
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 May 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1326976
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj1†
Abdul K. Siraj1† Nabil Siraj1
Nabil Siraj1 Saeeda O. Ahmed1
Saeeda O. Ahmed1 Maha Al-Rasheed1
Maha Al-Rasheed1 Zeeshan Qadri1
Zeeshan Qadri1 Khawar Siddiqui2
Khawar Siddiqui2 Saif S. Al-Sobhi3
Saif S. Al-Sobhi3 Fouad Al-Dayel4
Fouad Al-Dayel4 Khawla S. Al-Kuraya1*‡
Khawla S. Al-Kuraya1*‡Background: Radioactive iodine refractory differentiated thyroid cancer (RAIR-DTC) has received increasing attention due to its poor prognosis. However, outcomes may vary among patients with RAIR-DTC. The role of clinico-pathological and molecular prognostic factors in survival remains controversial, resulting in difficulty in selecting patients for new targeted therapies. We assessed mortality rate and DTC-specific survival in Middle Eastern RAIR-DTC to identify prognostic factors associated with survival.
Methods: This single center, retrospective study enrolled 268 patients with RAIR-DTC. Mortality rate and DTC-specific survival were analyzed to identify prognostic factors related to survival. Univariate and multivariate analysis were performed using Cox proportional hazards model.
Results: Of the 268 cases of RAIR-DTC, 40.3% (108/268) had absent 131I uptake (either on diagnostic or post-therapy whole body scan), 15.3% (41/268) had progressive disease (PD) despite 131I, 7.5% (20/268) had persistent disease despite cumulative activity of I131 of >600 mCi and 36.9% (n=99/268) developed distant metastasis. On multivariate analysis, age (more than 45 years), presence of metastatic disease and tumors harboring telomerase reverse transcriptase (TERT) promoter mutations were independent prognostic factors for poor DTC-specific survival. Subjects were divided into 3 groups according to the number of risk factors; low risk (no risk factors); intermediate (≤ 2 risk factors); and high risk (all the 3 risk factors). Ten-year DTC-specific survival rates in low, intermediate and high-risk groups were 100.0%, 92.9% and 53.6%, respectively.
Conclusions: The contribution of age greater than 45 years to RAIR-DTC mortality is impactful. Older age, presence of distant metastasis and TERT mutations could be used as early predictors of RAIR-DTC cases. The identification of prognostic factors for poor survival in RAIR-DTC may improve the selection of patients for more personalized surveillance and therapeutic modalities.
Recently, radioactive iodine refractory differentiated thyroid cancer (RAIR-DTC) has imposed a significant challenge as a result of increasing number of patients with DTC around the world (1). DTC, including Papillary Thyroid Carcinoma (PTC), Follicular Thyroid carcinoma (FTC), and Hurthle Cell Carcinoma (HCC), accounts for about 90% of all thyroid cancer (2–4). Most DTC patients can be treated successfully by surgery and radioactive iodine (RAI) with favorable outcome. Despite the favorable prognosis, recurrence and distant metastases occur in 2 – 30% of DTCs (5–7). Among these patients, unfortunately, a significant number show loss of iodine uptake (8). The efficacy of RAI therapy is largely influenced by the ability of tumors to take up radioiodine (9–11). A long-term study showed that 10- and 15-years survival rate in RAIR-DTC were much lower than those of DTC patients with RAI uptake (10% vs 56% and 6 vs 45%, respectively) (9). RAIR patients represent a great therapeutic challenge due to the limited alternative therapeutic options (12, 13). Therefore, understanding non-radioiodine avidity and identification of risk factor that help in early prediction of RAIR-DTC is of great clinical importance in avoiding unnecessary RAI therapy and help in the decision of subsequent feasible targeted therapy.
Patients’ age and other clinico-pathological as well as molecular risk factors for RAIR DTC have been explored in several studies with controversial results (14–18). Furthermore, the clinico-pathological associations, molecular features and prognostic impact of RAIR-DTC in Middle Eastern ethnicity has not been clarified. Therefore, we conducted this retrospective study to identify risk factors affecting DTC-specific survival in RAIR disease and risk stratification was also attempted based on the identified risk factors.
