
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 30 January 2024
Sec. Pituitary Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1332120
This article is part of the Research TopicInsights in Cushing’s Syndrome and Disease Volume IIView all 9 articles
 Rodrigo Rosa Giampietro1
Rodrigo Rosa Giampietro1 Marcos Vinicius Gama Cabral1
Marcos Vinicius Gama Cabral1 Elizandra Gomes Pereira1
Elizandra Gomes Pereira1 Marcio Carlos Machado2
Marcio Carlos Machado2 Lucio Vilar3
Lucio Vilar3 Vania dos Santos Nunes-Nogueira1*†
Vania dos Santos Nunes-Nogueira1*†We evaluated the accuracy of the 10 μg desmopressin test in differentiating Cushing disease (CD) from non-neoplastic hypercortisolism (NNH) and ectopic ACTH syndrome (EAS). A systematic review of studies on diagnostic test accuracy in patients with CD, NNH, or EAS subjected to the desmopressin test obtained from LILACS, PubMed, EMBASE, and CENTRAL databases was performed. Two reviewers independently selected the studies, assessed the risk of bias, and extracted the data. Hierarchical and bivariate models on Stata software were used for meta-analytical summaries. The certainty of evidence was measured using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation Working Group) approach. In total, 14 studies were included: 3 studies on differentiated CD versus NNH and 11 studies on differentiated CD versus EAS. Considering ΔACTH in 8 studies involving 429 patients, the pooled sensitivity for distinguishing CD from EAS was 0.85 (95% confidence interval [CI]: 0.80–0.89, I2 = 17.6%) and specificity was 0.64 (95% CI: 0.49–0.76, I2 = 9.46%). Regarding Δcortisol in 6 studies involving 233 participants, the sensitivity for distinguishing CD from EAS was 0.81 (95% CI: 0.74–0.87, I2 = 7.98%) and specificity was 0.80 (95% CI: 0.61–0.91, I2 = 12.89%). The sensitivity and specificity of the combination of ΔACTH > 35% and Δcortisol > 20% in 5 studies involving 511 participants were 0.88 (95% CI: 0.79–0.93, I2 = 35%) and 0.74 (95% CI: 0.55–0.87, I2 = 27%), respectively. The pooled sensitivity for distinguishing CD from NNH in 3 studies involving 170 participants was 0.88 (95% CI: 0.79–0.93) and the specificity was 0.94 (95% CI: 0.86–0.97). Based on the desmopressin test for differentiating CD from EAS, considering ΔACTH, Δcortisol, or both percent increments, 15%, 19%, or 20% of patients with CD, respectively, would be incorrectly classified as having EAS. For CD versus NNH, 11% of patients with CD would be falsely diagnosed as having NNH, whereas 7% of patients with NNH would be falsely diagnosed as having CD. However, in all hierarchical plots, the prediction intervals were considerably wider than the confidence intervals. This indicates low confidence in the estimated accuracy, and the true accuracy is likely to be different.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=85634, identifier CRD42018085634; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=68317, identifier CRD42017068317.
Evaluation of patients with suspected hypercortisolism is one of the most challenging investigations in endocrinology (1). This is due to the intermittent activation of the dynamic hypothalamic–pituitary–adrenal (HPA) axis, which results in clinical and biochemical characteristics that are indistinguishable between neoplastic and non-neoplastic forms of hypercortisolism. Furthermore, even in neoplastic cases, it is often difficult to distinguish between the two main differential diagnoses, namely, endogenous neoplastic hypercortisolism and non-neoplastic hypercortisolism (NNH) (1).
In adults, the most frequent etiology of endogenous neoplastic hypercortisolism is Cushing disease (CD), accounting for approximately 70% of Cushing syndrome (CS) cases (2). CD is caused by increased production of adrenocorticotropic hormone (ACTH) due to pituitary adenoma. It has an incidence and prevalence of 2–3 cases per 1,000,000 inhabitants/year and 40 cases per 1,000,000 inhabitants, respectively (3). The principal differential diagnosis of CD is endogenous neoplastic hypercortisolism secondary to ectopic production of ACTH (ectopic ACTH syndrome [EAS]), which accounts for 10%–20% of the causes of ACTH-dependent CS (4).
Patients with NNH (previously known as pseudo-Cushing syndrome) have been recognized for over 50 years (5). These individuals show mild-to-moderate ACTH-dependent hypercortisolism due to alcohol use disorder, neuropsychiatric disorders, chronic kidney disease, or poorly controlled diabetes mellitus (5–9).
When the prevalence of one of the conditions that characterize NNH increases, many patients with endogenous neoplastic hypercortisolism may not develop the most specific signs and symptoms associated with this hormonal disorder (e.g., easy bruising, capillary fragility, proximal weakness, and reddish-purple striae). Thus, there is an urgent need to distinguish these two clinical conditions. Additionally, as pituitary microadenomas may be present in 9.3% (range, 1.5%–26.7%) of pituitary incidentalomas in the general population (10) and in up to 38% of patients with EAS (11), the differential diagnosis between CD and EAS has been recommended (7, 12), especially when a lesion with a size of <6 mm is observed on pituitary magnetic resonance imaging (MRI).
Regarding differential diagnosis between CD and EAS, the gold standard examination is bilateral and simultaneous petrosal sinus sampling (BIPSS). This method exhibits a diagnostic accuracy of 90%–98% (13–15). However, BIPSS is invasive and should be performed by highly qualified professionals (7); these factors have limited its widespread use. Therefore, some dynamic tests have been developed for the differential diagnosis of endogenous CS.
The corticotropin-releasing hormone (CRH) test, dexamethasone-suppressed CRH stimulation test (DEX-CRH test), and desmopressin stimulation test have been widely used to distinguish neoplastic hypercortisolism from NNH as well as perform differential diagnosis between CD and EAS (16–18). However, the current lack of availability of CRH for diagnostic purposes, even in countries where it was previously used, has led to increased use of the desmopressin stimulation test to examine HPA axis function (1, 19).
Although these dynamic tests have been studied in detail in CS, no evidence synthesis with meta-analysis has focused on the desmopressin test. Thus, we aimed to evaluate the diagnostic accuracy of the desmopressin test at an intravenous dose of 10 μg to distinguish neoplastic hypercortisolism from NNH and perform differential diagnosis between CD and EAS.
A systematic review was conducted according to the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (20, 21), and the results were reported according to the PRISMA-diagnostic test accuracy (DTA) studies criteria (22). The protocol was registered in the International Prospective Registry of Systematic Reviews (IDs : CRD42018085634 and CRD42017068317).
We included the DTA studies that followed the PIRO structure described below.
Patients with clinical suspicion of endogenous CS who underwent at least two different screening tests for hypercortisolism: 24-h urinary free cortisol (UFC), late night salivary cortisol, no suppression of serum cortisol after the administration of 1 mg dexamethasone overnight, or no suppression after the administration of 2 mg dexamethasone for 48 h.
We considered desmopressin administered at an intravenous dose of 10 µg as the test index. Serum cortisol and plasma ACTH levels were measured at 15 and 0 min before and 15, 30, 45, 60, and 90 min after desmopressin administration.
Patients diagnosed with an ACTH-secreting pituitary adenoma during pathologic analysis after pituitary surgery were considered to have CD. Patients who did not undergo any surgery were considered to have CD if their plasma ACTH level was >10 pg/mL and if they met one of the following criteria: BIPSS with a central-to-peripheral ratio of plasma ACTH level of ≥2.0 pg/mL before or ≥3.0 pg/mL after CRH test or desmopressin administration, and the presence of a pituitary adenoma measuring >6 mm on MRI in a patient with concordant results suggestive of CD based on the high-dose dexamethasone suppression test (HDDST) and CRH or desmopressin stimulation tests (7).
EAS was diagnosed through immunohistochemical analysis of tumor tissues. In the absence of surgery or immunohistochemistry negative for ACTH expression, which can be noted in up to 30% of EAS cases (11, 23, 24), the absence of central gradient of ACTH at BIPSS (25) or improvement in hypercortisolism after surgery was considered.
A diagnosis of NNH was made in patients with major depression, obsessive–compulsive disorder, anxiety disorder, chronic alcoholism, or severe obesity as well as in those who exhibited hypercortisolism resolution at follow-up after the control of NNH-associated disease (8, 9, 26).
Using a 2 × 2 contingency table, the performance of the desmopressin test was compared with that of the reference test, in which true-positive, false-positive, false-negative, and true-negative cases were determined for CD diagnosis. Based on these data, the accuracy of the index test (sensitivity, specificity, positive likelihood ratio [LR+], and negative likelihood ratio [LR−]) was calculated.
Studies involving patients who were diagnosed with CD without presenting the abovementioned confirmatory criteria were excluded. Moreover, studies involving patients with NNH who did not undergo outpatient follow-up for evaluating hypercortisolism after the resolution of NNH-associated disease were excluded. Studies including patients who were diagnosed with CD or EAS without presenting the abovementioned confirmatory criteria were also excluded.
Four general search strategies were implemented for the EMBASE (1980-10/10/2017), PubMed (1966-10/10/2017), LILACS (1982-10/10/2017), and CENTRAL (Cochrane Collaboration Controlled Trials Registry-10/10/2017) electronic databases (Supplementary File). All databases were searched for the second time on September 25, 2023. The index terms “Cushing disease” and “desmopressin” were used to establish each search strategy with no language or year restrictions. EndNote X9 citation management software was used to download the references and remove duplicate entries. For initial screening of abstracts and titles, the free web application Rayyan QCRI was used (27).
