
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 February 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1332995
 Yangcheng Yao1,2†
Yangcheng Yao1,2† Wenjuan Liu2†
Wenjuan Liu2† Xiqian Zhang2
Xiqian Zhang2 Nianjun Su2
Nianjun Su2 Li Huang2
Li Huang2 Yingqi Nong2
Yingqi Nong2 Xiaomin Xiao1*
Xiaomin Xiao1* Fenghua Liu2*
Fenghua Liu2*Background: Body weight could be classified into underweight, normal weight and overweight according to percentage of body fat (%BF), and normal weight obesity (NWO) is defined as a normal BMI but a high %BF. While the impact of NWO in women fecundity remain unknow. Therefore, this study aimed to investigate the associations between %BF and reproductive outcomes among in vitro fertilization (IVF) women with normal BMI.
Methods: A total of 469 women were included in this study and were classified into low %BF, normal %BF and high %BF according to previous study. Multivariate generalized regression models were employed to evaluate the associations of %BF with ovarian reserve parameters, IVF outcomes and early pregnancy outcomes. We further run sensitivity analyses by restricted the analysis to young women and those only with tubal factor, respectively.
Results: About 32.2% of normal BMI women were misclassified according %BF, with 16.4% of them were low %BF and 15.8% were high %BF. The high %BF group had significantly lower antral follicle count (AFC) than normal %BF groups, and the AFC showed a tendency of decrease as %BF increased. In sensitivity analysis in young women, high %BF group also had significantly lower number of good-quality embryos when compared to normal %BF groups. The results expanded to all IVF outcomes when analysis restricted to tubal factor women.
Conclusion: In summary, misclassifications of body weight status based on BMI are common according to %BF, and NWO is associated with adverse reproductive outcomes.
The prevalence of overweight and obesity has globally increased during the last several decades in both men and women and both adults and children (1). Obesity is an independent risk factor for a myriad disease (e.g., type 2 diabetes, dyslipidemia, hypertension, coronary heart disease) and mortality (2). Obesity also exerts negative effects on reproduction and increases the risk of infertility (3). Epidemiological studies have demonstrated that an increased body mass index (BMI) is associated with lower ovarian responsiveness to ovulation induction, a lower number of retrieved oocytes and oocyte quality, and lower implantation, clinical pregnancy and live birth rates (4–7). The underlying mechanisms are extensive and include hormone changes, abnormal metabolism, ovulatory dysfunction, chronic inflammation and disorder, reactive oxygen species, mitochondrial dysfunction, and meiotic spindle disruption (8–10).
Obesity is defined by the WHO as abnormal or excessive fat accumulation that may impair health (11). Although BMI is a simple and commonly used index for assessing obesity, its accuracy is affected by some confounders, such as body fat-free mass, sex and age (12). Obesity misclassification is common and may lead to underestimating for health risks such as metabolic syndrome (13). Therefore, approaches for quantifying human body composition have been developed. Dual X-ray absorptiometry (DXA) is a well-accepted method for body composition assessment (e.g., bone density, fat mass, fat-free mass), which shows great consistency with MRI and CT and is regarded as a reference technique for body composition assessment in many studies (14, 15). However, DXA also has some disadvantages; for example, the machine is expensive, large, requires specialist staff and exposes the participant to radiation, which has limited its promotion.
Bioelectrical impedance analysis (BIA) devices use a harmless electrical current to assess human body composition by measuring the resistance and impedance and making predictions with its built-in algorithms (16). BIA demonstrated good agreement with DXA for fat mass and fat-free mass (17, 18), especially in the normal BMI range population (19). Studies suggest that BIA can be used as an alternative to DXA for body composition assessment due to its safety, inexpensiveness, convenience, reproducibility and efficiency (19, 20). Currently, BIA devices are widely used in clinical practice, public health, and research studies. With the application of BIA, based on the population of the National Health and Nutrition Examination Survey (NHANES) in the US, Zhu et al. developed cut-offs values for the percentage of body fat (%BF) by determining the metabolic syndrome risk equivalent to various BMI cut-off. The cut-off for %BF in women were set to 24%, 31%, 37% and 43%, which corresponded to BMI values of 18.5, 25, 30 and 35 kg/m², respectively (21).
With this %BF classification, misclassification of weight between BMI and %BF has been reported, and women are more likely to have an underestimated risk of overweight or obesity according to the BMI criteria (22). Therefore, the concept of normal weight obesity (NWO) was developed, defined as a normal BMI but high %BF (23). The associations between mismatched body weight and reproductive outcomes have rarely been researched. The present study aims to investigate the associations between %BF and reproductive outcomes among in vitro fertilization (IVF) women with normal BMI. We retrospectively collected and compared the clinical parameters between underweight, normal weight and overweight IVF women according to %BF.
