- 1Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Provincial Clinical Research Center for Diabetes and Metabolic Disorders, Department of Endocrinology, Union Hospital, Wuhan, China
- 3Department of Emergency Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Non-invasive prognostic predictors for rare pancreatic neuroendocrine tumors (PNETs) are lacking. We aimed to approach the prognostic value of preoperative systemic inflammatory markers in patients with PNETs.
Methods: The clinical data of 174 patients with PNETs undergoing surgical treatment were retrospectively analyzed to explore the correlation of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), and platelet to white blood cell ratio (PWR) with clinicopathological parameters and the progression of tumor after the operation. The optimal cutoff values for predictors and the area under the curve (AUC) of the receiver operating characteristic (ROC) were estimated. Univariate and multivariate Cox proportional hazards models were used to assess the relation between NLR, LMR, PLR, and progression-free survival (PFS), examined by the Kaplan–Meier and log-rank tests.
Results: The scores of the NLR (P = 0.039) and PLR (P = 0.011) in the progression group were significantly higher than those in the progression-free group, and the LMR was significantly lower than those in the progression-free group (P = 0.001). The best cutoff values of NLR, LMR, and PLR before operation were 2.28, 4.36, and 120.91. The proportions of tumor progression in the high NLR group (P = 0.007) and high PLR group (P = 0.013) obviously increased, and the proportion of tumor development in the low LMR group was higher than that in the high LMR group (P < 0.001). The K-M survival curve showed that the progression-free survival rate was lower in the high NLR group (P = 0.004), the low LMR group (P < 0.001), and the high PLR group (P = 0.018). The results of the multivariate Cox proportional hazards model suggested that preoperative LMR (HR = 3.128, 95% CI: 1.107~8.836, P = 0.031) was an independent predictor of PFS.
Conclusion: The markers of systemic inflammation, especially LMR, can predict the postoperative progression of PNETs.
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare tumors of pancreatic endocrine origin and show an increasing rate of incidence, accounting for approximately 2% of pancreatic tumors (1, 2). Survival rates have not kept pace with incidence, and improved understanding, diagnosis, and treatments are urgently required. Metastasis, malignant transformation, and significant heterogeneity are hallmarks, and surgery is the main therapeutic approach (3, 4). Patients remain at risk of recurrence and metastasis post-surgery, and preoperative prediction of prognosis would aid treatment strategies. Disease grading and staging are valuable predictors of PNET survival (5) but are complex, invasive, and expensive. Therefore, there is an urgent need for convenient and effective prognostic markers.
Cancer progression is acknowledged to be influenced by a complex interplay of tumor inflammation, including low-level chronic inflammation, characterized by a sustained increase in inflammatory cells and proinflammatory mediators (6–8). Systemic inflammation may be evaluated by the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), and platelet to white blood cell ratio (PWR), the assessments of which are simple, non-invasive, and low cost. Such ratios not only have predictive value in solid cancers but also are prognostic biomarkers for many malignancies, such as renal cell carcinoma, breast cancer, colorectal cancer, and pancreatic cancer (9–13). The above indexes are routinely measured; therefore, their clinical application value has gradually increased in recent years.
Due to the rarity of PNETs, there is a paucity of data exploring the relationship between systemic inflammatory factors and their prognosis. Several studies have confirmed NLR as a promising prognostic predictor for lymph node metastasis or recurrence in patients with PNETs. However, previous studies have mainly focused on NLR, and the effectiveness of other biomarkers, such as LMR, was not consistent (14–16). Therefore, it is necessary to further supplement clinical data for the prognostic value of markers of systemic inflammation in patients with PNETs after surgery.
This study intends to comprehensively explore the application value of NLR, PLR, LMR, and PWR in predicting the prognosis of PNETs so as to provide more abundant and comprehensive data support for clarifying the prognosis after tumor surgery.
Patients and methods
Study subjects
The data of PNET patients from the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from 2009 to 2021 were collected through the hospital’s electronic case system and retrospectively analyzed. One hundred eighty-two patients whose surgical specimens were pathologically examined and confirmed to be PNETs were included. The exclusion criteria were as follows: 1) presence of multiple endocrine neoplasia type 1 (MEN1), neurofibromatosis type 1 (NF1), or other genetic diseases; 2) hematological tests showed significant abnormalities such as too low or too high platelets, lymphocytes, etc.; 3) evidence of infection such as pyrexia, systemic inflammatory response, and other inflammatory conditions within 1 week before surgery; and 4) incomplete clinical data. Four patients were excluded due to incomplete data. One patient with primary hyperparathyroidism and pituitary tumors, one patient who had a blood routine that showed very low platelets, and two patients with severe preoperative inflection were excluded from the study. A total of 174 patients were ultimately included. This study was approved by the Committee for Medical Ethics of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Follow-up
Patients were followed up through outpatient visits or by telephone until June 2022. Study endpoint events were tumor progression, including metastasis and recurrence, or death from any cause. Progression-free survival (PFS) was defined as the number of months from surgical treatment to tumor progression or to the date of final follow-up.
