- 1Cardiovascular Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 2Department of Epidemiology, School of Public Health, Iran University of Medical Science, Tehran, Iran
- 3Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 4Social Determinants of Health Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 5Evidence-based Phytotherapy and Complementary Medicine Research Center, Alborz University of Medical Sciences, Karaj, Iran
Background: We conducted a systematic review and meta-analysis on dairy consumption and its association with anthropometric measurements, blood glucose status, insulin levels, and testosterone levels in women with Polycystic Ovary Syndrome.
Methods: This study conducted a comprehensive literature search using electronic databases like MEDLINE, Scopus, PubMed, Web of Science, and Google Scholar to identify observational and interventional studies investigating the relationship between dairy product consumption and Polycystic Ovary Syndrome. A meta-analysis was performed on clinical trial studies that examined the effect of a low starch/low dairy diet in Polycystic Ovary Syndrome subjects. Statistical analyses were performed using Stata version 16.0 (Stata Corporation, College Station, Texas, USA), and statistical significance was defined as p-value < 0.05.
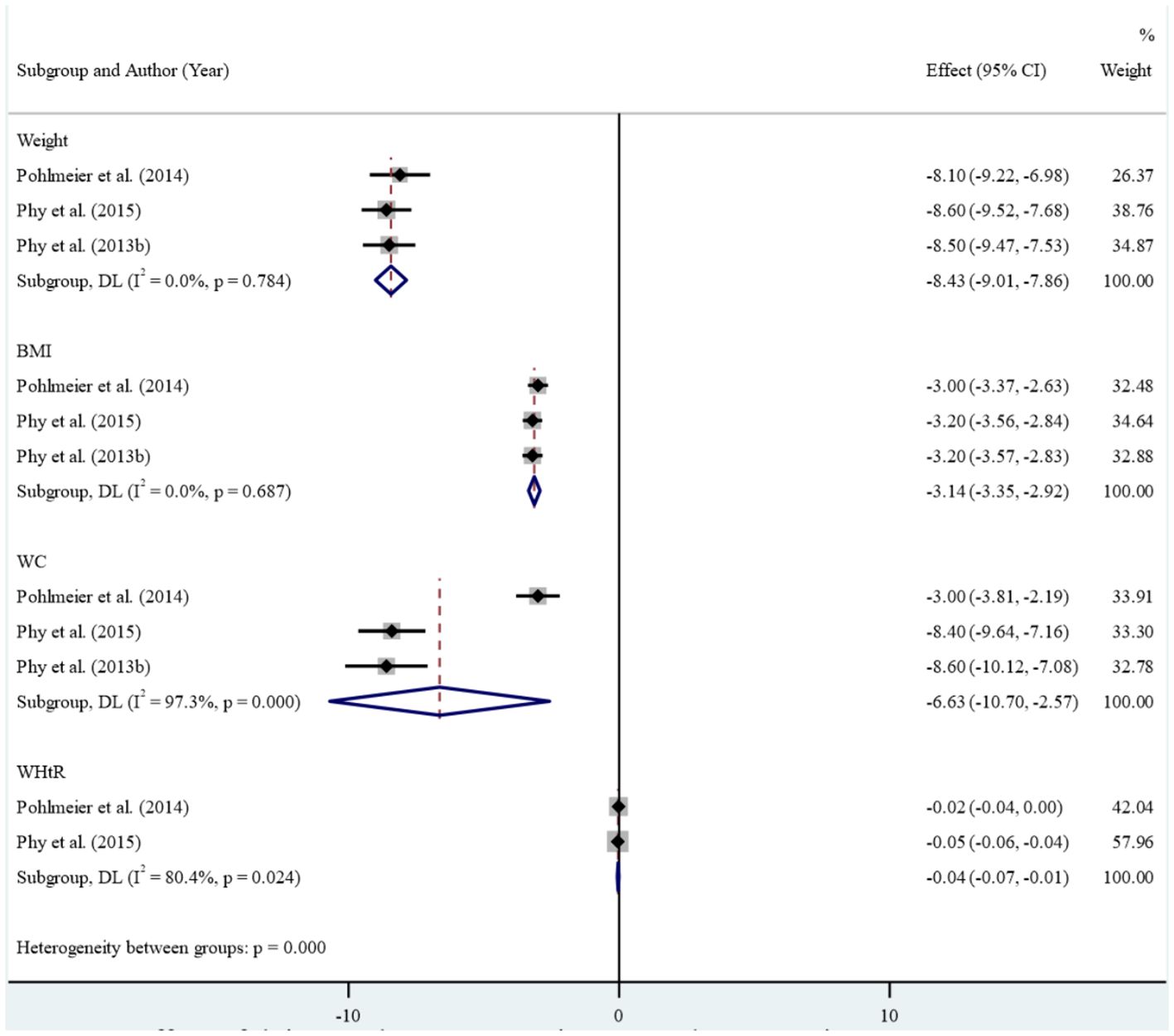
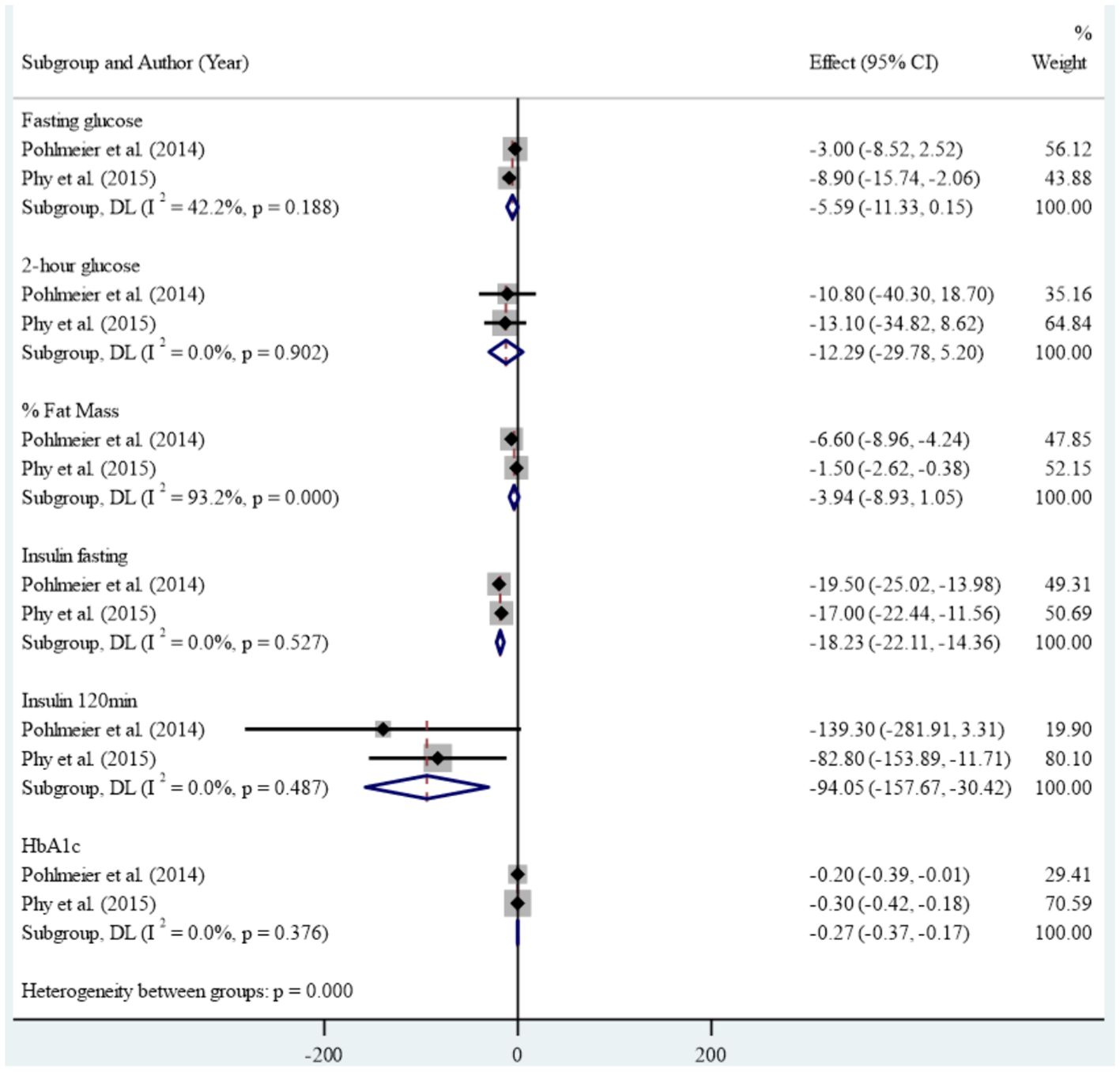
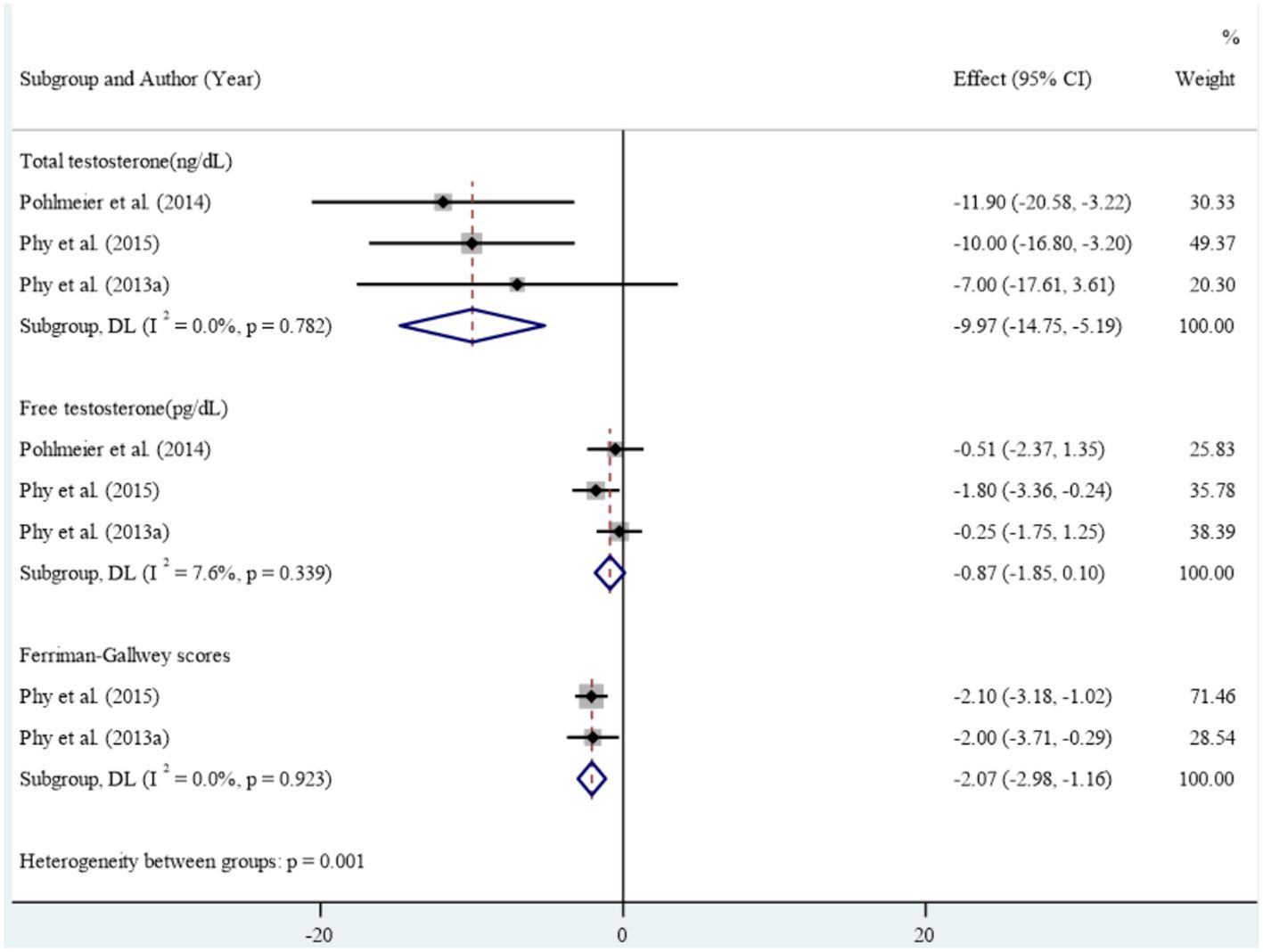
Results: Of the 1,313 citations reviewed, our systematic review identified 11 studies that met the inclusion criteria, comprising six case-control studies, four clinical trials, and one cross-sectional study. The case-control studies found limited evidence of an association between dairy consumption and Polycystic Ovary Syndrome. The result of the clinical trial studies in meta-analysis showed that reducing dairy intake along with reducing starch intake led to statistically significant improvements in anthropometric and metabolic measures including mean weight (Standardized mean difference: -8.43 (95% CI: -9.01, -7.86)), Body mass index (-3.14 (95% CI: -3.35, -2.92), waist circumference (-6.63 (95% CI: -10.70, -2.57)) and Waist-to-Height Ratio (-0.04 (95% CI: -0.07, -0.01), insulin fasting (-18.23 (95% CI: -22.11, -14.36)), insulin 120 minutes (-94.05 (95% CI: -157.67, -30.42)), HbA1c (-0.27 (95% CI: -0.37, -0.17)), Ferryman-Gallwey score (-2.07 (95% CI: -2.98, -1.16)) and total testosterone (-9.97 (95% CI: -14.75, -5.19)). No significant reduction was found in fasting glucose, 2 hours glucose, percent of fat mass, and mean free testosterone after intervention.
