- 1Department of Hepatobiliary and Pancreaticosplenic Surgery, Beijing Organ Transplant Center, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 2Department of Hepatobiliary Surgery, China-Japan Friendship Hospital, Beijing, China
Purpose: Whether the diagnosis of non-alcoholic fatty liver disease or metabolic dysfunction-associated fatty disease has a different impact on liver transplant recipients with hepatocellular carcinoma is not yet clear.
Methods: Data from a two-center retrospective cohort study were collected to compare and investigate the differences between non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease in clinicopathologic parameters and prognosis among liver transplant recipients with hepatocellular carcinoma.
Results: A total of 268 liver transplant recipients with hepatocellular carcinoma were included. The prevalence among pre- and post-transplant metabolic dysfunction-associated fatty liver disease was 10.82% and 30.22%, while for non-alcoholic fatty liver disease, it was 7.09% and 26.87%, respectively. The clinicopathological parameters were similar between the two pre-transplant groups. In contrast, the post-transplant group with metabolic dysfunction-associated fatty liver disease exhibited a higher prevalence of diabetes mellitus and a greater body mass index. However, the other parameters were similar between the two post-transplant groups (p > 0.05). Factors such as the largest tumor size > 4 cm, microvascular invasion, lack of tumor capsule, post-transplant metabolic dysfunction-associated fatty liver disease, and decreased post-transplant lymphocyte percentage were related to an increased risk of recurrence.
Conclusion: In patients undergone liver transplantation for hepatocellular carcinoma, the diagnosis of metabolic dysfunction-associated fatty disease is more strongly associated with metabolic abnormalities than the diagnosis of non-alcoholic fatty liver disease and is an independent predictor of hepatocellular carcinoma recurrence.
Introduction
Hepatocellular carcinoma (HCC), currently the sixth most common tumor worldwide, ranks fourth in terms of tumor mortality (1). Major risk factors contributing to the occurrence and development of HCC have been well established, including infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), excessive alcohol consumption, and exposure to fungal metabolite (2, 3). Moreover, growing evidence suggests that metabolic risk factors, such as steatosis, obesity, and metabolic syndrome, can collectively contribute to the development of HCC, with a rising prevalence of HCC related to metabolic dysfunction (4, 5). Additionally, metabolic dysfunction can lead to concurrent diseases such as type 2 diabetes mellitus (T2DM), chronic kidney disease, cardiovascular disease (CVD), certain extrahepatic cancers, and severe liver-related complications (6). Non-alcoholic fatty liver disease (NAFLD), which is associated with metabolic dysfunction (7, 8), has emerged as a significant contributor to liver-related morbidity and mortality globally, with a prevalence of approximately 25% (9, 10). Moreover, NAFLD is one of the major indications for liver transplantation (10). However, NAFLD stands out in its diagnostic approach, often failing to account for the influence of coexisting metabolic dysfunction and various liver disease etiologies (11).
Recently, the concept of metabolic dysfunction-associated fatty liver disease (MAFLD) has been introduced by international consensus, focusing on its relevance to the underlying conditions of systemic metabolic dysfunction (12, 13). The new criteria define MAFLD as hepatic steatosis together with the presence of metabolic conditions (T2DM, obesity/overweight, or at least two metabolic abnormalities). Although MAFLD is presumed to have a stronger association with metabolic syndrome than NAFLD (14), understanding the application of this new terminology in liver transplantation remains limited. Moreover, the differential impacts of MAFLD and NAFLD on the pathological characteristics and outcomes of liver transplant recipients (LTR) with HCC have not been thoroughly explored in the existing literature.
Hence, we conducted a two-center retrospective study on LTR with HCC to investigate their clinicopathological data and prognosis, aiming to assess the respective influences of MAFLD and NAFLD on these patients.
Materials and methods
Study design and participants
This retrospective cohort study was conducted at Beijing Chaoyang Hospital (March 2011 and December 2021) and China-Japan Friendship Hospital (February 2018 and December 2021) involving LTR with HCC who underwent liver transplantation. The study was approved by the Institutional Review Board of both hospitals (No.2022-D-115) in accordance with the 1964 Helsinki Declaration and its later amendments. Informed consent was not required, given the retrospective nature of the study.
Inclusion criteria and exclusion criteria
Inclusion criteria for LTR encompassed histopathologically confirmed HCC without distant metastases, undergoing liver transplantation, and a follow-up period of at least 6 months. Exclusion criteria consisted of combined-organ transplantation, liver retransplantation, presence of any other type of tumor, and missing data for a MAFLD diagnosis.
Definitions
MAFLD diagnosis was based on hepatic steatosis in conjunction with one of the following three conditions: a body mass index (BMI) ≥ 23 kg/m2 in Asians, T2DM, or metabolic dysregulation (12, 13). T2DM was defined as a history of diabetes, and/or fasting plasma glucose ≥ 7.0 mmol/L, and/or two-hour post-load plasma glucose ≥ 11.1 mmol/L, and/or HbA1c ≥ 6.5% (15). Metabolic dysregulation was characterized by having at least two of the following metabolic conditions: waist circumference ≥ 90 in Asian men and ≥ 80 cm in Asian women; blood pressure ≥ 130/85 mmHg or receiving drug treatment; plasma triglycerides ≥ 1.70 mmol/L or receiving drug treatment; plasma high-density lipoprotein (HDL)-cholesterol < 1.0 mmol/L for males and < 1.3 mmol/L for females or receiving drug treatment; prediabetes (fasting plasma glucose levels 5.6-6.9 mmol/L, or two-hour post-load plasma glucose levels 7.8-11.0 mmol/L or HbA1c 5.7% to 6.4%).
NAFLD was diagnosed when hepatic steatosis was present, and secondary causes, such as excessive alcohol intake, viral hepatitis, autoimmune liver disease, parenteral nutrition, genetic disorders, hepatic malignancies, hepatobiliary infections, biliary tract diseases, medications, and starvation, were excluded (16). Alcohol intake was limited to ≤ 20 g/d for males and ≤ 10 g/d for females to define non-alcohol related liver conditions.
Hepatic steatosis was assessed through histopathology post-surgery for the recipient’s liver and via abdominal ultrasonography during follow-up for LTR. The diagnosis was conducted by two experienced pathologists and sonologists, who were blinded to each other’s evaluation.
