
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 07 February 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1338420
This article is part of the Research TopicNew Insights Into Prostate Cancer: New Biomarkers, Molecular Mechanisms, and Therapeutic ApproachesView all 12 articles
A correction has been applied to this article in:
Corrigendum: Pretreatment level of serum sialic acid predicts both qualitative and quantitative bone metastases of prostate cancer
 Jingtao Sun1†
Jingtao Sun1† Tian Tian2†
Tian Tian2† Naiqiang Wang1
Naiqiang Wang1 Xuehui Jing1,3
Xuehui Jing1,3 Laiyuan Qiu1
Laiyuan Qiu1 Haochen Cui1
Haochen Cui1 Zhao Liu1
Zhao Liu1 Jikai Liu1
Jikai Liu1 Lei Yan1
Lei Yan1 Dawei Li1*
Dawei Li1*Background: Recently, serum sialic acid (SA) has emerged as a distinct prognostic marker for prostate cancer (PCa) and bone metastases, warranting differential treatment and prognosis for low-volume (LVD) and high-volume disease (HVD). In clinical settings, evaluating bone metastases can prove advantageous.
Objectives: We aimed to establish the correlation between SA and both bone metastasis and HVD in newly diagnosed PCa patients.
Methods: We conducted a retrospective analysis of 1202 patients who received a new diagnosis of PCa between November 2014 and February 2021. We compared pretreatment SA levels across multiple groups and investigated the associations between SA levels and the clinical parameters of patients. Additionally, we compared the differences between HVD and LVD. We utilized several statistical methods, including the non-parametric Mann-Whitney U test, Spearman correlation, receiver operating characteristic (ROC) curve analysis, and logistic regression.
Results: The results indicate that SA may serve as a predictor of bone metastasis in patients with HVD. ROC curve analysis revealed a cut-off value of 56.15 mg/dL with an area under the curve of 0.767 (95% CI: 0.703-0.832, P < 0.001) for bone metastasis versus without bone metastasis and a cut-off value of 65.80 mg/dL with an area under the curve of 0.766 (95% CI: 0.644-0.888, P = 0.003) for HVD versus LVD. Notably, PCa patients with bone metastases exhibited significantly higher SA levels than those without bone metastases, and HVD patients had higher SA levels than LVD patients. In comparison to the non-metastatic and LVD cohorts, the cohort with HVD exhibited higher levels of alkaline phosphatase (AKP) (median, 122.00 U/L), fibrinogen (FIB) (median, 3.63 g/L), and prostate-specific antigen (PSA) (median, 215.70 ng/mL), as well as higher Gleason scores (> 7). Multivariate logistic regression analysis demonstrated that an SA level of > 56.15 mg/dL was independently associated with the presence of bone metastases in PCa patients (OR = 2.966, P = 0.018), while an SA level of > 65.80 mg/dL was independently associated with HVD (OR = 1.194, P = 0.048).
Conclusion: The pretreatment serum SA level is positively correlated with the presence of bone metastases.
Prostate cancer (PCa) is a prevalent malignancy that originates from the male reproductive system. Despite a lower incidence rate among Asians compared to Americans and Europeans (1), the mortality and morbidity rates associated with PCa are on the rise in China due to improvements in living standards, lifestyle changes, and advancements in screening methods (2, 3). The use of prostate-specific antigen (PSA) for population screening, diagnosis, and monitoring of PCa has been a subject of controversy since its purification (4). Although PSA screening has some benefits, such as early detection of PCa, it also has several drawbacks, such as high costs, long waiting times, limited sensitivity, and low specificity. Current evidence suggests that the overall public health impact of PSA screening is not significantly greater than its benefits (5–7). Various treatment options are available for patients diagnosed with early-stage PCa, including surgical removal, chemotherapy, and castration therapy (8). Metastasis is the leading cause of death in PCa, with lymph nodes near the primary tumor being the initial sites of metastasis, followed by bone metastases (9). Currently, there exists no efficacious treatment for patients afflicted with bone metastases (10, 11). Multiple studies have demonstrated that individuals with bone metastases stemming from PCa have a poorer prognosis and a reduced quality of life (12). Consequently, the timely identification of bone metastases in PCa patients is of paramount importance. Although a bone scan is frequently employed for early detection, its specificity is limited, resulting in a high incidence of false-positive results. Given a thorough comprehension of PCa, emission computerized tomography (ECT) is regarded as one of the most effective techniques for detecting bone metastases in patients at present (13–15). Despite the expenses and radioactivity associated with the procedure, it is deemed a valuable investment. Thus, there is an urgent need for novel biomarkers to aid in the identification and long-term monitoring of bone metastases in PCa patients.
Acetylneuraminic acid (sialic acid [SA]), a nine-carbon monosaccharide initially reported in 1957, may serve as a potential candidate. The termination of glycoprotein side chains and glycolipid side chains, which are fundamental constituents of cellular membranes, occurs at this location (16). Furthermore, this agent not only provides cytoprotection but also safeguards cell membranes (17). Multiple studies have established a correlation between elevated levels of SA and cancer, as evidenced in patients (18) with ovarian cancer (19), breast cancer (20), oral cancer (21), and neck cancer (22). Additionally, certain chemical agents have been shown to enhance disease invasion by altering SA levels or impeding SA production, as demonstrated in some studies (23, 24). A noteworthy correlation has been established between SA levels and PCa (25, 26). Notably, patients with PCa who exhibit elevated SA levels are more prone to exhibit heightened levels of PSA, lactate dehydrogenase (LDH), and α-L-fucosidase (AFU), a higher Gleason score, and a higher metastasis incidence rate (26). At present, the primary treatment modalities for PCa include androgen deprivation therapy, radiation therapy, ablative therapy, chemotherapy, and emerging immunotherapies. In clinical settings, it may be necessary to employ multiple approaches simultaneously (27–29). However, this does not diminish the significance of thoroughly assessing the metastatic load of patients prior to treatment in order to determine appropriate treatment options and prognoses (30, 31). Currently, investigations into the correlation between pretreatment SA levels and the metastatic burden of PCa are limited. Therefore, our study was designed to examine this potential association through retrospective data analysis.
This retrospective analysis was performed on 1202 PCa patients diagnosed by prostate biopsy in our hospital’s Urology Department between January 2014 and January 2021. The inclusion criteria encompassed the following: (I) Absence of any prior cancer diagnosis; (II) Absence of hematologic disorders to prevent potential confounding of hematologic markers; (III) Availability of comprehensive postoperative pathology findings; (IV) Availability of comprehensive clinical data; (V) Diagnosed with PCa. Our study excluded patients with any one or more of the following conditions: (I) Patients with hematological diseases (Individuals diagnosed with hematological disorders, or prescribed medications known to potentially alter blood markers), known infections, and other malignancies; (II) Patients who had undergone prostate surgery (such as transurethral resection) before their biopsies; (III) Pathologically diagnosed patients with prostatic intraepithelial neoplasms and atypical small acinar proliferations; (IV) Patients with incomplete clinical data. Following that, we collected the following information from the medical records of eligible patients: age at the time of diagnosis, AKP, LDH, AFU, FIB, and PSA levels, pathological characteristics (including pathological grade and stage), and ECT results. The data screening process of the study is shown in Figure 1. Our study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (KYLL-202208-044-1).
Data were obtained from the database of Qilu Hospital of Shangdong University, including age at the time of diagnosis, AKP, LDH, AFU, FIB, and PSA levels, pathological characteristics (including pathological grade and stage), and ECT results. Pathological grades were evaluated using the Gleason system and pathological stages were evaluated according to TNM 2021. Assessment of bone metastasis was performed using ECT, where a positive test result indicated the presence of metastatic lesions. When an isolated condensed radionuclide spot was observed, this was considered as one metastatic lesion.
