
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 05 July 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1302436
 Yongguang Wei1,2,3†
Yongguang Wei1,2,3† Zedong Qin4†
Zedong Qin4† Xiwen Liao1,2,3
Xiwen Liao1,2,3 Xin Zhou1,2,3
Xin Zhou1,2,3 Huasheng Huang1,2,3
Huasheng Huang1,2,3 Chenlu Lan1,2,3
Chenlu Lan1,2,3 Wei Qin1,2,3
Wei Qin1,2,3 Guangzhi Zhu1,2,3
Guangzhi Zhu1,2,3 Hao Su1,2,3
Hao Su1,2,3 Tao Peng1,2,3*
Tao Peng1,2,3*Background: Pancreatic cancer (PC) is a prevalent malignancy within the digestive system, with diabetes recognized as one of its well-established risk factors.
Methods: Data on PC mortality attributed to high fasting blood sugar were retrieved from the Global Burden of Disease (GBD) study 2019 online database. To assess the temporal trends of PC burden attributable to high fasting plasma glucose (HFPG), estimated annual percentage changes (EAPCs) for age-standardized death rates (ASDRs) between 1990 and 2019 were determined using a generalized linear model. Furthermore, a Bayesian age-period-cohort (BAPC) model using the integrated nested Laplacian approximation algorithm was employed to project the disease burden over the next 20 years.
Results: Globally, the crude death number of PC attributable to HFPG almost tripled (from 13,065.7 in 1990 to 48,358.5 in 2019) from 1990 to 2019, and the ASDR increased from 0.36/100,000 to 0.61/100,000 with an EAPC of 2.04 (95% CI 1.91–2.16). The population aged ≥70 years accounted for nearly 60% of total deaths in 2019 and experienced a more significant increase, with the death number increasing approximately fourfold and the ASDR increasing annually by 2.65%. In regions with different sociodemographic indexes (SDIs), the highest disease burden was observed in the high-SDI region, whereas more pronounced increasing trends in ASDR were observed in the low to middle-SDI, low-SDI, and middle-SDI regions. Additionally, a significantly negative association was found between EAPCs and ASDRs of PC attributable to HFPG from 1990 to 2019. Moreover, the BAPC model predicts that ASDR and age-standardized disability-adjusted life-years (DALYs) rate for PC attributed to HFPG was projected to increase obviously for men and women from 2019 to 2040.
Conclusions: The burden of PC attributed to HFPG has increased globally over the past three decades, with the elderly population and high-SDI regions carrying a relatively greater disease burden, but more adverse trends observed in low-SDI areas. Furthermore, the burden is projected to continue increasing over the next 20 years. Hence, more tailored prevention methodologies should be established to mitigate this increasing trend.
Pancreatic cancer (PC), with a 5-year overall survival rate of only 4%–12%, presents one of the highest mortalities among all solid tumors (1, 2), particularly in developed nations and regions. Risk factors for PC, including inherited genetics, smoking, obesity, diabetes, and excessive drinking (1, 3), have been identified. Despite significant advancements in addressing PC over the past decades (4), its incidence continues to rise globally (3). This unfavorable trend is attributed to population expansion, aging populations, and changes in prevalent cancer-related risk factors, many of which are associated with societal and economic development. The growing prevalence of diabetes exerts a significant burden on society in terms of mortality, morbidity, and socioeconomic costs (5). Remarkably, the increasing prevalence of diabetes and obesity also contributes to the increase of PC incidence (6–8). Given the compelling links between diabetes and cancers (9, 10), the pandemic of diabetes portends an increase in the number of cancer diagnoses, including PC.
Precise prevention of PC can be advanced through targeted prevention approaches informed by a thorough understanding of PC mortality patterns and temporal trends. Adherence to type 2 diabetes mellitus (T2DM) prevention diets has been shown to lower risk of PC in the United States population (11). The Global Burden of Disease (GBD) 2019 study, with a wide range of data sources and scientific computational methodologies, has labored deliberately and systematically to disclose incidence, prevalence, mortality, years of life lost (YLLs), years lived with disability, and disability-adjusted life-years (DALYs) due to 369 diseases and injuries for two sexes, 20 age groups in seven super-regions, 21 regions, and 204 countries and territories, from 1990 to 2019. Currently, some studies have examined the burden of multiple cancers attributable to high fasting plasma glucose (HFPG), the most common indicator of diabetes within the GBD framework (12–14). However, research on the temporal and future trends of PC mortality attributable to HFPG is limited, especially concerning age and geographical dimensions (15, 16).
To explore the global burden of PC attributed to HFPG, we comprehensively analyzed the temporal trends of PC mortality globally and nationally from 1990 to 2019, based on data from GBD 2019. We further projected future disease burdens. These findings not only complement prior research but also offer guidance for designing and promoting targeted prevention strategies for PC attributed to HFPG (17).
The data on annual crude deaths, DALYs, and the corresponding age-standardized rates of PC with “HFPG” from 1990 to 2019, categorized by sex, region, and country, were extracted from the Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool) (18). Diabetes is commonly defined as a serum fasting plasma glucose (FPG) level exceeding 7 mmol/L (19, 20). In GBD 2019, the mean FPG, measured in mmol/L, was identified as the continuous exposure for HFPG and estimated from diabetes prevalence using an ensemble distribution established by GBD 2019. HFPG, as an individual risk factor, was defined as any FPG level beyond the theoretical minimum-risk exposure level (4.8 mmol/L–5.4 mmol/L) by the Institute for Health Metrics and Evaluation. Detailed methodologies have been described in previous publications (6, 12). The sociodemographic index (SDI), reflecting geographic closeness and epidemiological commonality, is a comprehensive indicator of socioeconomic development, ranging from 0 (worst) to 1 (best). SDI data for different countries were also retrieved from GBD 2019 (https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019). According to SDI, 204 countries and territories were divided into five areas and 21 regions. To predict the mortality of PC attributable to HFPG globally, the population estimates for 2017–2100 were also obtained from GBD 2019 (https://ghdx.healthdata.org/record/ihme-data/global-population-forecasts-2017-2100).
To further quantify the burden trend for PC attributable to HFPG globally and in subgroups, including sex, age, and SDI during 1990–2019, the estimated annual percentage change (EAPC), reflecting the annual change across a specific time period, was calculated (21, 22). We utilized a generalized linear regression model lnR = α + βT + ϵ, where R represents the amount or rate and T represents the calendar year. EAPC was determined as 100 − (exp[β]−1), with a 95% confidence interval (CI). An upward trend in age-standardized death rate (ASDR) occurs when both the EAPC measures and their corresponding lower limits of 95% CIs were > 0. On the contrary, if both EAPC values and their upper limits of 95% CIs were < 0, a downward trend would be indicated. Otherwise, ASDR was considered stable.