Data collection
Data collection was carried out through medical records and follow-up visits until December 2022. The following clinical variables were obtained: age, sex, alcohol intake, smoking (≥ 1 cigarette/d), body mass index, waist circumference, Child-Pugh grade, model of end-stage liver disease score (MELD score), immunosuppressive regimen, tumor-free survival, and overall survival in addition to histories of tumor therapy, CVD, hypertension and DM. CVD was comprised of coronary heart disease and stroke (17). Histopathological parameters included the largest tumor size, number of tumors, total tumor size, and tumor differentiation. The presence of tumor capsule, hepatic cirrhosis, hepatic capsule invasion, microvascular invasion, macrovascular invasion, and tumor within Milan criteria (18) was also noted. Laboratory measurements contained total bilirubin levels, aspartate aminotransferase levels, alanine transaminase levels, albumin levels, triglyceride levels, high-density lipoprotein cholesterol levels, fasting plasma glucose levels, alpha-fetoprotein levels, creatinine levels, neutrophil count, neutrophil percentage, lymphocyte count, lymphocyte percentage, neutrophil-to-lymphocyte count ratio.
Follow-up
Patient follow-up visits were scheduled at three-month intervals during the first postoperative year, semi-annually during the second postoperative year, and annually thereafter. The follow-up period began on the day of patient discharge and ended either on the date of tumor recurrence or the closing date of follow-up. The primary objective of the study was to investigate the occurrence of MAFLD and NAFLD post-transplantation, with the secondary aim being to evaluate tumor-free survival.
Statistical analysis
The normal distribution of continuous variables was tested using a Kolmogorov-Smirnov test. The independent samples t-test was employed for normally distributed variables, while the Wilcoxon ranksum test was selected for non-normally distributed variables. The Chi-square or Fisher’s exact test was used to compare categorical variables. The Kaplan-Meier method was used for survival analysis. The Cox regression model was employed for multifactor survival analysis. Data were analyzed using SPSS 19.0 computer software (IBM Corp., Armonk, NY, USA). The figures were generated using R 4.2.1 (https://www.R-project.org/). All statistical tests were two-sided, with statistical significance set at a P-value <0.05.
Results
Characteristics of LTR
A total of 268 patients with HCC, who underwent liver transplantation, were included in this study. The majority of patients were male (89.55%, n=234), with a mean age of 53.88 ± 9.05 years (range: 28~76 years). All LTR were of Asian descent. Prior to liver transplantation, 20.90% (n=56) of patients had DM while 17.91% (n=48) had hypertension. The mean BMI of LTR was 24.26 kg/m2. Following liver transplantation, the prevalence of DM and hypertension increased to 34.70% (n=93) and 27.61% (n=74), respectively, with a mean BMI of 23.42 kg/m2. During the follow-up period, 63 patients experienced tumor recurrence, while 204 LTR remained alive.
Overlap between pre- and post-transplant MAFLD and NAFLD
Before liver transplantation, 10.82% (29/268) of patients were diagnosed with MAFLD and 7.09% (19/268) with NAFLD. Among the 29 LTR with MAFLD, 15 also met the criteria for NAFLD. Following liver transplantation, the overall prevalence of MAFLD and NAFLD increased to 30.22% (81/268) and 26.87% (72/268), respectively. Among the 81 LTR with MAFLD, 41 also met the criteria for NAFLD. However, only a small number of LTR (n=3) fulfilled both criteria before and after liver transplantation (Figure 1).
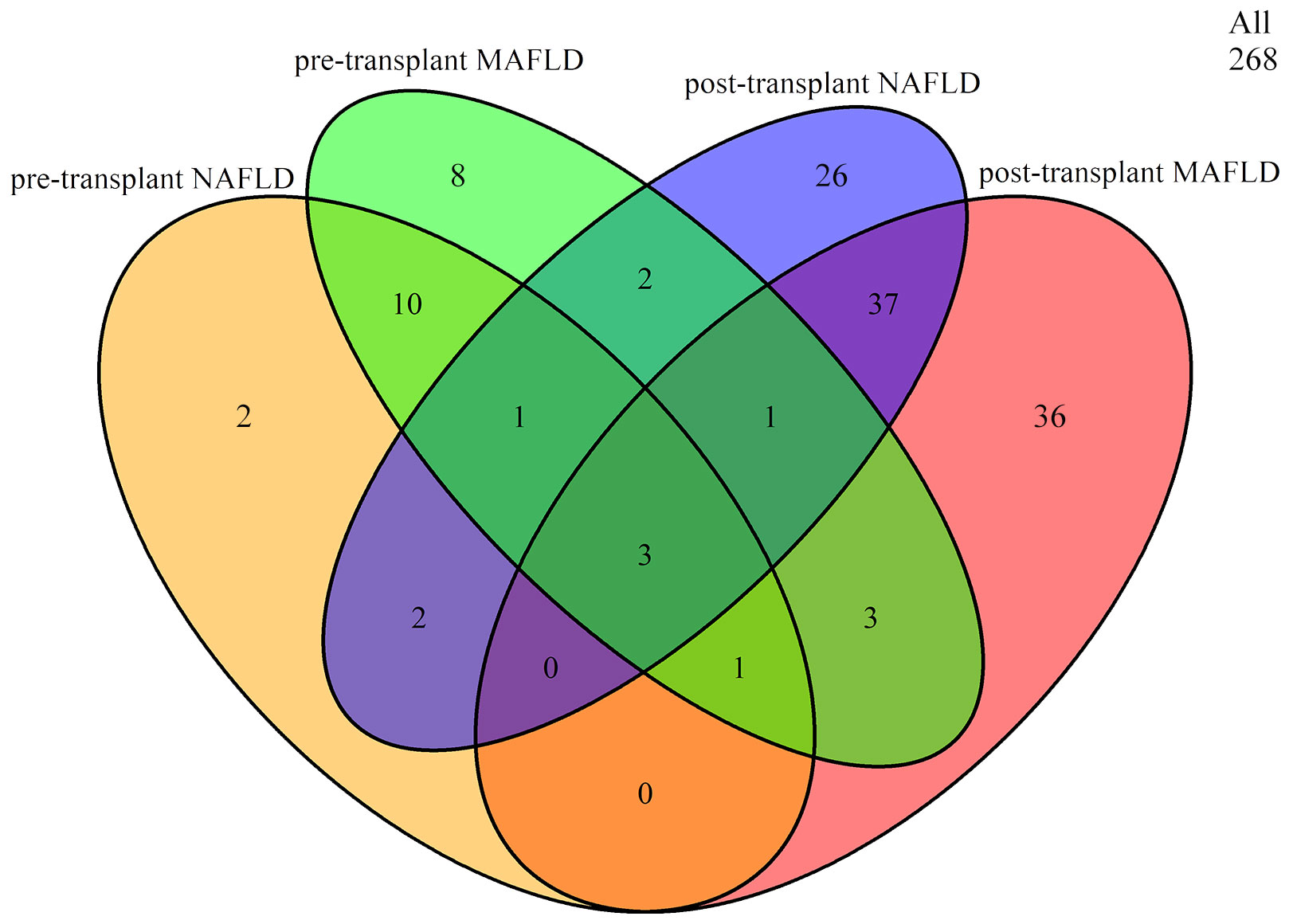
Figure 1 Overlap between pre- and post-transplant MAFLD and NAFLD. MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, non-alcoholic fatty liver disease.