Data collection
General patient data included age, sex, body mass index (BMI), smoking and drinking status, and history of past illness. Clinical data included routine blood examination and tumor markers such as carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), and neuron-specific enolase (NSE) within 1 week prior to surgery, tumor size and function, location, pathological information, and functioning status. Tumors that overproduce hormones may be associated with distinct clinical syndromes and are referred to as functional; those that do not secrete hormones, secrete them in minimal quantities, or secrete peptides that do not result in an obvious syndrome (e.g., pancreatic polypeptide) are termed non-functional (3). The cutoff values for normal CA125, CA199, and NSE are less than 35 U/mL, 37 U/mL, and 16.3 μg/L, respectively.
Statistical analysis
IBM SPSS Statistics software 26.0 and GraphPad Prism 7.0 were used for data analysis and plotting. For continuous variable data, such as age and BMI, the Kolmogorov–Smirnov test was used to analyze whether the data conformed to a normal distribution and expressed as mean ± standard deviation (x ± S). Comparisons were made by t-test. Non-normally distributed data such as inflammatory markers were expressed as median (M) and interquartile range (IQR) and compared by the Mann–Whitney U test. The chi-square test was utilized to evaluate count data, including gender, personal history, history of past illness, and tumor size. We used the receiver operating characteristic (ROC) curves, and the Youden index was calculated to find the optimal cutoff values for NLR, LMR, and PLR. The principle of the ROC curve is to assign multiple critical values to continuous variables, calculate the corresponding sensitivity and specificity at each critical value, and then plot a curve using sensitivity as the ordinate and 1-specificity as the abscissa. The best cutoff value refers to the best combination of sensitivity and specificity. The area under the curve (AUC) is defined as the area under the ROC curve. It is a measure of the model’s discriminatory power, where a higher AUC indicates better performance. The Kaplan–Meier curve and the log-rank test were used to analyze the relationship between NLR, LMR, and PLR with PFS rate. The Cox proportional hazards model was used to determine prognostic indicators by univariate analysis. Meaningful indicators were selected in the univariate analysis for multivariate analysis. A value of P <0.05 was considered to indicate statistical significance.
Result
General data and clinical pathological data
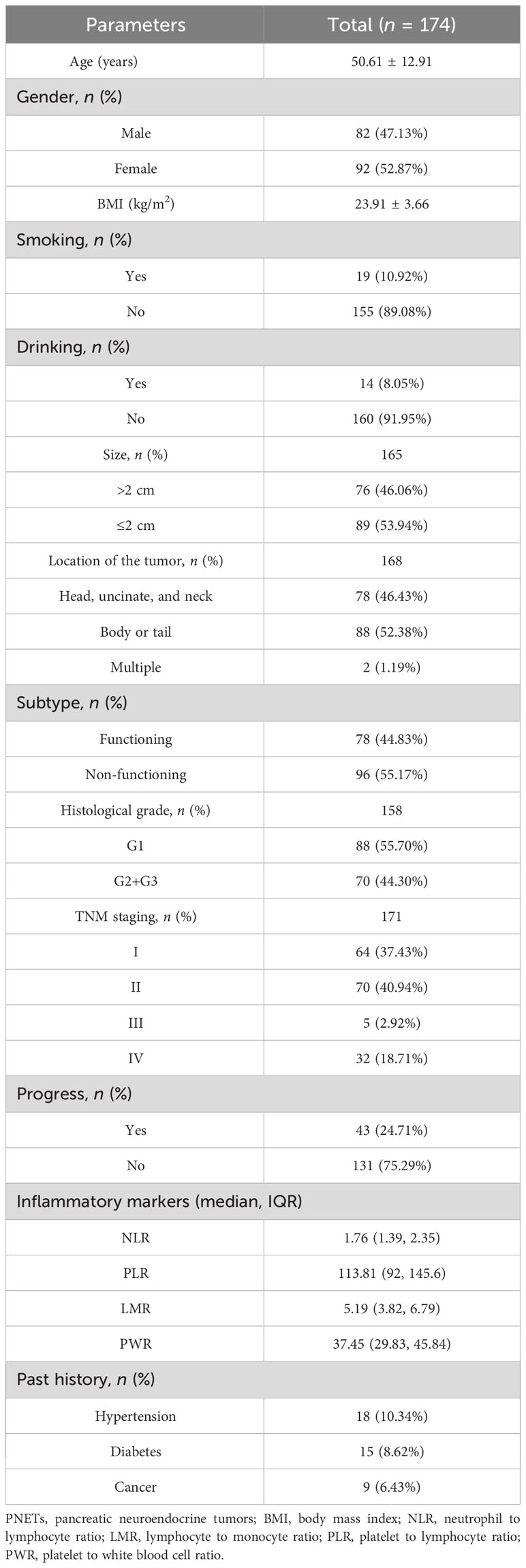
A total of 174 patients were eventually included in this study. The postoperative rates of tumor progression were 43/174 (24.71%), and 131/174 (75.29%) patients were progression-free. The mean patient age was 51.61, and 82 (47.13%) were men and 92 (52.87%) were women. The mean BMI was 23.91 kg/m2. Smokers and alcohol drinkers accounted for 10.92% and 8.05% of all patients, respectively. Eighteen patients (10.34%) had hypertension, 15 patients (8.62%) had diabetes, and 9 (6.43%) had a past history of other cancer types. Median NLR, PLR, LMR, and PWR were 1.76 (IQR, 1.39–2.35), 113.81 (IQR, 92–145.6), 5.19 (IQR, 3.82–6.79), and 37.45 (IQR, 29.83–45.84), respectively (Table 1).