Four reviewers independently and in pairs (RRG, MVGC, EGP, and VSN-N) selected titles and abstracts from the reference articles identified through bibliographic search. After selecting potentially eligible studies, the full-text was reviewed. The studies were evaluated for conformance to the proposed PIRO structure. In case of disagreements during the selection process, a consensus was achieved through discussion. The reasons for the exclusion of each study were justified.
Two reviewers extracted data regarding study characteristics and the corresponding participant-related information for each study. For each comparison between index and reference tests, data regarding the number of true-positive, true-negative, false-positive, and false-negative cases were extracted in the form of a 2 × 2 table.
The risk of bias associated with the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies tool (28).
The unit of analysis was the aggregate data extracted from the journal publications.
For each study, a 2 × 2 contingency table was constructed. Sensitivity, specificity, and LRs were calculated. When the primary study had a value of 0 in a cell of the 2 × 2 table, the value of 1 was added to facilitate calculations (29); this was observed in two of the included studies.
We performed meta-analyses using hierarchical and bivariate models, which account for variability in intrastudy accuracy as well as interstudy variations in test performance with the inclusion of random effects (30). Based on the results of heterogeneity investigations, the bivariate model was used to estimate summary sensitivity and specificity (summary points), and the hierarchical summary receiver operating characteristic (HSROC) model was applied to construct summary ROC curves.
Stata Statistical Software V.18 (StataCorp LLC), with metadta and metandi commands, was used for analyses.
Forest and HSROC plots were visually assessed for heterogeneity. If data allowed, we evaluated the sources of heterogeneity through subgroup analyses. Meta-regression could not be performed because of the limited number of studies available. Variability away from the summary ROC curve is likely to represent greater heterogeneity than variation along the summary ROC curve, which might correspond to simple threshold effects. If the number of studies included was adequate, we would assess the following potential heterogeneity sources: patient characteristics, test methods, and study design. A separate SROC curve would be fitted for each subgroup, and the results would be compared graphically across subgroups (30).
If the number of studies selected was adequate, we assessed the robustness of our results by conducting sensitivity analysis according to the threshold of ACTH level and cortisol percent increment after the desmopressin test.
For each outcome, the findings were summarized in a tabulated format to determine the effectiveness of the index test. The certainty of evidence was measured using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation Working Group) approach (31, 32).
The search strategies yielded 1,940 references. After removing duplicates, 1,838 studies remained (Figure 1). Thirty-three studies potentially eligible for inclusion in the full-text review were selected. However, of these, 19 studies were excluded for the following reasons. One study was a narrative review article (6), and seven studies did not use the desmopressin test as the index test (33–39). In another study, the authors used the desmopressin test to distinguish patients with CD from those with a clinical and laboratory suspicion of CS. In the same study, although most patients suspected of CS had undergone at least one positive screening test for hypercortisolism, they were not classified as carriers of NNH (40). Two studies compared the results of desmopressin test in patients with CD and those with depression; however, the patients with depression showed no clinical or laboratory features of CS (41, 42). Three studies involved patients who were previously included in a published series (43–45). Another study (46) had no patients with EAS in their series. Salgado et al. (47) evaluated the desmopressin test results in patients with EAS, and no patient with CD was included in their series. Sakai et al. and Suda et al. conducted the desmopressin test with 5 and 4 µg of desmopressin, respectively (48, 49). In another study, the criteria used for distinguishing CD from NNH were not described (50).
According to our eligibility criteria, we included the following 14 studies: 3 studies distinguishing CD from NNH (51–53) and 11 studies distinguishing CD from EAS (54–64). These studies included 979 participants (782 with CD, 79 with NNH, and 118 with EAS). Five of the included studies also involved a group of healthy individuals who underwent desmopressin tests. Tables 1, 2 present the descriptive data of the included studies on differential diagnosis of CD versus EAS and CD versus NNH respectively.
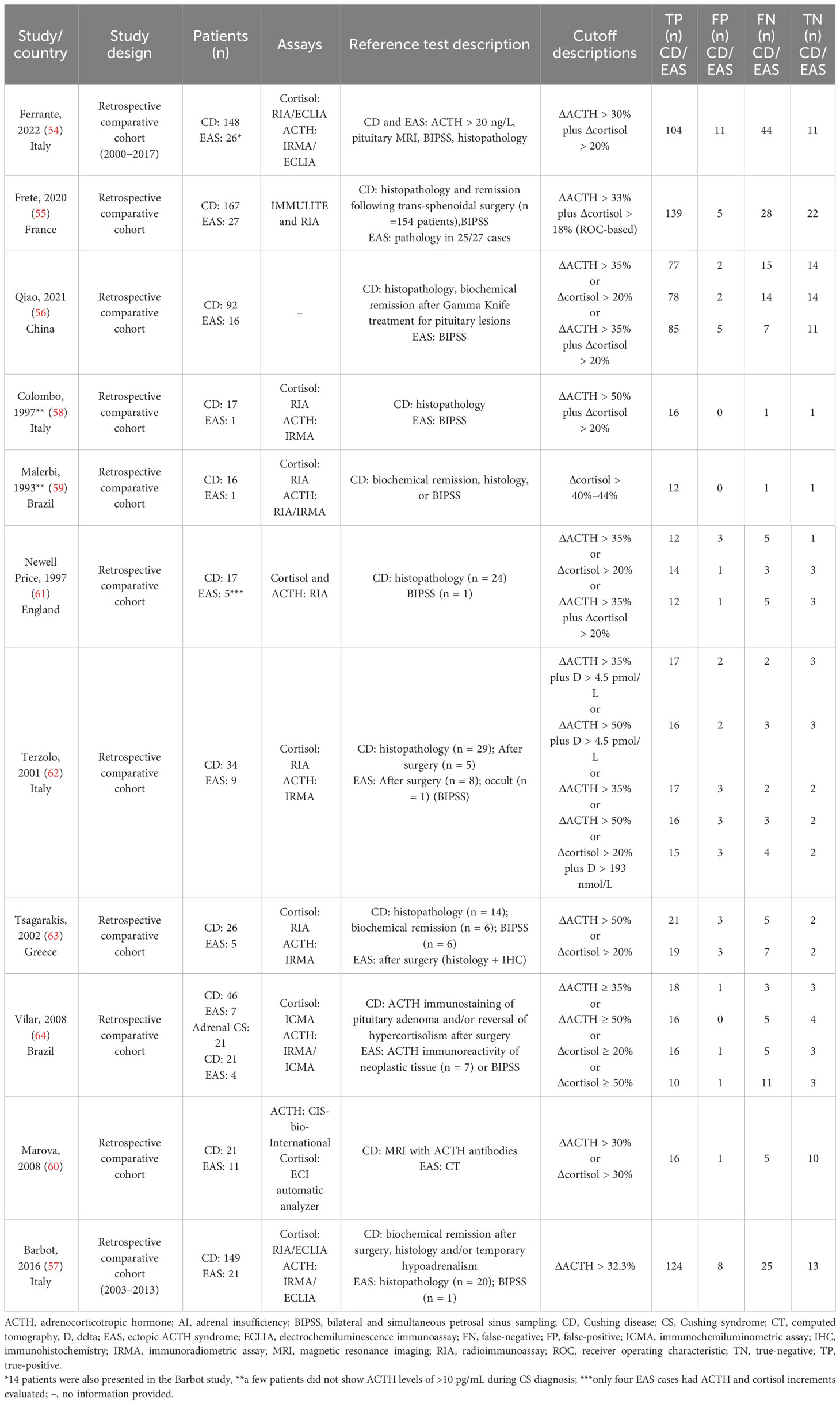
Table 1 Characteristics of the studies included in relation to the “PIRO” and the contingency table for the accuracy of the 10 µg desmopressin test to distinguish Cushing disease (CD) from ectopic ACTH syndrome (EAS).
