- 1School of Molecular Bioscience, Center for Reproductive Biology, Washington State University, Pullman, WA, United States
- 2Department of Pathology & Immunology, Baylor College of Medicine, Houston, TX, United States
- 3Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX, United States
Immune dysfunction is one of the central components in the development and progression of endometriosis by establishing a chronic inflammatory environment. Western-style high-fat diets (HFD) have been linked to greater systemic inflammation to cause metabolic and chronic inflammatory diseases, and are also considered an environmental risk factor for gynecologic diseases. Here, we aimed to examine how HFD cause an inflammatory environment in endometriosis and discern their contribution to endometriotic-associated hyperalgesia. Our results showed that HFD-induced obesity enhanced abdominal hyperalgesia that was induced by endometriotic lesions. Peritoneal inflammatory macrophages and cytokine levels increased by lesion induction were elevated by chronic exposure to HFD. Increased expression of pain-related mediators in the dorsal root ganglia was observed after lesion induction under the HFD condition. Although HFD did not affect inflammatory macrophages in the peritoneal cavity without lesion induction, the diversity and composition of the gut microbiota were clearly altered by HFD as a sign of low-grade systemic inflammation. Thus, HFD alone might not establish a local inflammatory environment in the pelvic cavity, but it can contribute to further enhancing chronic inflammation, leading to the exacerbation of endometriosis-associated abdominal hyperalgesia following the establishment and progression of the disease.
1 Introduction
Endometriosis is a chronic and incurable inflammatory disorder and affects approximately 10% of reproductive-aged women (1, 2). It is associated with debilitating chronic pelvic pain and infertility, which substantially reduce the quality of life of women and their families (3, 4). Because endometriosis is estrogen-dependent, current treatments focus on inhibiting estrogen production and function. However, hormonal treatments and surgical excision of lesions are often of limited efficacy with high recurrence rates, frequent side effects, additional costs, and potential morbidity (5). As nearly 70% of patients suffer unsolved chronic pain and other related conditions (6), the direct costs of endometriosis were estimated at $12,118 per patient per year in the US, and indirect costs were $15,737 (7). The pathogenesis of endometriosis is a complex process and remains to be fully understood. Retrograde menstruation has been widely accepted as the origin of endometriotic tissues (8). However, as retrograde menstruation occurs in more than 90% of menstruating women (9), the pathogenesis of the disease is not well understood, and other factors must contribute to establishing endometriotic lesions and disease progression (1, 4, 10).
Obesity is an epidemic health burden affecting nearly 40% of adults and 18% of children in the United States (11). Being overweight and obese are considered critical risk factors for chronic diseases, as fat accumulation causes low-grade chronic inflammation (12) characterized by immune cell infiltration into adipose tissues and elevated proinflammatory factors (13). Moreover, excessive fat consumption and accumulation in the body alter gut microbiota, resulting in dysbiosis to induce low-grade systemic inflammation (14). Obesity-induced inflammation is associated with metabolic and autoimmune disorders in women, causing reproductive dysfunctions such as polycystic ovary syndrome (PCOS), implantation and pregnancy failure, and pregnancy complications, including miscarriages (15–18). While endometriosis is a chronic inflammatory disease, several epidemiological studies have reported an inverse correlation between endometriosis and body mass index (BMI) (19). However, obesity does not protect against endometriosis (19), and BMI is correlated with the severity but not the frequency of disease diagnoses (20). Thus, BMI does not provide a simple risk factor for a heterogeneous endometriotic disease as it does not consider different components of excess weight, such as adipose deposit location and interaction with neighboring tissues (20, 21). Additionally, the correlation between diet-induced obesity and endometriosis-associated pain or hypersensitivity, one of the significant endometriosis symptoms, has not been addressed.
Rigorous prior research suggests that aberrant inflammation contributes to the onset and progression of endometriosis (22–27). Macrophages (MΦ) are considered to be key players in promoting disease progression (25, 28, 29), as abundant MΦ are present in ectopic lesions (30) and elevated in the pelvic cavity (31). These MΦ populations establish an inflammatory environment in the pelvic cavity by secreting cytokines and chemokines, which encourage lesion growth and progression (24, 28, 29, 32, 33) and contribute to endometriosis-associated pelvic pain (32, 34, 35). Diet-induced obesity dysregulates immune cells to induce cytokine secretion (13, 36, 37), increasing the risks of chronic pain. Therefore, the present study seeks to understand whether high-fat diets (HFD) affect the progression of endometriosis disease and immune dysfunctions and how HFD influence endometriosis-associated hyperalgesia.
The present results highlight that endometriosis-associated abdominal hyperalgesia was escalated in lesion-induced HFD mice according to the results of the behavior study and elevated pain-related mediators in the dorsal root ganglion (DRG). Increased proinflammatory MΦ (Ly6C+ MΦ) and cytokines by lesion induction were further enhanced by exposure to HFD. The results also indicate that gut microbiota dysbiosis under the HFD condition contributed to an aberrant inflammatory environment and sensitized endometriosis-associated hypersensitivity.
2 Results
2.1 Diet-induced obesity on endometriosis in mice
To examine the effect of diet-induced obesity on endometriosis, female mice were fed HFD containing 45% fat by calories or standard diets (SD) from the age of 5 weeks (defined as Week 0 of the 12-week as a baseline study or 18-week as an endometriosis study, Figure 1A). We chose to start the study at the age of 5 weeks, as this is the adolescent age of mice, corresponding to the teenage period for humans (38). Moreover, approximately 20% of this population is obese in humans (39). Mice on the 45% fat diets become obese and are considered physiologically similar to the typical Western diets that contain 36-40% fat by energy (40, 41). A standard rodent diet contains approximately 10% fat (40, 42). We assessed body weight (BW) increase, glucose, and insulin levels at 12 weeks after SD or HFD feeding as a baseline study and 18 weeks (6 weeks after endometriosis-like lesions (ELL) induction) as an endometriosis study (Figures 1A–D). BW, blood glucose, and plasma insulin levels were significantly increased in the group of HFD at 12 or 18 weeks, whereas they were not affected by lesion induction. BW, glucose, and insulin levels in the HFD group were similar to the previously reported levels (43, 44).

Figure 1 Diet-induced obesity in the mouse model of endometriosis. (A) Experimental study design as described in Material and Methods. (B) Body weight (BW) changes in mice during the feeding of standard diets (SD) or 45% high-fat diets (HFD) for the baseline study or the endometriosis study. Female mice were fed either SD or HFD starting at the age of 5 weeks (defined as Week 0 of the 12-week as a baseline study or 18-week as an endometriosis study). Two-way ANOVA was used to determine the significance between times and groups. (C) Blood glucose levels by cardiac puncture were measured by Contour Next (n=5 in each group). (D) Plasma insulin levels were quantified by ELISA (n=5 in each group). Data at 12 weeks were analyzed by two-tailed Student’s t-test comparing SD and HFD. Data at 18 weeks were analyzed through one-way ANOVA and Tukey’s post hoc test. Values in graphs are expressed as the mean ± SEM. Different letters indicated significant differences among the groups (P<0.05). ELL: endometrial-like lesion.
We next assessed lesion numbers in the endometriosis study at 18 weeks. Lesion numbers were not altered by HFD compared with SD (Figure 2A). We have previously reported that peritoneal MΦ or monocytes are infiltrated into the ELL (28). We thus addressed MΦ infiltration in the lesion staining with CD68, a macrophage marker. CD68+ MΦ were significantly increased within lesions from mice in the HFD group (Figures 2B, C), indicating MΦ infiltration was accelerated in the ELL-HFD mice, although this did not appear to affect lesion development.