We analyzed the characteristics of the tumor, such as size, location, and pathological stage. The median tumor diameter was 2 cm (range: 0.2–13 cm). Tumor diameter was >2 cm in 76 cases (46.06%) and ≤2 cm in 89 cases (53.94%). More patients had non-functional than functional tumors (55.17% vs. 44.83%). Seventy-eight (46.43%) were located in the pancreatic head and neck, and 88 (52.38%) were located in the body and tail. Two (1.19%) patients had multiple tumors. Differentiation was graded histologically according to the World Health Organization (WHO) pathological grading standard for gastrointestinal and pancreatic neuroendocrine tumors (17). Eighty-eight cases were poorly differentiated (G1), 65 moderately differentiated (G2), and 5 well-differentiated (G3). Comprehensive clinical staging was performed according to the TNM staging criteria of the 8th edition of the American Joint Commission on Cancer (AJCC) for PNETs (18). Sixty-four patients (37.43%) had stage I, 70 (40.94%) stage II, 5 (2.92%) stage III, and 32 (18.71%) stage IV (Table 1).
Markers of systemic inflammation and clinicopathological parameters
NLR was higher when tumor progression occurred relative to progression-free patients (median: 2.923 vs. 2.284, P = 0.039), as well as the PLR (median: 174.4 vs. 137.2, P = 0.011). LMR was lower in patients with progression than in those without (median: 3.733 vs. 4.857, P = 0.001). No significant difference in PWR was found (median: 40.28 vs. 38.41, P = 0.415; Figure 1). Cutoff values related