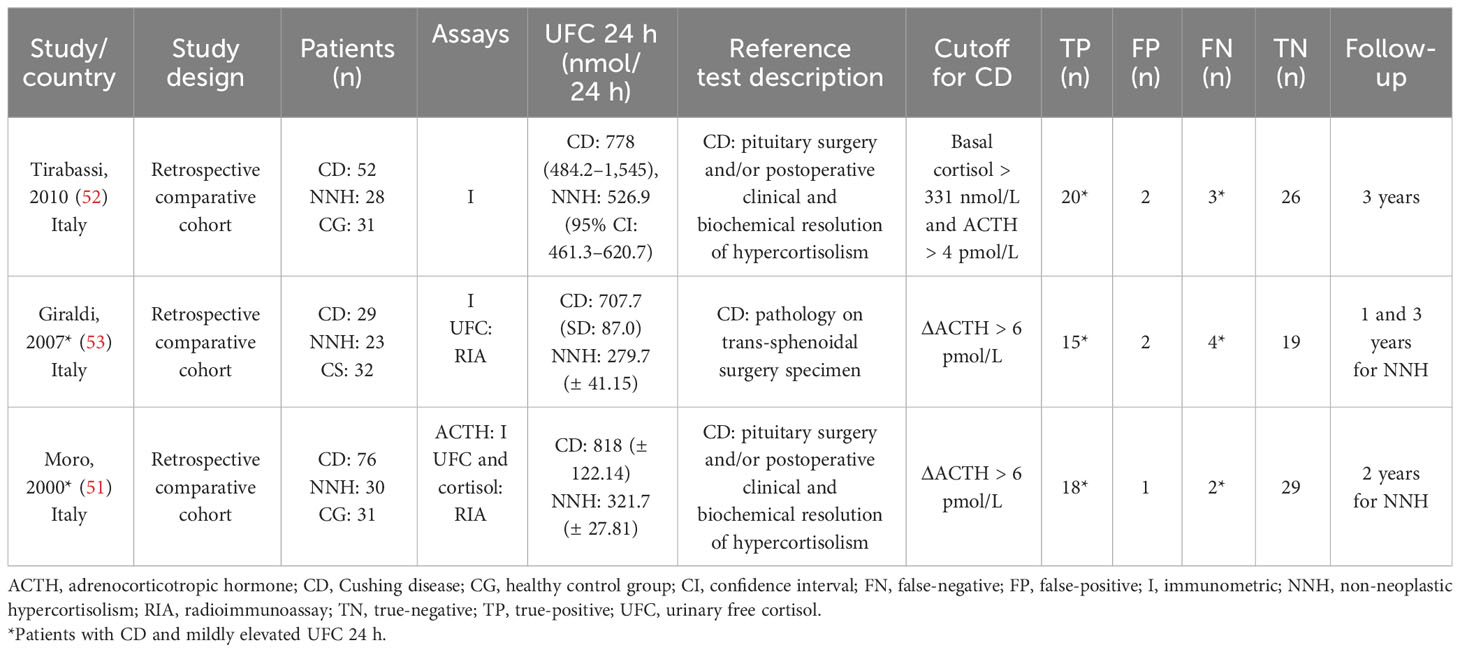
Table 2 Characteristics of the studies included in relation to the “PIRO” and the contingency table for the accuracy of the10 µg desmopressin test to differentiate Cushing disease from non-neoplastic hypercortisolism.
All studies conducted intravenous desmopressin tests, wherein a slow bolus of 10 μg desmopressin was injected into the antecubital vein of patients who had fasted overnight. This was followed by the measurement of plasma ACTH and serum cortisol levels at 15 and 0 min before and 10, 20, 30, 45, 60, 90, and 120 min after desmopressin administration. Only Terzolo et al. (62) excluded the 120-min time point from their protocol. Barbot et al. used the following time points: 15 and 0 min before and 15, 30, 45, 60, 90, and 120 min after desmopressin administration (57). The baseline ACTH and cortisol levels were expressed as the means of the respective measurements taken between 15 and 0 min before desmopressin administration. The absolute increase in plasma ACTH levels after desmopressin administration was defined as the difference between the value at 0 min and the highest value attained within 30 min (ΔACTH).
Regarding differential diagnosis of CD versus NNH, the patients in the included studies were suspected of having endogenous CS, and most of them had mild hypercortisolism. A similar definition of mild hypercortisolism was used in all included studies. Tirabassi et al. defined mild hypercortisolism as a 24-h UFC level of <771 nmol/day (~2 times of the upper limit of normal range [ULNR]), whereas Moro et al. (51) and Giraldi et al. (53) defined it as a 24 - h UFC level of <690 nmol/day (~3 times of the ULNR). Regarding the criteria for differentiating CD from NNH, a study defined CD as ΔACTH of >4 pmol/L along with a baseline serum cortisol threshold of >331 nmol/L (52), whereas other studies defined CD as ΔACTH of >6 pmol/L (51, 53).
To distinguish CD from EAS, eight studies calculated sensitivities and specificities based on ΔACTH percent increment, six studies calculated these values based on Δcortisol percent increment, whereas five studies calculated the sensitivity and specificity based on both, Δcortisol and ΔACTH percent increment. For CD diagnosis, the most used criteria were ΔACTH of >35% and Δcortisol of >20% (Table 1). Most criteria used in these studies were prespecified by the authors.
Figure 2 summarizes the overall methodological quality of all included studies. These studies retrospectively evaluated CS patient series that required differential diagnosis of CD versus EAS or CD versus NNH. However, these studies did not report whether participant recruitment was performed randomly or consecutively. Therefore, all included studies were considered as having an unclear risk of bias for patient selection. Barbot et al. (57) did not prespecify the threshold used; therefore, we considered that their study had an unclear applicability concern for the index test. We revealed that other studies and domains had a low risk of bias and applicability concern.

Figure 2 Risk of bias and applicability concerns: authors’ judgment on each domain for all included studies. (A) Desmopressin test to distinguish Cushing disease from non-neoplastic hypercortisolism. (B) Desmopressin test to distinguish Cushing disease from ectopic ACTH syndrome.
Considering ΔACTH, the pooled sensitivity for distinguishing CD from EAS was 0.85 (95% CI: 0.80–0.89, I2 = 17.6%) and pooled specificity was 0.64 (95% CI: 0.49–0.76, I2 = 9.46%); 8 studies, 429 patients, low certainty of evidence (Figure 3A; Table 3). The LR+ was 2.33 (95% CI: 1.58–3.45) and LR− was 0.24 (95% CI: 0.15–0.36).
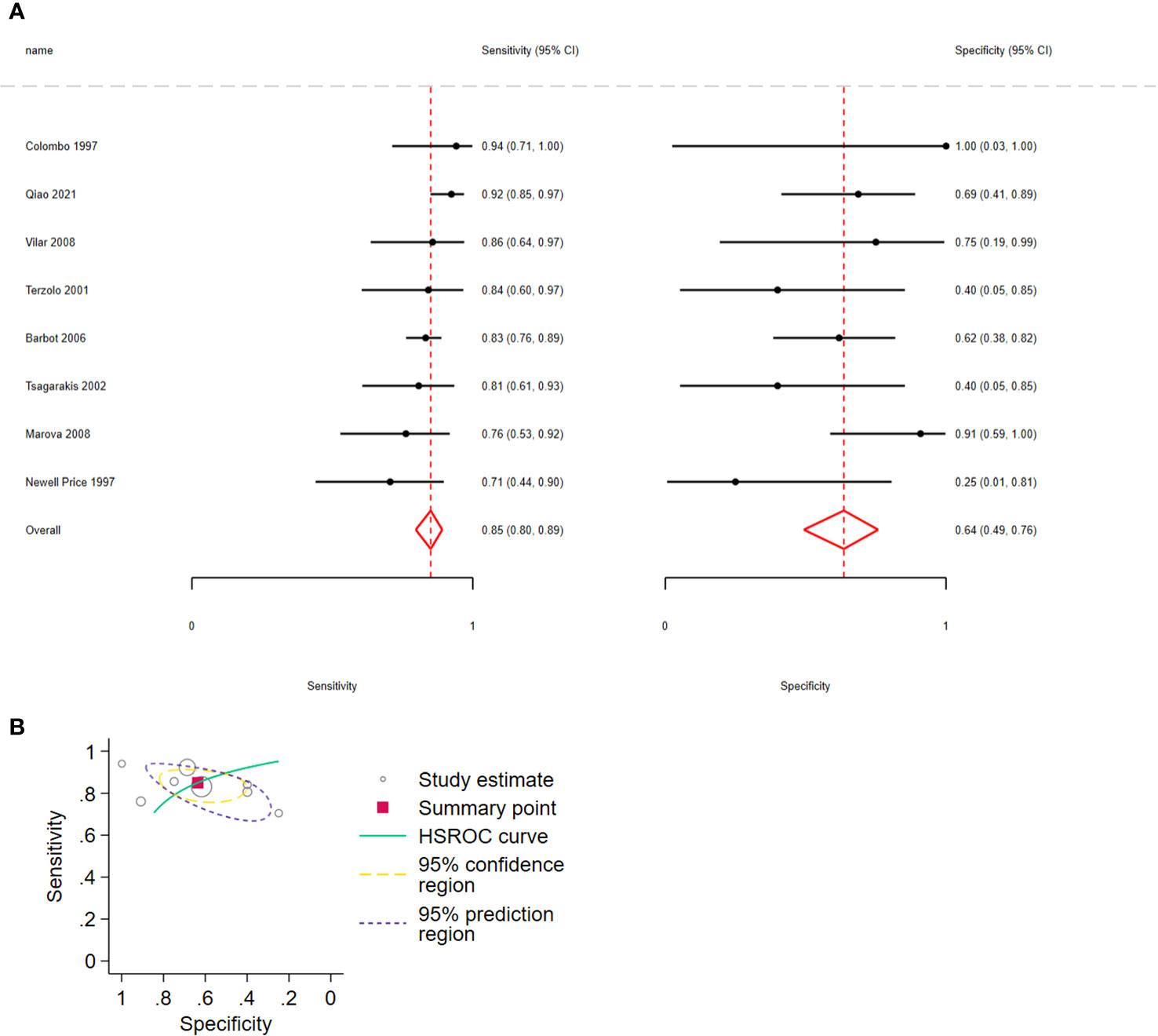
Figure 3 (A) Forest plot depicting the sensitivity and specificity considering ACTH percent increment after 10 µg desmopressin test to distinguish Cushing disease from ectopic ACTH syndrome. The figure indicates the estimated sensitivity and specificity of the study (black circle) and its 95% confidence interval (black horizontal line). (B) Summary ROC plots from Stata after fitting the hierarchical model to ACTH percent increment. The circles represent the estimates of individual primary studies, and square indicates the summary points of sensitivity and specificity. HSROC curve is plotted as a curvilinear line passing through summary point. The 95% confidence interval and 95% prediction interval are also provided. HSROC, hierarchical summary receiver operating characteristic.