Five milliliters of venous blood was drawn from each patient after they had fasted for 12 h in the early morning before any clinical intervention was given. During the experiment, test tubes containing a clot activator and gel were used to store blood samples, which coagulated naturally at room temperature. The samples were centrifuged for 10 min at 2000 rpm. After the serum was obtained, the AKP, LDH, AFU, and SA concentrations were determined using a Roche Cobas 8000 automatic analyzer. The normal concentration range for AKP was 45.00 to 125.00 U/L; the normal concentration range for LDH was 120.00 U/L to 230.00 U/L; the normal concentration range for AFU was < 40.00 U/L; and the normal concentration range for SA was 45.60 to 75.40 mg/dL. Before the prostate biopsy, plasma PSA levels and FIB concentrations were determined as well. To measure PSA and FIB levels, blood samples (about 3.50 mL) were collected. Samples were collected in plastic tubes with coagulant/separating glue for PSA analysis and in plastic tubes containing 3.80% sodium citrate for FIB detection. The normal concentration range for FIB was 2.00 to 4.00 g/L; the normal concentration range for PSA was < 4.00 ng/mL.
In total, 1202 cases of PCa were enrolled. Based on frequency analysis, AKP, LDH, AFU, FIB, and PSA did not have a normal distribution, so medians (percentiles) were used instead. To determine whether SA followed a normal distribution, the Kolmogorov-Smirnov test was employed on the means and standard deviations (SDs) in each group. Normally distributed data were analyzed using Student’s t test. Non-normally distributed data were analyzed using the Mann-Whitney U test or the Kruskal-Wallis H test. The correlation between two parameters was analyzed using the Spearman method. Categorical variables are presented as numbers (percentages), and the Chi-square test was used to compare the groups. The associations of different parameters with the bone metastasis status and HVD were evaluated by univariate and multivariate logistic regression analysis. To establish SA cut-off values, a receiver operating characteristic (ROC) curve was plotted and the resulting area under the curve (AUC) was calculated. The value with the largest Youden index (calculated as (sensitivity + specificity) + 1) was defined as the optimal cut-off value. Differences were considered to be statistically significant if P < 0.05. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 19.0 (SPSS, Inc., Chicago, IL, USA). The dynamic nomogram is introduced as a model, wherein the summation of individual patient scores on predictive factors enables the computation of their total score and subsequent determination of their risk for the outcome event. Variables exhibiting p values of 0.05 or lower in the logistic regression analysis were incorporated into the nomogram. Calibration curves and receiver operating characteristic (ROC) analysis were utilized to assess the predictive capabilities of the model. Additionally, decision curve analysis (DCA) was employed to evaluate the net benefits of clinical interventions guided by the model (R vision 4.1.3).
The characteristics of the patients are presented in Table 1. The location and quantity of bone metastases in PCa patients were assessed through ECT scanning as reported previously (32). The total number of patients diagnosed with bone metastases was 64.
The median SA level in the cohort with bone metastases was found to be 62.70 mg/dL, which was observed to be significantly higher than in the non-bone metastasis cohort (53.75 mg/dl) (P < 0.001) (Figure 2A). Further examination of the HVD and LVD groups indicated that patients with HVD exhibited elevated SA levels in comparison to patients with LVD (65.70 mg/dL) (P = 0.003) (Figure 2B).
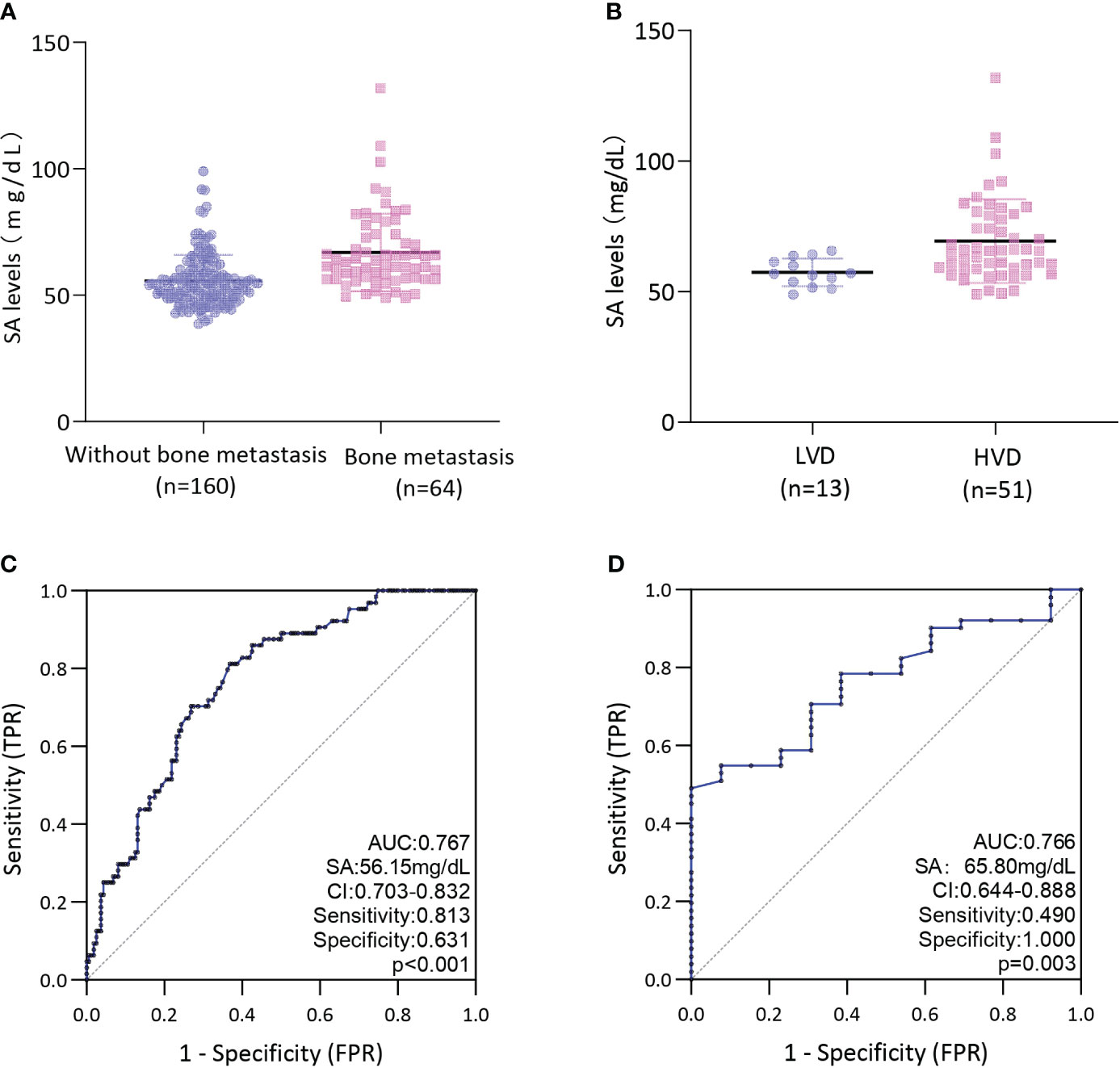
Figure 2 (A) Comparison of SA levels between bone metastases and without bone metastases in PCa patients; (B) Comparison of SA levels between LVD and HVD in PCa patients with bone metastases; (C) ROC curves for determination of cut-off value of SA levels regarding prediction of bone metastases in PCa patients; (D) ROC curves for determination of cut-off value of SA levels regarding prediction of HVD in PCa patients; HVD, high volume disease; LVD, low volume disease.