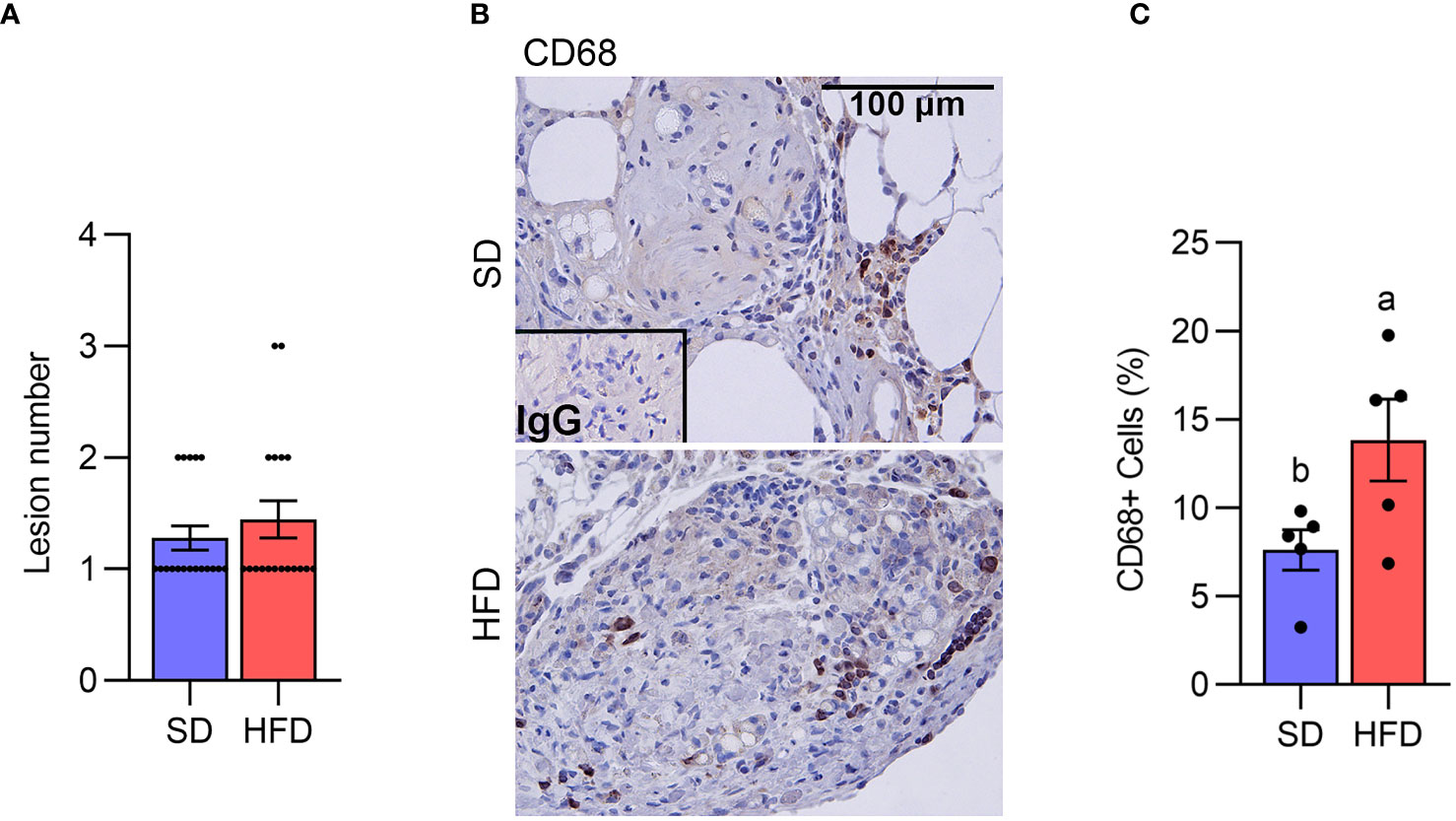
Figure 2 Diet-induced obesity increases macrophage infiltration in the lesion. (A) Lesion number (n=18 per group). (B) CD68 was stained to determine macrophage infiltration in the lesion. (C) The quantification of the percentage of CD68+ cells per total cells (n=5). Data were analyzed with the student t-test and are shown as mean ± SEM. Different letters indicated significant differences among the groups (P<0.05). SD: standard diets, and HFD: high-fat diets.
2.2 HFD accelerated endometriosis-associated abdominal hyperalgesia
Since HFD can induce chronic pain (12, 13, 45), we performed the von Frey test to examine the abdominal and hind paw retraction threshold to determine whether HFD affects endometriosis-associated hyperalgesia. We first assessed the abdominal and hind paw retraction threshold at 12 weeks after SD or HFD feeding. The abdominal and hind paw retraction threshold showed no differences under the SD or HFD diets for 12 weeks (Figures 3A, C).
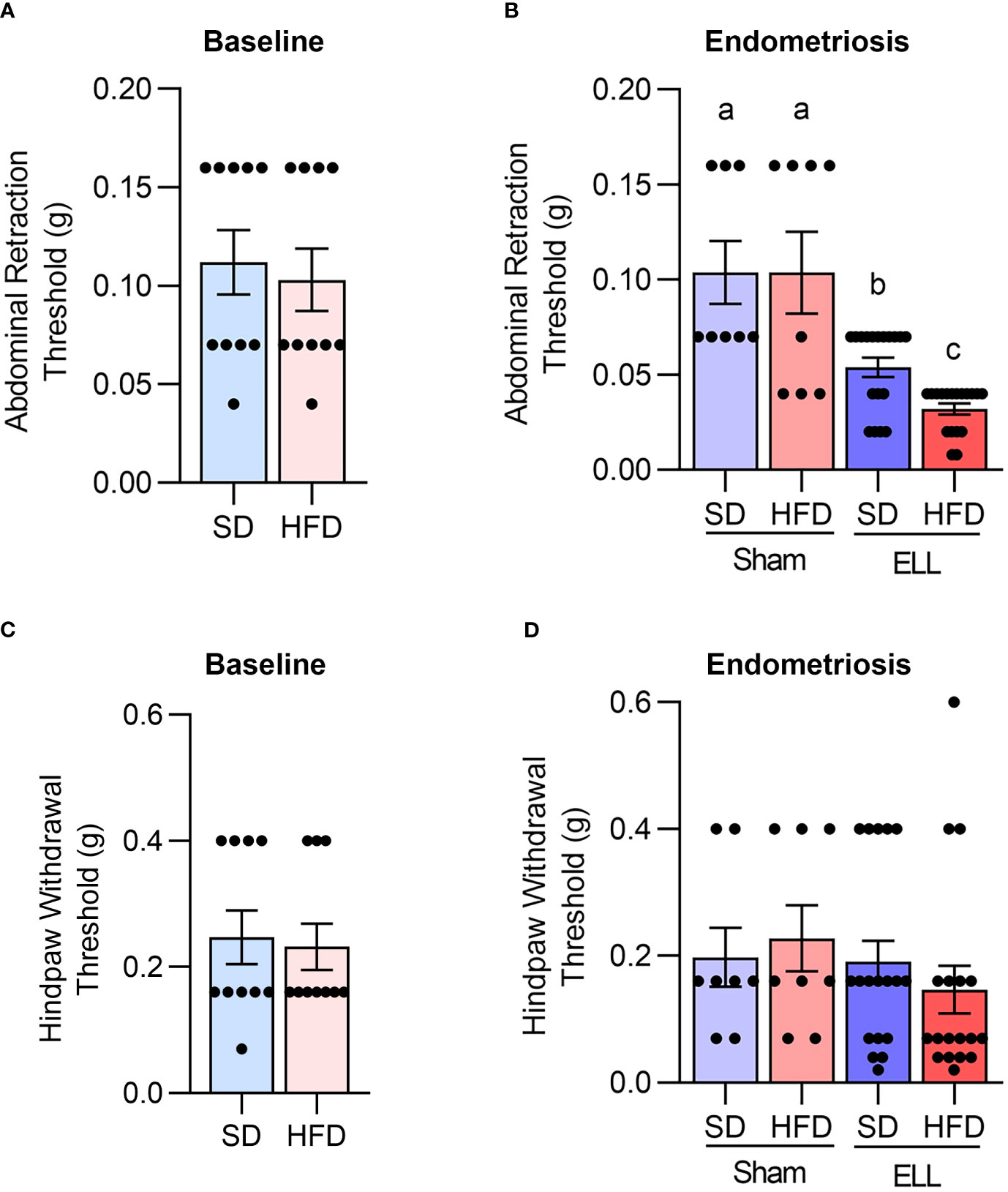
Figure 3 HFD accelerates endometriosis-associated abdominal hyperalgesia. Von Frey tests were performed on mice to the lower abdomen and hind paw in the bseline study after 12 weeks of SD or HFD feeding (A and C, n=10), or 6 weeks post-lesion induction in the endometriosis study (B, D, a total of 18 weeks of SD or HFD feeding, n=8 for Sham and n=18 for ELL groups). Data are shown as mean ± SEM. Statistical significance was determined by student t-test (A, C), or one-way ANOVA followed by Tukey’s post hoc test (B, D). Different letters indicated significant differences among the groups (P<0.05). ELL: endometrial-like lesion, SD: standard diets, and HFD: high-fat diets.
Before examining the effect of HFD on endometriosis-associated hyperalgesia, we examined how lesion induction time-dependently alters endometriosis-associated hyperalgesia in the mouse model. Three days after ELL induction, mice withdrew both abdominal and hind paw retraction thresholds with significantly lighter stimuli compared with those before ELL induction on Day -1 (Supplementary Figures S1A, B). The abdomen and hind paw retraction sensitivity continued until 3 weeks after ELL induction. By Day 42, 6 weeks after ELL induction, the hind paw retraction threshold was no longer significantly different from Day -1, indicating that systemic peripheral hyperalgesia gradually recovered, whereas the local abdomen was still sensitive. Since we examined the effect of chronic HFD exposure on endometriosis-associated hyperalgesia, we chose a chronic stage, 6 weeks after ELL induction, for further analysis, as endometriosis is a chronic disease, and most patients suffer chronic pelvic pain. Furthermore, the timing of disease onset in endometriosis is currently impossible to determine in patients, and the disease diagnosis typically relies on the woman noticing chronic symptoms.