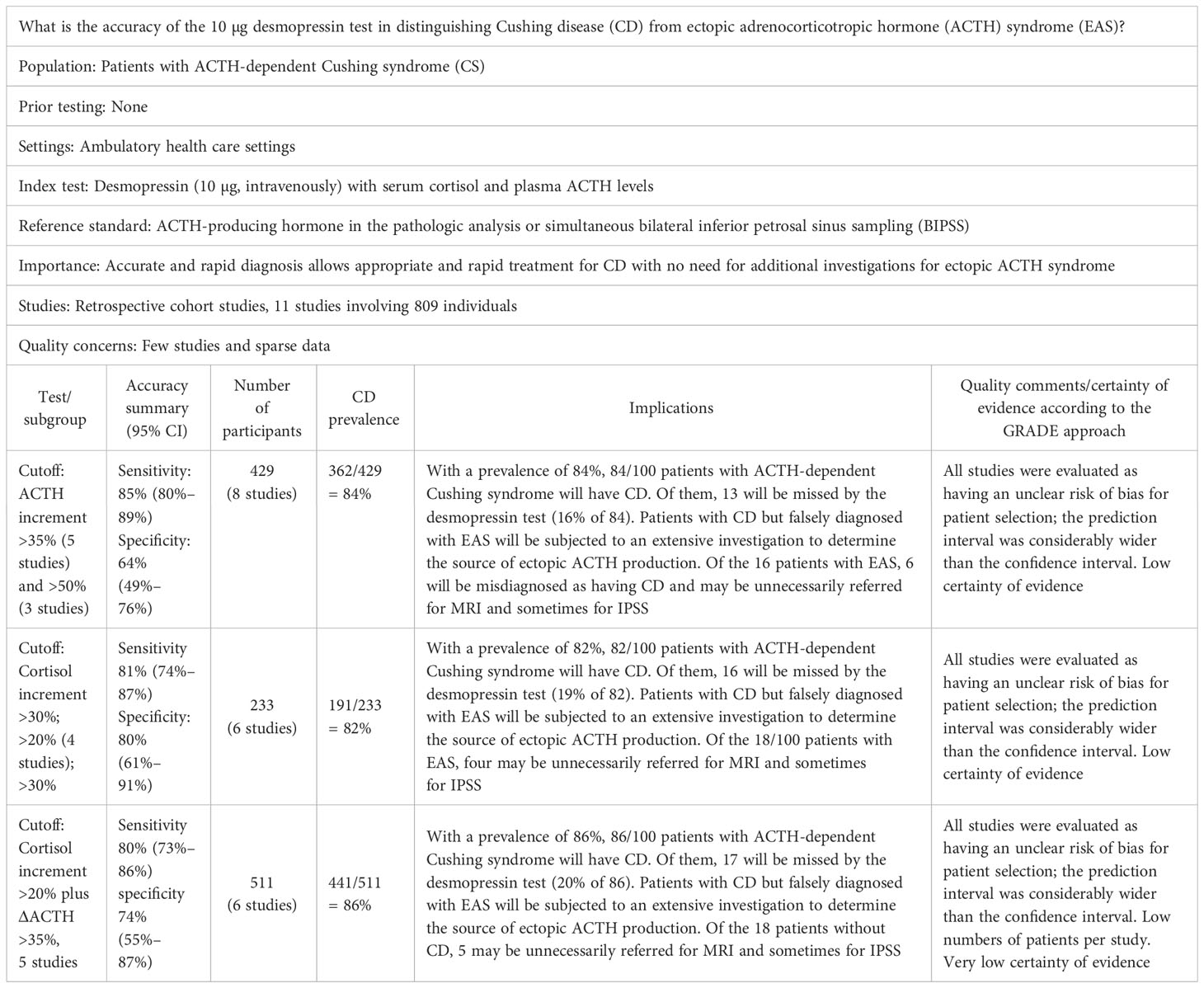
Table 3 Summary of the proposed “PIRO” and the pooled sensitivity and specificity results of the accuracy of the 10 µg desmopressin test to distinguish Cushing disease (CD) from ectopic ACTH syndrome (EAS) and certainty of evidence according to the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach.
Regarding Δcortisol, the pooled sensitivity for distinguishing CD from EAS was 0.81 (95% CI: 0.74–0.87, I2 = 7.98%) and pooled specificity was 0.80 (95% CI: 0.61–0.91, I2 = 12.89%); 6 studies, 233 participants, low certainty of evidence (Figure 4A; Table 3). The LR+ was 4.1 (95% CI: 1.9–8.94) and LR− was 0.23 (95% CI: 0.15–0.35).
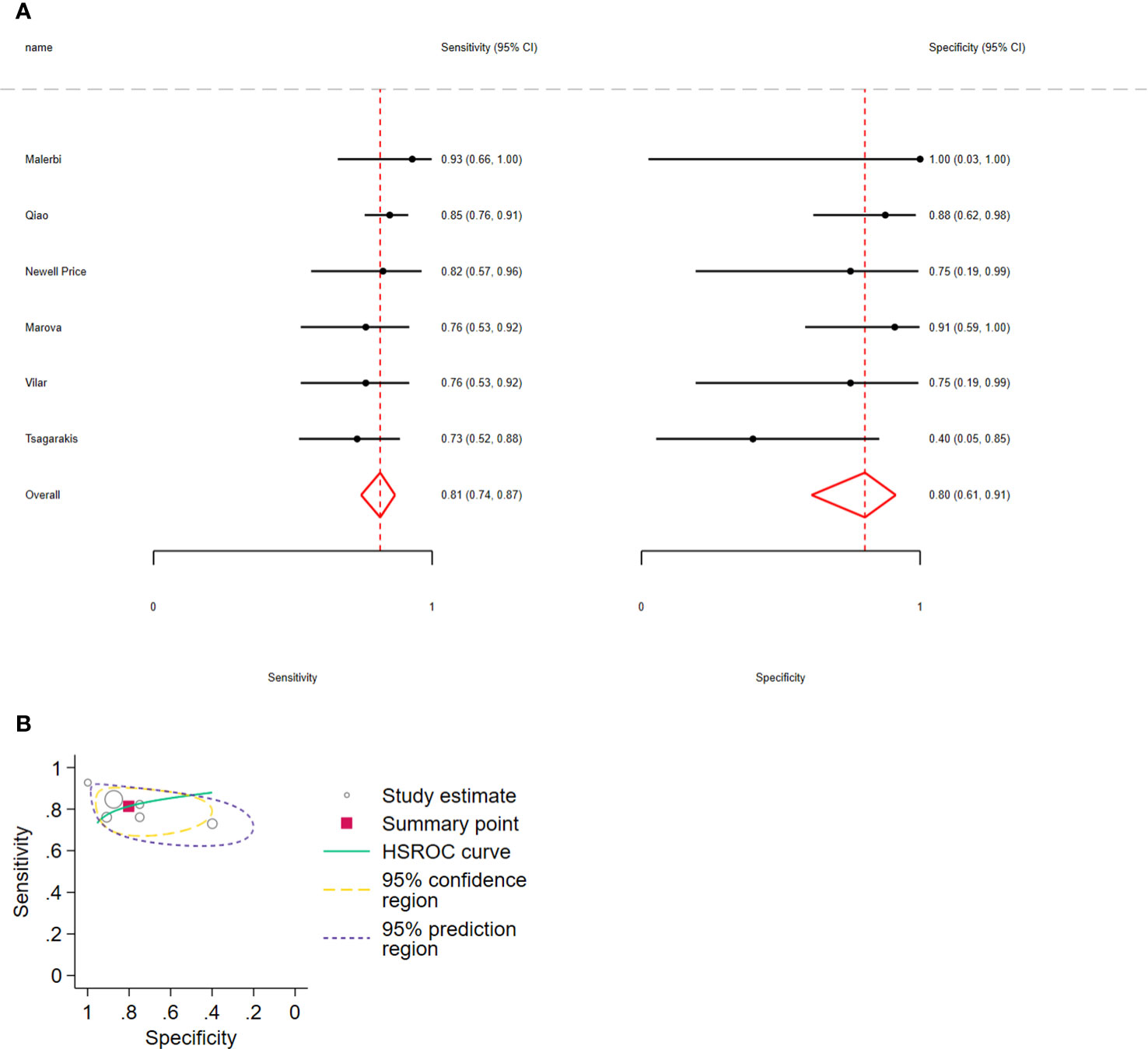
Figure 4 (A) Forest plot depicting the sensitivity and specificity considering the cortisol percent increment after the 10 µg desmopressin test to distinguish Cushing disease from ectopic ACTH syndrome. Estimated study sensitivity and specificity (black circle); 95% confidence interval (black horizontal line). (B) Summary ROC plots from Stata after fitting the hierarchical model to cortisol percent increment. Circles represent the estimates of individual primary studies, and squares indicate the summary points of sensitivity and specificity. HSROC curve is plotted as a curvilinear line passing through the summary point. The 95% confidence interval and 95% prediction interval are also provided. HSROC, hierarchical summary receiver operating characteristic.
