- 1Department of Ophthalmology, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, China
- 2Department of Ophthalmology, The Affiliated Shunde Hospital of Jinan University, Foshan, Guangdong, China
Introduction: The aim of this study was to better understand the efficacy of various drugs, such as glucocorticoids and anti-vascular endothelial growth factors (VEGF), in the treatment of diabetic macular edema (DME), and to evaluate various clinical treatment regimens consisting of different therapeutic measures.
Methods: This study included randomized controlled trials up to February 2023 comparing the efficacy of corticosteroid-related therapy and anti-VEGF therapy. PubMed, the Cochrane Library, and Embase were searched, and the quality of the studies was carefully assessed. Finally, 39 studies were included.
Results: Results at 3-month followup showed that intravitreal injection of bevacizumab (IVB) + triamcinolone acetonide (TA) was the most beneficial in improving best-corrected visual acuity and reducing the thickness of macular edema in the center of the retina in patients with DME. Results at 6-month follow-up showed that intravitreal dexamethasone (DEX) was the most effective in improving patients’ bestcorrected visual acuity and reducing the thickness of central macular edema.
Discussion: Overall, IVB+TA was beneficial in improving best-corrected visual acuity and reducing central macular edema thickness over a 3-month follow-up period, while DEX implants had a better therapeutic effect than anti-VEGF agents at 6 months, especially the patients with severe macular edema and visual acuity impaired.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=397100, identifier CRD42023397100.
1 Introduction
Diabetes mellitus is one of the common chronic diseases that affect the quality of life of middle-aged and older people. The high glucose state of diabetes can damage systemic blood vessels. When it acts on microvessels, it is mainly manifested as diabetic retinopathy, which is the main cause of newly diagnosed blindness in old people (1, 2). The number of people with visual impairment caused by diabetes is increasing year by year. It is estimated that by 2030, the number of patients with diabetic retinopathy worldwide will increase from 103 million in 2020 to 130 million (3). DME can occur at any stage of the non-proliferative and proliferative phases of diabetic retinopathy, showing progressive aggravation. Therefore, the treatment of diabetic retinopathy aims to delay the disease process, improve the existing visual quality of patients, and ameliorate the quality of life-related to vision.
There are two main mechanisms for the development of DME. First, the state of hyperglycemia promotes retinal vascular degeneration, ischemia and hypoxia in the posterior pole of the retina, and the local accumulation of large amounts of vascular endothelial growth factor (VEGF), resulting in the formation of large numbers of new vessels with high brittleness. Second, inflammation causes an increase in vascular permeability, leading to fluid exudation, which accumulates in the layers of ganglion cells in the macula of retina, causing macular edema (2). Clinically known as DME, it is one of the important causes of visual loss in patients with advanced diabetes. In view of the above-mentioned mechanism, in addition to surgical treatment such as laser photocoagulation and vitrectomy, the most commonly used drug therapy is intravitreal injection of vascular endothelial growth factor antibodies (such as abscisic, bevacizumab, conbercept, etc.) or glucocorticoid (such as triamcinolone acetonide, dexamethasone intravitreal implants, etc.) (4). However, there is no consensus on the efficacy and safety of various therapeutic regimens.
To date, there have been relevant network meta-analyses comparing the efficacy and safety of anti-VEGF drugs in diabetic macular edema, but they did not include glucocorticoids. Given the limitations of head-to-head RCTs or traditional meta-analyses, in order to obtain evidence for direct and indirect comparisons and cross-sectional analysis of the efficacy of various interventions in diabetic macular edema, thus providing the best clinical evidence, we conducted a network meta-analysis to comprehensively compare the efficacy of various drugs such as glucocorticoids and anti-VEGF in diabetic macular edema, and to evaluate clinical treatment regimens composed of different treatment measures.
2 Methods
This study followed the PRISMA statement for network meta-analysis (5), and has been previously registered with PROSPERO (Registration Code: CRD42023397100).
2.1 Literature screening
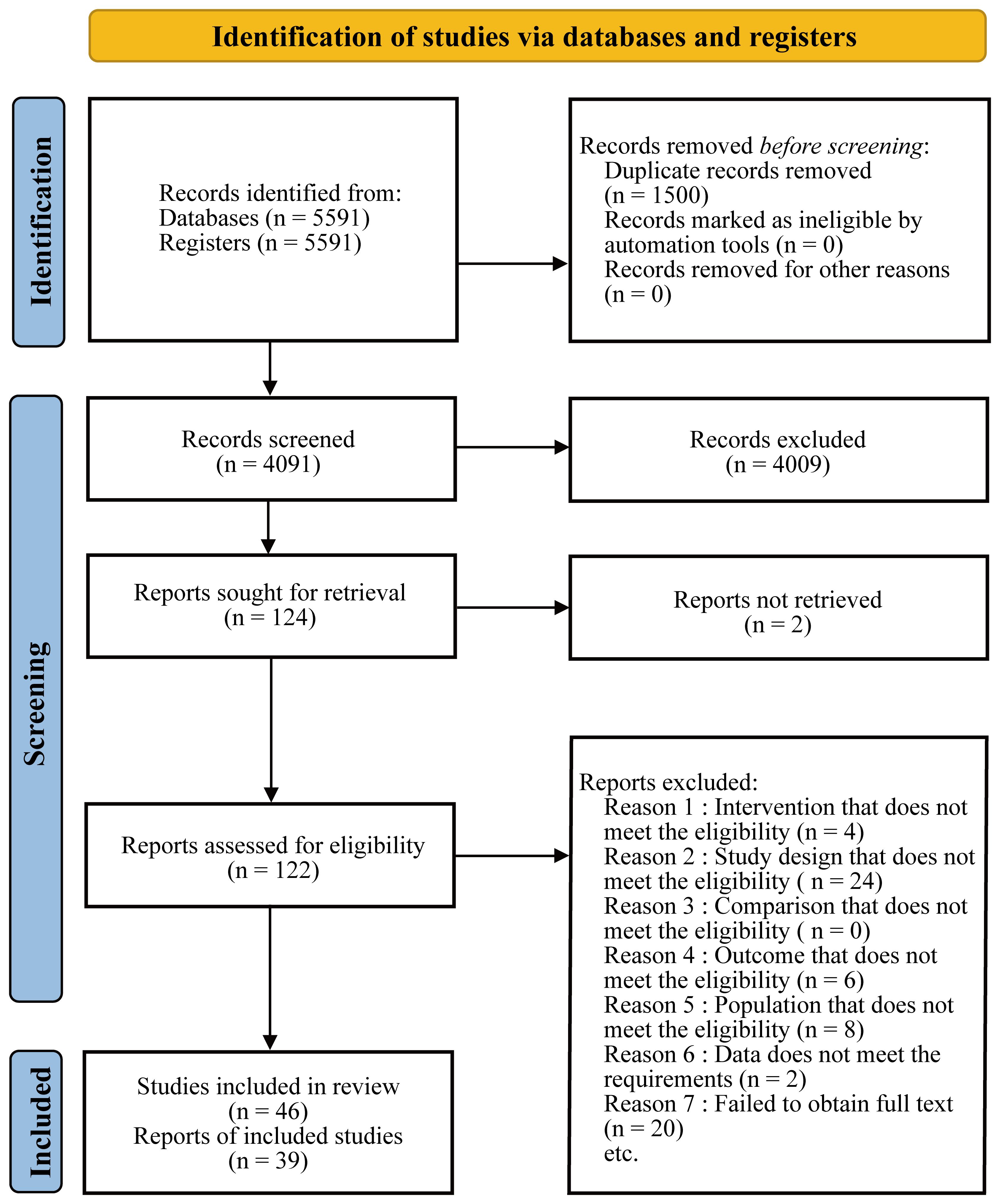
We searched PubMed, the Cochrane Library, and Embase for published articles from database inception to February 2023 without restrictions on time, date, language, and type of article. The search strategy and all data are detailed in the attachment. Two researchers conducted preliminary screening based on the title and abstract of the search results, and then obtained the full text for more detailed data screening. Disagreements were resolved through full discussion, and consultation with a third researcher if necessary. The process of this network meta-analysis was presented in the Figure 1.