to postoperative progression were determined from the ROC curve and gave optimal values of 2.28 for NLR, 4.36 for LMR, and 120.91 for PLR (Figure 2). Cutoff values enabled the patient cohort to be divided into high- and low-value groups. Higher proportions of tumors >2 cm (P = 0.001), more medium- to well-differentiated G2 and G3 tumors (P = 0.015), and significant correlation with TNM stage (P <0.001) were found among patients with high NLR. The low LMR group was associated with a greater number of smokers (P = 0.014) and worse tumor stage (P = 0.009). TNM stage was worse in the high PLR group (P = 0.016). No significant differences in gender, age, BMI, drinking history, tumor function, or tumor markers, such as CA125, CA199, and NSE, were present between the groups (Table 2).
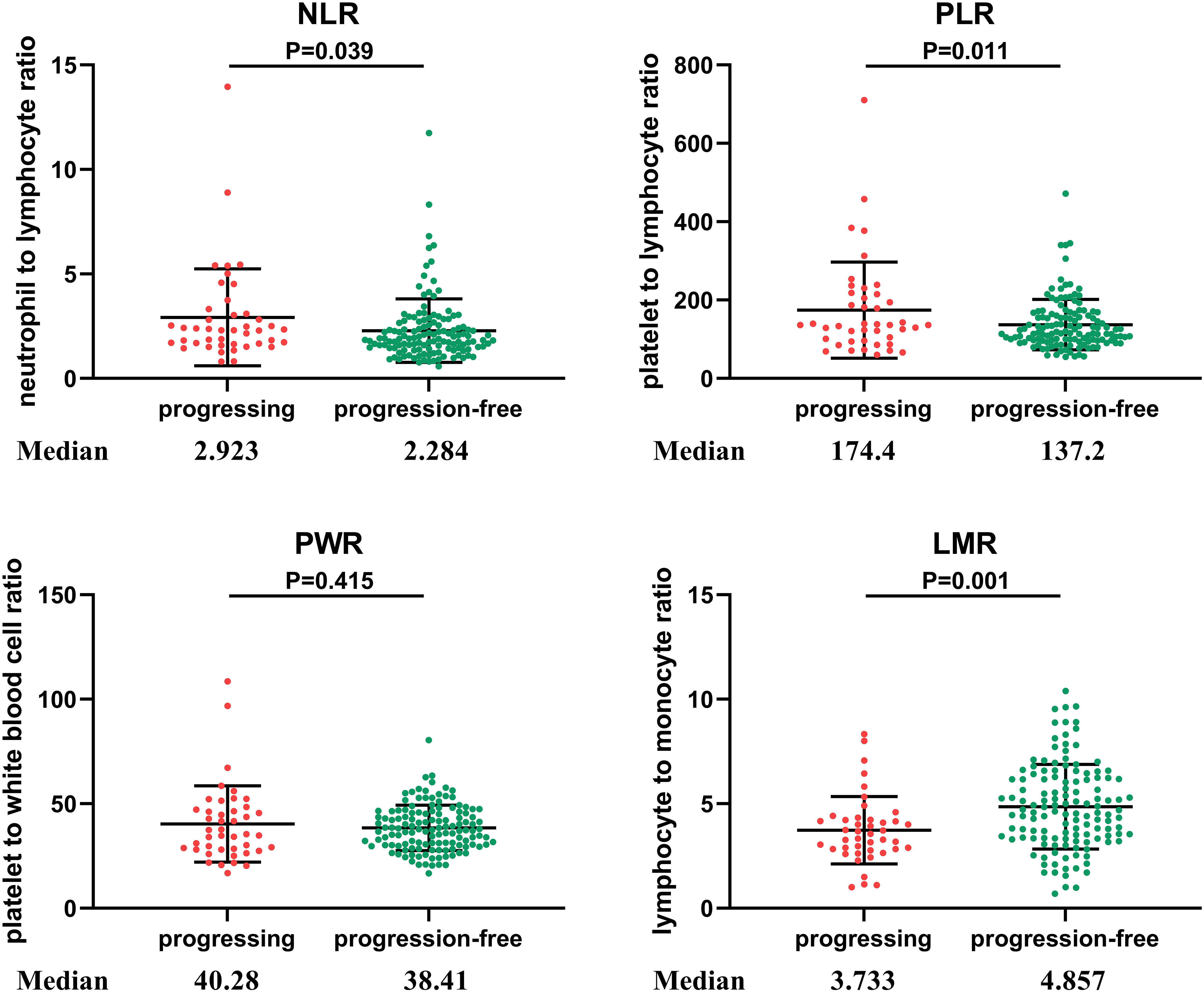
Figure 1 Distribution of inflammatory markers in PNETs. NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PWR, platelet to white blood cell ratio; LMR, lymphocyte to monocyte ratio. NLR was higher when tumor progression occurred relative to progression-free patients (P = 0.039), as was PLR (P = 0.011). LMR was lower in patients with progression than in those without progression (P = 0.001). No significant difference in PWR was found different between the two groups (P = 0.415).
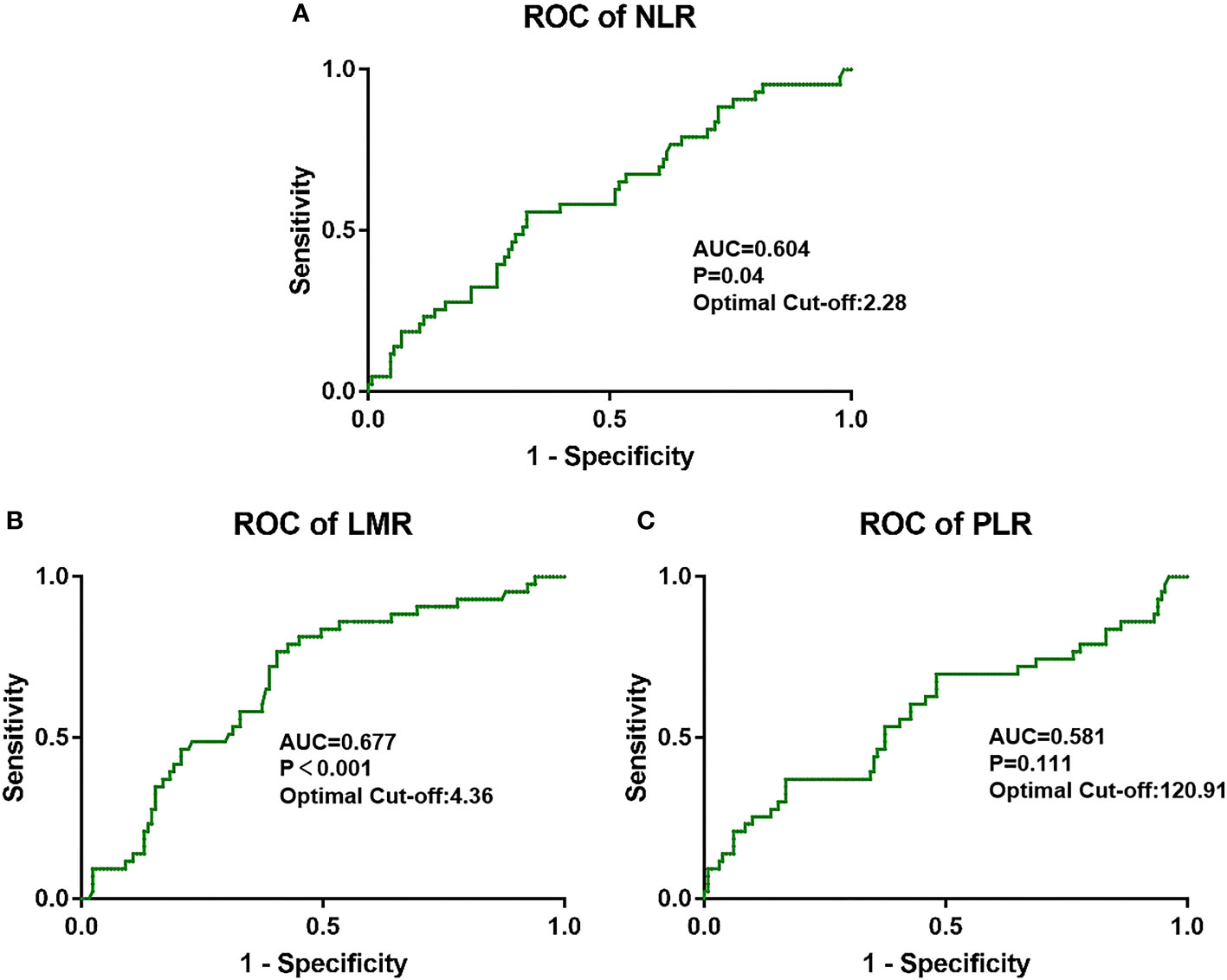
Figure 2 Optimal cutoff values for (A) NLR, (B) LMR, and (C) PLR and the defined area under the curve (AUC) were estimated from the receiver operating curve (ROC).