Regarding the combination of ΔACTH > 35% and Δcortisol > 20%, the pooled sensitivity and specificity were 0.80 (95% CI: 0.73–0.86, I2 = 35%) and 0.74 (95% CI: 0.55–0.87, I2 = 27%), respectively; 5 studies, 511 participants, low certainty of evidence (Figure 5A; Table 3). The LR+ was 3 (95% CI: 1.58–67) and LR− was 0.23 (95% CI: 0.17–0.43).
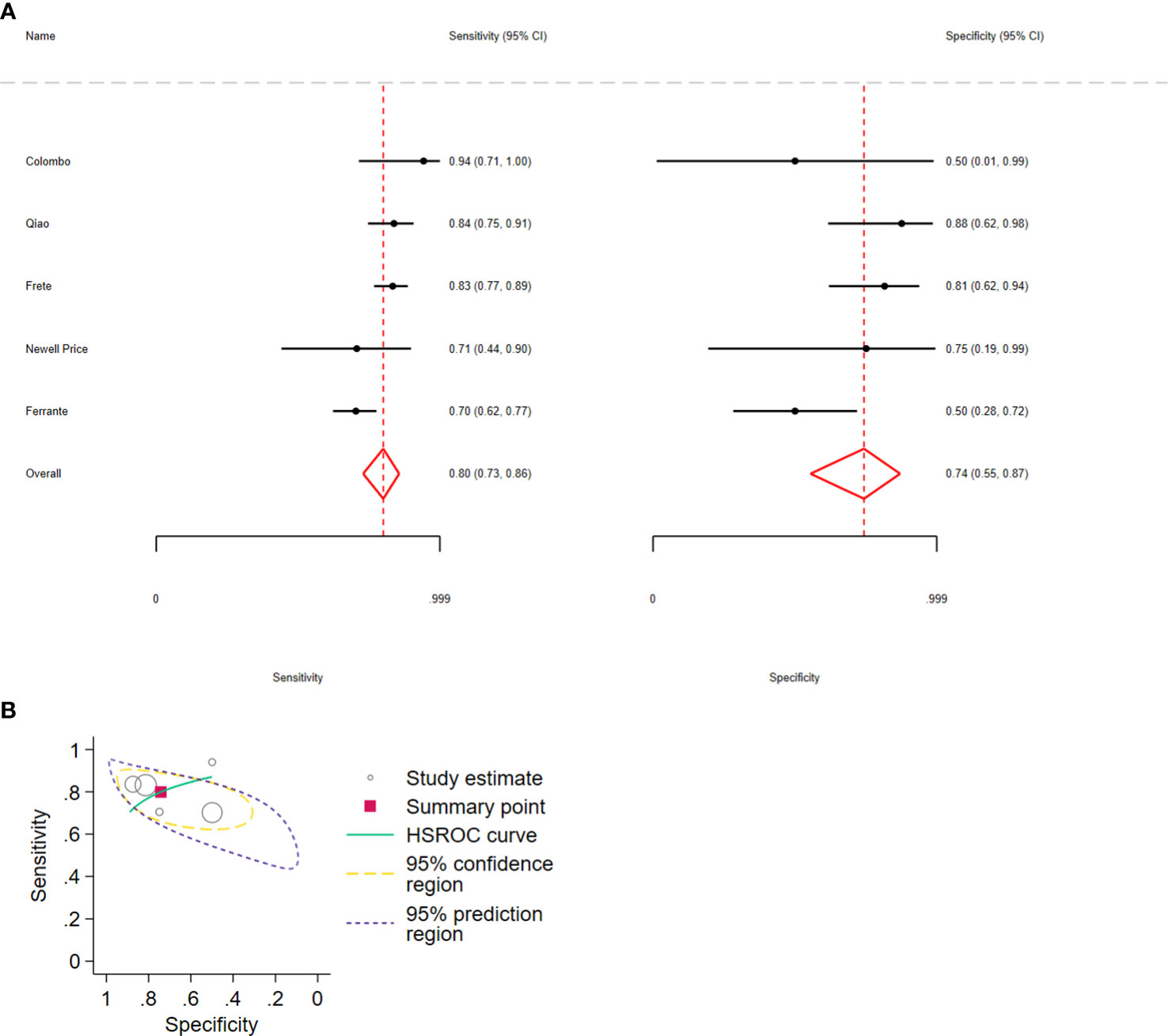
Figure 5 (A) Forest plot depicting the sensitivity and specificity considering the ACTH and cortisol percent increments after the 10 µg desmopressin test to distinguish Cushing disease from ectopic ACTH syndrome. Estimated study sensitivity and specificity (black circle); 95% confidence interval (black horizontal line). (B) Summary ROC plots from Stata after fitting the hierarchical model to ACTH and cortisol percent increment. Circles represent the estimates of individual primary studies, and squares indicate the summary points of sensitivity and specificity. HSROC curve is plotted as a curvilinear line passing through the summary point. The 95% confidence interval and 95% prediction interval are also provided. HSROC, hierarchical summary receiver operating characteristic.
In all analyses, compared with sensitivity, forest plots revealed greater variability in the estimated specificity across all studies. In addition, based on the graphical outputs obtained after fitting the hierarchical model, the 95% CIs were extremely wide, and the prediction intervals were wider than the CIs (Figures 3B, 4B, 5B).
The pooled sensitivity for distinguishing CD from NNH was 0.88 (95% CI: 0.79–0.93) and the specificity was 0.94 (95% CI: 0.86–0.97), 3 studies, 170 participants, very low certainty of evidence (Figure 6).
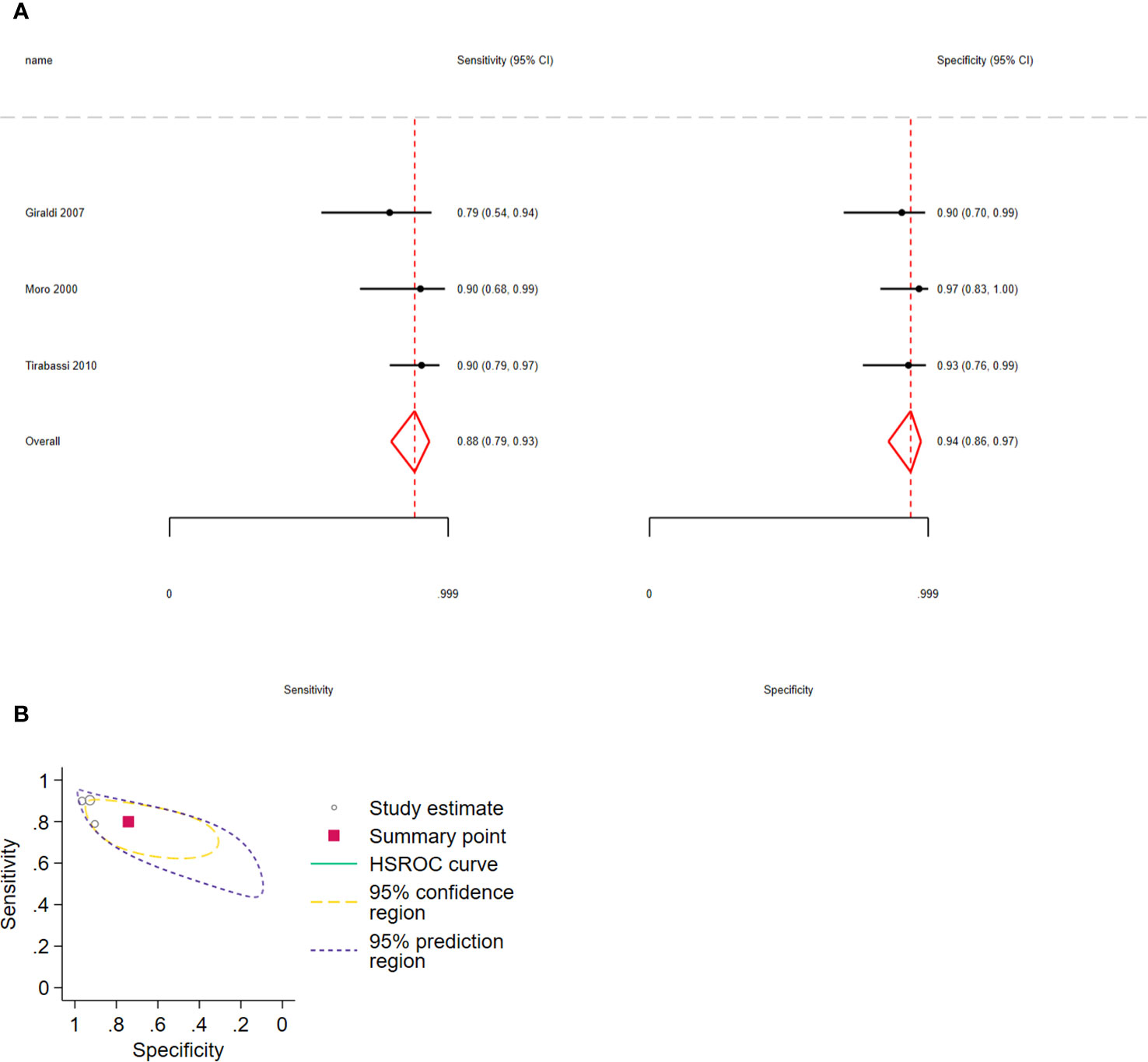
Figure 6 (A) Forest plot depicting the sensitivity and specificity considering the ACTH percent increment after the 10 µg desmopressin test to distinguish Cushing disease from non-neoplastic hypercortisolism. Estimated study sensitivity and specificity (black circle); 95% confidence interval (black horizontal line). (B) Summary ROC plots from Stata after fitting the hierarchical model to ACTH percent increment. Circles represent the estimates of individual primary studies, and squares indicate the summary points of sensitivity and specificity. HSROC curve is plotted as a curvilinear line passing through the summary point. The 95% confidence interval and 95% prediction interval are also provided. HSROC, hierarchical summary receiver operating characteristic.