The inclusion and exclusion criteria for screening were as follows, and the inclusion criteria were formulated according to the PICOS principle:
Participants: Patients with DME (more than 3 years); Age>40 years; Male or female; best-corrected visual acuity lower than 0.8; central macular thickness at least 320μm or more; no prior history of intravitreal or peribulbar injections of drugs or vitrectomy. The duration of patients with diabetic macular edema exceeds 1year; all patients involved in the center of the macula. We classify diabetic macular edema according to the thickness of the central macular area: 1) mild diabetic macular edema with the thickness of 320 to 450μm; 2) severe macular edema with the thickness >450μm (450μm not included).
Intervention measures: all intervention measures related to glucocorticoids such as DEX and TA; To provide more information for indirect comparison, laser photocoagulation combined with glucocorticoids and intravitreal injection of anti-VEGF drugs combined with glucocorticoids were also included in the study; There were no special requirements for treatment dose, frequency, time, mode of administration, treatment course, etc.
Comparison: laser photocoagulation or placebo group (including sham injection or sham laser).
Outcome measures: The primary outcome measures were the best corrected visual acuity and central macular thickness.
Study type: All included studies were RCTs.
Exclusion criteria were as follows:
All articles in languages other than English and Chinese were excluded; Articles with only abstract or preface available but missing full text were excluded; Research articles that had not been peer-reviewed were excluded; Non-RCTs were excluded, including literature review, systematic review, case report and retrospective study; When multiple study results were published for studies with long follow-up time, only the results with the longest follow-up time or the largest sample size were generally included; Studies that included patients with diabetic retinopathy, with or without macular edema, in the same trial should be excluded.
2.2 Data extraction and quality assessment
Two investigators jointly extracted the following information from the included studies: Study author and publication year, basic information of patients (such as age, gender, nationality, diagnosis, clinical stage of disease, severity of disease, study sample size, baseline visual acuity, central macular thickness, baseline intraocular pressure, etc.), information on intervention measures (including drug dose, injection frequency), information on outcome indicators (such as follow-up time, outcome data). The primary outcome indicator was the change in the best corrected visual acuity. Secondary outcome measures were changes in retinal anatomy such as central macular thickness. All outcome measures were recorded at 3- and 6-month follow-up. All disagreements during data extraction were resolved by thorough discussion.
We evaluated the quality of evidence for all included RCTs using the built-in ROB tool (version 1.0) in Review Manager (version 5.4.1), including the generation way of random sequences, allocation concealment, blinding of subjects, blinding of outcome measures, incomplete data on outcome measures, selective reporting, and other biases. All disagreements were resolved by thorough discussion.
2.3 Data analysis
Before the analysis, we already know that there were two forms of BCVA report in the existing research, namely LogMar and Letters. For the convenience of analysis, we use a formula to convert all Letters into LogMar. The formula was as follows:
We performed a Bayesian network meta-analysis using the GeMTC (version 1.0 - 1) package in R Studio 4.1.3. The two outcome measures included in the study, BCVA and CMT, were continuous variables and were therefore represented by means and standard deviations. The implementation of network meta-analysis needs to meet three basic assumptions, namely transitivity assumption, homogeneity assumption and consistency assumption. At present, there was no accepted statistical test method for transitivity hypothesis. In this study, the similarity of population characteristics included in the study was judged by using the data in the basic information table. If the population was generally similar, it was considered that the transitivity hypothesis is satisfied. Heterogeneity analysis was performed using the mtc.anohe function in the GeMTC package, and when the overall I2 was less than 50%, the heterogeneity of each included study within the same comparison was considered acceptable and the homogeneity assumption was met. The inconsistency between the direct comparison and the indirect comparison was checked using the mtc. nodessplit function in the GeMTC package by the node splitting method. When p>0.05, it was considered that there is no inconsistency between direct comparison and indirect comparison, and the consistency assumption is satisfied. In the network meta-analysis, although strict inclusion and exclusion criteria were established and heterogeneity analysis was passed, there were still inherent differences between studies that might affect the analysis results, so the random-effects model was directly used for analysis in this study.
After completing the test of the above assumptions, 1) the network was constructed with the interventions as nodes, and the lines between the nodes indicated the existence of direct comparison between the two interventions; 2) a forest plot of relative effects was drawn with the placebo group as a control by comparing the other interventions with placebo; 3) a league table was generated with the results of the analysis of relative effects, and the values in each cell indicated the difference in means between the intervention represented by its column and its row; 4) the cumulative probability ranking chart was analyzed, and all the cumulative ranking probabilities were estimated and reported as the surface area under the cumulative ranking curve (SUCRA).
3 Results
3.1 Basic characteristics of included studies
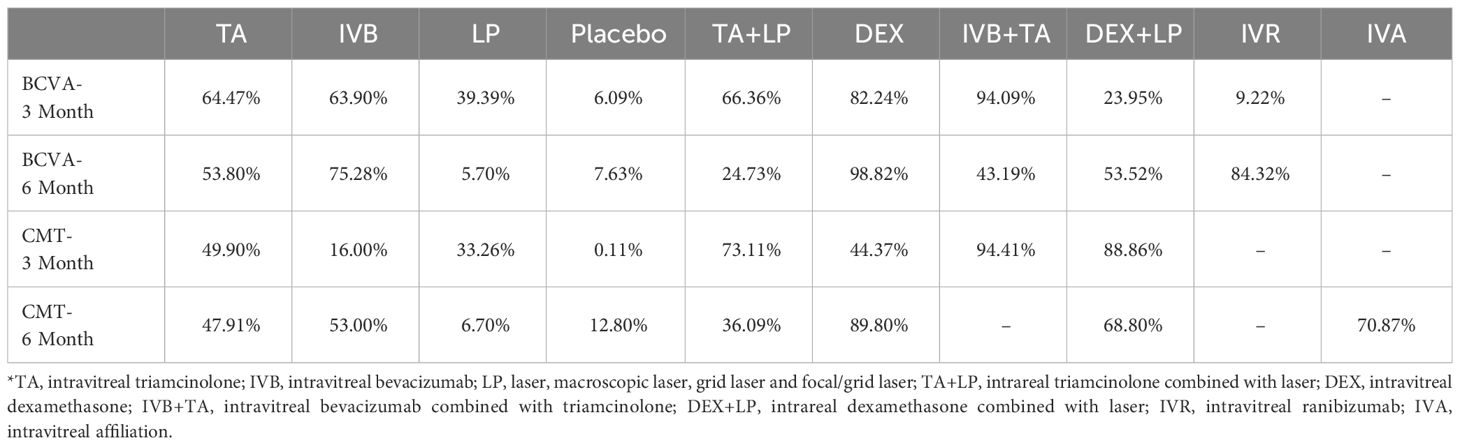
A total of 39 studies were included, including 5823 eyes of patients with diabetic macular edema and 10 interventions to treat the disease. Among them, TA was used in 19 studies (983 eyes), IVB in 12 studies (676 eyes), LP in 20 studies (2097 eyes), Placebo in 8 studies (2375 eyes), IVA in 3 studies (173 eyes), TA+LP in 7 studies (928 eyes), DEX+LP in 4 studies (487 eyes), DEX in 8 studies (2868 eyes), IVB+TA in 8 studies (508 eyes) and IVR in 1 study (363 eyes). The visual acuity fluctuation of the included patients before intervention was within the range of 0.1-0.8, and the macular thickness in the center of retina was within the range of 320-600 mm. The population characteristics of the included patients were highly similar, which could meet the transitivity hypothesis of network meta-analysis. The basic characteristics of the included studies are provided in Table 1 and Supplementary Table S1.

