- 1Department of Endocrinology and Metabolism, Peking University People’s Hospital, Beijing, ;China
- 2School of Automation, Beijing Institute of Technology, Beijing, ;China
Objective: The aim of this study is to determine the residual C-peptide level and to explore the clinical significance of preserved C-peptide secretion in glycemic control in Chinese individuals with type 1 diabetes (T1D).
Research design and methods: A total of 534 participants with T1D were enrolled and divided into two groups, low–C-peptide group (fasting C-peptide ≤10 pmol/L) and preserved–C-peptide group (fasting C-peptide >10 pmol/L), and clinical factors were compared between the two groups. In 174 participants who were followed, factors associated with C-peptide loss were also identified by Cox regression. In addition, glucose metrics derived from intermittently scanned continuous glucose monitoring were compared between individuals with low C-peptide and those with preserved C-peptide in 178 participants.
Results: The lack of preserved C-peptide was associated with longer diabetes duration, glutamic acid decarboxylase autoantibody, and higher daily insulin doses, after adjustment {OR, 1.10 [interquartile range (IQR), 1.06–1.14]; OR, 0.46 (IQR, 0.27–0.77); OR, 1.04 (IQR, 1.02–1.06)}. In the longitudinal analysis, the percentages of individuals with preserved C-peptide were 71.4%, 56.8%, 71.7%, 62.5%, and 22.2% over 5 years of follow-up. Preserved C-peptide was also associated with higher time in range after adjustment of diabetes duration [62.4 (IQR, 47.3–76.6) vs. 50.3 (IQR, 36.2–63.0) %, adjusted P = 0.003].
Conclusions: Our results indicate that a high proportion of Chinese patients with T1D had preserved C-peptide secretion. Meanwhile, residual C-peptide was associated with favorable glycemic control, suggesting the importance of research on adjunctive therapy to maintain β-cell function in T1D.
Introduction
Type 1 diabetes (T1D) is characterized by progressive autoimmune destruction of β cells. The loss of β cells leading to the diagnosis of T1D is gradual and continues after clinical onset. Initially, a significant number of β cells remain, and relatively low doses of exogenous insulin are required to limit glucose variability and hypoglycemia. Although it has been assumed that β cells are irreversibly lost after diagnosis, recent studies have shown that not all β cells are destroyed and that many people with T1D continue to produce insulin even after long-term disease course (1, 2). The Diabetes Control and Complications Trial showed that the persistence of residual β cells, as measured by C-peptide secretion, is associated with better glycemic control, reduced glycemic variability, and a lower incidence of microvascular complications (3, 4). Understanding the presence and trends of residual β-cell function and its relationship to the heterogeneity of glycemic control may provide insights into the natural history of the disease and facilitate possible interventions to modify disease progression.
Previous studies have suggested heterogeneity in preserved β-cell function in T1D across cohorts and according to the definition of “preserved C-peptide secretion.” In the Scottish Diabetes Research Network Type 1 Bioresource cohort, 37.7% of participants retain detectable non-fasting C-peptide (>5 pmol/L) (5). In addition, in the T1D Exchange Clinic Network, detectable non-fasting C-peptide (>17 pmol/L) was found in 29% of participants, and the frequency of non-fasting C-peptide ≥200 pmol/L was 10% (6). Meanwhile, even minimal levels of C-peptide have clinical significance in established T1D. Kuhtreiber et al. found that fasting C-peptide levels >10 pmol/L were associated with protection from complications (7), and Fraser et al. found that, under the same definition of preserved C-peptide, it was associated with fewer low glucose events and lower glucose variability on intermittently scanned continuous glucose monitoring (isCGM) (8). Although the maintenance of C-peptide secretion has been well studied in the Caucasian population, little is known about non-Caucasian populations, particularly East Asians. The aim of this study was to evaluate residual β-cell function, the underlying clinical factors contributing to the preservation of C-peptide secretion, and its impact on glycemic control in Chinese individuals with T1D.
Research design and methods
Study design and participants
A total of 631 individuals with T1D treated at Peking University People’s Hospital from January 2017 to October 2022 were screened for eligibility. The diagnosis of T1D was made independently by two endocrinologists based on clinical manifestations: diabetes ketoacidosis at the onset of disease, initiation of insulin therapy within 6 months of diagnosis and continued thereafter, or positive diabetes autoantibody [islet cell autoantibody (ICA)/insulin autoantibody (IAA)/glutamic acid decarboxylase (GAD) autoantibody]. Moreover, individuals with fasting C-peptide >1,500 pmol/L were excluded to limit the possibility of including people with diagnoses other than T1D (N = 4). Sixty-six participants lacking the data of C-peptide and 27 participants lacking the information of diabetes duration were excluded. Cross-sectional analysis were performed in the remained 534 participants. Of the participants, 174 people who returned to the clinic and had regular β-cell function assessments were included in the longitudinal analysis to determine the change in C-peptide secretion over the course of the disease. Meanwhile, 178 participants who wore professional isCGM were also included for analysis of glucose control according to C-peptide levels (Figure 1).
