
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 12 April 2024
Sec. Cellular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1343255
This article is part of the Research Topic Adipokines, batokines & cardiokines: crosstalk with metabolic organs View all 9 articles
Stem cell-based therapies exhibit considerable promise in the treatment of diabetes and its complications. Extensive research has been dedicated to elucidate the characteristics and potential applications of adipose-derived stromal/stem cells (ASCs). Three-dimensional (3D) culture, characterized by rapid advancements, holds promise for efficacious treatment of diabetes and its complications. Notably, 3D cultured ASCs manifest enhanced cellular properties and functions compared to traditional monolayer-culture. In this review, the factors influencing the biological functions of ASCs during culture are summarized. Additionally, the effects of 3D cultured techniques on cellular properties compared to two-dimensional culture is described. Furthermore, the therapeutic potential of 3D cultured ASCs in diabetes and its complications are discussed to provide insights for future research.
Stem cell-based therapy, including pluripotent stem cells (PSCs) and mesenchymal stromal/stem cells (MSCs), represents an innovative therapeutic strategy that capitalizes on the distinctive characteristics of stem cells, such as self-renewal and differentiation capabilities, to facilitate the regeneration of impaired cells and tissues within the body or the substitution of these cells with new, healthy, and fully functional cells by delivering exogenous cells (1).
PSCs are characterized as a type of self-renewing cells capable of differentiating into diverse cellular phenotypes originating from the three germ layers of the body (2). PSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), has revolutionized stem cell research and cell-based therapy (3). Nonetheless, the utilization of ESCs is constrained by ethical considerations, the possibility of immunological rejection, and the potential for tumorigenicity (1, 4). In contrast, iPSC technology overcomes ethical dilemmas associated with ESCs derived from human embryos, enabling the creation of patient-specific pluripotent stem cells. However, iPSCs are generated through the ectopic expression of pluripotency factors, often facilitated by viral vectors or non-viral reprogramming factors, which may lead to genomic instability (5, 6). Besides, iPSCs have been shown to elicit T cell-dependent immune response (7) and promote tumor formation (3, 8). Consequently, thorough safety assessments are imperative prior to iPSC transplantation.
Mesenchymal stromal/stem cells (MSCs) are adult stem cells with multipotent capabilities, including self-renewal (albeit limited in vitro) and differentiation into various mesenchymal lineages (9, 10). MSCs have been shown to overcome ethical concerns and mitigate the risk of mutational side effects associated with. Additionally, MSCs exhibit the lowest immunogenicity compared to other stem cell types, making them a favorable option for clinical use (11). In the field of organ and cell transplantation, MSCs have been utilized for their secretion of growth factors and immunoprotective cytokines. Their ability to differentiate into various cell types has been harnessed for applications in tissue engineering (12). Among these, adipose-derived MSCs (ASCs) are particularly advantageous due to their larger storage with less discomfort and damage to the donor site, easier accessibility without significant donor site morbidity, higher proliferation ability, fewer ethical concerns, and fewer immunological rejection (11, 13, 14). Furthermore, some growth factors and immunomodulators are more actively secreted in ASCs (13). Therefore, ASCs may be a better candidate for clinical application in theory.
Diabetes mellitus (DM) is a severe and chronic disease characterized by elevated blood glucose levels resulting from aberrant islet β-cell biology and insulin action (15). In 2021, the global population living with diabetes reached 529 million (15). Given β-cell dysfunction across various types of DM, most patients ultimately require insulin therapy (16–18). However, this therapy is frequently limited by individual factors, such as weight gain, fear of needles and lifestyle considerations, all of which contribute to poor glycemic control. Furthermore, insulin therapy cannot reverse β-cell damage and progress of diabetes, or replicate the normal physiological state. In recent clinical applications, pancreatic islet and cell transplantation have emerged as potential strategies (19). However, these procedures have numerous challenges, including the scarcity of suitable donors, surgical complexities, side effects associated with immunosuppressive agents as well as exhaustion of transplanted organs and cells (11). Furthermore, it is necessary to maintain β-cell function and blood glucose homeostasis, otherwise life-threatening complications are likely to occur (20).
In the treatment of diabetes and its complications, ASCs have been used due to their inherent attributes such as self-renewal capacity, differentiation potential, homing mechanism and immunosuppressive property (11, 21). Furthermore, three-dimensional (3D) cultured cells are studied to prolong the lifespan of transplanted cells and enhance their pro-healing functions in unfavorable environments (22–25). Recent literature provides numerous strategies for obtaining 3D cultured ASCs (26). These cells possess enhanced abilities to maintain their stemness and display multilineage plasticity compared to cells cultured in adhesion (26). Moreover, 3D cells more closely mirror biological processes compared to cells cultured in traditional monolayers, driving the need for the development of 3D culture, including spheroids, organoids, organ-on-a-chip models, and bioprinting (27–29).
Despite being an emerging and rapidly developing technology, there is currently no standardized method for ASC culture and no summary for the research of 3D cultured ASCs in diabetes and its complications. In this review, we summarize current knowledge about monolayer ASC culture techniques, with a particular emphasis on the influential factors during culture. Additionally, the effects for cellular properties of 3D cultured methods compared to two-dimensional (2D) culture is described. Furthermore, the therapeutic potential of 3D cultured ASCs in diabetes and its complications are discussed to provide insights for future research.
There is inconsistency in the nomenclature of this plastic adherent cell population isolated from adipose tissue (30). In 2006, the International Society for Cellular Therapy (ISCT) acknowledged the “inconsistencies and ambiguities” of the term “mesenchymal stem cells” and recommended a new designation: multipotent mesenchymal stromal cells (31). It is recommended to use the abbreviation “MSCs” in conjunction with extra information like AD-MSCs (9) (adipose tissue-derived MSCs) or MSC(AT) (32) and clearly define stem cells or stromal cells in terms of their function (9). Additionally, Caplan proposed the term “medicinal signaling cells” due to their therapeutic actions, which include homing to the site of injury and secreting regenerative and immunomodulatory factors (33). Despite the advocacy for standardization in nomenclature, it is still most common to refer to MSCs as “mesenchymal stem cells”, followed by “mesenchymal stromal cells” or a combined use of “stem/stromal” terms (34). In this review, following search terms for this kind of cells were adopted: “adipose-derived stromal cells”, “adipose-derived stem cells”, “adipose-derived stromal/stem cells”, “ASCs” and “ADSCs”, and having no limitation to the human or animal species.
In the 1960s, Rodbell and Jones pioneered the initial method of isolating cells from adipose tissue (35–37). The researchers isolated stromal vascular fraction (SVF) from rat fat pads, which contained heterogeneous cells. In the final step, adherent plastic cells within the SVF were selected and enriched for “preadipocytes”. In 2001, ZUK et al. obtained a fibroblast-like cell population or a processed lipoaspirate from human lipoaspirate. They determined these cells could differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells in vitro, which opened up new avenues for MSC research (38). The isolation and culture process of ASCs is shown in Figure 1.
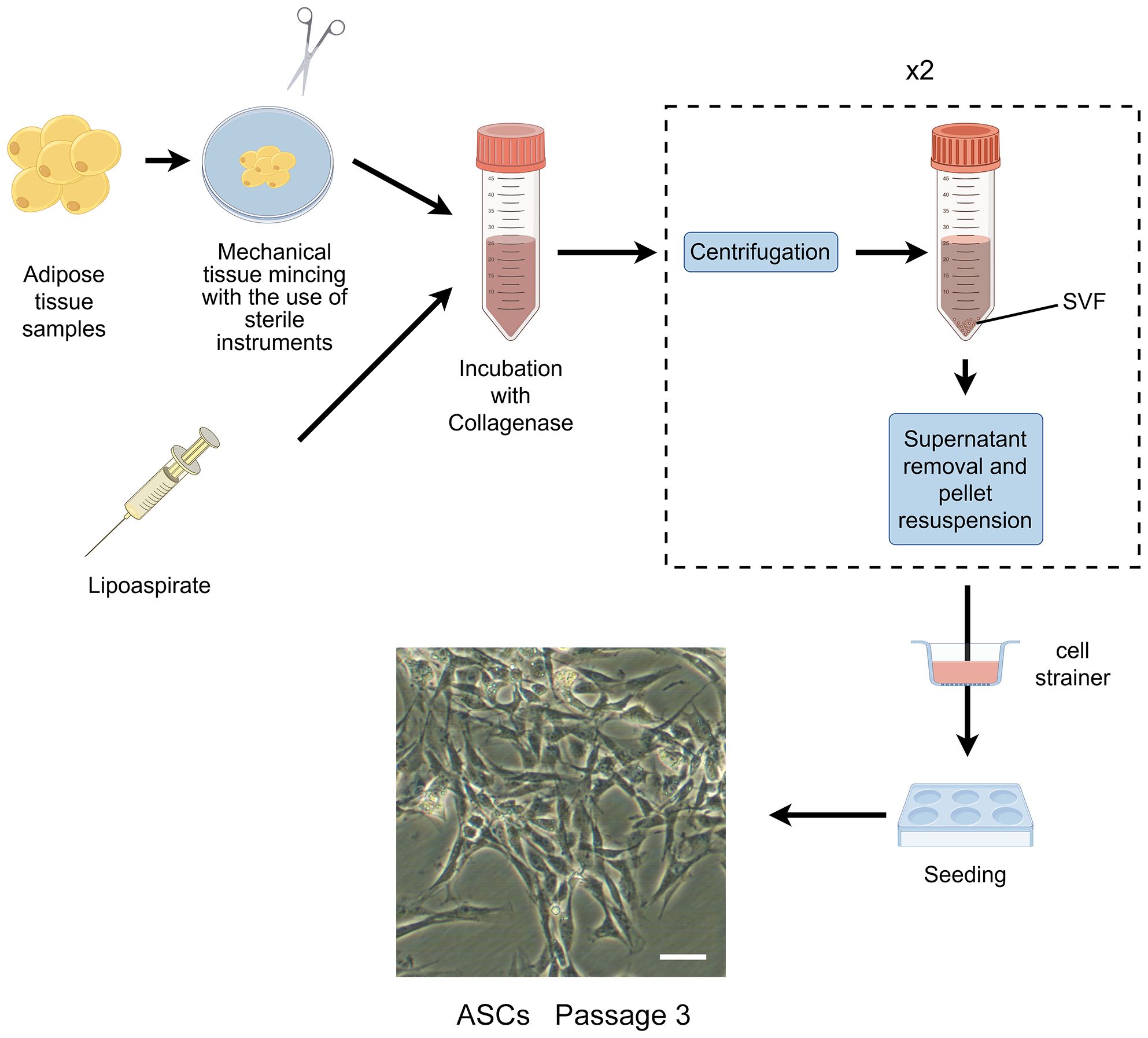
Figure 1 The isolation process of ASCs. The cells showed are isolated from rat’s inguinal adipose tissue. Scale bar, 200μm. SVF, stromal vascular fraction; ASCs, adipose-derived stromal/stem cells.
The characterization of ASCs involves fulfilling specific criteria related to cellular morphology (39, 40), immune-phenotypic (10), and differentiation capacity (10, 31). As high quality of cells is the prerequisite for their application, various factors that may influence their biological functions during culture have been proposed (Figure 2).
