- Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, China
Background: It has been demonstrated that in diabetic patients, an elevated neutrophil-lymphocyte ratio (NLR) is independently connected with higher cardiovascular and all-cause mortality. It is unclear, however, if the systemic immune-inflammatory index (SII) and the mortality rate among diabetic patients are related. Investigating the linkage between SII and diabetes patients’ risk of cardiovascular and all-cause death was the aim of the study.
Methods: 4972 diabetics who were chosen from six rounds of the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2016 were the study’s participants. The optimal SII threshold with the highest correlation with survival outcomes was identified by applying the Maximum Selection Ranking Statistical Method (MSRSM). To assess the relationship between SII and cardiovascular and all-cause mortality in diabetics, subgroup analysis and Cox regression modeling were employed. Furthermore, smoothed curve fitting was utilized to determine the nonlinear relationship of them.
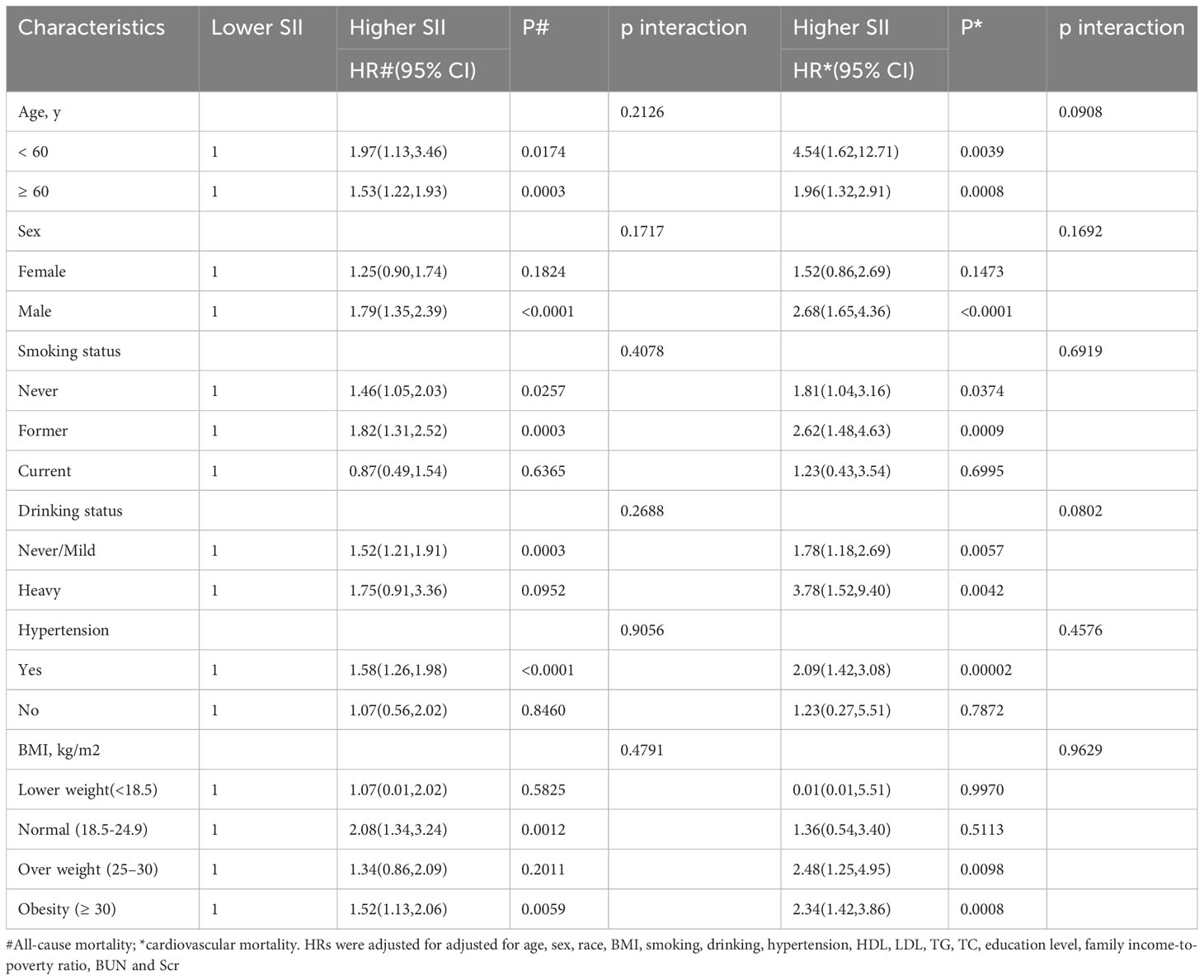
Results: Over the course of a median follow-up of 69 months (interquartile range [IQR], 54-123 months), 1,172 (23.6%) of the 4,972 diabetic patients passed away. These deaths included 332 (6.7%) cardiovascular deaths and 840 (16.9%) non-cardiovascular deaths. Individuals were categorized into higher (>983.5714) and lower (≤983.5714) SII groups according to MSRSM. In multi-variable adjusted models, subjects with higher SII had a significantly increased chance of dying from cardiovascular disease (HR 2.05; 95% confidence interval (CI):1.42,2.97) and from all causes (HR 1.60; 95% CI:1.22,1.99). Kplan-Meier curves showed similar results. Subgroup studies based on age, sex, BMI, drinking, smoking, and hypertension revealed that the connection maintained intact. The previously stated variables and SII did not significantly interact (p interaction > 0.05). In diabetic patients, smooth curve fitting revealed a nonlinear correlation between SII and mortality.