The prospective trend of a disease may help to establish appropriate health policies and promote the reasonable allocation of medical resources. Bayesian age-period-cohort (BAPC) models, using integrated nested Laplace approximations and assuming an inverse-gamma prior distribution of extant data, offer a practical approach for the prediction of cancer mortality rates (23–25). Using R packages “BAPC” and “INLA (integrated nested Laplace approximation)” and GBD data from 1990 to 2019, the BAPC model was employed to assess mortality and DALY loss due to PC attributed to HFPG globally, projecting future disease burdens until 2040.
All tests, calculations, and descriptive plotting were conducted using the R program (v4.1.1). The trend of disease burden across different regions and age groups was also assessed. To categorize temporal ASDR trends for each nation, a hierarchical cluster analysis was conducted, classifying countries and territories into four growth types (significant increase; minor increase; remained stable or minor decrease; significant decrease) based on EAPCs of ASDRs. Additionally, we examined the correlation between EAPCs and ASDRs (1990) globally using Pearson correlation analysis. Furthermore, Pearson rank-order correlation analysis was conducted to determine the strength and direction of the connection between SDI and ASDR at the regional and national levels, respectively. Unless otherwise indicated, a significance level of P < 0.05 was applied as the threshold cutoff value.
The crude death number of PC attributed to HFPG increased nearly threefold (from 13,065.7 in 1990 to 48,358.5 in 2019) from1990 to 2019 globally, accompanied by an increase in the ASDR from 0.36/100,000 to 0.61/100,000, with a global EAPC value of 2.04 (95% CI 1.91–2.16. That is to say, approximately 0.61% of all PC-related deaths in 2019 were attributed to HFPG (Table 1, Figure 1). Similarly, among the five SDI regions, an obvious increasing trend in crude deaths and ASDRs was observed across all age groups for men, women, and both sexes (Table 1, Figure 1). The increasing trends in ASDRs and DALYs for both sexes, women, and men were nearly consistent (Figure 1). Each gender contributed roughly half of all deaths. Although crude death number and ASDRs increased across all three age subgroups (Table 1), the subgroup aged ≥70 years, constituting nearly 60% of total deaths in 2019, experienced a more obvious increase in contrast to other age groups. Specifically, crude deaths in this subgroup increased approximately fourfold, with an annual ASDR increase of 2.65% between 1990 and 2019. In terms of SDI regions, the high SDI region had the highest ASDR in 2019 (0.97 [95% CI 0.23–2.07]), with ASDRs decreasing as SDI decreased. Despite that the high SDI region had a greater disease burden, more pronounced increasing trends in ASDRs were observed in the low to middle-SDI (EAPC: 3.75 [95% CI 3.67–3.82]), low-SDI (EAPC: 2.98 [95% CI 2.93–3.02]), and middle-SDI (EAPC: 2.73 [95% CI 2.63–2.83]) regions (Table 1, Figure 1). The distribution of ASDR and DALY rates across different age subgroups was largely consistent for men and women in 1990, 2010, and 2019 (Figure 2). In addition, the distribution of crude DALY rates peaked in the 75–79 age group and experienced an obvious rise across all SDI regions from 1990 to 2019 (Figure 2; Supplementary Figure 1). Conversely, crude death rate distributions in different age groups were unimodal for men in 1990, 2010, and 2019, and for women in 1990, with crude death rates peaking in the 85–95 age group. In addition, the crude death rate for women in 2010 and 2019 showed a skewed distribution, with a peak in the 95+ age group (Figure 2).
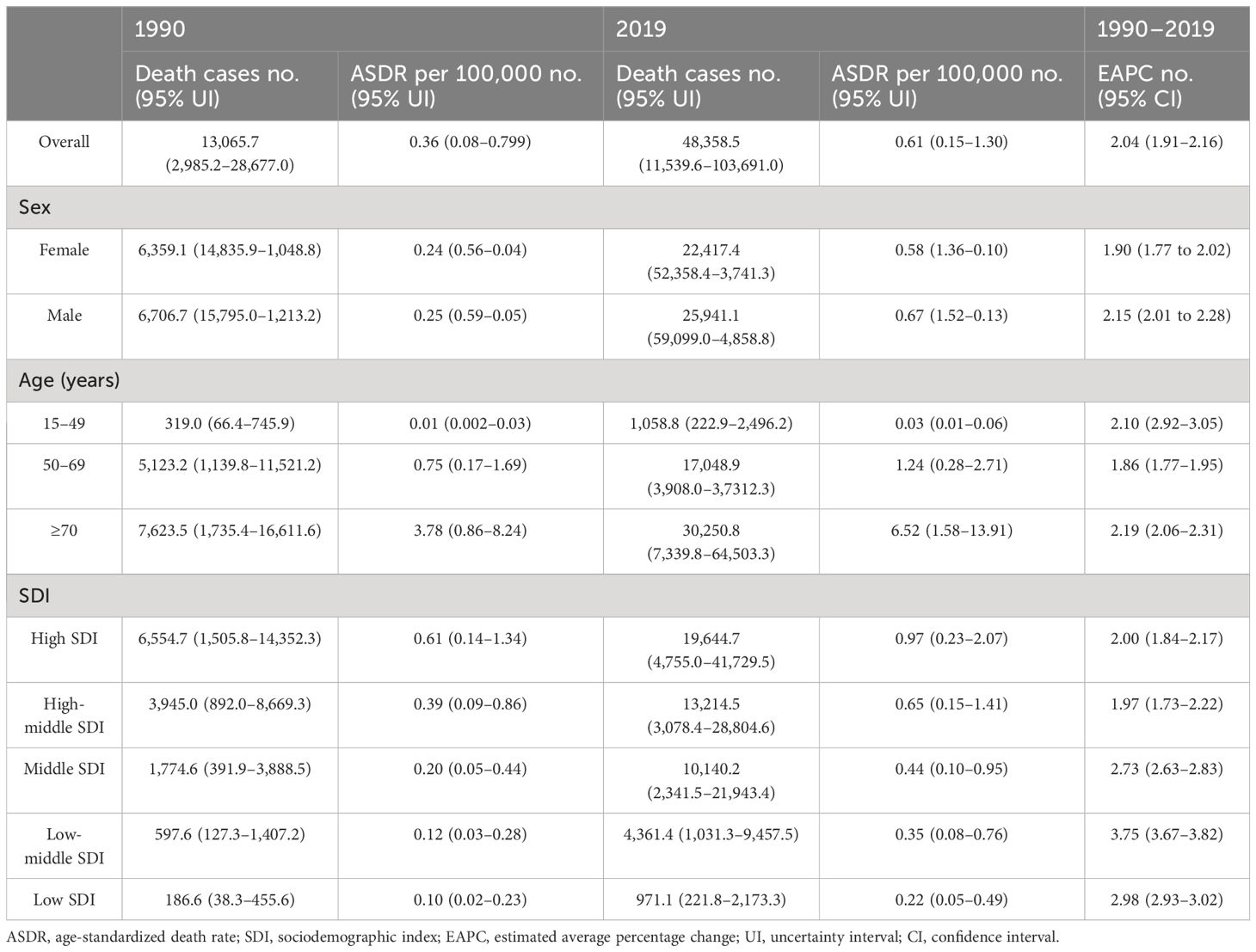
Table 1 The changing trends of number of deaths and age-standardized mortality rate of pancreatic cancer among patients with type 2 diabetes from 1990 to 2019.