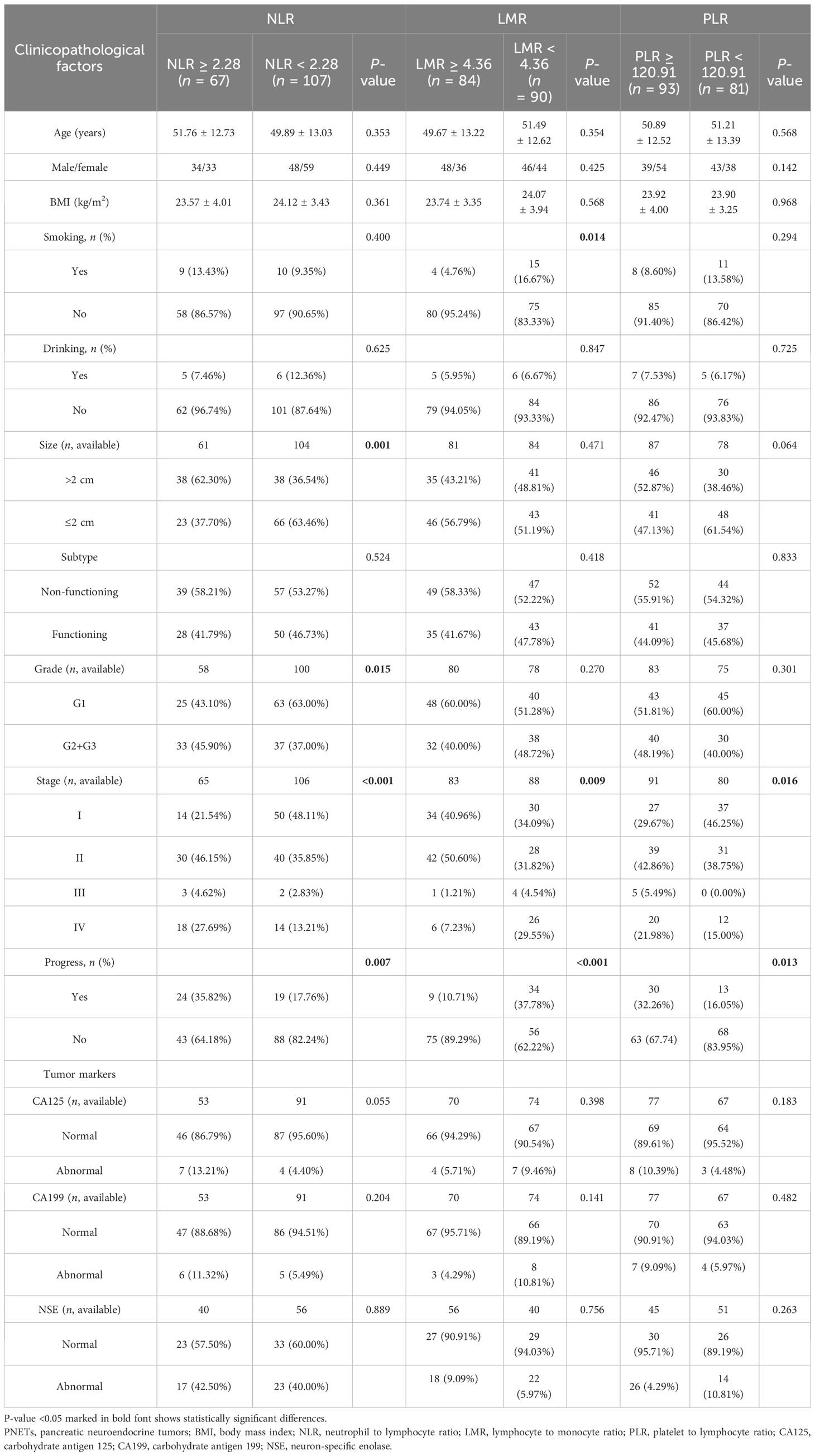
Table 2 Comparison of the clinicopathological factors between the two groups classified by NLR, LMR, and PLR.
NLR, LMR, PLR, and prognosis
The median follow-up time was 47 months and the mean PFS of the whole cohort was 54 months. Forty-three patients (24.71%) experienced tumor progression after surgery (Table 1). The proportion of tumor progression in the high NLR (35.82% vs. 17.76%, P = 0.007), low LMR (37.78% vs. 10.71%, P <0.001), and high PLR (32.26% vs. 16.05%, P = 0.013) groups was higher than in the opposing groups (Table 2). Kaplan–Meier survival analysis demonstrated decreased survival among patients with high NLR (P = 0.004), low LMR (P < 0.001), and high PLR (P = 0.018) (Figure 3).