ROC curve analysis was conducted to determine the predictive value of pretreatment SA levels for bone metastases. As depicted in Figure 2C, a cut-off level of 56.15 mg/dL SA was obtained, with SA levels above this threshold being associated with a higher risk of bone metastases (AUC = 0.767; 95% CI: 0.703-0.832; P < 0.001; sensitivity: 0.813, specificity:0.631). Our assessment of the ROC curve derived from SA levels of patients with LVD or HVD yielded an AUC of 0.766 (95% CI: 0.644-0.888), with an optimal threshold value of 65.80 mg/dL (sensitivity: 0.490, specificity:1.000) (Figure 2D).
As shown in Table 2, subjects in the present study were also divided into two groups based on SA levels (≤ 56.15 mg/dL vs. > 56.15 mg/dL). A significant association was found between pretreatment SA levels and AKP, LDH, FIB, and PSA levels, Gleason score, pT stage, and bone metastasis (all P < 0.050). Moreover, we divided the databases into two categories based on their SA levels (≤ 65.80 mg/dL vs. > 65.80 mg/dL) (Table 3). In addition to AKP, LDH, FIB, and PSA levels, Gleason score, pT stage, and HVD, pretreatment SA levels were significantly associated with these parameters (all P < 0.050).
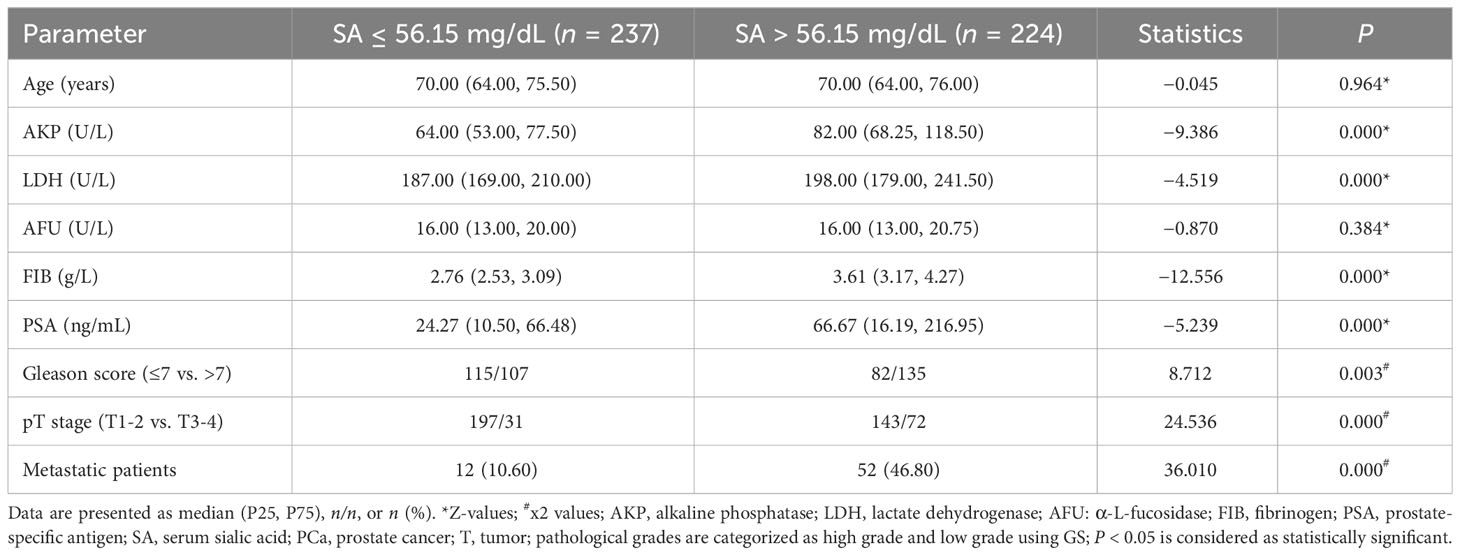
Table 2 Comparison of clinical parameters between groups presenting distinct ranges of SA levels (≤56.15 mg/dL vs. >56.15 mg/dL).
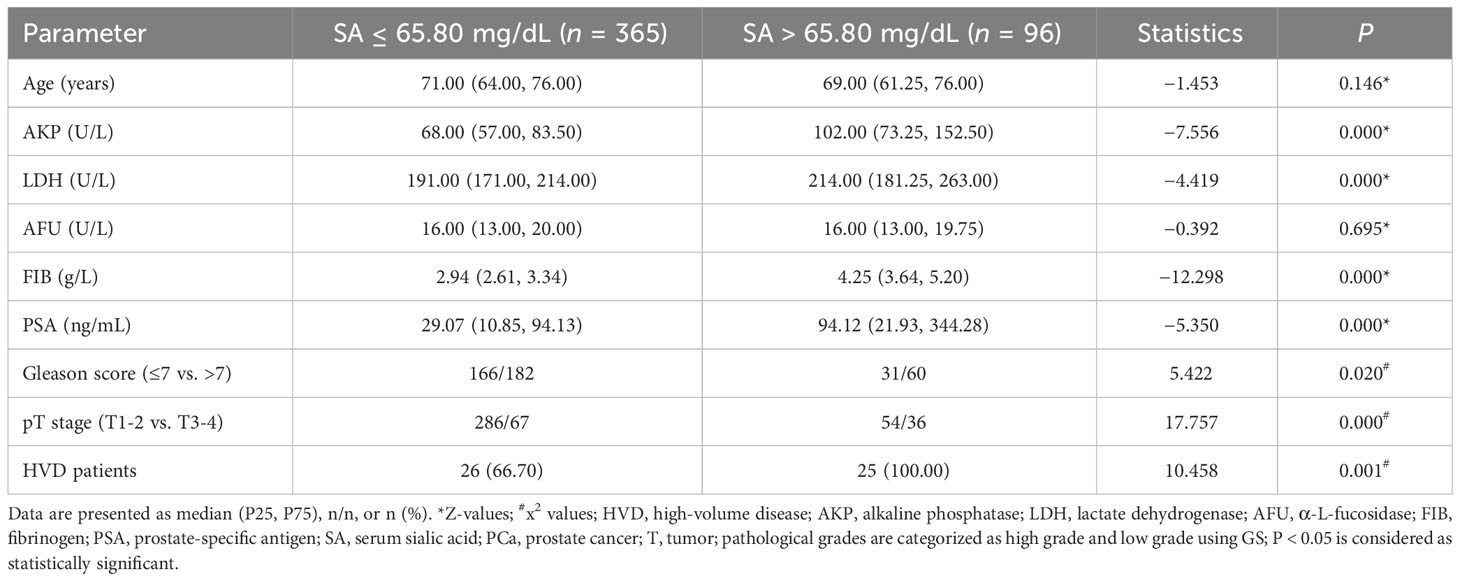
Table 3 Comparison of clinical parameters between groups presenting distinct ranges of SA levels (≤65.80 mg/dL vs. >65.80 mg/dL).