The quality of evidence regarding the desmopressin test for evaluating CD versus EAS was downgraded in two levels because of the risk of bias and uncertainty (all studies were evaluated as having an unclear risk of bias for patient selection, and prediction intervals in all pooled analyses were considerably wider than CIs). To evaluate CD versus NNH, the evidence was downgraded in three levels because of the risk of bias, uncertainty, and imprecision (a few participants per study). Publication bias could not be investigated because of the small number of studies included per meta-analysis (<10).
Considering the need to differentiate CD from EAS and NNH, we evaluated the accuracy of the desmopressin test in these two clinical scenarios. We conducted a systematic literature review and found 14 studies that met our eligibility criteria. Based on the studies included in this review, 84 of 100 patients with ACTH-dependent syndrome will have CD (362/429) and 16 will have EAS (67/429). Of the 84 patients with CD, 13 (15%) will be misdiagnosed as not having CD based on the desmopressin test. Of the 16 patients with EAS, 6 (36%) will be falsely considered as having CD. The patients with EAS falsely diagnosed as having CD may have to undergo MRI. In the absence of an adenoma with a size of >6 mm, BIPSS will be performed, and the diagnosis may be rectified. Conversely, the patients with CD falsely diagnosed as having EAS would have to undergo an extensive investigation to determine the presence of ectopic ACTH production. Conversely, for patients with mild hypercortisolism, 48 and 52 of the 100 patients will have respectively CD and NNH. Among 48 patients with CD, the desmopressin test may misdiagnose 5 (11%) patients; however, these patients can be re-tested. Of the 52 patients without NHH, 4 may be unnecessarily referred for MRI and occasionally for BIPSS.
Although separate meta-analyses of each summary point seem to be extremely accurate in distinguishing CD from EAS and NNH, we revealed that the specificity decreased when sensitivity increased in all analyses. This occurred because separate pooling overlooks the correlation between sensitivity and specificity (20). The results of the separate meta-analyses of sensitivity and specificity are valid when the same criteria have been used for a positive result in each study, and each study is of similar size and quality (30). If different criteria or thresholds have been used, a relationship exists between sensitivity and specificity across all studies. This is known as the threshold effect (30). In these cases, weighted averages do not reflect the overall accuracy of the test (65). Therefore, the methods recommended for summarizing sensitivity and specificity are hierarchical and bivariate models. The bivariate model is preferred for computing summary points, whereas the HSROC model is preferred for constructing the HSROC curve (20). Additionally, the bivariate method focuses on determining the summary estimates of sensitivity and specificity and how these values vary with study‐level covariates. In contrast, the HSROC approach focuses on evaluating the SROC curve as the basis for assessing the accuracy of the test and investigating how the position and shape of the curve may vary with study‐level covariates (30). The confidence interval is related to the joint summary estimates of sensitivity and specificity in the HSROC space; however, this region does not represent the between-study heterogeneity (66). Conversely, the prediction interval refers to the sensitivity and specificity values that might be observed in a future study by describing the full extent of the uncertainty of summary points, which can thus reflect the between-study heterogeneity. In the current review, the 95% prediction intervals of all calculated HSROCs were wider than the 95% confidence intervals. Therefore, the certainty of evidence regarding the accuracy of the desmopressin test to distinguish CD from EAS and NHH was low/very low. This indicated that we have very little confidence of the estimated accuracy, and the true accuracy is likely to be significantly different from the estimated result.
Dynamic tests other than the desmopressin test have been used to distinguish CD from EAS (7). Corticotropic pituitary tumors are sensitive to CRH stimulation, whereas ectopic secretory tumors of ACTH are usually not sensitive to the stimulation (65–67). Therefore, the CRH test is employed based on this purpose (67, 68). However, considering its cost and unavailability in Brazil and worldwide, the use of the CRH test has been decreasing (7).
Furthermore, the HDDST has been used to distinguish the abovementioned two diagnoses (44, 69, 70). Although HDDST is associated with low cost and is readily available, its diagnostic accuracy is low, with 5%–25% of patients with EAS exhibiting suppression (4, 11, 47, 71) and up to 20% of patients with CD not exhibiting suppression (15). The previously adopted value for the suppression of serum cortisol levels (collected between 8 am and 9 am after fasting following ingestion of 8 mg dexamethasone at night) was 50% in patients with CD (23, 72–74). To improve the specificity of the test, some authors have proposed suppression of 80% of cortisol levels as the cutoff value (64, 70). However, this may result in a low level of accuracy (64).
A systematic review evaluating the diagnostic accuracy of the CRH test, desmopressin test, and HDDST for establishing a CD or EAS diagnosis revealed that the CRH test had the highest sensitivity for detecting CD on the basis of ΔACTH (87%) and Δcortisol (86%), along with the highest specificity for detecting EAS on the basis of ΔACTH (94%) and Δcortisol (89%). However, I2 values suggested substantial heterogeneity for sensitivity (62% ACTH and 78% cortisol), and no HSROCs were calculated (17).
The Dexa-CRH test (a test combining CRH administration after 48 h with 2 mg/day low-dose dexamethasone suppression test) has been previously used to distinguish CS from NNH (49). Yanovski first used the Dexa-CRH test to detect CS and proposed that a serum cortisol level of >1.4 μg/dL (absolute value) observed 15 min after the test is suggestive of CS (33). Erickson et al. (16) and Giraldi et al. (53) used this test to distinguish CD from NNH; based on the abovementioned proposed cortisol cutoff, they achieved a sensitivity of 100% and specificities of 76% and 62.5%, respectively. Erickson et al. (16) reported 95% sensitivity and 97% specificity in the ROC analysis of ACTH values of >27 pg/mL (5.9 pmol/L) at 15 min after CRH stimulus.
The most crucial limitation of this review was the number of studies included and the number of patients included per study (<100 in most studies) (75). When the number of studies is small, deciding which terms should be included in a model and which is the best model may be difficult. For both bivariate and HSROC models, estimates of variances of the random effects can be subject to a high level of uncertainty (30). Additionally, because a low number of studies were included per meta-analysis (<10), the presence of publication bias could not be evaluated. Moreover, we could not evaluate the sources of heterogeneity through subgroup analyses or meta-regression. Furthermore, the evaluated outcomes were limited by the diagnostic accuracy, and evaluation of other crucial aspects from the patient’s viewpoint, such as quality of life, stress, and costs incurred due to a false-positive diagnosis, was lacking.
Although we did not specify remission of hypercortisolism as a criterion for pituitary or ectopic ACTH overproduction, no study was excluded based on this, and we included studies in which CD was confirmed by remission of hypercortisolism after trans-sphenoidal surgery. Regarding the diagnostic approach to distinguish ACTH-dependent CS from ACTH-independent CS, persistent ACTH levels of >15 or >20 pg/mL have been used to diagnose ACTH-dependent hypercortisolism, ACTH levels of <5 or <10 pg/mL have been used to diagnose ACTH-independent hypercortisolism, and ACTH levels of 5–15 or 10–20 pg/mL have been reported as indeterminate, indicating that new samples should be ordered (7, 76). Indeterminate ACTH levels usually indicate ACTH-dependent cortisol secretion. Thus, to avoid losing studies that did not order new samples but instead used BIPSS and the presence of a pituitary adenoma measuring >6 mm on MRI to diagnose CD, we used a cutoff value of 10 pg/mL as an indication of ACTH-dependent hypercortisolism. Although some included studies did not use the ACTH value to distinguish these two diagnoses, all of them considered histopathological analyses, remission of hypercortisolism after pituitary surgery, or BIPSS results when diagnosing CD.
When this review was being performed, two other systematic reviews were published on the same topic. However, none of them summarized sensitivity and specificity using hierarchical and bivariate methods and presented certainty of evidence according to the GRADE approach (17, 77). Additionally, our review focused on the desmopressin test, an inexpensive and readily available test in most countries, which has been used as a substitute for the CRH test.