Two-hundred and sixty eight RAIR DTC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. The main inclusion criteria were histology of DTC (papillary or follicular cancer) and disease classified as RAIR after at least one dose of 131I treatment. The Institutional Review Board of the hospital approved this study and since only retrospective patient data were used, the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031. The study was conducted in accordance with the Declaration of Helsinki.
Based on the recently published joint consensus from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches, and current literature (18–21), DTCs were classified as RAI refractory, if any of the following were fulfilled:
1. 131I uptake absent on diagnostic 131I scan of locoregional recurrence or distant metastasis.
2. 131I uptake absent on 131I scan, performed several days after 131I treatment.
3. 131I uptake present in some, but not all tumor foci.
4. Disease progression despite a cumulative 131I activity of ≥600mCi.
5. Metastatic disease progression despite 131I uptake.
6. Rising serum thyroglobulin levels ≥6 months after 131I treatment.
7. Structural disease progression after 131I treatment.
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Staging of DTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system (22). The patients were seen 6 to 8 weeks after surgery, having been prepared with thyroid hormone withdrawal for at least four weeks and low-iodine diet for one week, in order to achieve a target TSH level of >30µIU/mL. A diagnostic radioactive iodine (I-123) whole body scan (DxWBS) and neck ultrasonography were performed, and stimulated thyroglobulin (sTg), anti-Tg antibodies, TSH and free T4 were measured. Radioactive iodine (I-131) was administered at activities that averaged 30–100 mCi for thyroid remnant ablation and 100–200 mCi for patients with lymph node or distant metastases. The study endpoint for our analysis was DTC-specific survival, defined as the time (in years) from date of initial surgery to the date of death due to progression of DTC.
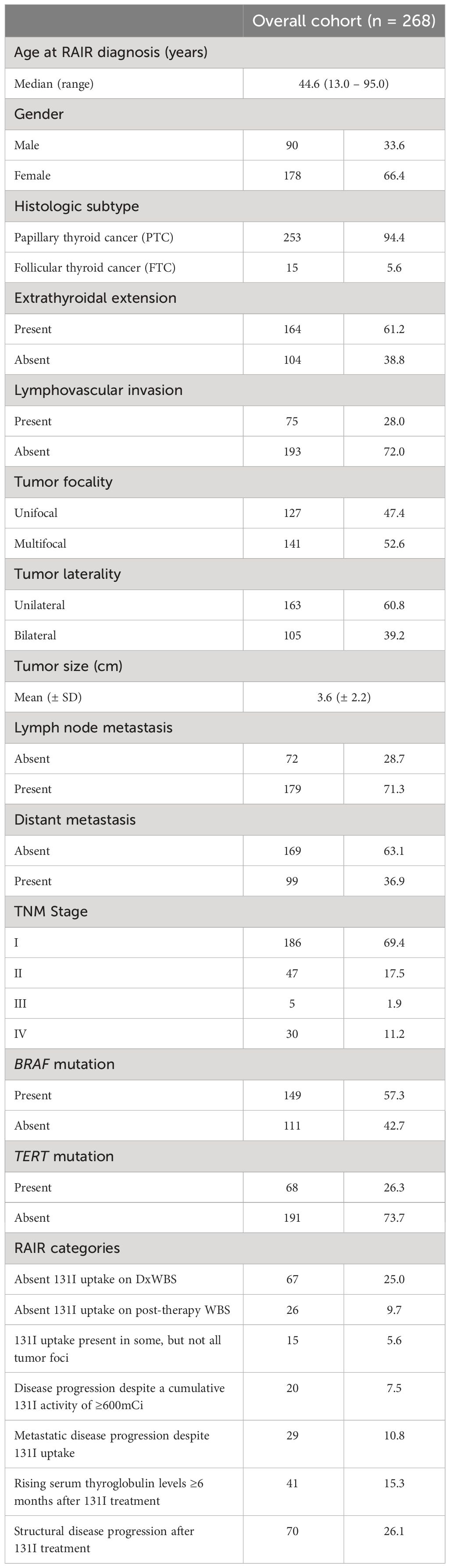
Table 1 Patient characteristics of the radioactive iodine refractory differentiated thyroid cancer (RAIR-DTC).