Comparison between pre-transplant MAFLD and NAFLD
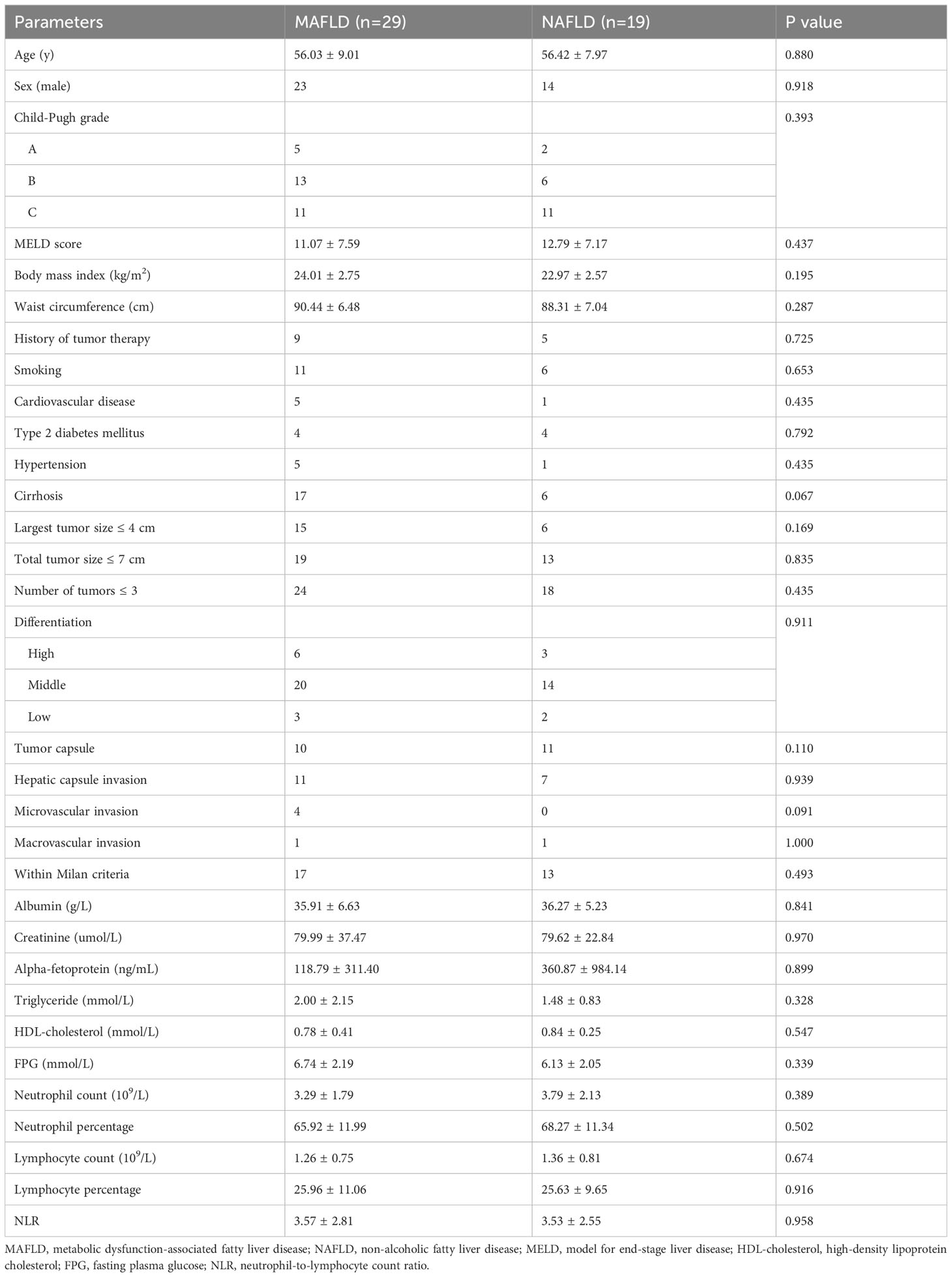
The clinical characteristics of the pre-transplant MAFLD and NAFLD groups are described in Table 1. The two groups exhibited similar histopathological and laboratory features, including age, sex, Child-Pugh grade, MELD score, body mass index, waist circumference, history of tumor therapy, smoking, CVD, T2DM, hypertension, cirrhosis, largest tumor size, total tumor size, number of tumors, tumor differentiation, presence of tumor capsule, hepatic capsule invasion, microvascular invasion, macrovascular invasion, HCC within Milan criteria, serum albumin levels, serum creatinine levels, serum alpha-fetoprotein levels, triglyceride levels, HDL-cholesterol levels, fasting plasma glucose levels, neutrophil count, neutrophil percentage, lymphocyte count, lymphocyte percentage and neutrophil-to-lymphocyte count ratio (p > 0.05).