Conclusion: In diabetic patients, elevated SII is linked to higher cardiovascular and all-cause mortality.
1 Introduction
Diabetes, one of the most serious and common chronic disorders in modern times, shortens life expectancy and can have disastrous, costly, debilitating, and even deadly effects (1, 2). It has also become a major public health issue (3). In addition, diabetes can lead to microvascular complications (such as nephropathy, retinopathy, neuropathy and so on) as well as macrovascular complications (such as coronary artery disease, stroke, and peripheral vascular disease and so on), which can reduce the patient’s quality of life (4). The 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas predicts that among people aged 20 to 79 years, 10.5% (or 536.6 million) would have diabetes globally by 2021. It is estimated that by 2045, that percentage will rise to 12.2% (783.2 million) instances (5). Research has demonstrated that individuals with diabetes mellitus bear a notably elevated risk of dying from cardiovascular disease(CVD) and other causes (6, 7). Thus, it’s critical to promptly identify new risk factors in order to stop, impede, or lessen the development of diabetes and the number of deaths associated with the disease.
The systemic immune-inflammation index (SII), a complete new immune-inflammation biomarker was got through platelet, neutrophil, and lymphocyte counts, was created by Hu et al. It accurately depicts both the local immune response and the body’s systemic inflammatory condition (8). One of the factors contributing to obesity and insulin resistance in obese human patients and animal models (such as db/db mice) has been suggested to be systemic inflammation (9, 10). Diabetes and low-grade systemic inflammation have been shown to be related (11). The SII was used to evaluate the prognosis of patients suffering from a variety of malignancies initially, such as non-small cell lung cancer, stomach cancer, and rectal cancer (8, 12–14). However, it has recently been discovered that SII is positively correlated with the incidence of comorbidities and sequelae, including diabetic nephropathy and depression, in individuals with diabetes and so on (15, 16). Furthermore, research has demonstrated that elevated levels of SII are highly predictive of cardiovascular disease (CVD) and all-cause mortality in the general individuals (17). The relationship between SII and the prevalence of diabetes has been clarified (18). Studies have also been conducted on the relationship between SII and mortality in diabetic patients. The study by Wei Q et al. (19) showed a U-shaped correlation between SII and all-cause mortality and a J-shaped association with CVD mortality. And the study of Liu Y et al. (20) showed a U-shaped relationship between SII and all-cause mortality in diabetic, prediabetic and non-diabetic populations, and a non-linear relationship between SII and CVD mortality in prediabetic and non-diabetic populations. However, in diabetic patients, there was a linear relationship between SII and cardiovascular mortality. The relationship between SII and mortality in diabetic patients remains unclear.