Data were missing for three PCa patients without bone metastases. To determine the parameters that differ between the three groups, we excluded patients with missing data. The first group had no bone metastasis, the second group had LVD, and the third group had HVD. P1 denotes the outcome of comparing the group without bone metastasis to the LVD group, whereas P2 represents the result of comparing the group without bone metastasis to the HVD group. Lastly, P3 signifies the outcome of comparing the LVD group to the HVD group. The three groups are compared in Table 4. Between any two groups, there was no statistically significant difference in age and AFU levels. In addition, there were statistically significant differences in PSA and AKP levels between any two groups. The HVD group had higher FIB levels compared with the non-metastatic and LVD groups (P < 0.050). The HVD group had higher Gleason scores compared with the non-metastatic (P = 0.000) and LVD groups (P = 0.001). There were no statistically significant differences in FIB levels (P = 0.417), and Gleason scores (P = 0.340) between the group without metastatic disease and LVD patients. When the LVD group was compared to non-metastatic patients (P = 0.143) and the HVD group (P = 0.409), there was no statistically significant difference in LDH levels. There was, however, a statistically significant difference in LDH levels between the patients without metastatic disease and the HVD group (P = 0.000).
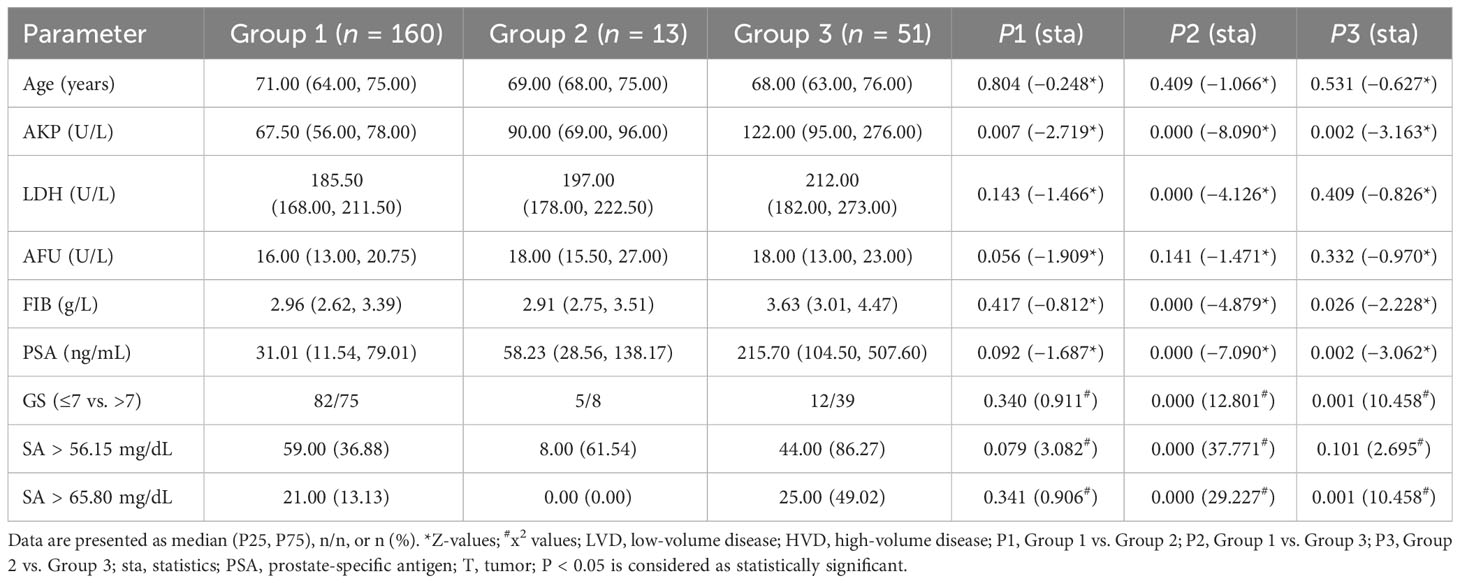
Table 4 Comparison of clinical parameters among non-metastatic (Group 1), LVD (Group 2), and HVD group (Group 3).
To further examine the clinical effects of pretreatment SA levels in the diagnosis of bone metastases and HVD, a logistic regression analysis was conducted. The final model selection was made using a backward stepdown selection process. As shown in Table 5, elevated SA levels significantly predicted poor diagnostic outcomes of bone metastases in the univariate and multivariate analysis (P < 0.050). In addition, elevated AKP levels, high PSA levels, and high Gleason score were significantly associated with the diagnosis of bone metastases based on univariate and multivariate analysis (AKP: univariate analysis: HR = 1.034, P = 0.000 and multivariate analysis: HR = 1.029, P = 0.000; PSA: univariate analysis: HR = 1.007, P = 0.000 and multivariate analysis: HR = 1.005, P = 0.000; Gleason score: univariate analysis: HR = 3.023, P = 0.001 and multivariate analysis: HR = 4.372, P = 0.003) (Table 5). Interestingly, in HVD patients, elevated SA levels were significantly predicted. Both univariate and multivariate logistic regressions showed that elevated SA levels, PSA levels and high Gleason score are independently associated with HVD patients (all P < 0.050) (Table 6). However, HVD was not significantly correlated with AKP based on univariate and multivariate logistic regressions (Table 6).
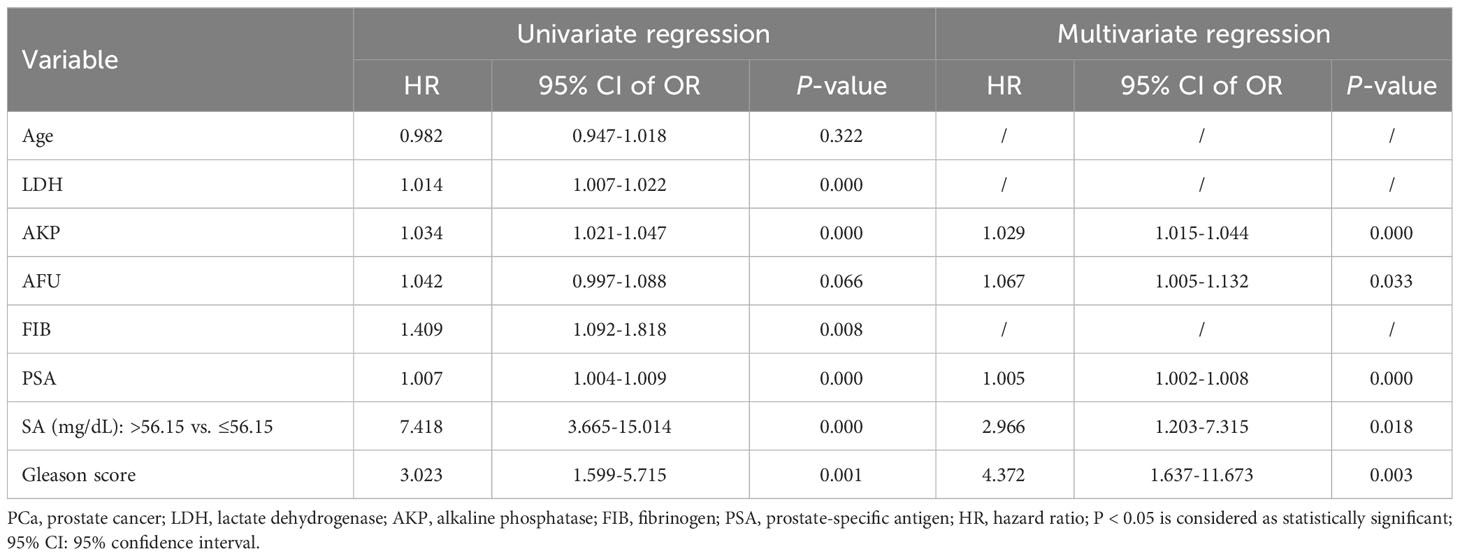
Table 5 Univariate and multivariate logistic regression analysis of selected parameters in PCa and its bone metastases.