The abdominal and hind paw sensitivity with SD or HFD were evaluated 6 weeks after lesion induction (18 weeks of SD or HFD feeding). As expected, a significant difference was observed in the abdominal retraction threshold between Sham (vehicle, PBS, control) and ELL, with ELL mice withdrawing from lighter stimuli than Sham mice (Figure 3B). Importantly, ELL-HFD mice were more sensitive than ELL-SD mice (Figure 3B). On the other hand, we did not observe any differences in hind paw retraction threshold among the post-induction groups (Figure 3D). Thus, HFD-induced obesity enhanced abdominal hyperalgesia that was induced by endometriotic lesions.
2.3 HFD increased Ly6C+ MΦ in the peritoneal fluid of ELL mice
As we observed increased MΦ infiltration in the lesions of the HFD group (Figures 2B, C) and ELL-HFD mice have increased hypersensitivity in the abdomen (Figure 3B) in the endometriosis study, we expected to observe differences in the inflammatory environment that is established in the peritoneal cavity. Therefore, we assessed immune cell profiles, MΦ, B- and T-cells, in the peritoneal cavity (Figure 4, Supplementary Figure S2). CD11b+ MΦ, CD3+ T-cells, and CD19+ B-cells were not altered by either HFD feeding or lesion induction (Figures 4A, B). We have previously reported that the presence of ELL enhanced the differentiation of recruited (=proinflammatory Ly6C+) MΦ and increased the ablation of embryo-derived resident MΦ (TIM4+ MΦ) (29). We thus examined Ly6C+ cells (monocytes and MΦ), Ly6C+ MΦ, and TIM4+ MΦ. High levels of Ly6C+ cells and Ly6C+ MΦ were observed in the ELL-HFD mice (Figures 4A–C). In particular, Ly6C+ MΦ were further increased in the ELL-HFD mice than those in ELL-SD mice (Figure 4C). In agreement with our previous study (29), TIM4+ MΦ were reduced in ELL-SD and ELL-HFD mice (Figure 4C). Ly6C+ cells, Ly6C+ MΦ and TIM4+ MΦ, as well as CD11b+ MΦ, CD19+ B-cells, and CD3+ T-cells were not affected by HFD feeding at 12 weeks in the baseline study (Supplementary Figure S2). These results suggest that ELL induction under the HFD condition further increases proinflammatory Ly6C+ MΦ in the peritoneal cavity.
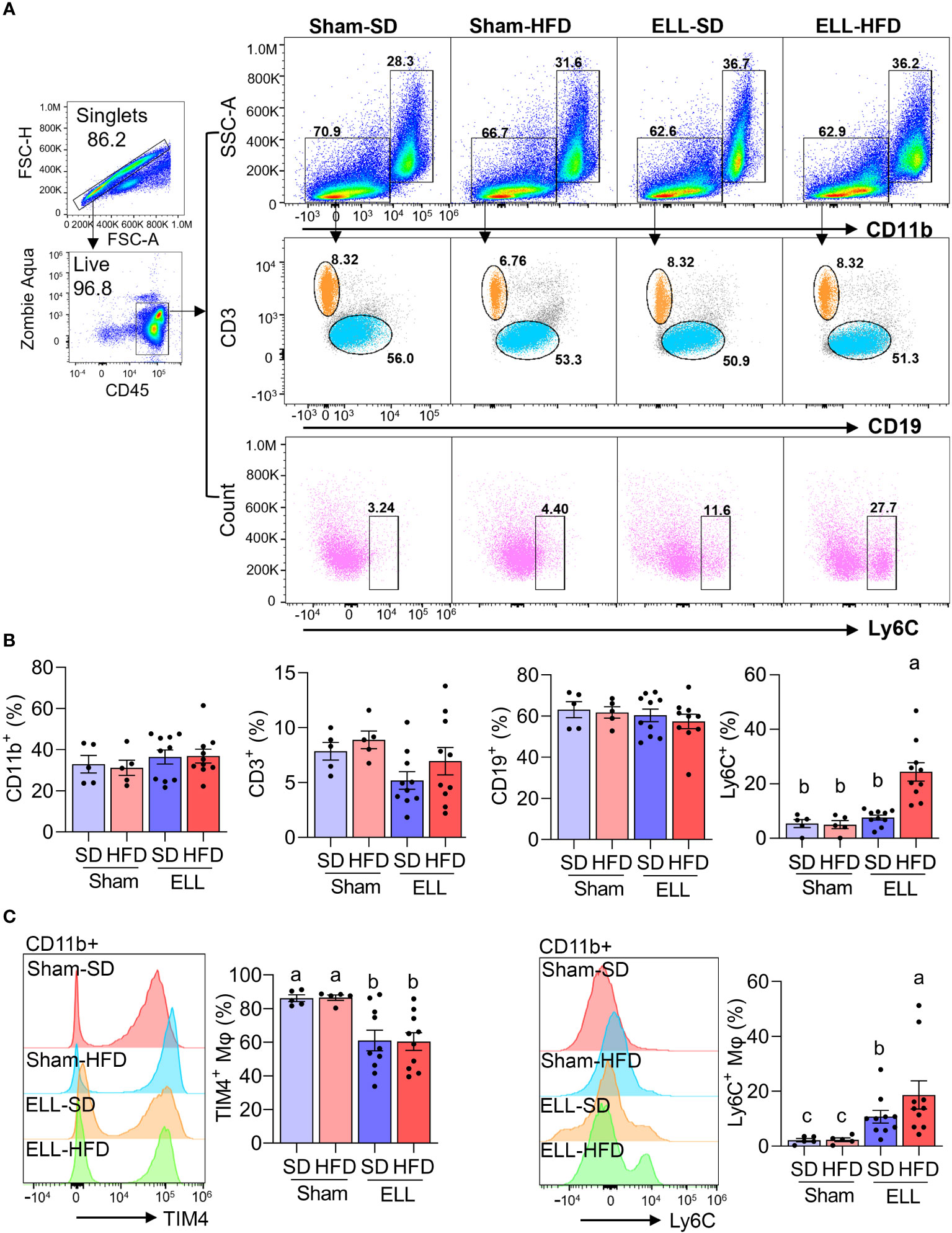
Figure 4 HFD increases Ly6C+ macrophages (MΦ) in the peritoneal fluid (PF) of ELL mice. (A) Flow cytometer analysis for CD11b+ (MΦ), CD3+ (T-cells), CD19+ (B-cells), and Ly6C+ (monocytes and MΦ) cells in the PF. FSC-H: Forward Scatter-Height; FSC-A: Forward Scatter-Area; SSC-A: Side Scatter-Area. (B) Quantification of CD11b+, CD3+, CD19+, and Ly6C+ cells in the groups of Sham-SD (n=5), Sham-HFD (n=5), ELL-SD (n=10) and ELL-HFD (n=10). (C) TIM4+ and Ly6C+ MΦ were quantified in the PF. Data were analyzed through One-way ANOVA followed by Tukey’s post hoc test and expressed as the mean ± SEM. Different letters indicated significant differences among the groups (P<0.05). ELL: endometrial-like lesion, SD: standard diets, and HFD: high-fat diets.
2.4 HFD altered peritoneal cytokines in the ELL mice
Proinflammatory MΦ secrete cytokines, chemokines, and growth factors that establish the inflammatory environment (27, 46). Abundant cytokines and chemokines have been observed in the pelvic cavity of endometriosis patients (24, 27). Specifically, the levels of TNFα, IL1β, and IL6 are increased in pelvic MΦ isolated from endometriosis patients (47). Thus, we next examined the secretion of proinflammatory factors, TNFα, IL1β, and IL6, as well as IL10, which is known to possess immunoregulatory function and anti-inflammatory properties (Figures 5A–D). In support of previous reports, TNFα and IL1β levels were elevated in the ELL groups compared with those in the Sham group, while TNFα was further increased in ELL-HFD mice. IL6 tended to be increased by lesion induction in both SD and HFD groups, though we did not see significant differences. IL10 levels were not significantly altered among the groups of Sham-SD, Sham-HFD, and ELL-SD mice, whereas it was significantly lower in the ELL-HFD mice compared with that of ELL-SD mice.
