
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 19 March 2024
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1333595
Introduction: Acetaldehyde dehydrogenase 2 (ALDH2) had reported as a prominent role in the development of cardiometabolic diseases among Asians. Our study aims to investigate the relationship between ALDH2 polymorphism and cardiometabolic risk factors in East Asian population.
Method: We searched databases of PubMed, Web of Science, and Embase updated to Oct 30th, 2023. We extracted data of BMI, Hypertension, SBP, DBP, T2DM, FBG, PPG, HbA1c, TG, TC, LDL-C and HDL-C.
Result: In total, 46 studies were finally included in our meta-analysis, containing, 54068 GG and, 36820 GA/AA participants. All outcomes related to blood pressure revealed significant results (hypertension OR=0.83 [0.80, 0.86]; SBP MD=-1.48 [-1.82, -1.14]; DBP MD=-1.09 [-1.58, -0.61]). FBG showed a significant difference (MD=-0.10 [-0.13, -0.07]), and the lipid resulted significantly in some outcomes (TG MD=-0.07 [-0.09, -0.04]; LDL-C MD=-0.04 [-0.05, -0.02]). As for subgroups analysis, we found that in populations without severe cardiac-cerebral vascular diseases (CCVDs), GG demonstrated a significantly higher incidence of T2DM (T2DM OR=0.88 [0.79, 0.97]), while the trend was totally opposite in population with severe CCVDs (T2DM OR=1.29 [1.00, 1.66]) with significant subgroup differences.
Conclusion: Our updated meta-analysis demonstrated that ALDH2 rs671 GG populations had significantly higher levels of BMI, blood pressure, FBG, TG, LDL-C and higher risk of hypertension than GA/AA populations. Besides, to the best of our knowledge, we first report GG had a higher risk of T2DM in population without severe CCVDs, and GA/AA had a higher risk of T2DM in population with severe CCVDs.
https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023389242.
Acetaldehyde dehydrogenase 2 (ALDH2) in humans is a 517-amino acid polypeptide encoded by a nuclear gene located at chromosome 12q24 (1). Among the 19 kinds of human ALDH isozymes, ALDH2 is the most efficient Isozyme for metabolizing ethanol-derived acetaldehyde (2). Although ALDH2 is well-known for its crucial role in ethanol metabolism, ALDH2 also has other diverse pathophysiological effects: ALDH2 could also metabolize many other short-chain fatty aldehydes and some aromatic and polycyclic aldehydes, which provides essential protective enzyme functions against these toxic substances and potential mechanism of participating in the development of cardiovascular disease (CVD) (3, 4). Besides, recent research has revealed non-enzymatic functions of ALDH2, participating in lipid metabolism in hepatocytes, regulating foam cell formation in macrophages, and modulating cellular senescence in endothelial cells and vascular smooth muscle cells (5). ALDH2 A carriers have low ALDH2 enzyme activity, characterized by nausea, facial flushing, headache, palpitations and dizziness after drinking. This alcohol-induced ALDH2 A individual flushing syndrome is caused by a single G to A nucleotide change (also known as SNP rs671 G>A), substituting the glutamate to lysine at position 487 (see Supplementary Figure S1) (6). The ALDH2 A mutation has a dominant effect on the ALDH2 G allele, so the enzyme activity of homozygous ALDH2 AA is expected to be 1-4% enzyme activity of the wild-type, and enzyme activity of heterozygous ALDH2 GA is significantly lower than that of the wild-type by 50% (3).
CVD is a non-communicable disease responsible for a large number of deaths worldwide, and genetic factors have been proven to be one of the main risk factors (7, 8). Besides, Asians are more susceptible to metabolic disorders than other racial groups, regardless of obesity (9). However, only a limited number of specific genetic loci variations have been found between individuals of European and East Asian ancestry. Recently, several previously unreported variants among East Asian individuals had been reported related to type 2 diabetes (T2DM) in a comprehensive meta-analysis, which included 433,540 East Asian subjects from genome-wide association studies (GWAS) (10). Of these variants, ALDH2 rs671, which was estimated to be present in about 30–50% of the East Asian population, compared to less than 5% of the European population, was found to be most prominently associated with T2DM (11, 12).
Cardiometabolic risk factors encompass lifestyle habits and health conditions, such as obesity, dyslipidemia, hypertension, diabetes, and hyperuricemia (13). The interaction among these various cardiometabolic risk factors ultimately leads to the progression of cardiovascular diseases. In China, more than 40% of deaths are attributable to CVDs, and the number of CVD deaths has almost doubled in the past decades (14). The increasing prevalence of cardiometabolic risk factors underlies the rise of CVDs, and thus curbing the rising cardiometabolic pandemic is imperative (13). ALDH2 gene polymorphism had been widely studied in many human diseases (5, 9, 15, 16), but only a few research discussed the relationship between ALDH2 and cardiometabolic risk factors. One meta-analysis (Li et al., 2017) discussed the correlation between ALDH2 and T2DM with data from only six studies (17). Although it resulted in a significant conclusion, the sensitivity analysis and Egger’s test were positive, and the results between subgroups demonstrated an opposite trend of odds ratio (OR) value. In addition, the conclusions of several recently published articles were inconsistent with the meta-analysis (18, 19). Besides, the most recent meta-analysis discussing ALDH2 and hypertension (Zheng et. al., 2020) did not conduct any subgroup analysis (20). Meanwhile, ALDH2 was also reported in previous GWAS to be associated with hypertension, triglyceride (TG) and body mass index (BMI) (21). This revealed the prominent role of ALDH2 in the development of CVDs among Asians. Therefore, the meta-analysis to investigate the relationship between ALDH2 polymorphism and cardiometabolic risk factors in East Asian population needed to be updated.
Our protocol was prospectively registered in the International prospective register of systematic reviews (PROSPERO) registry (CRD42023389242). We followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Table S1). Two independent reviewers (RL and PM) searched PubMed, Web of Science, and Embase (all updated to Oct 30th, 2023) and screened the titles and abstracts for potential included studies. The search strategy is available as Supplementary Material (Supplementary Table S2). We only full-text reviewed articles which met the inclusion criteria, and the reference lists of eligible articles were searched for additional citations.