In conclusion, this evidence synthesis demonstrates that using the desmopressin test for distinguishing CD from EAS results in up to 20% of patients with CD being incorrectly diagnosed as EAS. Additionally, the use of the desmopressin test to distinguish CD from NNH results in 11% of patients with CD being falsely diagnosed as NNH and 7% of patients with NNH being falsely diagnosed as CD. Thus, the use of the desmopressin test alone is not recommended to distinguish CD from EAS or CD from NNH.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RG: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – original draft. MC: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – review & editing. EP: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Validation, Writing – review & editing. LV: Data curation, Investigation, Methodology, Validation, Writing – review & editing. VN-N: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the São Paulo Research Foundation (FAPESP) [grant number: 2015/12531-6].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1332120/full#supplementary-material
1. Findling JW, Raff H. Recognition of nonneoplastic hypercortisolism in the evaluation of patients with Cushing syndrome. J Endocr Soc (2023) 7:bvad087. doi: 10.1210/jendso/bvad087
2. Hakami OA, Ahmed S, Karavitaki N. Epidemiology and mortality of Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab (2021) 35:101521. doi: 10.1016/j.beem.2021.101521
3. Steffensen C, Bak AM, Rubeck KZ, Jorgensen JO. Epidemiology of cushing’s syndrome. Neuroendocrinology (2010) 92 Suppl 1:1–5. doi: 10.1159/000314297
4. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab (2005) 90:4955–62. doi: 10.1210/jc.2004-2527
5. Findling JW, Raff H. DIAGNOSIS OF ENDOCRINE DISEASE: differentiation of pathologic/neoplastic hypercortisolism (Cushing’s syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing’s syndrome). Eur J Endocrinol (2017) 176:R205–16. doi: 10.1530/EJE-16-0946
6. Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev (1998) 19:647–72. doi: 10.1210/edrv.19.5.0346
7. MaChado MC, Fragoso MC, Moreira AC, Boguszewski CL, Vieira LN, Naves LA, et al. Recommendations of the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism for the diagnosis of Cushing’s disease in Brazil. Arch Endocrinol Metab (2016) 60:267–86. doi: 10.1590/2359-3997000000174
8. Chabre O. The difficulties of pseudo-Cushing’s syndrome (or ‘non-neoplastic hypercortisolism’). Ann Endocrinol (Paris) (2018) 79:138–45. doi: 10.1016/j.ando.2018.04.017
9. Lindholm J. Cushing’s disease, pseudo-Cushing states and the dexamethasone test: a historical and critical review. Pituitary (2014) 17:374–80. doi: 10.1007/s11102-013-0509-x
10. Orija IB, Weil RJ, Hamrahian AH. Pituitary incidentaloma. Best Pract Res Clin Endocrinol Metab (2012) 26:47–68. doi: 10.1016/j.beem.2011.07.003
11. Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab (2006) 91:371–7. doi: 10.1210/jc.2005-1542
12. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2008) 93:1526–40. doi: 10.1210/jc.2008-0125
13. Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J Clin Endocrinol Metab (2013) 98:2285–93. doi: 10.1210/jc.2012-3943
14. Matur AV, Body AM, Johnson MD, Smith MS, Bhabhra R, Lester EJ, et al. An algorithm to improve lateralization accuracy of inferior petrosal sinus sampling: procedural nuances for complex patterns of venous drainage. Patient series. J Neurosurg Case Lessons (2021) 2:CASE21374. doi: 10.3171/CASE21374
15. MaChado MC, de Sa SV, Domenice S, Fragoso MC, Puglia P Jr., Pereira MA, et al. The role of desmopressin in bilateral and simultaneous inferior petrosal sinus sampling for differential diagnosis of ACTH-dependent Cushing’s syndrome. Clin Endocrinol (Oxf) (2007) 66:136–42. doi: 10.1111/j.1365-2265.2006.02700.x
16. Erickson D, Natt N, Nippoldt T, Young WF Jr., Carpenter PC, Petterson T, et al. Dexamethasone-suppressed corticotropin-releasing hormone stimulation test for diagnosis of mild hypercortisolism. J Clin Endocrinol Metab (2007) 92:2972–6. doi: 10.1210/jc.2006-2662
17. Ceccato F, Barbot M, Mondin A, Boscaro M, Fleseriu M, Scaroni C. Dynamic testing for differential diagnosis of ACTH-dependent Cushing syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab (2023) 108:e178–88. doi: 10.1210/clinem/dgac686
18. Vassiliadi DA, Tsagarakis S. DIAGNOSIS OF ENDOCRINE DISEASE: the role of the desmopressin test in the diagnosis and follow-up of Cushing’s syndrome. Eur J Endocrinol (2018) 178:R201–14. doi: 10.1530/EJE-18-0007
19. Ceccato F, Di Dalmazi G. Shortage of hCRH for the diagnosis of endogenous CS: the end of an era or the beginning of a new journey? J Endocrinol Invest (2023) 46:2189–91. doi: 10.1007/s40618-023-02113-4
20. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol (2015) 16:1188–96. doi: 10.3348/kjr.2015.16.6.1188
21. Bossuyt PM, Deeks JJ, Leeflang MM, Takwoingi Y, Flemyng E. Evaluating medical tests: introducing the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Cochrane Database Syst Rev (2023) 7:ED000163. doi: 10.1002/14651858.ED000163
22. Sharifabadi AD, McInnes MDF, Bossuyt PMM. PRISMA-DTA: an extension of PRISMA for reporting of diagnostic test accuracy systematic reviews. Clin Chem (2018) 64:985–6. doi: 10.1373/clinchem.2018.289637
23. Grossman AB, Kelly P, Rockall A, Bhattacharya S, McNicol A, Barwick T. Cushing’s syndrome caused by an occult source: difficulties in diagnosis and management. Nat Clin Pract Endocrinol Metab (2006) 2:642–7. doi: 10.1038/ncpendmet0327
24. Kamp K, Alwani RA, Korpershoek E, Franssen GJ, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocrinol (2016) 174:271–80. doi: 10.1530/EJE-15-0968
25. Hayes AR, Grossman AB. The ectopic adrenocorticotropic hormone syndrome: rarely easy, always challenging. Endocrinol Metab Clin North Am (2018) 47:409–25. doi: 10.1016/j.ecl.2018.01.005
26. Batista DL, Courcoutsakis N, Riar J, Keil MF, Stratakis CA. Severe obesity confounds the interpretation of low-dose dexamethasone test combined with the administration of ovine corticotrophin-releasing hormone in childhood Cushing syndrome. J Clin Endocrinol Metab (2008) 93:4323–30. doi: 10.1210/jc.2008-0985
27. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev (2016) 5:210. doi: 10.1186/s13643-016-0384-4
28. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. and QUADAS-2 Group, QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
29. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ (2001) 323:157–62. doi: 10.1136/bmj.323.7305.157
30. Takwoingi Y, Dendukuri N, Schiller I, Rücker G, Jones HE, Partlett C, et al. Undertaking meta-analysis, cochrane handbook for systematic reviews of diagnostic test accuracy. Chichester (UK): John Wiley & Sons (2023). pp. 249–325.
31. Schunemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol (2020) 122:142–52. doi: 10.1016/j.jclinepi.2019.12.021
32. Schunemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J Clin Epidemiol (2020) 122:129–41. doi: 10.1016/j.jclinepi.2019.12.020
33. Yanovski JA, Cutler GB Jr., Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA (1993) 269:2232–8. doi: 10.1001/jama.1993.03500170062035
34. Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur J Endocrinol (2014) 170:477–86. doi: 10.1530/EJE-13-0702
35. Chrousos GP, Schuermeyer TH, Doppman J, Oldfield EH, Schulte HM, Gold PW, et al. NIH conference. Clinical applications of corticotropin-releasing factor. Ann Intern Med (1985) 102:344–58. doi: 10.7326/0003-4819-102-3-344
36. Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med (1986) 314:1329–35. doi: 10.1056/NEJM198605223142101
37. Grossman A, Howlett TA, Kopelman PG. The use of CRF-41 in the differential diagnosis of Cushing’s syndrome and obesity. Horm Metab Res Suppl (1987) 16:62–4.
38. Jaeckle RS, Lopez JF. Corticotropin releasing hormone: clinical endocrinology and implications for Cushing’s disease and endogenous depression. Psychiatr Med (1985) 3:111–34.