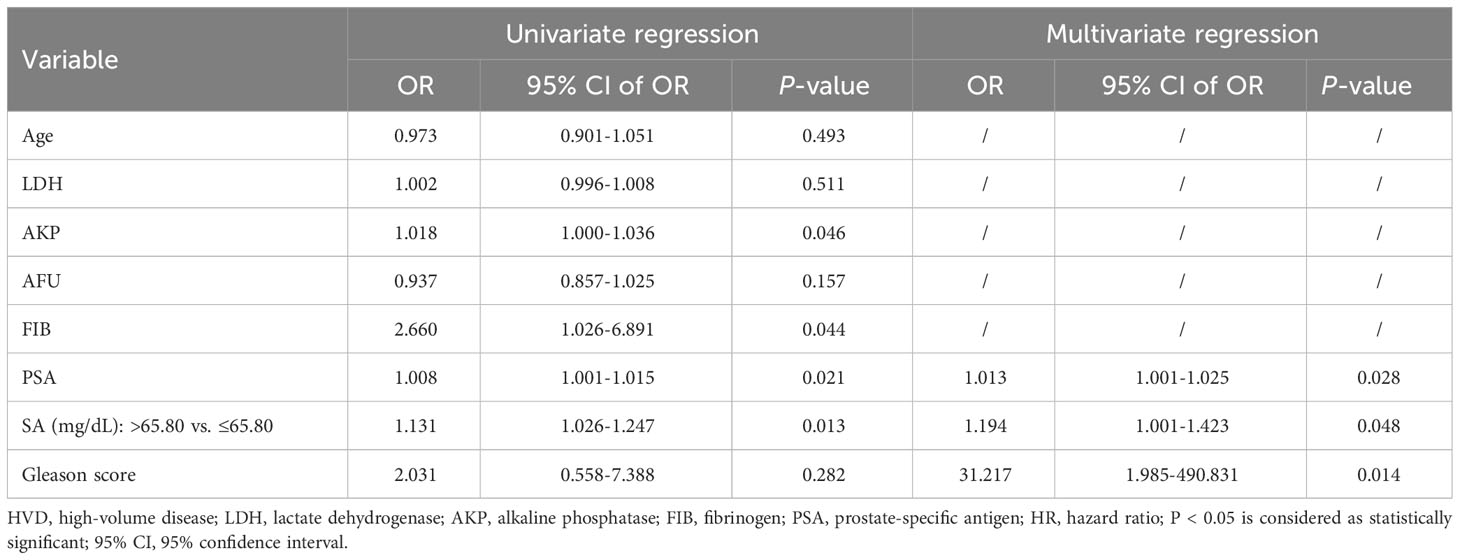
Table 6 Univariate and multivariate logistic regression analysis of selected parameters in LVD patients and HVD patients.
As shown in Figure 3, all significant factors for bone metastasis (Figure 3A) and HVD (Figure 3B) occurrence were integrated into the nomogram for predicting the probabilities of bone metastasis and HVD. Among them, age, SA levels, and AKP levels were divided according to their cut-off values. The ROC curve demonstrated good discrimination in predicting PCa (Figure 4A) and in predicting HVD (Figure 4D). In addition, the DCA curves and calibration curves indicated good agreement between the predicted and observed probabilities of bone metastasis (Figures 4B, C) and HVD (Figures 4E, F).
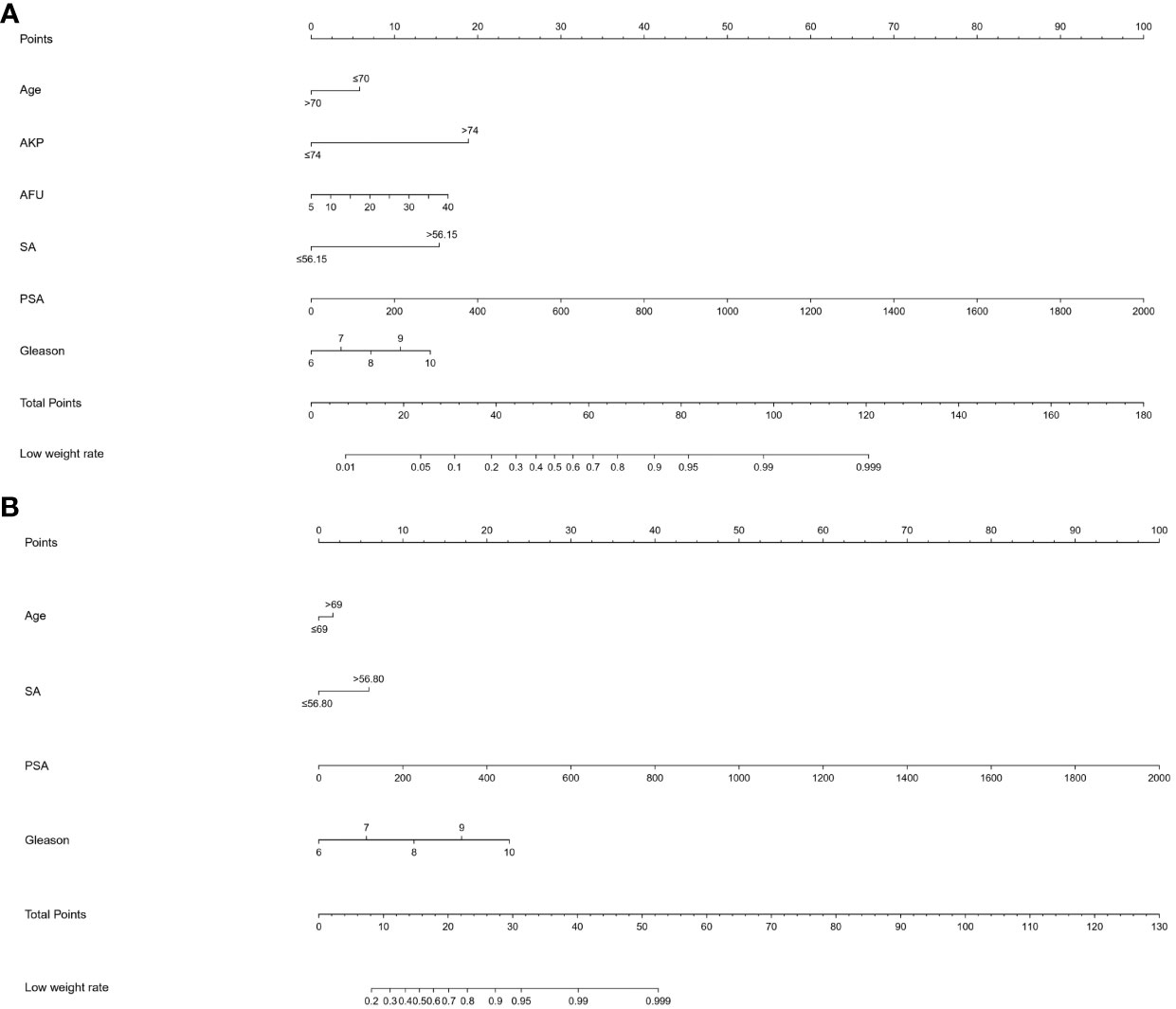
Figure 3 (A) A nomogram for predicting bone metastasis of PCa patients. (B) A nomogram for predicting HVD patients. The nomogram is used by summing all points identified on the scale for each variable. The total points projected on the bottom scales indicate the probabilities of bone metastasis and HVD. PCa, prostate cancer.