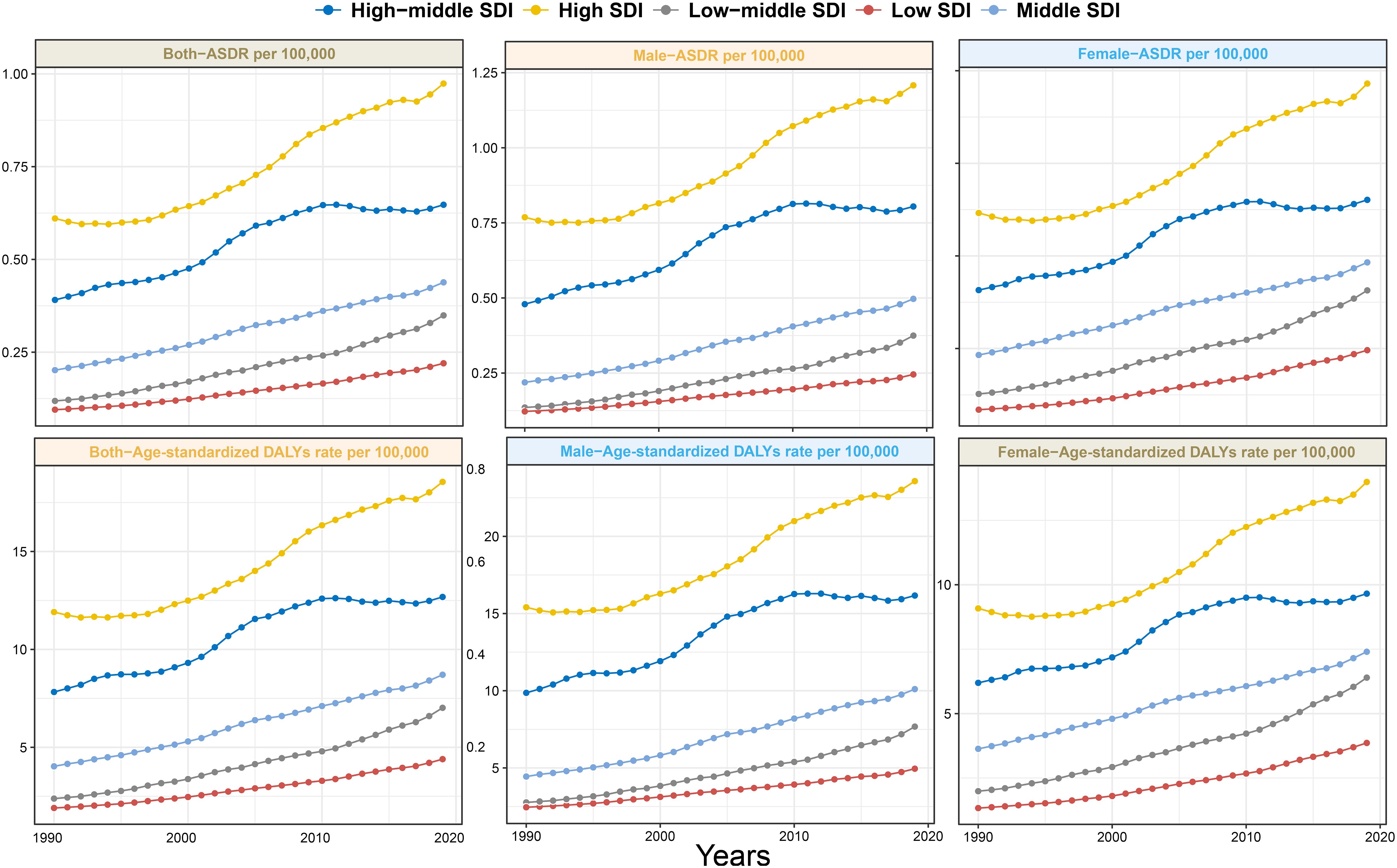
Figure 1 Trends in ASDR and age-standardized DALYs rate of pancreatic cancer attributed to HFPG among men, women, and both sexes in different SDI regions from 1990 to 2019. ASDR, age-standardized death rate; DALYs, disability-adjusted life-years; HFPG, high fasting plasma glucose; SDI, sociodemographic index.
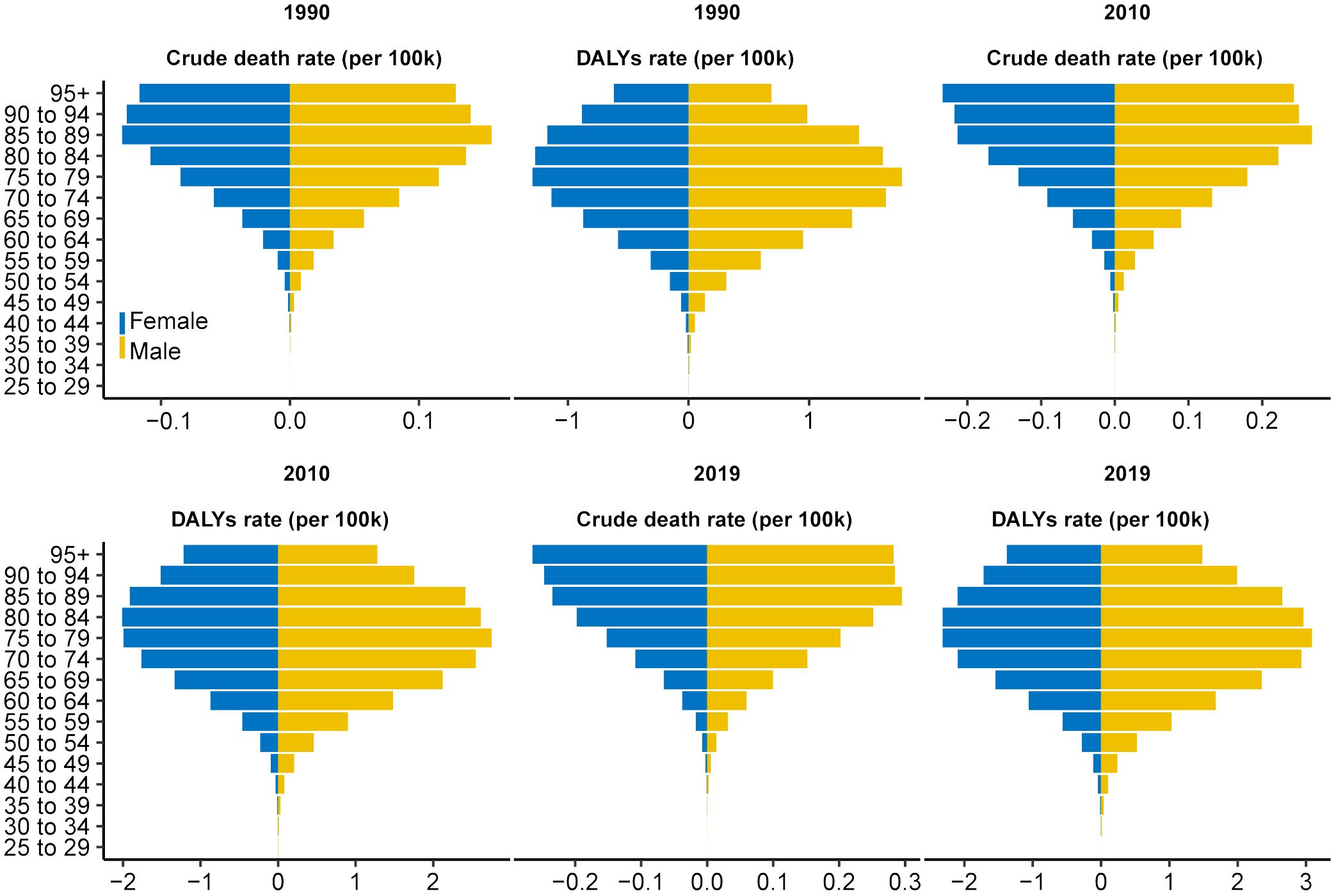
Figure 2 Distribution of ASDR and age-standardized DALY rate for pancreatic cancer attributed to HFPG by age group in 1990, 2010, and 2019. The horizontal axis represents the ASDR and age-standardized DALYs rate (per 100,000 persons), whereas the vertical axis represents different age groups. The blue stripe on the left represents women, whereas the yellow stripe on the right represents men. ASDR, age-standardized death rate; DALYs, disability-adjusted life-years; HFPG, high fasting plasma glucose.