Comparison between post-transplant MAFLD and NAFLD
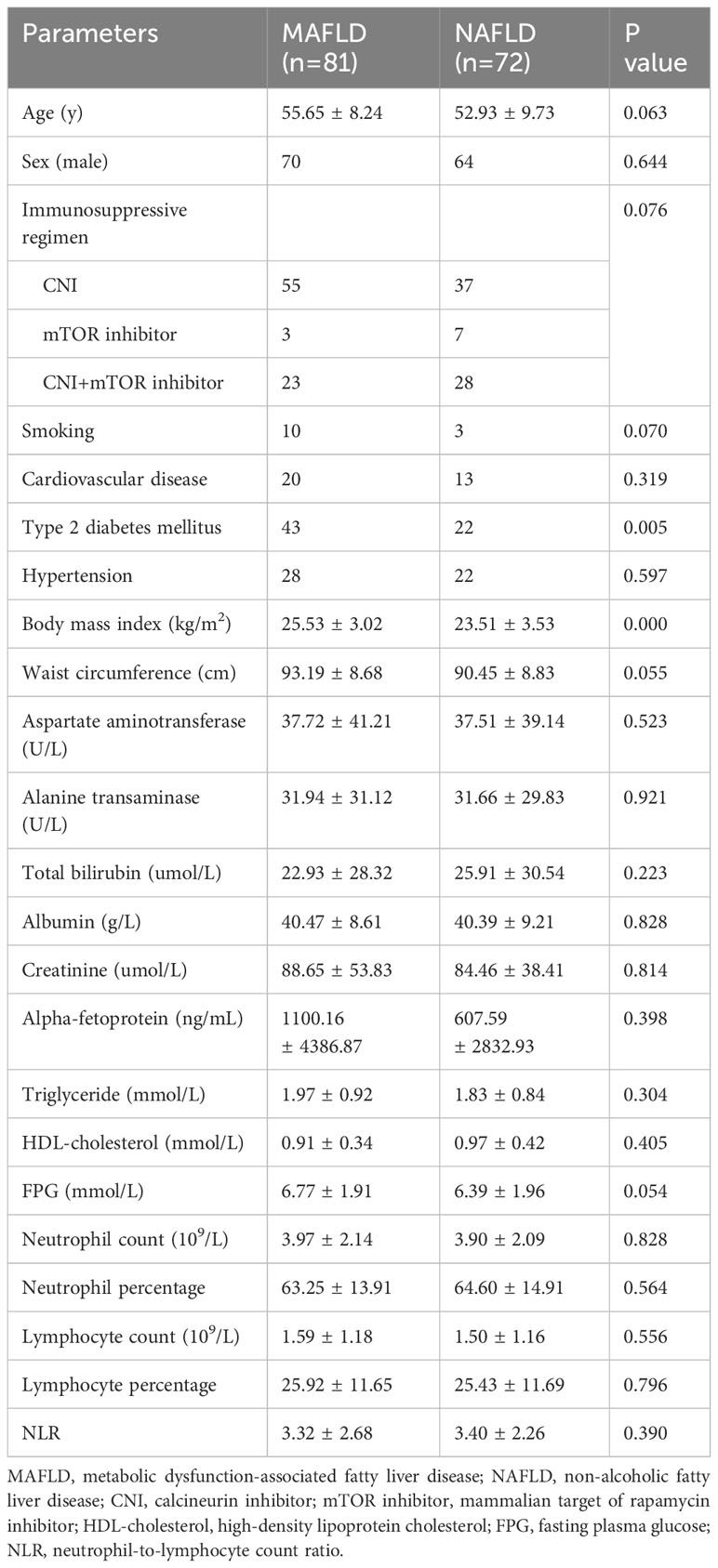
A comparison of clinical parameters between the post-transplant MAFLD and NAFLD groups is presented in Table 2. The MAFLD group had a higher prevalence of DM (53% vs. 31%) and a greater BMI (25.53 ± 3.02 kg/m2 vs. 23.51 ± 3.53 kg/m2). Conversely, the NAFLD group displayed decreased waist circumference and fasting plasma glucose levels, although these differences did not reach statistical significance (p > 0.05). Other parameters, including age, sex, immunosuppressive regimen, smoking, CVD, hypertension, aspartate aminotransferase levels, alanine transaminase levels, total bilirubin levels, serum albumin levels, serum creatinine levels, alpha-fetoprotein levels, triglyceride levels, high-density lipoprotein cholesterol levels, neutrophil count, neutrophil percentage, lymphocyte count, lymphocyte percentage and neutrophil-to-lymphocyte count ratio were similar between the two groups (p > 0.05). In addition, the tumor-free survival and overall survival of the post-transplant MAFLD and NAFLD cohorts also showed no significant differences (p > 0.05) as illustrated in Figure 2.