Conclusions: The findings of this systematic review show limited evidence about the association between dairy consumption and Polycystic Ovary Syndrome. The interventional studies suggest that a low-dairy/low-starch diet may improve some anthropometric and metabolic measures in women with Polycystic Ovary Syndrome.
Introduction
Polycystic ovary syndrome (PCOS) is a common hormonal disorder in women of reproductive age, characterized by irregular menstrual cycles, elevated androgen levels, and polycystic ovaries (1). The prevalence of PCOS varies between ranging from 4% to 18% (2). The wide range of prevalence can be attributed to the complex pathophysiology of PCOS, the variable clinical presentation, and the lack of adequate evidence-based data due to the existence of several diagnostic guidelines (3).
The exact causes of PCOS remain largely unknown, but it is believed that hormonal imbalances, including an excess of androgens and/or insulin, play a role (4). Additionally, environmental factors such as geography, diet, socioeconomic status, and environmental pollutants may also contribute to the development and management of PCOS (5).
Many women with PCOS are also overweight or obese, and weight loss can be challenging due to insulin resistance and compensatory hyperinsulinemia (6). The management of PCOS focuses on improving both the reproductive and metabolic symptoms (7), and lifestyle modifications, such as physical activity and diet, are critical for improving adverse outcomes associated with the condition (8, 9). Specifically, dietary modifications that reduce hyperinsulinemia may help improve fatty acid oxidation, facilitate weight loss, and prevent further weight gain (10).
Recent research has highlighted the potential significance of dairy products in the diet of women with PCOS, particularly in relation to carbohydrate metabolism disorders such as insulin resistance or type 2 diabetes. Dairy products contain valuable nutrients such as calcium, vitamin D, and high-quality proteins, which can have beneficial effects on overall health (11). Studies indicate that the consumption of carbohydrates from dairy and starch-based foods elicits a higher postprandial insulin response compared to carbohydrates from non-starchy vegetables and fruits (12, 13). To evaluate the differences in dairy consumption in women with and without PCOS and the effect of dairy diets on PCOS complications, a comprehensive systematic review and meta-analysis of all available studies was conducted.
Materials and methods
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. This study was approved by the ethics committee of Alborz University of Medical Sciences with the code IR.ABZUMS.REC.1401.061.