Table 1 Study characteristics of randomized controlled trials included in the network meta-analysis.
3.2 Evaluation of evidence quality and data extraction
As shown in Figures 2 and 3, 14 studies did not describe how to randomly allocate patients. Although these studies claimed to be randomized design, they did not elaborate on what kind of random allocation method was used. Nine studies were not assigned for concealment, one of which were open label. Seven studies had a high-risk bias, and 14 studies did not have a double-blinded or triple-blinded design for patients and researchers, with an open label design. For all included studies, no bias was found in the outcome evaluation blind method. Eight studies had incomplete outcome indicators, and one of them had a high-risk bias. Selective reporting bias occurred in 2 studies, of which 17 had medium-risk biases. For the evaluation of bias items not mentioned above, it can be summarized as other biases. No serious defects such as unreasonable design of patient inclusion criteria were found, such as patients receiving laser or other treatment prior to inclusion in this study, which may affect the results of clinical trials and cause a certain degree of bias risk (6–49).

Figure 2 Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
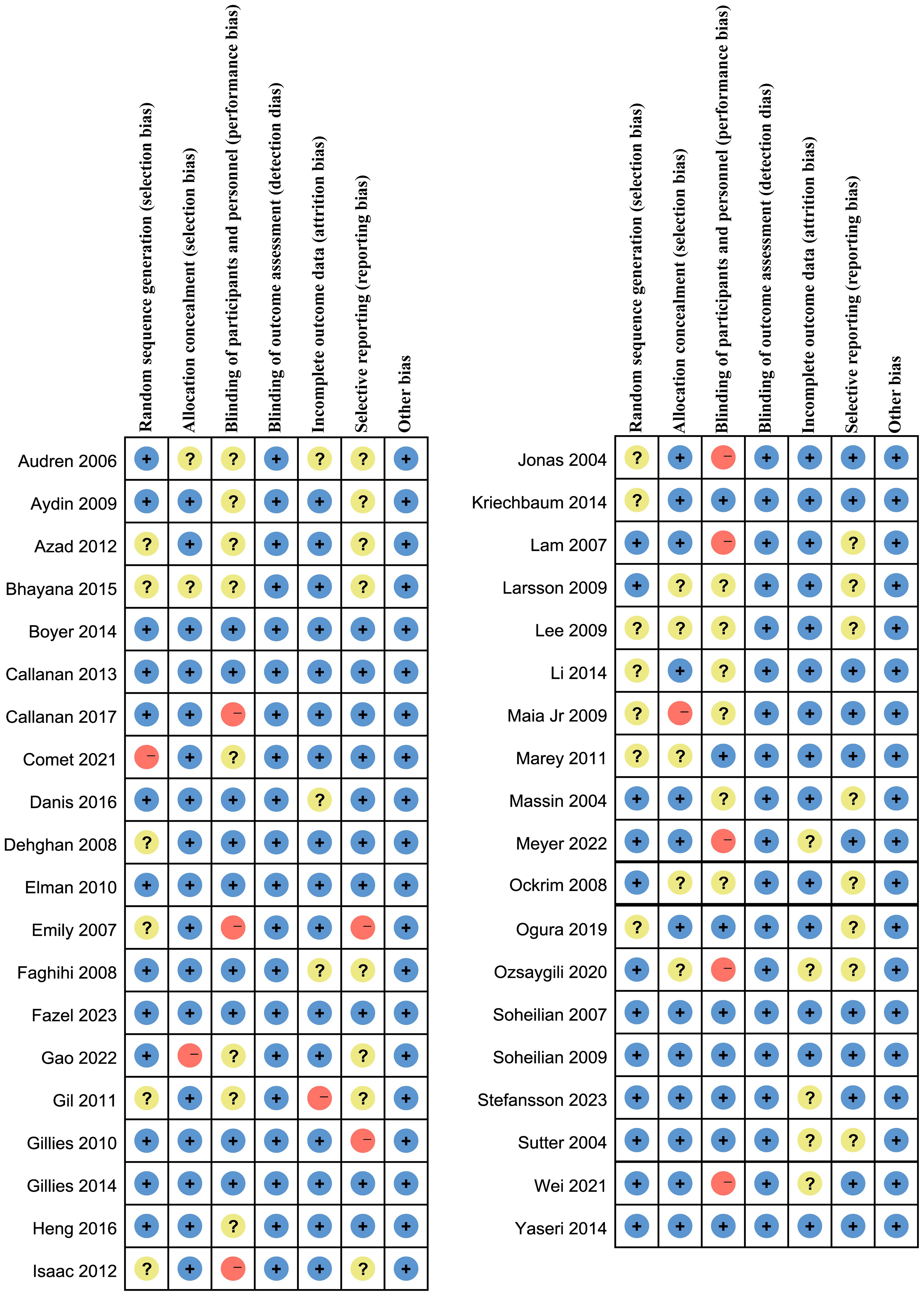
Figure 3 Assessment of the risk of bias in included studies. Risk of bias summary: review authors’ judgments about each risk of bias items for each included study. +: Low risk of bias; -: High risk of bias; ?: Clear risk of bias.
3.3 Inconsistency and heterogeneity analysis of network model
The heterogeneity and inconsistency of the network meta-analysis were tested. In the inconsistency test, the P value of the inconsistency test of all studies was greater than 0.05, indicating that there is no significant inconsistency between the direct comparison and the indirect comparison of the included studies, and the consistency hypothesis is satisfied. The analysis results were shown in Annex 1. According to heterogeneity analysis, the I2 value of most studies was less than 50%. Although there was heterogeneity in a few studies, it had little impact on the homogeneity hypothesis of this study. Overall, there was no significant heterogeneity, indicating that our network meta-analysis met the homogeneity hypothesis. The analysis results were shown in Annex 2.
3.4 Mean change in best-corrected visual acuity at 3 months
A total of 27 studies involving 3770 eyes were included in this study, with a total of 9 interventions including TA, IVB, LP, Placebo, TA + LP, DEX, IVB + TA, DEX + LP and IVR. A network meta-analysis was performed and a network plot was constructed (Figure 4A). In the relative effect forest plot, IVB + TA had the best effect on improving visual acuity. TA + LP, IVB and TA had similar effects on improving visual acuity, and Placebo was the worst. The values for each intervention compared with placebo were shown in the league table (Table 2). The results showed that there were significant differences in the improvement of the best corrected visual acuity among the interventions. The SUCRA values of each intervention were shown in the Table 3, and IVB + TA has a maximum value of 94.39% compared with other interventions. According to the rank chart and league table, IVB + TA was the best treatment plan to improve the best corrected visual acuity at 3 months (Figure 5).