Figure 4 ROC curve (A), DCA curve (B) and calibration curve (C) for assessing the discrimination and calibration of the nomogram in predicting the probabilities of bone metastasis. ROC curve (D), DCA curve (E) and calibration curve (F) for assessing the discrimination and calibration of the nomogram in predicting the probabilities of HVD.
PCa is a prevalent malignancy among men and is a significant contributor to mortality (33). PCa ranks as the third most frequently detected cancer in males, with an estimated 1.4 million global diagnoses projected for 2021, and it stands as the fifth primary contributor to cancer-related mortality. Various risk factors, encompassing genetic predisposition, advanced age, ethnic background, elevated testosterone levels, and lifestyle choices, significantly influence the initiation of PCa. Moreover, the incidence of PCa exhibits notable variation across diverse geographical regions, with developed and industrialized nations demonstrating a heightened prevalence (34, 35). Furthermore, research suggests that the incidence of PCa is positively correlated with advancing age among patients (34, 36). The overall incidence rate stands at 31 cases per 100,000 males across all age groups, while the lifetime cumulative risk is estimated at 3.9%. Notably, the prevalence of PCa among men aged 75 and above exceeds one in four individuals (37, 38). While the majority of cases are indolent and do not result in death, a substantial number of cases present with intermediate- or high-risk localized, locally advanced, or metastatic cancer despite treatment (39). The primary cause of PCa-related mortality is metastatic disease, which occurs when PCa cells proliferate beyond the prostate and disseminate to other organs, particularly the lungs, liver, bones, and lymph nodes (40). The likelihood of bone metastasis is significantly higher in patients with PCa who present with both osteoblastic and osteolytic lesions (41). The treatment of PCa has become increasingly diverse due to the wide range of available techniques (42). Local therapy is advantageous for many individuals, and patients who undergo radical prostatectomy have a better prognosis than those who receive radiation therapy (43). Presently, androgen deprivation therapy is the standard treatment for metastatic hormone-sensitive PCa. Furthermore, the combination of second-generation antiandrogens has been shown to increase the survival rate of men with metastatic castration-resistant PCa (M0 CRPC) (44, 45). However, aggressive treatment methods for PCa can result in significant adverse effects on continence and erectile function (46, 47). Therefore, it is imperative to accurately determine the clinical type and stage of PCa prior to initiating treatment.
Notably, clinically significant disparities between HVD and LVD have been observed in PCa, with LVD often being characterized as oligo-metastatic based on numerous studies (32, 48). Numerous clinical trials have established a correlation between (49–51). Furthermore, a consensus has yet to be reached on the definitions of HVD and LVD. Notably, both CHARTED and LATITUDE scores, which share many similarities, have been identified as significant predictors of survival. Consequently, we have opted to differentiate between HVD and LVD based on the CHARTED experiment’s definition (52).
Research has indicated that SA molecules are integral to the process of cancer metastasis. The progression of malignant tumors towards migration, invasion, and metastasis involves alterations in the levels of numerous intracellular and extracellular proteins, as well as modifications to normal cellular behavior, including extracellular matrix degradation, reduced cell adhesion, heightened cell motility, and augmented local microvessel formation, ultimately culminating in metastasis.
Prior research has demonstrated the significant involvement of adhesive glycoproteins in tumor metastasis, whereby the upregulation of 2-6-sialyltransferase and fibronectin expression influences the migratory capacity of mouse liver cancer cells (53). Furthermore, SA has been identified as a mediator of tumor cell adhesion to platelets, white blood cells, and vascular endothelial cells, while also reducing cell adhesion to collagen IV (a constituent of the basement membrane) and intercellular adhesion (54). Moreover, it has been demonstrated that the capacity to interact with nerve cell adhesion molecules can be augmented, thereby promoting the invasion, migration, and metastasis of neoplastic cells (55).
Despite the currently available antitumor pharmacological regimens, bone metastases originating from PCa have yet to be effectively managed. In comparison to antiangiogenic agents and matrix metalloproteinase inhibitors, pharmacotherapy targeting aberrant SA receptors may prove to be a more efficacious strategy in the future for combating tumor metastasis. AL10, a novel sialyltransferase inhibitor, has been shown to impede the adhesion, migration, actin expression, and invasion of malignant cells (56). Furthermore, SA, a molecule exhibiting a strong attraction towards anticancer drugs, presents a promising avenue for the advancement of antitumor therapy. Notably, highly metastatic tumor cells express certain adhesion molecules, including CD22 (57), and exhibit a heightened capacity for binding with SA. Consequently, anticancer drugs bound to SA can accumulate on the surface of tumor or metastatic cells, thereby augmenting the efficacy of the drugs. The development of such drugs has emerged as a prominent area of research.
The present study investigated the levels of SA in 461 patients prior to treatment, in conjunction with other pertinent indices and clinical characteristics. Furthermore, ROC curves were generated to predict bone metastases and HVD in PCa patients based on preoperative SA levels. The optimal diagnostic cut-off value for discriminating between patients with and without bone metastases was determined to be 56.15 mg/dL, while the optimal diagnostic cut-off for distinguishing between LVD and HVD was 65.80 mg/dL. We also presented AUC, sensitivity, and specificity as measures of accuracy. Our findings indicate that an elevated level of SA exhibits high sensitivity and specificity in the diagnosis of bone metastases in patients with PCa. Notably, pretreatment SA levels were significantly higher in patients with HVD compared to non-metastatic patients and patients with LVD. Multivariate regression analysis revealed that SA is independently associated with HVD. Therefore, elevated SA levels may serve as a valuable diagnostic tool for identifying osteometastases and HVD in PCa patients.
In this study, an examination was conducted on pretreatment SA levels in a cohort of 461 patients, alongside other pertinent assay indices and clinical characteristics. Consistent with previous research by Goswami K (58) and Cong Zhang (26) et al., our findings indicate a significant elevation in SA levels among patients with bone metastasis compared to those without, as well as higher SA levels in patients with HVD compared to those with LVD. Subsequently, patients diagnosed with PCa were subsequently stratified based on age, AKP, LDH, FIB, and PSA levels. The findings revealed a strong association between SA levels and LDH, AKP, FIB, and PSA. These results align with the conclusions drawn by Crook MA et al. (59), as no statistically significant correlation was observed between SA levels and age. Furthermore, a notable correlation was observed between SA levels and pathological grade, whereby higher pathological grades were accompanied by elevated SA levels. The patients were categorized into three groups based on the results of ECT: those without bone metastases, those with LVD, and those with HVD. The levels of SA exhibited a gradual increase across the three groups, with SA levels being lowest in the group without bone metastases, followed by the LVD group, and highest in the HVD group. ROC curves were then generated to assess the predictive value of preoperative SA levels for bone metastases and HVD. The optimal diagnostic cut-off points, determined by the maximum Younden index (60), were found to be > 56.15 mg/dL for distinguishing between bone metastases and the absence of bone metastases, and > 65.80 mg/dL for distinguishing between HVD and LVD. The picture also displayed the AUC, sensitivity, and specificity values for determining accuracy. It was evident that elevated SA levels exhibited high sensitivity and specificity in diagnosing bone metastases and HVD in PCa patients. Furthermore, SA levels were included in both univariate and multivariate analyses, which revealed their significance in diagnosing bone metastases and HVD. Consequently, elevated SA levels hold promise in the diagnosis of bone metastases and HVD in PCa patients.