In 2019, both crude death and DALY rats exhibited an increasing trend not only from young to old individuals but also from high to low SDI regions (Supplementary Figure 2). In addition, the ratio of male to female crude death rates across different SDI regions showed a generally consistent distribution across different age groups globally, with the main peak occurring in the 30–40 age group (Supplementary Figure 3). Moreover, from the 35–39 age group onward, the ratio showed a decreasing trend but remained above 1 with increasing age. The world map showed highly heterogeneous ASDRs and corresponding EAPCs for PC attributed to HFPG across different countries and territories in 2019 (Figure 3). Particularly severe ASDRs were observed in a few countries bordering the Mediterranean, as well as in most countries in North America, Europe, and South America (Figure 3A). Based on EAPC values, almost all countries experienced varying degrees of increase in disease burden from 1990 to 2019, with more pronounced increases primarily detected in developed countries, reaching a peak in Kazakhstan and Uzbekistan. In cluster analysis, 204 countries and territories were categorized into four subgroups with different increasing rates: significant increase, increase, remained stable or minor decrease, and significant decrease (Supplementary Figure 4).
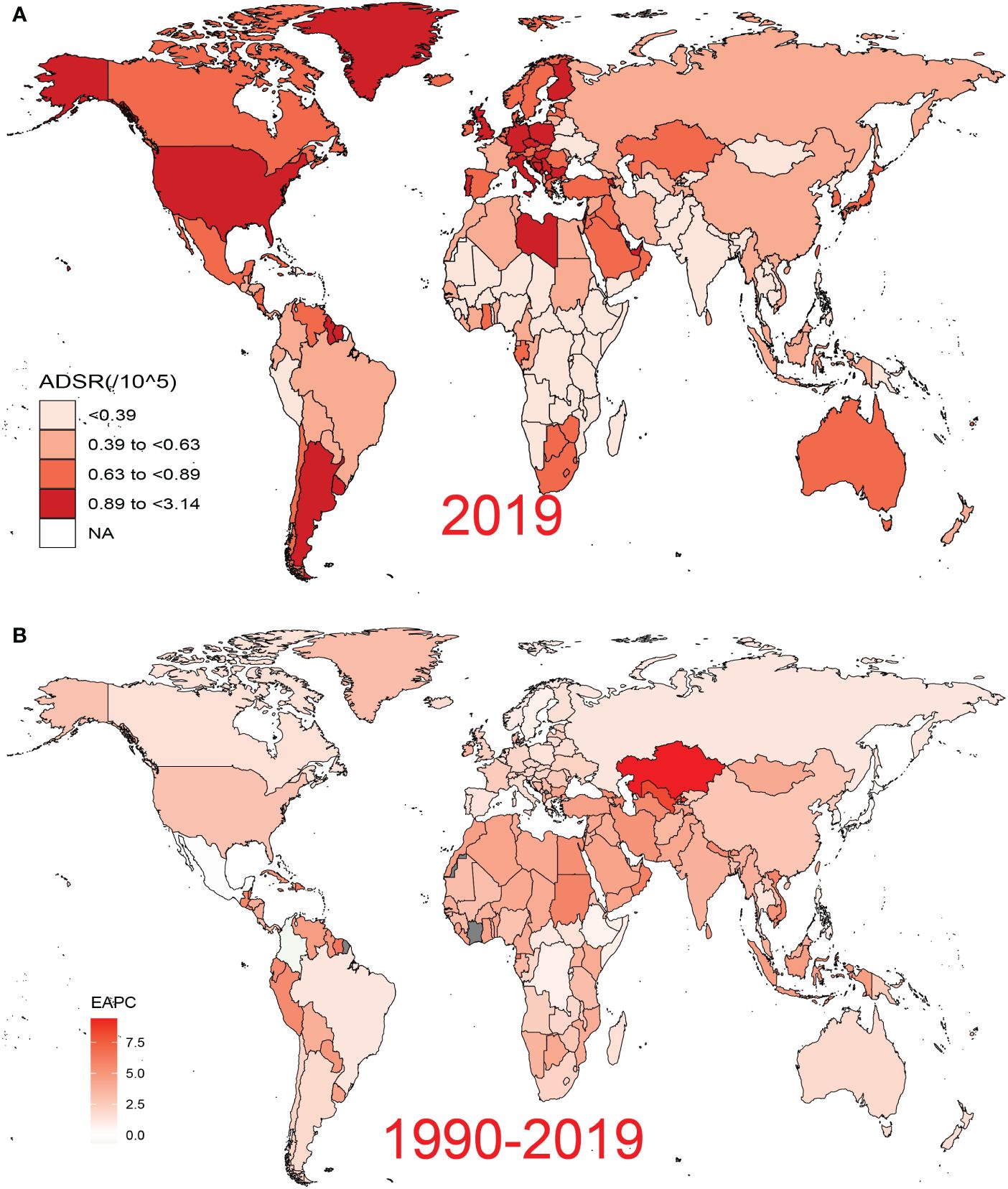
Figure 3 Global disease burden of pancreatic cancer attributed to HFPG for both sexes across 195 countries and territories. (A) ASDR of pancreatic cancer attributed to HFPG in 2019. (B) EAPC of ASDR for pancreatic cancer attributed to HFPG from 1990 to 2019. HFPG, high fasting plasma glucose; ASDR, age-standardized death rate; EAPC, estimated annual percentage change.