There was no restriction on age, sex, and language. The inclusion criteria were (1): studies included subgroup data of ALDH2 gene rs671 polymorphism (GG, GA, AA type). (2) studies included at least one of the cardiometabolic risk factors: BMI, Hypertension, systolic blood pressure (SBP), diastolic blood pressure (DBP), T2DM, fasting blood glucose (FBG), postprandial blood glucose (PPG), hemoglobin A1c (HbA1c), TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C). (3) studies only included the East Asian population. We excluded studies discussing other single nucleotide polymorphisms of ALDH2.
Two researchers (RL and KQ) independently extracted general characteristics (number of participants, age, sex) and outcomes of cardiometabolic risk factors from eligible articles. Decisions were made by consulting another reviewer LC, when RL and KQ met disagreements and failed to reach a consensus. We emailed the corresponding authors for additional information when the data was incomplete.
Newcastle Ottawa Scale (NOS) was used for quality assessment of cohort and case-control studies (22), and the Agency for Healthcare Research and Quality (AHRQ) was used for cross-sectional studies (23). Two researchers (JZ and TZ) independently assessed the quality of included studies. The decision will be reached by consulting a third reviewer LC, when met disagreements and failed to reach a consensus. The precise Hardy Weinberg Equilibrium (HWE) was used to test the distribution of genotypes and evaluated by chi-square tests. High quality is considered NOS ≥6 points or AHRQ ≥8 points, and PHWE>0.05.
Several types of subgroup analysis were conducted in our study: (1) quality of study; (2) publication type (cross-sectional, cohort, case-control); (3) nationality (Chinese, Korean, Japanese); (4) population (with or without severe cardiac-cerebral vascular diseases (CCVDs), which including myocardial infarct, coronary artery disease, ischemic stroke and hemorrhagic stroke).
Revman 5.3 and Stata 16.0 software were used to analyze Meta-analysis data. All forest plots and funnel plots were produced by Revman 5.3 software. Continuous data would be calculated by mean difference (MD) with 95% confidence intervals (CI). Dichotomous data would be calculated by OR with 95% CIs. Heterogeneity in the result of the meta-analysis was assessed by Cochrane Q and I2 statistics. I2 ≤ 50% demonstrated a low heterogeneity, and the fixed-effect model would be used for analysis; I2>50% demonstrated a high heterogeneity, and the random-effect model would be used for analysis. All statistical tests were two-tailed, and P ≤ 0.05 was regarded as a statistically significant difference. Publication bias was assessed by funnel plots and Egger’s tests. Sensitivity analysis was performed in the meta-analysis by excluding each study once at a time to check whether the effectiveness of the outcome was determined by individual studies.
The detailed steps of the literature search are shown in Figure 1: 373 studies were reviewed from three databases, and eight studies were reviewed through the references of eligible full-text articles; 263 studies were excluded after screening titles and abstracts, and the remaining 118 studies were reviewed in full text. After excluding 72 studies according to the selection criteria, 46 studies with, 54068 GG participants and, 36820 GA/AA participants were included in our meta-analysis (19, 20, 24–67).
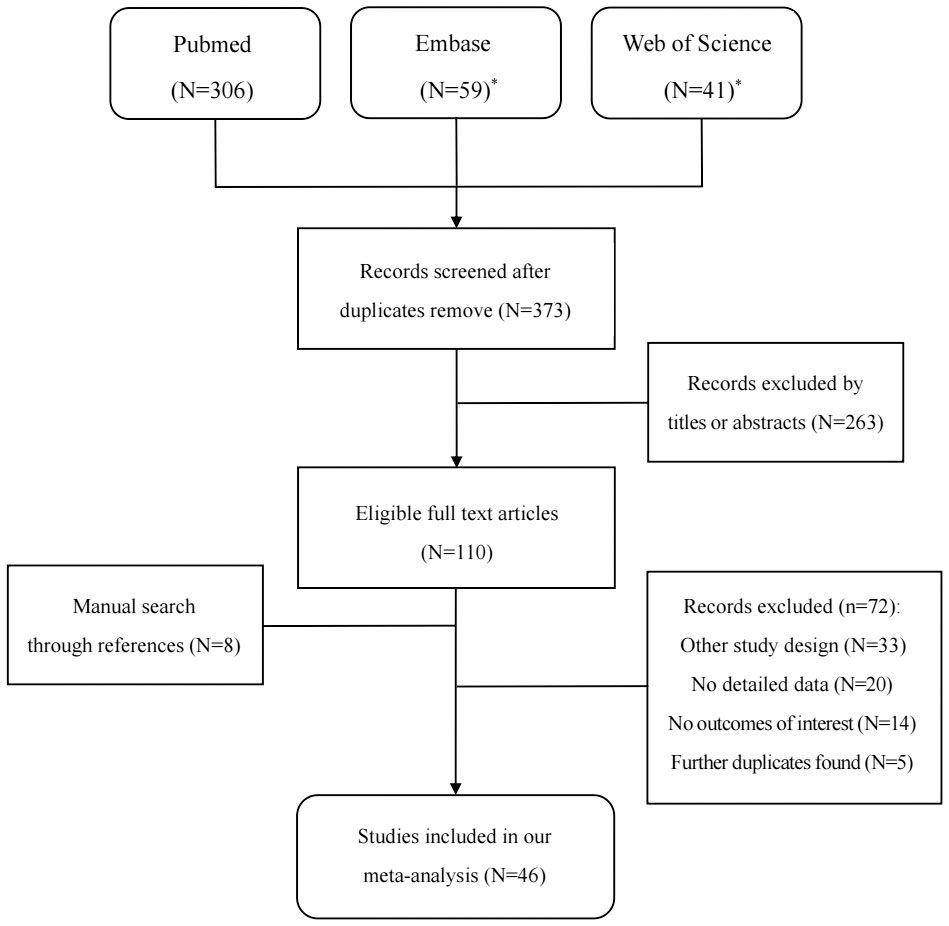
Figure 1 Flowchart of selection of studies for inclusion in our meta-analysis. (*Outcomes from Embase and Web of science were not included studies from Medline).