This retrospective study was performed at Guangdong Women and Children’s Hospital, and was approved by the institutional review board of the hospital. Women who attended the clinic of the reproductive medicine center from January 2018 to September 2020 and completed the body composition assessment were eligible for this study. The inclusion criteria were as follows: 1) women aged 20-45 years, 2) a BMI of 18.5-24.9 kg/m2, 3) available body composition assessment data, and 4) IVF or ICSI treatment. The exclusion criteria were as follows: 1) parental chromosomal or genetic abnormalities, 2) no eggs retrieved or oocyte cryopreservation, 3) donated oocytes, 4) attempted enrolment during a thawing cycle, and 5) a history of iatrogenic ovarian injury. The demographic information of the participants was collected from the medical system (e.g., ethnicity, smoking status, alcohol consumption and parity) and checked by clinical staff.
Body composition was measured by a BIA device (IOI353, HONGTAISHENG Co., Beijing, China) under the guidance of trained staff. The measurement was implemented in a standardized environment, during which the subject was instructed to remain motionless and relaxed. The analyzer measured the whole-body electrical resistance and impedance, operated through built-in algorithms, and then exported the results, including height, weight, body fat mass, %BF, degreased body weight and body water content.
The reproductive data were abstracted from the electronic medical records. Patients received the appropriate treatment at the discretion of their primary physician. The diagnosis of infertility included female factors, male factors, mix factors and unexplained reasons. Female factors included tubal factor, ovulation disorders, diminished ovarian reserve, endometriosis, and uterine factors. Male factors included semen abnormalities and coital infertility. The ovarian stimulation protocol in this study included long GnRH agonists, GnRH antagonists, luteal phase, progestin primed ovarian stimulation and mild stimulation. Reproductive data included ovarian reserve parameters (serum anti-Mullerian hormone, AMH; day 3 follicle stimulating hormone, FSH; and antral follicle count, AFC), IVF outcomes (the number of oocytes retrieved, fertilized oocytes, cleaved embryos, and good-quality embryos on day 3) and early pregnancy outcomes (implantation, biochemical pregnancy, and clinical pregnancy). Good-quality embryos were defined as grade I and II embryos according to the Cummins criteria (24).
For early pregnancy outcomes, we included only the next fresh embryo transfer cycle for the current analysis. We defined implantation as serum β-HCG ≥ 5 IU/L 12 days after embryo transfer, clinical pregnancy as the presence of at least one gestational sac on ultrasound 5 weeks after embryo transfer, and biochemical pregnancy as confirmed implantation with failure to achieve clinical pregnancy (25). Additionally, the implantation rate was defined as the number of intrauterine sacs divided by the number of embryos transferred, the clinical pregnancy rate as the number of clinical pregnancy cycles divided by the number of embryo transfer cycles, and the biochemical pregnancy rate as the number of biochemical pregnancy cycles plus the number of clinical pregnancy cycles divided by the number of embryo transfer cycles.
Demographic and clinical characteristics are expressed as the means ± SDs or number (%) where appropriate. Subjects were classified into low %BF (underweight), normal %BF (normal weight) and high %BF (overweight) according to cut-off values of 24% and 31% (21). The differences for baseline between three groups were tested using one-way ANOVA for continuous variable and chi-square test for categorical variable. Multivariate generalized linear regression models were used to evaluate the associations of %BF with FSH and AMH levels. Multivariate Poisson regression models were conducted to explore the associations of %BF with AFC, retrieved oocytes, fertilized oocytes, cleaved embryos, and good-quality embryos. Multivariate logistic regression models were employed to estimate the associations of %BF with implantation rate, biochemical pregnancy rate and clinical pregnancy rate. Furthermore, we included %BF as a continuous variable in the models to investigate the trends in the associations of %BF with reproductive outcomes.
Covariates were selected according to biological relevance or prior knowledge (26). For the ovarian reserve parameters, the covariates were age (continuous), BMI (continuous), ethnicity (Han, other), smoking status (ever, never), alcohol consumption (ever, never) and infertility diagnosis. For IVF outcomes, the covariates were mentioned above plus the ovarian stimulation protocol and insemination technique (IVF, ICSI). For early pregnancy outcomes, the covariates were age, BMI, ethnicity, smoking status, alcohol consumption and parity history (0, ≥ 1). Age is an important independent risk factor for adverse pregnancy outcomes (27, 28). To explore the potential effect modification of age, we ran sensitivity analyses by included women aged 20-35 years. As infertility factors may affect ovarian function, we also ran sensitivity analyses by restricting the analysis to women with tubal factor infertility. Statistical significance was indicated if the P value < 0.05. Data were analysed by Statistical Package for the Social Sciences (SPSS, version 22.0).