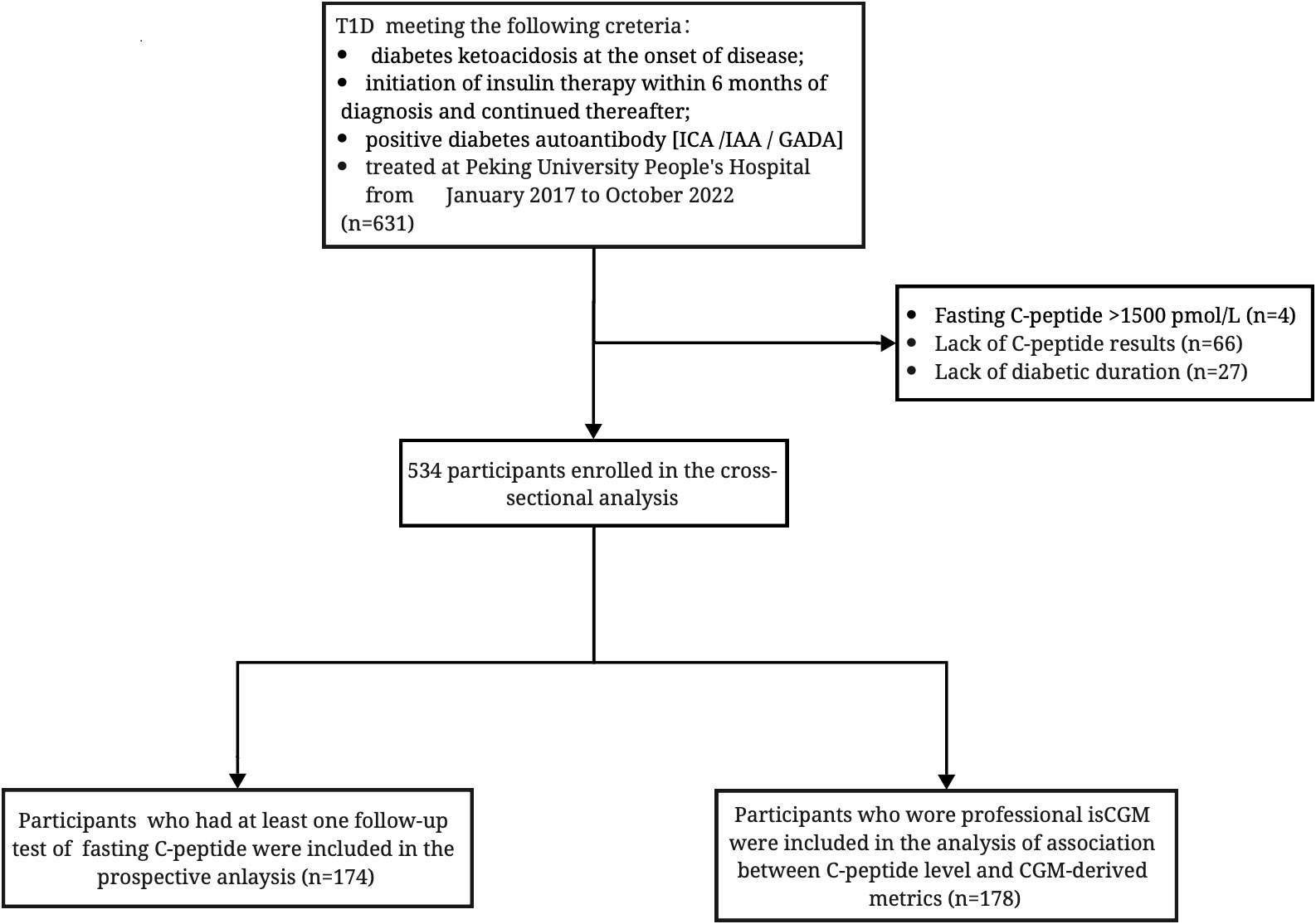
Figure 1 Inclusion flowchart of 534 participants with diabetic duration information and fasting C-peptide data; 174 participants had a at least one follow-up test of fasting C-peptide test; 178 participants had CGM-derived data. ICA, islet cell autoantibody; IAA, insulin autoantibody; GADA, glutamic acid decarboxylase autoantibody.
The study was conducted in accordance with the ethical principles in the Declaration of Helsinki and was approved by the Peking University People’s Hospital Ethics Committee (2022PHB407-001). Informed consent was obtained from all participants.
Physical and laboratory measurements
Blood samples were taken in the morning after an 8-h to 10-h fast, and a mixed meal tolerance test (MMTT) was performed (9). During the MMTT, participants consumed a standardized breakfast calculated on the basis of total caloric requirements (25%–30% of daily caloric intake; 50% of calories as carbohydrates, 33% of calories as lipids, and 17% of calories as proteins). Glucose, low-density lipoprotein cholesterol (LDL-C), triglycerides, and uric acid were measured using an automated biochemistry analyzer. HbA1c was measured by high-performance liquid chromatography (Primus Ultra 2, Trinity Biotech, Bray, Co-Wicklow, Ireland). Insulin and C-peptide were assayed by electrochemiluminescence immunoassay on a Roche autoanalyzer (Cobas e601, Germany) using Elecsys C-Peptide (Roche Diagnostics GmbH, Mannheim, Germany). The inter-assay CVs for the low–, medium–, and high–C-peptide controls were 3.4%, 2.6%, and 1.8%, respectively.