The general characteristics of included studies in our meta-analysis are shown in Table 1. 23 studies were Chinese, 22 were Japanese, and one was Korean. As for publication type, 17 were cross-sectional studies, 13 were cohort studies, and 16 were case-control studies. Among all 46 studies, 36 studies (78.3%) included populations without severe CCVDs, and 35 studies (76.1%) were assessed as high quality according to HWE and NOS or AHRQ. Detailed procedures of NOS and AHRQ are shown in Supplementary Table S3.
Summary findings of outcomes are shown in Table 2. Firstly, the differences of all confounders in our study were insignificant. It was worth mentioning that ALDH2 had a significantly different effect on T2DM, so the Pgender>0.05 could eliminate the bias caused by gender. As for outcomes, the GG population had demonstrated a significantly higher BMI level than the GA/AA population (MD=-0.26 [-0.32, -0.19], P<0.001, detailed forest plot see Figure 2). Besides, all outcomes related to blood pressure revealed significant results (incidence of hypertension OR=0.83 [0.80, 0.86], P<0.001; SBP MD=-1.48 [-1.82, -1.14], P<0.001; DBP MD=-1.09 [-1.58, -0.61], P<0.001; detailed forest plot of hypertension see Figure 3). However, only FBG showed a significant difference (MD=-0.10 [-0.13, -0.07], P<0.001), while the incidence of T2DM resulted in an insignificant difference (detailed forest plot see Figure 4). Moreover, the lipid resulted significantly in some of the outcomes (TG MD=-0.07 [-0.09, -0.04], P<0.001; LDL-C MD=-0.04 [-0.05, -0.02]). In all, ALDH2 is significantly associated with the most cardiometabolic risk factors in the total population. All detailed forest plots are displayed in Supplemental Files (see Supplementary Figures S2-S12). Funnel plots of BMI, hypertension and T2DM are also showed in Supplementary Figures S13-15. Egger’s test was only significant in the outcome of HbA1c. All outcomes of the sensitivity analysis were also insignificant.
There were four types of subgroup analyses in our study. Supplementary Table S4 shows that all subgroup differences between different qualities of included studies were insignificant, so the studies of low qualities were not excluded from our meta-analysis. Supplementary Table S5 shows that all subgroup differences between different nationalities of included studies were also insignificant. Besides, Supplementary Table S6 demonstrates that all subgroup differences between different article types were insignificant. However, we found that in populations without severe CCVDs, GG demonstrated a significantly higher incidence of T2DM and FBG level (GA/AA vs. GG: T2DM OR=0.88 [0.79, 0.97]; MD=-0.11 [-0.14, -0.08]), while the trend was totally opposite in population with severe CCVDs (GA/AA vs. GG: OR=1.29 [1.00, 1.66]; MD=0.25 [0.09, 0.41]) with significant subgroup differences (PT2DM=0.006, PFBG<0.001, detailed data see Supplementary Table S7). Moreover, other outcomes revealed insignificant subgroup differences. It is worth mentioning that the subgroup analysis between severe CCVDs and without severe CCVDs was the first time reported.
Our meta-analysis indicated that ALDH2 rs671 GG gene type was not only a risk factor for promoting the development of hypertension, but also in increasing BMI, blood pressure, FBG, TG, and LDL-C. In addition, the impact of ALDH2 on T2DM and FBG varies significantly among different populations. In the population without severe CCVDs, the incidence of T2DM and FBG levels in the GG population were significantly higher than the GA/AA population. In contrast, the conclusion was totally opposite in the population with severe CCVDs. Our results were more comprehensive and convincing based on the following aspects: Firstly, our meta-analysis included more eligible studies, providing sufficient statistical efficacy. Secondly, confounding factors were examined in our meta-analysis to avoid the potential intervention. Thirdly, we conducted four subgroup analyses on factors that may affect the reliability to reduce the heterogeneity between different studies. Fourthly, sensitivity analysis and Egger’s test showed that the results were stable and reliable, and no significant publication bias was found.
Currently, there is no meta-analysis discussing the relationship between ALDH2 and BMI, and our meta-analysis demonstrated that ALDH2 GG populations had a significantly higher BMI than GA/AA populations in the outcome of all subgroups. Some clinical articles have reported lower BMI due to healthier dietary habits caused by flushing syndrome in the GA/AA population (68, 69). Besides, it should be clarified that the correlation between ALDH2 and BMI varies among different genders. Gender differences also exist in other cardiometabolic outcomes (10, 70). Several studies have reported the activation effect of estradiol on ALDH2 (71, 72). Besides, we cannot ignore the differences in alcohol consumption between different genders due to social factors in East Asian culture, as well as the protective effect of estrogen itself on insulin resistance (73).
T2DM and hypertension are common microvascular and macrovascular diseases in East Asian populations (74). ALDH2 mutants have lower levels of BMI, providing a partial explanation for the correlation between ALDH2 polymorphism and T2DM and hypertension. In addition, other literature also discussed the mechanisms between ALDH2 and cardiometabolic risk factors. Previous studies on ALDH2 rs671 polymorphism and T2DM had provided controversial results (25, 35, 38, 40, 57, 63, 67). This may be due to the sample size of most ALDH2 studies being relatively small (28 included studies of our study had participants less than, 1000), and ALDH2 was interfered with other confounding factors. Our study included abundant studies and analyzed the confounders (age, gender, and smoker) to avoid the potential bias. Besides, it was worth mentioning that ALDH2 rs671 polymorphism was reported to modify the association between dietary behaviors and BMI independently of drinking habits. Therefore, lower BMI and alcohol intake of GA/AA individuals were supposed to be the mechanism of ALDH2 on T2DM in populations without severe CCVDs. However, people with severe CCVDs revealed a higher risk of T2DM and FPG levels in the GA/AA population. Some mechanisms may explain it (1): Tan et al. demonstrated that ALDH2 alleviated the ischemia and reperfusion injury in diabetic cardiomyopathy through inhibition of mitoPTP opening and activation of PI3K/AKT/mTOR pathway (75); (2) low ALDH2 activity exacerbated 4HNE-mediated coronary endothelial cell injury and thereby cardiac dysfunction and ischemia-reperfusion injury (76, 77). A meta-analysis by Xu et al. also concluded the correlation between the higher risk of ischemic stroke and ALDH2 rs671-variant, especially in AA populations. Therefore, for CAD populations, GA/AA individuals had a higher risk of progressing to severe CCVDs, which explained the opposite outcomes in the severe CCVDs subgroup. Moreover, Chang et al. demonstrated that male Aldh2 knock-in mice were prone to develop glucose intolerance, insulin resistance, and fatty liver under diet-induced obesity. Proteomic analyses of the brown adipose tissue from the male Aldh2 knock-in mice identified increased 4-hydroxynonenal-adducted proteins involved in mitochondrial fatty acid oxidation and electron transport chain, leading to markedly decreased fatty acid oxidation rate and mitochondrial respiration. Similar phenomena were also reproduced in other studies of mice experiments (78, 79). In summary, we inferred that in the normal population, ALDH2 GG has a higher risk of T2DM due to higher BMI and alcohol consumption, but severe CCVDs contributed to exacerbating central muscle cell damage in the GA/AA individuals with a higher FBG level.