Thus, we examined the linkage between SII and the risk of cardiovascular and all-cause death in a sizable, nationally representative patient cohort with diabetes.
2 Materials and methods
2.1 Data source and study population
The NHANES is a comprehensive research study that assesses the nutritional and physical health of adults and children in the United States. The NCHS Research Ethics Review Board gave it official approval, and each study participant completed an informed consent form. This study included data from six NHANES cycles (2005-2016) with a total of 60,936 participants. We removed respondents under the age of 20, as well as those who lacked complete survival and laboratory test information,eventually, 4,972 people were enrolled in this study (Figure 1).
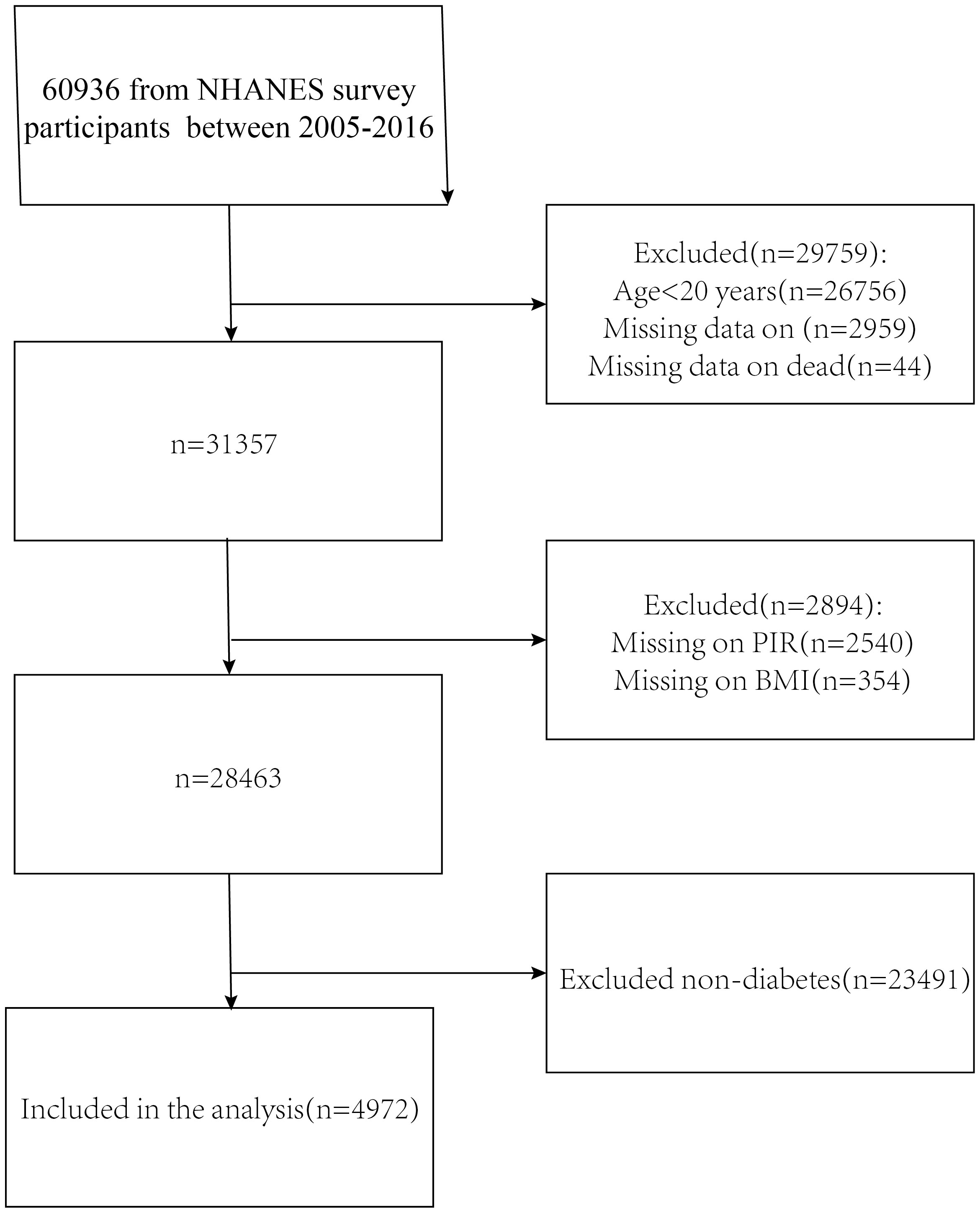
Figure 1 Flowchart for choosing participants. National Health and Nutrition Examination Survey, or NHANES.
2.2 Definition of SII and diabetes
Diabetes mellitus patients satisfied one or more of the following requirements (21): (1) Fasting blood glucose level of 7.0 mmol/L and above, or an oral glucose tolerance test result greater than or equal to 11.1 mmol/L hours after two hours; (2) Random blood glucose greater than or equal to 11.1 mmol/L; (3) Glycohemoglobin (HbA1c) level greater than or equal to 6.5%; (4) use of insulin or diabetic medicines; and (5) diabetes self-reported as diagnosed by a medical professional.
A routine blood test called a complete blood count is used to identify several illnesses and evaluate a person’s general health. The Beckman Coulter technique is used to calculate a full blood count. SII is computed use platelet× neutrophil/lymphocyte. It was first proposed by Bo Hu et al. in 2014 for assessing the prognosis of hepatocellular carcinoma (8).
2.3 Mortality outcomes of the study population
We used specific study identifiers (SEQN) and probability matching to the National Death Index (NDI) as of December 31, 2019, to ascertain the mortality status of our participants. Apart from the records of deaths due to all causes, we additionally collected mortality data, primarily associated with cardiovascular diseases, under the term “Underlying Leading Cause of Death”.
2.4 Covariates
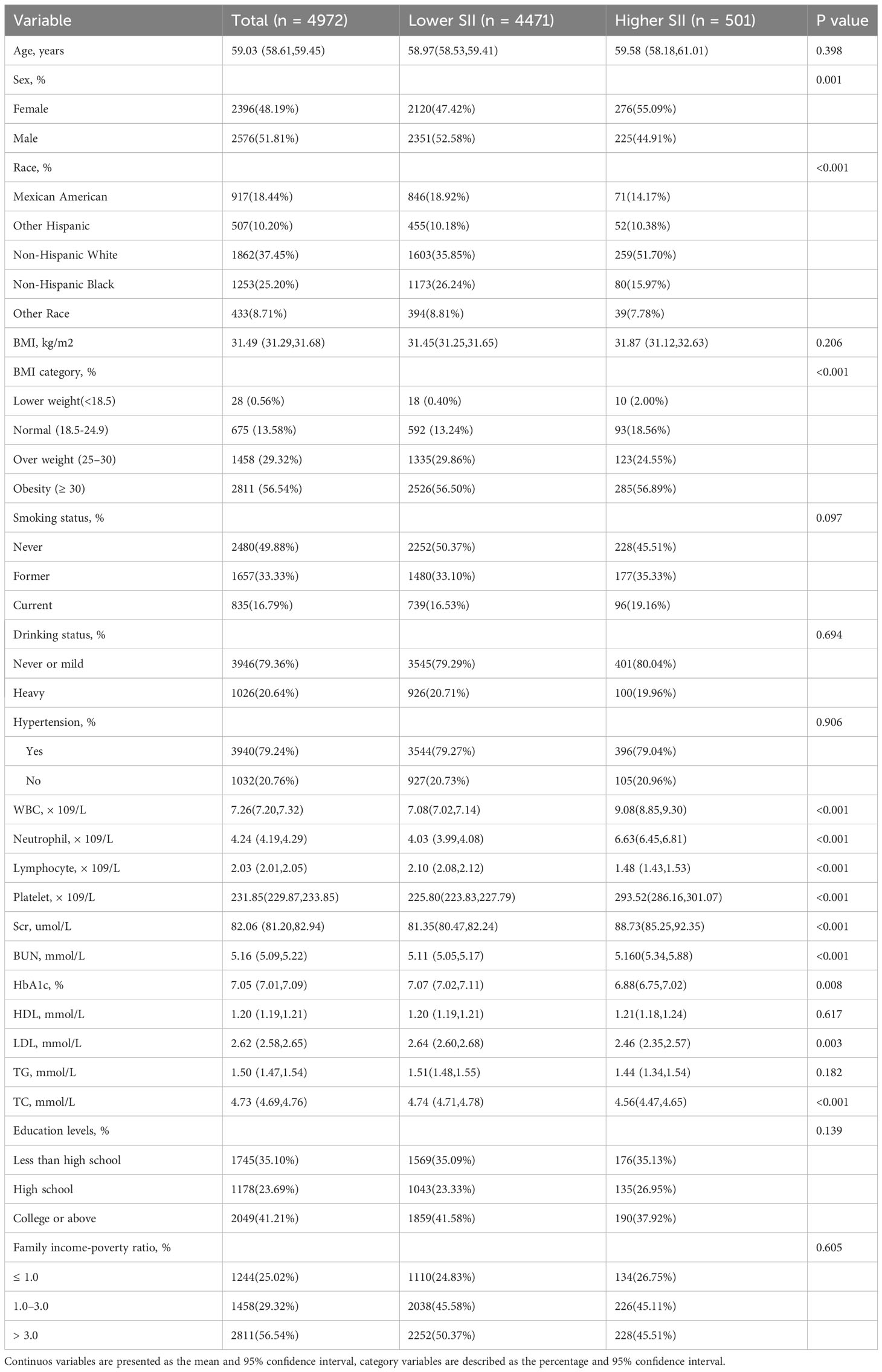