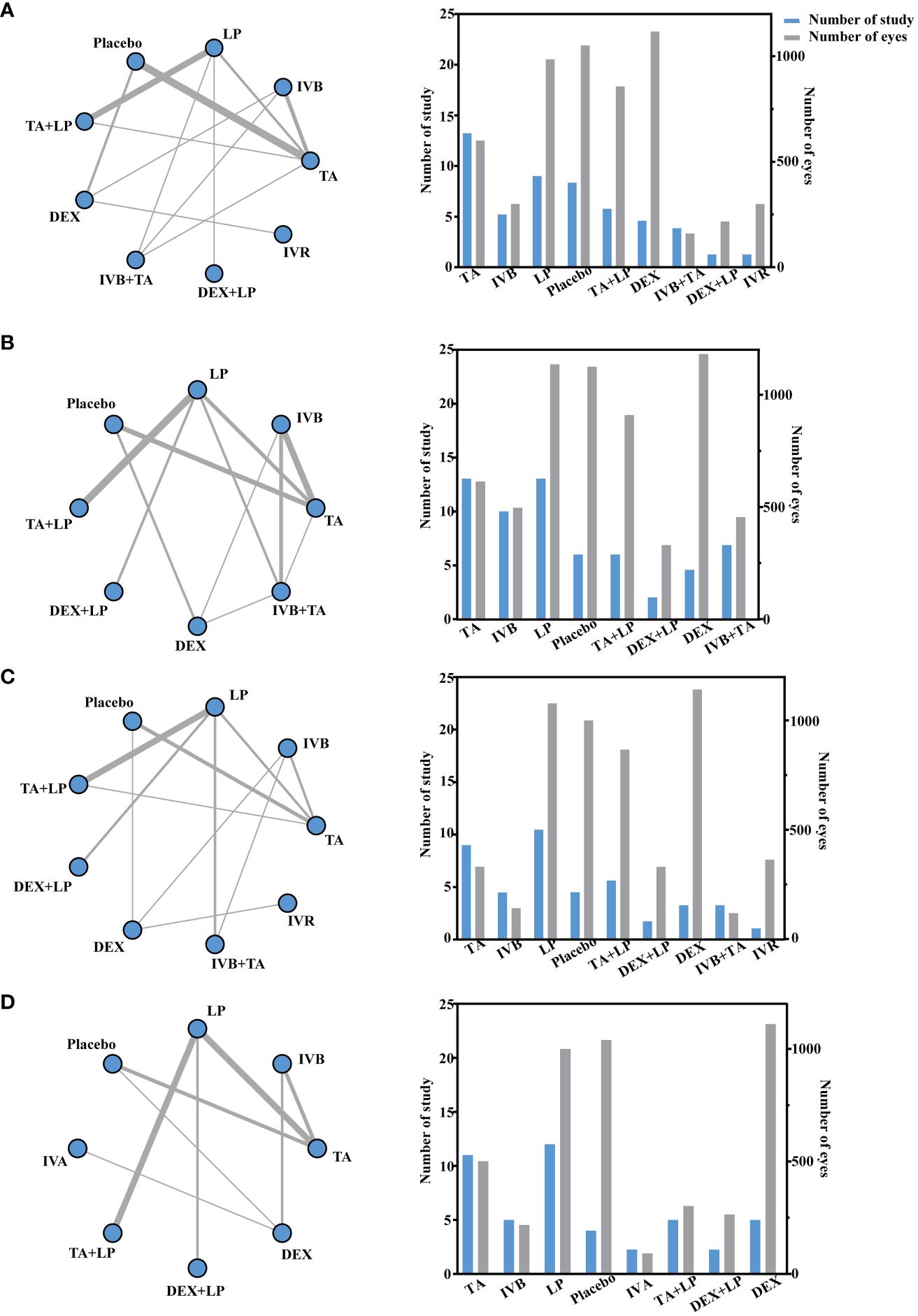
Figure 4 Network geometry for BCVA mean change from baseline. All populations at 3-months follow-up [(A) 25 trials] and 6-months follow-up [(C) 21 trials]. Network geometry for CMT mean change from baseline. All populations at 3-months follow-up [(B) 30 trials] and 6-months follow-up [(D) 23 trials]. BCVA, best-corrected visual acuity; CMT, central macular thickness. TA, intravitreal triamcinolone; IVB, intravitreal bevacizumab; LP, laser, macroscopic laser, grid laser and focal/grid laser; IVA, intravitreal affiliation; TA+LP, intrareal triamcinolone combined with laser; DEX, intravitreal dexamethasone; IVB+TA, intravitreal bevacizumab combined with triamcinolone; DEX+LP, intrareal dexamethasone combined with laser; IVR, intravitreal ranibizumab. Direct comparisons are represented by the black lines connecting the different interventions. Line width is proportional to the number of trials including every pair of interventions.
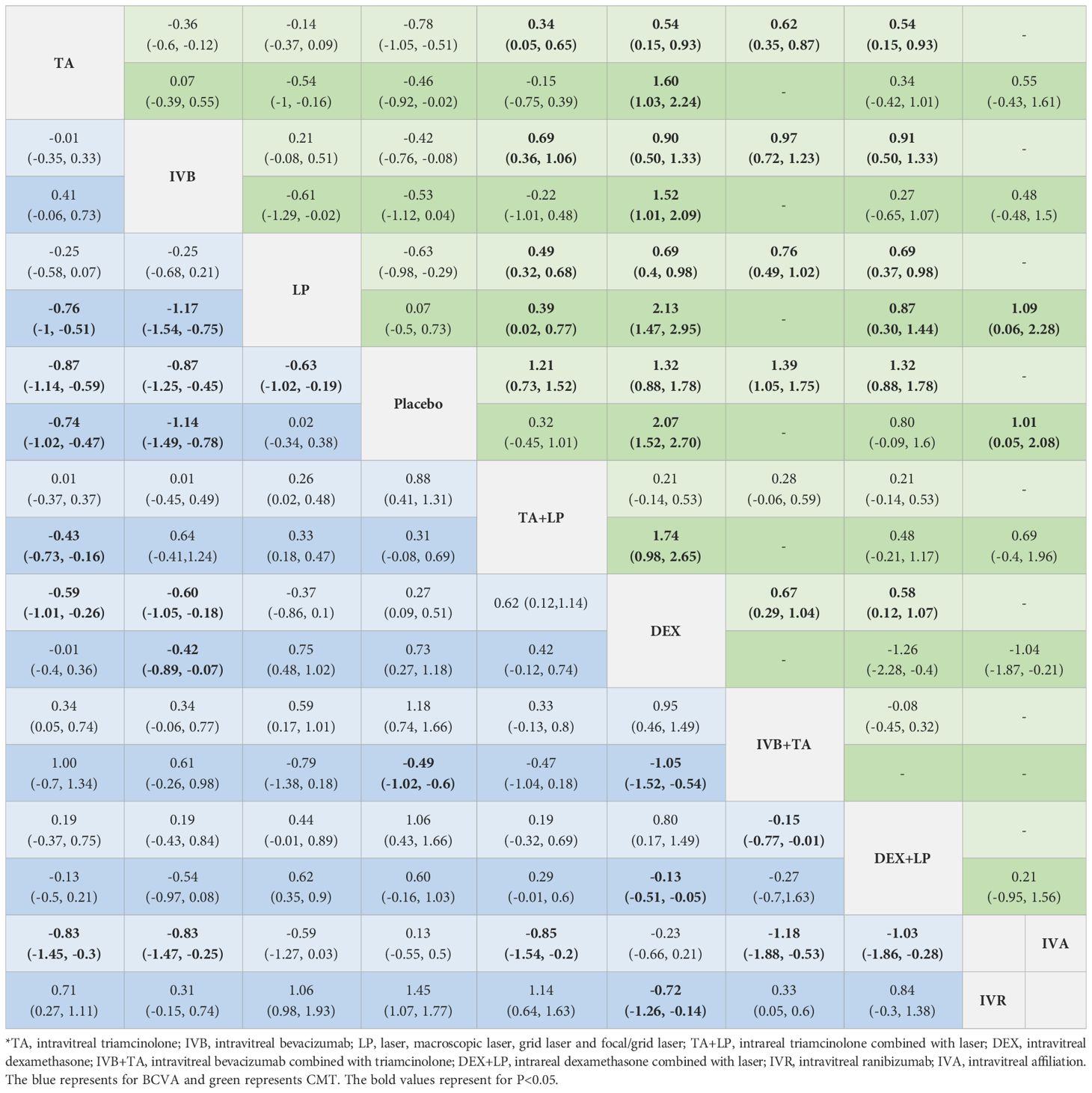
Table 2 Network meta-analysis results in BCVA and CMT at 3 months (lower part) and 6 months (upper part).

Figure 5 Forest plots for BCVA at 3 months and 6 months. TA, intravitreal triamcinolone; IVB, intravitreal bevacizumab; LP, laser, macroscopic laser, grid laser and focal/grid laser; TA+LP, intrareal triamcinolone combined with laser; DEX, intravitreal dexamethasone; IVB+TA, intravitreal bevacizumab combined with triamcinolone; DEX+LP, intrareal dexamethasone combined with laser; IVR, intravitreal ranibizumab.