DNAs were extracted from PTC formalin-fixed and paraffin-embedded (FFPE) tumor tissues utilizing Gentra DNA isolation kit (Gentra, Minneapolis, MN, USA) according to manufacturer’s protocols as elaborated in the previous studies (23).
PCR and Sanger sequencing analysis of the promoter region in TERT gene were carried out as described previously (24). Primer 3 online software was utilized to design the primers (available upon request). Reference sequences were downloaded from NCBI GenBank. Sequencing results were compared with the reference sequence by Mutation Surveyor V4.04 (Soft Genetics, LLC, State College, PA).
DTC-specific survival rates were calculated by the Kaplan-Meier method. Cox proportional hazards model was used for analyzing the impact of prognostic factors on DTC-specific survival in univariate and multivariate manner. Risk stratification was performed according to the factors related to survival. Limit of significance was defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Median age at RAIR diagnosis for the entire cohort was 44.6 years (range = 13 – 95 years), with a male: female ratio of 1:2. Majority of the tumors were PTC (94.4%; 253/268). Extrathyroidal extension was noted in 61.2% (164/268) of cases and lymphovascular invasion in 28.0% (75/268). 52.6% (141/268) of PTCs were multifocal and 39.2% (105/268) were bilateral. Lymph node metastasis was noted in 71.3% (179/251) cases. BRAF mutation analysis was performed in 260 cases and TERT mutation analysis in 259 cases, with mutations noted in 57.3% (149/260) and 26.3% (68/259) cases, respectively (Table 1).
The DTC-specific mortality rate in the entire cohort was 6.7% (18/268). Figure 1 shows the DTC-specific mortality rate for different age groups. As shown in Figure 2, before the age of 45 years, the mortality rates (percentages of deaths in the cohort) were low in the entire cohort. After the age of 45 years, mortality rates increased as patient age increased in all patients (Figure 2A). Accumulated mortality rates also increased continuously after age 45 years in all patients (Figure 2B).
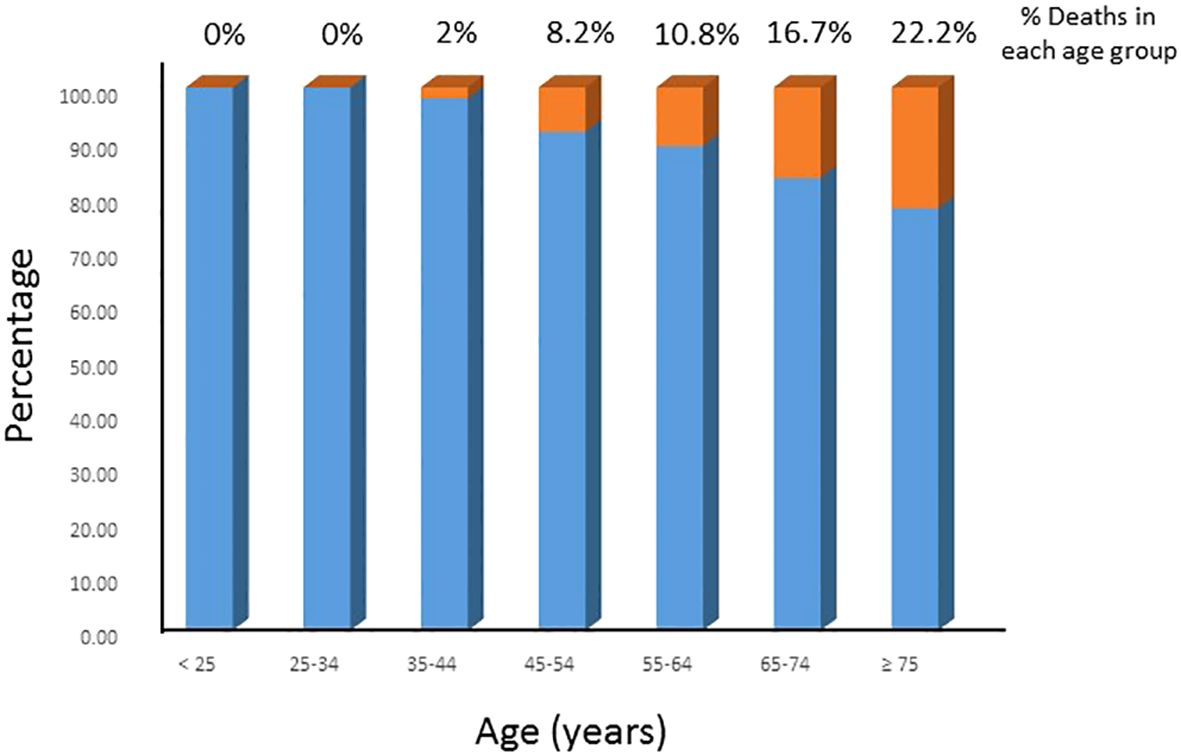
Figure 1 DTC-specific mortality rate in different age groups. The DTC-specific mortality rates increased with increasing age in the entire cohort.