The baseline disease pool of PC attributable to HFPG is represented by the ASDRs in 1990. In our study, significantly negative associations were found between EAPCs and ASDRs (Figure 4; Pearson’s r = −0.50; P < 0.001).
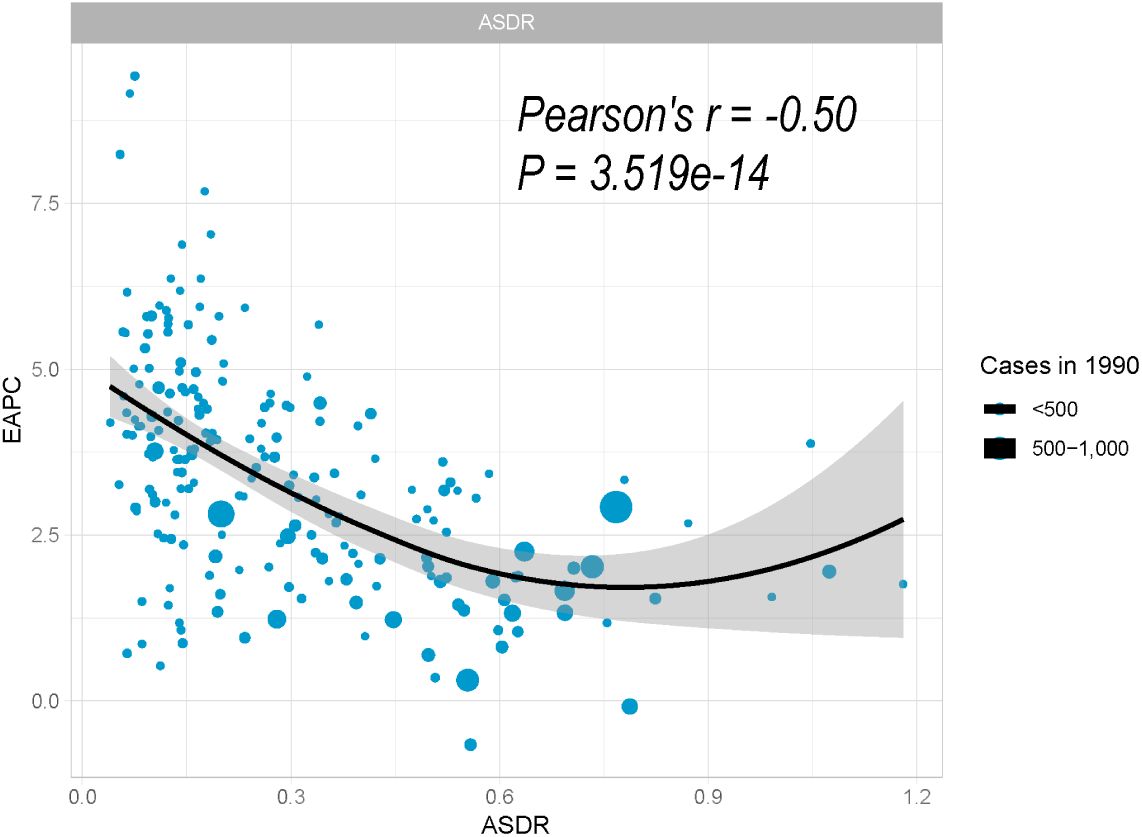
Figure 4 Correlation between EAPC and ASDR of pancreatic cancer attributed to HFPG in 1990. The size of the circle is proportional to the number of pancreatic cancer cases. EAPC, estimated annual percentage change; ASDR, age-standardized death rate; HFPG, high fasting plasma glucose.
Among the 21 regions of different SDI, most regions exhibited ascending trends of ASDRs along with increases in SDI from 1990 to 2019 (Figure 5A; R = 0.785; P < 0.001). In this context, most high-income regions exceeded the expected level in all years, whereas many low-income regions, despite experiencing an upward trend, remained below the expected level (Figure 5A). Figure 5B illustrates the connection between ASDRs and SDI across different countries and territories in 2019. There was also a general upward tendency of ASDRs along with increases in SDI at the national level, which was similar to regional pattern. However, this upward trend leveled off after SDI exceeded 0.7 (Figure 5B; R = 0.632; P < 0.001).
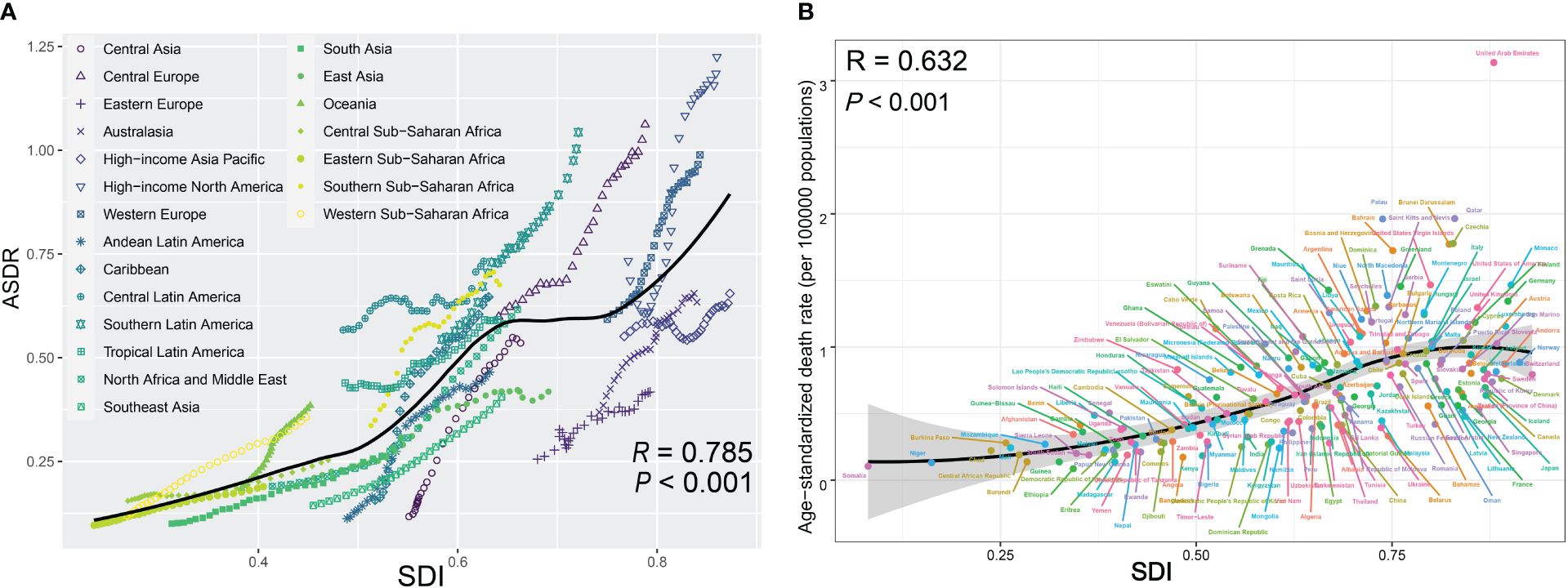
Figure 5 (A) Trend in ASDRs of pancreatic cancer attributed to high fasting plasma glucose (HFPG) among/across 21 regions based on SDI in 2019. For each region, points from left to right depict estimates from each year from 1990 to 2017, with expected values shown as the black line. (B) ASDRs of pancreatic cancer attributed to HFPG across 195 countries and territories by SDI in both sexes, 2019. Expected values are shown as the black line. Each point shows observed age-standardized DALY rate for a certain country in 2019. ASDRs, age-standardized death rates; HFPG, high fasting plasma glucose; SDI, sociodemographic index.