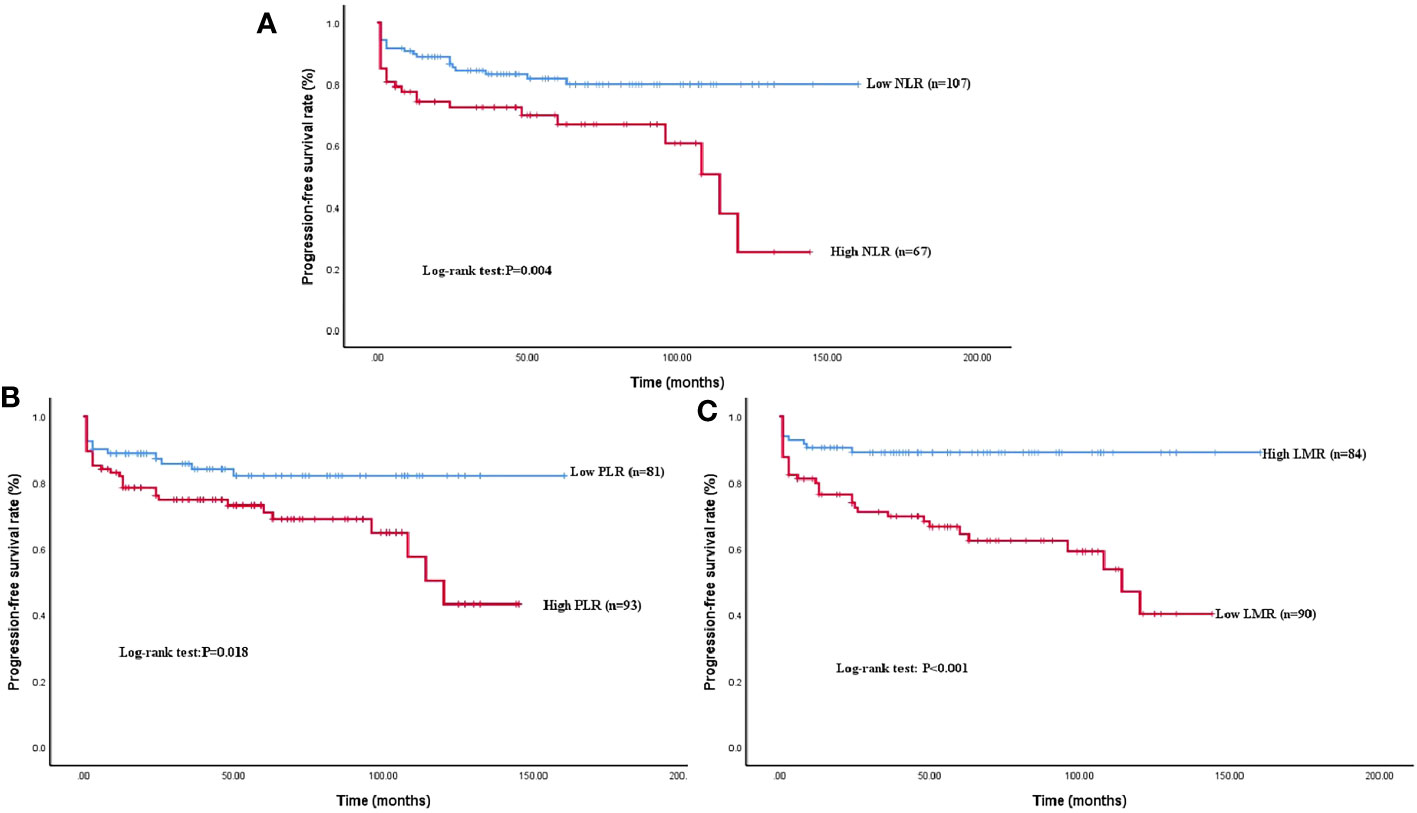
Figure 3 Kaplan–Meier survival curve analysis of PFS rate grouped by NLR, LMR, and PLR levels. A higher NLR (A), a higher PLR (B), and a low LMR (C) showed a significant correlation with shorter PFS (P = 0.004; P < 0.001; P = 0.018).
Single-factor Cox regression analysis showed that preoperative NLR, LMR, PLR, age, tumor function, tumor size, pathological grade, and CA199 were all significantly correlated with PFS. The risk of tumor progression was increased 2.352 times (95% CI: 1.286~4.303, P = 0.006) by high NLR, 3.742 times (95% CI: 1.794~7.804, P < 0.001) by low LMR, and 2.076 times by high PLR (95% CI: 1.083~3.982, P = 0.028). Statistically significant indicators were examined by multivariate Cox regression analysis. Preoperative LMR (HR: 3.128, 95% CI: 1.107~8.836, P = 0.031), pathological grade (HR: 5.433, 95% CI: 1.964~15.026, P = 0.001), and age (HR: 3.178, 95% CI: 1.214~8.320, P = 0.019) were all associated with PFS (Table 3). After adjustment for confounding factors, the postoperative risk of tumor progression was still greater in the low LMR than in the high LMR group.