A total of 469 subjects were included in this study, as shown in Table 1. The average age of the study population was 31.3 years; 77 (16.4%) of them had low %BF (< 24%), and 74 (15.8%) had high %BF (≥ 31%), according to a previous study (21). The mean AFC and number of retrieved oocytes, fertilized oocytes, cleaved embryos, and good-quality embryos on day 3 were 14.5, 12.8, 7.5, 7.4 and 5.0, respectively (Table 2). A total of 275 women underwent fresh embryo transfer, 142 of whom achieved clinical pregnancy.
The ovarian reserve parameters and IVF outcomes categorized by %BF are presented in Table 3. The mean AFCs of the low %BF, normal %BF and high %BF groups were 14.62, 14.57 and 14.11, respectively. Subjects in the high %BF group had significantly lower AFC than those in the normal %BF group. Moreover, the AFC tended to decrease as %BF increased across the three groups (P < 0.001). Regarding the remaining ovarian reserve parameters, the high %BF group showed slightly lower values than the normal and low groups, but the differences were not significant. Additionally, there were no significant differences in IVF outcome parameters among the groups.
In the sensitivity analysis with women aged 20-35 years, the above results were also observed. Moreover, the high %BF group had a significantly lower number of good-quality embryos on day 3 than the normal %BF group (Table 4). In the analysis restricted to IVF women with tubal factor infertility, differences were also observed in other IVF outcomes. Women with high %BF had significantly lower numbers of retrieved oocytes, fertilized oocytes, and cleaved embryos. Furthermore, the AFC and the numbers of retrieved oocytes, fertilized oocytes, cleaved embryos, and good-quality embryos on day 3 significantly tended to decrease as %BF increased across the three groups (Table 5).
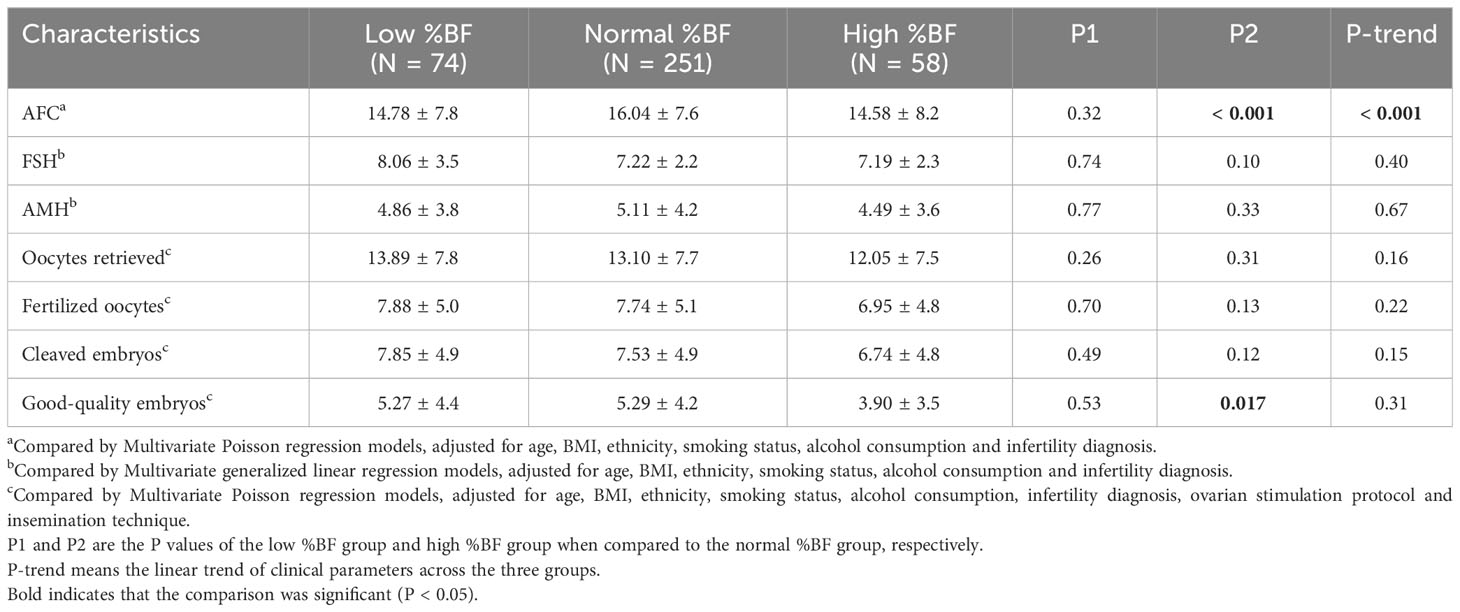
Table 4 Reproductive outcomes between groups according to %BF among women aged 20-35 years (N = 383).