Notwithstanding, there exist several constraints that necessitate consideration in this study. First, a retrospective study was conducted in a single location, which may have led to a statistically significant degree of selection bias. Second, despite the recognition of ECT as the “golden standard” for identifying skeletal metastases, this technique may still yield an erroneous diagnosis for patients with PCa. Third, despite the implementation of rigorous enrollment criteria, it was not feasible to entirely eliminate conditions that could impact plasma SA levels, such as varicose veins in the lower extremities and atherosclerosis. Finally, certain HVD patients may have had undetected visceral metastases due to inadequate assessment. To validate these findings, it will be imperative to conduct multicenter prospective studies with larger sample sizes.
In summary, pretreatment plasma SA levels exhibit a positive correlation with the number of bone metastases and are independently linked to HVD. These results suggest that SA levels in the bloodstream may serve as a potential marker for more accurate diagnosis and treatment of advanced PCa.
The present study established a correlation between SA levels and the clinicopathological characteristics of patients with PCa and HVD. Elevated SA levels prior to surgery could serve as an indicator of increased malignancy risk and advanced cancer stages. The findings of this study suggested that SA levels could serve as a valuable screening and prognostic marker for PCa and HVD.
JS: Data curation, Software, Writing – original draft. TT: Data curation, Writing – review & editing. NW: Software, Writing – original draft. XJ: Software, Writing – original draft. LQ: Writing – review & editing. HC: Methodology, Writing – review & editing. ZL: Methodology, Writing – review & editing. JL: Methodology, Writing – review & editing. LY: Data curation, Writing – review & editing. DL: Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partly supported by financial grants from the National Nature Science Foundation of China (grant Nos: 82172743, 81502213 and 82102999), the Natural Science Foundation of Shandong Province (grant Nos: ZR2020QH068 and ZR2021MH029).
The authors thank Cong Zhang (Fudan University, China) for the critical reading of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bernard B, Muralidhar V, Chen Y-H, Sridhar SS, Mitchell EP, Pettaway CA, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer (2017) 123:1536–44. doi: 10.1002/cncr.30503
2. Prostate Cancer Working Group of China Anti-Cancer A, Genitourinary Cancer CExpert consensus on genetic testing in Chinese prostate cancer patientedition). China Oncol (2018) 28:627–33.
3. Huang Q, Zi H, Luo L, Li X, Zhu C, Zeng X. Secular trends of morbidity and mortality of prostate, bladder, and kidney cancers in Chinto 2019 and their predictions to 2030. BMC Cancer (2022) 22. doi: 10.1186/s12885-022-10244-9
4. Chung J-W, Kim HT, Ha Y-S, Lee EH, Chun SY, Lee C-H, et al. Identification of a novel non-invasive biological marker to overcome the shortcomings of PSA in diagnosis and risk stratification for prostate cancer: Initial prospective study of developmental endothelial locus-1 protein. PloS One (2021) 16. doi: 10.1371/journal.pone.0250254
5. Scott E, Munkley J. Glycans as biomarkers in prostate cancer. Int J Mol Sci (2019) 20. doi: 10.3390/ijms20061389
6. Boerrigter E, Groen LN, Van Erp NP, Verhaegh GW, Schalken JA. Clinical utility of emerging biomarkers in prostate cancer liquid biopsies. Expert Rev Mol Diagnostics (2020) 20:219–30. doi: 10.1080/14737159.2019.1675515
7. Wang J, Ni J, Beretov J, Thompson J, Graham P, Li Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit Rev Oncol Hematol (2020) 145. doi: 10.1016/j.critrevonc.2019.102860
8. Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol (2019) 75:88–99. doi: 10.1016/j.eururo.2018.03.028
9. Datta K, Muders M, Zhang H, Tindall DJ. Mechanism of lymph node metastasis in prostate cancer. Future Oncol (2010) 6:823–36. doi: 10.2217/fon.10.33
10. Norum J, Nieder C. Treatments for metastatic prostate cancer (mPC): A review of costing evidence. Pharmacoeconomics (2017) 35:1223–36. doi: 10.1007/s40273-017-0555-8
11. Body J-J, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol (2015) 12:340–56. doi: 10.1038/nrurol.2015.90
12. Mollica V, Rizzo A, Rosellini M, Marchetti A, Ricci AD, Cimadamore A, et al. Bone targeting agents in patients with metastatic prostate cancer: state of the art. Cancers (Basel) (2021) 13:546. doi: 10.3390/cancers13030546
13. Liu J, Dong Y, Xu D, Zhang C, Lan T, Chang D. Progress in diagnosis of bone metastasis of prostate cancer. Zhong nan da xue xue bao Yi xue ban = J Cent South Univ Med Sci (2021) 46:1147–52. doi: 10.11817/j.issn.1672-7347.2021.200999
14. Campana LG, Edhemovic I, Soden D, Perrone AM, Scarpa M, Campanacci L, et al. Electrochemotherapy - Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Ejso (2019) 45:92–102. doi: 10.1016/j.ejso.2018.11.023
15. Kaku H, Saika T, Tsushima T, Nagai A, Yokoyama T, Abarzua F, et al. Combination chemotherapy with estramustine phosphate, ifosfamide and cisplatin for hormone-refractory prostate cancer. Acta Med Okayama (2006) 60:43–9. doi: 10.18926/AMO/30759
16. Chittemsetti S, Manchikatla PK, Guttikonda V. Estimation of serum sialic acid in oral submucous fibrosis and oral squamous cell carcinoma. J Oral Maxillofac Pathol JOMFP (2019) 23:156–. doi: 10.4103/jomfp.JOMFP_239_18
17. Schauer R, Kamerling JP. Exploration of the sialic acid world. Adv Carbohydr Chem Biochem (2018) 75:1–213. doi: 10.1016/bs.accb.2018.09.001
18. Zhang Z, Wuhrer M, Holst S. Serum sialylation changes in cancer. Glycoconj J (2018) 35:139–60. doi: 10.1007/s10719-018-9820-0
19. Dedova T, Braicu EI, Sehouli J, Blanchard V. Sialic acid linkage analysis refines the diagnosis of ovarian cancer. Front Oncol (2019) 9. doi: 10.3389/fonc.2019.00261
20. Acikgoz E, Duzagac F, Guven U, Yigitturk G, Kose T, Oktem G. "Double hit" strategy: Removal of sialic acid from the dendritic cell surface and loading with CD44+/CD24-/low cell lysate inhibits tumor growth and metastasis by targeting breast cancer stem cells. Int Immunopharmacol (2022) 107. doi: 10.1016/j.intimp.2022.108684
21. Guruaribam VD, Sarumathi T. Relevance of serum and salivary sialic acid in oral cancer diagnostics. J Cancer Res Ther (2020) 16:401–4. doi: 10.4103/jcrt.JCRT_512_19
22. Mikkonen JJW, Singh SP, Akhi R, Salo T, Lappalainen R, Gonzalez-Arriagada WA, et al. Potential role of nuclear magnetic resonance spectroscopy to identify salivary metabolite alterations in patients with head and neck cancer. Oncol Lett (2018) 16:6795–800. doi: 10.3892/ol.2018.9419
23. Schmidt CQ, Ederveen ALH, Harder MJ, Wuhrer M, Stehle T, Blaum BS. Biophysical analysis of sialic acid recognition by the complement regulator Factor H. Glycobiology (2018) 28:765–73. doi: 10.1093/glycob/cwy061
24. Moons SJ, Adema GJ, Derks MTGM, Boltje TJ, Bull C. Sialic acid glycoengineering using N-acetylmannosamine and sialic acid analogs. Glycobiology (2019) 29:433–45. doi: 10.1093/glycob/cwz026
25. Pihikova D, Pakanova Z, Nemcovic M, Barath P, Belicky S, Bertok T, et al. Sweet characterisation of prostate specific antigen using electrochemical lectin-based immunosensor assay and MALDI TOF/TOF analysis: Focus on sialic acid. Proteomics (2016) 16:3085–95. doi: 10.1002/pmic.201500463
26. Zhang C, Yan L, Song H, Ma Z, Chen D, Yang F, et al. Elevated serum sialic acid levels predict prostate cancer as well as bone metastases. J Cancer (2019) 10:449–57. doi: 10.7150/jca.27700
27. Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer (2017) 24:T297–310. doi: 10.1530/ERC-17-0145
28. Evans AJ. Treatment effects in prostate cancer. Modern Pathol (2018) 31:110–21. doi: 10.1038/modpathol.2017.158
29. Achard V, Putora PM, Omlin A, Zilli T, Fischer S. Metastatic prostate cancer: treatment options. Oncology (2022) 100:48–59. doi: 10.1159/000519861
30. Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol (2018) 36:1080. doi: 10.1200/JCO.2017.75.3657
31. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (2018) 392:2353–66. doi: 10.1016/S0140-6736(18)32486-3
32. Xie G-S, Li G, Li Y, Pu J-X, Huang Y-H, Li J-H, et al. Clinical association between pre-treatment levels of plasma fibrinogen and bone metastatic burden in newly diagnosed prostate cancer patients. Chin Med J (2019) 132:2684–9. doi: 10.1097/CM9.0000000000000506
33. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules (2022) 27. doi: 10.3390/molecules27175730
34. Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol (2021) 4:877–92. doi: 10.1016/j.euo.2021.09.006
35. Bray F, Parkin DM, African Canc Registry N. Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol (2022) 23:719–28. doi: 10.1016/S1470-2045(22)00270-4
36. Perdana NR, Mochtar CA, Umbas R, Hamid ARA. The risk factors of prostate cancer and its prevention: A literature review. Acta Med Indonesiana (2016) 48:228–38.