Demographic features include things like age, sex (male and female), ethnic (Mexican Americans, other (Hispanics, non-Hispanic Whites, non-Hispanic Blacks and others), educational attainment (Less than high schoo, high school, College or above), and the household poverty-to-income ratio (PIR) (these ratios are classed as follows: ≤1.0%, between >1 and ≤3.0%, and >3.0%). Body mass index (BMI) was divided into four categories: <18.5, 18.5-24.9, 25.0-30, and ≥ 30kg/m2 (22). Risk variables were chosen from the health questionnaire and included history of hypertension(it was defined as follows: 1)a self-reported history of hypertension; 2) the use of anti-hypertensive medication; 3) a mean systolic blood pressure of at least 130 mmHg and/or a mean diastolic blood pressure of at least 80 mmHg), smoking, and alcohol consumption. The participants were classified into three groups based on their current smoking habits: current (those who smoke over 100 cigarettes per day or on occasion), former (those who have smoked over 100 cigarettes but no longer do so), and never (those who have smoked less than 100 cigarettes in their lifetime). Participants were then divided into two categories: heavy drinkers (>2 drinks for males and >1 drink for women per week) and light or non-drinkers (meaning abstaining from alcohol entirely or consuming no more than one drink per week for women and two drinks per week for men) (23). The laboratory variables were blood cell counts, HbA1c, serum creatinine (Scr), low-density lipoprotein cholesterol (LDL), triglycerides (TG), total cholesterol (TC), blood urea nitrogen (BUN) high-density lipoprotein cholesterol (HDL). In conclusion, the NHANES website provides detailed instructions for collecting blood biochemical measurements. To sum up, comprehensive instructions for gathering blood biochemical measures are available on the NHANES website.
2.5 Statistical analysis
We employed survey analysis techniques and adhered to NHANES analytical reporting guidelines to guarantee nationally representative estimates (24). The Student t test and the Mann-Whitney U test were used in this study to describe the variations in continuous variables between the two groups. When evaluating group differences in categorical data, the chi-square test was employed.
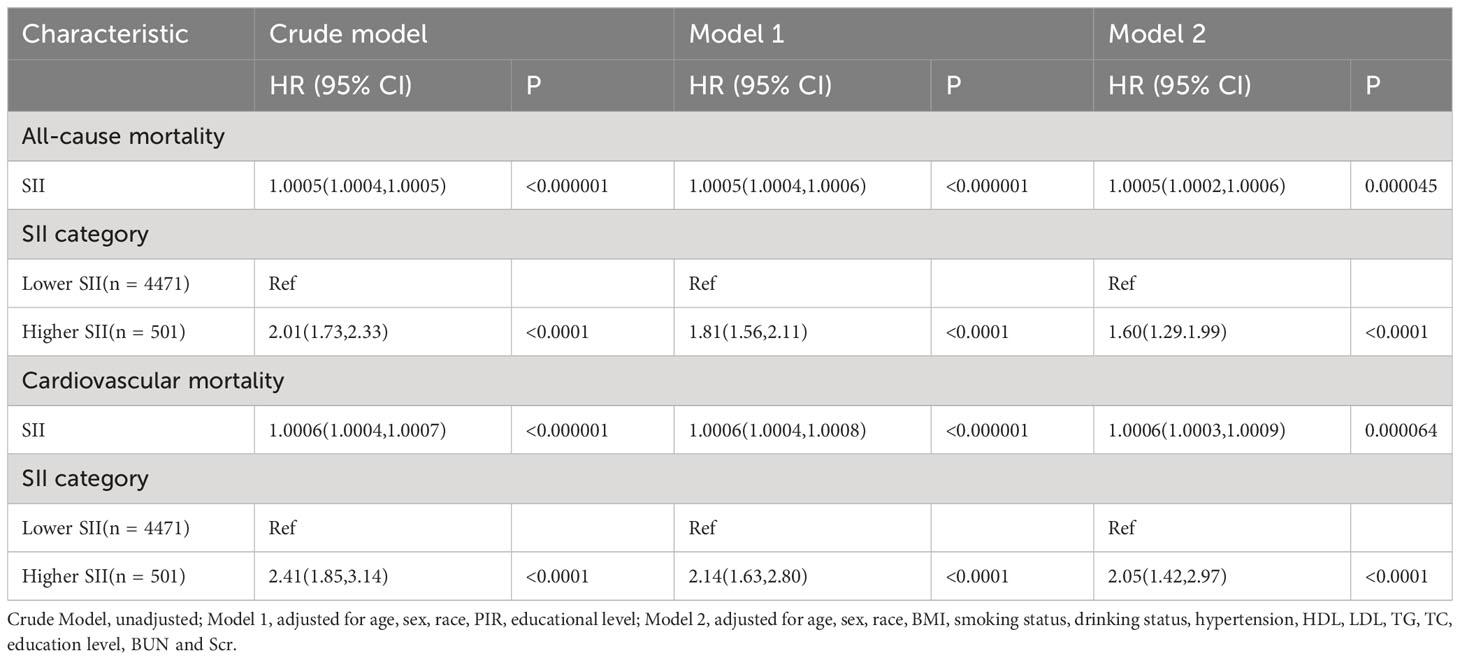
Using the “maxstat” package (https://CRAN.R-project.org/package=maxstat), the maximum selection rank statistic was utilized to ascertain the optimal SII cut-off point that was most significantly associated with survival outcomes. Participants were then split up into two groups: high and low SII. Survey-weighted Cox regression analysis was used in the study to evaluate the correlation between SII and cardiovascular and all-cause mortality in people with diabetes. Three models were built with potential confounders taken into account. While Model 1 was left unadjusted Model 2 underwent adjustments for gender,age, ethnicity, educational level, and PIR. BMI, drinking and smoking habits, hypertension, TG, TC, HDL, LDL, Scr, and BUN levels were further adjusted for in Model 3.