All eligible studies that assessed the relationship between dairy consumption and PCOS, were included in this systematic review.
Search strategy
The review question was formulated using the PECO framework in observational study and PICO in interventional study. Population (P) was women, Exposure (E) in observational study and intervention study (I) was dairy product, Comparison (C) was control group (if applicable) and Outcome (O) was Polycystic Ovary Syndrome. the Search strategy was performed based on PECO and PICO.
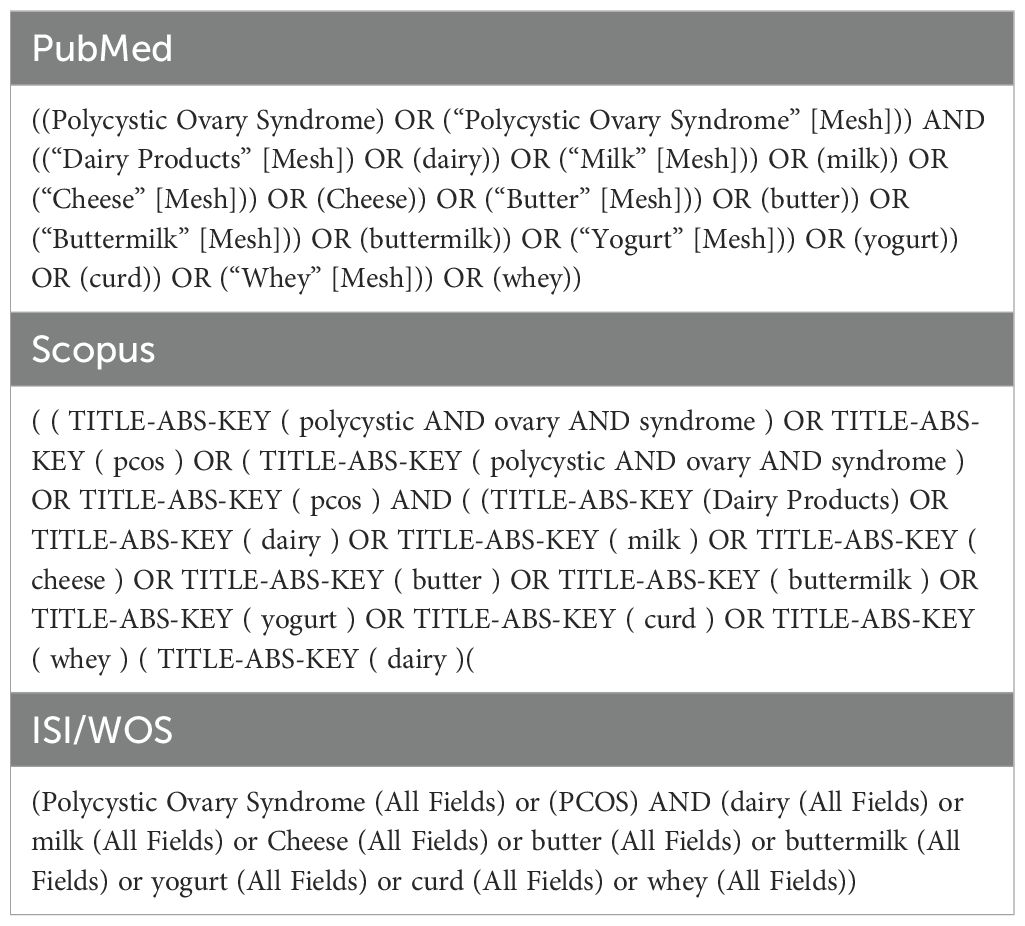
A comprehensive literature search was performed in the electronic databases (including MEDLINE/PubMed, Scopus, Web of Science, and Google Scholar) until 17 June 2023 to identify eligible studies investigating the association of PCOS with dairy product consumption. The search was conducted in each database using a combination of the key terms in two domains (1): PCOS and (2) Dairy product (Table 1).
Selection process
All retrieved articles were imported into EndNote X8.2, and duplicates were automatically removed from the list. Two researchers independently screened titles, abstracts, and full texts, respectively. Furthermore, they examined the reference list of eligible studies to identify any potentially eligible citations. Disagreements between researchers were resolved by discussing the full texts.
Eligibility criteria
Our systematic review included interventional studies that examined the effects of dairy product consumption on PCOS-related complications and met the following criteria (1): the intervention involved a dietary intervention with dairy products, and (2) the study participants were women with PCOS. In addition, observational studies comparing the dairy product intake in women with and without PCOS were also included. Studies that were not written in English, as well as letters to the editor and editorials, were excluded from our analysis.”
Data extraction
Data extraction from the included studies was conducted by two researchers using Microsoft Excel 2013. The following information was extracted: author details, country of origin, study design, sample size (for both intervention and control groups), age of participants (mean and standard deviation (SD)), anthropometric measurements (including weight, Body mass index (BMI), waist circumference (WC), and Waist-to-Height Ratio (WHtR)), blood glucose tests (including fasting glucose, 2-hour glucose, percentage of fat mass, fasting insulin, insulin at 120 minutes, and HbA1c), variables used for confounding adjustment, PCOS diagnosis, eligibility criteria, assessment tool, time to event (in weeks), and effect size (mean and SD). Any discrepancies between the two researchers were resolved through discussion and review of the full-text articles.
Quality assessment
Two researchers assessed the quality of the studies using the National Institutes of Health (NIH) quality assessment scales for before-and-after studies without control groups, cross-sectional, and case-control studies (14). Any discrepancies between the researchers were resolved through discussion and review of the full-text articles.
Statistical analysis
To assess heterogeneity among the studies, we used both the Chi-squared and I2 tests. A random-effects model was employed if the heterogeneity was statistically significant (Chi-squared; P-value<0.10); otherwise, a fixed-effects model was used. We conducted a meta-analysis of clinical trial studies that investigated the impact of a low starch/low dairy diet on PCOS patients before and after the intervention. For these studies, we summarized the mean and standard deviation using the standard mean difference (SMD) and 95% confidence interval (CI). Meta-analysis was performed for the following endpoints: anthropometric measurements, blood glucose levels, Ferryman-Gallwey score, and testosterone levels. All statistical analyses were conducted using Stata version 16.0 (Stata Corporation, College Station, Texas, USA
Results
In the initial literature search, a total of 1,313 citations were retrieved from electronic databases, including 50 from PubMed, 320 from Scopus, and 256 from ISI/Web of Science. An additional 687 citations were identified through a manual search on Google Scholar. After removing the duplicates automatically, 776 studies were assessed using titles and abstracts. Then, 107 studies were evaluated for full texts. Finally, 11 articles were found to be relevant to our topic. Out of the 11 studies, 6 were case-control, 4 were clinical trials, and 1 was cross-sectional. The detailed selection process is illustrated in Figure 1.

Figure 1. PRISMA flowchart of literature search and selection process. Adapted from PRISMA 2020 flow diagram templates, licensed under CC BY 4.0.
The reasons for excluding the studies were as follows: not investigating the intended outcomes of this review study (n= 68), study design (n=15)), not examining dairy products as a separate item (n= 9), being repetitive (n=3), and using non-English language in the text.