As for the hypertension-related results, a statistically significant correlation between ALDH2 and hypertension was observed in all subgroups. Therefore, we could confirm that the GG population had a higher risk of hypertension and higher levels of SBP and DBP as well. Although GA/AA individuals had more acetaldehyde accumulation, Zhang et al. reported in their cohort study that ethanol, rather than acetaldehyde, played a key role in alcohol-induced hypertension (80). Therefore, GA/AA individuals were protected from hypertension due to their lower ethanol intake than GG individuals. In addition, though our conclusion was consistent with the recently published meta-analysis by Zheng et al., we had updated hypertension-related data by adding from twelve new studies than their study (20). Moreover, we analyzed confounders (age and smoker) and three new subgroup analyses, which they did not include, so we believed our article was updated compared to previous studies.
ALDH2 also revealed a significant correlation with TG and LDL-C. TG is the primary dietary lipid, so the level of TG is almost only influenced by dietary habits (81). The higher TG level of GG populations demonstrated unhealthier dietary habits than GA/AA populations. In recent years, many researchers have tried to explain the differences between ALDH2 gene types and lipids in other mechanisms. Gibb et al. found that the ALDH2 rs671 mutant could repress the transcription of a lysosomal H+ pump subunit in nucleus, which is crucial for lipid degradation and foam cell formation (82). Besides, Zhong et al. also discovered an unexpected interaction of ALDH2 with the LDL receptor, which may directly act on ATP6V0E2 (a critical substance for maintaining lysosomal function and degradation of oxidized LDL-C) and increase foam cell formation (83). However, the correlation between ALDH2 and TG or LDL-C was not strong enough that not all outcomes were significant in the subgroups. Further studies of ALDH2 including lipid-related data were needed.
In all, ALDH2 is primarily involved in the degradation of acetaldehyde which reduced ALDH2 activity could lead to “Asian flush syndrome,” affecting alcohol intake. Additionally, ALDH2 could also metabolize endogenous lipid aldehydes. Reduced ALDH2 activity results in lipid aldehyde accumulation, generating reactive oxygen species and activating various oxidative stress pathways. Over the past few decades, numerous studies have shown the dual effect between ALDH2 gene polymorphisms and cardiovascular diseases: ALDH2 mutation could increase the risk of coronary artery disease, peripheral artery disease and stroke; ALDH2 mutation could also decrease the risk of hypertension and aortic aneurysms or dissections (5). Besides, recent research has revealed non-enzymatic functions of ALDH2, participating in lipid metabolism in hepatocytes, regulating foam cell formation in macrophages, and modulating cellular senescence in endothelial cells and vascular smooth muscle cells. Moreover, our meta-analysis concludes that ALDH2 mutants had lower BMI levels. In summary, ALDH2 has diverse pathophysiological effects, and ALDH2 mutations can variably influence cardiovascular metabolic risk factors through multiple pathways.
Our article included sufficient studies that there was no publication bias (Egger’s test P>0.05) in almost all outcomes. Besides, we first reported the significant subgroup differences in T2DM and FBG in severe CCVD subgroup analysis, which could explain the controversial conclusion in the previously published articles. However, there were several limitations in our study: (1) Meta-regression is unavailable to subgroup data from less than ten studies, leading to a low R-squared of regression model. Therefore, our article only analyzed confounding factors through subgroup analysis rather than adjusting the effect of alcohol consumption or BMI on outcomes of cardiometabolic risk factors. (2) We did not conduct the subgroup analysis of drinker/non-drinker, male/female, and different genotypes (for example, GG vs. AA) due to the insufficient (less than five studies) subgroup data in the included studies. Even though we analyzed these subgroup analyses, the heterogeneity was too high to obtain a credible conclusion. (3) Although we believed that alcohol consumption, dietary habits, and BMI were the critical factors between ALDH2 and cardiometabolic risk factors, due to the lack of original data of included studies, we could not conduct the mediating analysis and interaction effects in our study. Future studies were needed to confirm the cardiometabolic mechanism of ALDH2.