In diabetic patients, potential nonlinear relationships between SII and cardiovascular and all-cause death were visualized using smooth curve fitting. The log-rank test was utilized to compare the odds of survival, which were calculated using the Kaplan-Meier technique. Furthermore, we investigated the interaction between SII values and mortality by utilizing subgroups based on age, gender, BMI, drinking status, and smoking status to examine the relationship between the two. The data were examined using licensed statistics (http://www.empowerstats.com) and the statistical software R (http://www.R-project.org). P values indicating statistical significance for differences were less than 0.05.
3 Results
3.1 Baseline characteristics of participants
For this research, a total of 4972 diabetes individuals were included. The maximum selection-based rank statistic (Figure 2) was used to determine the ideal SII threshold (983.5714), which divided the participants into two groups: a lower group (SII ≤983.5714, n = 4471) and a higher group (SII >, n = 501). Individuals in the higher SII group were more likely to be white than those in the lower SII group. They also had lower levels of lymphocytes, LDL, TG, TC, HbA1c and higher levels of neutrophils, white blood cells(WBC), Scr, BUN, and BMI. Table 1 displays further participant characteristics.

Figure 2 The cutoff point was calculated using the maximally selected rank statistics based on the ‘maxstat’ package.
3.2 Association of SII with mortality
During a median follow-up of 87 months (interquartile range [IQR], 55-124 months), 1,172 (23.57%) of the 4,972 patients with diabetes mellitus passed away. 840 (16.89%) of them were non-cardiovascular fatalities, and 332 (6.68%) were cardiovascular deaths. We discovered that when the SII values increased, there was a substantial increase in the risk of all-cause death in the crude model (HR 2.01; 95% CI:1.73,2.33). After multi-factorial adjustment, there was a corresponding increase in all-cause mortality in patients with diabetes of 81% (model 1, HR 1.81;95% CI:1.56,2.11) and 60% (model 2, HR 1.60; 95% CI:1.29,1.99) for every unit increase in SII value. Furthermore, we discovered that there was a strong correlation between high SII and the risk of cardiovascular death. In the crude model, we discovered that the risk of cardiovascular death rose significantly with rising SII levels (HR 2.41;95%CI:1.85,3.14). Diabetes patients experienced an increase in cardiovascular mortality with each unit increase in SII value of 114% (model 1, HR 2.14;95%CI:1.63,2.80) and 105% (model 2, HR 2.05; 95%CI:1.42,2.97), even after controlling for a number of other variables (Table 2). Kaplan-Meier data indicated that the high SII group had a poorer survival rate (Figure 3).