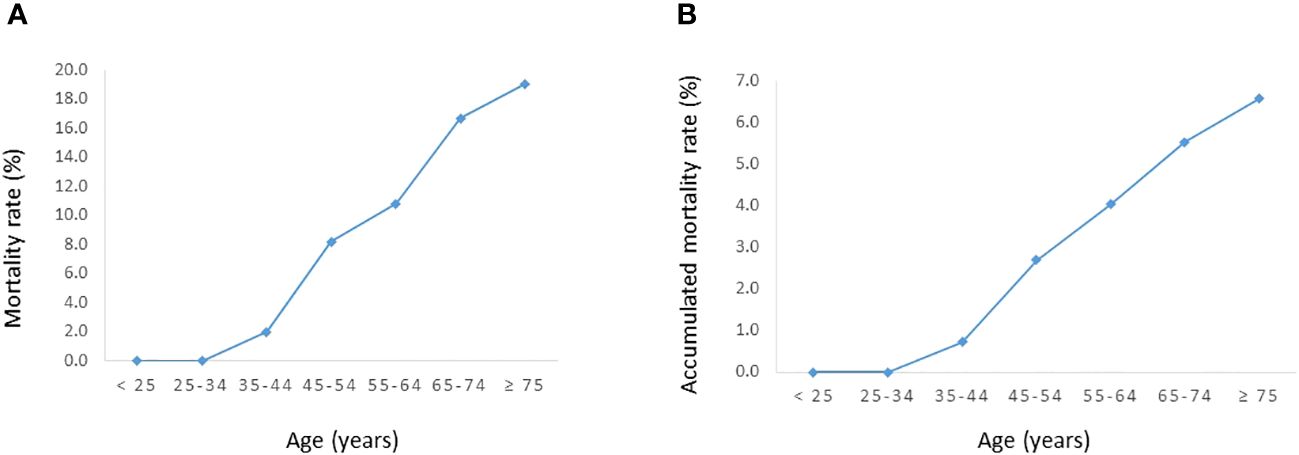
Figure 2 Relationship between age and DTC-specific mortality rates in the entire cohort. (A) Mortality rate. (B) Accumulated mortality rate.
We further sought to determine the optimal age cutoff for predicting DTC-specific mortality. Using the Contal and O’Quigley method for DTC-specific mortality, the optimal age cutoff was determined to be 44 years (Figure 3). Based on the above findings and since the median age of our cohort was also close to 45 years, we used the age cut-off of 45 years for subsequent univariate and multivariate analysis.