Professional isCGM
isCGM was placed at clinic by care givers. A professional CGM (Freestyle Libre H, Abbott, US) was used to collect glucose data every 15 min for 14 days. The glucose metrics were calculated using data from 174 participants who had sensor activation over 90% during the 14 days period. Standard deviation (SD), mean glucose (MG), coefficient of variance (CV), interquartile range (IQR), mean amplitude of glucose excursions (MAGE), time below range (TBR), time above range (TAR), and time in range (TIR) were calculated according to isCGM data.
Statistical analyses
Unless explicitly stated otherwise, statistical analysis for this study was performed as follows. Continuous variables that followed a normal distribution were expressed as mean ± SD, whereas non-normally distributed variables were presented as median with IQR. Categorical variables were reported as proportions.
Participants were divided into a low–C-peptide group (fasting C-peptide ≤10 pmol/L) and a preserved–C-peptide group (fasting C-peptide >10 pmol/L). One-way ANOVA and Mann–Whitney U-tests were used to compare continuous variables between the two cohorts, depending on the distribution of the variables. Chi-squared tests were used for categorical variables.
Factors identified in the univariate analysis, including age at diagnosis, duration of diabetes, body mass index (BMI), positive GAD autoantibody, estimated glomerular filtration rate (eGFR), daily insulin dose, and HbA1c category were then examined using binary logistic regression analysis. In the longitudinal cohort, the change in C-peptide levels from baseline to last follow-up (ΔC-peptide last follow-up - baseline) was used to define individuals with sustained and failed β-cell function. Cox regression analysis was also performed to determine the influence of age at diagnosis, duration of diabetes, HbA1c, and positive GAD autoantibodies on β-cell function. Diabetes duration was adjusted in the logistic model to assess the association between C-peptide level and CGM metric.
Statistical analysis was performed using SPSS software (version 26), and a p-value<0.05 was considered statistically significant. R (version 4.3.2) and GraphPad Prism (version 9.3.1) were used to generate the figures.
Results
A total of 534 people were included in the study, 46.1% of whom were men. The average age of the participants was 50 years, and the average duration of diabetes was 9 years. The average HbA1c level of the participants was 8.9%, and 21.9% of the participants were under euglycemic control (HbA1c ≤7%).
Preserved C-peptide was common even with long duration of diabetes