Based on the comprehensive GBD data from 1990 to 2019, we utilized the BAPC model (26) to project that both ASDR and age-standardized DALY rates for PC attributed to HFPG would significantly increase for both sexes over the period 2019–2040 (Figures 6A–D). The shaded areas in the figure indicate that the mortality could fluctuate dramatically as the corresponding rates rise or fall by 1% per year.
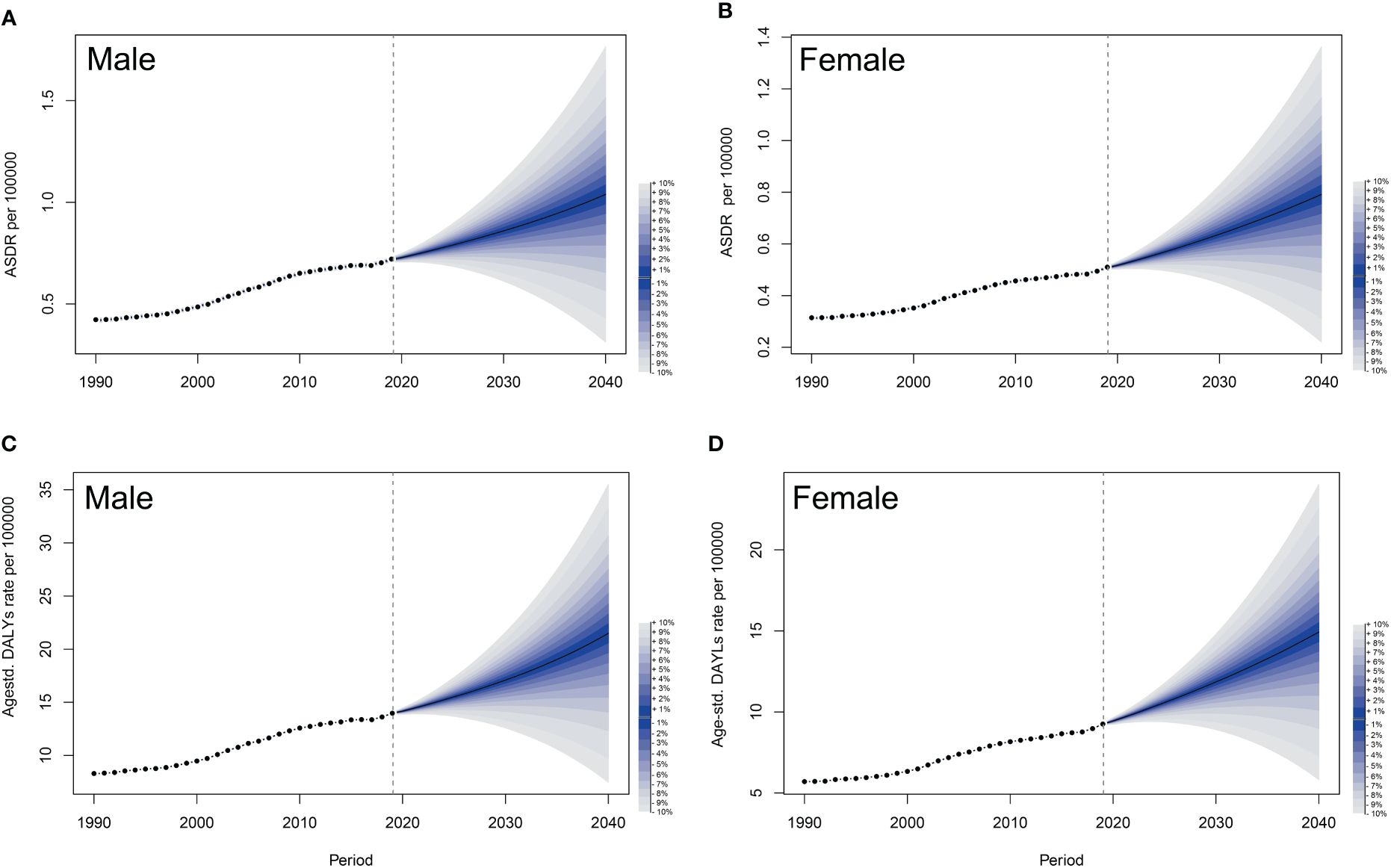
Figure 6 Observed and predicted trends of ASDRs and age-standardized DALYs of pancreatic cancer attributed to HFPG by sex globally from 1990 to 2040 using the BAPC model. (A) ASDRs of male. (B) ASDRs of female. (C) Age-standardized DALYs of male. (D) Age-standardized DALYs of female. The shadow in the figure represents uncertainty intervals, suggesting that mortality could fluctuate dramatically as the corresponding rates rise or fall by 1% per year, with each shade corresponding to a change of 1%. ASDRs, age-standardized death rates; DALYs, disability-adjusted life-years; HFPG, high fasting plasma glucose; BAPC, Bayesian age-period-cohort; UIs, uncertainty intervals.
T2DM is a significant contributing factor to PC (27). Insulin resistance, induced by either a paraneoplastic syndrome or pancreatic β-cell dysfunction, serves as a pivotal mechanism in the development of diabetes mellitus (DM) (28). In the pathogenesis of PC induced by DM, exosomes and miR-19a emerge as crucial mediators and effector molecules (29). Furthermore, there are several potential driving mechanisms in the proliferation and invasion of PC cells, including activation of the high glucose-activated p38 MAPK pathway, enhanced expression of GDNF and RET, induction of EGF expression, and EGFR transactivation (28, 30, 31). The high-glucose microenvironment in PC can further promote cancer development by affecting the SREBP1–autophagy axis (32). In addition, type 3c diabetes, also known as pancreatogenic diabetes, which arises when the pancreas suffers chronic injury, may increase the risk of pancreatic ductal adenocarcinoma (33). These findings shed light on the pathogenesis of PC associated with hyperglycemia or DM (34).