Case-control studies
Description
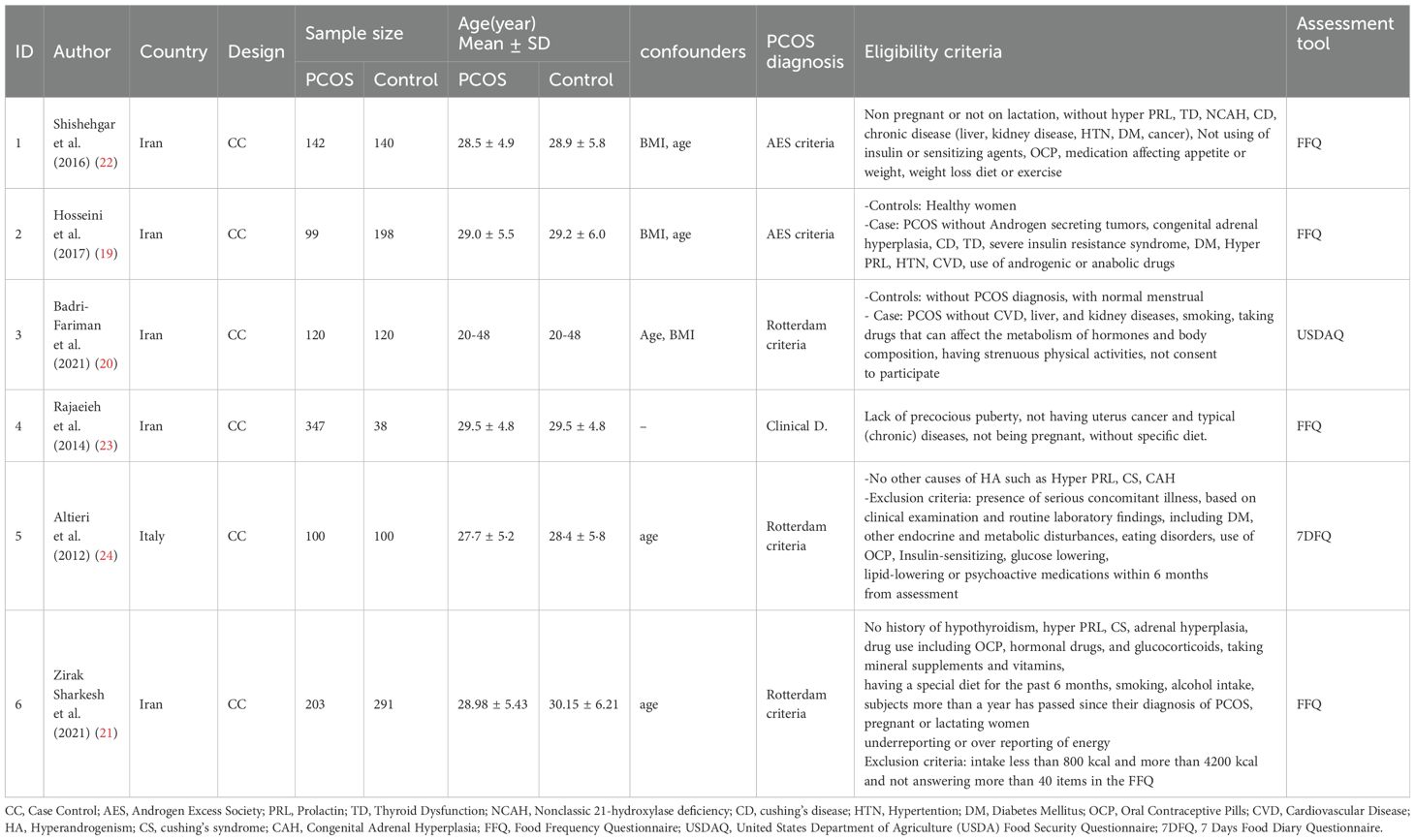
In this systematic review, six case-control studies were reviewed, of which five studies were conducted in Iran and one study in Italy. A total of 1,011 individuals with PCOS and 887 controls with different diagnostic criteria participated in these studies (Table 2).
Among the included studies, two studies used the Androgen Excess Society (AES) criteria for diagnosing PCOS. One study used a clinical diagnosis, while the remaining four studies used the Rotterdam criteria. The dietary intake assessment tool varied among the studies, with four using the Food Frequency Questionnaire (FFQ), one using the United States Department of Agriculture (USDAQ), and another using the 7 Days Food Diary Questionnaire (7DFQ).
The number of participants with PCOS ranged from 99 to 347, while the number of controls ranged from 38 to 291 across the included studies. The eligibility criteria considered in each study are listed in Table 2. The quality assessment of the case-control studies is presented in Table 3. Two studies fulfilled 10 out of the 12 assessment criteria, while four studies fulfilled 9 out of the 12 criteria. All studies fulfilled at least 9 out of the 12 assessment criteria, indicating a high level of quality across the studies.
Main findings
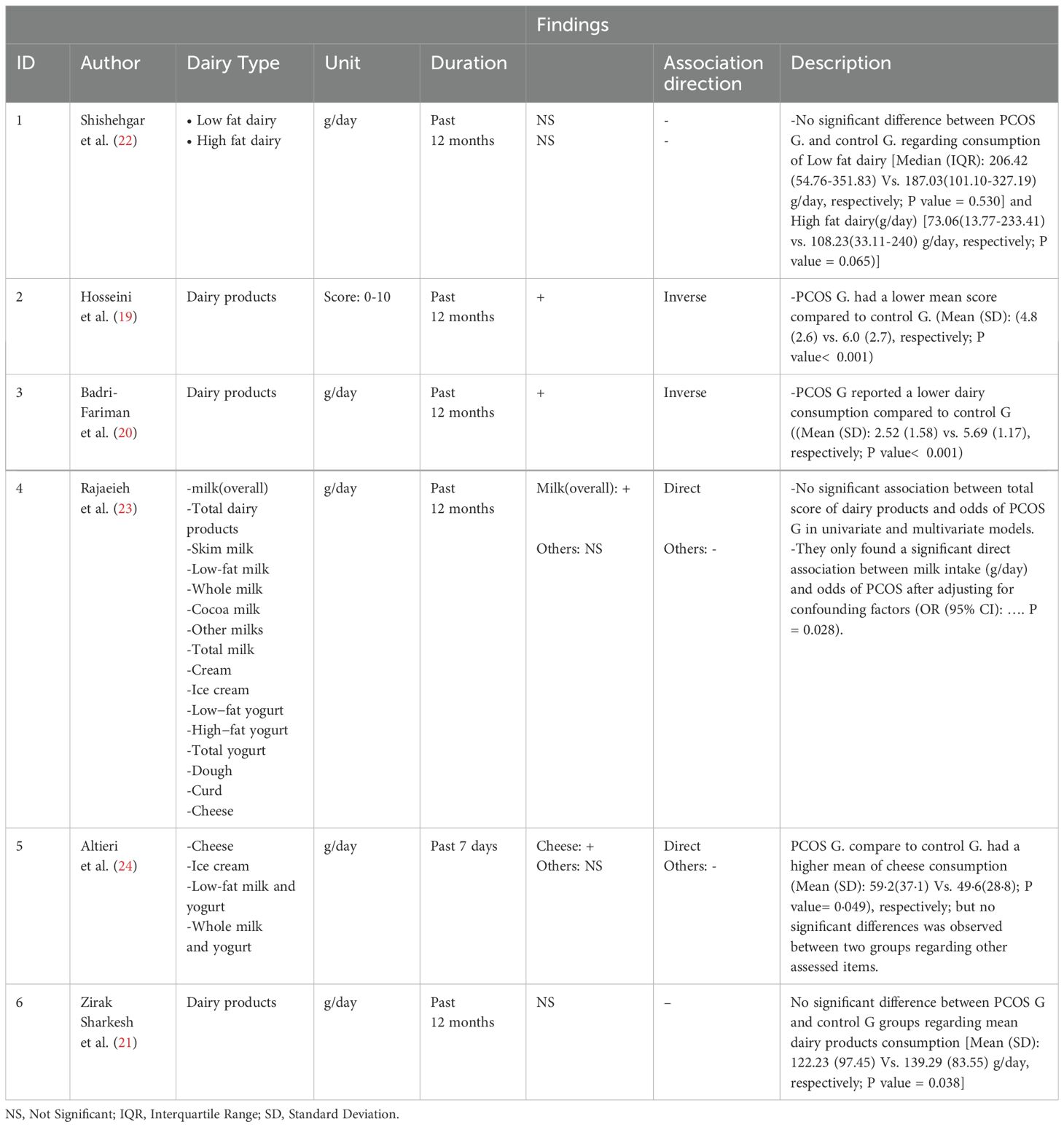
Out of the six case-control studies that were included in study, four of them measured overall dairy consumption. Of these, two studies reported lower dairy consumption in individuals with PCOS compared to non-infected individuals, while the other two studies reported no significant difference. One study examined the consumption of high-fat (p=0.065) and low-fat (p=0.530) dairy products separately, but there was no significant difference between the two groups. The findings from the case-control studies, including those related to dairy and cheese consumption, are presented in Table 4.