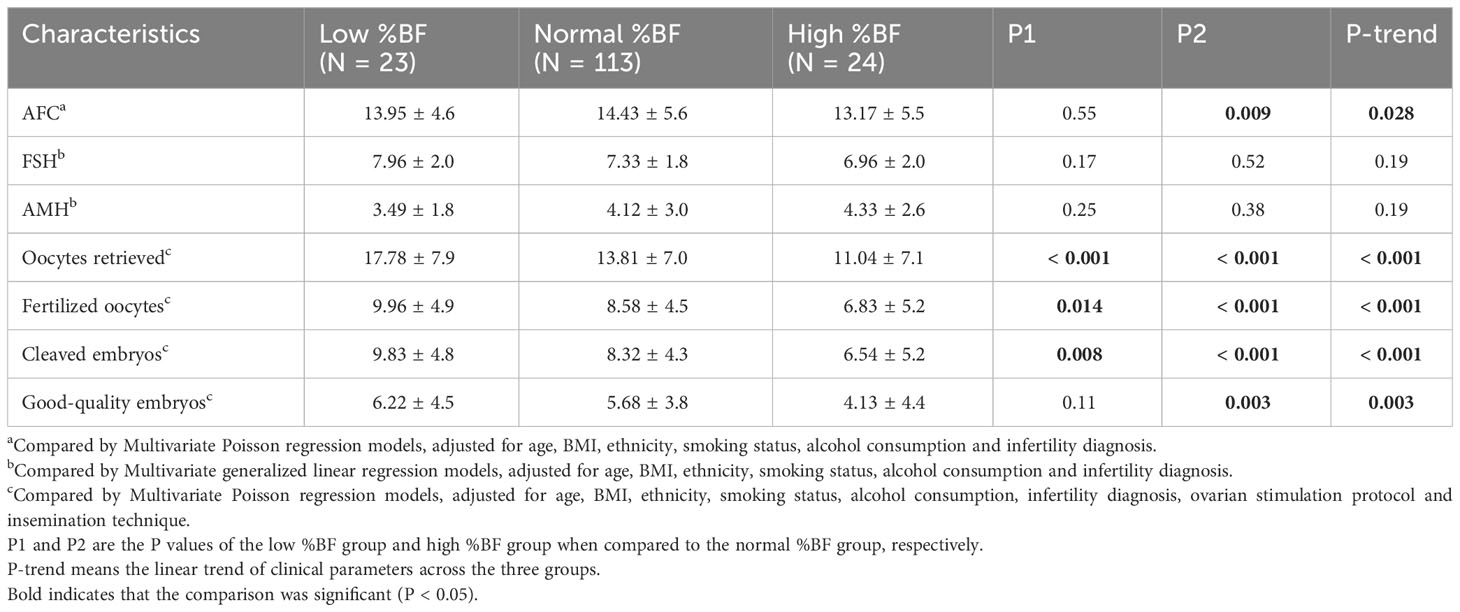
Table 5 Reproductive outcomes between groups according to %BF among women with tubal factor infertility (N = 160).
For the early pregnancy outcomes, compared with the low %BF and normal %BF groups, the high %BF group had a lower implantation rate, biochemical pregnancy rate and clinical pregnancy rate, but the differences were not significant (Table S1). There were also no significant differences in implantation rate, biochemical pregnancy rate or clinical pregnancy rate among the three groups in the sensitivity analyses (Tables S2, S3).
In this study of 469 women with normal BMI (18.5-24.9 kg/m2), a total of 151 subjects (32.2%) were misclassified according to %BF, with 16.4% of them being underweight (%BF < 24%) and 15.8% being overweight (%BF ≥ 31%). Consistent with our results, Kim et al. found that 28.6% (283 of 989) of normal BMI women (18.5-24.9 kg/m2) were overweight according to %BF (%BF ≥ 31%), but the proportion considered underweight was not estimated (22). Peterson et al. reported that nearly half of women who were misclassified as normal weight (BMI < 25 kg/m2) were actually obese according to %BF (≥ 35%) (13). These results demonstrate that misclassification based on BMI criteria is common. The accuracy is affected by the BMI and age; as the BMI increases, the sensitivity decreases and the specificity increases, and accuracy also decreases with increasing age (29). The mismatch rate in the previous studies was much higher than that in the present study, which may be due to the relatively lower mean BMI of the study population in this research.