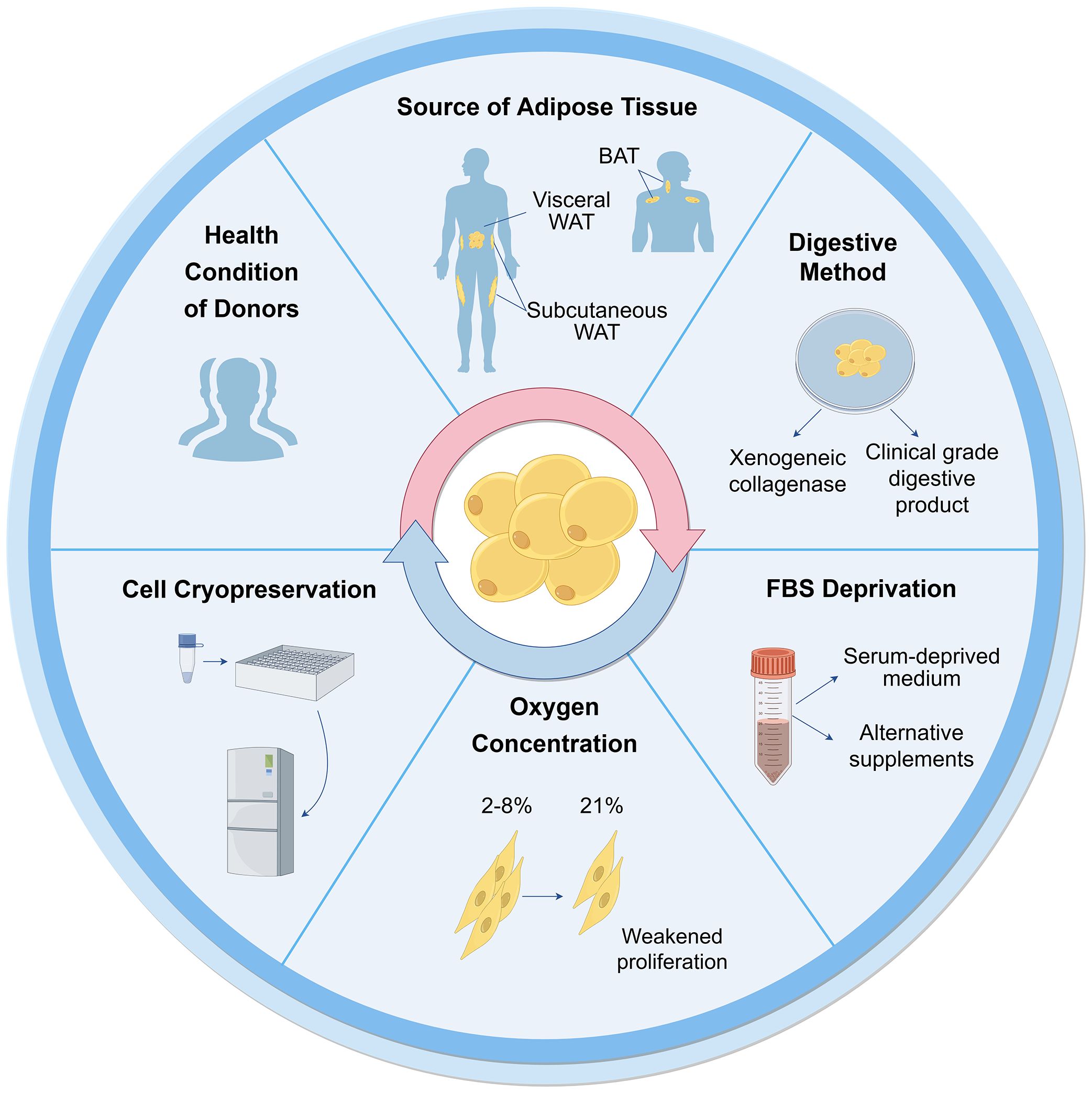
Figure 2 Influential factors on biological functions of ASCs during culture. Many aspects are reported to influence ASC culture and their biological functions. These can broadly be divided into the sources of tissues and cells, techniques of isolation, culture and cryopreservation. WAT, white adipose tissue; BAT, brown adipose tissue; FBS, fetal bovine serum.
Cells can be obtained from healthy donors or individuals with varying degrees of diabetes, obesity, and other chronic diseases. The use of autologous and allogeneic ASCs should be carefully considered. Autologous cells have advantages in terms of histocompatibility and infectious concerns (41), but their functionality may be compromised in an unhealthy environment. ASCs derived from diabetic donors have shown reduced proliferation ability and paracrine activity compared to autologous ASCs from healthy individuals (42–44), but they still hold potential in cell therapies (45–47). Additionally, Obesity has an adverse impact on ASCs, resulting in defective functionalities and properties (48). ASCs from individuals with obesity exhibit decreased telomerase activity and telomere length (49). There are no significant differences observed in ASCs between oncological patients and healthy subjects (50, 51). However, ASCs from donors exposed to radiotherapy and chemotherapy exhibit altered cell migration, proliferation, and differentiation capacity (48). The outcomes are also correlated with other demographics, such as age, gender, and ethnicity (52).
White adipose tissue (WAT) mainly exists in two types: subcutaneous and visceral adipose tissue. ASCs obtained from subcutaneous (S-ASCs) and visceral adipose tissue (V-ASCs) share similar cell viability and surface markers but differ in motility, secretory function, and expression of stemness-related genes (53). However, S-ASCs have a greater differentiation capacity to adipogenic and osteogenic cells, and V-ASCs proliferate slower, require stronger stimulation for differentiation (54), and secrete higher levels of inflammatory cytokine such as interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α (55). Wada et al. (56). also found that V-ASCs and S-ASCs release inflammatory and angiogenesis cytokines differently. Moreover, ASCs in the superficial layer, located closer to the dermis, exhibited hyperplastic and angiogenic capacities, while ASCs in the deep layer were characterized by inflammatory properties similar to V-ASCs (27).
Furthermore, studies have shown the presence of ASCs derived from brown adipose tissue (BAT) (57, 58). The characteristics of ASCs derived from BAT differ from those of WAT, particularly, the expression of myogenic factor 5 (Myf5) and myogenic origin. In these cells, gene expression profiles are unique, particularly the higher expression of genes associated with BAT including uncoupling protein-1 (UCP1), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), PR domain containing 16 (PRDM16), and CAMP responsive element binding protein one (CREB1). Therefore, the tissue and cell sources should be considered for further application.
The first crucial step in obtaining cells from adipose tissue is cell isolation. Currently collagenase digestion remains the most common method to obtain cells due to its simplicity and high cell purity (41, 48). However, the use of xenogeneic collagenase may lead to pathogen transmission and immune response in vivo. To be considered safe, the development of clinical grade digestive products is crucial for the isolation of ASCs. Carvalho et al. demonstrated that several alternative enzyme products, including Collagenase NB 4 Standard Grade (NB4) [Serva], Collagenase Type 1 (CLS1) [Worthington], Collagenase (Animal Origin Free)-A (CLSAFA) [Worthington], and Liberase [Roche], were equally effective as research-grade products (59). Kølle et al. implemented a clinical trial using cells which were isolated by clinical collagenase NB 6 (60).
Fetal bovine serum (FBS) is another important consideration for ASC culture and application, similar to xenogeneic collagenases. The available studies showed that human ASCs (hASCs) maintain their stemness in serum-deprived medium (61). In the absence of FBS for 48 hours, hASCs showed reduced metabolism and proliferation, but maintained the expression of crucial surface markers, without undergoing apoptosis or necrosis (51). Human ASCs cultured in STK2 (a chemically-defined serum-free medium) exhibited enhanced proliferation, elevated expression of MSC surface markers, and diminished cell aging compared to those cultivated in media supplemented with FBS (62). According to these observations, FBS deprivation does not cause impacts that would prevent cellular clinical application.
Other alternative supplements have been investigated as potential substitutes for FBS. Human platelet lysates (HPLs) could serve as a superior supplement. They were found to augment the proliferative capacity of hASCs in comparison to FBS, while simultaneously preserving their untransformed state and differentiation ability (63, 64). Kocaoemer et al. observed that hASCs cultured in medium supplemented with either thrombin-activated platelet rich plasma (tPRP) or pooled human serum (HS) exhibited similar properties, although a reduction in adhesion was observed in cells cultured in tPRP-supplemented medium (65). According to the whole genome gene analysis, 90 genes were significantly expressed more in hASCs cultured in FBS-supplemented medium (66).
As the oxygen concentration of adipose tissue in vivo is 2%-8%, ASCs exist in a relatively low-oxygen microenvironment (67, 68). However, most ASCs are cultured under normal oxygen conditions (21% oxygen concentration) in vitro. Human subcutaneous ASCs cultured in hypoxic conditions in vitro exhibited increased proliferation rates and secretion of growth factors (69). Tirza et al. discovered weakened proliferation ability, increased accumulation of reactive oxygen species (ROS), and genetic instability of rat visceral ASCs cultured under normal oxygen experienced, which could be improved by lowering the culture temperature (67).
Despite the diminished cell viability and lower colony-forming-unit percentages observed in cells derived from cryopreserved lipoaspirate compared to fresh lipoaspirate-derived cells, the viable cells that remained exhibited preserved adhesive and proliferative properties (70), which could counteract the negative effect with continued cell growth (71). After prolonged cryopreservation at 70°C, the number of viable cells decreased as well as their viability (71). A cryopreservation medium containing HS, HS albumin, or knockout serum replacement did not affect the gene expression, differentiation ability, and immunophenotype of hASCs for a duration of 3-4 freeze-thaw cycles, but significantly reduced the proliferation. Thus, it has been recommended that cells for clinical application should not undergo more than two freeze-thaw cycles (72).
In summary, isolation and culture methods can affect ASCs properties, therefore, there is still a need to look for appropriate culture protocol that will provide the right number and characteristics of ASCs without affecting their therapeutic potential for clinical application.
Cellular senescence, also called aging, has always been an obstacle to the development of MSC therapy. Some studies confirmed the stability of ASCs during a certain period (usually up to the sixth or seventh passage) (51, 73, 74). However, Yin et al. found that hASCs rapidly underwent replicative senescence and lost stem cell properties over 21 days by current 2D culture (75). During long-term culture, senescent cells experience a cessation in proliferation, and exhibit distinct morphological and physiological features, including enlarged nuclear and cytoplasmic volumes, heightened β-galactosidase enzyme activity, decreased expression of β cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1), telomere shortening and accumulation of ROS (76, 77). The stability and safety of ASCs should be considered in application, thus, many research efforts have been enhanced to address the problem of cellular aging.
Immortalization techniques have been shown to overcome senescence in primary cells (78). Kang et al. discovered that ectopic expression of telomerase reverse transcriptase (TERT) in non-human primates ASCs enabled cells to maintain proliferative potential and multipotent differentiation ability (79). Tchkonia et al. generated preadipocyte strains from single abdominal subcutaneous, mesenteric and omental human preadipocytes through stable expression of human TERT (hTERT). These strains were capable of repeated subculturing and maintained the capacity for differentiation, as well as the specific dynamic characteristics of fat depot cells (80). Wolbank et al. found hTERT overexpression generated ASC lines (ASCshTERT) exhibited continuous growth and showed minimal changes in morphology, surface marker profile, karyotype, immunosuppressive capacity and differentiation potential (81). Similarly, Shamsi and Tseng developed protocols for immortalizing brown and white preadipocytes (82). Furthermore, researchers have cultured ASCs with TERT expression to conduct further researches in regenerative medicine and other medical fields (79, 83–85).
Furthermore, Tátrai et al. found that human ASCshTERT and ASCs generated by the co-transduction of hTERT and Bmi-1 retained MSC features and did not senesce, whereas ASCs generated by the overexpression of Bmi-1 exhibited limited replicative potential. Notably, a subpopulation of ASCshTERT also acquired aberrant karyotype and showed signs of transformation after long-term culture (86).
However, Balducci et al. found that hTERT alone failed to immortalize hASCs. Moreover, hASCs that were co-transduced with hTERT and human papillomavirus (HPV)-E6/E7 were successfully immortalized and could secrete significant amount of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), albeit with reduced differentiation properties and some chromosomal aberrations (87). Darimont et al. demonstrated that co-transduction of hTERT and HPV-E7 enabled human preadipocytes to extend their lifespan and maintain their capacity for differentiation (88).
The overexpression of simian virus 40 large T antigen (SV40T) has been widely employed as a strategy to overcome replicative senescence in human primary cells. However, it was found that the adipogenic differentiation process was blocked by SV40T expression in 3T3-F442A cells (89). Human ASC lines operated by co-transduction of hTERT and SV40T underwent chromosome aberration, deviated from the normal MSC phenotype, and lose the ability of differentiate (86, 87).
Although there were variations in results across different studies, it is generally established that cell immortalization can be achieved through gene editing technology (Table 1). Notably, the possibility of karyotype variation should be taken into consideration in these immortalized cells constructed by gene editing technology.
Significantly, advancements in stem cell and 3D culture technologies have enabled the creation of cellular models that accurately mimic the histological, molecular, and physiological characteristics of tissues and organs (29). The formation of 3D cultures relies on the self-organization and differentiation of cells, as well as signaling cues from the extracellular matrix (ECM) and conditioned media (90).
3D cultures are typically self-assembled in vitro 3D structures derived from primary tissues or various types of stem cells, including MSCs, iPSCs, and ESCs. Various cell types exhibit distinct developmental pathways, underscoring the importance of selecting an appropriate initial cell population for the successful establishment of organoid cultures. 3D culture of ESCs has not been a priority due to their ethical concerns. 3D culture models derived from MSCs have been shown to highly recapitulate the homeostasis and regenerative capacity of the tissue of origin (91). Conversely, models derived from iPSCs often hardly recapitulate the adult tissue stage, instead resembling the fetal tissue stage (92, 93). 3D culture models derived from ASCs are generated without genetic modification by transcription factors, unlike those derived from iPSCs (94). Moreover, ASC exhibit immune privileged properties, and accordingly show excellent safety for allogeneic transplantation in multiple human clinical trials (4). Therefore, ASCs is a cell type with great potential and advantages in 3D culture technology.
Despite numerous studies, there is no standardized method for 3D ASC culture. It is necessary to comprehend the impact of different 3D culture techniques on cellular properties in contrast to traditional 2D culture.
The stemness properties of MSCs are retained in the in vivo microenvironment, which includes soluble growth factors, cell-cell interactions and cell-matrix interactions (95, 96). Increasing evidence has indicated that the cellular microenvironment significantly influences stemness properties (95, 97). In comparison to conventional monolayer cultures, 3D cultured methods provide a cellular niche that more closely resembles the in vivo microenvironment (98).