Our updated meta-analysis demonstrated that ALDH2 rs671 GG populations had significantly higher levels of BMI, blood pressure, FBG, TG, LDL-C and higher risk of hypertension than GA/AA populations. Besides, to the best of our knowledge, we first report GG had a higher risk of T2DM in population without severe CCVDs, and GA/AA had a higher risk of T2DM in population with severe CCVDs. Future studies were warranted to confirm the cardiometabolic mechanism of ALDH2.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RL: Methodology, Project administration, Software, Writing – original draft. MP: Data curation, Funding acquisition, Investigation, Supervision, Writing – original draft. JZ: Data curation, Visualization, Writing – review & editing. KQ: Formal Analysis, Software, Visualization, Writing – review & editing. TZ: Formal Analysis, Supervision, Writing – review & editing. LC: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (LC, Grant no., 82170822; MP, Grant no., 81900734).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1333595/full#supplementary-material
Supplementary Figure S1 | Tetramer structure of ALDH2 enzyme
Supplementary Figure S2 | Forest plot of ALDH2 GA/AA vs. GG on Age
Supplementary Figure S3 | Forest plot of ALDH2 GA/AA vs. GG on Gender
Supplementary Figure S4 | Forest plot of ALDH2 GA/AA vs. GG on Smoker
Supplementary Figure S5 | Forest plot of ALDH2 GA/AA vs. GG on SBP
Supplementary Figure S6 | Forest plot of ALDH2 GA/AA vs. GG on DBP
Supplementary Figure S7 | Forest plot of ALDH2 GA/AA vs. GG on FBG
Supplementary Figure S8 | Forest plot of ALDH2 GA/AA vs. GG on HbA1c
Supplementary Figure S9 | Forest plot of ALDH2 GA/AA vs. GG on TC
Supplementary Figure S10 | Forest plot of ALDH2 GA/AA vs. GG on TG
Supplementary Figure S11 | Forest plot of ALDH2 GA/AA vs. GG on LDL-C
Supplementary Figure S12 | Forest plot of ALDH2 GA/AA vs. GG on HDL-C
Supplementary Figure S13 | Funnel plot of outcome of BMI
Supplementary Figure S14 | Funnel plot of outcome of Hypertension
Supplementary Figure S15 | Funnel plot of outcome of T2DM
Supplementary Table 1 | PRISMA Checklist of our study
1. Raghunathan L, Hsu LC, Klisak I, Sparkes RS, Yoshida A, Mohandas T. Regional localization of the human genes for aldehyde dehydrogenase-1 and aldehyde dehydrogenase-2. Genomics. (1988) 2:267–9. doi: 10.1016/0888-7543(88)90012-2
2. Eriksson CJ. Acetaldehyde metabolism in vivo during ethanol oxidation. Adv Exp Med Biol. (1977) 85A:319–41. doi: 10.1007/978-1-4899-5181-6_21
3. Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. (2014) 94:1–34. doi: 10.1152/physrev.00017.2013
4. Klyosov AA. Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydes. Biochemistry. (1996) 35:4457–67. doi: 10.1021/bi9521102
5. Zhang J, Guo Y, Zhao X, Pang J, Pan C, Wang J, et al. The role of aldehyde dehydrogenase 2 in cardiovascular disease. Nat Rev Cardiol. (2023) 20:495–509. doi: 10.1038/s41569-023-00839-5
6. Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci USA. (1984) 81:258–61. doi: 10.1073/pnas.81.1.258
7. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
8. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. European Society of Cardiology: cardiovascular disease statistics 2021: Executive Summary. Eur Heart J Qual Care Clin Outcomes. (2022) 8:377–82. doi: 10.1093/ehjqcco/qcac014
9. Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, et al. Diabetes in asia and the pacific: Implications for the global epidemic. Diabetes Care. (2016) 39:472–85. doi: 10.2337/dc15-1536
10. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. (2020) 582:240–5. doi: 10.1038/s41586-020-2263-3
11. Dandre F, Cassaigne A, Iron A. The frequency of the mitochondrial aldehyde dehydrogenase I2 (atypical) allele in Caucasian, Oriental and African black populations determined by the restriction profile of PCR-amplified DNA. Mol Cell Probes. (1995) 9:189–93. doi: 10.1006/mcpr.1995.0030
12. Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. (1992) 88:344–6. doi: 10.1007/BF00197271
13. Li J, Liu H, Li S. Landscape of cardiometabolic risk factors in Chinese population: a narrative review. Cardiovasc Diabetol. (2022) 21:113. doi: 10.1186/s12933-022-01551-3
14. Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, et al. Burden of cardiovascular diseases in China, 1990-2016: Findings from the 2016 global burden of disease study. JAMA Cardiol. (2019) 4:342–52. doi: 10.1001/jamacardio.2019.0295
15. Ueno M, Yoshino Y, Mori H, Funahashi Y, Kumon H, Ochi S, et al. Association study and meta-analysis of polymorphisms and blood mRNA expression of the ALDH2 gene in patients with alzheimer's disease. J Alzheimers Dis. (2022) 87:863–71. doi: 10.3233/JAD-215627
16. Tajiri A, Ishihara R, Sakurai H, Nakamura T, Tani Y, Inoue T, et al. Clinical features of superficial esophagus squamous cell carcinoma according to alcohol-degrading enzyme ADH1B and ALDH2 genotypes. J Gastroenterol. (2022) 57:630–9. doi: 10.1007/s00535-022-01892-6
17. Li GY, Li ZB, Li F, Dong LP, Tang L, Xiang J, et al. Meta-analysis on the association of ALDH2 polymorphisms and type 2 diabetic mellitus, diabetic retinopathy. Int J Environ Res Public Health. (2017) 14:165. doi: 10.3390/ijerph14020165
18. Li MJ, Ren J, Zhang WS, Jiang CQ, Jin YL, Lam TH, et al. Association of alcohol drinking with incident type 2 diabetes and pre-diabetes: The Guangzhou Biobank Cohort Study. Diabetes Metab Res Rev. (2022) 38:e3548. doi: 10.1002/dmrr.3548
19. Kogiso T, Sagawa T, Kodama K, Taniai M, Hashimoto E, Tokushige K. Outcomes of Japanese patients with non-alcoholic fatty liver disease according to genetic background and lifestyle-related diseases. Ann Hepatol. (2021) 21:100260. doi: 10.1016/j.aohep.2020.09.004