In one study, milk consumption was found to be significantly higher in individuals with PCOS after adjusting for confounding factors (P=0.028), but there was no significant difference between the two groups in terms of milk consumption by milk type. In five of the studies reviewed, dairy consumption in the last 12 months was measured using a scale of g/day, while in one study, it was measured over the last 7 days using the same scale. Additionally, the amount of dairy consumption was measured using a scale of g/day in all studies reviewed, except for one study in which it was measured by scoring on a scale of 0-10.
Two studies examined cheese consumption separately. In one study, there was a significant difference in cheese consumption between individuals with PCOS and controls. In the other study, there was no significant difference observed.
Interventional studies
Description
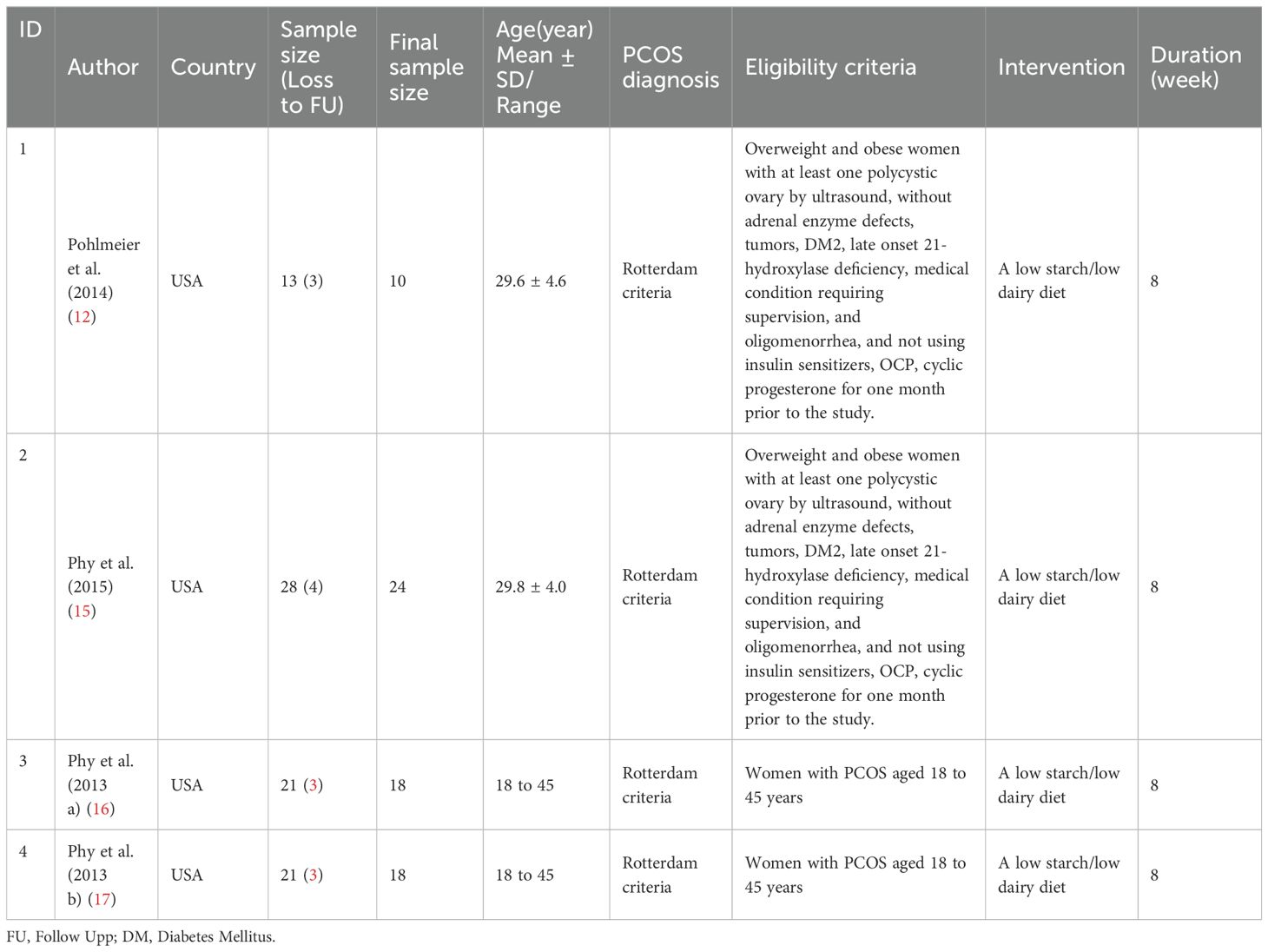
No studies were identified that specifically examined the effectiveness of reducing or increasing the amount or type of dairy consumption separately. However, four interventional studies were found that evaluated the effect of simultaneously reducing dairy and starch consumption (12, 15–17). The eligible women in these studies were between 18 and 45 years of age. In these studies, participants consumed a high-fat, low-carbohydrate liquid meal (HSFLM) consisting of 8 floz (237 ml) of chocolate Ensure (Abbott Laboratories, Chicago, Illinois), which contained a total of 6 g of fat (1 g of saturated fat (SFA), 3 g of polyunsaturated fat, and 2 g of monounsaturated fat), 40 g of carbohydrate, and 9 g of protein. To increase the proportion of SFA and make the liquid meal an SFA-rich high-fat meal, 32 g of butter, 5 g of coconut oil, and 19 g of palm oil were added to the chocolate Ensure. After the addition of dietary fat and lecithin, the total grams of fat in the high-fat meal equaled 56 g, which constituted 68% of total calories.
All four studies were conducted in the United States and included a total of 51 participants, with a range of 10 to 24 participants per study. In all four studies, PCOS was diagnosed using the Rotterdam criteria. The eligibility criteria for each study are summarized in Table 5. The quality assessment of the before-and-after intervention studies with no control group is outlined in Table 6. All studies fulfilled 10 out of the 12 assessment criteria, indicating a high level of quality across the studies.