Previous studies have shown that age-standardized PC deaths were primarily attributable to smoking (21.1%), HFPG (8.9%), and high body mass index (BMI) (6.2%) worldwide in 2017 (6). The proportion of PC deaths attributed to tobacco consumption declined, whereas those attributed to HFPG and high BMI increased during 1990–2019 (7). Notably, the 65–69 age group had the greatest proportion of incident cases and deaths from PC, whereas the disease burden peaked in the 75–79 age subgroup in women (6). The increasing burden of diabetes brings a cumulative risk of human cancers (10, 27).
Based on global investigation and statistics, the burden of T2DM increased by 1.56% per year (1.64% in men and 1.51% in women) worldwide from 2000 to 2019. The most significant incidence increases took place in the Eastern Mediterranean, the United States of America, and Southeast Asia, whereas the smallest increases were observed in the Western Pacific, Africa, and Europe. The most pronounced increases in age-standardized T2DM prevalence were observed in high-SDI countries, followed by low-medium and low SDI countries (35). Diabetes was more common in individuals over the age of 60 and had no significant sex differences (5). Alarmingly, global estimates predict that the number of adults with impaired glucose tolerance will rise from 374 million in 2017 to 587 million by 2045 (5). Obesity, which can decrease internal sensitivity to insulin, may induce impaired glucose tolerance and even diabetes (36). Moreover, metabolic changes due to obesity and diabetes may directly or indirectly contribute to cancer progression (37). High BMI is causally linked to various cancers, including PC (38, 39). Cumulative risk effects for developing PC may arise from diabetes accompanied by weight loss (40).
In our study, we demonstrated an increasing trend in the global disease burden for PC mortality attributed to HFPG from 1990 to 2019, and this trend is expected to continue over the next 20 years in both sexes. Furthermore, we found that the death burden was significantly concentrated in the elderly population, which also experienced a more pronounced increase. Additionally, the mortality difference between men and women was not obvious, which was similar to the prevalence characteristics of diabetes (5). Moreover, higher SDI regions had higher ASDRs of PC attributed to HFPG from 1990 to 2019. This could result from factors such as aging populations, inherited conditions, and lifestyles with long-term exposure to risk factors more prevalent in high SDI countries, including obesity or malnutrition, alcohol abuse, and high-calory diets. The Western diet, comprising high red meat, refined grains, and sugar-sweetened beverages, may contribute to enhancing systemic inflammation agents and, consequently, developing PC (28). However, our study observed more pronounced increasing trends in ASDRs in low to middle-SDI, low-SDI, and middle-SDI regions. In fact, in the Asia-Pacific region, particularly in nations experiencing rapid economic development, the burden of PC is also substantially increasing (41). The variation in increasing trends could stem from exposure to environmental risk factors and lifestyle transformations in the context of globalization. Moreover, the lack of effective screening techniques and poorer medical conditions in low-SDI and middle-SDI countries should also be considered (3, 6, 28, 42). Globally, 87.5% of all undiagnosed diabetes cases occur in low- and middle-income countries, with low-income countries accounting for the largest proportion (50.5%), and even in high-income countries, approximately 28.8% of those with diabetes remain undiagnosed (5).
The present study had several limitations. Firstly, our data source was derived from the GBD 2019, an online database providing predictive information on disease burden, rather than actual data from monitoring and surveillance. Secondly, the etiology of PC is complex, and single attribution is often inadequate to explain the development of this malignancy, despite GBD 2019 excluding other risk factors when processing HFPG exposure data. Thirdly, the projection of disease burden for PC attributable to HFPG relied on mathematical algorithms, and our findings require confirmation through actual epidemiological investigation and data generalization.
Fortunately, the major risk factors related to PC are potentially modifiable, offering an opportunity to prevent this deadly cancer. Positive lifestyle interventions have been shown to delay the onset of diabetes by 4 years, cause all-cause death by 4.82 years, and increase average life expectancy by 1.44 years in populations with impaired glucose tolerance (36). Additionally, intentional weight loss may help prevent the onset of cancer, and disease control of diabetes could serve as a resultful adjuvant to inhibit tumor progression (37). Changes in disease attribution affect the direction of health promotion efforts. Our study emphasized the importance of addressing HFPG, prompting policymakers to allocate resources more efficiently for early diagnosis and reduction of this modifiable risk factor for PC. Strategies to prevent and ameliorate the unhealthy status of HFPG through dietary adjustments, appropriate daily exercise, and medication use as appropriate could help mitigate the risk of PC.
In conclusion, the burden of PC attributed to HFPG has increased globally over the past three decades, with the elderly population and high-SDI regions bearing a relatively greater disease burden. However, more unfavorable trends were observed in low-SDI areas. Moreover, the burden is projected to continue increasing over the next 20 years. Thus, tailored methodologies should be established for prevention to mitigate this increasing trend.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YW: Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ZQ: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XL: Data curation, Formal analysis, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XZ: Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing. HH: Methodology, Software, Validation, Visualization, Writing – original draft. CL: Investigation, Software, Validation, Writing – review & editing. WQ: Formal analysis, Visualization, Writing – review & editing. GZ: Conceptualization, Data curation, Project administration, Writing – review & editing. HS: Data curation, Project administration, Writing – review & editing. TP: Conceptualization, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Firstly, I would like to express my gratitude to the authors of this article and thank them for their help and dedication from the end. Second, we would like to thank the GBD 2019 collaborators and thanks to Xiao Ming (Xiaoming_room@hotmail.com) for his work in the GBD database. Finally, the authors would like to thank all the staff in the editorial department, and thanks for all your valuable comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1302436/full#supplementary-material
1. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. (2011) 378:607–20. doi: 10.1016/S0140-6736(10)62307-0