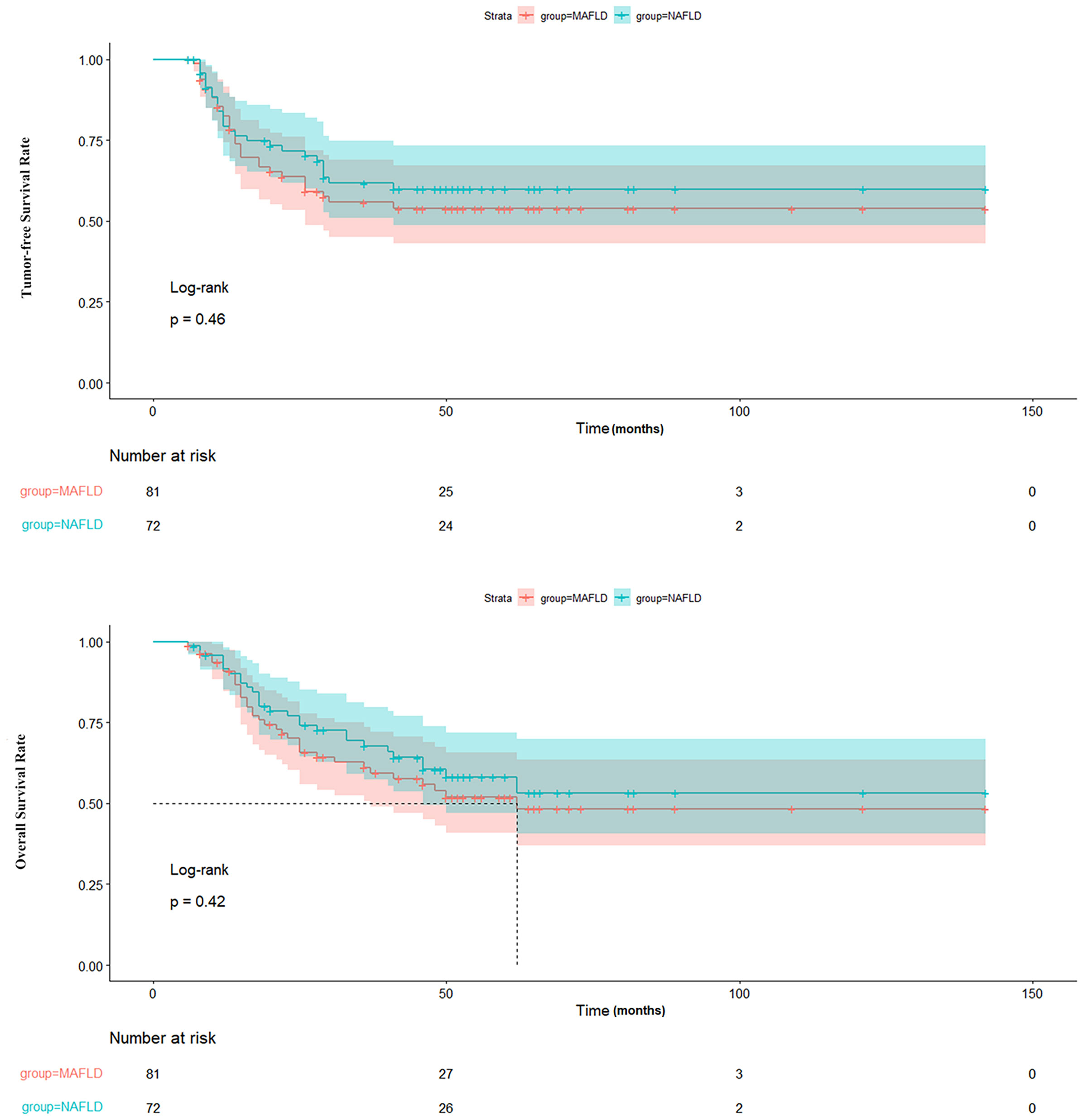
Figure 2 Comparison of TFS and OS between patients with MAFLD and NAFLD. TFS, tumor-free survival; OS, overall survival; MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, non-alcoholic fatty liver disease.
Risk factors associated with HCC recurrence
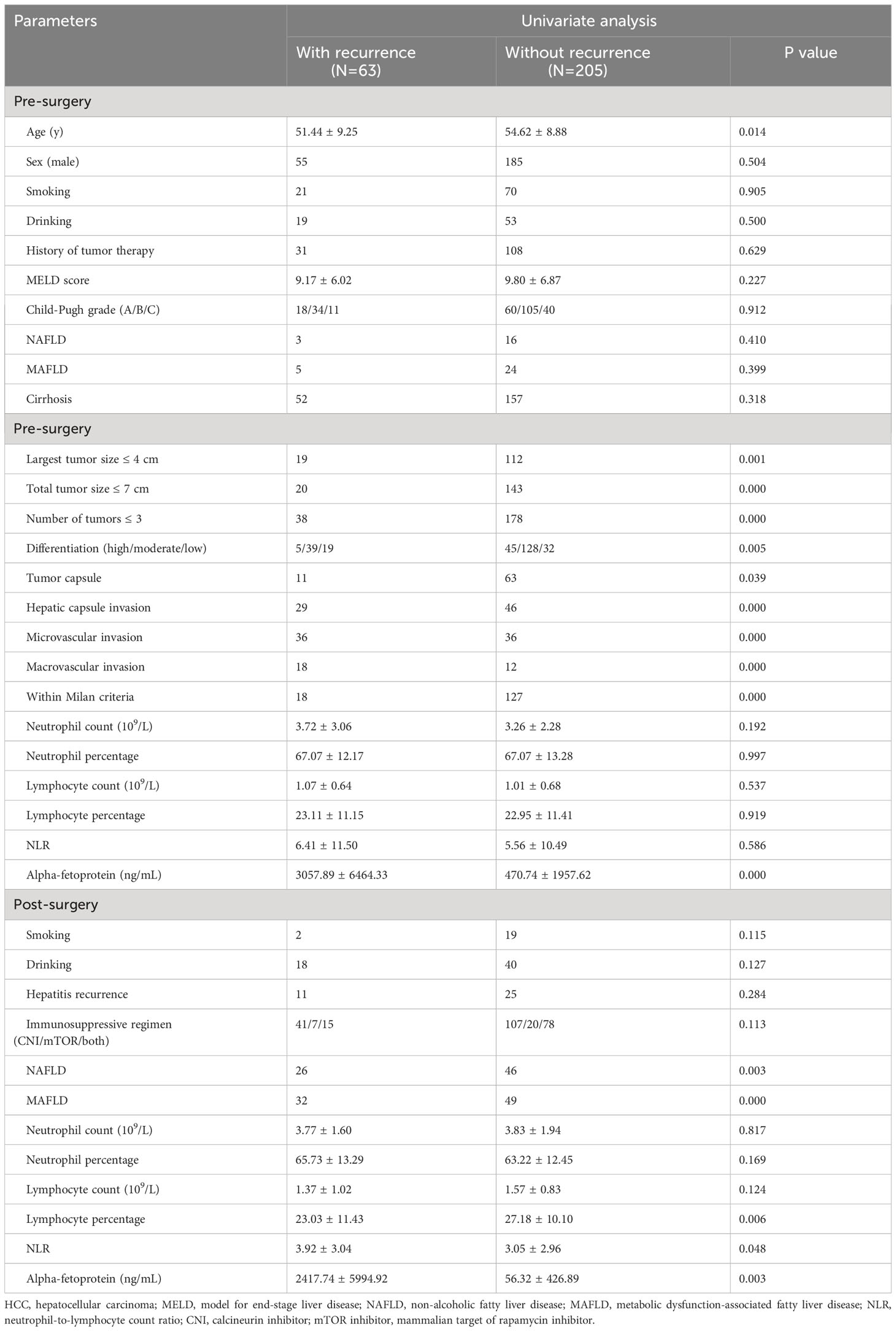
Table 3 outlines the outcomes of the univariate analysis concerning risk factors linked with HCC recurrence. Individuals who experienced liver transplant rejection with recurrence were notably younger and demonstrated a higher incidence of tumors exceeding > 4 cm in size, total tumor size surpassing > 7 cm, and more than three tumors. Other risk factors included poor tumor differentiation, absence of tumor encapsulation, hepatic capsule invasion, microvascular invasion, macrovascular invasion, deviation from Milan criteria, post-transplant MAFLD and NAFLD, elevated levels of pre- and post-transplant alpha-fetoprotein, NLR, and decreased post-transplant lymphocyte percentage.