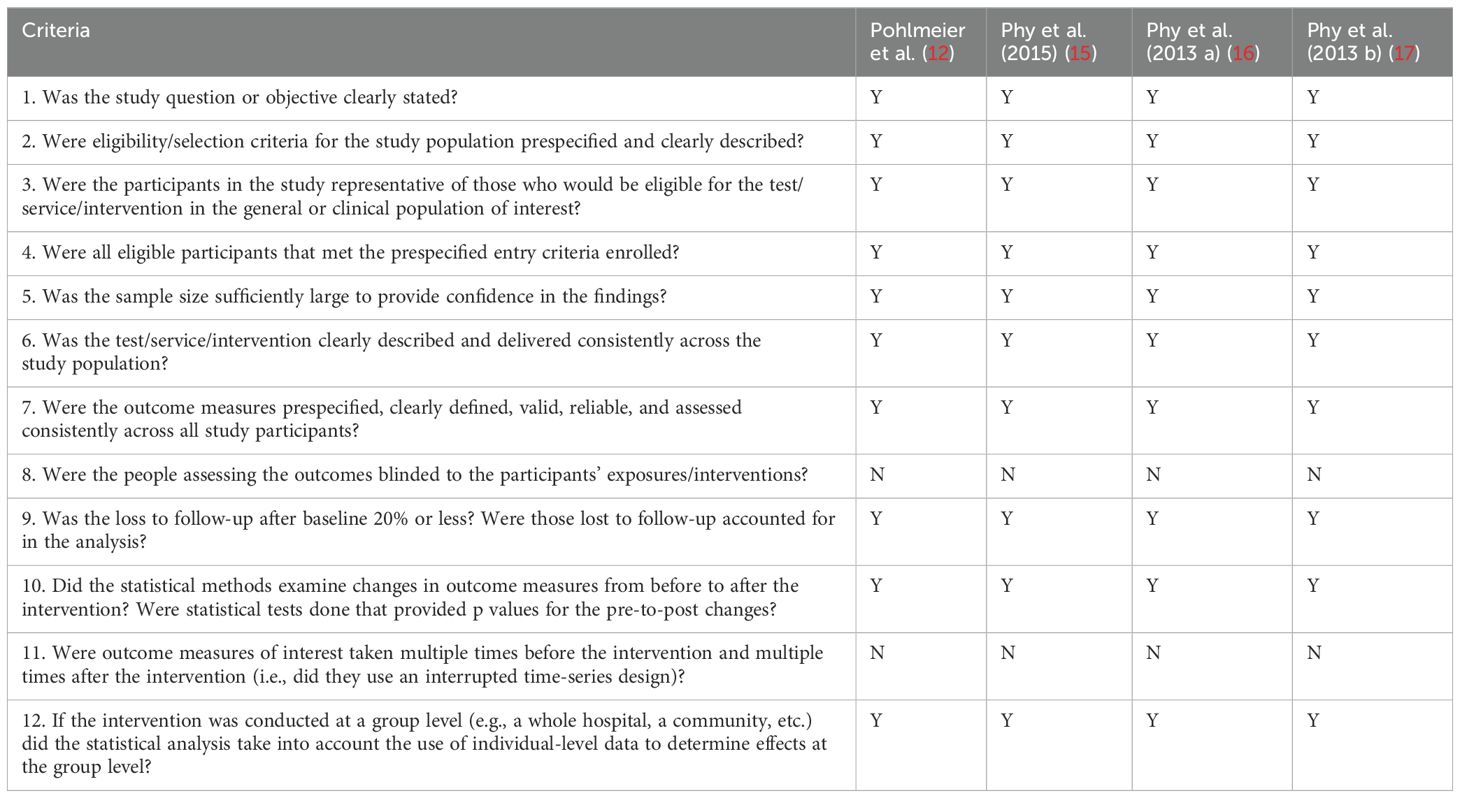
Table 6. Quality of the Included Interventional Studies (14).
Main findings
In the clinical studies included in the meta-analysis, high levels of heterogeneity were observed in the association between reducing dairy product consumption and the mean of anthropometric, blood glucose, Ferryman-Gallwey score, and testosterone level components (p-value<0.001). Therefore, a random-effect model was used to analyze the data. After reducing dairy product consumption, the results demonstrated statistically significant reductions in mean weight (SMD: -8.43 (95% CI: -9.01, -7.86)), BMI (-3.14 (95% CI: -3.35, -2.92)), WC (-6.63 (95% CI: -10.70, -2.57)), and WHtR (-0.04 (95% CI: -0.07, -0.01)) compared to pre-intervention levels. (Figure 2). Furthermore, a notable association was found between reducing dairy product consumption and the mean levels of fasting insulin (-18.23 (95% CI: -22.11, -14.36)), insulin at 120 minutes (-94.05 (95% CI: -157.67, -30.42)), and HbA1c (-0.27 (-0.37, -0.17)). However, no significant reduction was observed in the mean levels of fasting glucose, 2-hour glucose, and percentage of fat mass before and after the intervention (Figure 3). Regarding the Ferryman-Gallwey score and testosterone level components, there was a statistically significant decrease in the mean of total testosterone (-9.97 (95% CI: -14.75, -5.19)) and Ferryman-Gallwey score (-2.07 (95% CI: -2.98, -1.16)) after the intervention compared to before. However, there was no significant association observed between the two groups in terms of the mean of free testosterone after the intervention (Figure 4).
Cross-sectional studies
Description
This cross-sectional study assessed the dietary quality of 100 women diagnosed with PCOS. All participants met the Rotterdam criteria for PCOS diagnosis and were within the reproductive age range of 18–45 years. Anthropometric measurements, including weight, height, WC, BMI, and hip circumference (HC) were obtained. Dietary intake was evaluated using the Brazilian Healthy Eating Index – Revised (BHEI-R), where higher scores reflect better dietary quality (18). The quality of this study was assessed and outlined in Table 7. It fulfilled 10 out of the 14 assessment criteria, indicating a high level of quality.
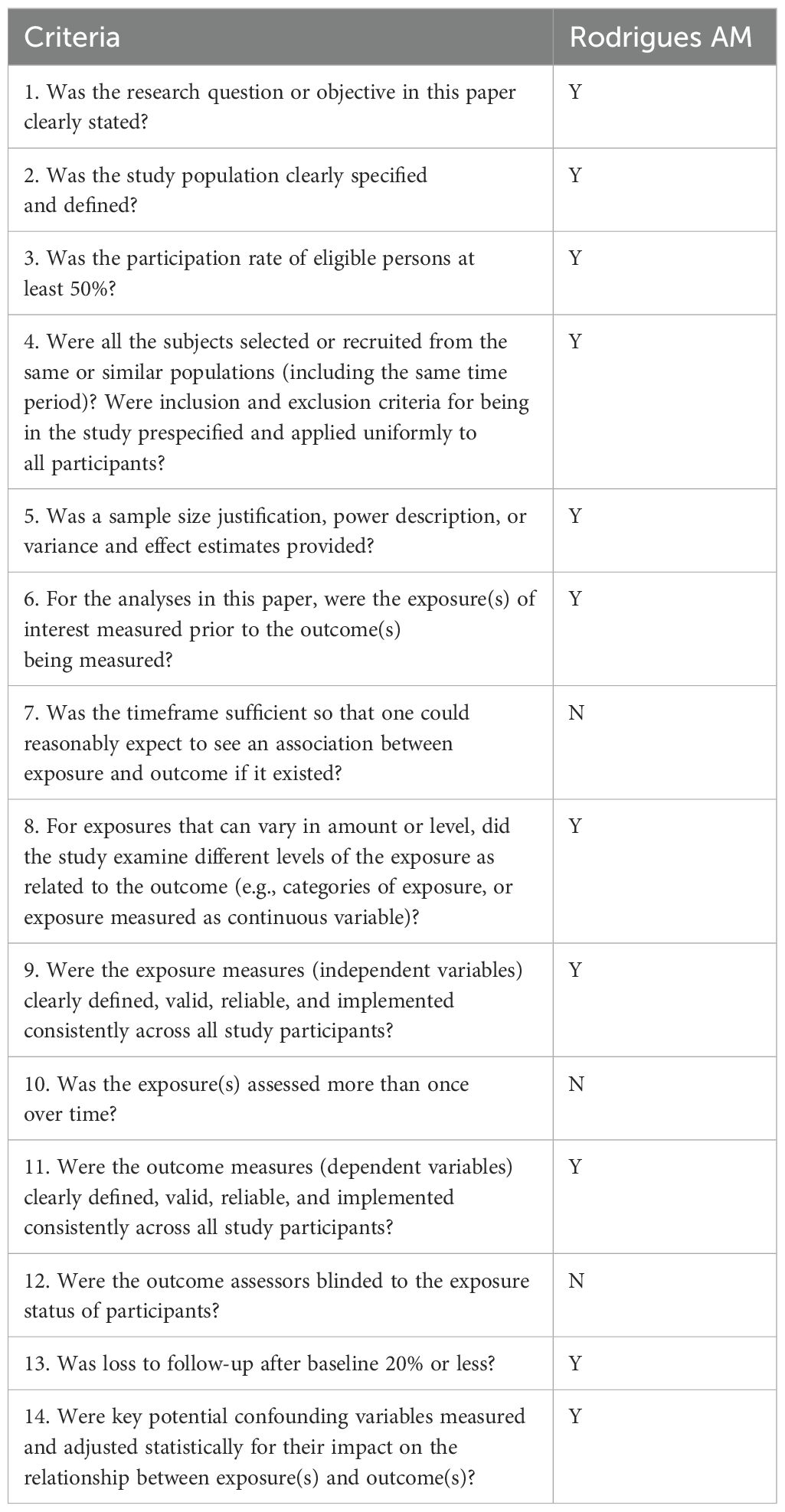
Table 7. Quality of the Included Cross-Sectional Studies (14).