3.5 Mean change in central macular thickness of retina at 3 months
A total of 32 studies involving 4209 eyes were included in this study, with a total of 8 interventions, namely TA, IVB, LP, Placebo, TA+LP, DEX+LP, DEX and IVB+TA. A network meta-analysis was constructed (Figure 4B). In the forest plot of relative effect, DEX+LP and IVB+TA reduced the central macular thickness of patients with diabetic macular edema to approximately the same level, and their treatment effects were the best compared with other interventions. TA+LP was less effective in reducing macular edema. TA and DEX improved macular edema to a similar extent, and were only inferior to the first two levels of intervention; LP have similar effects, ranking fourth; IVB showed the least improvement in macular edema compared with placebo. Convergence tests were performed between interventions with head-to-head studies. The results of iterative convergence tests were expressed as PSRF values, and the closer the value to 1, the better the convergence. The PSRF of the network meta-analysis was 1, indicating good convergence. The values of each intervention compared with the placebo group were shown in Table 2. These results show that there was a significant difference in the reduction of central macular thickness after each intervention. The SUCRA values for each intervention were in Table 3. The SUCRA values and league table above showed that IVB+TA were the best interventions in reducing central macular thickness in patients with DME, followed by DEX+LP. IVB and Placebo had the worst results (Figure 6).

Figure 6 Forest plots for CMT at 3 months and 6 months. TA, intravitreal triamcinolone; IVB, intravitreal bevacizumab; LP, laser, macroscopic laser, grid laser and focal/grid laser; IVA, intravitreal affiliation; TA+LP, intrareal triamcinolone combined with laser; DEX, intravitreal dexamethasone; IVB+TA, intravitreal bevacizumab combined with triamcinolone; DEX+LP, intrareal dexamethasone combined with laser.
3.6 Mean change in best-corrected visual acuity at 6 months
A total of 9 interventions, TA, IVB, LP, Placebo, TA+LP, DEX+LP, DEX, IVB+TA and IVR, were included in this analysis, and 23 studies with a total of 3362 eyes were used to conduct a network meta-analysis and construct a network plot (Figure 4C). According to the forest plot of relative effect (placebo group as control), the effect of each intervention on improving patients’ vision can be divided into three levels: The effective schemes including DEX and IVR; The programs with moderate effect including IVB, TA, DEX+LP and IVB+TA; Less effective schemes including TA+LP and LP. Convergence tests were performed between interventions with head-to-head comparisons, and the results of iterative convergence tests were expressed as PSRF values, with values closer to 1 indicating better convergence. The PSRF of the network meta-analysis was 1, indicating good convergence. The values of interventions compared with the placebo group and the SUCRA values for each of the above interventions were shown in Tables 2 and 3, respectively. Combining the league table and SUCRA values, among these interventions, DEX were the best in improving best-corrected visual acuity at 6 months of treatment, LP and Placebo were relatively the worst (Figure 5).
3.7 Mean change in central macular thickness of retina at 6 months
A total of 25 studies involving 2858 eyes were included in this study, with a total of 8 interventions including TA, IVB, LP, Placebo, IVA, TA+LP, DEX+LP and DEX. A network meta-analysis was performed (Figure 4D). In the forest plot of relative effect constructed with the placebo group as control, at the 6-month treatment cycle, each intervention was better than the placebo group in reducing the central macular thickness of DME. The effect of DEX was the best, followed by IVA and DEX+LP, then followed by IVB and TA, while the effects of TA+LP and LP were not ideal. The PSRF of the mesh meta-analysis was 1, indicating good convergence. In the effect league table, the values of each intervention compared with the placebo group were in Table 2. The SUCRA values for each intervention were shown in Table 3. According to SUCRA value and league table analysis, DEX was the best in reducing central macular thickness and the effects of TA+LP and LP were relatively the worst (Figure 6).
3.8 Subgroup analysis of best-corrected visual acuity
According to best-corrected visual acuity, diabetic macular edema was divided into two groups: best-corrected visual acuity impaired (<0.6) and best-corrected visual acuity not impaired (0.6~0.8). The network meta-analysis was performed separately (Figure 7). The values of each intervention were shown in the effect league table (Supplementary 1, 2). At 3-month, IVB+TA had a best therapeutic effect on patients with intact vision. At 6-month, DEX was the best way to treat patients with impaired vision, while IVB is the best for patients without impaired vision.

Figure 7 Network geometry for BCVA mean change from baseline. All populations of best-corrected visual acuity impaired and not impaired at 3-months follow-up [(A, B)] and 6-months follow-up [(C, D)]. BCVA, best-corrected visual acuity; CMT, central macular thickness. TA, intravitreal triamcinolone; IVB, intravitreal bevacizumab; LP, laser, macroscopic laser, grid laser and focal/grid laser; TA+LP, intrareal triamcinolone combined with laser; DEX, intravitreal dexamethasone; IVB+TA, intravitreal bevacizumab combined with triamcinolone; DEX+LP, intrareal dexamethasone combined with laser; IVR, intravitreal ranibizumab. Notes: Direct comparisons are represented by the black lines connecting the different interventions. Line width is proportional to the number of trials including every pair of interventions. The values of SUCRA are represented by the point size.
3.9 Subgroup analysis of central macular thickness of retina
According to central macular thickness, diabetic macular edema was divided into two groups: mild macular edema (320~450μm) and severe macular edema (>450μm). The network meta-analysis was performed separately (Figure 8). The values of each intervention were shown in the effect league table (Supplementary 3, 4). At 3-month, IVB+TA had a best therapeutic effect on patients no matter mild or severe macular edema. At 6-month, DEX was the best way to treat patients with severe macular edema, while the data of mild macular edema was unable to construct a network structure and be performed further analysis.

Figure 8 Network geometry for CMT mean change from baseline. All populations of mild macular edema and severe macular edema at 3-months follow-up [(A, B)]. All populations of severe macular edema at 6-months follow-up [(C)]. BCVA, best-corrected visual acuity; CMT, central macular thickness. macroscopic laser, grid laser and focal/grid laser; TA+LP, intrareal triamcinolone combined with laser; DEX, intravitreal dexamethasone; IVB+TA, intravitreal bevacizumab combined with triamcinolone; DEX+LP, intrareal dexamethasone combined with laser; IVR, intravitreal ranibizumab. Notes: Direct comparisons are represented by the black lines connecting the different interventions. Line width is proportional to the number of trials including every pair of interventions. The values of SUCRA are represented by the point size.
In addition, this study presents a risk table for the side effects associated with various interventions (Supplementary Table S2). It was observed that both TA and DEX have the potential to increase intraocular pressure. However, this adverse effect can be mitigated through the administration of intraocular pressure-lowering eye drops.
In terms of improving the best corrected visual acuity and reducing macular edema, the patients with diabetic macular edema who received IVB+TA had the best therapeutic effect at the 3-month follow-up. At the 6-month follow-up, DEX was the most effective treatment in improving central macular thickness. Glucocorticoids combined with laser therapy improved the efficacy at 3 and 6 months both, but LP alone had a poor effect. Furthermore, IVB+TA had a best therapeutic effect on patients with mild macular edema and best-corrected visual acuity not impaired at 3-month, while DEX was the best way to treat patients with severe macular edema and best-corrected visual acuity impaired.
4 Discussion
This network meta-analysis included 39 RCTs involving 5823 eyes. Through this study, we found that intravitreal injection of IVB + TA was the most beneficial for improving BCVA and reducing CMT compared with other treatments during the 3-month follow-up period. During the 6-month follow-up period, intravitreal DEX had the best effect in improving the BCVA and reducing the CMT.