We found that the AFC tended to decrease across the three groups as %BF increased, and women with high %BF had a significantly lower AFC than normal %BF women; this result persisted in the sensitivity analysis. Moreover, the decreasing tendency expanded to the IVF outcomes in the sensitivity analysis of women with tubal factor infertility (the numbers of retrieved oocytes, fertilized oocytes, cleaved embryos, and good-quality embryos on day 3). In Kim’s research, no difference was found between %BF normal weight (BMI < 25 kg/m2, %BF < 31%) and %BF overweight (BMI < 25 kg/m2, %BF ≥ 31%) women for AFC or the number of oocytes retrieved (22). This difference may be attributed to the differences in the study population, e.g., race, region, age, and study design. For the early pregnancy outcomes (i.e., implantation rate, biochemical pregnancy rate and clinical pregnancy rate), although decreasing trends were observed as %BF increase, no significance were reached. In line with our results, a previous study also reported no differences in pregnancy outcomes among IVF women stratified by %BF (30).
Obesity has been reported to impair ovarian responsiveness and is associated with lower ovarian reserve, smaller oocyte size, a lower oocyte yield, impaired oocyte quality, and suboptimal pregnancy outcomes (31–34). Obesity induces metabolic disorders, chronic low-grade inflammatory status and hormone alterations (e.g., higher levels of leptin, insulin, androgen, estrogen), which could further impair ovarian folliculogenesis (35–38). Obesity has also been associated with the altered follicular fluid, the critical environment for oocyte development and granulosa cell steroidogenesis, with elevated concentrations of leptin, insulin, triglycerides, inflammation markers (e.g., lactate and C-reactive protein), and oxidative stress (37, 39–41). Elevated levels of free fatty acids in follicular fluid are correlated with low-grade cumulus-oocyte complexes and low-quality oocytes (42). Excess free fatty acids can also induce mitochondrial and endoplasmic reticulum stress by increasing reactive oxygen species (43). In addition, obesity also appears to alter the meiotic spindles, mitochondrial distribution and function in the oocyte (10, 44). In contrast, a lower %BF generally indicates more physical activity, which may have beneficial effects for reproductive health in women (45).
The results of sensitivity analysis suggest that the associations of %BF with reproductive outcomes may modified by age and infertility diagnosis. High %BF group had less good-quality embryos on day 3 than the normal %BF group in younger women. Age is a critical independent risk factor for ovarian function, oocyte developmental potential drops in advanced age women (28), which may cover the associations of %BF with reproductive outcomes. This was also observed in sensitivity analysis of women with tubal factor infertility. Tubal factor includes hydrosalpinx, fallopian tube obstruction, and so on. Women with hydrosalpinx were generally undergoing surgical treatment before IVF. Therefore, the tubal factor was mainly included fallopian tube obstruction in the present study. And they were chosen for sensitivity analyses because tubal factor is the most common reason of women infertile, with largest number of subjects among these subgroups. The number of infertile women diagnosed with ovulation disorders, diminished ovarian reserve, endometriosis, mix factors and unexplained factors were lower, and was associated with lower oocyte quality and embryo developmental potential (46–48).
Body fat is a storage site for excessive energy, and obesity is associated with increased serum free fatty acid levels (49). Adipose tissue is also an endocrine organ and involved in coordinating a variety of biological processes including energy metabolism, neuroendocrine function, and immune function (50). Adipose tissue dysfunction is associated with insulin resistance, hyperglycemia, dyslipidemia, and hypertension (51). The cut-offs values for %BF were determined based on metabolic syndrome risk (21). For NWO individuals, although their BMI were in normal range, the body fat was exceeded and may have induce health risks. It has been reported that NWO increased risk for abnormal blood glucose, cardiometabolic morbidity and mortality (52, 53). Our research further suggests that the excessive body fat may threat to reproductive health. Metabolic alterations in serum were reflected in the follicular fluid, which is critical for oocyte development (54). Elevated levels of free fatty acids in follicular fluid could alter the granulosa cells functions by affecting steroidogenesis, proliferation, and apoptotic processes (55), affect oocytes maturation and developmental competence via induce mitochondrial dysfunction and endoplasmic reticulum stress (56). Infertility has become an ongoing reproductive health problem around the world (57), the potential adverse effect of obesity has rising concern and more and more reproductive centers are setting weight management clinics (32). However, currently it mainly serves the obese population or women with PCOS. Our research suggests that NWO infertility women may also benefit from weight management. Therefore, we recommend a body composition assessment for all new infertility patients if permit.