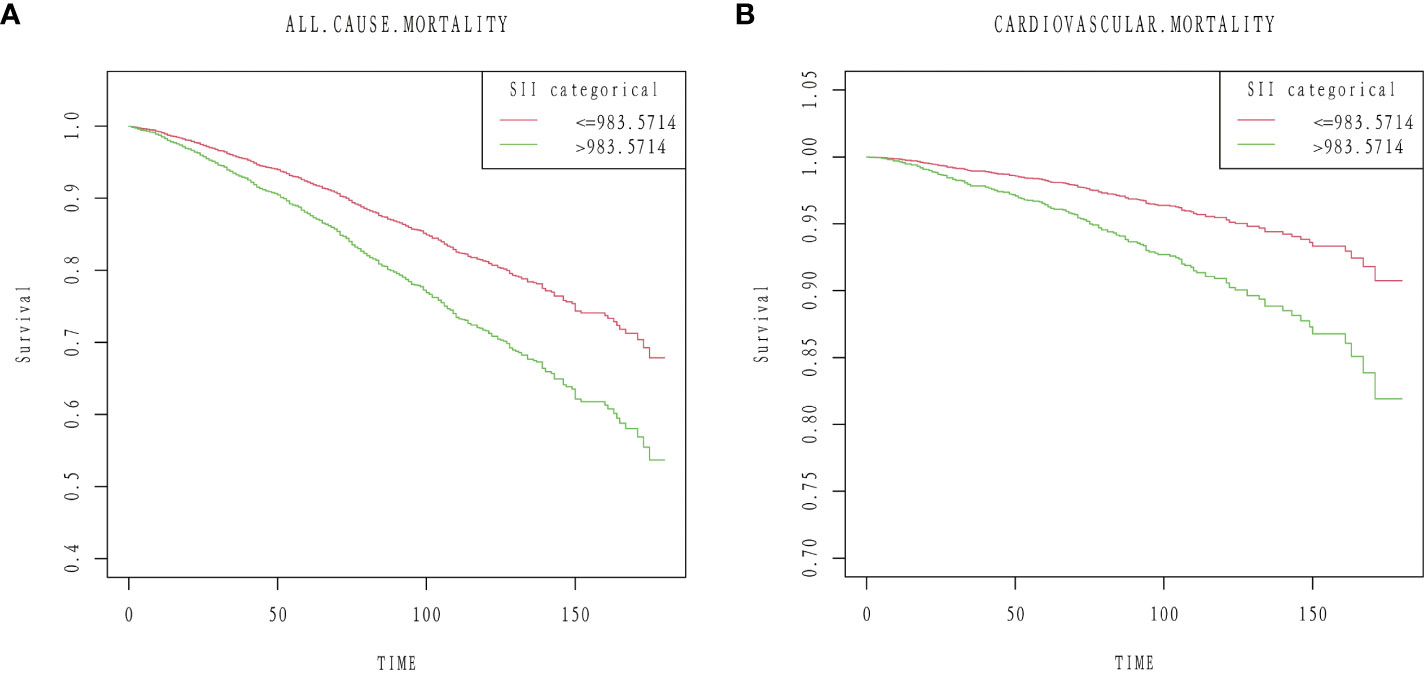
Figure 3 Kaplan–Meier curves of the survival rate: (A) All-cause mortality. (B) Cardiovascular mortality.
3.3 Smooth curve fitting
After fully accounting for covariates, we fitted a smooth curve of SII with cardiovascular and all-cause mortality in diabetics (model 3). In diabetic patients, we discovered a “L” shaped correlation between SII and CVD and all-cause mortality (Figure 4).
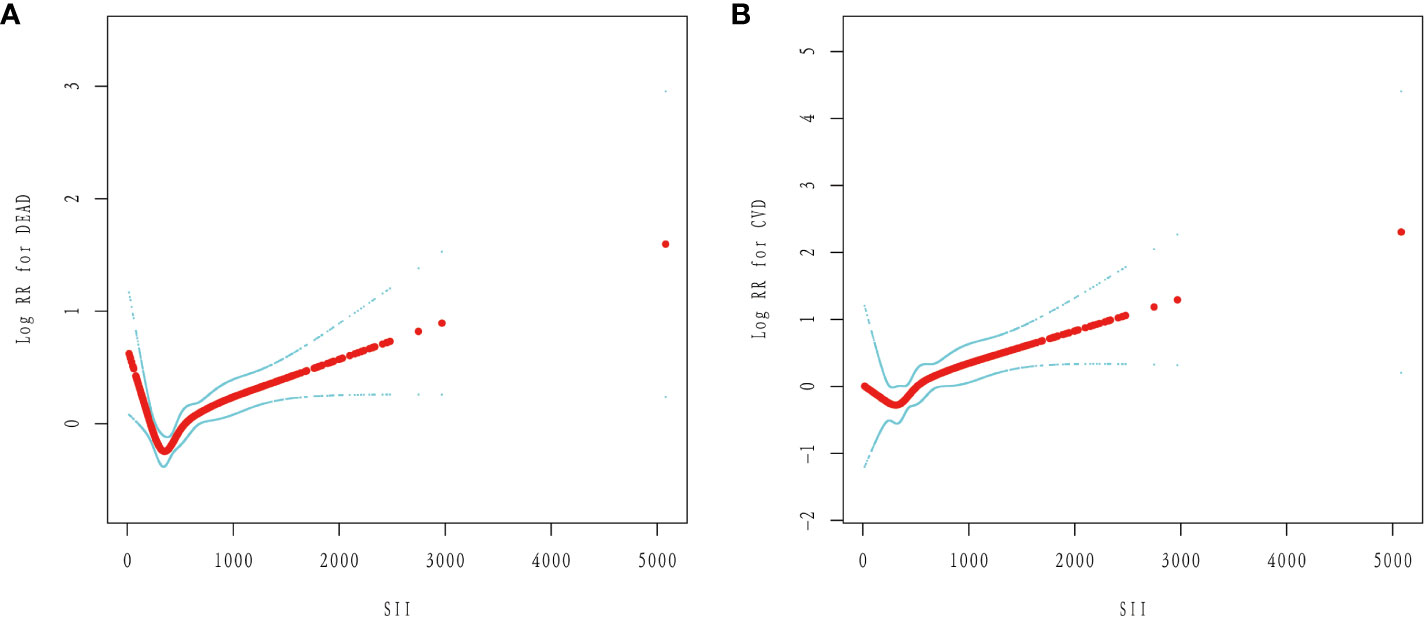
Figure 4 The non-linear relationship between SII and the risk of mortality in diabetes: (A) All-cause mortality. (B) Cardiovascular mortality.
