, Tan H and Li J (2024) Systemic treatments for radioiodine-refractory thyroid cancers. Front. Endocrinol. 15:1346476. doi: 10.3389/fendo.2024.1346476
Received: 20 February 2024; Accepted: 27 September 2024;
Published: 15 October 2024.
Edited by:
Maria João Bugalho, Santa Maria Hospital, PortugalReviewed by:
Erika Abelleira, Hospital de Clínicas José de San Martín, ArgentinaCopyright © 2024 Chen, Yao, Tan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiwen Tan, aHVpd2VudDIwMTZAc2N1LmVkdS5jbg==; Jianwei Li, amVycnlsaTY3OEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.