We found that, in patients with diabetic macular edema at 3 months of follow-up, IVB+TA combined regimen improved the outcome of diabetic macular edema patients best, while TA, IVB alone regimen also achieved good clinical treatment effect. At the same time, glucocorticoids (TA or DEX) combined with LP achieved good results, too. At 6 months of follow-up, patients treated with the DEX regimen achieved the most significant improvement in visual quality and restoration of edematous macular anatomy. The combination of IVB+TA significantly improved vision and restored macular anatomy in patients with diabetic macular edema. This is consistent with the results of systematic review studies (50, 51). It might because the therapeutic effects of IVB + TA might be related to the antagonism of IVB against neovascularization and the antagonism of TA against macular local inflammation. The fundus retina of patients with diabetic retinopathy is in a state of ischemia and hypoxia due to local retinal microvascular damage. VEGF is highly concentrated in this area, which stimulates neovascularization around the lesion to compensate for ischemia and hypoxia. However, the neovascularization is fragile and easy to leak into the outer plexiform layer of the retina, resulting in macular edema (2, 52, 53). At the same time, bevacizumab, as a human monoclonal antibody targeting VEGF, inhibits angiogenesis by specifically binding to VEGF and blocking the signal transduction pathway of angiogenesis (54–56). In addition, inflammation plays an important role in the mechanism of DME. Chronic hyperglycemia in patients with DME induces oxidative stress and inflammation, resulting in retinal pericyte separation and structural changes in capillary tight junctions, causing blood-retinal barrier damage. Triamcinolone acetonide, a long-acting glucocorticoid, reduces inflammation by inducing the synthesis of anti-inflammatory cytokines and inhibiting the migration of inflammatory cells out of blood vessels. It stabilizes mast cells, reduces histamine release, shrinks capillary, and reduces vascular permeability (57, 58), thereby reducing inflammation-induced changes in the anatomy of the retina. Studies found that glucocorticoids (TA, DEX, etc.) can down-regulate the expression of various inflammatory factors including VEGF (57). Some studies showed that anti-VEGF was less effective in reducing foveal edema than other treatments, while glucocorticoid can eliminate macular foveal edema, and over time, the patient’s macular foveal regression effect is significant (59), thereby improving the central vision damage caused by retinal macular edema. On the other hand, previous meta-analyses did not compare the efficacy of TA alone with that of anti-VEGF alone, but we confirmed that the efficacy of TA alone was similar to that of anti-VEGF alone; The combination of IVB and TA was significantly superior to other anti-VEGF or glucocorticoid therapies during the 3-month follow-up period. TA and IVB, acting on separate pathways to combat inflammation and neovascularization, were less effective than the combination, but TA or IVB alone can also achieve significant efficacy and had a greater advantage over conventional LP. In addition, TA combined with LP had a significant therapeutic effect on central macular thickness in patients with DME, which was statistically different from LP. Therefore, clinicians should be aware of the benefits of TA+LP in improving visual acuity and reducing edema in the early treatment of DME. We also found that the effect of LP on macular edema was not significantly different from that of placebo at 3 and 6 months, suggesting that the role of LP in the treatment of DME should be re-evaluated; This differs from the findings of previous systematic reviews (60). It can be used as a supplement to combat macular edema, taking into account the economic burden of patients.
In this study, although there was a trend toward improvement in the efficacy of each intervention compared with the placebo group, the significance of the difference gradually decreased and the reasons are listed as below. On the one hand, the number of studies of the various interventions was insufficient, and the random effects model expanded the 95% confidence interval, leading to interventions other than TA were not statistically significant. At 3 and 6 months of follow-up, there was a trend of improvement in each treatment regimen compared with Placebo, but the difference was not statistically significant, which may be related to the lack of study size or the use of random effects model resulting in a wider 95%CI. Our results indicated that all regimens except placebo can improve the best corrected visual acuity and reduce macular thickness. In addition, the efficacy of DEX in improving the best corrected visual acuity of patients at 3 and 6 months of follow-up was comparable to that of placebo. Therefore, the effect of DEX on improving the best corrected visual acuity needs to be re-evaluated to eliminate the error caused by the risk of bias, and to clarify the actual impact of DEX on BCVA at 3 and 6 months, so as to provide a reliable basis for clinical selection of treatment regimens. On the other hand, most interventions showing statistical differences at 3 months and most interventions showing no statistical differences at 6 months. The reason may be that the efficacy of the intervention diminished at 6 months, with only DEX remaining effective.
This might be related to the duration of DEX action and the progression of regression of macular edema in the central retina. Compared with triamcinolone acetonide, DEX implants have a delayed onset of action, slower drug release, and longer duration of action after intravitreal injection (61). DEX exhibits a biphasic sustained release with high concentrations for the first 2 months and low concentrations for several months, resulting in a longer period of modulation of VEGF expression and anti-inflammatory effects in the vitreous (62, 63). We also found that intravitreal injection of DEX also provided significant improvement in patients treated with LP within 3 months before and after injection, regardless of the order of treatment of LP and DEX. In our study of 6-month CMT, although no study of IVB combined with TA was included, it can still provide a reference for clinical treatment. In addition, anti-VEGF treatment resistance may affect the results. Some studies showed that patients with certain types of diabetic macular edema were resistant to anti-VEGF drug therapy and did not achieve significant visual and anatomical improvement (64). Therefore, future research may focus on these patients who are resistant to anti-VEGF drugs during clinical treatment.
This network meta-analysis studied the common drugs used in the treatment of DME in clinical practice, including anti-VEGF drugs, glucocorticoids and laser. Unlike previous network meta-analyses, which only paid attention to the study of anti-VEGF drugs, and ignored the important effect of glucocorticoids, such as TA and DEX, in improving the visual acuity and central macular edema thickness, our study evaluated the improvement of visual acuity and macular structure recovery in patients with glucocorticoids in 3-month and 6-month short cycle treatment, thus providing more references for clinicians to choose. In addition, we combined the data of single or combination therapy and found that DEX combined with LP can also achieve satisfactory clinical efficacy. Previous meta-analyses did not assess the effect of DEX in combination with LP, focusing only on monotherapy (65), but our research made up for this deficiency.
Our study also had some limitations. First, a total of 39 studies were included in the network meta-analysis, but it was not assessed whether the number of included studies met the requirements for testing publication bias. In addition, the central macular thickness of the retina did not form a closed loop at the 6-month follow-up, and the inconsistency test could not be completed. Second, there were some contradictory phenomena in our study. For example, LP was found to be less effective than placebo in reducing macular edema at 3 months. Besides, the results of LP and TA+LP in improving foveal edema at 6 months were implausible and inconsistent with the efficacy outcomes of drugs in clinical practice. It was speculated that there may be two reasons. On the one hand, the number and sample size of the research projects were small. On the other hand, the included studies on the single treatment of some measures may have large heterogeneity, which may impair the reliability of the network meta-analysis results of this group. Third, we did not pay attention to the type of diabetes, the stage of diabetic retinopathy, and the effects of other syndrome. Whether these conditions can change the outcome of medical treatment of diabetic macular edema needs further study. Fourth, this study only focused on the efficacy of glucocorticoid alone, and the evaluation of the efficacy and safety of glucocorticoid combined with anti-VEGF treatment regimen is not perfect. Side effects such as endophthalmitis caused by hormone therapy were not mentioned in all included studies, so the safety evaluation of adverse reactions caused by drugs should be paid continuous attention. In addition, the number of studies on DEX+LP is insufficient, and there are limitations in evaluating its effectiveness. Further studies are needed to address these gaps and provide a more comprehensive understanding of its therapeutic potential. Therefore, in order to more accurately analyze the efficacy and safety of drug treatment of diabetic macular edema, more multi-center, multi-ethnic, multi-regional, high-quality prospective randomized controlled trials are required.