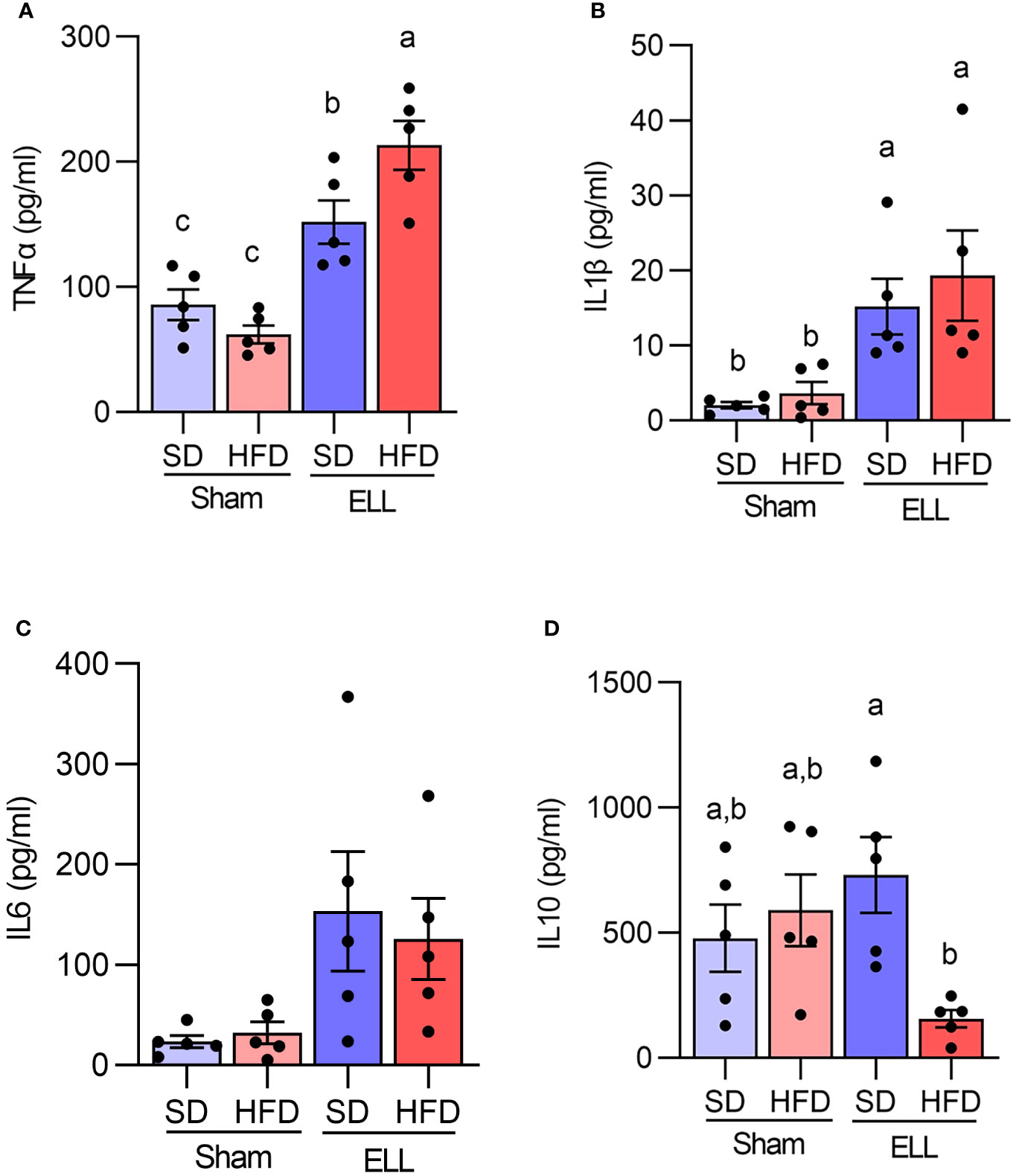
Figure 5 Quantification of TNFα, IL1β, IL6, and IL10 in the peritoneal fluid (PF). Peritoneal (A) TNFα, (B) IL1β, (C) IL6, and (D) IL10 were measured with IQELISA and analyzed with ANOVA followed by Tukey’s post hoc test. Values in graphs are expressed as the mean ± SEM (n=5). Different letters indicated significant differences among the groups (P<0.05). ELL: endometrial-like lesion, SD: standard diets, and HFD: high-fat diets.
2.5 HFD stimulated pain-related mediators in the DRG of ELL mice
Aberrant accumulation of inflammatory factors can stimulate peripheral nerve terminals of nociceptor neurons innervating different tissues in peripheral organs (48), resulting in an increase in the expression of transient receptor potential channels e.g., TRPV1. Activation of peripheral nerves is also associated with the increased release of neurotransmitters and neuromodulators such as SP, CGRP, and BDNF. BDNF is known to regulate both initiation and maintenance of chronic endometriosis-associated pain (49, 50) involving neuroangiogenesis (51) and innervation in the pelvic organs (48). We thus examined the inflammatory mediators, neurotransmitters, and neuromodulators in the L4-6 DRG, which are the primary spinal ganglia receiving sensory input from pelvic organs (Figures 6A, B, Supplementary Figure S3). Significantly more BDNF+ neurons were observed in mice fed HFD. BDNF+ neurons were higher in mice when ELL were present and most abundant in the HFD-ELL group. In contrast, CGRP+ neurons were only significantly elevated in the ELL-HFD mice. SP+ neurons were elevated by lesion induction, while HFD further increased SP+ neurons after ELL induction. Although the numbers of TRPV1+ neurons were relatively consistent between Sham- and ELL-mice, there was a significant difference between ELL-HFD mice and ELL-SD mice. These results suggest that lesion induction and/or HFD feeding stimulate endometriosis-associated peripheral pain mediators.
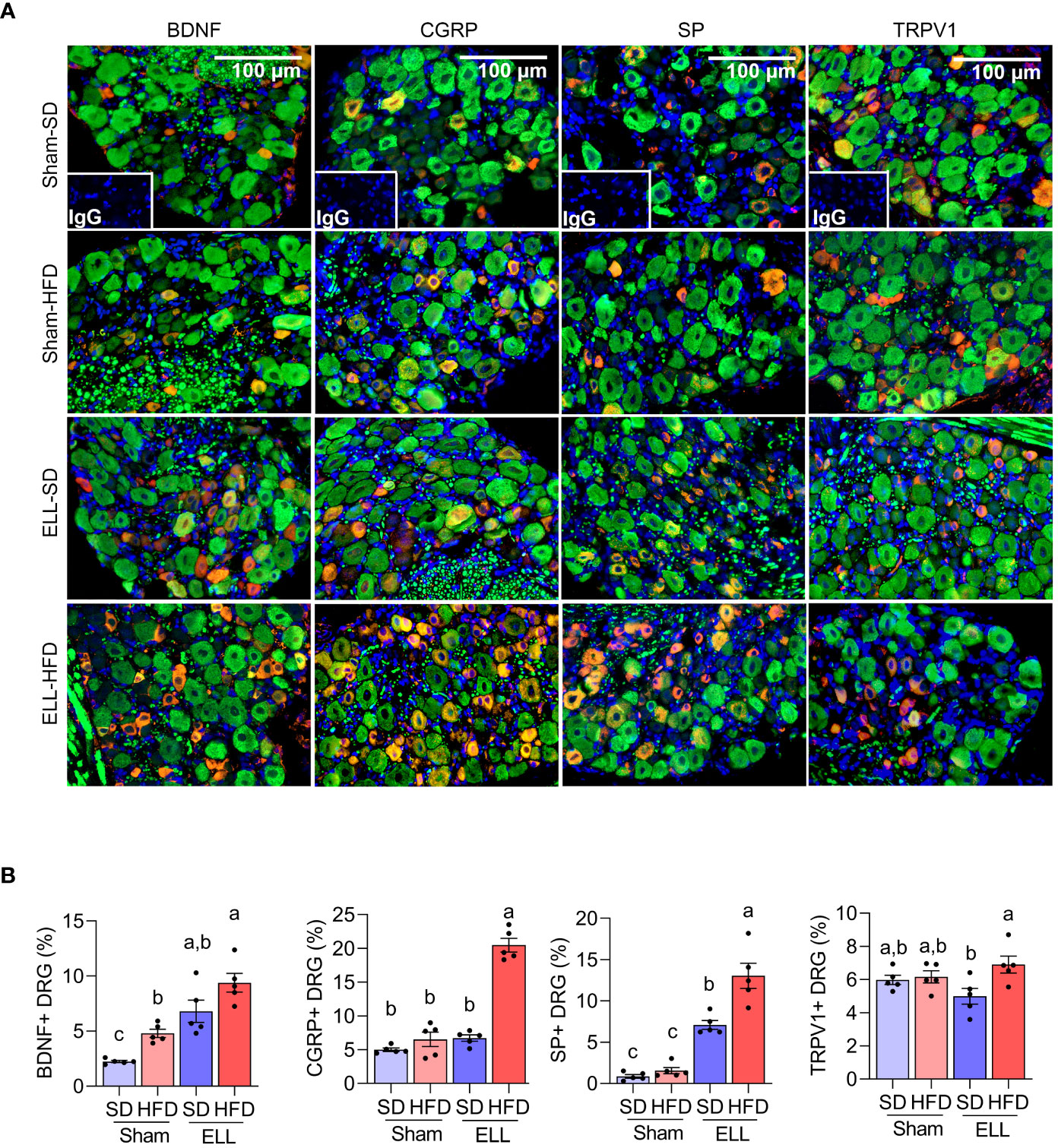
Figure 6 HFD stimulates pain-related mediators in the DRG of ELL mice. (A) Immunofluorescence results of BDNF, CGRP, SP, TRPV1, and neurofilament (NF, green) in DRG. NF was used as a marker of DRG cell body and was co-stained with BDNF, CGRP, SP, or TRPV1. (B) BDNF, CGRP, SP, or TRPV1 positive DRG per NF positive DRG was counted and quantified (n=5 per group). One-way ANOVA followed by Tukey’s post hoc test was used for statistical analysis. Data were shown as mean ± SEM. Different letters indicated significant differences among the groups (P<0.05). ELL: endometrial-like lesion, SD: standard diets, and HFD: high-fat diets. DRG: dorsal root ganglia.