The prevalence of overweight and obesity was 30.0% and 66.0%, respectively, with 90.0% exhibiting increased visceral fat mass, indicating a heightened risk of metabolic complications. Additionally, 64.0% were categorized as sedentary, while 36.0% were classified as less active (18).
Main finding
The mean BHEI-R score was 56.1 ± 12.0 points, with 56.0% of participants reporting an inadequate diet and 44.0% reporting a diet that needs improvement’. The BHEI-R score showed negative correlations with measures of obesity, including BMI (r = 0.248; P = 0.013), body weight (r = 0.220; P = 0.028), and WC (r = 0.278; P = 0.005).
Discussion
In this study, we reviewed 11 studies that met our inclusion criteria out of the 1,313 citations reviewed. These studies consisted of six case-control studies, four clinical trials, and one cross-sectional study. The meta-analysis of the clinical trials studies revealed statistically significant improvements in various clinically relevant measures when compared to the pre-intervention period. These included reductions in weight, BMI, waist circumference, waist-to-height ratio, fasting insulin, insulin at 120 minutes, HbA1c, and Ferryman-Gallwey score. However, there were no significant changes observed in fasting glucose, 2-hour glucose, percent of fat mass, and mean free testosterone levels between the two groups following the intervention.
Main findings
In general, our research suggests that there is controversy regarding association between dairy consumption and PCOS occurrence. Some studies, such as Hosseini et al. and Badri-Fariman et al., detected an inverse correlation between dairy consumption and PCOS (19, 20), while others, such as Zirak Sharkesh et al. and Shishehgar et al., found no significant relationship (21, 22). Rajaeieh et al.’s study showed a direct relationship between milk consumption and PCOS, while Altieri et al. found a direct relationship with cheese consumption (23, 24).
Overall, it is impossible to conclude that dairy consumption is a risk factor for PCOS, and further research is needed to determine whether reducing dairy consumption can improve PCOS symptoms.
All four interventional studies reviewed found that a low-dairy/low-starch diet had a positive impact on anthropometric factors and glycemic control, as demonstrated in the studies conducted by Pohlmeier et al., Phy et al., and Phy et al. b (1, 16, 17). Additionally, the diet was shown to have positive effects on testosterone levels in the studies conducted by Phy et al., Phy et al. a, and Pohlmeier et al. (1, 15, 17) Overall, these studies suggest that a low-dairy/low-starch diet may be effective in improving laboratory and anthropometric factors in patients with PCOS. The results suggest that reducing the intake of dairy and starch in the diet may lead to beneficial impacts on laboratory measures in people with polycystic ovary syndrome.
Janiszewska et al.’s study showed that including whole milk and dairy products in the diet of women with polycystic ovary syndrome could be beneficial due to their positive effect on the risk of developing type 2 diabetes mellitus in women (25). However, this finding was not supported by experimental and clinical studies, which instead showed that reducing dairy and starch consumption had a positive effect on glycemic control, as demonstrated in our study.
Temperament as the final homogeneous quality results from the interactions of opposite qualities- heat, coldness, moisture, and dryness - the four philosophical elements when they combine and form the composite materials of the world. Based on this, everything, including food and medicine and even conditions such as weather and climate, has its temperament, which is determined by the change it imposes on human temperament. Due to this effect, their temperament can cause human disease or be used to maintain health or treat diseases in different people. Large groups of diseases are caused by bad temperaments provoked in a part or the whole human body. Therefore, disease prevention or treatment may be facilitated by recognizing and treating the underlying temperament disorder. The temperament of the whole body or specific organs has been observed by sages with scenarios of signs and symptoms accumulated over the centuries (26, 27).
Iranian medical texts describe milk as consisting of three parts: fat, water, and cheese (carbohydrates and protein). The fat part of milk has a hot and dry temperament, while the cheese part has a cold and dry temperament, and the water has a cold and wet temperament. This means that as milk fat content increases, its temperament becomes warmer, and if the fat content is low, its coldness increases. For example, high-fat milk and dairy products are warmer than low-fat options, and buttermilk and yogurt are colder than milk (28, 29).
In traditional Iranian medicine, PCOS is considered a disease with a cold and wet temperament (30). Therefore, it is important to carefully observe the avoidance of dairy products with a cold and wet temperament in people with PCOS (31).
Chavarro’s study suggested that high intake of low-fat dairy foods may increase the risk of anovulatory infertility, while intake of high-fat dairy foods may decrease this risk. Lactose, the main carbohydrate in milk and dairy products is not believed to affect fertility within the usual range of intake levels in humans (32, 33).
Shishehgar’s study found that PCOS cases consumed less high-fat dairy than controls, but the difference was not statistically significant (22). However, in other studies, the temperament of the consumed dairy product has not been considered, which may be a reason for the different and contradictory results.
In observational studies, in most cases, the consumption of dairy products has been lower in the polycystic ovary syndrome group, but in interventional studies, reducing dairy consumption has had a positive effect on improving anthropometric and laboratory factors.
To make more accurate and informed decisions regarding the consumption of dairy products in PCOS patients, it is important to conduct both interventional and descriptive studies that take into account the temperament of dairy products. This may help to clarify the relationship between dairy consumption and PCOS symptoms and provide more specific recommendations regarding the types and amounts of dairy products that are safe and beneficial for PCOS patients.
Limitations
One limitation of our study was the absence of intervention studies that solely investigated the impact of reducing or increasing dairy consumption. All four interventional studies that we analyzed focused on the effect of simultaneously reducing dairy and starch consumption. Another limitation was the lack of studies that considered the type of dairy products consumed and the temperament of patients with PCOS. These factors are important in assessing the impact of dairy products on the disease and could influence the results of the studies.
Conclusion
In summary, the findings of this systematic review show limited evidence about the association between dairy consumption and PCOS. The interventional studies suggest that reducing dairy consumption in combination with reducing starch intake may improve some anthropometric and metabolic measures in PCOS women, including weight, BMI, waist circumference, Waist-to-Height Ratio, insulin fasting, insulin 120 minutes, HbA1c. However, more research with larger sample sizes and more diverse populations is needed to fully understand the effects of dairy consumption on PCOS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HR: Writing – original draft, Writing – review & editing, Formal Analysis, Methodology, Validation, Visualization. ES: Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, Software. HH: Methodology, Validation, Writing – original draft, Writing – review & editing, Visualization. MM: Writing – original draft, Writing – review & editing, Data curation, Investigation, Project administration, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The author (s) received financial support for the research, authorship, and publication of this article from Alborz University of Medical Science.