Of the participants, 55.4% still had preserved C-peptide (fasting C-peptide >10 pmol/L). Among those who had diabetes for more than 20 years (n = 131), 38.9% still had detectable C-peptide levels (fasting C-peptide >3 pmol/L, Supplementary Figure 1). Fasting C-peptide levels decreased with diabetes duration, and the fitted curve suggested a non-linear association between C-peptide and disease duration (Supplementary Figure 2).
C-peptide levels independently associated with diabetes duration and positive GAD autoantibody
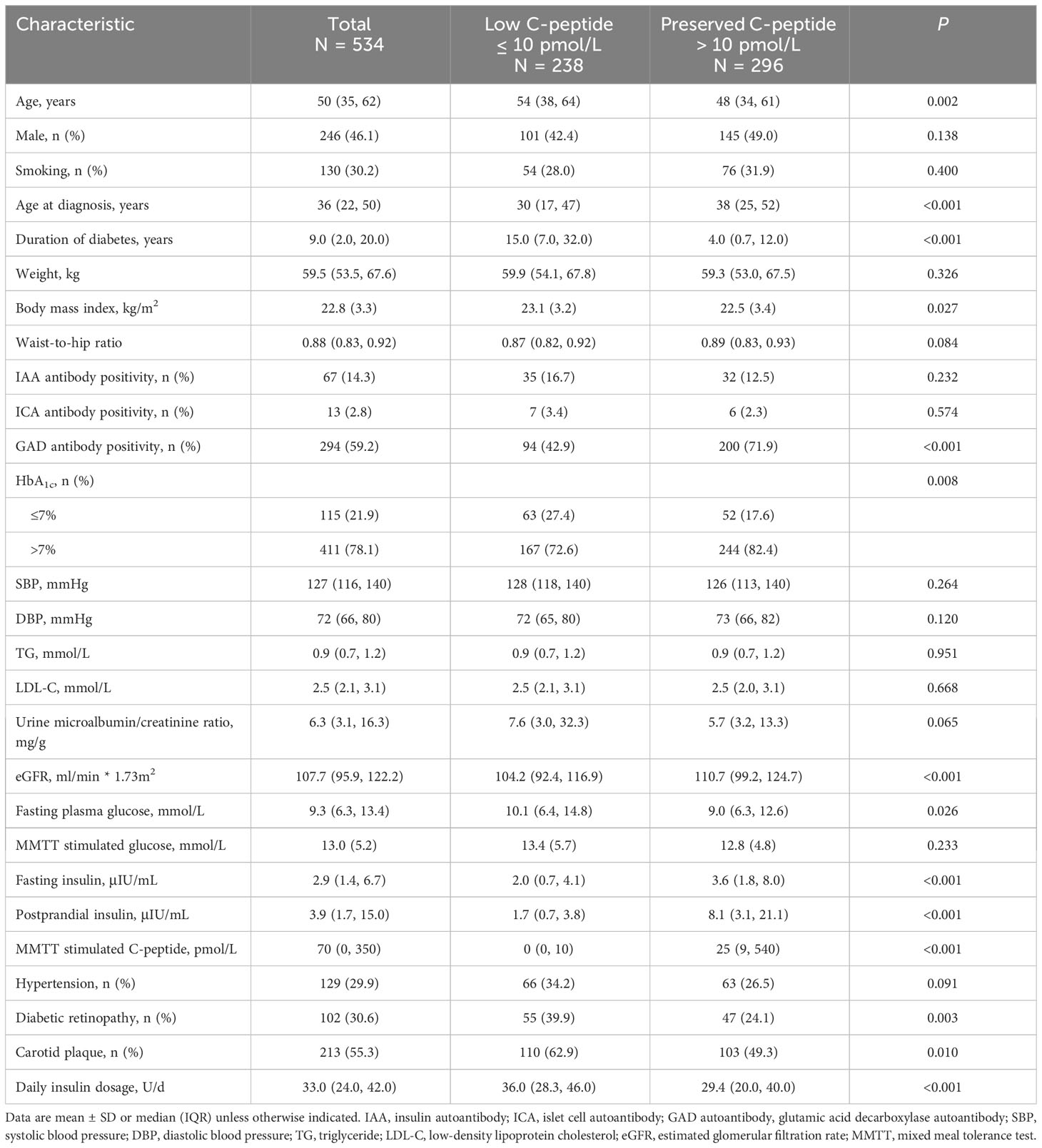
Participants in the preserved–C-peptide group were younger [48 (IQR, 34–61) vs. 54 (IQR, 38–64) years, P = 0.002], had a shorter diabetes duration [4.0 (IQR, 0.7–12.0) vs. 15.0 (IQR, 7.0–32.0) years, P 0.001], and had a lower insulin dose [29.4 (IQR, 20.0–40.0) vs. 36.0 (IQR, 28.3–46.0) U/d, P <0.001] compared with those in the low–C-peptide group. Meanwhile, BMI was lower in the preserved–C-peptide group than that in the low–C-peptide group (22.5 ± 3.4 vs. 23.1± 3.2 kg/m2, P = 0.027). Positive GAD autoantibody was detected in 71.9% of participants in the preserved C-peptide and 42.9% in the low–C-peptide group (P 0.001). The eGFR was also higher in the preserved–C-peptide group [110.7 (IQR, 99.2–124.7) vs. 104.2 (IQR, 92.4–116.9) ml/min * 1.73 m2, P< 0.001]. In addition, the rates of diabetic retinopathy and carotid plaque were lower in the preserved–C-peptide group (24.1% vs. 39.9%, P = 0.003; 49.3% vs. 62.9%, P = 0.010) Table 1.
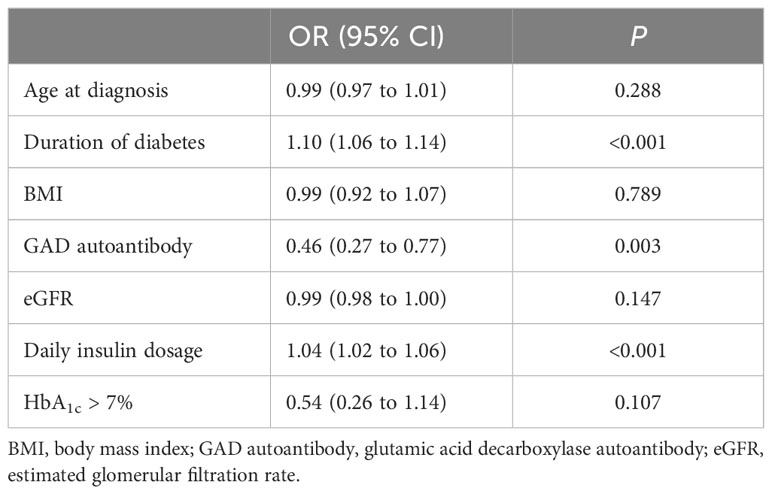
After adjustment for age at diagnosis, duration of diabetes, BMI, GAD autoantibodies, eGFR, and HbA1c, three factors including duration of diabetes, GAD autoantibodies, and daily insulin dosage were still associated with lack of preserved C-peptide [OR 1.10 (IQR, 1.06–1.14); OR, 0.46 (IQR, 0.27–0.77); OR, 1.04 (IQR, 1.02–1.06)] Table 2.
Sustained β-cell function associated with diabetes duration in the longitudinal cohort
The longitudinal analysis included 174 participants who had at least one follow-up visit with a fasting C-peptide test. The median follow-up was 2.0 years. Supplementary Figure 3A shown that β-cell function declined with increasing duration of diabetes. The proportions of participants with C-peptide >10 pmol/L were 71.4%, 56.8%, 71.7%, 62.5%, and 22.2% at baseline, < 1 year, 1 to 2 years, 2 to 3 years, 3 to 4 years, and 4 to 5 years follow-up, respectively (Supplementary Figure 3B). We divided these participants into two cohorts: those with failed β-cell function (ΔC-peptide the last follow-up - baseline ≤ 0) and those with sustained β-cell function (ΔC-peptide the last follow-up - baseline 0). Cox regression analysis showed that duration of diabetes was independently associated with sustained β-cell function (Supplementary Table 1).