2.6 HFD altered the composition of the gut microbiota
As increased fat accumulation alters gut microbiota and causes low-grade systemic inflammation (14), we next examined 16S rRNA gene sequencing of DNA isolated from fecal samples in SD or HFD with/without ELL-induced mice (Figure 7). Microbial alpha diversity was lower in the feces of HFD-fed mice than in SD-fed mice (Figure 7A). Principal coordinates analysis (PCoA) showed uniquely clustered microbial variance induced by HFD (Figure 7B). However, ELL induction did not alter microbial diversity or variance, indicating that long-term systemic alterations induced by HFD affect the composition of the gut microbiota more than lesion induction in mice.
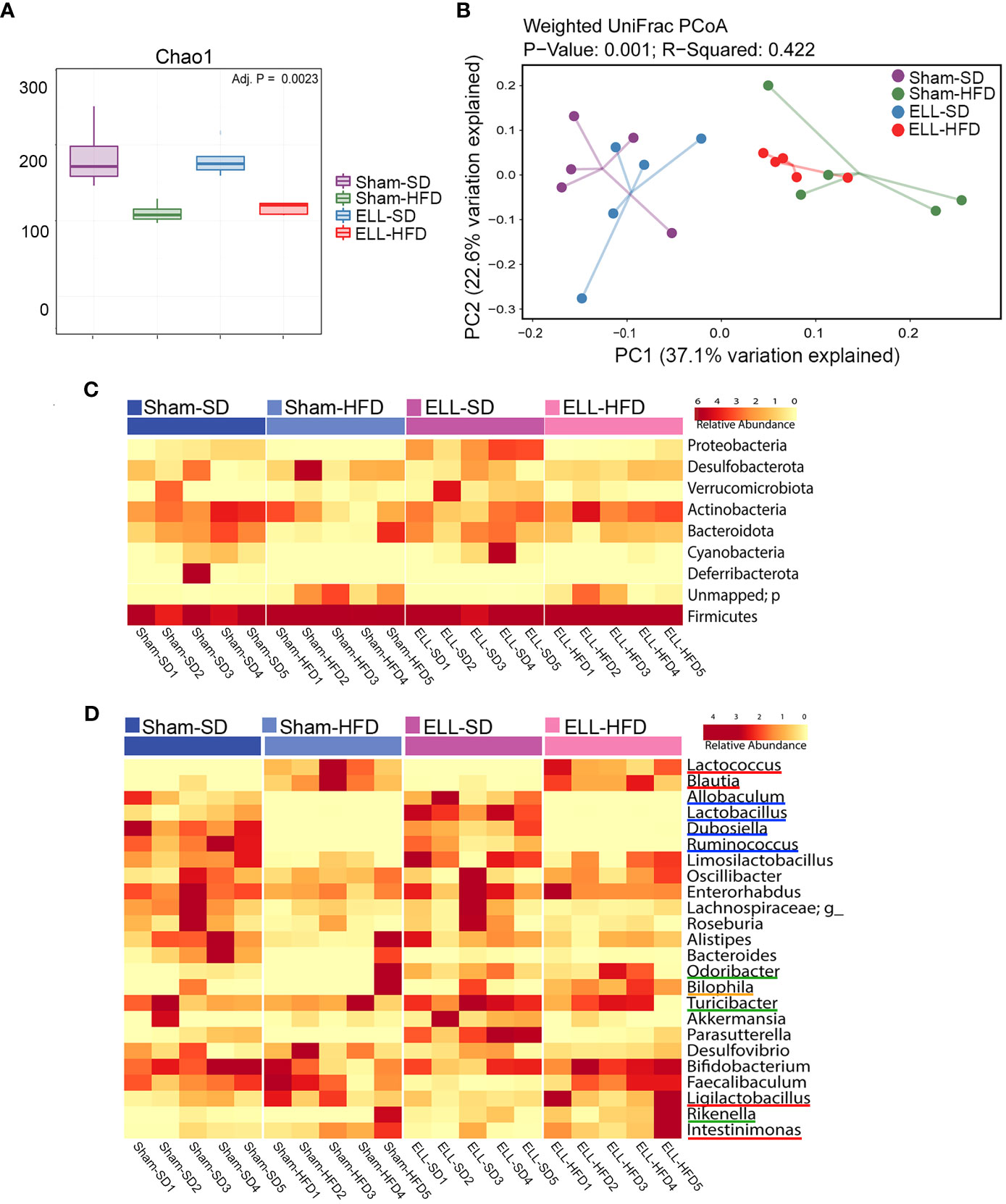
Figure 7 HFD altered the composition of the gut microbiota. (A) Box plots corresponding to the Chao1 diversity index (alpha diversity). (B) Principal Coordinates Analysis (PCoA) of beta-diversity based on weighted Unifrac dissimilarities in fecal samples. P = 0.001, R=0.422. (n=5 per group). (C) Heatmap representation of relative abundances of the phyla in feces. (D) Heatmap depiction of the relative abundances of the genera in feces (n=5 per group). ELL: endometrial-like lesion, SD: standard diets, and HFD: high-fat diets.
To assess whether the unique enteric bacterial profiles were attributed to specific taxa, the phyla among samples in the group were profiled (Figure 7C). The proportions of Proteobacteria and Cyanobacteria were reduced under the HFD condition, while feces in ELL-SD mice contained a higher abundance of Proteobacteria than those in Sham-SD mice. However, increased Proteobacteria were not observed in ELL-HFD mice compared to Sham-HFD mice, suggesting that the effect of HFD on Proteobacteria was stronger than lesion induction. Firmicutes and Bacteroidetes, which constitute the majority of the gut microbiota, are known to be affected by obesity, as obesity induces a reduction in the abundance of Bacteroidetes and an increase of Firmicutes proportion (52). Although the increase of Firmicutes was minor under the HFD condition, the Bacteroidetes proportion was clearly reduced in Sham-HFD mice. The Bacteroidetes population was retained in ELL-HFD mice, similar to its abundance in Sham-SD and ELL-SD mice, indicating that lesion induction increased Bacteroidetes even though mice were under the HFD condition. This result supports the study from Chadchan et al. that lesion induction increases the abundance of Bacteroidetes (53), while lesion induction with SD did not show a noticeable increase of Bacteroidetes in our study (Sham-SD vs ELL-SD). When we examined bacteria at the genus level, HFD clearly altered several genera among the groups (Figure 7D). In agreement with previous studies (54, 55), HFD strongly elevated Lactococcus and Blautia genera (red lines). HFD slightly increased Ligilactobacillus and Intestinimonas genera (red lines), whereas HFD mice contained negligible abundances of Allobaculum, Lactobacillus, Dubosiella, and Ruminococcus genera (blue lines). Odoribacter, Turicibacter, and Rikenella genera (green lines) were increased in ELL mice, and the Bilophila genus (orange line) was only higher in ELL-HFD mice. These data suggest that HFD or ELL alter the bacteria diversity and composition associated with endometriosis.
3 Discussion
Endometriosis is generally classified into four stages according to the revised criteria from the American Society of Reproductive Medicine (rASRM) and the American Fertility Society (AFS) based on lesion size, location, and the extent of adhesions (4, 56). However, disease symptoms, such as endometriosis-associated pain, are not correlated with the staging system (4, 57). Patients with stage I disease can have severe pain, while stage IV patients can be asymptomatic (1, 58), indicating that several other factors contribute to disease symptoms. Due to the chronic inflammatory nature of endometriosis, the disease progression and symptoms can be affected by environmental factors. In the present study, our results highlight that Western-style HFD-induced obesity did not alter endometriotic lesion numbers (=disease progression) but enhanced disease-related hyperalgesia (=endometriosis-associated pain). The important findings are: 1) Peritoneal inflammatory (Ly6C+) MΦ and cytokine levels, especially TNFα, increased by lesion induction were elevated by chronic exposure to HFD. 2) Pain-related mediators, such as neurotransmitters CGRP and SP, in the DRG were further stimulated after lesion induction under the HFD condition. 3) Although HFD alone did not affect peritoneal Ly6C+ MΦ without lesion induction, the diversity and composition of the gut microbiota were clearly altered by HFD as a sign of low-grade systemic inflammation (14). Thus, HFD might not be able to establish solely a local inflammatory environment in the pelvic cavity but can contribute to further enhancing chronic inflammation associated with disease symptoms after the disease is established.