39. Arnaldi G, Tirabassi G, Papa R, Furlani G, Trementino L, Cardinaletti M, et al. Human corticotropin releasing hormone test performance in the differential diagnosis between Cushing’s disease and pseudo-Cushing state is enhanced by combined ACTH and cortisol analysis. Eur J Endocrinol (2009) 160:891–8. doi: 10.1530/EJE-09-0125
40. Rollin GA, Costenaro F, Gerchman F, Rodrigues TC, Czepielewski MA. Evaluation of the DDAVP test in the diagnosis of Cushing’s disease. Clin Endocrinol (Oxf) (2015) 82:793–800. doi: 10.1111/cen.12661
41. Malerbi DA, Fragoso MC, Vieira Filho AH, Brenlha EM, Mendonca BB. Cortisol and adrenocorticotropin response to desmopressin in women with Cushing’s disease compared with depressive illness. J Clin Endocrinol Metab (1996) 81:2233–7. doi: 10.1210/jcem.81.6.8964857
42. Tsagarakis S, Vasiliou V, Kokkoris P, Stavropoulos G, Thalassinos N. Assessment of cortisol and ACTH responses to the desmopressin test in patients with Cushing’s syndrome and simple obesity. Clin Endocrinol (Oxf) (1999) 51:473–7. doi: 10.1046/j.1365-2265.1999.00830.x
43. Tirabassi G, Papa R, Faloia E, Boscaro M, Arnaldi G. Corticotrophin-releasing hormone and desmopressin tests in the differential diagnosis between Cushing’s disease and pseudo-Cushing state: a comparative study. Clin Endocrinol (Oxf) (2011) 75:666–72. doi: 10.1111/j.1365-2265.2011.04096.x
44. Vilar L, Freitas Mda C, Faria M, Montenegro R, Casulari LA, Naves L, et al. Pitfalls in the diagnosis of Cushing’s syndrome. Arq Bras Endocrinol Metabol (2007) 51:1207–16. doi: 10.1590/S0004-27302007000800006
45. Vilar L, Naves LA, Freitas Mda C, Moura E, Canadas V, Leal E, et al. [Endogenous Cushing’s syndrome: clinical and laboratorial features in 73 cases]. Arq Bras Endocrinol Metabol (2007) 51:566–74. doi: 10.1590/S0004-27302007000400010
46. Testa RM, Albiger N, Occhi G, Sanguin F, Scanarini M, Berlucchi S, et al. The usefulness of combined biochemical tests in the diagnosis of Cushing’s disease with negative pituitary magnetic resonance imaging. Eur J Endocrinol (2007) 156:241–8. doi: 10.1530/eje.1.02332
47. Salgado LR, Fragoso MC, Knoepfelmacher M, MaChado MC, Domenice S, Pereira MA, et al. Ectopic ACTH syndrome: our experience with 25 cases. Eur J Endocrinol (2006) 155:725–33. doi: 10.1530/eje.1.02278
48. Suda T, Kageyama K, Nigawara T, Sakihara S. Evaluation of diagnostic tests for ACTH-dependent Cushing’s syndrome. Endocr J (2009) 56:469–76. doi: 10.1507/endocrj.K08E-353
49. Sakai Y, Horiba N, Tozawa F, Sakai K, Kuwayama A, Demura H, et al. Desmopressin stimulation test for diagnosis of ACTH-dependent Cushing’s syndrome. Endocr J (1997) 44:687–95. doi: 10.1507/endocrj.44.687
50. Coiro V, Volpi R, Capretti L, Caffarri G, Chiodera P. Desmopressin and hexarelin tests in alcohol-induced pseudo-Cushing’s syndrome. J Intern Med (2000) 247:667–73. doi: 10.1046/j.1365-2796.2000.00676.x
51. Moro M, Putignano P, Losa M, Invitti C, Maraschini C, Cavagnini F. The desmopressin test in the differential diagnosis between Cushing’s disease and pseudo-Cushing states. J Clin Endocrinol Metab (2000) 85:3569–74. doi: 10.1210/jcem.85.10.6862
52. Tirabassi G, Faloia E, Papa R, Furlani G, Boscaro M, Arnaldi G. Use of the desmopressin test in the differential diagnosis of pseudo-Cushing state from Cushing’s disease. J Clin Endocrinol Metab (2010) 95:1115–22. doi: 10.1210/jc.2009-1146
53. Pecori Giraldi F, Pivonello R, Ambrogio AG, De Martino MC, De Martin M, Scacchi M, et al. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test and the desmopressin test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. Clin Endocrinol (Oxf) (2007) 66:251–7. doi: 10.1111/j.1365-2265.2006.02717.x
54. Ferrante E, Barbot M, Serban AL, Ceccato F, Carosi G, Lizzul L, et al. Indication to dynamic and invasive testing in Cushing’s disease according to different neuroradiological findings. J Endocrinol Invest (2022) 45:629–37. doi: 10.1007/s40618-021-01695-1
55. Frete C, Corcuff JB, Kuhn E, Salenave S, Gaye D, Young J, et al. Non-invasive diagnostic strategy in ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab (2020) 105:3273–84. doi: 10.1210/clinem/dgaa409
56. Qiao J, Li J, Zhang W, Wang C, Li J, Jiang S, et al. The usefulness of the combined high-dose dexamethasone suppression test and desmopressin stimulation test in establishing the source of ACTH secretion in acth-dependent Cushing’s syndrome. Endocr J (2021) 68:839–48. doi: 10.1507/endocrj.EJ20-0837
57. Barbot M, Trementino L, Zilio M, Ceccato F, Albiger N, Daniele A, et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome. Pituitary (2016) 19:488–95. doi: 10.1007/s11102-016-0729-y
58. Colombo P, Passini E, Re T, Faglia G, Ambrosi B. Effect of desmopressin on ACTH and cortisol secretion in states of ACTH excess. Clin Endocrinol (Oxf) (1997) 46:661–8. doi: 10.1046/j.1365-2265.1997.1330954.x
59. Malerbi DA, Mendonca BB, Liberman B, Toledo SP, Corradini MC, Cunha-Neto MB, et al. The desmopressin stimulation test in the differential diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf) (1993) 38:463–72. doi: 10.1111/j.1365-2265.1993.tb00341.x
60. Marova EI, Goncharov NP, Kolesnikova GS, Arapova SD, Lapshina AM. The response of corticotropin and adrenal steroids to desmopressin stimulation in patients with various forms of hypercortisolism. Hormones (Athens) (2008) 7:243–50. doi: 10.14310/horm.2002.1204
61. Newell-Price J, Perry L, Medbak S, Monson J, Savage M, Besser M, et al. A combined test using desmopressin and corticotropin-releasing hormone in the differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab (1997) 82:176–81. doi: 10.1210/jcem.82.1.3674
62. Terzolo M, Reimondo G, Ali A, Borretta G, Cesario F, Pia A, et al. The limited value of the desmopressin test in the diagnostic approach to Cushing’s syndrome. Clin Endocrinol (Oxf) (2001) 54:609–16. doi: 10.1046/j.1365-2265.2001.01260.x
63. Tsagarakis S, Tsigos C, Vasiliou V, Tsiotra P, Kaskarelis J, Sotiropoulou C, et al. The desmopressin and combined CRH-desmopressin tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab (2002) 87:1646–53. doi: 10.1210/jcem.87.4.8358
64. Vilar L, Freitas MC, Naves LA, Canadas V, Albuquerque JL, Botelho CA, et al. The role of non-invasive dynamic tests in the diagnosis of Cushing’s syndrome. J Endocrinol Invest (2008) 31:1008–13. doi: 10.1007/BF03345640
65. Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg (2005) 79:16–20. doi: 10.1016/j.athoracsur.2004.09.040
66. Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess (2005) 9:1–113. doi: 10.3310/hta9120
67. Newell-Price J, Morris DG, Drake WM, Korbonits M, Monson JP, Besser GM, et al. Optimal response criteria for the human CRH test in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab (2002) 87:1640–5. doi: 10.1210/jcem.87.4.8357
68. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB Jr. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab (1993) 77:1308–12.
69. Elenius H, McGlotten R, Nieman LK. Ovine CRH stimulation and 8 mg dexamethasone suppression tests in 323 patients with ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab (2023) 109:e182–9. doi: 10.1210/clinem/dgad454
70. Aytug S, Laws ER Jr., Vance ML. Assessment of the utility of the high-dose dexamethasone suppression test in confirming the diagnosis of Cushing disease. Endocr Pract (2012) 18:152–7. doi: 10.4158/EP11179.OR
71. Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab (1997) 82:1780–5. doi: 10.1210/jc.82.6.1780
72. Hermus AR, Pieters GF, Pesman GJ, Smals AG, Benraad TJ, Kloppenborg PW. The corticotropin-releasing-hormone test versus the high-dose dexamethasone test in the differential diagnosis of Cushing’s syndrome. Lancet (1986) 2:540–4. doi: 10.1016/S0140-6736(86)90113-3
73. Tyrrell JB, Findling JW, Aron DC, Fitzgerald PA, Forsham PH. An overnight high-dose dexamethasone suppression test for rapid differential diagnosis of Cushing’s syndrome. Ann Intern Med (1986) 104:180–6. doi: 10.7326/0003-4819-104-2-180
74. Howlett TA, Drury PL, Perry L, Doniach I, Rees LH, Besser GM. Diagnosis and management of ACTH-dependent Cushing’s syndrome: comparison of the features in ectopic and pituitary ACTH production. Clin Endocrinol (Oxf) (1986) 24:699–713. doi: 10.1111/j.1365-2265.1986.tb01667.x
75. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol (2011) 64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011
76. Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol (2021) 9:847–75. doi: 10.1016/S2213-8587(21)00235-7
Keywords: Cushing syndrome, Cushing disease, pseudo-Cushing syndrome, non-neoplastic hypercortisolism, desmopressin test, systematic review
Citation: Giampietro RR, Cabral MVG, Pereira EG, Machado MC, Vilar L and Nunes-Nogueira VdS (2024) Accuracy of the 10 μg desmopressin test for differential diagnosis of Cushing syndrome: a systematic review and meta-analysis. Front. Endocrinol. 15:1332120. doi: 10.3389/fendo.2024.1332120
Received: 02 November 2023; Accepted: 09 January 2024;
Published: 30 January 2024.
Edited by:
Fabienne Langlois, Centre Hospitalier Universitaire de Sherbrooke, CanadaReviewed by:
Laurence Katznelson, Stanford University, United StatesCopyright © 2024 Giampietro, Cabral, Pereira, Machado, Vilar and Nunes-Nogueira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vania dos Santos Nunes-Nogueira, dmFuaWEubnVuZXMtbm9ndWVpcmFAdW5lc3AuYnI=
†ORCID: Vania dos Santos Nunes-Nogueira, orcid.org/0000-0001-9316-4167
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.