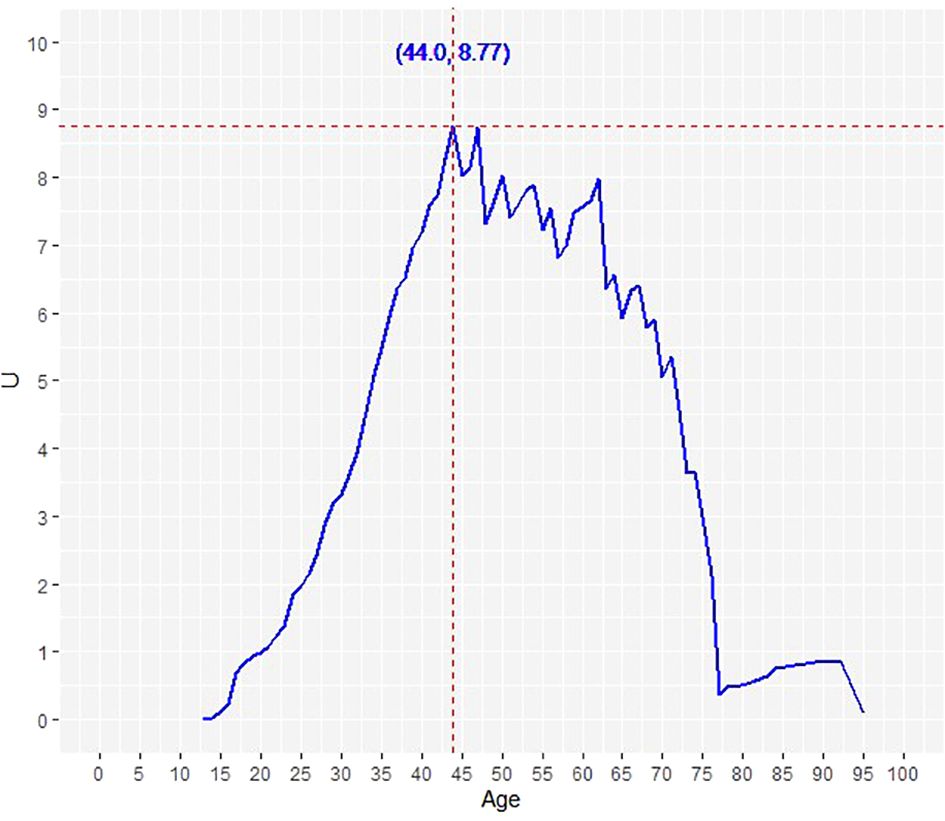
Figure 3 Determination of the optimal age cut-off point for DTC-specific survival using Contal and O’Quigley’s method. The dashed line demarcates the optimal age cut-off point: 44.0 years.
The 5- and 10-year DTC-specific survival rates for the entire cohort were 93.4% and 89.3%, respectively (Figure 4A). On univariate analysis, age ≥45 years (p < 0.0001), tumor size (p = 0.0052), distant metastasis (p < 0.0001) and TERT mutation (p < 0.0001) were significantly related to DTC-specific survival (Table 2). However, on multivariant analysis, age ≥45 years (Hazard ratio (HR) = 7.41; 95% confidence interval (CI) = 1.20 – 142.58; p = 0.0295), distant metastasis (HR = 3.43; 95% CI = 1.31 – 10.74; p = 0.0110) and TERT mutation (HR = 3.96; 95% CI = 1.53 – 12.14; p = 0.0036) were found to be independent predictive markers of poor DTC-specific survival in this cohort (Table 2).
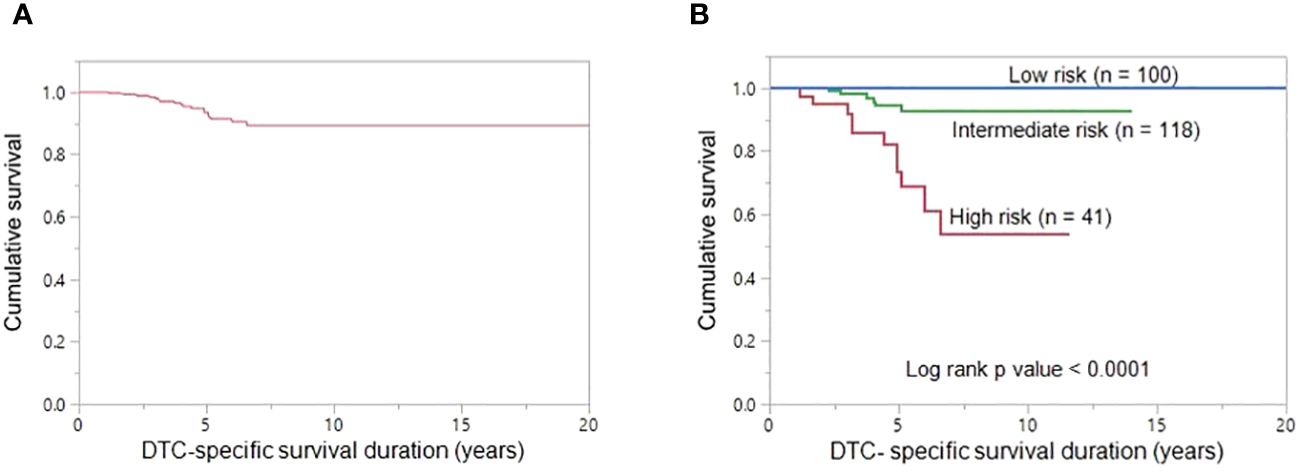
Figure 4 DTC-specific survival. (A) The 5- and 10-year DTC-specific survival rates in the entire cohort (n = 268) were 93.4% and 89.3%, respectively. (B) The 10-year DTC-specific survival rates in the low-, intermediate-, and high-risk groups are 100.0%, 92.9% and 53.6%, respectively. DTC-specific survival is significantly better in low-risk group patients than in high-risk group patients and intermediate-risk group patients (p < 0.0001).