The stepwise Cox proportional hazard model depicted in Figure 3 summarizes the prognostic factors associated with HCC recurrence in this cohort. The largest tumor size (OR = 0.27, p = 0.012), microvascular invasion (OR = 3.50, p = 0.006), absence of tumor capsule (OR = 0.31, p = 0.024), post-transplant MAFLD (OR = 4.96, p = 0.001), and decreased post-transplant lymphocyte percentage (OR = 0.95, p = 0.032) were identified as factors associated with a higher risk of recurrence.

Figure 3 Multivariate analysis of prognostic factors related to HCC recurrence. HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; NAFLD, non-alcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; NLR, neutrophil-to-lymphocyte count ratio.
To further ascertain the impact of post-transplant MAFLD on tumor recurrence, the tumor-free survival of liver transplant recipients with and without recurrence was evaluated. The findings revealed that post-transplant MAFLD was indicative of patients at a heightened risk of recurrence, resulting in significantly decreased tumor-free survival rates (p < 0.001) and overall survival rates (p < 0.001) as depicted in Figure 4.
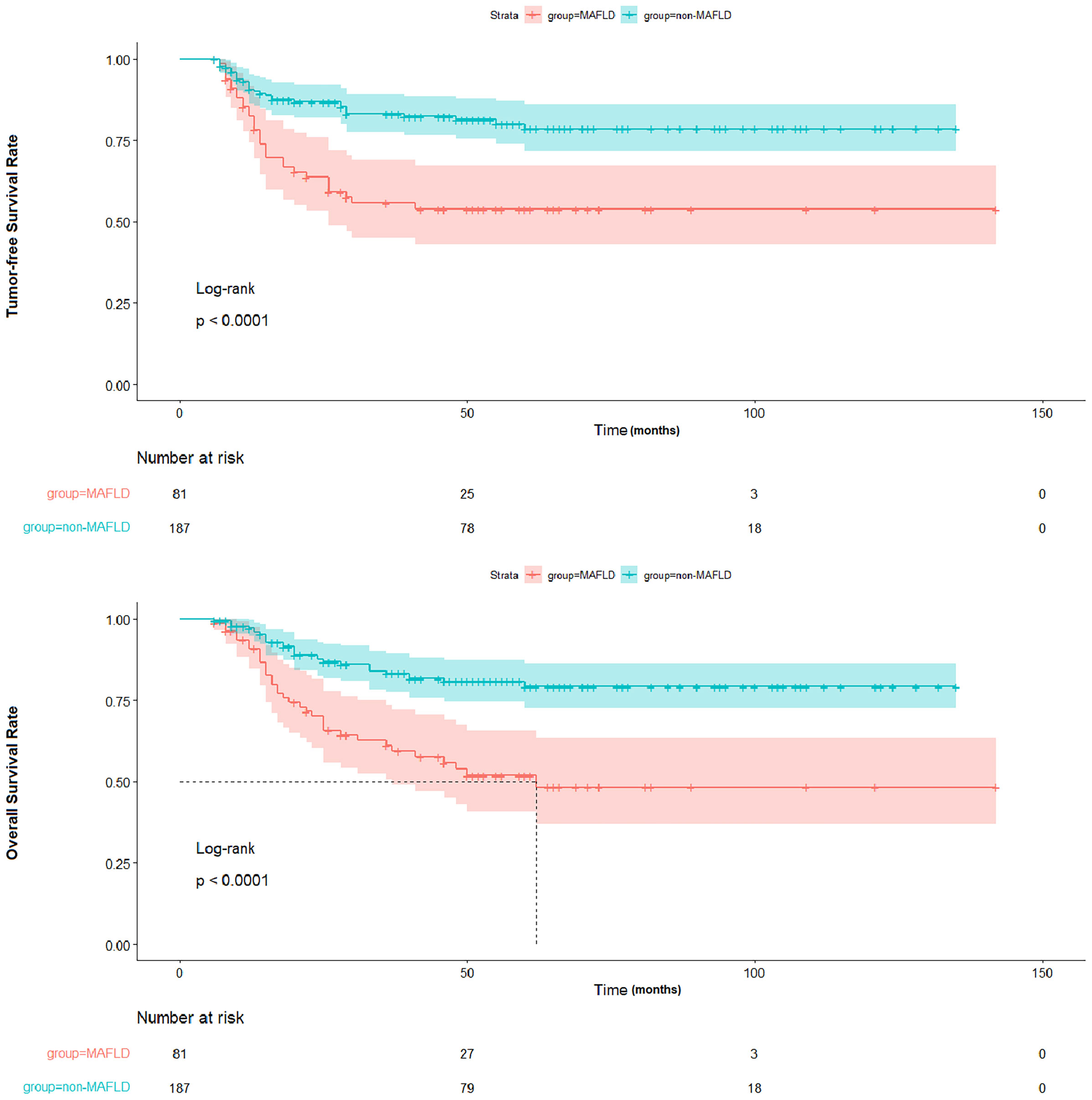
Figure 4 Comparison of TFS and OS between patients with and without MAFLD. TFS, tumor-free survival; OS, overall survival; MAFLD, metabolic dysfunction-associated fatty liver disease.
Discussion
We conducted a comparative analysis between LTR with MAFLD and NAFLD in terms of prevalence and characteristics. Our findings indicate that LTR with post-transplant MAFLD are significantly associated with metabolic risk factors such as T2DM and obesity. Additionally, we identified several independent risk factors for HCC recurrence in LTR with HCC, including post-transplant MAFLD, the largest tumor size > 4 cm, microvascular invasion, absence of tumor capsule, and decreased post-transplant lymphocyte percentage.