Preserved C-peptide was associated with higher TIR
Mean glucose was lower in the preserved–C-peptide group compared with that in the low–C-peptide group [8.4 (IQR, 7.0–10.2) vs. 9.9 (IQR, 8.3–11.7) mmol/L, P <0.001]. In addition, TIR was higher, and TAR was lower in the preserved–C-peptide group [62.4 (IQR, 47.3–76.6) vs. 50.3 (IQR, 36.2–63.0) %, P <0.001; 27.4 (IQR, 14.0–49.3) vs. 44.4 (IQR, 31.0–62.5), P <0.001). Glucose metrics indicating variability, including SD, IQR, and MAGE, were lower in the preserved–C-peptide group [3.1 .9 vs.3.8 .9 mmol/L, P <0.001; 4.2 ± 1.4 vs. 5.4 ± 1.5 mmol/L, P < 0.001; 7.1 (IQR, 4.2–13.3) vs. 10.8 (IQR, 4.9–16.3), P = 0.029]. After adjustment of diabetes duration, preserved C-peptide was still associated with higher TIR and lower TAR, SD, and IQR (P =0.003, P =0.003, P 0.03, P <0.001, and P <0.001) (Figure 2; Supplementary Table 2).
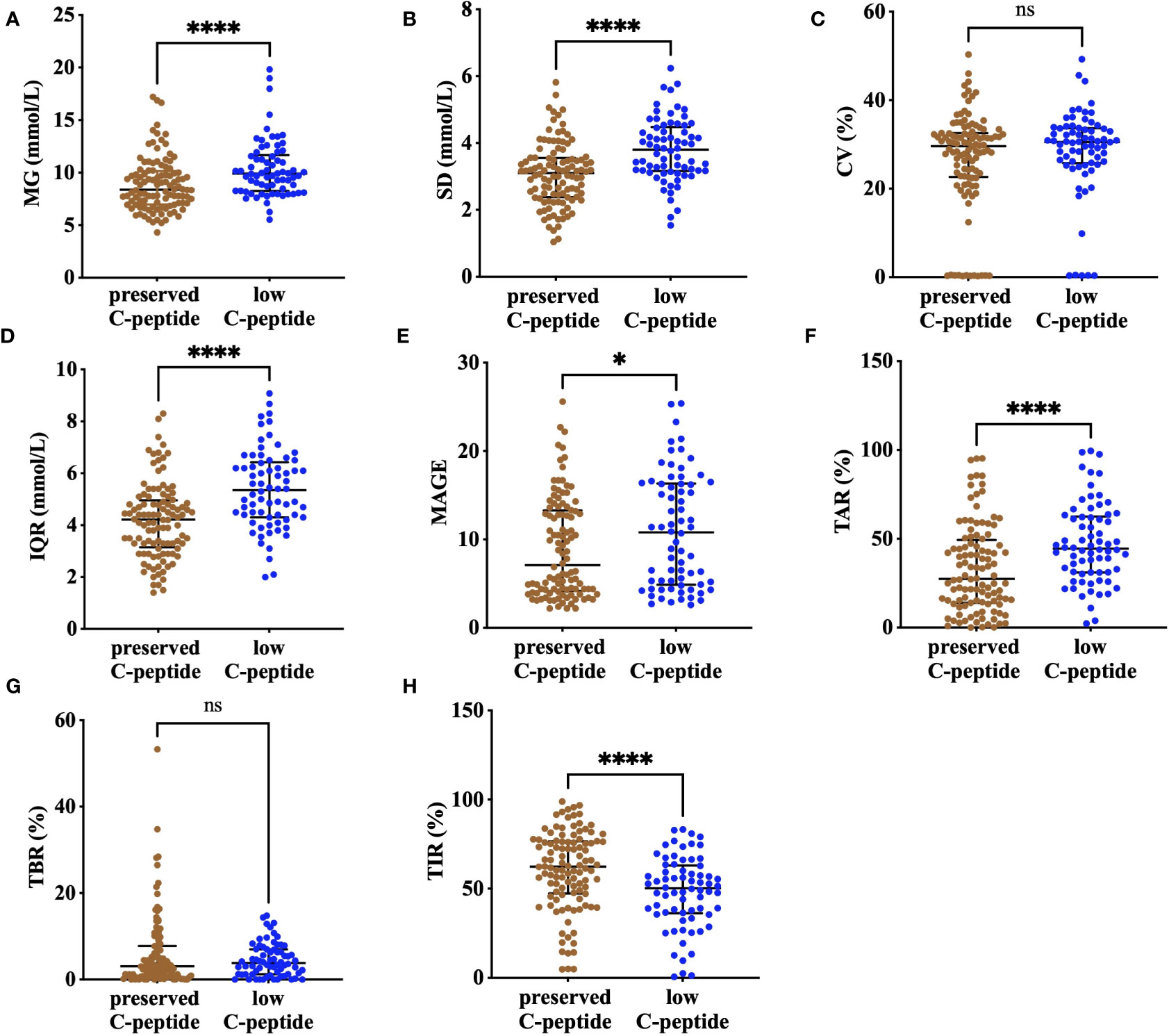
Figure 2 The comparison of CGM metrics, including MG (A), SD (B), CV (C), IQR (D), MAGE (E), TAR (F), TBR (G), and TIR (H), between the preserved–C-peptide group and the low–C-peptide group. **** means P<0.001, * means P<0.05, and ns means non-significant; MG, mean glucose; CV, coefficient of variance; IQR, interquartile range; SD, standard deviation; MAGE, mean amplitude of glycemia excursions; TBR, time below range (glucose concentrations below 3.9 mmol/L); TIR, time in range (glucose concentrations of 3.9–10.0 mmol/L); TAR, time above range (glucose concentrations over 10.0 mmol/L).