3.4 Subgroup analysis
Additionally, we used subgroup analyses based on gender, age, BMI, drinking, smoking, and hypertension. However, we were unable to determine whether SII and the previously mentioned variables interacted significantly (p interaction > 0.05) (Table 3).
4 Discussion
The prevalence of diabetes has dramatically expanded in tandem with the expansion of the global economy. A number of chronic comorbidities and problems brought on by diabetes drastically shorten life expectancy (1). Therefore, reducing diabetes complications and improving patient quality of life can be achieved by having a complete grasp of the underlying causes that influence the development of lesions. Previous studies have shown a 4% increase in the likelihood of developing diabetes for each unit increase in SII. However, there are fewer studies evaluating SII scores and the prognosis of diabetic patients, and the aim of this study was to evaluate the relationship between SII scores and mortality in diabetic patients (18).
There are not many studies on SII and diabetes prognosis. In 4,972 diabetic patients from six NHANES cycles (2005-2016), our analysis demonstrated that increased SII was substantially linked with all-cause and cardiovascular mortality. The connection held true even after controlling for covariates. Furthermore, in individuals with diabetes, smooth curve fitting demonstrated a non-linear connection between SII and cardiovascular and all-cause mortality. To sum up, SII is a reliable indicator for estimating the chances of survival for individuals with diabetes. This screening method can be used to rapidly identify people who are at a high risk of dying or suffering from unfavorable health outcomes at a relatively low cost.
Insulin resistance is associated with immune system activation and persistent low-grade inflammation, and it is connected to both obesity and type 2 diabetes mellitus (T2DM) (25). Risk factors for the onset of T2DM and its macrovascular consequences include systemic inflammatory indicators.Numerous prospective and cross-sectional studies have reported elevated levels of cytokines and chemokines, sialic acid, and circulating acute-phase proteins (such as, fibrinogen, haptoglobin, C-reactive protein (CRP), fibrinogen activator inhibitor, and serum amyloid A) in patients with type 2 diabetes. Moreover, increased CRP, interleukin-1β (IL-1β) and IL-6 T2DM-predictive values are found (11, 26–28). Based on the available data, diabetes is thought to be influenced by inflammation. Furthermore, a multitude of studies have demonstrated that persistent inflammation can also give rise to a wide range of vascular and diabetic problems (25). In individuals with type 2 diabetes, greater levels of SII were linked to diabetic kidney disease (DKD), according to a cross-sectional study by Shao L et al (29). SII might be a straightforward and affordable way to identify DKD. Zhang J et al. shown in a prospective cohort analysis a substantial correlation between high SII and elevated cardiovascular and all-cause mortality in the overall populace (17). The finding that a greater SII is substantially linked to an increased risk of cardiovascular diseases (CVDs) is supported by a recent meta-analysis (30). Additionally, it has been noted that elevated SII raises the hazard of all-cause death as well as the subtypes of ischemic and hemorrhagic stroke (31). In conclusion, the above evidence suggests that SII is a valid predictor for assessing the prognosis of diabetic complications and CVDs in the general population. SII levels can be used as an easily detectable biomarker of systemic inflammatory activity. They are based on the counts of three circulating immune cells (i.e., neutrophils, lymphocytes, and platelets) and represent the inflammatory state (16, 29). After adjusting for confounders, our study’s findings indicated that diabetes individuals with higher SII had worse overall survival. The optimally selected rank statistic, an outcome-oriented method that yields the cutoff point most substantially associated with survival outcomes, identified the best threshold (983.5714). Furthermore, smoothed curve fitting revealed a substantial nonlinear connection between SII and diabetes-related survival outcomes.
Our study’s huge sample size and long follow-up period allow us to confidently make conclusions, which is only one of its many benefits. Secondly, in order to examine the potential use of a unique inflammatory index for diabetes patient mortality prediction, we adjusted for a wide range of established risk factors to rule out potential confounders. Third, selection bias was avoided because all participants were taken from the NHANES survey, which uses a sophisticated multistage probability sampling technique. There are a few restrictions to be aware of, though. First off, even though we took a number of possible confounders into account when conducting our studies, it is still possible that other unidentified factors had an impact on SII. Secondly, American patients with diabetes participated in this study. To determine whether the results can be applicable to other populations, further research is necessary. Third, while SII is easily measured in clinical settings, it is frequently lost along with neutrophil, lymphocyte, and platelet counts, which might result in bias in the selection process.