Existing techniques for ASC culture can be categorized into scaffold-free and scaffold systems (26, 99). The conventional scaffold-free culture techniques, such as the use of low adhesion plates, hanging drops, and spinner flasks, have been shown to impact the viability and stemness of ASCs.
Low adhesion plate culture method involves the formation of spheroids by suspending cells on a surface with low adhesion properties. Guo et al. successfully generated 3D spheroids using non-adhesive agarose Petri dishes. This method was found to overcome poor post thaw cells and improve the viability and neural differentiation potential of hASCs (100). Similarly, Coyle et al. conducted a study examining hASC spheroids with various sizes and demonstrated the enhanced viability of spheroids was achieved through anaerobic glycolysis in conditions of increased glucose availability and decreased oxygen levels (101). Di Stefano et al. conducted a comparative analysis of hASCs cultured in ultralow culture flasks and hASCs with 2D primary cultures. Their study identified distinct molecular expression patterns of genes associated with stemness, as well as genes related to anti-aging, oxidative stress, and telomeres maintenance of hASCs (102). Rybkowska et al. conducted a study in which 3D hASC spheroids were cultured using antiadhesive plates. However, they observed that the spheroids exhibited slightly lower viability, reduced proliferation rates, but higher expression of stemness-related transcriptional factors compared to cells cultured in monolayer. Additionally, the 3D culture resulted in increased mitochondrial DNA content, oxygen consumption rate, and extracellular acidification rate. Elevated levels of ROS and decreased intracellular lactic acid levels were also detected (103).
The hanging drop method technique capitalizes on the intrinsic tendency of cells to self-assemble into three-dimensional aggregates needless of scaffolding. A drop is formed within an inverted plate and held in place due to surface tension. Jin et al. utilized the hanging-drop technique to produce hASC microtissues in a smooth muscle inductive medium supplemented with human transforming growth factor β1, and subsequently bioprinted these induced microtissues onto a 3D framework. The microtissues retained their phenotypic characteristics post-bioprinting. Cell viability and proliferation within the 3D microtissues were consistently superior in comparison to the traditional single-cell bioprinting approach (104).
The spinner flask facilitates the generation of fluid flow, which discourages cellular adhesion and facilitates cellular aggregation. Bangh et al. placed hASC spheroids in spinner flasks under 1% oxygen. The spheroids exhibited faster growth rates compared to monolayer cultures. Additionally, they observed an upregulation of survival factors in response to the spheroid size (105).
Another commonly employed approach involves seeding stem cells into scaffolds that mimic the ECM of native tissues, which can be fabricated using biologically derived or synthetic materials. Natural scaffolds consist predominantly of collagen, fibrin, gelatin, vitronectin, laminin, alginate, hyaluronic acid (HA), or decellularized materials, while synthetic scaffolds may consist of materials such as polyesters, polyethers, polyethylene glycol, and polylactic acid (PLLA) (106). Several studies have investigated the use of different hydrogels to create ASC spheroids, utilizing commonly used materials in tissue engineering such as HA and chitosan, resulting in enhanced stemness gene expression compared to traditional adhesion plate cultures (107–110). A poly(ethylene glycol) (PEG) hydrogel microwell pattern was fabricated on a poly(N-isopropylacrylamide) hydrogel substrate to regulate the size of spheroids. The viability of hASC spheroids exceeded 97.5% (111).
Based on mechanical structure or new systems, various novel techniques were devised to create 3D ASC structures with enhanced viability, increased stemness, and enhanced differentiation capabilities, such as the following: A switchable water-adhesive, super-hydrophobic nanowire surface (112); microgravity bioreactors (113); microwell plates employed with gelatin microparticles (114); microfabricated porous tissue strands (pTSs) (115); a method defined “all-in-one platform” with hydrogels with an embossed surface (HES) (116); gelatin hydrogels with microbial transglutaminase (mTG) (117); and TeSR-E8 medium (a highly chemically defined medium) in conventional tissue culture polystyrene dishes (118). Furthermore, Labriola et al. utilized polymer-based, cell mimicking microparticles (CMMPs) to deliver distinct, stable mechanical cues to hASCs in 3D spheroid culture. Mechanically tuned CMMPs controlled whole-spheroid mechanical phenotype and stability but minimally affected differentiation response (119).
Based on the findings of these studies, it is evident that the majority of research indicates that 3D culture enhances cell viability, stemness, proliferation rate, and metabolic functions, with only a few exceptions showing a decrease in cell viability.
Multilineage differentiation potential of ASCs towards both mesenchymal and non-mesenchymal lineage cells have been reported, particularly towards adipogenic, chondrogenic, and osteogenic lineages, which can be facilitated by the introduction of lineage-specific factors (120).
Adipogenic differentiation: Decellularized adipose tissue (DAT) based hydrogels have been demonstrated to closely replicate the native ECM environment, effectively inducing adipogenic differentiation and promoting the proliferation of hASCs (121, 122). In a study by Zhang et al., hASC spheroids cultured in a microgravity bioreactor exhibited enhanced stemness properties and adipogenic differentiation potential compared to monolayer culture (113). Hoefner et al. cultured hASC spheroids in growth cell media under agitation at 50 revolutions per minute. After a brief 2-day induction period for adipogenic lineages, it was observed that ASC spheroids exhibited enhanced differentiation capacity within their own ECM when compared to traditional 2D cultures (123). These findings suggest that utilizing 3D ASC culture may be a promising approach for adipose tissue engineering applications.
However, Rumiński et al. reported that hASC spheroids seeded in 96-well sterile round-bottom culture plates and subjected to gentle rotation on a rotary shaker displayed reduced adipocyte differentiation (124). The elastin-like polypeptide (ELP)-polyethyleneimine (PEI) coated surface was demonstrated a suitable cell culture material (125). However, the study conducted by Turner et al. revealed that triglyceride accumulation was less pronounced in hASC spheroids seeded on ELP-PEI coated surfaces compared to 3T3-L1 adipocytes, correlated with smaller average spheroids, suggesting a relatively slower differentiation process (126).
Chondrogenic differentiation: Yoon et al. employed the spinner flask method to illustrate that 3D hASC spheroids exhibit enhanced chondrogenic capabilities when cultured in a specific differentiation medium as opposed to monolayer culture (127). Tsai et al. employed mTG, an enzyme with high specificity across a broad temperature range, to crosslink gelatin. The evaluation of differentiation potential revealed that hASC spheroids within the 3D gelatin/mTG hydrogel demonstrated heightened activity, particularly in adipogenesis and chondrogenesis, in comparison to the cell suspension group (117). Furthermore, when comparing hASC spheroids cultured using microwell techniques to ASCs cultured in a 2D monolayer, it was observed that cell survival and chondrogenic potential were enhanced, while apoptosis was diminished. Injecting hASC spheroids exerted enhanced regenerative capabilities for articular cartilage and effectively halted the advancement of surgically induced osteoarthritis through the paracrine mechanism of action, when compared to ASCs in single-cell suspension (128).
Osteogenic differentiation: Gurumurthy et al. illustrated that 3D hASCs cultivated on ELP-PEI scaffolds exhibited a heightened propensity for differentiation towards the osteogenic lineage in comparison to 2D cultures (129). Human ASCs were cultured in 3D systems devoid of bioactive material components: spheroids and polystyrene scaffolds. Alkaline phosphatase activity, a marker of early osteogenesis, exhibited increased levels in ASC spheroids and ASC-seeded scaffolds in comparison to 2D cultures. The expression of the osteoblast marker, including Runt-related transcription factor 2, and osterix and integrin binding sialoprotein was significantly up-regulated in spheroids compared to polystyrene scaffolds and 2D culture (124). Kim et al. conducted a study to evaluate the osteogenic potential of hASCs in 2D and 3D culture environments. Through comprehensive analysis of transcriptome sequencing data, they identified an upregulation of genes associated with skeletal development, bone formation, and bone remodeling processes in hASCs cultured in concave microwells (130).
Differentiation into other lineages: Cheng et al. utilized chitosan films to form hASC spheroids, which, when cultured in appropriate induction media, exhibited enhanced differentiation capabilities, including differentiation into neuron and hepatocyte-like cells (131). Guo et al. observed an increased capacity for neural differentiation in 3D hASC spheroids cultured in agarose 3D Petri dishes (100). Amirpour et al. employed a defined neural induction medium with small molecules to directly differentiate hASCs into anterior neuroectodermal cells using hanging drop protocols (132). Additionally, Salehi et al. conducted a comparison between two differentiation protocols for the generation of retinal precursor-like cells in vitro: hASCs monolayer culture and hanging drop culture with a defined medium. The study indicated that the hanging drop method led to an enhanced yield of retinal precursor differentiation, resulting in precursor-like cells that exhibited responsiveness to the glutamate neurotransmitter (133). Moreover, the hanging drop method was found to enhance the efficiency of hASC smooth muscle differentiation and improve cell viability within a 3D bioprinted structure (104). Bagheri-Hosseinabadi et al. observed a higher rate of cardiomyogenic differentiation in hASCs cultured in a 3D hanging drop system with 5-azacytidine compared to the 2D culture (134).
These findings suggest that the 3D environment may offer enhanced stimuli for the differentiation of ASCs into various lineages. These results have implications for the development of protocols for preparing ASCs for use in clinical studies focused on regeneration.
The paracrine secretion of cytokines such as angiogenic factors, adipokines, neurotrophic factors, and interleukin plays a crucial role in the therapeutic application of ASCs by promoting tissue regeneration and repair (120).
3D cultured ASCs possess distinct and inherent characteristics independent of the method of formation. The size of 3D cultured ASCs is a critical factor, as larger cells exhibit higher levels of hypoxic factors that stimulate angiogenesis and antiapoptotic gene expression (26). small spheroids of average spherical shape were generated in 96-well plates. The 3D condition of the hASCs was found to be correlated with elevated levels of VEGF-A and IL-8 expressions in relation to wound healing (135). Kim et al. introduced HES as a comprehensive platform capable of facilitating the rapid formation and cultivation of a substantial quantity of size-adjustable 3D hASC spheroids. Notably, HES-derived spheroids exhibited a higher VEGF secretion compared to spheroids cultured on a commercially available low-attachment culture plate. Utilizing these advantages, HES-based spheroids were employed for 3D bioprinting, resulting in enhanced retention and VEGF secretion within the 3D-printed construct compared to a similar structure containing single cell suspension (116). Yu et al. utilized agarose microwells to seed hASCs, generating uniform cell spheroids with adjustable size, and stimulated ECM deposition through the use of ascorbic acid 2-phosphate to form ASC sheets. Transcriptome sequencing analysis indicated upregulation of angiogenesis-related genes in ASC spheroids compared to monolayer ASCs. The study illustrated the stimulatory impact of spheroid formation on ASCs towards endothelial lineage by observing increased expression of cluster of differentiation (CD) 31, which persisted following the seeding of ASC spheroids on cell sheets. Furthermore, compared to ASC sheets, ASC spheroid sheets exhibited heightened expression of VEGF and HGF, and the conditioned medium from ASC spheroid sheets significantly promoted tube formation of endothelial cells in vitro (136).
Seo et al. innovatively created a switchable water-adhesive, super-hydrophobic nanowire surface to enhance cell-cell and cell-matrix interaction, leading to improved cell viability and paracrine secretion of VEGF in hASC spheroids. The size of hASC spheroids can be easily manipulated on this surface. Accordingly, the spheroids generated on this surface demonstrate significantly heightened angiogenic effectiveness in comparison to spheroids produced through traditional methods such as spinner flask suspension culture and hanging drop culture on a petri dish (112). The successful establishment of a 3D co-culture model utilizing HA gel and a 10:1 ratio of late-passage hASCs and endothelial colony-forming cells resulted in increased secretion of cytokines, including HGF, VEGF, and epidermal growth factor (EGF), compared to single-cell 3D culture or monolayer culture (109). These findings suggest potential applications of 3D strategies in angiogenesis and regeneration therapies.
Furthermore, Zhang et al. utilized a low-adhesion cell culture plate to generate rat ASCs (rASCs) into microtissues in vitro. They employed grafts composed of microtissues and polycaprolactone nerve conduit for the purpose of repairing sciatic nerve defects in rats. Their study revealed that microtissues promote the secretion of nerve regeneration-related cytokines, including brain-derived neurotrophic factor, and nerve growth factor, the angiogenic factor such as VEGF, as well as anti-inflammatory cytokines such as IL-4, IL-10, and IL-13. This secretion ultimately facilitated the growth of axons when compared to an equivalent number of cells cultured in a 2D manner (137). Zhou et al. utilized a hanging drop method to generated murine ASCs-based microtissues, which were subsequently injected into streptozotocin (STZ)-induced diabetic rats for the treatment of erectile dysfunction. The findings demonstrated elevated expression of VEGF, nerve growth factor, and TNF-stimulated gene-6 within the microtissues, indicating neuroprotective and anti-inflammatory properties (138).