Discussion
PNETs are rare and account for less than 3% of all primary pancreatic tumors (1, 2). The rarity has limited the availability of data regarding clinical features and prognosis. A greater number of male PNET patients than female patients has been reported in the United States (19), but the current study suggests a slightly greater number of female patients with PNETs in Hubei Province, China. The proportion of the current cohort with comorbid diabetes was also not consistent with the conclusion of Zhuge et al. (20), suggesting an influence of race and region. Complications, including hypertension and other tumors, are also summarized by the current report and may give data useful for the treatment of this rare tumor. The heterogeneous nature of PNETs leads to great variation in individual prognoses, illustrating the importance of identifying prognostic indicators.
Inflammation is linked to tumor initiation, transformation, invasion, and metastasis (8, 21). Increased numbers of neutrophils, platelets, and monocytes and decreased lymphocytes are non-specific manifestations of tumor-related inflammatory reactions, associated with poor prognosis for a variety of malignant tumors (22–25). The inflammatory role of neutrophils promotes tumor growth, invasion, angiogenesis, and metastasis by secreting reactive nitrogen species (RNS), proteases, reactive oxygen species (ROS), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and other cytokines (26–28). The systemic non-specific inflammatory response of thrombocytosis has long been recognized as an indicator of poor prognosis. Tumor cells interact with platelets to elicit hematogenous metastasis (29, 30). Macrophages derived from tumor-infiltrating monocytes secrete IL-10 and TGF-β to promote immunosuppression and mediate T lymphocyte dysfunction, allowing immune escape and tumor growth (31). The levels of peripheral blood lymphocytes reflect immune system activation, which acts to inhibit tumor cell proliferation and migration by activating cytotoxic lymphocytes (32, 33). Thus, diverse populations of blood cells regulate the balance between cancer, inflammation, and immunity. NLR and PLR are combined markers of inflammation and immune status, while LMR reflects antitumor immune activity. It can be seen that the level of NLR and PLR may be more susceptible to confounding factors. LMR may be more sensitive to the physiological state of the tumor.
The few studies on the markers of systemic inflammation in PNETs have focused on NLR, which has been shown to be an independent predictor of relapse-free survival (34). However, studies on the prognostic value of LMR have produced inconsistent findings. The current study analyzed the prognostic value of NLR, PLR, LMR, and PWR for PNET patients undergoing surgery. Single-factor Cox regression analysis showed high NLR and high PLR to be associated with tumor progression, reflecting the negative influence of a state of inflammation. However, NLR and PLR were not independently related to prognosis, suggesting the presence of confounding factors. Multivariate Cox analysis showed LMR to be an independent risk factor for predicting postoperative PNET progression. A cutoff value of LMR >4.36 had prognostic significance for identifying high-risk PNET patients.
We acknowledge some limitations to the current study. First, NLR, PLR, and LMR may be affected by inflammation, drugs, complications, and other factors. PNET patients are prone to surgical stress reactions and infection, which may affect the above indicators, but only preoperative levels were considered to minimize the interference of confounding factors. An in-depth exploration of complications and other confounding factors was outside the scope of the study. Secondly, this was a single-center, retrospective study, and the results may be biased. Finally, the sample size was small due to the low prevalence of PNETs. Therefore, a multicenter, large-sample prospective study is required for verification.
In conclusion, pathological tumor characteristics and predictors of postoperative PNET progression via accessible and low-cost blood tests were explored. The high risk of tumor progression and the merits of individualized treatment should be emphasized for patients with elevated preoperative inflammatory markers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Committee for Medical Ethics of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin.
Author contributions
LYa: Writing – original draft. MF: Writing – original draft. LYu: Writing – original draft. HW: Writing – original draft. XC: Writing – original draft. HS: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Perri G, Prakash LR, Katz MHG. Pancreatic neuroendocrine tumors. Curr Opin Gastroenterol (2019) 35(5):468–77. doi: 10.1097/MOG.0000000000000571
2. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589
3. Scott AT, Howe JR. Evaluation and management of neuroendocrine tumors of the pancreas. Surg Clinics North America (2019) 99(4):793–814. doi: 10.1016/j.suc.2019.04.014
4. Kawasaki K, Fujii M, Sato T. Gastroenteropancreatic neuroendocrine neoplasms: genes, therapies and models. Dis Models Mech (2018) 11(2):dmm029595. doi: 10.1242/dmm.029595
5. Deng BY, Liu F, Yin SN, Chen AP, Xu L, Li B. Clinical outcome and long-term survival of 150 consecutive patients with pancreatic neuroendocrine tumors: A comprehensive analysis by the World Health Organization 2010 grading classification. Clinics Res Hepatol Gastroenterol (2018) 42(3):261–8. doi: 10.1016/j.clinre.2017.09.004
6. Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer (2022) 8(11):901–14. doi: 10.1016/j.trecan.2022.07.002
7. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
8. Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: A troika governing cancer and its treatment. Cell (2016) 166(2):288–98. doi: 10.1016/j.cell.2016.05.051
9. Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin Chim Acta (2018) 481:142–6. doi: 10.1016/j.cca.2018.03.008
10. Song H, Jeong MJ, Cha J, Lee JS, Yoo JG, Song MJ, et al. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int J Med Sci (2021) 18(16):3712–7. doi: 10.7150/ijms.64658
11. Li Y, Wang C, Xu M, Kong C, Qu A, Zhang M, et al. Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. J Cancer Res Clin Oncol (2017) 143(5):861–71. doi: 10.1007/s00432-016-2330-1
12. Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J Gastroenterol (2019) 25(31):4383–404. doi: 10.3748/wjg.v25.i31.4383
13. Chen Y, Liao Y, Lam LM, He L, Tsang YS, Di YS, et al. Pretreatment biomarkers as prognostic predictors of survival in patients with Pancreatic Cancer treated with Gemcitabine-based Therapy and 5-Fluorouracil: Neutrophil-to-lymphocyte ratio vs Platelet-to-lymphocyte ratio. Int J Med Sci (2020) 17(10):1449–57. doi: 10.7150/ijms.46254
14. Chan DL, Yao JC, Carnaghi C, Buzzoni R, Herbst F, Ridolfi A, et al. Markers of systemic inflammation in neuroendocrine tumors: A pooled analysis of the RADIANT-3 and RADIANT-4 studies. Pancreas (2021) 50(2):130–7. doi: 10.1097/MPA.0000000000001745
15. Miura T, Ohtsuka H, Aoki T, Aoki S, Hata T, Takadate T, et al. Increased neutrophil-lymphocyte ratio predicts recurrence in patients with well-differentiated pancreatic neuroendocrine neoplasm based on the 2017 World Health Organization classification. BMC Surg (2021) 21(1):176. doi: 10.1186/s12893-021-01178-3
16. Panni RZ, Lopez-Aguiar AG, Liu J, Poultsides GA, Rocha FG, Hawkins WG, et al. Association of preoperative monocyte-to-lymphocyte and neutrophil-to-lymphocyte ratio with recurrence-free and overall survival after resection of pancreatic neuroendocrine tumors (US-NETSG). J Surg Oncol (2019) 120(4):632–8. doi: 10.1002/jso.25629
17. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975
18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA: Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
19. Greenberg JA, Ivanov NA, Egan CE, Lee YJ, Zarnegar R, Fahey TJ, et al. Sex-based clinicopathologic and survival differences among patients with pancreatic neuroendocrine tumors. J Gastrointestinal Surg (2022) 26(11):2321–9. doi: 10.1007/s11605-022-05345-6
20. Zhuge X, Wang Y, Chen X, Guo C. Diabetes in patients with pancreatic neuroendocrine neoplasms. Front Endocrinol (2020) 11:615082. doi: 10.3389/fendo.2020.615082
21. Aguilar-Cazares D, Chavez-Dominguez R, Marroquin-Muciño M, Perez-Medina M, Benito-Lopez JJ, Camarena A, et al. The systemic- level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front Endocrinol (2022) 13:929572. doi: 10.3389/fendo.2022.929572
22. Segal BH, Giridharan T, Suzuki S, Khan ANH, Zsiros E, Emmons TR, et al. Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells. Immunol Rev (2023) 314(1):13–35. doi: 10.1111/imr.13178
23. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52
24. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939
25. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev (2018) 32(19-20):1267–84. doi: 10.1101/gad.314617.118
26. Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci (2023) 24(4):4002. doi: 10.3390/ijms24044002
27. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci (2019) 20(3):529. doi: 10.3390/ijms20030529
28. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol (2022) 22(3):173–87. doi: 10.1038/s41577-021-00571-6
29. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol (2018) 11(1):125. doi: 10.1186/s13045-018-0669-2
30. Palacios-Acedo AL, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol (2019) 10:1805. doi: 10.3389/fimmu.2019.01805
31. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol (2019) 19(6):369–82. doi: 10.1038/s41577-019-0127-6
32. Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res (2015) 21(22):5047–56. doi: 10.1158/1078-0432.CCR-15-0685
33. Pruneri G, Vingiani A, Denkert C. Tumor infiltrating lymphocytes in early breast cancer. Breast (Edinburgh Scotland) (2018) 37:207–14. doi: 10.1016/j.breast.2017.03.010
Keywords: pancreatic neuroendocrine tumors, markers of systemic inflammation, Lymphocyte to monocyte ratio, biomarker, prognosis
Citation: Yang L, Fu M, Yu L, Wang H, Chen X and Sun H (2024) Value of markers of systemic inflammation for the prediction of postoperative progression in patients with pancreatic neuroendocrine tumors. Front. Endocrinol. 15:1293842. doi: 10.3389/fendo.2024.1293842
Received: 19 September 2023; Accepted: 15 January 2024;
Published: 01 February 2024.
Edited by:
Theodoros Michelakos, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Marco Ventin, Massachusetts General Hospital and Harvard Medical School, United StatesShahrzad Arya, Cedars Sinai Medical Center, United States
Lei Cai, Massachusetts General Hospital and Harvard Medical School, United States
Anastasios Karneris, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Yang, Fu, Yu, Wang, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Sun, c3Vubnk2OEBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Liu Yang
Liu Yang Mengfei Fu
Mengfei Fu Li Yu
Li Yu Hanyu Wang
Hanyu Wang Xiao Chen1,2
Xiao Chen1,2 Hui Sun
Hui Sun