Based on the number of independent risk factors, patients were divided into 3 groups: low risk (no risk factors); intermediate risk (≤ two risk factors); and high risk (all three risk factors). Risk stratification was performed for 259 patients for whom TERT mutation data was available. 38.6% (100/259), 45.6% (118/259) and 15.8% (41/259) of patients were classified as low-, intermediate- and high-risk, respectively. 10-year DTC-specific survival rates in the low, intermediate and high risk groups were 100.0%, 92.9% and 53.6%, respectively (p < 0.0001) (Figure 4B).
Recently, RAIR-DTC has received increasing attention due to its impact on patient survival. Better understanding of early predictors of RAIR-DTC is of great clinical importance to prevent unnecessary repeated use of radioactive iodine therapy and help physicians in tailoring patients’ surveillance and exploring other alternative modalities.
Age is a well-established prognosticator and mortality risk factor in general (25–27). Upon exploring the relationship between patient age and disease-specific survival in DTC, emerging data have appeared to question the appropriateness of dichotomizing age, given the inconsistency of published data on this subject (25–31). A recent study has analyzed the influence of age on survival of patients with RAIR-DTC and identified cutoff age of 45 years as being predictive of overall survival (14).
Our current study explored the relationship between age and DTC-specific mortality in RAIR-DTC. In unadjusted analysis, increasing patient age was associated with progressive increase in mortality. Before age of 45 years, the mortality rates were low in all age groups. However, after the age of 45 years, mortality rate and accumulated mortality rate increased continuously in all patients. We used a cutoff age of 45, which is close to the median age of our cohort. Furthermore, using statistical adjustments between young and older group, we attempted to establish the cutoff point for DTC-specific survival using Contal and O’Quigley’s method. Interestingly, the statistically suggested cutoff point for DTC-specific survival was 44 years, which further supports our suggested cutoff point of 45 years. Using this value, we performed multivariate analysis to identify clinico-pathological and molecular risk factors for reduced DTC-specific survival.
In this series, we identified older age (> 45 years), presence of distant metastasis and TERT promoter mutations as independent predictors for poor DTC-specific survival. Previous studies have shown the correlation between older age and poor patient outcome in RAIR-DTC (14, 32). Aggressive tumors, especially the presence of metastasis, is a useful feature for indicating worse prognosis in DTC (33, 34). TERT promoter mutation has been reported by us and other groups to be associated with aggressive clinico-pathological characteristics, including its association with RAIR-DTC (35–37). A recent study examined the status of TERT mutation in distant metastatic DTC and its association with RAI uptake as well as therapy response, and identified TERT mutation as having a greater negative influence on RAI uptake compared to BRAF mutation (38).
According to the number of significant risk factors related to poor DTC-specific survival, we attempted to perform risk stratification; low risk group were defined as patients having no risk factor, intermediate risk as patients having one or two risk factors and patients having all three risk factors combined were classified as high risk. Interestingly, based on the risk stratification, there was a significant difference in mortality between low-, intermediate- and high-risk groups, with the 10-year DTC-specific survival rates being 100.0%, 92.9% and 53.6%, respectively.