Overall, the use of specific culture media and 3D cultured techniques can enhance the differentiation potential and paracrine secretion of ASCs. Hence, it is imperative to carefully deliberate on the selection and refinement of techniques for producing 3D cultured ASCs, as they have the potential to impact the characteristics of cells.
Diabetes as a multi-organ disease, is a significant cause of increased morbidity and mortality worldwide. In the treatment of diabetes and its complications, ASCs have been used due to their inherent attributes such as self-renewal capacity, differentiation potential, homing mechanism and immunosuppressive property (11, 21, 139). Currently, the clinical trials of ASCs for treating diabetes and its associated complications, including the diabetic foot ulcer (DFU), diabetic critical limb ischemia, and diabetic nephropathies, are still in the preliminary research stage (Table 2). There is a lack of agreement regarding the optimal method of administration to achieve enhanced therapeutic outcomes. Potential routes of administration include intravascular injection, local tissue injection, and thymus injection. In diabetic patients, the most commonly used administration routes are intraportal injection and intravenous infusion (140, 141, 143, 144). For diabetic angiopathy conditions like DFU, common delivery methods include local injection of ASCs and direct application of 3D ASC grafts onto the wound site (145–147).
Unfortunately, their efficacy is primarily impeded by the limited expansion and survival of transplanted stem cells and their inability for proper functional integration in response to the physical environment (21, 149). Moreover, only a fraction of MSCs successfully home to the pancreas and express insulin (150). 3D culture technology provides an opportunity to fill this knowledge gap. While there is a scarcity of research on the clinical application of 3D cultured ASCs, findings from animal and cellular studies suggest the potential benefits and advantages of 3D cultured ASCs in the treatment of diabetes (Table 3).
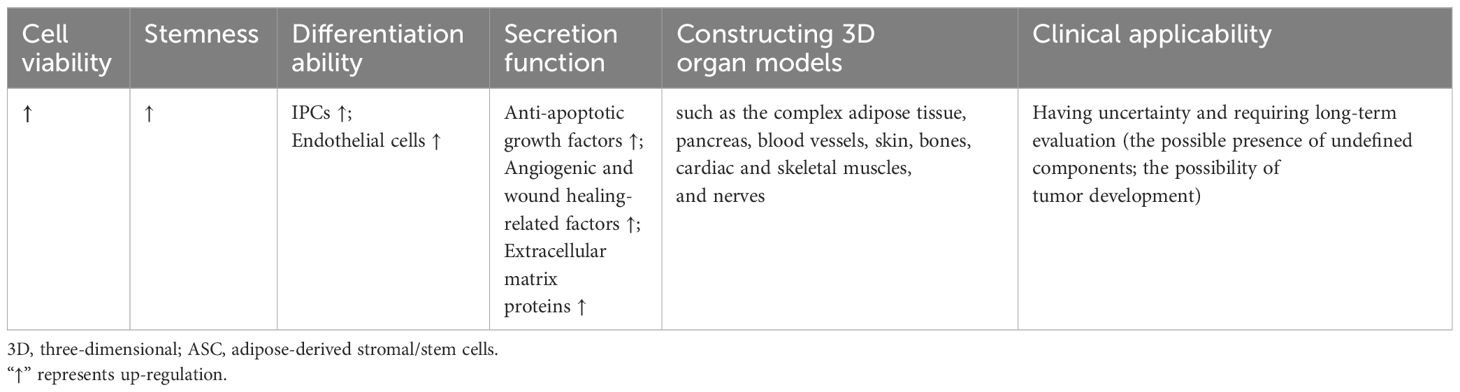
Table 3 The characteristics of 3D cultured ASCs in diabetes and diabetic complications compared to monolayer cells.
Type 2 diabetes mellitus (T2DM) is the most common type of diabetes, characterized by two interrelated metabolic defects: insulin resistance and pancreatic islet β-cell dysfunction. The development of T2DM is influenced by a complex interplay of genetic, environmental, emotional, and behavioral factors (151). Individuals with T2DM typically exhibit insulin resistance and gradual β-cell deterioration, resulting in insufficient insulin secretion, and consequent hyperglycemia and elevated free fatty acid levels. The resulting glucotoxicity and lipotoxicity exacerbate the dysfunction of β-cells to secrete insulin in response to hyperglycemia or oral hypoglycemic agents (16).
Type 1 diabetes mellitus (T1DM) is a chronic disease characterized by insulin deficiency resulting from autoimmune destruction of pancreatic islet β-cells, ultimately leading to hyperglycemia. Although the mechanism of T1DM in still not completely understood, it is believed to involve abnormalities in multiple immune cells, including T cells, B cells, regulatory T cells, monocytes and macrophages, and dendritic cells (152).
Given their similar outcome of pancreatic islet β-cell dysfunctions, the cell therapy as a potential strategy has attracted increased research attention. However, the transplantation of functional β-cells as a therapeutic strategy is impeded by the significant challenge of generating an adequate quantity of β-cells ex vivo and subsequently maintaining their viability post-transplantation. β-cells are susceptible to hypoxia and are prone to rapid apoptosis or damage as a result of the host immune response (153). Notably, the use of 3D cultured ASCs significantly promotes the construction and transplantation of islets and promote the insulin production.
Firstly, 3D cultured ASCs are capable to differentiate into insulin-producing cells (IPCs) to promote insulin production. For example, Khorsandi et al. found that the collagen/HA scaffold could enhance the differentiation of IPCs from rASCs. Compared to the 2D culture, the insulin release from 3D ASCs-derived IPCs showed up-regulation when exposed to a high glucose medium. The percentage of insulin-positive cells in 3D culture showed an approximately 4-fold increase compared to the 2D cultured cells (154). Ikemoto et al. developed a human recombinant peptide petaloid μ-piece 3D culture method to generate IPCs from hASCs. Following transplantation of 96 IPCs under the kidney capsule or intra-mesentery in STZ-induced diabetic nude mice, the hyperglycemic state was restored to normoglycemia (155). Ohta et al. found that blood glucose levels of STZ-induced diabetic nude mice were normalized after transplantation of 3D-cultured IPCs (156).
Secondly, the immunomodulatory action of 3D cultured ASCs can improve the micro-environment of islets. Abadpour et al. developed 3D-printed bioactive scaffolds containing islets and hASCs by combining alginate and nano-fibrillated cellulose bioink. Bioink diffusion properties were demonstrated, as well as benefits of hASCs for glucose sensing, insulin secretion, islet viability, and the reduction of pro-inflammatory cytokines, including growth-regulated protein-α and interferon gamma-induced protein-10 (157).
Furthermore, the 3D culture methods can also facilitate the survival of pancreatic islets and increase the functionality of grafts before transplantation. Jun et al. introduced a method of transplantation by co-culturing single primary islet cells with rASCs in concave microwells. These spheroids exhibited distinct ultrastructural morphologies, increased viability, and enhanced insulin secretion compared to mono-cultured islet spheroids, suggesting that ASCs may protect islet cells from damage by releasing anti-apoptotic growth factors. Additionally, the co-encapsulation of islets with additional ASCs within microfibers could further prolong graft survival through the anti-inflammatory properties of ASCs (22). Wang et al. effectively produced viable and functional heterocellular islet micro-tissues by combining islet cells, human umbilical vein endothelial cells, and hASCs within porcine decellularized ECM. These 3D islet micro-tissues exhibited sustained viability and normal secretory function, as well as heightened drug sensitivity during testing. Additionally, the utilization of 3D islet micro-tissues resulted in improved survival rates and enhanced graft function in murine models of diabetes (158).
In conclusion, 3D cultured ASC grafts play more significant role of insulin production through differentiating into IPCs, improving the micro-environment of islets, and enhancing survival and functionality.
Diabetic complications are mainly caused by high-glucose-induced cellular and molecular impairments and dysfunctions of cardiovascular and neural systems. While monolayer ASCs have demonstrated efficacy in treating a range of diabetic complications (139), the current studies about treatment of 3D ASCs are mainly focused on the DFU.
The diabetic foot ulcer, considered among the most severe types of diabetic wounds, has significant challenges to healing due to diabetic neuropathy, reduced blood flow, and infections (159). Non-healing ulcers may progress to gangrene, requiring foot amputations.
The normal wound healing process is characterized by four stages: hemostasis, inflammation, proliferation, and remodeling. In the hemostasis stage, vasoconstriction, platelet aggregation, and recruitment of circulating coagulation factors occur. The inflammation stage involves the gathering of inflammatory cells that secrete inflammatory factors like matrix metalloproteinase (MMP) and neutrophil extracellular traps (NETs). During the proliferation stage, the inflammation diminishes, and skin cells such as keratinocytes secrete EGF, proliferate, and migrate to the wound bed. During the process of tissue remodeling, new tissue is restructured and deposited via ECM and neovascularization, facilitated by fibroblasts secreting FGF and vascular endothelial cells secreting VEGF (160–162).
In diabetic wounds, tissue ischemia, hypoxia, and a high glucose microenvironment disrupt the normal progression of these healing stages, leading to delayed or non-healing of wounds and various clinical complications (163). Currently, DFUs are treated with vascular intervention therapy, drugs and other non-surgical therapies, such as dressing adjuvant therapy, hyperbaric oxygen therapy, hyperthermia and growth factor therapy (164). However, the efficacy of these approaches remains limited (159). Therefore, future research endeavors are anticipated to concentrate on more effective treatment strategies, with a particular emphasis on advancing stem cell-based therapies.
ASCs exhibit significant promise in the treatment of diabetic foot ulcers. Basically, the effects of ASCs rely on their promotion of immunomodulation, neovascularization and fibro synthesis (165, 166). The routes of delivery of ASCs into the wound vary between direct injection (such as intradermal injection around the wound, intra-fascial, and intramuscular injection), topical gel treatment, engineered skin graft sheet, and with scaffolds. The survival rate and potency of expansion of ASCs in wound bed are limited in traditional injection. Therefore, scaffolds cell delivery systems are necessary which offer optimal environments for cell adhesion, proliferation, and differentiation (167, 168).
A common solution involves seeding cells into hydrogels. Zeng et al. proposed that gelatin microcryogels (GMs) presented a novel method of cell delivery that could not only enhance wound bed healing but also directly influence the basal layer of the wound. They demonstrated that GMs provided an enhanced microenvironment for inducing endothelial cell differentiation of hASCs, thereby offering potential in vivo applications for angiogenic regeneration. Additionally, they demonstrated the priming effects of GMs on the upregulation of stemness genes and improved secretion of crucial growth factors in hASCs for wound healing, such as VEGF, HGF, basic fibroblast growth factor (bFGF), and platelet-derived growth factor BB (PDGFbb) (169). Feng et al. examined the therapeutic potential of hASCs cultured as micro-spheroids in the HA gel. Diabetic ulcers in mice with hASC spheroids resulted in accelerated wound epithelialization and increased dermal thickness, surpassing the outcomes observed with vehicle alone or monolayer-cultured ASCs (170). An injectable hydrogel system based on PEG and gelatin was examined for delivering hASCs into diabetic wounds. The stemness-linked transcription factor expression of hASCs was preserved in vitro and cell retention was significantly enhanced in vivo by this gel. In diabetic mice, this ASC-hydrogel treatment reduced inflammatory cell infiltration, enhanced neovascularization, and sped up wound closure (23).
There are also other bioengineering approaches for constructing 3D cultured ASCs. For example, Tyeb et al. introduced a combinatorial method involving the utilization of gelatin-sericin (GS) scaffolds coated with laminin (GSL). GS scaffolds provided enhanced protection against free radical-induced damage compared to gelatin scaffolds and consequently improved cell viability and metabolic function. The utilization of rASCs loaded onto GSL scaffolds resulted in enhanced regeneration, collagen remodeling, and increased expression of CD31 in diabetic ulcer rat models (171).
However, the broad use of matrix components aiding in the formation of 3D structures may impose constraints on the clinical applicability owing to the presence of undefined components. The implementation of hASCs formulated as multicellular aggregates without scaffolds also facilitated the healing wounds of diabetic mice. These aggregates exhibited a noteworthy increase in the production of extracellular matrix proteins including tenascin C, collagen VI α3, and fibronectin, as well as the secretion of soluble factors including HGF, MMP-2, and MMP-14 when compared to monolayer culture (172).
Considering that the main mechanism of cell action involves the paracrine effect, the characterization of components secreted by cells is vital, which indicates that ASCs can also function through their conditioned media. Lee et al. successfully fabricated an alginate-based scaffold using 3D printing and electrospinning techniques, which served as a structure to encapsulate hASC spheroids. This structure not only securely entrapped the spheroids but also facilitated the stable release of factors associated with angiogenesis and wound healing, such as CD31, VEGF, HGF, C-X-C chemokine receptor type 5 (CXCR5), IL-8, and MMP-1. They also demonstrated the role of these factors through a tube-forming assay and found that conditioned media from the spheroid-scaffold group enhanced the formation of capillary-like structures in human umbilical vein endothelial cells when compared to the single cell-scaffold group (173).