The term MAFLD was introduced as a replacement for NAFLD to more accurately reflect the pathology of liver disease, recognizing the limitations of the NAFLD definition (12, 13). Since then, substantial efforts have been devoted to exploring the relationship between NAFLD and MAFLD. Lim et al. found significant differences in the natural progression of MAFLD and NAFLD (19), while Lin et al. revealed that patients with MAFLD had a higher risk of disease progression (14). However, it remains unknown whether NAFLD and MAFLD can be used interchangeably to characterize LTR with HCC. This study revealed that the prevalence of MAFLD was higher than that of NAFLD before and after transplantation. Surprisingly, only a small proportion of patients with pre-transplant MAFLD (10.82%) and NAFLD (7.09%) received a diagnosis, resulting in an overlap of 52% among LTR with MAFLD. However, a meta-analysis of 379,801 patients reported a pooled prevalence of 39.22% for MAFLD and 33.86% for NAFLD, with regional variations. Interestingly, among patients with MAFLD, the pooled prevalence of those with both MAFLD and NAFLD was remarkably high at 81.59% (19). However, LTR constituted a distinct patient population with unique pathophysiologic characteristics. It is evident that the majority of LTR with HCC in this study were caused by hepatitis infection and excessive alcohol intake. Consequently, upon admission, certain patients exhibited severe cirrhosis and liver function decompensation, as determined by high MELD scores and Child-Pugh grade B or C ratios. These factors might result in malnutrition and a decrease in lymphocyte subset percentages (20), thereby significantly reducing the number of both MAFLD and NAFLD cases. Therefore, the characteristics of pre-transplant MAFLD and NAFLD in LTR with HCC were found to be similar upon comparison, suggesting the interchangeability of these two terms in a clinical setting.
Following transplantation, the prevalence of MAFLD and NAFLD increased due to the amelioration of malnutrition with the introduction of a healthier liver. Furthermore, urbanization and the use of immunosuppressant drugs can also contribute to the elevated prevalence (9, 21–23). while the routine practice at our center for LTR with HCC involved early corticosteroid withdrawal, high doses of corticosteroids were administered along with other immunosuppressant drugs in cases of acute rejection, causing insulin resistance and weight gain (24, 25). Nevertheless, concurrent recurrence of hepatitis and alcohol consumption may lead to an increase in MAFLD cases while reducing the number of NAFLD cases based on their respective definitions. Hepatitis C recurrence in some LTR can increase the risk of dyslipidemia, hepatic steatosis, and insulin resistance, whereas alcohol intake is strongly associated with hepatic steatosis and dyslipidemia, although further research is needed to investigate the link between HBV and metabolic syndrome (26–28). Moreover, compared to the NAFLD group, LTR with MAFLD were more strongly associated with metabolic disorders, including a higher proportion of individuals with T2DM and a higher BMI, which are key criteria for diagnosing MAFLD. Other characteristics did not exhibit significant differences, as MAFLD and NAFLD share a similar pathophysiology involving the metabolic functions of the liver, particularly an extended endoplasmic reticulum network (29). Consequently, there was an overlap of 41 patients (51%) among LTR with MAFLD, which is still lower than the prevalence (81.59%) reported in a meta-analysis (19).
Finally, we identified five independent prognostic factors for HCC recurrence in this study. Metabolic syndrome has been found to be a significant risk factor for HCC (4, 5). Since the novel definition of MAFLD was proposed based on metabolic syndrome, post-transplant MAFLD can effectively stratify LTR at a high risk of HCC recurrence. Thus MAFLD was revealed as an independent risk factor for HCC recurrence in our study. Additionally, our study further confirmed the significant association between the largest tumor size and microvascular invasion, traditionally indicators of aggressive biology (30, 31), with HCC recurrence. Furthermore, the presence of the tumor capsule exhibited a protective effect by acting as a barrier against vascular and local invasion (32, 33). Lastly, LTR with a low post-transplant lymphocyte percentage showed a worse prognosis, as lymphocytes play a critical role in tumor surveillance (34, 35). Consequently, decreased post-transplant lymphocyte percentages weakened the antitumor response in these patients, leading to HCC recurrence.
There are several limitations in this study. Firstly, the sample size of patients with pre- and post-transplant MAFLD and NAFLD is small, considering the substantial overlap between the two conditions. This study involved a unique population with a low prevalence of pre-transplant MAFLD and NAFLD, strongly associated with the presence of cirrhosis. Regarding post-transplant MAFLD and NAFLD, there are several conceivable explanations. LTR paid more attention to their health post-transplantation, adhering to regular follow-ups and timely treatment of emerging diseases. Besides, patients with missing data required for MAFLD classification were excluded from the study, which further decreased the prevalence of MAFLD. Hence, a two-center cohort study was conducted to increase patient enrollment. Moreover, with an increase in the number of LTR experiencing post-transplant MAFLD or NAFLD, the occurrence of LTR with overlapping diseases also escalated. Secondly, ultrasonography-based assessment was utilized instead of biopsy-based assessment for diagnosing post-transplant MAFLD and NAFLD because the latter is invasive and carries the risk of severe complications. However, the diagnosis based on ultrasound features may have certain limitations due to its dependency on operator skills. Consequently, two experienced sonologists, blinded to each other’s evaluations, performed the diagnosis to minimize potential bias. Thirdly, a significant number of LTR with HCC exceeding Milan criteria had a history of tumor therapy, potentially impacting hepatic steatosis. Additionally, the MAFLD definition was based on Asian criteria. Therefore, the results of this study should be interpreted with caution for Caucasian men/women. The retrospective design of this study calls for future randomized controlled trials in multiple centers to validate these findings and assess reproducibility in Caucasian populations.
In summary, patients with MAFLD have a stronger association with fatty liver disease compared to those with NAFLD following liver transplantation. Post-transplant MAFLD has the potential to stratify patients based on tumor progression.
Data availability statement
Data analyzed in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Beijing Chaoyang Hospital and the Institutional Review Board of China-Japan Friendship Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a two-center retrospective cohort study.