Due to the limited number of patients, our risk stratification should be interpreted with caution. However, risk adapted management of RAIR-DTC should be explored. Limitations caused by retrospective design of our study and modification to RAIR-DTC management over extensive follow-up period of more than 20 years cannot be ruled out. A prospective well-standardized study may help in providing more accurate information. In conclusion, older age, distant metastasis and TERT mutation are independent predictors of RAIR-DTC in Middle Eastern ethnicity. These markers could improve assessment of prognosis in RAIR-DTC patients and thus help clinicians in the selection of optimum therapeutic modalities, such as aggressive treatment and follow-up for those with worse DTC-specific survival.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Research Advisory Council, King Faisal Specialist Hospital and Research Centre. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SP: Conceptualization, Data curation, Investigation, Validation, Writing – review & editing. AS: Conceptualization, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. NS: Investigation, Methodology, Writing – review & editing. SA: Data curation, Investigation, Methodology, Writing – review & editing. MA: Investigation, Methodology, Writing – review & editing. ZQ: Formal Analysis, Methodology, Software, Writing – review & editing. KS: Formal Analysis, Software, Writing – review & editing. SA: Resources, Writing – review & editing. FA: Resources, Writing – review & editing. KA: Conceptualization, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Kaleem Iqbal and Padmanaban Annaiyappanaidu for their technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
2. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. New Engl J Med. (2016) 375:1054–67. doi: 10.1056/NEJMra1501993
3. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. Jama. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
4. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes endocrinol. (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9
5. Goffredo P, Sosa JA, Roman SA. Differentiated thyroid cancer presenting with distant metastases: a population analysis over two decades. World J surge. (2013) 37:1599–605. doi: 10.1007/s00268-013-2006-9
6. Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. (2015) 112:149–54. doi: 10.1002/jso.23967
7. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin endocrinol. (2005) 63:87–93. doi: 10.1111/j.1365-2265.2005.02304.x
8. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes endocrinol. (2014) 2:356–8. doi: 10.1016/S2213-8587(13)70215-8
9. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli J, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
10. Zhao D, Jin X, Li F, Liang J, Lin Y. Integrin αvβ3 imaging of radioactive iodine–refractory thyroid cancer using 99mTc-3PRGD2. J Nucl Med. (2012) 53:1872–7. doi: 10.2967/jnumed.112.107821
11. De la Vieja A, Riesco-Eizaguirre G. Radio-iodide treatment: from molecular aspects to the clinical view. Cancers. (2021) 13:995. doi: 10.3390/cancers13050995
12. Satapathy S, Bal C. Theranostic options for radioiodine-refractory differentiated thyroid carcinoma: recent advances, challenges, and road ahead. Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.924841
13. Silaghi H, Lozovanu V, Georgescu CE, Pop C, Nasui BA, Cătoi AF, et al. State of the art in the current management and future directions of targeted therapy for differentiated thyroid cancer. Int J Mol Sci. (2022) 23:3470. doi: 10.3390/ijms23073470
14. Saïe C, Wassermann J, Mathy E, Chereau N, Leenhardt L, Du Montcel ST, et al. Impact of age on survival in radioiodine refractory differentiated thyroid cancer patients. Eur J Endocrinol. (2021) 184:667–76. doi: 10.1530/EJE-20-1073
15. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
16. Liu Y, Wang Y, Zhang W. Scoring system and a simple nomogram for predicting radioiodine refractory differentiated thyroid cancer: a retrospective study. EJNMMI Res. (2022) 12:1–14. doi: 10.1186/s13550-022-00917-8
17. Meng C, Song J, Long W, Mu Z, Sun Y, Liang J, et al. A user-friendly nomogram for predicting radioiodine refractory differentiated thyroid cancer. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1109439
18. Hurst Z, Liyanarachchi S, He H, Brock P, Sipos J, Nabhan F, et al. Risk haplotypes uniquely associated with radioiodine-refractory thyroid cancer patients of high African ancestry. Thyroid. (2019) 29:530–9. doi: 10.1089/thy.2018.0687
19. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
20. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. 140 Huguenot Street, 3rd Floor New: Mary Ann Liebert, Inc., publishers (2019) p. 461–70.