5 Conclusion
This study showed that intravitreal injection of IVB+TA was most beneficial in improving best-corrected visual acuity and reducing the thickness of macular edema in the center of the retina compared with other treatments during the 3-month follow-up period. Intravitreal injection of DEX was the most effective in improving best-corrected visual acuity and reducing central macular edema thickness over a 6-month follow-up period, especially the patients with severe macular edema and visual acuity impaired. Given the limitations of this study, the results need to be interpreted with caution, and more well-designed RCTs are warranted in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Z’AC: Writing – original draft, Software, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. XL: Writing – review & editing, Visualization, Supervision, Project administration, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1342530/full#supplementary-material
References
1. Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. (2013) 4:151–69. doi: 10.1177/2042018813512360
2. Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with diabetic retinopathy. New Engl J Med. (2020) 382:1629–37. doi: 10.1056/NEJMra1909637
3. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
4. Zhang J, Zhang J, Zhang C, Zhang J, Gu L, Luo D, et al. Diabetic macular edema: current understanding, molecular mechanisms and therapeutic implications. Cells. (2022) 11(21):3362. doi: 10.3390/cells11213362
5. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med. (2015) 162:777–84. doi: 10.7326/M14-2385
6. Audren F, Erginay A, Haouchine B, Benosman R, Conrath J, Bergmann JF, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular oedema: 6-month results of a prospective controlled trial. Acta Ophthalmol Scand. (2006) 84:624–30. doi: 10.1111/j.1600-0420.2006.00700.x
7. Aydin E, Demir HD, Yardim H, Erkorkmaz U. Efficacy of intravitreal triamcinolone after or concomitant with laser photocoagulation in nonproliferative diabetic retinopathy with macular edema. Eur J Ophthalmol. (2009) 19:630–7. doi: 10.1177/112067210901900418
8. Azad R, Sain S, Sharma YR, Mahajan D. Comparison of intravitreal bevacizumab, intravitreal triamcinolone acetonide, and macular grid augmentation in refractory diffuse diabetic macular edema: A prospective, randomized study. Oman J Ophthalmol. (2012) 5:166–70. doi: 10.4103/0974-620X.106100
9. Bhayana S, Sood S, Narang S, Sethi NK. Intravitreal bevacizumab versus posterior subtenon triamcinolone in diffuse diabetic macular edema. Int Ophthalmol. (2015) 35:519–25. doi: 10.1007/s10792-014-9978-9
10. Boyer DS, Yoon YH, Belfort R Jr., Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. (2014) 121:1904–14. doi: 10.1016/j.ophtha.2014.04.024
11. Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. (2013) 120:1843–51. doi: 10.1016/j.ophtha.2013.02.018
12. Callanan DG, Loewenstein A, Patel SS, Massin P, Corcóstegui B, Li XY, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefe's Arch Clin Exp Ophthalmol = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. (2017) 255:463–73. doi: 10.1007/s00417-016-3472-1
13. Comet A, Gascon P, Ramtohul P, Donnadieu B, Denis D, Matonti F. INVICTUS: Intravitreal anti-VEGF and dexamethasone implant comparison for the treatment of diabetic macular edema: A 12 months follow-up study. Eur J Ophthalmol. (2021) 31:754–8. doi: 10.1177/1120672120930603
14. Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. (2016) 100:796–801. doi: 10.1136/bjophthalmol-2015-306823
15. Dehghan MH, Ahmadieh H, Ramezani A, Entezari M, Anisian A. A randomized, placebo-controlled clinical trial of intravitreal triamcinolone for refractory diabetic macular edema. Int Ophthalmol. (2008) 28:7–17. doi: 10.1007/s10792-007-9097-y
16. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. (2010) 117:1064–77.e35. doi: 10.1016/j.ophtha.2010.02.031
17. Faghihi H, Roohipoor R, Mohammadi SF, Hojat-Jalali K, Mirshahi A, Lashay A, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. Eur J Ophthalmol. (2008) 18:941–8. doi: 10.1177/112067210801800614
18. Fazel F, Malekahmadi M, Feizi A, Oliya B, Tavakoli M, Fazel M. Suprachoroidal injection of triamcinolone acetonide plus intravitreal bevacizumab in diabetic macular edema: a randomized pilot trial. BMC Ophthalmol. (2023) 23:40. doi: 10.1186/s12886-023-02790-y
19. Gil AL, Azevedo MJ, Tomasetto GG, Muniz CH, Lavinsky J. Treatment of diffuse diabetic maculopathy with intravitreal triamcinolone and laser photocoagulation: randomized clinical trial with morphological and functional evaluation. Arquivos Brasileiros Oftalmol. (2011) 74:343–7. doi: 10.1590/S0004-27492011000500007
20. Gillies MC, McAllister IL, Zhu M, Wong W, Louis D, Arnold JJ, et al. Pretreatment with intravitreal triamcinolone before laser for diabetic macular edema: 6-month results of a randomized, placebo-controlled trial. Invest Ophthalmol Visual Sci. (2010) 51:2322–8. doi: 10.1167/iovs.09-4400
21. Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. (2014) 121:2473–81. doi: 10.1016/j.ophtha.2014.07.002
22. Heng LZ, Sivaprasad S, Crosby-Nwaobi R, Saihan Z, Karampelas M, Bunce C, et al. A prospective randomised controlled clinical trial comparing a combination of repeated intravitreal Ozurdex and macular laser therapy versus macular laser only in centre-involving diabetic macular oedema (OZLASE study). Br J Ophthalmol. (2016) 100:802–7. doi: 10.1136/bjophthalmol-2015-307136
23. Isaac DL, Abud MB, Frantz KA, Rassi AR, Avila M. Comparing intravitreal triamcinolone acetonide and bevacizumab injections for the treatment of diabetic macular oedema: a randomized double-blind study. Acta Ophthalmol. (2012) 90:56–60. doi: 10.1111/j.1755-3768.2009.01817.x
24. Jonas JB, Harder B, Kamppeter BA. Inter-eye difference in diabetic macular edema after unilateral intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. (2004) 138:970–7. doi: 10.1016/j.ajo.2004.07.007
25. Kriechbaum K, Prager S, Mylonas G, Scholda C, Rainer G, Funk M, et al. Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: one-year results. Eye (London England). (2014) 28:9–15. doi: 10.1038/eye.2013.242
26. Lam DS, Chan CK, Mohamed S, Lai TY, Lee VY, Liu DT, et al. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema: six-month outcomes. Ophthalmology. (2007) 114:2162–7. doi: 10.1016/j.ophtha.2007.02.006
27. Larsson J, Kifley A, Zhu M, Wang JJ, Mitchell P, Sutter FK, et al. Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol. (2009) 87:275–80. doi: 10.1111/j.1755-3768.2008.01245.x
28. Lee HY, Lee SY, Park JS. Comparison of photocoagulation with combined intravitreal triamcinolone for diabetic macular edema. Korean J Ophthalmol KJO. (2009) 23:153–8. doi: 10.3341/kjo.2009.23.3.153
29. Li S, Miao L, Chen H, Liu S. Efficacy and safety of combination treatment with triamcinolone acetonide retrobulbar inj ection and panretinal photocoagulation in diabetic macular edema. J Jilin Univ. (2014) 40(6):1289–92.
30. Maia OO Jr., Takahashi BS, Costa RA, Scott IU, Takahashi WY. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am J Ophthalmol. (2009) 147:291–7.e2. doi: 10.1016/j.ajo.2008.08.024
31. Marey HM, Ellakwa AF. Intravitreal bevacizumab alone or combined with triamcinolone acetonide as the primary treatment for diabetic macular edema. Clin Ophthalmol (Auckland NZ). (2011) 5:1011–6. doi: 10.2147/OPTH
32. Massin P, Audren F, Erginay A, Haouchine B, Bergmann JF, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: results of a prospective controlled trial. Invest Ophthalmol Visual Sci. (2004) 45:3463–.