20. Zheng Y, Ning C, Zhang X, Zhao Y, Li Y, Qian L, et al. Association between ALDH-2 rs671 and essential hypertension risk or blood pressure levels: A systematic review and meta-analysis. Front Genet. (2020) 11:685. doi: 10.3389/fgene.2020.00685
21. Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet. (2015) 24:865–74. doi: 10.1093/hmg/ddu478
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Cuello-Garcia CA, Schunemann HJ. Update of the agency for healthcare research and quality guidance on using nonrandomized studies in evidence syntheses. J Clin Epidemiol. (2022) 152:307–8. doi: 10.1016/j.jclinepi.2022.10.010
24. Hisamatsu T, Tabara Y, Kadota A, Torii S, Kondo K, Yano Y, et al. Alcohol consumption and cerebral small- and large-vessel diseases: A mendelian randomization analysis. J Atheroscler Thromb. (2023) 31(2):135–47. doi: 10.5551/jat.64222
25. Hayashida H, Matsumoto A, Nanri H, Nishida Y, Takagi Y, Hara M. ALDH2 rs671 variant allele is associated with higher energy intake in middle-aged and elderly Japanese who routinely consume alcohol. Environ Health Prev Med. (2023) 28:29. doi: 10.1265/ehpm.22-00276
26. Lan X, Wang Z, Zeng Z, Yao H, Xu W, Zhang Y. Association of Different Combinations of ALDH2 rs671, APOE rs429358, rs7412 Polymorphisms with Hypertension in Middle-Aged and Elderly People: A Case-Control Study. Int J Gen Med. (2023) 16:915–27. doi: 10.2147/IJGM.S402437
27. Okura T, Nakamura R, Anno M, Ito Y, Kitao S, Endo S, et al. Aldehyde dehydrogenase 2 polymorphism is an important gene for insulin resistance in Japanese patients with type 2 diabetes. Metabol Open. (2023) 18:100242. doi: 10.1016/j.metop.2023.100242
28. Zhang S, Luo W, Pan T, Xie J, Xu Z, Fang Y. ALDH2 rs671 polymorphism likely a risk factor for hemorrhagic stroke: A hospital-based study. Int J Gen Med. (2023) 16:1471–8. doi: 10.2147/IJGM.S409183
29. Wu H, Huang Q, Yu Z, Zhong Z. Association of ALDH2 rs671 and MTHFR rs1801133 polymorphisms with hypertension among Hakka people in Southern China. BMC Cardiovasc Disord. (2022) 22:128. doi: 10.1186/s12872-022-02577-x
30. He Q, Pan J, Wang L, Fang Y, Hu R. Prospective study: Aldehyde dehydrogenase 2 gene is associated with cardio-cerebrovascular complications in type 2 diabetes patients. J Diabetes Investig. (2021) 12:1845–54. doi: 10.1111/jdi.13538
31. Takeno K, Tamura Y, Kakehi S, Kaga H, Kawamori R, Watada H. ALDH2 rs671 is associated with elevated FPG, reduced glucose clearance and hepatic insulin resistance in Japanese men. J Clin Endocrinol Metab. (2021) 106:e3573–81. doi: 10.1210/clinem/dgab324
32. Ishida T, Arima Y, Mizuno Y, Harada E, Yamashita T, Sueta D, et al. East Asian variant aldehyde dehydrogenase type 2 genotype exacerbates ischemia/reperfusion injury with ST-elevation myocardial infarction in men: possible sex differences. Heart Vessels. (2022) 37:184–93. doi: 10.1007/s00380-021-01907-x
33. Zhu LP, Yin WL, Peng L, Zhou XH, Zhou P, Xuan SX, et al. Association of aldehyde dehydrogenase 2 gene polymorphism with myocardial infarction. J Inflammation Res. (2021) 14:3039–47. doi: 10.2147/JIR.S311885
34. Hou JY, Zhong ZX, Deng QT, Liu SD, Lin LF. Association between the polymorphism of aldehyde dehydrogenase 2 gene and cerebral infarction in a hakka population in Southern China. Biochem Genet. (2020) 58:322–34. doi: 10.1007/s10528-020-09950-5
35. Kim HY, Choi CK, Kweon SS, Lee YH, Nam HS, Park KS, et al. Effect modification of acetaldehyde dehydrogenase 2 rs671 polymorphism on the association between alcohol intake and blood pressure: The dong-gu study. J Korean Med Sci. (2020) 35:e14. doi: 10.3346/jkms.2020.35.e14
36. Xia CL, Chu P, Liu YX, Qu XL, Gao XF, Wang ZM, et al. ALDH2 rs671 polymorphism and the risk of heart failure with preserved ejection fraction (HFpEF) in patients with cardiovascular diseases. J Hum Hypertens. (2020) 34:16–23. doi: 10.1038/s41371-019-0182-2
37. Zhu Z, Jiang Y, Cui M, Wang Y, Li S, Xu K, et al. ALDH2 rs671 polymorphisms and the risk of cerebral microbleeds in Chinese elderly: the Taizhou Imaging Study. Ann Transl Med. (2020) 8:229. doi: 10.21037/atm.2020.01.01
38. Wang D, Zou Y, Yu S, Lin S, Li H, Yin Y, et al. The effect of ALDH2 rs671 gene mutation on clustering of cardiovascular risk factors in a big data study of Chinese population: associations differ between the sexes. BMC Cardiovasc Disord. (2020) 20:509. doi: 10.1186/s12872-020-01787-5
39. Han S, Zhao X, Zhang X, Xu Y, Geng J, Wang Y. Acetaldehyde dehydrogenase 2 rs671 polymorphism affects hypertension susceptibility and lipid profiles in a Chinese population. DNA Cell Biol. (2019) 38:962–8. doi: 10.1089/dna.2019.4647
40. Li ZM, Kong CY, Sun KY, Wang LS. The ALDH2 gene rs671 polymorphism is not associated with essential hypertension. Clin Exp Hypertens. (2017) 39:691–5. doi: 10.1080/10641963.2017.1299749
41. Ma C, Yu B, Zhang W, Wang W, Zhang L, Zeng Q. Associations between aldehyde dehydrogenase 2 (ALDH2) rs671 genetic polymorphisms, lifestyles and hypertension risk in Chinese Han people. Sci Rep. (2017) 7:11136. doi: 10.1038/s41598-017-11071-w
42. Zhu Y, Zhang D, Zhou D, Li Z, Li Z, Fang L, et al. Susceptibility loci for metabolic syndrome and metabolic components identified in Han Chinese: a multi-stage genome-wide association study. J Cell Mol Med. (2017) 21:1106–16. doi: 10.1111/jcmm.13042
43. Mizuno Y, Hokimoto S, Harada E, Kinoshita K, Nakagawa K, Yoshimura M, et al. Variant aldehyde dehydrogenase 2 (ALDH2*2) is a risk factor for coronary spasm and ST-segment elevation myocardial infarction. J Am Heart Assoc. (2016) 5:e003247. doi: 10.1161/JAHA.116.003247
44. Yin G, Naito M, Wakai K, Morita E, Kawai S, Hamajima N, et al. ALDH2 polymorphism is associated with fasting blood glucose through alcohol consumption in Japanese men. Nagoya J Med Sci. (2016) 78:183–93.