Conclusions
Our study showed that preserved C-peptide secretion was common in Chinese individuals with T1D and was associated with diabetes duration, positive GAD autoantibody, and insulin dosage. Meanwhile, preserved C-peptide was also associated with favorable glycemic control as represented by TIR.
Persistent C-peptide secretion, reflecting some degree of intrinsic β-cell function, is now recognized to be common in T1D (5, 8, 10). In the Joslin Medalist Study, residual C-peptide secretion was detected in a large proportion of Medalists, even after more than 50 years of follow-up (9). However, such studies were mainly conducted in Caucasian populations, and few studies have focused on Chinese, with the currently available studies having relatively short diabetes duration or small populations. In a cohort of 446 participants with T1D with a mean duration of 2.36 years, more than 80% of them had detectable C-peptide, but the percentage decreased rapidly with disease progression (11). In another study of 109 participants with T1D followed for at least 10 years, Cheng et al. showed that 38.5% of participants had detectable C-peptide secretion (random C-peptide ≥16.7 pmol/L) (12). Miao and colleagues reported that, in 443 participants with T1D for 2.38 years, stimulated C-peptide ≥200 pmol/L was detected in 64.3% of participants (13). To our knowledge, our study was the largest with a relatively long duration of diabetes in the Chinese population with T1D and suggested that more than half of them still had preserved insulin secretion.
Previous studies have suggested that age at diagnosis, duration of diabetes, autoantibody positivity, and Human Leukocyte antigen (HLA) genotype may influence serum C-peptide level (10, 14, 15). Our results were consistent with the previous studies that diabetes duration was negatively associated with residual β-cell function and that autoantibody positivity was correlated with sustained intrinsic insulin production (1, 13, 16). The relationship between longer disease duration and lower C-peptide is widely recognized according to previous studies, whereas the finding of a strong relationship between higher autoantibody levels and higher C-peptide levels is difficult to interpret. Autoantibodies are generally good predictors of disease onset but are not specific for disease outcome (17, 18). Our previous findings showed that 17.1% of Chinese patients with T1D with long duration of diabetes were with GAD autoantibody positive, and 14.7% had fasted serum C-peptide higher than 75 pmol/L (19). Further investigation of GAD autoantibody is clearly required. Meanwhile, our study suggested that residual β-cell function was associated with lower daily insulin dose, which was in line with previous studies (11, 20–23). Because C-peptide levels represent intrinsic β-cell function (24), a possible explanation is that participants with higher C-peptide levels had more endogenous insulin production and required lower doses of exogenous insulin. As accumulating evidence suggests that preserved C-peptide is associated with a lower likelihood of diabetes microvascular complications (5, 10, 25), the association between autoantibody positivity, residual β-cell function, and favorable diabetes outcomes should be further investigated and the underlying mechanisms explored.
Understanding how the residual β-cell function relates to the heterogeneity of glycemic control is important for people with diabetes and their clinicians. Moreover, a more personalized approach to diabetes care may be possible with a better understanding of the contribution of residual β-cell function to CGM-derived metrics such as TBR, TIR, TAR, and CV. Previous studies have investigated the impact of residual insulin secretion in T1D, as measured by the MMTT, on the maintenance of glycemic control, as measured by HbA1c (20, 26, 27). Previous studies in Caucasian populations have investigated the association between residual β-cell function and TIR. Researchers found that, in the T1D Exchange participants, fasting C-peptide was correlated with higher TIR (28). In addition, in a recent study recruiting participants from The Netherlands, Coco et al. suggested that residual insulin secretion, as measured by urinary C-peptide to creatinine ratio, was associated with longer TIR, shorter TBR and TAR, and lower CV (23). Although a study conducted in Chinese patients with diabetes including T1D, type 2 diabetes, and latent autoimmune diabetes in adults showed a continuous spectrum of glycemic variability pattern (29), no large-scale study focusing on T1D population in Chinese has been reported. Our study provided a relatively large sample size covering the entire duration of T1D in Chinese and showed that residual β-cell function was associated with TIR after adjustment for potential confounders.