Utilizing diverse 3D culture techniques and materials such as hydrogels, bioactive scaffolds, scaffold-free methods, and conditioned media from 3D cultured cells, ASCs have the potential to facilitate diabetic wound healing by the promotion of immunomodulation, neovascularization and fibro synthesis.
Aside from their application in diabetic therapy through transplantation, 3D cultured ASCs are crucial in the development of in vitro models that mimic the pathophysiology of different tissues and organs linked to diabetes and its associated complications. These models also potentially serve as valuable tools for screening novel therapeutic interventions and minimizing the reliance on animal experimentation.
Adipose tissue is a significant location of insulin resistance in individuals with type 2 diabetes mellitus (T2DM) and is linked to heightened chronic inflammation. The establishment of in vitro models for investigating the pathogenesis of adipose tissue in metabolic diseases would offer significant benefits. Numerous efforts have been made to create 3D adipose cultures utilizing ASCs. For instance, hASCs were cultivated on plates coated with ELP–PEI copolymer, as the PEI component promotes spheroid formation and the ELP component facilitates the attachment of spheroids to the surface. This culture platform enabled the production of functional adipocytes that exhibited a favorable response to fatty acid stimulation (126). Moreover, Gerlach et al. utilized multicompartment hollow fiber-based bioreactor technology to generate 3D adipose tissue. In vitro, 3D bioreactors allowed greater metabolic activity compared with traditional 2D cultured hASCs and enabled the generation of adipose tissue as long as two months (174). Yang et al. created a 3D human adipose microtissue engineered within a microfluidic system (175). Furthermore, culture technologies have been employed in the generation of beige or brown adipose tissue (176–178). As the characterization of ASCs can be influenced by the source of adipose tissue, the availability of such tools presents a wide range of opportunities in vitro studies. By utilizing these models, it becomes feasible to compare relative metabolic responses of adipose depots under different health conditions to metabolic researches.
An ideal and comprehensive adipose tissue models should include all in vivo components, such as adipocytes, connective tissues, veins and nerves. For example, Lau et al. described an adipose micro-physiological system that involved sandwiching human WAT between tissue-engineered sheets of ASCs. The use of ASCs provided a structural ECM framework to encompass and support the mature adipocytes as well as paracrine growth factors (179). One common approach in the generation of vascularized adipose tissue involves the inclusion of exogenous endothelial cells through co-culture (180–182). The utilization of vascularized adipose models presents a promising avenue for developing novel drugs to treat metabolic diseases by modulation of the adipose vasculature. Moreover, adipose depots could be infiltrated with inflammatory and immune cells during preparation or after differentiation into adipocytes (183), offering a valuable tool for immune–metabolic research.
Furthermore, through differentiation and secretory capabilities of ASCs, it becomes possible to connect them with micro-physiological systems representing other organs. Despite being in the early stages of development, 3D models simulating organs such as the pancreas, blood vessels, skin, bones, cardiac and skeletal muscles, and nerves (Table 4), exhibit promising potential in mimicking the effects of diabetes and its complications, as well as evaluating the efficacy of cell transplantation therapy.
3D cultured cells have been advantageous in various biomedical fields. This technology is still in the early stages. The isolation, culture, and identification of cells are the basis of 3D culture. Therefore, large-scale manufacturing methods incorporating quality control are necessary for producing cells and 3D cultured transplantations.
Indeed, 2D adherent cell culture of ASCs is still conventionally used for both in vitro and in vivo studies. These cells have been extensively characterized, whereas many factors have not been analyzed on 3D cultured ASCs yet. Additionally, while monolayer ASC cells have shown effects for the treatment of diabetes and its complications in both clinical trials and animal experiments, current research status on 3D cultured ASCs mainly concentrates on T1DM and DFU only. Thus, further research is required to better understand the function and underlying mechanisms of 3D cultured ASC therapy.
The potential side effects of ASCs for tumor development should not be disregarded in studies; however, they may also serve as a potential tool for antitumor therapies. While tumor cells altering the phenotype and function of in vitro cultured ASCs through paracrine mechanisms (203), ASCs can also serve as a factor that promotes tumor growth (203–205). By contrast, ASC exosomes were shown to possess immunomodulatory properties and can inhibit cancer growth, migration, and colony formation (206). A strategy for tumor therapy used ASCs which loaded gold nanorod (AuNR)-PEG-poly(ethyleneimine) (APP) and Chlorin e6 (Ce6). Following activation of the APP/Ce6 agents through irradiation, ASCs were shown to play a role in tumor migration, tropism, and exhibit anticancer properties (207). These findings underscore the importance of exercising caution in the utilization of 3D cultured ASCs, with long-term experiments necessary to assess their safety. Specifically, careful consideration should be given to the potential of 3D culture to induce tumorigenesis while enhancing cell viability and stemness.
YS: Conceptualization, Writing – original draft. XY: Conceptualization, Writing – original draft. JM: Writing – review & editing. WK: Writing – review & editing. XH: Writing – review & editing. JZ: Writing – review & editing. LC: Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82170822, 82070809, 82300895, and 81900734).
The authors thank Figdraw for providing access to create figures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. (2022) 7:272. doi: 10.1038/s41392-022-01134-4
2. Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res. (2002) 91:866–76. doi: 10.1161/01.res.0000041435.95082.84
3. Cyranoski D. How human embryonic stem cells sparked a revolution. Nature. (2018) 555:428–30. doi: 10.1038/d41586-018-03268-4
4. Ho J, Yue D, Cheema U, Hsia HC, Dardik A. Innovations in stem cell therapy for diabetic wound healing. Adv Wound Care (New Rochelle). (2023) 12:626–43. doi: 10.1089/wound.2021.0104
5. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. (2007) 318:1917–20. doi: 10.1126/science.1151526
6. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. (2009) 324:797–801. doi: 10.1126/science.1172482
7. Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. (2011) 474:212–5. doi: 10.1038/nature10135
8. Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. (2013) 19:998–1004. doi: 10.1038/nm.3267
9. Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International society for cell & Gene therapy (Isct®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. (2019) 21:1019–24. doi: 10.1016/j.jcyt.2019.08.002
10. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the international federation for adipose therapeutics and science (Ifats) and the international society for cellular therapy (Isct). Cytotherapy. (2013) 15:641–8. doi: 10.1016/j.jcyt.2013.02.006
11. Qi Y, Ma J, Li S, Liu W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. (2019) 10:274. doi: 10.1186/s13287-019-1362-2
12. Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. (2013) 10:77–89. doi: 10.1021/mp3005148
13. Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. (2018) 36:1111–26. doi: 10.1016/j.bioteChadv.2018.03.011
14. Lin PC, Chiou TW, Lin ZS, Huang KC, Lin YC, Huang PC, et al. A proposed novel stem cell therapy protocol for liver cirrhosis. Cell Transplant. (2015) 24:533–40. doi: 10.3727/096368915x687228
15. Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet. (2023) 402:203–34. doi: 10.1016/s0140-6736(23)01301-6
16. Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: Evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. (2011) 60:1–23. doi: 10.1016/j.metabol.2010.09.010
17. Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. (2019) 13:364–72. doi: 10.1016/j.dsx.2018.10.008
18. Lorberbaum DS, Sarbaugh D, Sussel L. Leveraging the strengths of mice, human stem cells, and organoids to model pancreas development and diabetes. Front Endocrinol (Lausanne). (2022) 13:1042611. doi: 10.3389/fendo.2022.1042611
19. Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation. (2014) 98:593–9. doi: 10.1097/tp.0000000000000288
20. Hu C, Jia W. Therapeutic medications against diabetes: What we have and what we expect. Adv Drug Delivery Rev. (2019) 139:3–15. doi: 10.1016/j.addr.2018.11.008
21. Lin HP, Chan TM, Fu RH, Chuu CP, Chiu SC, Tseng YH, et al. Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell Transplant. (2015) 24:521–32. doi: 10.3727/096368915x686977
22. Jun Y, Kang AR, Lee JS, Park SJ, Lee DY, Moon SH, et al. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials. (2014) 35:4815–26. doi: 10.1016/j.biomaterials.2014.02.045
23. Dong Y, Rodrigues M, Kwon SH, Li X, Sigen A, Brett EA, et al. Acceleration of diabetic wound regeneration using an in situ-formed stem-cell-based skin substitute. Adv Healthc Mater. (2018) 7:e1800432. doi: 10.1002/adhm.201800432
24. Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Funct Mater. (2015) 25:1344–51. doi: 10.1002/adfm.201403631
25. Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. (2012) 18:806–15. doi: 10.1089/ten.TEA.2011.0391
26. Di Stefano AB, Urrata V, Trapani M, Moschella F, Cordova A, Toia F. Systematic review on spheroids from adipose-derived stem cells: Spontaneous or artefact state? J Cell Physiol. (2022) 237:4397–411. doi: 10.1002/jcp.30892
27. Baptista LS, Silva KR, Jobeili L, Guillot L, Sigaudo-Roussel D. Unraveling white adipose tissue heterogeneity and obesity by adipose stem/stromal cell biology and 3d culture models. Cells. (2023) 12:1583. doi: 10.3390/cells12121583
29. Hu W, Lazar MA. Modelling metabolic diseases and drug response using stem cells and organoids. Nat Rev Endocrinol. (2022) 18:744–59. doi: 10.1038/s41574-022-00733-z
30. Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. (2018) 561:455–7. doi: 10.1038/d41586-018-06756-9
31. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
32. Viswanathan S, Ciccocioppo R, Galipeau J, Krampera M, Le Blanc K, Martin I, et al. Consensus international council for commonality in blood banking automation-international society for cell & Gene therapy statement on standard nomenclature abbreviations for the tissue of origin of mesenchymal stromal cells. Cytotherapy. (2021) 23:1060–3. doi: 10.1016/j.jcyt.2021.04.009
33. Caplan AI. Mesenchymal stem cells: Time to change the name! Stem Cells Transl Med. (2017) 6:1445–51. doi: 10.1002/sctm.17-0051
34. Renesme L, Pierro M, Cobey KD, Mital R, Nangle K, Shorr R, et al. Definition and characteristics of mesenchymal stromal cells in preclinical and clinical studies: A scoping review. Stem Cells Transl Med. (2022) 11:44–54. doi: 10.1093/stcltm/szab009
35. Rodbell M. Metabolism of Isolated Fat Cells. Ii. The Similar Effects of Phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. (1966) 241:130–9. doi: 10.1016/S0021-9258(18)96967-X
36. Rodbell M, Jones AB. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. (1966) 241:140–2. doi: 10.1016/S0021-9258(18)96968-1
37. Rodbell M. The metabolism of isolated fat cells. Iv. Regulation of release of protein by lipolytic hormones and insulin. J Biol Chem. (1966) 241:3909–17. doi: 10.1016/S0021-9258(18)99793-0
38. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for Cell-Based Therapies. Tissue Eng. (2001) 7:211–28. doi: 10.1089/107632701300062859
39. Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-Derived Stem Cell: A Better Stem Cell Than Bmsc. Cell Biochem Funct. (2008) 26:664–75. doi: 10.1002/cbf.1488
40. Al-Ghadban S, Bunnell BA. Adipose Tissue-Derived Stem Cells: Immunomodulatory effects and therapeutic potential. Physiol (Bethesda). (2020) 35:125–33. doi: 10.1152/physiol.00021.2019
41. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. (2007) 100:1249–60. doi: 10.1161/01.Res.0000265074.83288.09
42. Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther. (2014) 5:79. doi: 10.1186/scrt468
43. Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regener. (2013) 21:545–53. doi: 10.1111/wrr.12051
44. El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. (2009) 123:475–85. doi: 10.1097/PRS.0b013e3181954d08
45. Wang M, Song L, Strange C, Dong X, Wang H. Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol Ther. (2018) 26:1921–30. doi: 10.1016/j.ymthe.2018.06.013
46. An R, Zhang Y, Qiao Y, Song L, Wang H, Dong X. Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Res Ther. (2020) 11:120. doi: 10.1186/s13287-020-01621-x
47. Uzun E, Güney A, Gönen ZB, Özkul Y, Kafadar İH, Günay M, et al. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. (2021) 27:636–42. doi: 10.1016/j.fas.2020.08.002
48. Deptuła M, Brzezicka A, Skoniecka A, Zieliński J, Pikuła M. Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges. Med Res Rev. (2021) 41:2130–71. doi: 10.1002/med.21789
49. Louwen F, Ritter A, Kreis NN, Yuan J. Insight into the development of obesity: Functional alterations of adipose-derived mesenchymal stem cells. Obes Rev. (2018) 19:888–904. doi: 10.1111/obr.12679
50. García-Contreras M, Vera-Donoso CD, Hernández-Andreu JM, García-Verdugo JM, Oltra E. Therapeutic potential of human adipose-derived stem cells (Adscs) from cancer patients: A pilot study. PLoS One. (2014) 9:e113288. doi: 10.1371/journal.pone.0113288
51. Mieczkowska A, Schumacher A, Filipowicz N, Wardowska A, Zieliński M, Madanecki P, et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci Rep. (2018) 8:11339. doi: 10.1038/s41598-018-29477-5
52. Bunnell BA. Adipose tissue-derived mesenchymal stem cells. Cells. (2021) 10:3433. doi: 10.3390/cells10123433
53. Ritter A, Friemel A, Roth S, Kreis NN, Hoock SC, Safdar BK, et al. Subcutaneous and visceral adipose-derived mesenchymal stem cells: Commonality and diversity. Cells. (2019) 8:1288. doi: 10.3390/cells8101288
54. Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. (2012) 61:1691–9. doi: 10.2337/db11-1753
55. Tang Y, Pan ZY, Zou Y, He Y, Yang PY, Tang QQ, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. (2017) 21:2153–62. doi: 10.1111/jcmm.13138
56. Wada Y, Ikemoto T, Morine Y, Imura S, Saito Y, Yamada S, et al. The differences in the characteristics of insulin-producing cells using human adipose-tissue derived mesenchymal stem cells from subcutaneous and visceral tissues. Sci Rep. (2019) 9:13204. doi: 10.1038/s41598-019-49701-0
57. Silva FJ, Holt DJ, Vargas V, Yockman J, Boudina S, Atkinson D, et al. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. (2014) 32:572–81. doi: 10.1002/stem.1595
58. Di Franco A, Guasti D, Squecco R, Mazzanti B, Rossi F, Idrizaj E, et al. Searching for classical brown fat in humans: Development of a novel human fetal brown stem cell model. Stem Cells. (2016) 34:1679–91. doi: 10.1002/stem.2336
59. Carvalho PP, Gimble JM, Dias IR, Gomes ME, Reis RL. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Methods. (2013) 19:473–8. doi: 10.1089/ten.TEC.2012.0465
60. Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet. (2013) 382:1113–20. doi: 10.1016/s0140-6736(13)61410-5
61. Follin B, Tratwal J, Haack-Sørensen M, Elberg JJ, Kastrup J, Ekblond A. Identical effects of vegf and serum-deprivation on phenotype and function of adipose-derived stromal cells from healthy donors and patients with ischemic heart disease. J Transl Med. (2013) 11:219. doi: 10.1186/1479-5876-11-219
62. Lee MS, Youn C, Kim JH, Park BJ, Ahn J, Hong S, et al. Enhanced cell growth of adipocyte-derived mesenchymal stem cells using chemically-defined serum-free media. Int J Mol Sci. (2017) 18:1779. doi: 10.3390/ijms18081779
63. Dessels C, Ambele MA, Pepper MS. The effect of medium supplementation and serial passaging on the transcriptome of human adipose-derived stromal cells expanded in vitro. Stem Cell Res Ther. (2019) 10:253. doi: 10.1186/s13287-019-1370-2
64. Riis S, Nielsen FM, Pennisi CP, Zachar V, Fink T. Comparative analysis of media and supplements on initiation and expansion of adipose-derived stem cells. Stem Cells Transl Med. (2016) 5:314–24. doi: 10.5966/sctm.2015-0148
65. Kocaoemer A, Kern S, Klüter H, Bieback K. Human ab serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. (2007) 25:1270–8. doi: 10.1634/stemcells.2006-0627
66. Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, et al. Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue Eng Part A. (2010) 16:3467–84. doi: 10.1089/ten.TEA.2009.0727
67. Tirza G, Solodeev I, Sela M, Greenberg I, Pasmanik-Chor M, Gur E, et al. Reduced culture temperature attenuates oxidative stress and inflammatory response facilitating expansion and differentiation of adipose-derived stem cells. Stem Cell Res Ther. (2020) 11:35. doi: 10.1186/s13287-019-1542-0
68. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. (2010) 7:150–61. doi: 10.1016/j.stem.2010.07.007
69. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of vegf and bfgf. Wound Repair Regener. (2009) 17:540–7. doi: 10.1111/j.1524-475X.2009.00499.x
70. Zanata F, Bowles A, Frazier T, Curley JL, Bunnell BA, Wu X, et al. Effect of cryopreservation on human adipose tissue and isolated stromal vascular fraction cells: In vitro and in vivo analyses. Plast Reconstr Surg. (2018) 141:232e–43e. doi: 10.1097/prs.0000000000004030
71. Devitt SM, Carter CM, Dierov R, Weiss S, Gersch RP, Percec I. Successful isolation of viable adipose-derived stem cells from human adipose tissue subject to long-term cryopreservation: positive implications for adult stem cell-based therapeutics in patients of advanced age. Stem Cells Int. (2015) 2015:146421. doi: 10.1155/2015/146421
72. Park S, Lee DR, Nam JS, Ahn CW, Kim H. Fetal bovine serum-free cryopreservation methods for clinical banking of human adipose-derived stem cells. Cryobiology. (2018) 81:65–73. doi: 10.1016/j.cryobiol.2018.02.008
73. Søndergaard RH, Follin B, Lund LD, Juhl M, Ekblond A, Kastrup J, et al. Senescence and quiescence in adipose-derived stromal cells: Effects of human platelet lysate, fetal bovine serum and hypoxia. Cytotherapy. (2017) 19:95–106. doi: 10.1016/j.jcyt.2016.09.006
74. Neri S, Bourin P, Peyrafitte JA, Cattini L, Facchini A, Mariani E. Human adipose stromal cells (Asc) for the regeneration of injured cartilage display genetic stability after in vitro culture expansion. PLoS One. (2013) 8:e77895. doi: 10.1371/journal.pone.0077895
75. Yin Q, Xu N, Xu D, Dong M, Shi X, Wang Y, et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res Ther. (2020) 11:226. doi: 10.1186/s13287-020-01744-1
76. Estrada JC, Torres Y, Benguría A, Dopazo A, Roche E, Carrera-Quintanar L, et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. (2013) 4:e691. doi: 10.1038/cddis.2013.211
77. Efimenko AY, Kochegura TN, Akopyan ZA, Parfyonova YV. Autologous stem cell therapy: How aging and chronic diseases affect stem and progenitor cells. Biores Open Access. (2015) 4:26–38. doi: 10.1089/biores.2014.0042
78. Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. (2008) 27:5975–87. doi: 10.1038/onc.2008.213
79. Kang SK, Putnam L, Dufour J, Ylostalo J, Jung JS, Bunnell BA. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells. Stem Cells. (2004) 22:1356–72. doi: 10.1634/stemcells.2004-0023
80. Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. (2006) 55:2571–8. doi: 10.2337/db06-0540
81. Wolbank S, Stadler G, Peterbauer A, Gillich A, Karbiener M, Streubel B, et al. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: maintenance of differentiation and immunomodulatory characteristics. Tissue Eng Part A. (2009) 15:1843–54. doi: 10.1089/ten.tea.2008.0205
82. Shamsi F, Tseng YH. Protocols for generation of immortalized human brown and white preadipocyte cell lines. Methods Mol Biol. (2017) 1566:77–85. doi: 10.1007/978-1-4939-6820-6_8
83. Comas F, Latorre J, Ortega F, Oliveras-Cañellas N, Lluch A, Ricart W, et al. Permanent cystathionine-Β-synthase gene knockdown promotes inflammation and oxidative stress in immortalized human adipose-derived mesenchymal stem cells, enhancing their adipogenic capacity. Redox Biol. (2021) 42:101668. doi: 10.1016/j.redox.2020.101668
84. Huang M, Claussnitzer M, Saadat A, Coral DE, Kalamajski S, Franks PW. Engineered allele substitution at ppargc1a rs8192678 alters human white adipocyte differentiation, lipogenesis, and pgc-1α Content and turnover. Diabetologia. (2023) 66:1289–305. doi: 10.1007/s00125-023-05915-6
85. Iacomi DM, Rosca AM, Tutuianu R, Neagu TP, Pruna V, Simionescu M, et al. Generation of an immortalized human adipose-derived mesenchymal stromal cell line suitable for wound healing therapy. Int J Mol Sci. (2022) 23:8925. doi: 10.3390/ijms23168925
86. Tátrai P, Szepesi Á, Matula Z, Szigeti A, Buchan G, Mádi A, et al. Combined introduction of bmi-1 and htert immortalizes human adipose tissue-derived stromal cells with low risk of transformation. Biochem Biophys Res Commun. (2012) 422:28–35. doi: 10.1016/j.bbrc.2012.04.088
87. Balducci L, Blasi A, Saldarelli M, Soleti A, Pessina A, Bonomi A, et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res Ther. (2014) 5:63. doi: 10.1186/scrt452
88. Darimont C, Zbinden I, Avanti O, Leone-Vautravers P, Giusti V, Burckhardt P, et al. Reconstitution of telomerase activity combined with hpv-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ. (2003) 10:1025–31. doi: 10.1038/sj.cdd.4401273
89. Cherington V, Brown M, Paucha E, St Louis J, Spiegelman BM, Roberts TM. Separation of simian virus 40 large-T-antigen-transforming and origin-binding functions from the ability to block differentiation. Mol Cell Biol. (1988) 8:1380–4. doi: 10.1128/mcb.8.3.1380-1384.1988
90. Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell. (2016) 38:590–600. doi: 10.1016/j.devcel.2016.08.014
91. Kaushik G, Ponnusamy MP, Batra SK. Concise review: Current status of three-dimensional organoids as preclinical models. Stem Cells. (2018) 36:1329–40. doi: 10.1002/stem.2852
92. Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, Mukherjee S, et al. High-content screening in hpsc-neural progenitors identifies drug candidates that inhibit zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell. (2017) 21:274–83.e5. doi: 10.1016/j.stem.2017.06.017
93. Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U.S.A. (2015) 112:15672–7. doi: 10.1073/pnas.1520760112
94. Pain B. Organoids in domestic animals: With which stem cells? Vet Res. (2021) 52:38. doi: 10.1186/s13567-021-00911-3
95. Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. (2002) 298:597–600. doi: 10.1126/science.1072530
96. Watt FM, Hogan BL. Out of eden: stem cells and their niches. Science. (2000) 287:1427–30. doi: 10.1126/science.287.5457.1427
97. Yan XZ, van den Beucken JJ, Both SK, Yang PS, Jansen JA, Yang F. Biomaterial strategies for stem cell maintenance during in vitro expansion. Tissue Eng Part B Rev. (2014) 20:340–54. doi: 10.1089/ten.TEB.2013.0349
99. Gibler P, Gimble J, Hamel K, Rogers E, Henderson M, Wu X, et al. Human adipose-derived stromal/stem cell culture and analysis methods for adipose tissue modeling in vitro: A systematic review. Cells. (2021) 10:1378. doi: 10.3390/cells10061378
100. Guo X, Li S, Ji Q, Lian R, Chen J. Enhanced viability and neural differential potential in poor post-thaw hadscs by agarose multi-well dishes and spheroid culture. Hum Cell. (2015) 28:175–89. doi: 10.1007/s13577-015-0116-4