37. Crocetto F, Barone B, D’Aguanno G, Falcone A, de Vivo R, Rienzo M, et al. Vitamin D, a regulator of androgen levels, is not correlated to PSA serum levels in a cohort of the middle Italy region participating to a prostate cancer screening campaign. J Clin Med (2023) 12(5):1831. doi: 10.3390/jcm12051831
38. Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control (2005) 16:83–95. doi: 10.1007/s10552-004-1661-4
39. Klotz L. Active surveillance in intermediate-risk prostate cancer. BJU Int (2020) 125:346–54. doi: 10.1111/bju.14935
40. Pin F, Prideaux M, Bonewald LF, Bonetto A. Osteocytes and cancer. Curr Osteoporosis Rep (2021) 19:616–25. doi: 10.1007/s11914-021-00712-9
41. Cui Y-X, Evans BAJ, Jiang WG. New roles of osteocytes in proliferation, migration and invasion of breast and prostate cancer cells. Anticancer Res (2016) 36:1193–201.
42. Wallace TJ, Torre T, Grob M, Yu J, Avital I, Bruecher B, et al. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer (2014) 5:3–24. doi: 10.7150/jca.7709
43. Sebesta EM, Anderson CB. The surgical management of prostate cancer. Semin Oncol (2017) 44:347–57. doi: 10.1053/j.seminoncol.2018.01.003
44. Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res (2018) 7. F1000 Faculty Rev-513. doi: 10.12688/f1000research.15382.1
45. Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci (2018) 19. doi: 10.3390/ijms19051359
46. Fossati N, Willemse P-PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: A systematic review. Eur Urol (2017) 72:84–109. doi: 10.1016/j.eururo.2016.12.003
47. Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med (2016) 375:1425–37. doi: 10.1056/NEJMoa1606221
48. Zhang Y, Ding L, Zheng Y, Wang K, Xia W, Wang J, et al. Retrospective validation of bone risk stratification criteria for men with de novo metastatic hormone-naive prostate cancer in China. Peerj (2023) 11. doi: 10.7717/peerj.14500
49. Ibe IK, Sahlstrom A, White A, Henderson SE, Lee FY. Metastatic cancers to bone: an overview and cancer-induced bone loss. Instructional course lectures (2019) 68:547–56.
50. Patanaphan V, Salazar OM, Risco R. Breast-cancer - metastatic patterns and their prognosis. South Med J (1988) 81:1109–12. doi: 10.1097/00007611-198809000-00011
51. Burlaka AA, Makhmudov DE, Lisnyi II, Paliichuk AV, Zvirych VV, Lukashenko AV. Parenchyma-sparing strategy and oncological prognosis in patients with colorectal cancer liver metastases. World J Surg Oncol (2022) 20. doi: 10.1186/s12957-022-02579-1
52. Buelens S, Poelaert F, Dhondt B, Fonteyne V, De Visschere P, Ost P, et al. Metastatic burden in newly diagnosed hormone-naive metastatic prostate cancer: Comparing definitions of CHAARTED and LATITUDE trial. Urologic Oncology-Seminars Original Investigations (2018) 36. doi: 10.1016/j.urolonc.2017.12.009
53. Yu S, Fan J, Liu L, Zhang L, Wang S, Zhang J. Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett (2013) 587:782–7. doi: 10.1016/j.febslet.2013.02.002
54. Rivadeneyra L, Falet H, Hoffmeister KM. Circulating platelet count and glycans. Curr Opin Hematol (2021) 28:431–7. doi: 10.1097/MOH.0000000000000682
55. Crespo HJ, Lau JTY, Videira PA. Dendritic cells: a spot on sialic acid. Front Immunol (2013) 4. doi: 10.3389/fimmu.2013.00491
56. Chiang C-H, Wang C-H, Chang H-C, More SV, Li W-S, Hung W-C. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J Cell Physiol (2010) 223:492–9. doi: 10.1002/jcp.22068
57. Pluvinage JV, Sun J, Claes C, Flynn RA, Haney MS, Iram T, et al. The CD22-IGF2R interaction is a therapeutic target for microglial lysosome dysfunction in Niemann-Pick type C. Sci Trans Med (2021) 13. doi: 10.1126/scitranslmed.abg2919
58. Goswami K, Nandeesha H, Koner BC, Nandakumar DN. A comparative study of serum protein-bound sialic acid in benign and Malignant prostatic growth: possible role of oxidative stress in sialic acid homeostasis. Prostate Cancer Prostatic Dis (2007) 10:356–9. doi: 10.1038/sj.pcan.4500965
59. Crook MA, Tutt P, Pickup JC. Elevated serum sialic-acid concentration in niddm and its relationship to blood-pressure and retinopathy. Diabetes Care (1993) 16:57–60. doi: 10.2337/diacare.16.1.57
Keywords: serum sialic acid, bone metastases, prostate cancer, low-volume disease, high-volume disease
Citation: Sun J, Tian T, Wang N, Jing X, Qiu L, Cui H, Liu Z, Liu J, Yan L and Li D (2024) Pretreatment level of serum sialic acid predicts both qualitative and quantitative bone metastases of prostate cancer. Front. Endocrinol. 15:1338420. doi: 10.3389/fendo.2024.1338420
Received: 14 November 2023; Accepted: 18 January 2024;
Published: 07 February 2024.
Edited by:
Anna Perri, Magna Græcia University of Catanzaro, ItalyReviewed by:
Biagio Barone, Azienda Ospedaliera di Caserta, ItalyCopyright © 2024 Sun, Tian, Wang, Jing, Qiu, Cui, Liu, Liu, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawei Li, bGlkYXdlaW1kQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.