This study had several limitations. First, the cross-sectional design made it impossible to establish causality. However, it is most likely that preserved β-cell function has a positive effect on glycemic control and not vice versa, as recent studies have shown that tight glycemic control, even with an artificial pancreas, does not preserve β-cell function even in newly diagnosed T1D subjects (30, 31). Second, although fasted serum may be a good representation of β-cell function, it is not considered the gold standard for measuring β-cell function. Therefore, we cannot exclude the possibility that our study underestimates the contribution of β-cell function to glycemic control. Third, our study was a single-center study, and the number of young patients with T1D was limited; we are planning on elaborate with some specialized children’s hospitals in the future study. Finally, as the CGM data were not blinded to the participants, other important confounders related to glycemic control, such as diabetes management skills, emotional factors could also contribute to the individual’s CGM metrics. However, as we used professional CGM in the study and all participants received standard T1D care from our specialists, the impact of individual procedures was minimized. Nevertheless, we point out that this observation further supports the concept that β-cell function contributes to better daily control, as we found strong and consistent associations with both TIR and TAR.
In conclusion, residual β-cell function was common in people with T1D, and preservation of C-peptide secretion was associated with shorter duration, positive GAD autoantibody, and lower insulin dosage. As glucose control measured by CGM is at least partly influenced by residual β-cell function, personalized glucose targets should be considered on the basis of individual C-peptide level. Furthermore, disease-modifying therapies aiming to preserve β-cell function should also be considered in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Peking University People’s Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WL: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YF: Data curation, Formal analysis, Visualization, Writing – original draft. XC: Conceptualization, Methodology, Writing – review & editing. YZ: Investigation, Writing – review & editing. MZ: Investigation, Writing – review & editing. XH: Investigation, Writing – review & editing. JL: Investigation, Writing – review & editing. SY: Investigation, Writing – review & editing. DC: Software, Writing – review & editing. JC: Software, Writing – review & editing. LW: Software, Writing – review & editing. DS: Software, Writing – review & editing. LJ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Peking University People’s Hospital Scientific Research Development Funds (RDY2019-05), Peking University Medical Science and Technology Fund (2158000040), National Natural Science Foundation of China (82000770).
Acknowledgments
The authors would like to thank all the patients and staff who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1335913/full#supplementary-material
References
1. Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care (2012) 35(3):465–70. doi: 10.2337/dc11-1236
2. Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. (2014) 57(1):187–91. doi: 10.1007/s00125-013-3067-x
3. Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. (2014) 63(2):739–48. doi: 10.2337/db13-0881
4. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care (2003) 26(3):832–6. doi: 10.2337/diacare.26.3.832
5. Jeyam A, Colhoun H, McGurnaghan S, Blackbourn L, McDonald TJ, Palmer CNA, et al. Clinical impact of residual C-peptide secretion in type 1 diabetes on glycemia and microvascular complications. Diabetes Care (2021) 44(2):390–8. doi: 10.2337/dc20-0567
6. Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care (2015) 38(3):476–81. doi: 10.2337/dc14-1952
7. Kuhtreiber WM, Washer SL, Hsu E, Zhao M, Reinhold P 3rd, Burger D, et al. Low levels of C-peptide have clinical significance for established type 1 diabetes. Diabetes Med (2015) 32(10):1346–53. doi: 10.1111/dme.12850
8. Gibb FW, McKnight JA, Clarke C, Strachan MWJ. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia. (2020) 63(5):906–14. doi: 10.1007/s00125-020-05099-3
9. Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, et al. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. (2010) 59(11):2846–53. doi: 10.2337/db10-0676
10. Harsunen M, Haukka J, Harjutsalo V, Mars N, Syreeni A, Härkönen T, et al. Residual insulin secretion in individuals with type 1 diabetes in Finland: longitudinal and cross-sectional analyses. Lancet Diabetes Endocrinol (2023) 11(7):465–73. doi: 10.1016/S2213-8587(23)00123-7
11. Wang Y, Qin Y, Gu H, Zhang L, Wang J, Huang Y, et al. High residual β-cell function in Chinese patients with autoimmune type 1 diabetes. J Clin Endocrinol Metab (2022) 107(6):e2348–e58. doi: 10.1210/clinem/dgac077
12. Cheng J, Yin M, Tang X, Yan X, Xie Y, He B, et al. Residual β-cell function after 10 years of autoimmune type 1 diabetes: prevalence, possible determinants, and implications for metabolism. Ann Transl Med (2021) 9(8):650. doi: 10.21037/atm-20-7471