In non-human primates, rhesus macaque females exposed to testosterone (T) and/or consumed Western-style diets (WSD) at the time of menarche for 7 years developed endometriosis, especially T+WSD resulted in earlier onset of disease with high stages and large chocolate cysts (59). In a mouse model of endometriosis, HFD-induced obese mice increased lesion number and weight, which depended on leptin or leptin receptor (60). Another mouse study of endometriosis showed that HFD increased lesion number and MΦ infiltration and proinflammatory and prooxidative stress-related genes in the lesion when Klf9 null donor endometrial fragments were inoculated as a donor tissue (61). This group further reported reduced lesion number and weight when wild-type donor tissues were used, whereas enhanced signs of inflammation were not observed in this study, indicating variability of distinct genetic dysfunctions and lesion environment for endometriosis progression (62).
One of the hallmarks of diet-induced obesity is low-grade chronic inflammation (12). Chronic consumption of HFD leads to the accumulation of MΦ and T-cells in adipose tissues to secrete proinflammatory cytokines (13). We have previously reported that lesion induction enhances the process of differentiation and maturation of monocyte-derived MΦ and increases Ly6C+ proinflammatory MΦ in the peritoneal cavity while reducing the maintenance of embryo-derived resident TIM4+ MΦ (29). The present study showed that Ly6C+ MΦ were higher in ELL mice and further increased in mice exposed to HFD, indicating the impact of HFD contribution to peritoneal inflammation after disease onset. In support of our findings, an HFD-induced proinflammatory environment promotes the differentiation of Ly6C+ monocyte into inflammatory MΦ, which migrate to the lung and worsen its pathophysiology (63). TIM4+ residential MΦ were reduced in both ELL-SD and ELL-HFD mice, whereas HFD did not further alter TIM4+ MΦ. Peritoneal inflammation can induce the macrophage disappearance reaction (MDR), by which the reduction of residential MΦ occurs. We have previously shown that extreme MDR of TIM4+ MΦ was induced 3 days after lesion induction, and it gradually recovered. However, it remains slightly diminished 6 weeks after disease onset (29). Thus, the recovery of residential TIM4+ MΦ from MDR, which includes replenishment and proliferation, is less likely affected by exposure to HFD. On the other hand, an alteration in the distribution of peritoneal T-cells by lesion induction and HFD was not observed in the study, suggesting aberrant MΦ functions might be a crucial event for establishing the chronic inflammatory state of endometriosis, as increased MΦ infiltration was also observed in the lesions under the HFD condition. However, heterogeneous T-cell functions and interaction between T-cells and MΦ remain to be studied.
In the present study, abdominal endometriosis-associated hyperalgesia was induced by lesion induction and further sensitized in ELL-HFD mice. This result was supported by the signs of sensitization of peripheral DRG, which was mediated by increased proinflammatory cytokines, TNFα, IL1β, and IL6, that are known to be increased pelvic MΦ in endometriosis patients (47) and have been targeted for pathological pain (64). Our previous studies show that PF from ELL mice stimulated DRG outgrowth, which was reduced by inhibiting cytokine and chemokine secretion in the peritoneal cavity (28). Thus, the inflammatory environment established in the pelvic cavity is critical for chronic endometriosis-associated hypersensitivity. The elevated sensitivity is not systemic, as our results showed only signs of abdominal hyperalgesia but not hind paw sensitivity by either lesion induction or HFD. Thus, it remains to study how chronic abdominal pain stimulus is delivered and maintained to the central nervous system. As endometriosis-associated pain is one of the significant problems in this disease, its mechanisms with the pathophysiology of endometriosis need to be further studied to enhance the quality of life in patients.
Our study showed that gut microbiota dysbiosis was induced by chronic exposure to HFD. HFD have been known to reduce the diversity of gut microbiota (65). The phyla Firmicutes increase while Bacteroidetes decrease, though there are variations depending on the differences in diet compositions and exposure duration (66, 67). Interestingly, our results showed a lower abundance of Proteobacteria in HFD mice, whereas increased Proteobacteria abundance with HFD consumption has been reported (68). Increased Allobaculum abundance has been shown under the HFD condition (69), though the abundance of Allobaculum was reduced in our HFD mice. However, this inconsistency is likely due to different types of diet, fat, and other environmental factors in the various studies (14). Despite having variable alterations of gut microbiota, HFD-induced dysbiosis increases gut permeability and creates chronic inflammation, affecting inflammatory diseases directly or indirectly (70).
The present study showed that endometriosis-associated abdominal hyperalgesia was escalated under exposure to HFD. These results include increased proinflammatory MΦ and cytokine levels in the peritoneal cavity, neuromodulators in the DRG, and dysbiosis of gut microbiota. There was no significant difference in mean lesion numbers between control and HFD mice, suggesting that the low-grade pre-induction inflammatory state of HFD mice may not significantly alter the mechanism that allows tissue adherence and survival. However, it is clear that once the ELL is established, the HFD lesions exhibit more MΦ infiltration with a more severe pain phenotype. Retrograde menstruation causes massive inflammatory responses in the pelvic cavity, which involves the recruitment of monocytes that differentiate into proinflammatory MΦ and secrete cytokines and chemokines (27). However, the acute inflammation associated with retrograde menstruation typically resolves by the next menstrual cycle. If women are under systemic low-grade inflammation induced by environmental factors like HFD, it is expected to be hard to solve this acute incidence. As menstrual cycles repeatedly occur in women, each retrograde menstruation induces composite inflammation in the pelvic cavity, and unsolved inflammation can worsen to develop chronic conditions further. Thus, the present results suggest that diet-induced obesity could be a risk factor for establishing a chronic inflammatory environment and severe endometriosis-associated pain, which can be independent of disease progression.
4 Materials and methods
4.1 Animals
All animal experiments were performed at Washington State University according to the NIH guidelines for the care and use of laboratory animals (protocol #6751). C57BL/6 (JAX: 000664) breeder pairs were obtained from the Jackson Laboratory, bred in-house, and maintained in the vivarium with a 12:12 light-dark (LD) cycle under ad libitum conditions of food and water. Female C57BL/6 mice at the age of 5 weeks were used for the studies.
4.2 Mouse model of endometriosis
An experimental mouse model of endometriosis was established by adopting procedures described previously (28, 29, 51, 71–73) and Supplementary Method. Briefly, a ‘menses-like’ event was induced in ovariectomized estradiol-17β (E2)- and progesterone-primed donor mice following an established protocol (74). Then, mouse menses-like endometrium scraped from myometrium and cut into fragments (1-2 mm per side) were introduced as the source of syngeneic mouse endometrium (donor) via injection (in 0.2 mL PBS) into the peritoneal cavity of untreated naive mice (recipient) under anesthesia via inhaled isoflurane.
4.3 Study design
To induce diet-dependent obese mice, female mice were fed Teklad Rodent Diet (#2019, Envigo) as SD (Washington State University regular diet) that contain 9-10% of total calories from fat or HFD (D12451, Research Diets) that contain 45% of total calories from fat starting at the age of 5 weeks (defined as Week 0 of the 12-week as a baseline study or 18-week as an endometriosis study, Figure 1A). BW was recorded once a week. In the baseline study, mice were fed with SD (n=10) or HFD (n=10) for 12 weeks. After 12 weeks of feeding, a von Frey behavior test was performed, and peripheral blood and peritoneal lavage were collected. In the endometriosis study, mice were further assigned to sham control without lesion induction or ELL-induced groups twelve weeks after SD or HFD feeding. Thus, there were a total of 4 groups with Sham (vehicle, PBS, control)-SD (n=8), Sham-HFD (n=8), ELL-SD (n=18) and ELL-HFD (n=18). Six weeks after induction (a total of 18 weeks), a behavior test was performed, and fresh feces were collected and immediately frozen at -80C. Mice were then euthanized for sample collections: blood was collected via cardiac puncture, PF was recovered by lavage (4 mL x 2 of ice-cold PBS), and ELL and bilateral lumbar (L4-6) DRG were collected for further analysis. Blood glucose levels (n=5) were measured by Contour Next (Ascensia Diabetes Care), and plasma insulin (n=5) was analyzed by ELISA (EZRMI-13K, Sigma Aldrich), according to the manufacturer’s instructions.
4.4 Von Frey test
A behavioral (mechanical sensitivity) test was performed before sample collection (34, 73). Mice were allowed to acclimate in the testing room for 30 min, and then the von Frey test was performed using von Frey Filaments (BIO-VF-M, Bioseb). Filaments were applied 10 times to the skin perpendicular to the lower abdomen and bilateral hind paws. The force in grams (g) of the filament evoking a withdrawal response (50% response count as sensitive) was recorded. Three behaviors were considered positive responses to filament stimulation: 1) sharp retraction of the abdomen, 2) immediate licking and/or scratching of the area of filament stimulation, or 3) jumping. All behavioral tests were performed without describing the identity and details of treatment groups to investigators. The data were analyzed by another investigator. Mice without ELL or sham induction after 12 weeks of SD or HFD feeding were included as a baseline result.