21. Gulec SA, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Draganescu C, et al. A joint statement from the American thyroid association, the European association of nuclear medicine, the European thyroid association, the society of nuclear medicine and molecular imaging on current diagnostic and theranostic approaches in the management of thyroid cancer. Thyroid. (2021) 31:1009–19. doi: 10.1089/thy.2020.0826
22. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
23. Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. (2008) 93:611–8. doi: 10.1210/jc.2007-1717
24. Bu R, Siraj AK, Al-Obaisi KA, Beg S, Al Hazmi M, Ajarim D, et al. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int J Cancer. (2016) 139:1091–7. doi: 10.1002/ijc.30143
25. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. (2015) 25:1106–14. doi: 10.1089/thy.2015.0104
26. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. (2016) 34:4415. doi: 10.1200/JCO.2016.68.9372
27. Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. (2015) 25:125–32. doi: 10.1089/thy.2014.0116
28. Shi R-l, Qu N, Liao T, Wei W-j, Wang Y-L, Ji Q-h. The trend of age-group effect on prognosis in differentiated thyroid cancer. Sci Rep. (2016) 6:1–8. doi: 10.1038/srep27086
29. Bischoff LA, Curry J, Ahmed I, Pribitkin E, Miller JL. Is above age 45 appropriate for upstaging well-differentiated papillary thyroid cancer? Endocr Pract. (2013) 19:995–7. doi: 10.4158/EP13029.OR
30. Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E, et al. Patient age–associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. (2018) 36:438. doi: 10.1200/JCO.2017.74.5497
31. Trimboli P, Piccardo A, Signore A, Valabrega S, Barnabei A, Santolamazza G, et al. Patient age is an independent risk factor of relapse of differentiated thyroid carcinoma and improves the performance of the American Thyroid Association stratification system. Thyroid. (2020) 30:713–9. doi: 10.1089/thy.2019.0688
32. Ito Y, Miyauchi A, Ito M, Yabuta T, Masuoka H, Higashiyama T, et al. Prognosis and prognostic factors of differentiated thyroid carcinoma after the appearance of metastasis refractory to radioactive iodine therapy. Endocr J. (2014) 61:821–4. doi: 10.1507/endocrj.EJ14-0181
33. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. (2012) 22:884–9. doi: 10.1089/thy.2011.0535
34. Gillanders S, O’Neill J. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur J Surg Oncol. (2018) 44:286–96. doi: 10.1016/j.ejso.2017.07.013
35. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase reverse transcriptase mutations are independent predictor of disease-free survival in M iddle E astern papillary thyroid cancer. Int J cancer. (2018) 142:2028–39. doi: 10.1002/ijc.31225
36. Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine. (2020) 67:44–57. doi: 10.1007/s12020-019-02117-2
37. Meng Z, Matsuse M, Saenko V, Yamashita S, Ren P, Zheng X, et al. TERT promoter mutation in primary papillary thyroid carcinoma lesions predicts absent or lower 131I uptake in metastases. IUBMB Life. (2019) 71:1030–40. doi: 10.1002/iub.2056
Keywords: differentiated thyroid cancer, radioactive iodine refractory, risk factors, TERT mutation, DTC-specific survival
Citation: Parvathareddy SK, Siraj AK, Siraj N, Ahmed SO, Al-Rasheed M, Qadri Z, Siddiqui K, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2024) Radioactive iodine refractoriness in Middle Eastern differentiated thyroid cancer: clinical outcome and risk factor analysis. Front. Endocrinol. 15:1326976. doi: 10.3389/fendo.2024.1326976
Received: 24 October 2023; Accepted: 30 April 2024;
Published: 15 May 2024.
Edited by:
Yin Detao, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Flávia Dornelas Kurkowski, Pontifical Catholic University of Rio Grande do Sul, BrazilCopyright © 2024 Parvathareddy, Siraj, Siraj, Ahmed, Al-Rasheed, Qadri, Siddiqui, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
‡ORCID: Khawla S. Al-Kuraya, orcid.org/0000-0002-4126-3419
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.