33. Meyer J, Fry C, Turner A, Razavi H. Intravitreal dexamethasone versus bevacizumab in Aboriginal and Torres Strait Islander patients with diabetic macular oedema: The OASIS study (a randomised control trial). Clin Exp Ophthalmol. (2022) 50:522–33. doi: 10.1111/ceo.14079
34. Ockrim ZK, Sivaprasad S, Falk S, Roghani S, Bunce C, Gregor Z, et al. Intravitreal triamcinolone versus laser photocoagulation for persistent diabetic macular oedema. Br J Ophthalmol. (2008) 92:795–9. doi: 10.1136/bjo.2007.131771
35. Ogura Y, Shimura M, Iida T, Sakamoto T, Yoshimura N, Yamada M, et al. Phase II/III clinical trial of sub-tenon injection of triamcinolone acetonide (WP-0508ST) for diabetic macular edema. Ophthalmol J Int d'ophtalmol Int J Ophthalmol Z Fur Augenheilkunde. (2019) 241:161–9. doi: 10.1159/000492135
36. Ozsaygili C, Duru N. Comparison of intravitreal dexamethasone implant and aflibercept in patients with treatment-naive diabetic macular edema with serous retinal detachment. Retina. (2020) 40:1044–52. doi: 10.1097/IAE.0000000000002537
37. Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, et al. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. (2007) 27:1187–95. doi: 10.1097/IAE.0b013e31815ec261
38. Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. (2009) 116:1142–50. doi: 10.1016/j.ophtha.2009.01.011
39. Stefansson E, Loftsson T, Larsen M, Papp A, Kaarniranta K, Munk MR, et al. Topical treatment of diabetic macular edema using dexamethasone ophthalmic suspension: A randomized, double-masked, vehicle-controlled study. Acta Ophthalmol. (2023) 101:22–33. doi: 10.1111/aos.15215
40. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. (2004) 111:2044–9. doi: 10.1016/j.ophtha.2004.05.025
41. Wei W, Chen Y, Hu B, Zhao M, Han M, Dai H, et al. Multicenter, prospective, randomized study of dexamethasone intravitreal implant in patients with center-involved diabetic macular edema in the asia-pacific region. Clin Ophthalmol (Auckland NZ). (2021) 15:4097–108. doi: 10.2147/OPTH.S325618
42. Yaseri M, Zeraati H, Mohammad K, Soheilian M, Ramezani A, Eslani M, et al. Intravitreal bevacizumab injection alone or combined with triamcinolone versus macular photocoagulation in bilateral diabetic macular edema; application of bivariate generalized linear mixed model with asymmetric random effects in a subgroup of a clinical trial. J Ophthalmic Vision Res. (2014) 9:453–60. doi: 10.4103/2008-322X.150818
43. Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefe's Arch Clin Exp Ophthalmol = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. (2008) 246:483–9. doi: 10.1007/s00417-007-0688-0
44. Anwar F, Khan AA, Majhu TM, Javaid RMM, Ghaffar MT, Bokhari MH. Comparison of suprachoroidal injection of triamcinolone acetonide versus intravitreal bevacizumab in primary diabetic macular odema. Pakistan J Med Health Sci. (2022) 16:304–. doi: 10.53350/pjmhs2216
45. Rongyu G, Jiandong L, Fangxing Z, Juanjuan Y, Enpei X, Xinyan X. Comparison of the efficacy of conbercept and intravitreal dexamethasone implant Ozurdex in the treatment of diabetic macular edema. Chin J Exp Ophthalmol. (2022) 40(7)658–63.
46. Norlaili M, Bakiah S, Zunaina E. Intravitreal triamcinolone versus laser photocoagulation as a primary treatment for diabetic macular oedema–a comparative pilot study. BMC Ophthalmol. (2011) 11:36. doi: 10.1186/1471-2415-11-36
47. Verma LK, Vivek MB, Kumar A, Tewari HK, Venkatesh P. A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocular Pharmacol Ther Off J Assoc Ocular Pharmacol Ther. (2004) 20:277–84. doi: 10.1089/1080768041725308
48. Network DRCR. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology. (2007) 114:1190–6. e3. doi: 10.1016/j.ophtha.2007.02.010
49. Zhu Y, Li J, Yu S, Mao B, Ying J. Clinical comparative study of intravitreal injection of triamcinolone acetonide and aflibercept in the treatment of diabetic retinopathy cystoid macular edema. Emergency Med Int. (2022) 2022:1348855. doi: 10.1155/2022/1348855
50. Liu X, Zhou X, Wang Z, Li T, Jiang B. Intravitreal bevacizumab with or without triamcinolone acetonide for diabetic macular edema: a meta-analysis of randomized controlled trials. Chin Med J. (2014) 127:3471–6.
51. Goyal S, Lavalley M, Subramanian ML. Meta-analysis and review on the effect of bevacizumab in diabetic macular edema. Graefe's Arch Clin Exp Ophthalmol = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. (2011) 249:15–27. doi: 10.1007/s00417-010-1452-4
52. Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retinal Eye Res. (2013) 34:19–48. doi: 10.1016/j.preteyeres.2013.02.001
53. Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: Beyond the surface. Prog Retinal Eye Res. (2018) 63:20–68. doi: 10.1016/j.preteyeres.2017.10.006
54. Miller JW. VEGF: from discovery to therapy: the champalimaud award lecture. Trans Vision Sci Technol. (2016) 5:9. doi: 10.1167/tvst.5.2.9
55. Ehlers JP, Yeh S, Maguire MG, Smith JR, Mruthyunjaya P, Jain N, et al. Intravitreal pharmacotherapies for diabetic macular edema: A report by the american academy of ophthalmology. Ophthalmology. (2022) 129:88–99. doi: 10.1016/j.ophtha.2021.07.009
56. Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. (1997) 57:4593–9.
57. Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Survey Ophthalmol. (2007) 52:503–22. doi: 10.1016/j.survophthal.2007.06.004
58. Sommer A, Veraart J, Neumann M, Kessels A. Evaluation of the vasoconstrictive effects of topical steroids by laser-Doppler-perfusion-imaging. Acta Dermato-venereol. (1998) 78:15–8. doi: 10.1080/00015559850135751
59. He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. (2018) 18:121. doi: 10.1186/s12886-018-0779-1
60. Rittiphairoj T, Mir TA, Li T, Virgili G. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. (2020) 11:Cd005656. doi: 10.1002/14651858.CD005656.pub3
61. Rose MA, Vukicevic M, Koklanis K, Rees G, Sandhu S, Itsiopoulos C. Experiences and perceptions of patients undergoing treatment and quality of life impact of diabetic macular edema: a systematic review. Psychol Health Med. (2019) 24:383–401. doi: 10.1080/13548506.2018.1533249
62. Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Visual Sci. (2011) 52:80–6. doi: 10.1167/iovs.10-5285
63. Hu K, Hui Y, Du H. Research progress of dexamethasone intravitreal implant in the treatment of diabetes Macular edema. Int Eye Sci. (2022) 22:1992–6.
64. Namba R, Kaneko H, Suzumura A, Shimizu H, Kataoka K, Takayama K, et al. In vitro epiretinal membrane model and antibody permeability: relationship with anti-VEGF resistance in diabetic macular edema. Invest Ophthalmol Visual Sci. (2019) 60:2942–9. doi: 10.1167/iovs.19-26788
Keywords: diabetic macular edema, network meta-analysis, anti-vascular endothelial growth factor, triamcinolone acetonide, dexamethasone, best corrected visual acuity, central macular thickness
Citation: Cheng Z and Liu X (2024) Comparing the efficacy of glucocorticoids and anti-VEGF in treating diabetic macular edema: systematic review and comprehensive analysis. Front. Endocrinol. 15:1342530. doi: 10.3389/fendo.2024.1342530
Received: 22 November 2023; Accepted: 07 March 2024;
Published: 22 March 2024.
Edited by:
Lei Ye, University of Alabama at Birmingham, United StatesCopyright © 2024 Cheng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyong Liu, am5keGx4eUAxNjMuY29t
 Zhi’ang Cheng
Zhi’ang Cheng Xiaoyong Liu1,2*
Xiaoyong Liu1,2*