This study has several limitations. The first was its retrospective design, and the stringent inclusion criteria and the exclusion of candidates may have introduced bias in the study population. Second, the sample size was relatively small, especially in the sensitivity analysis for early pregnancy outcomes. Third, we included only women who underwent fresh embryo transfer for early pregnancy outcome analysis due to data limitations, and since %BF has no associations with early pregnancy outcomes, body composition assessment may have limited benefits. Despite these limitations, by measuring body composition, we found that NWO was common in women, and a higher %BF was negatively associated with AFC and IVF outcomes. To our knowledge, this is the first study to reseal these findings.
Our data suggest that the classification of normal weight according to BMI may be inaccurate according to %BF, and NWO is associated with adverse reproductive outcomes. Reducing body fat may benefit to the reproduction among normal BMI women. More research is required to further validate the findings of this study.
The data analyzed in this study is subject to the following licenses/restrictions: the dataset analysed during the current study are not publicly available because they contain multiple sensitive information. Data are however available only upon reasonable and necessary request. Requests to access these datasets should be directed to Fenghua Liu,bGl1c2hpbmUyMDA2QDE2My5jb20=.
The studies involving humans were approved by the Ethics Committee of Guangdong Women and Children Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this research is a retrospective study.
YY: Conceptualization, Formal analysis, Writing – original draft. WL: Investigation, Resources, Writing – original draft. XZ: Data curation, Writing – review & editing. NS: Writing – review & editing. LH: Resources, Writing – original draft. YN: Investigation, Writing – review & editing. XX: Supervision, Writing – review & editing. FL: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Guangdong Province, China [grant number 2022A1515010776].
The authors thank for the support of the staff of the Department of Reproductive Medical Center in Guangdong Women and Children Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1332995/full#supplementary-material
AFC, Antral follicle count; AMH, Anti-Mullerian hormone; BIA, Bioelectrical impedance analysis; DXA, Dual X-ray absorptiometry; FSH, Follicle stimulating hormone; IVF, In vitro fertilization; NOW, Normal weight obesity; %BF, Percentage of body fat.
1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8.
2. Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. (2020) 370:m3324. doi: 10.1136/bmj.m3324.
3. Hunter E, Avenell A, Maheshwari A, Stadler G, Best D. The effectiveness of weight-loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: A systematic review update of evidence from randomized controlled trials. Obes Rev. (2021) 22:e13325. doi: 10.1111/obr.13325.
4. Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, et al. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril (2016) 105:663–9. doi: 10.1016/j.fertnstert.2015.11.008.
5. Goldman RH, Farland LV, Thomas AM, Zera CA, Ginsburg ES. The combined impact of maternal age and body mass index on cumulative live birth following in vitro fertilization. Am J Obstet gynecol. (2019) 221:617 e1– e13. doi: 10.1016/j.ajog.2019.05.043.
6. Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet gynecol. (2011) 118:63–70. doi: 10.1097/AOG.0b013e31821fd360.
7. Pinborg A, Gaarslev C, Hougaard CO, Nyboe Andersen A, Andersen PK, Boivin J, et al. Influence of female bodyweight on IVF outcome: a longitudinal multicentre cohort study of 487 infertile couples. Reprod biomed online. (2011) 23:490–9. doi: 10.1016/j.rbmo.2011.06.010.
8. Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet gynaecol. (2015) 29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014.
9. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. (2019) 158:R79–r90. doi: 10.1530/REP-18-0583.
10. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS One. (2012) 7:e49217. doi: 10.1371/journal.pone.0049217.
11. Ding C, Chan Z, Magkos F. Lean, but not healthy: the ‘metabolically obese, normal-weight’ phenotype. Curr Opin Clin Nutr Metab Care. (2016) 19:408–17. doi: 10.1097/MCO.0000000000000317.
12. Jablonowska-Lietz B, Wrzosek M, Wlodarczyk M, Nowicka G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiologia polska. (2017) 75:1185–91. doi: 10.5603/KP.a2017.0149.
13. Peterson MD, Al Snih S, Stoddard J, Shekar A, Hurvitz EA. Obesity misclassification and the metabolic syndrome in adults with functional mobility impairments: Nutrition Examination Survey 2003-2006. Prev Med. (2014) 60:71–6. doi: 10.1016/j.ypmed.2013.12.014.
14. Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Invest Med. (2018) 66:1–9. doi: 10.1136/jim-2018-000722.
15. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. (2017) 104:101–5. doi: 10.1016/j.bone.2017.06.010.
16. Smith S, Madden AM. Body composition and functional assessment of nutritional status in adults: a narrative review of imaging, impedance, strength and functional techniques. J Hum Nutr Dietetics. (2016) 29:714–32. doi: 10.1111/jhn.12372.
17. Wang JG, Zhang Y, Chen HE, Li Y, Cheng XG, Xu L, et al. Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J strength conditioning Res. (2013) 27:236–43. doi: 10.1519/JSC.0b013e31824f2040.
18. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res. (2012) 32:479–85. doi: 10.1016/j.nutres.2012.05.009.
19. Day K, Kwok A, Evans A, Mata F, Verdejo-Garcia A, Hart K, et al. Comparison of a bioelectrical impedance device against the reference method dual energy X-ray absorptiometry and anthropometry for the evaluation of body composition in adults. Nutrients. (2018) 10:1469. doi: 10.3390/nu10101469.
20. McLester CN, Nickerson BS, Kliszczewicz BM, McLester JR. Reliability and agreement of various inBody body composition analyzers as compared to dual-energy X-ray absorptiometry in healthy men and women. J Clin Densitom. (2020) 23:443–50. doi: 10.1016/j.jocd.2018.10.008.
21. Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S. Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988-1994). Am J Clin Nutr. (2003) 78:228–35. doi: 10.1093/ajcn/78.2.228.
22. Kim J, Juneau C, Patounakis G, Morin S, Neal S, Seli E, et al. The appraisal of body content (ABC) trial: obesity does not significantly impact gamete production in infertile men and women. J Assist Reprod Genet. (2020) 37:2733–42. doi: 10.1007/s10815-020-01930-3.
23. Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. (2014) 56:426–33. doi: 10.1016/j.pcad.2013.10.003.
24. Zhou R, Zhang X, Huang L, Wang S, Li L, Dong M, et al. The impact of different cycle regimens on birthweight of singletons in frozen-thawed embryo transfer cycles of ovulatory women. Fertil Steril. (2022) 117:573–82. doi: 10.1016/j.fertnstert.2021.09.033.
25. Zhou R, Zhang X, Dong M, Huang L, Zhu X, Wang S, et al. Association between endogenous LH level prior to progesterone administration and live birth rate in artificial frozen-thawed blastocyst transfer cycles of ovulatory women. Hum Reprod. (2021) 36:2687–96. doi: 10.1093/humrep/deab172.
26. Yao QY, Yuan XQ, Liu C, Du YY, Yao YC, Wu LJ, et al. Associations of sleep characteristics with outcomes of IVF/ICSI treatment: a prospective cohort study. Hum Reprod. (2022) 37:1297–310. doi: 10.1093/humrep/deac040.
27. Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET). Sci China Life Sci. (2012) 55:694–8. doi: 10.1007/s11427-012-4357-0.
28. Vitagliano A, Paffoni A, Viganò P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil Steril. (2023) 120:251–65. doi: 10.1016/j.fertnstert.2023.02.036.
29. Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int J Obes. (2016) 40:761–7. doi: 10.1038/ijo.2015.243.
30. Kim J, Patounakis G, Juneau C, Morin S, Neal S, Bergh P, et al. The Appraisal of Body Content (ABC) trial: Increased male or female adiposity does not significantly impact in vitro fertilization laboratory or clinical outcomes. Fertil Steril. (2021) 116:444–52. doi: 10.1016/j.fertnstert.2020.12.037.
31. Xiong Y, Wang J, Huang S, Liu C, Liu Y, Qi Y, et al. Association between maternal prepregnancy body mass index and pregnancy outcomes following assisted reproductive technology: A systematic review and dose-response meta-analysis. Obes Rev. (2021) 22:e13219. doi: 10.1111/obr.13219.
32. Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the American Society for Reproductive M. Obesity and reproduction: a committee opinion. Fertil Steril. (2021) 116:1266–85. doi: 10.1016/j.fertnstert.2021.08.018
33. Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause. (2018) 25:1046–55. doi: 10.1097/GME.0000000000001116.
34. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod update. (2019) 25:439–51. doi: 10.1093/humupd/dmz011.
35. Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet gynecol. (2010) 203:525–30. doi: 10.1016/j.ajog.2010.06.043.
36. Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. (2010) 91:258S–61S. doi: 10.3945/ajcn.2009.28449C.