101. Coyle R, Yao J, Richards D, Mei Y. The effects of metabolic substrate availability on human adipose-derived stem cell spheroid survival. Tissue Eng Part A. (2019) 25:620–31. doi: 10.1089/ten.TEA.2018.0163
102. Di Stefano AB, Grisafi F, Perez-Alea M, Castiglia M, Di Simone M, Meraviglia S, et al. Cell quality evaluation with gene expression analysis of spheroids (3d) and adherent (2d) adipose stem cells. Gene. (2021) 768:145269. doi: 10.1016/j.gene.2020.145269
103. Rybkowska P, Radoszkiewicz K, Kawalec M, Dymkowska D, Zabłocka B, Zabłocki K, et al. The metabolic changes between monolayer (2d) and three-dimensional (3d) culture conditions in human mesenchymal stem/stromal cells derived from adipose tissue. Cells. (2023) 12:178. doi: 10.3390/cells12010178
104. Yipeng J, Yongde X, Yuanyi W, Jilei S, Jiaxiang G, Jiangping G, et al. Microtissues enhance smooth muscle differentiation and cell viability of hadscs for three dimensional bioprinting. Front Physiol. (2017) 8:534. doi: 10.3389/fphys.2017.00534
105. Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. (2011) 32:2734–47. doi: 10.1016/j.biomaterials.2010.12.035
106. Al-Ghadban S, Artiles M, Bunnell BA. Adipose stem cells in regenerative medicine: looking forward. Front Bioeng Biotechnol. (2021) 9:837464. doi: 10.3389/fbioe.2021.837464
107. Hsu SH, Ho TT, Huang NC, Yao CL, Peng LH, Dai NT. Substrate-dependent modulation of 3d spheroid morphology self-assembled in mesenchymal stem cell-endothelial progenitor cell coculture. Biomaterials. (2014) 35:7295–307. doi: 10.1016/j.biomaterials.2014.05.033
108. Chen ST, Wu CY, Chen HY. Enhanced growth activities of stem cell spheroids based on a durable and chemically defined surface modification coating. ACS Appl Mater Interfaces. (2018) 10:31882–91. doi: 10.1021/acsami.8b09103
109. Hu W, Zhu S, Fanai ML, Wang J, Cai J, Feng J. 3d co-culture model of endothelial colony-forming cells (Ecfcs) reverses late passage adipose-derived stem cell senescence for wound healing. Stem Cell Res Ther. (2020) 11:355. doi: 10.1186/s13287-020-01838-w
110. Lin IC, Wang TJ, Wu CL, Lu DH, Chen YR, Yang KC. Chitosan-cartilage extracellular matrix hybrid scaffold induces chondrogenic differentiation to adipose-derived stem cells. Regener Ther. (2020) 14:238–44. doi: 10.1016/j.reth.2020.03.014
111. Kim G, Jung Y, Cho K, Lee HJ, Koh WG. Thermoresponsive poly(N-isopropylacrylamide) hydrogel substrates micropatterned with poly(Ethylene glycol) hydrogel for adipose mesenchymal stem cell spheroid formation and retrieval. Mater Sci Eng C Mater Biol Appl. (2020) 115:111128. doi: 10.1016/j.msec.2020.111128
112. Seo J, Lee JS, Lee K, Kim D, Yang K, Shin S, et al. Switchable water-adhesive, superhydrophobic palladium-layered silicon nanowires potentiate the angiogenic efficacy of human stem cell spheroids. Adv Mater. (2014) 26:7043–50. doi: 10.1002/adma.201402273
113. Zhang S, Liu P, Chen L, Wang Y, Wang Z, Zhang B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials. (2015) 41:15–25. doi: 10.1016/j.biomaterials.2014.11.019
114. Kim Y, Baipaywad P, Jeong Y, Park H. Incorporation of gelatin microparticles on the formation of adipose-derived stem cell spheroids. Int J Biol Macromol. (2018) 110:472–8. doi: 10.1016/j.ijbiomac.2018.01.046
115. Wu Y, Hospodiuk M, Peng W, Gudapati H, Neuberger T, Koduru S, et al. Porous tissue strands: avascular building blocks for scalable tissue fabrication. Biofabrication. (2018) 11:015009. doi: 10.1088/1758-5090/aaec22
116. Kim SJ, Park J, Byun H, Park YW, Major LG, Lee DY, et al. Hydrogels with an embossed surface: an all-in-one platform for mass production and culture of human adipose-derived stem cell spheroids. Biomaterials. (2019) 188:198–212. doi: 10.1016/j.biomaterials.2018.10.025
117. Tsai CC, Hong YJ, Lee RJ, Cheng NC, Yu J. Enhancement of human adipose-derived stem cell spheroid differentiation in an in situ enzyme-crosslinked gelatin hydrogel. J Mater Chem B. (2019) 7:1064–75. doi: 10.1039/C8TB02835D
118. Zhao Y, Xiao E, Lv W, Dong X, He L, Wang Y, et al. A chemically defined serum-free culture system for spontaneous human mesenchymal stem cell spheroid formation. Stem Cells Int. (2020) 2020:1031985. doi: 10.1155/2020/1031985
119. Labriola NR, Sadick JS, Morgan JR, Mathiowitz E, Darling EM. Cell mimicking microparticles influence the organization, growth, and mechanophenotype of stem cell spheroids. Ann BioMed Eng. (2018) 46:1146–59. doi: 10.1007/s10439-018-2028-4
120. Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP. Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int J Mol Sci. (2018) 19:2200. doi: 10.3390/ijms19082200
121. Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. (2014) 35:1914–23. doi: 10.1016/j.biomaterials.2013.11.067
122. Mohiuddin OA, O'Donnell BT, Poche JN, Iftikhar R, Wise RM, Motherwell JM, et al. Human adipose-derived hydrogel characterization based on in vitro asc biocompatibility and differentiation. Stem Cells Int. (2019) 2019:9276398. doi: 10.1155/2019/9276398
123. Hoefner C, Muhr C, Horder H, Wiesner M, Wittmann K, Lukaszyk D, et al. Human adipose-derived mesenchymal stromal/stem cell spheroids possess high adipogenic capacity and acquire an adipose tissue-like extracellular matrix pattern. Tissue Eng Part A. (2020) 26:915–26. doi: 10.1089/ten.TEA.2019.0206
124. Rumiński S, Kalaszczyńska I, Długosz A, Lewandowska-Szumieł M. Osteogenic differentiation of human adipose-derived stem cells in 3d conditions - comparison of spheroids and polystyrene scaffolds. Eur Cell Mater. (2019) 37:382–401. doi: 10.22203/eCM.v037a23
125. Fitzgerald SJ, Cobb JS, Janorkar AV. Comparison of the formation, adipogenic maturation, and retention of human adipose-derived stem cell spheroids in scaffold-free culture techniques. J BioMed Mater Res B Appl Biomater. (2020) 108:3022–32. doi: 10.1002/jbm.b.34631
126. Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV. Adipogenic differentiation of human adipose-derived stem cells grown as spheroids. Process Biochem. (2017) 59:312–20. doi: 10.1016/j.procbio.2017.02.003
127. Yoon HH, Bhang SH, Shin JY, Shin J, Kim BS. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng Part A. (2012) 18:1949–56. doi: 10.1089/ten.TEA.2011.0647
128. Ko JY, Park JW, Kim J, Im GI. Characterization of adipose-derived stromal/stem cell spheroids versus single-cell suspension in cell survival and arrest of osteoarthritis progression. J BioMed Mater Res A. (2021) 109:869–78. doi: 10.1002/jbm.a.37078
129. Gurumurthy B, Bierdeman PC, Janorkar AV. Spheroid model for functional osteogenic evaluation of human adipose derived stem cells. J BioMed Mater Res A. (2017) 105:1230–6. doi: 10.1002/jbm.a.35974
130. Kim BC, Kwack KH, Chun J, Lee JH. Comparative transcriptome analysis of human adipose-derived stem cells undergoing osteogenesis in 2d and 3d culture conditions. Int J Mol Sci. (2021) 22:7939. doi: 10.3390/ijms22157939
131. Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. (2012) 33:1748–58. doi: 10.1016/j.biomaterials.2011.11.049
132. Amirpour N, Razavi S, Esfandiari E, Hashemibeni B, Kazemi M, Salehi H. Hanging drop culture enhances differentiation of human adipose-derived stem cells into anterior neuroectodermal cells using small molecules. Int J Dev Neurosci. (2017) 59:21–30. doi: 10.1016/j.ijdevneu.2017.03.002
133. Salehi H, Razavi S, Esfandiari E, Kazemi M, Amini S, Amirpour N. Application of hanging drop culture for retinal precursor-like cells differentiation of human adipose-derived stem cells using small molecules. J Mol Neurosci. (2019) 69:597–607. doi: 10.1007/s12031-019-01388-8
134. Bagheri-Hosseinabadi Z, Seyedi F, Mollaei HR, Moshrefi M, Seifalian A. Combination of 5-azaytidine and hanging drop culture convert fat cell into cardiac cell. Biotechnol Appl Biochem. (2021) 68:92–101. doi: 10.1002/bab.1897
135. Furuhata Y, Kikuchi Y, Tomita S, Yoshimoto K. Small spheroids of adipose-derived stem cells with time-dependent enhancement of il-8 and vegf-a secretion. Genes Cells. (2016) 21:1380–6. doi: 10.1111/gtc.12448
136. Fuku A, Taki Y, Nakamura Y, Kitajima H, Takaki T, Koya T, et al. Evaluation of the usefulness of human adipose-derived stem cell spheroids formed using spherering(®) and the lethal damage sensitivity to synovial fluid in vitro. Cells. (2022) 11:337. doi: 10.3390/cells11030337
137. Zhang J, Li C, Meng F, Guan Y, Zhang T, Yang B, et al. Functional tissue-engineered microtissue formed by self-aggregation of cells for peripheral nerve regeneration. Stem Cell Res Ther. (2022) 13:3. doi: 10.1186/s13287-021-02676-0
138. Zhou F, Hui Y, Xin H, Xu YD, Lei HE, Yang BC, et al. Therapeutic effects of adipose-derived stem cells-based microtissues on erectile dysfunction in streptozotocin-induced diabetic rats. Asian J Androl. (2017) 19:91–7. doi: 10.4103/1008-682x.182817
139. Yan D, Song Y, Zhang B, Cao G, Zhou H, Li H, et al. Progress and application of adipose-derived stem cells in the treatment of diabetes and its complications. Stem Cell Res Ther. (2024) 15:3. doi: 10.1186/s13287-023-03620-0
140. Vanikar AV, Dave SD, Thakkar UG, Trivedi HL. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. (2010) 2010:582382. doi: 10.4061/2010/582382
141. Dave SD, Vanikar AV, Trivedi HL, Thakkar UG, Gopal SC, Chandra T. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin Exp Med. (2015) 15:41–5. doi: 10.1007/s10238-013-0266-1
142. Thakkar UG, Trivedi HL, Vanikar AV, Dave SD. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. (2015) 17:940–7. doi: 10.1016/j.jcyt.2015.03.608
143. Araujo DB, Dantas JR, Silva KR, Souto DL, Pereira MFC, Moreira JP, et al. Allogenic adipose tissue-derived stromal/stem cells and vitamin D supplementation in patients with recent-onset type 1 diabetes mellitus: A 3-month follow-up pilot study. Front Immunol. (2020) 11:993. doi: 10.3389/fimmu.2020.00993
144. Dantas JR, Araújo DB, Silva KR, Souto DL, de Fátima Carvalho Pereira M, Luiz RR, et al. Adipose tissue-derived stromal/stem cells + Cholecalciferol: A pilot study in recent-onset type 1 diabetes patients. Arch Endocrinol Metab. (2021) 65:342–51. doi: 10.20945/2359-3997000000368
145. Nilforoushzadeh MA, Sisakht MM, Amirkhani MA, Seifalian AM, Banafshe HR, Verdi J, et al. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: A clinical study for diabetic wound healing. J Tissue Eng Regener Med. (2020) 14:424–40. doi: 10.1002/term.3003
146. Moon KC, Suh HS, Kim KB, Han SK, Young KW, Lee JW, et al. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes. (2019) 68:837–46. doi: 10.2337/db18-0699
147. Carstens MH, Quintana FJ, Calderwood ST, Sevilla JP, Ríos AB, Rivera CM, et al. Treatment of Chronic Diabetic Foot Ulcers with Adipose-Derived Stromal Vascular Fraction Cell Injections: Safety and Evidence of Efficacy at 1 year. Stem Cells Transl Med. (2021) 10:1138–47. doi: 10.1002/sctm.20-0497
148. Soria-Juan B, Garcia-Arranz M, Llanos Jiménez L, Aparicio C, Gonzalez A, Mahillo Fernandez I, et al. Efficacy and safety of intramuscular administration of allogeneic adipose tissue derived and expanded mesenchymal stromal cells in diabetic patients with critical limb ischemia with no possibility of revascularization: study protocol for a randomized controlled double-blind phase ii clinical trial (the noma trial). Trials. (2021) 22:595. doi: 10.1186/s13063-021-05430-2
149. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. (2014) 10:29–37. doi: 10.4161/org.27405
150. Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. (2003) 111:843–50. doi: 10.1172/jci16502
151. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2009) 32(Suppl 1):S62–7. doi: 10.2337/dc09-S062
152. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. (2014) 383:69–82. doi: 10.1016/s0140-6736(13)60591-7
153. Mohandas S, Gayatri V, Kumaran K, Gopinath V, Paulmurugan R, Ramkumar KM. New frontiers in three-dimensional culture platforms to improve diabetes research. Pharmaceutics. (2023) 15:725. doi: 10.3390/pharmaceutics15030725