45. Oniki K, Morita K, Watanabe T, Kajiwara A, Otake K, Nakagawa K, et al. The longitudinal effect of the aldehyde dehydrogenase 2*2 allele on the risk for nonalcoholic fatty liver disease. Nutr Diabetes. (2016) 6:e210. doi: 10.1038/nutd.2016.17
46. Sung YF, Lu CC, Lee JT, Hung YJ, Hu CJ, Jeng JS, et al. Homozygous ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese men. Stroke. (2016) 47:2174–9. doi: 10.1161/STROKEAHA.116.013204
47. Idewaki Y, Iwase M, Fujii H, Ohkuma T, Ide H, Kaizu S, et al. Association of genetically determined aldehyde dehydrogenase 2 activity with diabetic complications in relation to alcohol consumption in Japanese patients with type 2 diabetes mellitus: The fukuoka diabetes registry. PLoS One. (2015) 10:e143288. doi: 10.1371/journal.pone.0143288
48. Taylor AE, Lu F, Carslake D, Hu Z, Qian Y, Liu S, et al. Exploring causal associations of alcohol with cardiovascular and metabolic risk factors in a Chinese population using Mendelian randomization analysis. Sci Rep. (2015) 5:14005. doi: 10.1038/srep14005
49. Yokoyama A, Yokoyama T, Matsui T, Mizukami T, Kimura M, Matsushita S, et al. Alcohol dehydrogenase-1B (rs1229984) and aldehyde dehydrogenase-2 (rs671) genotypes are strong determinants of the serum triglyceride and cholesterol levels of Japanese alcoholic men. PLoS One. (2015) 10:e133460. doi: 10.1371/journal.pone.0133460
50. Qu Y, Zhang HL, Yu LM, Sun Y, Wu HL, Chen YG. Aldehyde dehydrogenase 2 polymorphism as a protective factor for intracranial vascular stenosis in ischemic stroke in Han Chinese. Int J Neurosci. (2016) 126:342–7. doi: 10.3109/00207454.2015.1017760
51. Wang Y, Zhang Y, Zhang J, Tang X, Qian Y, Gao P, et al. Association of a functional single-nucleotide polymorphism in the ALDH2 gene with essential hypertension depends on drinking behavior in a Chinese Han population. J Hum Hypertens. (2013) 27:181–6. doi: 10.1038/jhh.2012.15
52. Morita K, Saruwatari J, Miyagawa H, Uchiyashiki Y, Oniki K, Sakata M, et al. Association between aldehyde dehydrogenase 2 polymorphisms and the incidence of diabetic retinopathy among Japanese subjects with type 2 diabetes mellitus. Cardiovasc Diabetol. (2013) 12:132. doi: 10.1186/1475-2840-12-132
53. Yokoyama A, Mizukami T, Matsui T, Yokoyama T, Kimura M, Matsushita S, et al. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol Clin Exp Res. (2013) 37:1391–401. doi: 10.1111/acer.12108
54. Lv Y, Hu X, Wang Y, Xu W, Tao P. A study on the association between gene polymorphism of ALDH2 (Glu504Lys) and hypertension among Chinese han people in Zhejiang. Zhejiang Prev Med. (2013) 25:4–7.
55. Feng J, Wang C, Ye Q, Yin Z, Guo A, Huang M, et al. Relationship between gene polymorphism of acetaldehyde dehydrogenase 2 and hypertension in aged patients. Chin J Cardiovasc Rehabil Med. (2012) 21:143–6. doi: 10.3969/j.issn.1008-0074.2012.02.10
56. Hasi T, Hao L, Yang L, Su XL. Acetaldehyde dehydrogenase 2 SNP rs671 and susceptibility to essential hypertension in Mongolians: a case control study. Genet Mol Res. (2011) 10:537–43. doi: 10.4238/vol10-1gmr1056
57. Xu F, Chen Y, Lv R, Zhang H, Tian H, Bian Y, et al. ALDH2 genetic polymorphism and the risk of type II diabetes mellitus in CAD patients. Hypertens Res. (2010) 33:49–55. doi: 10.1038/hr.2009.178
58. Dakeishi M, Murata K, Sasaki M, Tamura A, Iwata T. Association of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 genotypes with fasting plasma glucose levels in Japanese male and female workers. Alcohol Alcohol. (2008) 43:143–7. doi: 10.1093/alcalc/agm173
59. Nagasawa H, Wada M, Arawaka S, Kawanami T, Kurita K, Daimon M, et al. A polymorphism of the aldehyde dehydrogenase 2 gene is a risk factor for multiple lacunar infarcts in Japanese men: the Takahata Study. Eur J Neurol. (2007) 14:428–34. doi: 10.1111/j.1468-1331.2007.01700.x
60. Hui P, Nakayama T, Morita A, Sato N, Hishiki M, Saito K, et al. Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens Res. (2007) 30:585–92. doi: 10.1291/hypres.30.585
61. Suzuki Y, Muramatsu T, Taniyama M, Atsumi Y, Kawaguchi R, Higuchi S, et al. Association of aldehyde dehydrogenase with inheritance of NIDDM. Diabetologia. (1996) 39:1115–8. doi: 10.1007/BF00400662
62. Murata C, Suzuki Y, Muramatsu T, Taniyama M, Atsumi Y, Matsuoka K, et al. Inactive aldehyde dehydrogenase 2 worsens glycemic control in patients with type 2 diabetes mellitus who drink low to moderate amounts of alcohol. Alcohol Clin Exp Res. (2000) 24:S5–S11. doi: 10.1111/j.1530-0277.2000.tb00003.x
63. Takagi S, Iwai N, Yamauchi R, Kojima S, Yasuno S, Baba T, et al. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens Res. (2002) 25:677–81. doi: 10.1291/hypres.25.677
64. Amamoto K, Okamura T, Tamaki S, Kita Y, Tsujita Y, Kadowaki T, et al. Epidemiologic study of the association of low-Km mitochondrial acetaldehyde dehydrogenase genotypes with blood pressure level and the prevalence of hypertension in a general population. Hypertens Res. (2002) 25:857–64. doi: 10.1291/hypres.25.857