5 Conclusions
Overall, we analyzed 4972 diabetic patients over 6 cycles (2005-2016) and concluded that (1) high SII levels were significantly associated with all-cause and cardiovascular mortality in diabetic patients, and (2) there was a nonlinear association between SII and both all-cause and cardiovascular mortality in diabetic patients. Our findings emphasize the significance of using SII as a prognostic indicator for diabetes mellitus in daily clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
CM: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. KL: Conceptualization, Data curation, Funding acquisition, Methodology, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the authors of the included studies. A special thanks to all of the NHANES participants who freely gave their time to make this and other studies possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heald AH, Stedman M, Davies M, Livingston M, Alshames R, Lunt M, et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab (2020) 9(4):183–5. doi: 10.1097/XCE.0000000000000210
2. Cannon A, Handelsman Y, Heile M, Shannon M. Burden of illness in type 2 diabetes mellitus. J Manag Care Spec Pharm (2018) 24(9-a Suppl):S5–S13. doi: 10.18553/jmcp.2018.24.9-a.s5
3. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract (2010) 87(1):4–14. doi: 10.1016/j.diabres.2009.10.007
4. Neves RG, Tomasi E, Duro SMS, Saes-Silva E, de Oliveira Saes M. [Complications due to diabetes mellitus in Brazil: 2019 nationwide study]. Cien Saude Colet (2023) 28(11):3183–90. doi: 10.1590/1413-812320232811.11882022
5. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
6. Raghavan S, Vassy JL, Ho Y-L, Song RJ, Gagnon DR, Cho K, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc (2019) 8(4):e011295. doi: 10.1161/JAHA.118.011295
7. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med (2011) 364(9):829–41. doi: 10.1056/NEJMoa1008862
8. Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442
9. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest (2003) 112(12):1821–30. doi: 10.1172/JCI200319451
10. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest (2003) 112(12):1796–808. doi: 10.1172/JCI200319246
11. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol (2011) 11(2):98–107. doi: 10.1038/nri2925
12. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med (2015) 236(4):297–304. doi: 10.1620/tjem.236.297
13. Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer biomark (2016) 17(1):33–40. doi: 10.3233/CBM-160614
14. Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: A propensity score-matched analysis. Sci Rep (2016) 6:39482. doi: 10.1038/srep39482
15. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging (2021) 16:97–105. doi: 10.2147/CIA.S285000
16. Elbeyli A, Kurtul BE, Ozcan SC, Ozcan DO. The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin Exp Optom (2022) 105(8):831–5. doi: 10.1080/08164622.2021.1994337
17. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J, et al. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J Clin Med (2023) 12(3). doi: 10.3390/jcm12031128
18. Nie Y, Zhou H, Wang J, Kan H. Association between systemic immune-inflammation index and diabetes: a population-based study from the NHANES. Front Endocrinol (Lausanne) (2023) 14:1245199. doi: 10.3389/fendo.2023.1245199
19. Chen C, Chen Y, Gao Q, Wei Q. Association of systemic immune inflammatory index with all-cause and cause-specific mortality among individuals with type 2 diabetes. BMC Cardiovasc Disord (2023) 23(1):596. doi: 10.1186/s12872-023-03638-5
20. Tang Y, Feng X, Liu N, Zhou Y, Wang Y. Relationship between systemic immune inflammation index and mortality among US adults with different diabetic status: Evidence from NHANES 1999-2018. Exp Gerontol (2023) 185:112350. doi: 10.1016/j.exger.2023.112350
21. Dong G, Gan M, Xu S, Xie Y, Zhou M, Wu L, et al. The neutrophil-lymphocyte ratio as a risk factor for all-cause and cardiovascular mortality among individuals with diabetes: evidence from the NHANES 2003-2016. Cardiovasc Diabetol (2023) 22(1):267. doi: 10.1186/s12933-023-01998-y
22. Zhang J, Chen Y, Zou L, Gong R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol (2023) 60(2):235–45. doi: 10.1007/s00592-022-01985-x
23. Gao M, Liu M, Chen J, Zhu Z, Chen H. Association of serum 25-hydroxyvitamin D concentrations with all-cause mortality among individuals with kidney stone disease: the NHANES database prospective cohort study. Front Endocrinol (Lausanne) (2023) 14:1207943. doi: 10.3389/fendo.2023.1207943
24. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2 (2013) 161):1–24.
25. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia (1997) 40(11):1286–92. doi: 10.1007/s001250050822
26. Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, et al. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care (2009) 32(3):421–3. doi: 10.2337/dc08-1161
27. Song Y, Zhao Y, Shu Y, Zhang L, Cheng W, Wang L, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: A cross-sectional study. Front Endocrinol (Lausanne) (2023) 14:1100453. doi: 10.3389/fendo.2023.1100453
28. Dhande IS, Doris PA. Genomics and inflammation in cardiovascular disease. Compr Physiol (2021) 11(4):2433–54. doi: 10.1002/cphy.c200032
29. Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front Endocrinol (Lausanne) (2022) 13:1071465. doi: 10.3389/fendo.2022.1071465
30. Ye Z, Hu T, Wang J, Xiao R, Liao X, Liu M, et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: A systematic review and meta-analysis. Front Cardiovasc Med (2022) 9:933913. doi: 10.3389/fcvm.2022.933913
Keywords: systemic immune-inflammation index, all-cause mortality, cardiovascular mortality, diabetes, neutrophil, lymphocyte
Citation: Meng C and Liu K (2024) Nonlinear association of the systemic immune-inflammatory index with mortality in diabetic patients. Front. Endocrinol. 15:1334811. doi: 10.3389/fendo.2024.1334811
Received: 07 November 2023; Accepted: 18 January 2024;
Published: 13 February 2024.
Edited by:
Bo Zhu, Harvard Medical School, United StatesReviewed by:
Vladimir M. Pisarev, Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitation, RussiaMarija Vavlukis, Ss. Cyril and Methodius University in Skopje, North Macedonia
Copyright © 2024 Meng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Liu, TGl1YnVzaW5lc3NAMTYzLmNvbQ==
 Chunli Meng
Chunli Meng Kai Liu
Kai Liu