4.5 Flow cytometry
Peritoneal lavages were centrifuged to collect peritoneal exudate cells. After lysing red blood cells by 1x RBC Lysis Buffer (BioLegend), cells were incubated at room temperature for 20 minutes with Zombie Aqua™ Fixable Viability dye (BioLegend) and blocked on ice for 20 minutes with Fc Block anti-CD16/CD32 (Thermo Fisher). Then, cells were stained with fluorochrome-conjugated monoclonal antibodies (Supplementary Table S1) for 1 hour. Samples were acquired with the Attune NxT Acoustic Focusing Cytometer using Attune NxT software (Thermo Fisher), and data were analyzed with FlowJo v10.9. For analysis, only singlets (determined by forward scatter height vs. area) and live cells (Zombie Aqua negative) were used.
4.6 Immunofluorescence
Immunostaining of BDNF, CGRP, SP, TRPV1, neurofliment (NF), and CD68 was performed with cross-sections (5 μm) of paraffin-embedded tissues using specific commercially available primary antibodies (Supplementary Table S1) and AlexaFluor 488 and 568-conjugated F(ab’) secondary antibody (Molecular Probe) or VECTASTAIN ABC kit (Vector lab). Immunostaining images were acquired by Leica DM4 B. Cell-specific CD68 positive and total cell numbers were counted by Image J in the area of 0.07244 mm2, and the percentage of CD68+ cells was shown. NF was used as a pan-neuronal marker and was co-stained with BDNF, CGRP, SP, or TRPV1. BDNF, CGRP, SP, or TRPV1 positive cells in the DRG were counted by Image J in the area of 0.07244 mm2. The percentages of BDNF, SP, CGRP, or TRPV1 positive cells per NF-positive DRG were shown.
4.7 IQELISA
Protein yield from PF was quantitated by BCA assay (Pierce), and TNFα (IQM-TNFA-1), IL1β (IQM-IL1b-1), IL6 (IQM-IL6-1), and IL10 (IQM-IL10-1) were further quantified by IQELISA kits (Ray Biotech) according to the manufacturer’s instructions.
4.8 16S rRNA gene sequencing and analysis
DNA was extracted from fecal pellets (100 mg, n=5 per group) by the QIAmp Power Fecal DNA kit (12850-50, Qiagen). The V4 region of 16S rRNA gene was amplified, and sequencing was performed on an Illumina platform by the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine. Demultiplexed reads were quality filtered after initial trimming, and taxonomic information was retrieved by mapping against SILVA version 138.1 (75) using an identity threshold of 70% in Quantitative Insights Into Microbial Ecology (76). Raw data in FASTQ format were uploaded to the NCBI Sequence Read Archive (PRJNA1007658). This dataset was used for downstream alpha and beta diversity analysis, and top taxa were identified using a mean abundance threshold of ≥ 0.05, as described previously (53). The alpha diversity was measured using Chao1 distances, while the beta diversity was estimated using weighted UniFrac measures (77).
4.9 Statistical analysis
Data at 18 weeks were subjected to one-way ANOVA and Tukey’s post hoc test to identify differences among the groups using Prism software (Ver. 9.1.0, GraphPad). Data at 12 weeks and lesions at 18 weeks were analyzed by two-tailed Student’s t-test comparing SD and HFD. Two-way ANOVA was used to determine the significance between times and groups. All experimental data are presented as mean with standard error of the mean (SEM). Unless otherwise indicated, a P value less than 0.05 was considered to be statistically significant. Different letters indicated significant differences among the groups (P<0.05).
Ethics statement
The animal study was approved by Institutional animal care and use committee (IACUC) at Washington State University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TH-W: Formal analysis, Investigation, Methodology, Writing – review & editing. MS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. MH: Investigation, Methodology, Writing – review & editing. CT: Data curation, Formal analysis, Investigation, Writing – review & editing. RK: Funding acquisition, Resources, Supervision, Writing – review & editing. JM: Data curation, Supervision, Writing – review & editing. KH: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by NIH/NICHD, R01HD104619 (to KH) and R01HD102680 (to RK).
Acknowledgments
We thank Logan C. Butler for feeding diets and caring for mice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1336496/full#supplementary-material
References
1. Zondervan KT, Becker CM, Missmer SA. Endometriosis. New Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
2. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. (2018) 51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001
3. Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertility sterility. (2011) 96:366–373 e8. doi: 10.1016/j.fertnstert.2011.05.090
4. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. (2018) 4:9. doi: 10.1038/s41572-018-0008-5
5. As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. (2019) 221:86–94. doi: 10.1016/j.ajog.2019.02.033
6. Agarwal SK, Foster WG, Groessl EJ. Rethinking endometriosis care: applying the chronic care model via a multidisciplinary program for the care of women with endometriosis. Int J Womens Health. (2019) 11:405–10. doi: 10.2147/IJWH.S207373
7. Soliman AM, Yang H, Du EX, Kelley C, Winkel C. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod. (2016) 31:712–22. doi: 10.1093/humrep/dev335
8. Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. (1927) 3:93–110 43.
9. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstetrics gynecology. (1984) 64:151–4.
10. Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocrine Rev. (2019) 40:1048–79. doi: 10.1210/er.2018-00242
11. Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. (2018) 319:2419–29. doi: 10.1001/jama.2018.7270
12. Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. (2019) 10:1607. doi: 10.3389/fphys.2019.01607
13. Artemniak-Wojtowicz D, Kucharska AM, Pyrzak B. Obesity and chronic inflammation crosslinking. Cent Eur J Immunol. (2020) 45:461–8. doi: 10.5114/ceji.2020.103418
14. Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 10. (2021) 10(11), 3164. doi: 10.3390/cells10113164
15. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertility sterility. (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
16. McPherson NO, Bell VG, Zander-Fox DL, Fullston T, Wu LL, Robker RL, et al. When two obese parents are worse than one! Impacts on embryo and fetal development. Am J Physiol Endocrinol Metab. (2015) 309:E568–81. doi: 10.1152/ajpendo.00230.2015
17. Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod. (2016) 31:385–92. doi: 10.1093/humrep/dev298
18. Bodnar LM, Parks WT, Perkins K, Pugh SJ, Platt RW, Feghali M, et al. Maternal prepregnancy obesity and cause-specific stillbirth. Am J Clin Nutr. (2015) 102:858–64. doi: 10.3945/ajcn.115.112250
19. Pantelis A, Machairiotis N, Lapatsanis DP. The formidable yet unresolved interplay between endometriosis and obesity. ScientificWorldJournal. (2021) 2021:6653677. doi: 10.1155/2021/6653677
20. Holdsworth-Carson SJ, Dior UP, Colgrave EM, Healey M, Montgomery GW, Rogers PAW, et al. The association of body mass index with endometriosis and disease severity in women with pain. J Endometr Pelvic Pain Disord. (2018) 10:79–87. doi: 10.1177/2284026518773939
21. Gutierrez-Rojas CA, Cruz-Soto R, Sanchez-Munoz V, Romero A, Mosti-Molina M, Sanchez-Aguilar HA, et al. Does FMI correlate better than BMI with the occurrence of metabolic changes in obese patients? Study Based on 2007 Consecutive Mexican Patients. Obes Surg. (2020) 30:1324–31. doi: 10.1007/s11695-019-04289-2
22. Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. (2003) 50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x
23. Capobianco A, Monno A, Cottone L, Venneri MA, Biziato D, Di Puppo F, et al. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. (2011) 179:2651–9. doi: 10.1016/j.ajpath.2011.07.029
24. Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertility sterility. (1996) 65:925–30. doi: 10.1016/S0015-0282(16)58262-4
25. Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol (Lausanne). (2020) 11:7. doi: 10.3389/fendo.2020.00007
26. Hogg C, Panir K, Dhami P, Rosser M, Mack M, Soong D, et al. Macrophages inhibit and enhance endometriosis depending on their origin. Proc Natl Acad Sci U.S.A. (2021) 118 (6):e2013776118. doi: 10.1073/pnas.2013776118
27. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, et al. The immunopathophysiology of endometriosis. Trends Mol Med. (2018) 24:748–62. doi: 10.1016/j.molmed.2018.07.004
28. Shi M, Sekulovski N, Whorton AE, MacLean JA 2nd, Greaves E, Hayashi K. Efficacy of niclosamide on the intra-abdominal inflammatory environment in endometriosis. FASEB J. (2021) 35:e21584. doi: 10.1096/fj.202002541RRR