Author contributions
J-QZ: Data curation, Writing – original draft. J-ZL: Data curation, Writing – original draft. S-WY: Data curation, Writing – original draft. Z-YR: Formal analysis, Writing – review & editing. X-YY: Formal analysis, Writing – review & editing. ZL: Investigation, Writing – review & editing. X-LL: Investigation, Writing – review & editing. D-DH: Conceptualization, Writing – review & editing. QH: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; NAFLD, non-alcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; LTR, liver transplant recipients; BMI, body mass index.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. (2004) 127:S35–50. doi: 10.1053/j.gastro.2004.09.014
3. Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2016) 2:16018. doi: 10.1038/nrdp.2016.18
4. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. (2012) 10:1342–59 e2. doi: 10.1016/j.cgh.2012.10.001
5. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. (2021) 73 Suppl 1:4–13. doi: 10.1002/hep.31288
6. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. (2020) 111S:154170. doi: 10.1016/j.metabol.2020.154170
7. Muthiah MD, Cheng Han N, Sanyal AJ. A clinical overview of non-alcoholic fatty liver disease: A guide to diagnosis, the clinical features, and complications-What the non-specialist needs to know. Diabetes Obes Metab. (2022) 24 Suppl 2:3–14. doi: 10.1111/dom.14521
8. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
9. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. (2018) 69:896–904. doi: 10.1016/j.jhep.2018.05.036
10. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
11. Bedossa P. Current histological classification of NAFLD: strength and limitations. Hepatol Int. (2013) 7 Suppl 2:765–70. doi: 10.1007/s12072-013-9446-z
12. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
13. Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014 e1. doi: 10.1053/j.gastro.2019.11.312
14. Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. (2020) 40:2082–9. doi: 10.1111/liv.14548
15. American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
16. Farrell GC, Chitturi S, Lau GK, Sollano JD, Asia-Pacific Working Party on N. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. (2007) 22:775–7. doi: 10.1111/j.1440-1746.2007.05002.x
17. Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: A 4.6-year cohort study in China. J Clin Endocrinol Metab. (2022) 107:88–97. doi: 10.1210/clinem/dgab641
18. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. (1996) 334:693–9. doi: 10.1056/NEJM199603143341104
19. Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. (2023) 21:619–29 e7. doi: 10.1016/j.cgh.2021.11.038
20. Pan F, Cao S, Li XL, Jia YN, Wang RL, He Q, et al. The model for end-stage liver disease score and the follow-up period can cause the shift of circulating lymphocyte subsets in liver transplant recipients. Front Med (Lausanne). (2021) 8:779443. doi: 10.3389/fmed.2021.779443
21. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl. (2001) 7:S13–21. doi: 10.1053/jlts.2001.29167
22. Watt KD, Charlton MR. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. (2010) 53:199–206. doi: 10.1016/j.jhep.2010.01.040
23. Kurdi A, Martinet W, De Meyer GRY. mTOR inhibition and cardiovascular diseases: dyslipidemia and atherosclerosis. Transplantation. (2018) 102:S44–S6. doi: 10.1097/TP.0000000000001693
24. Xu WL, Wang RL, Liu Z, Wu Q, Li XL, He Q, et al. Granzyme B-producing B cells function as a feedback loop for T helper cells in liver transplant recipients with acute rejection. Inflammation. (2021) 44:2270–8. doi: 10.1007/s10753-021-01498-9
25. Zhu JQ, Wang J, Li XL, Xu WL, Lv SC, Zhao X, et al. A combination of the percentages of IFN-gamma(+)CD4(+)T cells and granzyme B(+)CD19(+)B cells is associated with acute hepatic rejection: a case control study. J Transl Med. (2021) 19:187. doi: 10.1186/s12967-021-02855-w
26. Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol. (2015) 30:14–20. doi: 10.1111/jgh.12700
27. Shen Z, Munker S, Wang C, Xu L, Ye H, Chen H, et al. Association between alcohol intake, overweight, and serum lipid levels and the risk analysis associated with the development of dyslipidemia. J Clin Lipidol. (2014) 8:273–8. doi: 10.1016/j.jacl.2014.02.003
28. Serfaty L. Metabolic manifestations of hepatitis C virus: diabetes mellitus, dyslipidemia. Clin Liver Dis. (2017) 21:475–86. doi: 10.1016/j.cld.2017.03.004
29. Flessa CM, Kyrou I, Nasiri-Ansari N, Kaltsas G, Kassi E, Randeva HS. Endoplasmic reticulum stress in nonalcoholic (metabolic associated) fatty liver disease (NAFLD/MAFLD). J Cell Biochem. (2022) 123:1585–606. doi: 10.1002/jcb.30247
30. Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. (2022) 157:779–88. doi: 10.1001/jamasurg.2022.2800
31. Vitale A, Peck-Radosavljevic M, Giannini EG, Vibert E, Sieghart W, Van Poucke S, et al. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol. (2017) 66:412–23. doi: 10.1016/j.jhep.2016.09.012
32. Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. (2014) 21:147–54. doi: 10.1245/s10434-013-3211-3
33. Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. (1992) 70:45–9. doi: 10.1002/1097-0142(19920701)70:1<45::AID-CNCR2820700108>3.0.CO;2-7
34. Li C, Wen TF, Yan LN, Li B, Yang JY, Xu MQ, et al. Scoring selection criteria including total tumour volume and pretransplant percentage of lymphocytes to predict recurrence of hepatocellular carcinoma after liver transplantation. PloS One. (2013) 8:e72235. doi: 10.1371/journal.pone.0072235
Keywords: hepatocellular carcinoma, liver transplantation, non-alcoholic fatty liver disease, metabolic dysfunction-associated fatty liver disease, prognosis
Citation: Zhu J-Q, Liu J-Z, Yang S-W, Ren Z-Y, Ye X-Y, Liu Z, Li X-L, Han D-D and He Q (2024) Impact of the diagnosis of metabolic dysfunction-associated fatty liver disease and non-alcoholic fatty liver disease in patients undergoing liver transplantation for hepatocellular carcinoma. Front. Endocrinol. 15:1306091. doi: 10.3389/fendo.2024.1306091
Received: 03 October 2023; Accepted: 29 March 2024;
Published: 15 April 2024.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Ioannis I. Lazaridis, University Hospital of Basel, SwitzerlandShaghayegh Khanmohammadi, Tehran University of Medical Sciences, Iran
Copyright © 2024 Zhu, Liu, Yang, Ren, Ye, Liu, Li, Han and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Dong Han, c3VyZ2hhbmRkQDE2My5jb20=; Qiang He, aGVxaWFuZ3N1cmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ji-Qiao Zhu
Ji-Qiao Zhu Jia-Zong Liu
Jia-Zong Liu Shi-Wei Yang2†
Shi-Wei Yang2† Zhang-Yong Ren
Zhang-Yong Ren Xian-Liang Li
Xian-Liang Li Dong-Dong Han
Dong-Dong Han Qiang He
Qiang He