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. (2021) 18:493–502. doi: 10.1038/s41575-021-00457-x
4. Tempero MA. NCCN guidelines updates: Pancreatic cancer. J Natl Compr Cancer Netw: JNCCN. (2019) 17:603–5. doi: 10.6004/jnccn.2019.5007
5. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
6. GBD 2017 Pancreatic Cancer collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2019) 4:934–47. doi: 10.1016/s2468-1253(19)30347-4
7. Yu J, Yang X, He W, Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int J Cancer. (2021) 149:993–1001. doi: 10.1002/ijc.33617
8. Khalaf N, El-Serag HB, Abrams HR, Thrift AP. Burden of pancreatic cancer: From epidemiology to practice. Clin Gastroenterol hepatology: Off Clin Pract J Am Gastroenterological Assoc. (2021) 19:876–84. doi: 10.1016/j.cgh.2020.02.054
9. Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev. (2020) 41(1):bnz014. doi: 10.1210/endrev/bnz014
10. Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: The role of pharmacotherapy. J Clin oncology: Off J Am Soc Clin Oncol. (2016) 34:4261–9. doi: 10.1200/JCO.2016.67.4044
11. Huang Y, Liu F, Chen AM, Yang PF, Peng Y, Gong JP, et al. Type 2 diabetes prevention diet and the risk of pancreatic cancer: A large prospective multicenter study. Clin Nutr (Edinburgh Scotland). (2021) 40:5595–604. doi: 10.1016/j.clnu.2021.09.037
12. Ge XJ, Du YX, Zheng LM, Wang M, Jiang JY. Mortality trends of liver cancer among patients with type 2 diabetes at the global and national level. J Diabetes its Complications. (2020) 34:107612. doi: 10.1016/j.jdiacomp.2020.107612
13. Safiri S, Nejadghaderi SA, Karamzad N, Kaufman JS, Carson-Chahhoud K, Bragazzi NL, et al. Global, regional and national burden of cancers attributable to high fasting plasma glucose in 204 countries and territories, 1990-2019. Front Endocrinol. (2022) 13:879890. doi: 10.3389/fendo.2022.879890
14. Tondro Anamag F, Noori M, Nejadghaderi SA, Sullman MJM, Grieger JA, Kolahi AA, et al. Burden of cancers attributable to high fasting plasma glucose in the Middle East and North Africa region, 1990-2019. Cancer Med. (2023) 12:10031–44. doi: 10.1002/cam4.5743
15. Murray CJL. The Global Burden of Disease Study at 30 years. Nat Med. (2022) 28:2019–26. doi: 10.1038/s41591-022-01990-1
16. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
17. Menini S, Iacobini C, de Latouliere L, Manni I, Ionta V, Blasetti Fantauzzi C, et al. The advanced glycation end-product N(ϵ) -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention. J Pathol. (2018) 245:197–208. doi: 10.1002/path.5072
18. GBD 2019 Viewpoint Collaborators. Five insights from the global burden of disease study 2019. Lancet. (2020) 396:1135–59. doi: 10.1016/S0140-6736(20)31404-5
19. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/s0140-6736(20)30752-2
20. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1923–94. doi: 10.1016/s0140-6736(18)32225-6
21. Hankey BF, Ries LA, Kosary CL, Feuer EJ, Merrill RM, Clegg LX, et al. Partitioning linear trends in age-adjusted rates. Cancer causes control: CCC. (2000) 11:31–5. doi: 10.1023/A:1008953201688
22. Jiang L, Wang A, Yang S, Fang H, Wang Q, Li H, et al. The burden of gastric cancer attributable to high sodium intake: A longitudinal study from 1990 to 2019 in China. Nutrients. (2023) 15(24):5088. doi: 10.3390/nu15245088
23. Hu W, Fang L, Ni R, Zhang H, Pan G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer. (2022) 22:836. doi: 10.1186/s12885-022-09940-3
24. Riebler A, Held L. Projecting the future burden of cancer: Bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biometrical J Biometrische Z. (2017) 59:531–49. doi: 10.1002/bimj.201500263
25. Jürgens V, Ess S, Cerny T, Vounatsou P. A Bayesian generalized age-period-cohort power model for cancer projections. Stat Med. (2014) 33:4627–36. doi: 10.1002/sim.6248
26. Li S, Chen H, Man J, Zhang T, Yin X, He Q, et al. Changing trends in the disease burden of esophageal cancer in China from 1990 to 2017 and its predicted level in 25 years. Cancer Med. (2021) 10:1889–99. doi: 10.1002/cam4.3775
27. Andersen DK, Korc M, Petersen GM, Eibl G, Li D, Rickels MR, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. (2017) 66:1103–10. doi: 10.2337/db16-1477
28. Quoc Lam B, Shrivastava SK, Shrivastava A, Shankar S, Srivastava RK. The Impact of obesity and diabetes mellitus on pancreatic cancer: Molecular mechanisms and clinical perspectives. J Cell Mol Med. (2020) 24:7706–16. doi: 10.1111/jcmm.15413
29. Pang W, Yao W, Dai X, Zhang A, Hou L, Wang L, et al. Pancreatic cancer-derived exosomal microRNA-19a induces β-cell dysfunction by targeting ADCY1 and EPAC2. Int J Biol Sci. (2021) 17:3622–33. doi: 10.7150/ijbs.56271
30. Han L, Ma Q, Li J, Liu H, Li W, Ma G, et al. High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS One. (2011) 6:e27074. doi: 10.1371/journal.pone.0027074
31. Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem. (2011) 347:95–101. doi: 10.1007/s11010-010-0617-0
32. Zhou C, Qian W, Li J, Ma J, Chen X, Jiang Z, et al. High glucose microenvironment accelerates tumor growth via SREBP1-autophagy axis in pancreatic cancer. J Exp Clin Cancer research: CR. (2019) 38:302. doi: 10.1186/s13046-019-1288-7
33. Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. (2016) 1:226–37. doi: 10.1016/S2468-1253(16)30106-6
34. Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, et al. Hyperglycemia, insulin resistance, impaired pancreatic β-cell function, and risk of pancreatic cancer. J Natl Cancer Institute. (2013) 105:1027–35. doi: 10.1093/jnci/djt123
35. Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. (2023) 35:414–428.e413. doi: 10.1016/j.cmet.2023.02.003
36. Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. (2019) 7:452–61. doi: 10.1016/S2213-8587(19)30093-2
37. Gallagher EJ, LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol Rev. (2015) 95:727–48. doi: 10.1152/physrev.00030.2014
38. Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: An analysis of the Global Burden of Disease Study. PLoS Med. (2020) 17:e1003198. doi: 10.1371/journal.pmed.1003198
39. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
40. Yuan C, Babic A, Khalaf N, Nowak JA, Brais LK, Rubinson DA, et al. Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol. (2020) 6:e202948. doi: 10.1001/jamaoncol.2020.2948
41. Ranganath R, Chu Q. Global trends in pancreas cancer among Asia-Pacific population. J gastrointestinal Oncol. (2021) 12:S374–s386. doi: 10.21037/jgo-20-118
Keywords: pancreatic cancer, high fasting plasma glucose, diabetes, global trend, projection
Citation: Wei Y, Qin Z, Liao X, Zhou X, Huang H, Lan C, Qin W, Zhu G, Su H and Peng T (2024) Pancreatic cancer mortality trends attributable to high fasting blood sugar over the period 1990–2019 and projections up to 2040. Front. Endocrinol. 15:1302436. doi: 10.3389/fendo.2024.1302436
Received: 26 September 2023; Accepted: 13 June 2024;
Published: 05 July 2024.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Sudhanshu Kumar Bharti, Patna University, IndiaCopyright © 2024 Wei, Qin, Liao, Zhou, Huang, Lan, Qin, Zhu, Su and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Peng, cGVuZ3Rhb2dtdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.