29. Zhao L, Shi M, Winuthayanon S, MacLean JA 2nd, Hayashi K. Niclosamide targets the dynamic progression of macrophages for the resolution of endometriosis in a mouse model. Commun Biol. (2022) 5:1225. doi: 10.1038/s42003-022-04211-0
30. Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. (2013) 4:9. doi: 10.3389/fimmu.2013.00009
31. Hill JA, Faris HM, Schiff I, Anderson DJ. Characterization of leukocyte subpopulations in the peritoneal fluid of women with endometriosis. Fertility sterility. (1988) 50:216–22. doi: 10.1016/S0015-0282(16)60062-6
32. Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. (2009) 175:547–56. doi: 10.2353/ajpath.2009.081011
33. Prather GR, MacLean JA 2nd, Shi M, Boadu DK, Paquet M, Hayashi K. Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model. Biol Reprod. (2016) 95:76. doi: 10.1095/biolreprod.116.140236
34. Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW, et al. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. (2019) 33(10):11210–11222. doi: 10.1096/fj.201900797R
35. Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflamm. (2017) 14:53. doi: 10.1186/s12974-017-0828-3
36. Kiran S, Kumar V, Murphy EA, Enos RT, Singh UP. High fat diet-induced CD8(+) T cells in adipose tissue mediate macrophages to sustain low-grade chronic inflammation. Front Immunol. (2021) 12:680944. doi: 10.3389/fimmu.2021.680944
37. Kiran S, Rakib A, Kodidela S, Kumar S, Singh UP. High-fat diet-induced dysregulation of immune cells correlates with macrophage phenotypes and chronic inflammation in adipose tissue. Cells. (2022) 11(8), 1327. doi: 10.3390/cells11081327
38. Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. (2016) 152:244–8. doi: 10.1016/j.lfs.2015.10.025
39. Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and adolescent obesity in the United States: A public health concern. Glob Pediatr Health. (2019) 6:2333794X19891305. doi: 10.1177/2333794X19891305
40. Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes (Lond). (2019) 43:1491–2. doi: 10.1038/s41366-019-0363-7
41. Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H, diet W. but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. (2007) 406:457–67. doi: 10.1042/BJ20070392
42. Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. (2004) 53 Suppl 3:S215–9. doi: 10.2337/diabetes.53.suppl_3.s215
43. van der Heijden RA, Sheedfar F, Morrison MC, Hommelberg PP, Kor D, Kloosterhuis NJ, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY). (2015) 7:256–68. doi: 10.18632/aging.100738
44. Gallou-Kabani C, Vige A, Gross MS, Rabes JP, Boileau C, Larue-Achagiotis C, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obes (Silver Spring). (2007) 15:1996–2005. doi: 10.1038/oby.2007.238
45. Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, et al. Inflammatory links between high fat diets and diseases. Front Immunol. (2018) 9:2649. doi: 10.3389/fimmu.2018.02649
46. Cassado Ados A, D'Imperio Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front Immunol. (2015) 6:225. doi: 10.3389/fimmu.2015.00225
47. Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, et al. Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertility sterility. (2008) 90:156–64. doi: 10.1016/j.fertnstert.2006.11.200
48. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discovery. (2014) 13:533–48. doi: 10.1038/nrd4334
49. Ding S, Zhu T, Tian Y, Xu P, Chen Z, Huang X, et al. Role of brain-derived neurotrophic factor in endometriosis pain. Reprod Sci. (2017) 25(7):1045–1057. doi: 10.1177/1933719117732161
50. Kobayashi H, Yamada Y, Morioka S, Niiro E, Shigemitsu A, Ito F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch Gynecol Obstet. (2014) 289:13–21. doi: 10.1007/s00404-013-3049-8
51. Greaves E, Temp J, Esnal-Zufiurre A, Mechsner S, Horne AW, Saunders PT. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol. (2015) 185:2286–97. doi: 10.1016/j.ajpath.2015.04.012
52. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U.S.A. (2005) 102:11070–5. doi: 10.1073/pnas.0504978102
53. Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod. (2019) 34:1106–16. doi: 10.1093/humrep/dez041
54. Hases L, Stepanauskaite L, Birgersson M, Brusselaers N, Schuppe-Koistinen I, Archer A, et al. High-fat diet and estrogen modulate the gut microbiota in a sex-dependent manner in mice. Commun Biol. (2023) 6:20. doi: 10.1038/s42003-022-04406-5
55. Lin H, An Y, Tang H, Wang Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J Agric Food Chem. (2019) 67:3624–32. doi: 10.1021/acs.jafc.9b00249
56. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility sterility. (1997) 67:817–21. doi: 10.1016/S0015-0282(97)81391-X
57. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. (2007) 22:266–71. doi: 10.1093/humrep/del339
58. Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. (2017) 32:315–24. doi: 10.1093/humrep/dew293
59. Bishop CV, Takahashi DL, Luo F, Sidener H, Martin LD, Gao L, et al. The combined impact of testosterone and Western-style diet on endometriosis severity and progression in rhesus macaquesdagger. Biol Reprod. (2023) 108:72–80. doi: 10.1093/biolre/ioac183
60. Kim TH, Bae N, Kim T, Hsu AL, Hunter MI, Shin JH, et al. Leptin stimulates endometriosis development in mouse models. Biomedicines. (2022) 10(9), 2160. doi: 10.3390/biomedicines10092160
61. Heard ME, Melnyk SB, Simmen FA, Yang Y, Pabona JM, Simmen RC. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology. (2016) 157:2870–82. doi: 10.1210/en.2016-1092
62. Heard-Lipsmeyer ME, Alhallak I, Simmen FA, Melnyk SB, Simmen RCM. Lesion genotype modifies high-fat diet effects on endometriosis development in mice. Front Physiol. (2021) 12:702674. doi: 10.3389/fphys.2021.702674
63. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. (2019) 12:843–50. doi: 10.1038/s41385-019-0160-6
64. Machairiotis N, Vasilakaki S, Thomakos N. Inflammatory mediators and pain in endometriosis: A systematic review. Biomedicines. (2021) 9(1), 54. doi: 10.3390/biomedicines9010054
65. Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. (2019) 68:1417–29. doi: 10.1136/gutjnl-2018-317609
66. Fan S, Chen S, Lin L. Research progress of gut microbiota and obesity caused by high-fat diet. Front Cell Infect Microbiol. (2023) 13:1139800. doi: 10.3389/fcimb.2023.1139800
67. Velazquez KT, Enos RT, Bader JE, Sougiannis AT, Carson MS, Chatzistamou I, et al. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J Hepatol. (2019) 11:619–37. doi: 10.4254/wjh.v11.i8.619
68. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
69. Zheng Z, Lyu W, Ren Y, Li X, Zhao S, Yang H, et al. Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front Nutr. (2021) 8:690138. doi: 10.3389/fnut.2021.690138
70. Gill PA, Inniss S, Kumagai T, Rahman FZ, Smith AM. The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front Immunol. (2022) 13:866059. doi: 10.3389/fimmu.2022.866059
71. Greaves E, Collins F, Esnal-Zufiaurre A, Giakoumelou S, Horne AW, Saunders PT. Estrogen receptor (ER) agonists differentially regulate neuroangiogenesis in peritoneal endometriosis via the repellent factor SLIT3. Endocrinology. (2014) 155:4015–26. doi: 10.1210/en.2014-1086
72. Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. (2014) 184:1930–9. doi: 10.1016/j.ajpath.2014.03.011
73. Greaves E, Horne AW, Jerina H, Mikolajczak M, Hilferty L, Mitchell R, et al. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci Rep. (2017) 7:44169. doi: 10.1038/srep44169
74. Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PloS One. (2014) 9:e86378. doi: 10.1371/journal.pone.0086378
75. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
76. Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinf Chapter. (2011) 10:10 7 1–10 7 20. doi: 10.1002/0471250953.bi1007s36
Keywords: endometriosis, pain, high-fat diets, inflammation, macrophages, microbiota
Citation: Herup-Wheeler T, Shi M, Harvey ME, Talwar C, Kommagani R, MacLean JA II and Hayashi K (2024) High-fat diets promote peritoneal inflammation and augment endometriosis-associated abdominal hyperalgesia. Front. Endocrinol. 15:1336496. doi: 10.3389/fendo.2024.1336496
Received: 10 November 2023; Accepted: 28 February 2024;
Published: 15 March 2024.
Edited by:
Iveta Yotova, Medical University of Vienna, AustriaReviewed by:
Valentina Caputi, University College Cork, IrelandStacy McAllister, Emory University, United States
Zhexin Ni, Second Military Medical University, China
Copyright © 2024 Herup-Wheeler, Shi, Harvey, Talwar, Kommagani, MacLean and Hayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanako Hayashi, ay5oYXlhc2hpQHdzdS5lZHU=
†These authors contributed equally to this work
 Tristin Herup-Wheeler1†
Tristin Herup-Wheeler1† Mingxin Shi
Mingxin Shi Madeleine E. Harvey
Madeleine E. Harvey Chandni Talwar
Chandni Talwar James A. MacLean II
James A. MacLean II Kanako Hayashi
Kanako Hayashi