Acknowledgments
Researchers would like to express our sincere appreciation and our deepest gratitude to the Clinical Research Development Center of Kamali and Rajaee Hospitals in Alborz University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pfieffer ML. Polycystic ovary syndrome: Diagnosis and management. Nurse Pract. (2019) 44:30–5. doi: 10.1097/01.NPR.0000553398.50729.c0
2. Papavasiliou K PE. Nutritional support and dietary interventions for women with polycystic ovary syndrome. Nutr Dietary Supplements. (2017) 9:63–85. doi: 10.2147/NDS
3. Nicolaides NC, Matheou A, Vlachou F, Neocleous V, Skordis N. Polycystic ovarian syndrome in adolescents: From diagnostic criteria to therapeutic management. Acta bio-medica: Atenei Parmensis. (2020) 91:e2020085. doi: 10.23750/abm.v91i3.10162
4. Xu Y, Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): A review of literature. J healthcare engineering. (2022) 2022:9240569. doi: 10.1155/2022/9240569
5. Merkin SS, Phy JL, Sites CK, Yang D. Environmental determinants of polycystic ovary syndrome. Fertility sterility. (2016) 106:16–24. doi: 10.1016/j.fertnstert.2016.05.011
6. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin endocrinology. (2021) 95:531–41. doi: 10.1111/cen.14421
7. Rocha AL, Oliveira FR, Azevedo RC, Silva VA, Peres TM, Candido AL, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Research. (2019) 8. doi: 10.12688/f1000research
8. Shahid R, Iahtisham Ul H, Mahnoor, Awan KA, Iqbal MJ, Munir H, et al. Diet and lifestyle modifications for effective management of polycystic ovarian syndrome (PCOS). J Food Biochem. (2022) 46:e14117. doi: 10.1111/jfbc.14117
9. Gu Y, Zhou G, Zhou F, Wu Q, Ma C, Zhang Y, et al. Life modifications and PCOS: old story but new tales. Front Endocrinol (Lausanne). (2022) 13:808898. doi: 10.3389/fendo.2022.808898
10. Cutillas-Tolín A, Arense-Gonzalo JJ, Mendiola J, Adoamnei E, Navarro-Lafuente F, Sánchez-Ferrer ML, et al. Are dietary indices associated with polycystic ovary syndrome and its phenotypes? A Preliminary Study. Nutrients. (2021) 13:313. doi: 10.3390/nu13020313
11. Janiszewska J, Ostrowska J, Szostak-Węgierek D. Milk and dairy products and their impact on carbohydrate metabolism and fertility—A potential role in the diet of women with polycystic ovary syndrome. Nutrients. (2020) 12:3491. doi: 10.3390/nu12113491
12. Pohlmeier AM, Phy JL, Watkins P, Boylan M, Spallholz J, Harris KS, et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Applied physiology, nutrition, and metabolism. = Physiologie appliquee Nutr metabolisme. (2014) 39:1237–44. doi: 10.1139/apnm-2014-0073
13. Gannon MC, Nuttall FQ, Westphal SA, Fang S, Ercan-Fang N. Acute metabolic response to high-carbohydrate, high-starch meals compared with moderate-carbohydrate, low-starch meals in subjects with type 2 diabetes. Diabetes Care. (1998) 21:1619–26. doi: 10.2337/diacare.21.10.1619
14. National Heart L, and Blood Institute. Study quality assessment tools (2021). Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. accessed [June 30, 2023].
15. Phy JL, Pohlmeier AM, Cooper JA, Watkins P, Spallholz J, Harris KS, et al. Low starch/low dairy diet results in successful treatment of obesity and co-morbidities linked to polycystic ovary syndrome (PCOS). J Obes weight loss Ther. (2015) 5. doi: 10.4172/2165-7904.1000259
16. Phy J, Pohlmeier A, Cooper J, Harris K, Watkins P, Boylan M. REDUCTION OF CLINICAL HYPERANDROGENISM IN POLYCYSTIC OVARY SYNDROME PATIENTS AFTER 8-WEEK LOW STARCH/LOW DAIRY DIET. Fertility sterility. (2013) 100:S350–S. doi: 10.1016/j.fertnstert.2013.07.816
17. Phy JL, Pohlmeier A, Cooper J, Harris K, Watkins P, Boylan M. POLYCYSTIC OVARY SYNDROME PATIENTS ACHIEVE SUCCESSFUL WEIGHT LOSS AND DECREASED WAIST AND HIP CIRCUMFERENCE AFTER 8-WEEK LOW STARCH/LOW DAIRY DIET. Fertility sterility. (2013) 100:S337–S. doi: 10.1016/j.fertnstert.2013.07.889
18. Rodrigues AM, Martins LB, Franklin AM, Candido AL, dos Santos LC, Ferreira AV. Poor quality diet is associated with overweight status and obesity in patients with polycystic ovary syndrome. J Hum Nutr dietetics: Off J Br Dietetic Assoc. (2015) 28 Suppl 2:94–101. doi: 10.1111/jhn.12205
19. Hosseini MS, Dizavi A, Rostami H, Parastouei K, Esfandiari S. Healthy eating index in women with polycystic ovary syndrome: A case-control study. Int J Reprod BioMedicine. (2017) 15:575–82. doi: 10.29252/ijrm.15.9.575
20. Badri-Fariman M, Naeini AA, Mirzaei K, Moeini A, Hosseini M, Bagheri SE, et al. Association between the food security status and dietary patterns with polycystic ovary syndrome (PCOS) in overweight and obese Iranian women: a case-control study. J Ovarian Res. (2021) 14:134. doi: 10.1186/s13048-021-00890-1
21. Zirak Sharkesh E, Keshavarz SA, Nazari L, Abbasi B. The dietary inflammatory index is directly associated with polycystic ovary syndrome: A case-control study. Clin endocrinology. (2022) 96:698–706. doi: 10.1111/cen.14672
22. Shishehgar F, Ramezani Tehrani F, Mirmiran P, Hajian S, Baghestani AR, Moslehi N. Comparison of dietary intake between polycystic ovary syndrome women and controls. Global J Health science. (2016) 8:54801. doi: 10.5539/gjhs.v8n9p302
23. Rajaeieh G, Marasi M, Shahshahan Z, Hassanbeigi F, Safavi SM. The relationship between intake of dairy products and polycystic ovary syndrome in women who referred to isfahan university of medical science clinics in 2013. Int J Prev Med. (2014) 5:687–94.
24. Altieri P, Cavazza C, Pasqui F, Morselli AM, Gambineri A, Pasquali R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin endocrinology. (2013) 78:52–9. doi: 10.1111/j.1365-2265.2012.04355.x
25. Janiszewska J, Ostrowska J, Szostak-Węgierek D. Milk and dairy products and their impact on carbohydrate metabolism and fertility-A potential role in the diet of women with polycystic ovary syndrome. Nutrients. (2020) 12. doi: 10.3390/nu12113491
26. Parvinroo S, Kamalinejad M, Sabetkasaei M. Pharmacological concepts of temperament in Iranian traditional medicine. Iran J Public Health. (2014) 43:1463–5.
27. Laila S, Azadeh Z, Ayeh N, Mahdi Alizadeh V. The concept of temperaments in traditional persian medicine. Traditional Integr Med. (2017) 2:143–56.
28. Ibn-Sina H. General Principles of Medicine Assessment Regimen in Health and Disease. New Delhi, India: Jamia Hamdard (1993).
29. Moeini R, Gorji N. Persian medicine in the world of research; review of articles on Iranian traditional medicine. Iran J Med Sci. (2016) 41:S7.
30. Hosseinkhani A, Asadi N, Pasalar M, Zarshenas MM. Traditional Persian Medicine and management of metabolic dysfunction in polycystic ovary syndrome. J Tradit Complement Med. (2018) 8:17–23. doi: 10.1016/j.jtcme.2017.04.006
31. Tansaz M, Bahmani M. Principles of nutrition in patients with polycystic ovary syndrome in Iranian traditional medicine and comparison with modern medicine. Iran J Med Sci. (2016) 41:S49.
32. Chavarro JE, Rich-Edwards JW, Rosner B, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reproduction. (2007) 22:1340–7. doi: 10.1093/humrep/dem019
Keywords: dairy consumption, polycystic ovary syndrome (PCOS), anthropometric measurements, blood glucose, insulin levels, women, systematic review, meta-analysis
Citation: Rastad H, Shahrestanaki E, Heydarian HR and Maarefvand M (2024) Dairy consumption and its association with anthropometric measurements, blood glucose status, insulin levels, and testosterone levels in women with polycystic ovary syndrome: a comprehensive systematic review and meta-analysis. Front. Endocrinol. 15:1334496. doi: 10.3389/fendo.2024.1334496
Received: 07 November 2023; Accepted: 14 August 2024;
Published: 03 October 2024.
Edited by:
Thozhukat Sathyapalan, Hull York Medical School, United KingdomReviewed by:
Kaijian Hou, Shantou University, ChinaAnca Pantea Stoian, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Rastad, Shahrestanaki, Heydarian and Maarefvand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mina Maarefvand, ZHIubS5tYWFyZWZ2YW5kQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hadith Rastad
Hadith Rastad Ehsan Shahrestanaki
Ehsan Shahrestanaki Hamid Reza Heydarian4
Hamid Reza Heydarian4 Mina Maarefvand
Mina Maarefvand