65. Saito K, Yokoyama T, Yoshiike N, Date C, Yamamoto A, Muramatsu M, et al. Do the ethanol metabolizing enzymes modify the relationship between alcohol consumption and blood pressure? J Hypertens. (2003) 21:1097–105. doi: 10.1097/00004872-200306000-00009
66. Suzuki Y, Taniyama M, Muramatsu T, Higuchi S, Ohta S, Atsumi Y, et al. ALDH2/ADH2 polymorphism associated with vasculopathy and neuropathy in type 2 diabetes. Alcohol Clin Exp Res. (2004) 28:S111–6. doi: 10.1097/01.alc.0000133583.44581.99
67. Xu F, Chen YG, Geng YJ, Zhang H, Jiang CX, Sun Y, et al. The polymorphism in acetaldehyde dehydrogenase 2 gene, causing a substitution of Glu>Lys(504), is not associated with coronary atherosclerosis severity in Han Chinese. Tohoku J Exp Med. (2007) 213:215–20. doi: 10.1620/tjem.213.215
68. Igarashi M, Nogawa S, Hachiya T, Furukawa K, Takahashi S, Jia H, et al. Association between dietary behaviors and BMI stratified by sex and the ALDH2 rs671 polymorphism in Japanese adults. Nutrients. (2022) 14:5116. doi: 10.3390/nu14235116
69. Liu YR, Tantoh DM, Lin CC, Hsiao CH, Liaw YP. Risk of gout among Taiwanese adults with ALDH-2 rs671 polymorphism according to BMI and alcohol intake. Arthritis Res Ther. (2021) 23:115. doi: 10.1186/s13075-021-02497-9
70. Kimura M, Miyakawa T, Matsushita S, So M, Higuchi S. Gender differences in the effects of ADH1B and ALDH2 polymorphisms on alcoholism. Alcohol Clin Exp Res. (2011) 35:1923–7. doi: 10.1111/acer.2011.35.issue-11
71. Kishimoto R, Ogishi Y, Ueda M, Matsusaki M, Amako K, Goda K, et al. Gender-related differences in mouse hepatic ethanol metabolism. J Nutr Sci Vitaminol (Tokyo). (2002) 48:216–24. doi: 10.3177/jnsv.48.216
72. Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. (2010) 106:1681–91. doi: 10.1161/CIRCRESAHA.109.213645
73. Ciarambino T, Crispino P, Guarisco G, Giordano M. Gender differences in insulin resistance: New knowledge and perspectives. Curr Issues Mol Biol. (2023) 45:7845–61. doi: 10.3390/cimb45100496
74. Zhang X, Deng X, Zhang L, Wang P, Tong X, Mo Y, et al. Single-cell RNA sequencing analysis of lung cells in COVID-19 patients with diabetes, hypertension, and comorbid diabetes-hypertension. Front Endocrinol (Lausanne). (2023) 14:1258646. doi: 10.3389/fendo.2023.1258646
75. Tan X, Chen YF, Zou SY, Wang WJ, Zhang NN, Sun ZY, et al. ALDH2 attenuates ischemia and reperfusion injury through regulation of mitochondrial fusion and fission by PI3K/AKT/mTOR pathway in diabetic cardiomyopathy. Free Radic Biol Med. (2023) 195:219–30. doi: 10.1016/j.freeradbiomed.2022.12.097
76. Pan G, Roy B, Giri S, Lanfear DE, Thandavarayan RA, Guha A, et al. Aldehyde dehydrogenase 2 activator augments the beneficial effects of empagliflozin in mice with diabetes-associated HFpEF. Int J Mol Sci. (2022) 23:10439. doi: 10.3390/ijms231810439
77. Pan G, Roy B, Palaniyandi SS. Diabetic aldehyde dehydrogenase 2 mutant (ALDH2*2) mice are more susceptible to cardiac ischemic-reperfusion injury due to 4-hydroxy-2-nonenal induced coronary endothelial cell damage. J Am Heart Assoc. (2021) 10:e21140. doi: 10.1161/JAHA.121.021140
78. Yang SS, Chen YH, Hu JT, Chiu CF, Hung SW, Chang YC, et al. Aldehyde dehydrogenase mutation exacerbated high-fat-diet-induced nonalcoholic fatty liver disease with gut microbiota remodeling in male mice. Biol (Basel). (2021) 10:737. doi: 10.3390/biology10080737
79. Mali VR, Ning R, Chen J, Yang XP, Xu J, Palaniyandi SS. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp Biol Med (Maywood). (2014) 239:610–8. doi: 10.1177/1535370213520109
80. Zhang WS, Xu L, Schooling CM, Jiang CQ, Cheng KK, Liu B, et al. Effect of alcohol and aldehyde dehydrogenase gene polymorphisms on alcohol-associated hypertension: the Guangzhou Biobank Cohort Study. Hypertens Res. (2013) 36:741–6. doi: 10.1038/hr.2013.23
81. Li X, Liu Q, Pan Y, Chen S, Zhao Y, Hu Y. New insights into the role of dietary triglyceride absorption in obesity and metabolic diseases. Front Pharmacol. (2023) 14:1097835. doi: 10.3389/fphar.2023.1097835
82. Gibb AA, Elrod JW. Not just correlative: a new pathway defines how an ALDH2 SNP contributes to atherosclerosis. J Clin Invest. (2019) 129:63–5. doi: 10.1172/JCI125433
Keywords: ALDH2, meta-analysis, cardiometabolic risk factor, T2DM, hypertension
Citation: Liu R, Peng M, Zhang J, Qiu K, Zeng T and Chen L (2024) The ALDH2 gene rs671 polymorphism is associated with cardiometabolic risk factors in East Asian population: an updated meta-analysis. Front. Endocrinol. 15:1333595. doi: 10.3389/fendo.2024.1333595
Received: 05 November 2023; Accepted: 06 March 2024;
Published: 19 March 2024.
Edited by:
Eva Kassi, National and Kapodistrian University of Athens Medical School, GreeceReviewed by:
Chunheng Mo, Sichuan University, ChinaCopyright © 2024 Liu, Peng, Zhang, Qiu, Zeng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lulu Chen, Y2hlcmlhX2NoZW5AMTI2LmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.