37. Hill MJ, Uyehara CF, Hashiro GM, Frattarelli JL. The utility of serum leptin and follicular fluid leptin, estradiol, and progesterone levels during an in vitro fertilization cycle. J Assist Reprod Genet. (2007) 24:183–8. doi: 10.1007/s10815-007-9106-0.
38. Zhou L, Li K, Liu Y, Zhang R, Yao Y, Chen Q, et al. Living cell-derived intelligent nanobots for precision oncotherapy. Adv Funct Mater. (2023), 2311857. doi: 10.1002/adfm.202311857.
39. Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. (2009) 94:1533–40. doi: 10.1210/jc.2008-2648.
40. Bausenwein J, Serke H, Eberle K, Hirrlinger J, Jogschies P, Hmeidan FA, et al. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol Hum Reprod. (2010) 16:117–24. doi: 10.1093/molehr/gap078.
41. Zhou L, Lyu J, Liu F, Su Y, Feng L, Zhang X. Immunogenic PANoptosis-initiated cancer sono-immune reediting nanotherapy by iteratively boosting cancer immunity cycle. Adv Mater. (2023) 36:2305361. doi: 10.1002/adma.202305361.
42. Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. (2011) 95:1970–4. doi: 10.1016/j.fertnstert.2011.01.154.
43. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017.
44. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS One. (2010) 5:e10074. doi: 10.1371/journal.pone.0010074.
45. Mena GP, Mielke GI, Brown WJ. The effect of physical activity on reproductive health outcomes in young women: a systematic review and meta-analysis. Hum Reprod update. (2019) 25:541–63. doi: 10.1093/humupd/dmz013.
46. Xia Q, Wang W, Liu Z, Xiao J, Qiao C, Zhao Y, et al. New insights into mechanisms of berberine in alleviating reproductive disorders of polycystic ovary syndrome: Anti-inflammatory properties. Eur J Pharmacol. (2023) 939:175433. doi: 10.1016/j.ejphar.2022.175433.
47. Horton J, Sterrenburg M, Lane S, Maheshwari A, Li TC, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod update. (2019) 25:592–632. doi: 10.1093/humupd/dmz012.
48. Jaswa EG, McCulloch CE, Simbulan R, Cedars MI, Rosen MP. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril. (2021) 115:966–73. doi: 10.1016/j.fertnstert.2020.10.051.
49. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. (2011) 18:139–43. doi: 10.1097/MED.0b013e3283444b09.
50. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. (2004) 89:2548–56. doi: 10.1210/jc.2004-0395.
51. Bluher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. (2013) 27:163–77. doi: 10.1016/j.beem.2013.02.005.
52. Wijayatunga NN, Dhurandhar EJ. Normal weight obesity and unaddressed cardiometabolic health risk-a narrative review. Int J Obes. (2021) 45:2141–55. doi: 10.1038/s41366-021-00858-7.
53. Jo A, Mainous AG 3rd. Informational value of percent body fat with body mass index for the risk of abnormal blood glucose: a nationally representative cross-sectional study. BMJ Open. (2018) 8:e019200. doi: 10.1136/bmjopen-2017-019200.
54. Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, et al. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. (2012) 27:3531–9. doi: 10.1093/humrep/des350.
55. Baddela VS, Sharma A, Vanselow J. Non-esterified fatty acids in the ovary: friends or foes? Reprod Biol Endocrinol. (2020) 18:60. doi: 10.1186/s12958-020-00617-9.
56. Sutton-McDowall ML, Wu LLY, Purdey M, Abell AD, Goldys EM, MacMillan KL, et al. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence1. Biol Reprod. (2016) 94:23. doi: 10.1095/biolreprod.115.131862.
Keywords: body mass index, percentage of body fat, normal weight obesity, antral follicle count, reproductive outcomes
Citation: Yao Y, Liu W, Zhang X, Su N, Huang L, Nong Y, Xiao X and Liu F (2024) Normal weight obesity is associated with lower AFC and adverse IVF outcomes. Front. Endocrinol. 15:1332995. doi: 10.3389/fendo.2024.1332995
Received: 04 November 2023; Accepted: 08 February 2024;
Published: 22 February 2024.
Edited by:
Laura Gray, The University of Sheffield, United KingdomReviewed by:
Wang-Yu Cai, Zhejiang Chinese Medical University, ChinaCopyright © 2024 Yao, Liu, Zhang, Su, Huang, Nong, Xiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Liu, bGl1c2hpbmUyMDA2QDE2My5jb20=; Xiaomin Xiao, eGlhb3hpYW9taW41NUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.