154. Khorsandi L, Khodadadi A, Nejad-Dehbashi F, Saremy S. Three-dimensional differentiation of adipose-derived mesenchymal stem cells into insulin-producing cells. Cell Tissue Res. (2015) 361:745–53. doi: 10.1007/s00441-015-2140-9
155. Ikemoto T, Feng R, Iwahashi SI, Yamada S, Saito Y, Morine Y, et al. In vitro and in vivo effects of insulin-producing cells generated by xeno-antigen free 3d culture with rcp piece. Sci Rep. (2019) 9:10759. doi: 10.1038/s41598-019-47257-7
156. Ohta S, Ikemoto T, Wada Y, Saito Y, Yamada S, Imura S, et al. A change in the zinc ion concentration reflects the maturation of insulin-producing cells generated from adipose-derived mesenchymal stem cells. Sci Rep. (2019) 9:18731. doi: 10.1038/s41598-019-55172-0
157. Abadpour S, Niemi EM, Orrhult LS, Hermanns C, de Vries R, Nogueira LP, et al. Adipose-derived stromal cells preserve pancreatic islet function in a transplantable 3d bioprinted scaffold. Adv Healthc Mater. (2023) 12:e2300640. doi: 10.1002/adhm.202300640
158. Wang X, Jin L, Liu W, Stingelin L, Zhang P, Tan Z. Construction of engineered 3d islet micro-tissue using porcine decellularized ecm for the treatment of diabetes. Biomater Sci. (2023) 11:5517–32. doi: 10.1039/D3BM00346A
159. Zhao X, Guo J, Zhang F, Zhang J, Liu D, Hu W, et al. Therapeutic application of adipose-derived stromal vascular fraction in diabetic foot. Stem Cell Res Ther. (2020) 11:394. doi: 10.1186/s13287-020-01825-1
160. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. (2008) 453:314–21. doi: 10.1038/nature07039
161. Wicks K, Torbica T, Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol. (2014) 26:341–53. doi: 10.1016/j.smim.2014.04.006
162. Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: A review. Jama. (2023) 330:62–75. doi: 10.1001/jama.2023.10578
163. Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: New crossroads. Curr Diabetes Rep. (2018) 18:2. doi: 10.1007/s11892-018-0970-z
164. Lipsky BA. Diabetic foot infections: Current treatment and delaying the 'Post-antibiotic era'. Diabetes Metab Res Rev. (2016) 32 Suppl 1:246–53. doi: 10.1002/dmrr.2739
165. Liu R, Dong R, Chang M, Liang X, Wang HC. Adipose-derived stem cells for the treatment of diabetic wound: From basic study to clinical application. Front Endocrinol (Lausanne). (2022) 13:882469. doi: 10.3389/fendo.2022.882469
166. Schneider I, Calcagni M, Buschmann J. Adipose-derived stem cells applied in skin diseases, wound healing and skin defects: A review. Cytotherapy. (2023) 25:105–19. doi: 10.1016/j.jcyt.2022.08.005
167. Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. (2016) 7:37. doi: 10.1186/s13287-016-0303-6
168. Turner NJ, Badylak SF. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care (New Rochelle). (2015) 4:490–500. doi: 10.1089/wound.2014.0604
169. Zeng Y, Zhu L, Han Q, Liu W, Mao X, Li Y, et al. Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater. (2015) 25:291–303. doi: 10.1016/j.actbio.2015.07.042
170. Feng J, Mineda K, Wu SH, Mashiko T, Doi K, Kuno S, et al. An injectable non-cross-linked hyaluronic-acid gel containing therapeutic spheroids of human adipose-derived stem cells. Sci Rep. (2017) 7:1548. doi: 10.1038/s41598-017-01528-3
171. Tyeb S, Shiekh PA, Verma V, Kumar A. Adipose-derived stem cells (Adscs) loaded gelatin-sericin-laminin cryogels for tissue regeneration in diabetic wounds. Biomacromolecules. (2020) 21:294–304. doi: 10.1021/acs.biomac.9b01355
172. Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: Impact of cell formulation and delivery. Tissue Eng Part A. (2010) 16:1595–606. doi: 10.1089/ten.TEA.2009.0616
173. Lee JS, Chae S, Yoon D, Yoon D, Chun W, Kim GH. Angiogenic factors secreted from human asc spheroids entrapped in an alginate-based hierarchical structure via combined 3d printing/electrospinning system. Biofabrication. (2020) 12:045028. doi: 10.1088/1758-5090/abaf9a
174. Gerlach JC, Lin YC, Brayfield CA, Minteer DM, Li H, Rubin JP, et al. Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors. Tissue Eng Part C Methods. (2012) 18:54–61. doi: 10.1089/ten.TEC.2011.0216
175. Yang F, Carmona A, Stojkova K, Garcia Huitron EI, Goddi A, Bhushan A, et al. A 3d human adipose tissue model within a microfluidic device. Lab Chip. (2021) 21:435–46. doi: 10.1039/D0LC00981D
176. Tharp KM, Stahl A. Bioengineering beige adipose tissue therapeutics. Front Endocrinol (Lausanne). (2015) 6:164. doi: 10.3389/fendo.2015.00164
177. Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, et al. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes. (2015) 64:3713–24. doi: 10.2337/db15-0728
178. Yang JP, Anderson AE, McCartney A, Ory X, Ma G, Pappalardo E, et al. Metabolically active three-dimensional brown adipose tissue engineered from white adipose-derived stem cells. Tissue Eng Part A. (2017) 23:253–62. doi: 10.1089/ten.TEA.2016.0399
179. Lau FH, Vogel K, Luckett JP, Hunt M, Meyer A, Rogers CL, et al. Sandwiched white adipose tissue: A microphysiological system of primary human adipose tissue. Tissue Eng Part C Methods. (2018) 24:135–45. doi: 10.1089/ten.TEC.2017.0339
180. Kang JH, Gimble JM, Kaplan DL. In vitro 3d model for human vascularized adipose tissue. Tissue Eng Part A. (2009) 15:2227–36. doi: 10.1089/ten.tea.2008.0469
181. Paek J, Park SE, Lu Q, Park KT, Cho M, Oh JM, et al. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano. (2019) 13:7627–43. doi: 10.1021/acsnano.9b00686
182. Kim SJ, Byun H, Lee S, Kim E, Lee GM, Huh SJ, et al. Spatially arranged encapsulation of stem cell spheroids within hydrogels for the regulation of spheroid fusion and cell migration. Acta Biomater. (2022) 142:60–72. doi: 10.1016/j.actbio.2022.01.047
183. Shen K, Vesey DA, Hasnain SZ, Zhao KN, Wang H, Johnson DW, et al. A cost-effective three-dimensional culture platform functionally mimics the adipose tissue microenvironment surrounding the kidney. Biochem Biophys Res Commun. (2020) 522:736–42. doi: 10.1016/j.bbrc.2019.11.119
184. Yu J, Hsu YC, Lee JK, Cheng NC. Enhanced angiogenic potential of adipose-derived stem cell sheets by integration with cell spheroids of the same source. Stem Cell Res Ther. (2022) 13:276. doi: 10.1186/s13287-022-02948-3
185. Leong J, Hong YT, Wu YF, Ko E, Dvoretskiy S, Teo JY, et al. Surface tethering of inflammation-modulatory nanostimulators to stem cells for ischemic muscle repair. ACS Nano. (2020) 14:5298–313. doi: 10.1021/acsnano.9b04926
186. Jiao W, Liu C, Shan J, Kong Z, Wang X. Construction and evaluation of small-diameter bioartificial arteries based on a combined-mold technology. Polymers (Basel). (2022) 14:3089. doi: 10.3390/polym14153089
187. Fu H, Zhang D, Zeng J, Fu Q, Chen Z, Sun X, et al. Application of 3d-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int J Bioprint. (2023) 9:674. doi: 10.18063/ijb.v9i2.674
188. Li M, Ma J, Gao Y, Dong M, Zheng Z, Li Y, et al. Epithelial differentiation of human adipose-derived stem cells (Hascs) undergoing three-dimensional (3d) cultivation with collagen sponge scaffold (Css) via an indirect co-culture strategy. Stem Cell Res Ther. (2020) 11:141. doi: 10.1186/s13287-020-01645-3
189. Alamán-Díez P, García-Gareta E, Arruebo M, Pérez M. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering. J BioMed Mater Res A. (2023) 111:88–105. doi: 10.1002/jbm.a.37448
190. Sun W, Zhang J, Qin Y, Tang H, Chen Y, Lin W, et al. A simple and efficient strategy for preparing a cell-spheroid-based bioink. Adv Healthc Mater. (2022) 11:e2200648. doi: 10.1002/adhm.202200648
191. Lee J, Lee S, Huh SJ, Kang BJ, Shin H. Directed regeneration of osteochondral tissue by hierarchical assembly of spatially organized composite spheroids. Adv Sci (Weinh). (2022) 9:e2103525. doi: 10.1002/advs.202103525
192. Fujimoto R, Murata D, Nakayama K. Bio-3d printing of scaffold-free osteogenic and chondrogenic constructs using rat adipose-derived stromal cells. Front Biosci (Landmark Ed). (2022) 27:52. doi: 10.31083/j.fbl2702052
193. Celik N, Kim MH, Yeo M, Kamal F, Hayes DJ, Ozbolat IT. Mirna induced 3d bioprinted-heterotypic osteochondral interface. Biofabrication. (2022) 14:10. doi: 10.1088/1758-5090/ac7fbb
194. Landau S, Szklanny AA, Machour M, Kaplan B, Shandalov Y, Redenski I, et al. Human-engineered auricular reconstruction (Hear) by 3d-printed molding with human-derived auricular and costal chondrocytes and adipose-derived mesenchymal stem cells. Biofabrication. (2021) 14:1–14. doi: 10.1088/1758-5090/ac3b91
195. Jang CH, Koo Y, Kim G. Asc/chondrocyte-laden alginate hydrogel/pcl hybrid scaffold fabricated using 3d printing for auricle regeneration. Carbohydr Polym. (2020) 248:116776. doi: 10.1016/j.carbpol.2020.116776
196. Ibrahim A, Rodriguez-Florez N, Gardner OFW, Zucchelli E, New SEP, Borghi A, et al. Three-dimensional environment and vascularization induce osteogenic maturation of human adipose-derived stem cells comparable to that of bone-derived progenitors. Stem Cells Transl Med. (2020) 9:1651–66. doi: 10.1002/sctm.19-0207
197. Ewa-Choy YW, Pingguan-Murphy B, Abdul-Ghani NA, Jahendran J, Chua KH. Effect of alginate concentration on chondrogenesis of co-cultured human adipose-derived stem cells and nasal chondrocytes: A biological study. Biomater Res. (2017) 21:19. doi: 10.1186/s40824-017-0105-7
198. Guerrero J, Pigeot S, Müller J, Schaefer DJ, Martin I, Scherberich A. Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification. Acta Biomater. (2018) 77:142–54. doi: 10.1016/j.actbio.2018.07.004
199. Ergene E, Sezlev Bilecen D, Kaya B, Yilgor Huri P, Hasirci V. 3d cellular alignment and biomimetic mechanical stimulation enhance human adipose-derived stem cell myogenesis. BioMed Mater. (2020) 15:055017. doi: 10.1088/1748-605X/ab95e2
200. Liu BH, Yeh HY, Lin YC, Wang MH, Chen DC, Lee BH, et al. Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan. Biores Open Access. (2013) 2:28–39. doi: 10.1089/biores.2012.0285
201. Gomila Pelegri N, Stanczak AM, Bottomley AL, Cummins ML, Milthorpe BK, Gorrie CA, et al. Neural marker expression in adipose-derived stem cells grown in peg-based 3d matrix is enhanced in the presence of B27 and cultureone supplements. Int J Mol Sci. (2023) 24:16269. doi: 10.3390/ijms242216269
202. Wang Y, Lee JH, Shirahama H, Seo J, Glenn JS, Cho NJ. Extracellular matrix functionalization and huh-7.5 cell coculture promote the hepatic differentiation of human adipose-derived mesenchymal stem cells in a 3d icc hydrogel scaffold. ACS Biomater Sci Eng. (2016) 2:2255–65. doi: 10.1021/acsbiomaterials.6b00487
203. Scioli MG, Storti G, D'Amico F, Gentile P, Kim BS, Cervelli V, et al. Adipose-derived stem cells in cancer progression: New perspectives and opportunities. Int J Mol Sci. (2019) 20:3296. doi: 10.3390/ijms20133296
204. Strong AL, Burow ME, Gimble JM, Bunnell BA. Concise review: The obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells. (2015) 33:318–26. doi: 10.1002/stem.1857
205. Freese KE, Kokai L, Edwards RP, Philips BJ, Sheikh MA, Kelley J, et al. Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: A systematic review. Cancer Res. (2015) 75:1161–8. doi: 10.1158/0008-5472.Can-14-2744
206. Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang CC, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. (2015) 2015:853506. doi: 10.1155/2015/853506
Keywords: adipose-derived stem/stromal cells, monolayer culture, three-dimensional culture, diabetes mellitus, diabetic foot ulcer
Citation: Shi Y, Yang X, Min J, Kong W, Hu X, Zhang J and Chen L (2024) Advancements in culture technology of adipose-derived stromal/stem cells: implications for diabetes and its complications. Front. Endocrinol. 15:1343255. doi: 10.3389/fendo.2024.1343255
Received: 23 November 2023; Accepted: 29 March 2024;
Published: 12 April 2024.
Edited by:
Toshio Takahashi, Suntory Foundation for Life Sciences, JapanCopyright © 2024 Shi, Yang, Min, Kong, Hu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lulu Chen, Y2hlcmlhX2NoZW5AMTI2LmNvbQ==; Jiaoyue Zhang, emhhbmdqaWFveXVlQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.