13. Miao H, Zhang J, Gu B, Gao A, Hong J, Zhang Y, et al. Prognosis for residual islet β-cell secretion function in young patients with newly diagnosed type 1 diabetes. J Diabetes (2019) 11(10):818–25. doi: 10.1111/1753-0407.12912
14. Bogun MM, Bundy BN, Goland RS, Greenbaum CJ. C-Peptide levels in subjects followed longitudinally before and after type 1 diabetes diagnosis in TrialNet. Diabetes Care (2020) 43(8):1836–42. doi: 10.2337/dc19-2288
15. McKeigue PM, Spiliopoulou A, McGurnaghan S, Colombo M, Blackbourn L, McDonald TJ, et al. Persistent C-peptide secretion in type 1 diabetes and its relationship to the genetic architecture of diabetes. BMC Med (2019) 17(1):165. doi: 10.1186/s12916-019-1392-8
16. Marren SM, Hammersley S, McDonald TJ, Shields BM, Knight BA, Hill A, et al. Persistent C-peptide is associated with reduced hypoglycaemia but not HbA(1c) in adults with longstanding type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabetes Med (2019) 36(9):1092–9. doi: 10.1111/dme.13960
17. Brorsson C, Vaziri-Sani F, Bergholdt R, Eising S, Nilsson A, Svensson J, et al. Correlations between islet autoantibody specificity and the SLC30A8 genotype with HLA-DQB1 and metabolic control in new onset type 1 diabetes. Autoimmunity. (2011) 44(2):107–14. doi: 10.3109/08916934.2010.509120
18. Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. (2005) 54 Suppl 2:S32–9. doi: 10.2337/diabetes.54.suppl_2.s32
19. Liu W, Han X, Wang Y, Gong S, Ma Y, Zhang S, et al. Characteristics and ongoing autoimmunity of patients with long-standing type 1 diabetes living in China. Diabetes Care (2018) 41(6):e97–e8. doi: 10.2337/dc18-0046
20. Sørensen JS, Johannesen J, Pociot F, Kristensen K, Thomsen J, Hertel NT, et al. Residual β-Cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care (2013) 36(11):3454–9. doi: 10.2337/dc13-0418
21. Suh J, Lee HI, Lee M, Song K, Choi HS, Kwon A, et al. Insulin requirement and complications associated with serum C-peptide decline in patients with type 1 diabetes mellitus during 15 years after diagnosis. Front Endocrinol (Lausanne) (2022) 13:869204. doi: 10.3389/fendo.2022.869204
22. McGee P, Steffes M, Nowicki M, Bayless M, Gubitosi-Klug R, Cleary P, et al. Insulin secretion measured by stimulated C-peptide in long-established type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabetes Med (2014) 31(10):1264–8. doi: 10.1111/dme.12504
23. Fuhri Snethlage CM, McDonald TJ, Oram RD, de Groen P, Rampanelli E, Schimmel AWM, et al. Residual β-Cell function is associated with longer time in range in individuals with type 1 diabetes. Diabetes Care (2023) 46:1–8. doi: 10.2337/dc23-0776
24. McDonald TJ, Perry MH. Detection of C-peptide in urine as a measure of ongoing beta cell function. Methods Mol Biol (2016) 1433:93–102. doi: 10.1007/7651_2016_330
25. Gabbay MAL, Crispim F, Dib SA. Residual β-cell function in Brazilian Type 1 diabetes after 3 years of diagnosis: prevalence and association with low presence of nephropathy. Diabetol Metab Syndr (2023) 15(1):51. doi: 10.1186/s13098-023-01014-z
26. Gubitosi-Klug RA, Braffett BH, Hitt S, Arends V, Uschner D, Jones K, et al. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest (2021) 131(3):e143011. doi: 10.1172/JCI143011
27. Sorensen JS, Birkebaek NH, Bjerre M, Pociot F, Kristensen K, Hoejberg AS, et al. Residual β-cell function and the insulin-like growth factor system in Danish children and adolescents with type 1 diabetes. J Clin Endocrinol Metab (2015) 100(3):1053–61. doi: 10.1210/jc.2014-3521
28. Rickels MR, Evans-Molina C, Bahnson HT, Ylescupidez A, Nadeau KJ, Hao W, et al. High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest (2020) 130(4):1850–62. doi: 10.1172/JCI134057
29. Zhang L, Guo K, Tian Q, Ye J, Ding Z, Zhou Q, et al. The continuous spectrum of glycaemic variability changes with pancreatic islet function: A multicentre cross-sectional study in China. Diabetes Metab Res Rev (2022) 38(8):e3579. doi: 10.1002/dmrr.3579
30. McVean J, Forlenza GP, Beck RW, Bauza C, Bailey R, Buckingham B, et al. Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: A randomized clinical trial. Jama. (2023) 329(12):980–9. doi: 10.1001/jama.2023.2063
Keywords: type 1 diabetes, preserved C-peptide, beta cell, CGM, glycemic control
Citation: Liu W, Fang Y, Cai X, Zhu Y, Zhang M, Han X, Li J, Yin S, Cai D, Chen J, Wang L, Shi D and Ji L (2024) Preserved C-peptide is common and associated with higher time in range in Chinese type 1 diabetes. Front. Endocrinol. 15:1335913. doi: 10.3389/fendo.2024.1335913
Received: 09 November 2023; Accepted: 23 January 2024;
Published: 09 February 2024.
Edited by:
Joseph M. Pappachan, Lancashire Teaching Hospitals NHS Foundation Trust, United KingdomReviewed by:
Mohammad Jeeyavudeen, University of Edinburgh, United KingdomHoward Davidson, University of Colorado, United States
Anoop Mohamed Iqbal, Advocate Aurora Health, United States
Dayakshi Abeyaratne, University of Ruhuna, Sri Lanka
Copyright © 2024 Liu, Fang, Cai, Zhu, Zhang, Han, Li, Yin, Cai, Chen, Wang, Shi and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Cai, ZHJfanVuZWxAc2luYS5jb20=; Linong Ji, amlsbkBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Wei Liu1†
Wei Liu1† Yayu Fang
Yayu Fang Xiaoling Cai
Xiaoling Cai Dawei Shi
Dawei Shi Linong Ji
Linong Ji