- 1Graduate School, Xinjiang Medical University, Urumqi, China
- 2Department of Endocrinology, People’s Hospital of Xinjiang Uygur Autonomous Region, Xinjiang Clinical Research Center for Diabetes, Urumqi, China
Context: Although the role of insulin-like growth factor I (IGF-1) in nonalcoholic fatty liver disease (NAFLD) has garnered attention in recent years, few studies have examined both reduced and elevated levels of IGF-1.
Objective: The aim of this study was to examine the potential relationship between IGF-1 levels and the risk of new-onset NAFLD in patients with pituitary neuroendocrine tumors (PitNET).
Methods: We employed multivariable Cox regression models and two-piecewise regression models to assess the association between IGF-1 and new-onset NAFLD. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated to quantify this association. Furthermore, a dose-response correlation between lgIGF-1 and the development of NAFLD was plotted. Additionally, we also performed subgroup analysis and a series sensitivity analysis.
Results: A total of 3,291 PitNET patients were enrolled in the present study, and the median duration of follow-up was 65 months. Patients with either reduced or elevated levels of IGF-1 at baseline were found to be at a higher risk of NAFLD compared to PitNET patients with normal IGF-1(log-rank test, P < 0.001). In the adjusted Cox regression analysis model (model IV), compared with participants with normal IGF-1, the HRs of those with elevated and reduced IGF-1 were 2.33 (95% CI 1.75, 3.11) and 2.2 (95% CI 1.78, 2.7). Furthermore, in non-adjusted or adjusted models, our study revealed a U-shaped relationship between lgIGF-1 and the risk of NAFLD. Moreover, the results from subgroup and sensitivity analyses were consistent with the main results.
Conclusions: There was a U-shaped trend between IGF-1 and new-onset NAFLD in patients with PitNET. Further evaluation of our discoveries is warranted.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as a leading cause of chronic liver disease globally and is likely to be a major contributor to end-stage liver failure in the future (1–3). NAFLD is a complex liver disease characterized by steatosis, hepatic inflammation, fibrosis (4–6) and is closely associated with other metabolic disorders (7, 8). In addition, individuals with NAFLD are more likely to experience all-cause mortality, with cardiovascular events being the primary cause among those affected (9, 10).
Pituitary neuroendocrine tumors (PitNET) include functional tumors that autonomously secrete pituitary hormones and nonfunctional tumors that are not associated with excess hormones (11, 12). Growth hormone (GH)-secreting pituitary adenomas, marked by elevated levels of GH and IGF-1, account for approximately 12% of functional pituitary tumors (13). Persistently increased secretion of GH and IGF-1 can result in liver hypertrophy, which is a form of visceral hypertrophy, and can disrupt glucose and lipid metabolism, leading to multiple comorbidities (12). A meta-analysis has reported a 2-fold increase in all-cause mortality among patients with functional GH cell adenomas (14). However, one recent study has shown that patients who receive appropriate treatment and maintain normal serum IGF-1 levels are not at increased risk of death (15).
PitNET can also cause hypopituitarism due to its occupying effect (16), and the deficiency of pituitary hormones usually begins with the GH/IGF-1 axis (17). However, GH/IGF-1 deficiency is often not adequately addressed in hormone replacement therapy (18). Indeed, several reports have defined a significant connection between NAFLD and the GH/IGF-1 axis (4, 19). Patients with NAFLD have reduced levels of IGF-1 (20), and IGF-1 deficiency can facilitate the development and advancement of NAFLD (21). In adolescents and adults with NAFLD patients, recombinant human growth hormone (rhGH) supplementation therapy has been shown to significantly improve hepatic steatosis and fibrosis (22, 23). Additionally, IGF-1 has demonstrated anti-fibrotic properties in rodent models of NAFLD (24).
GH-secreting pituitary adenomas can result in overproduction of IGF-1, while other types of PitNET can lead to IGF-1 deficiency due to their occupying effect. This makes individuals with PitNET an ideal group to study the connection between IGF-1 levels and NAFLD. Generally, reduced IGF-1 levels are associated with NAFLD. However, there may also be positive connections between elevated IGF-1 and NAFLD, indicating a potential nonlinear relationship between IGF-1 levels and NAFLD occurrence. Consequently, we hypothesize that in individuals with PitNET, both elevated and reduced levels of IGF-1 are linked to an increased risk of developing NAFLD.
2 Materials and methods
2.1 Study population
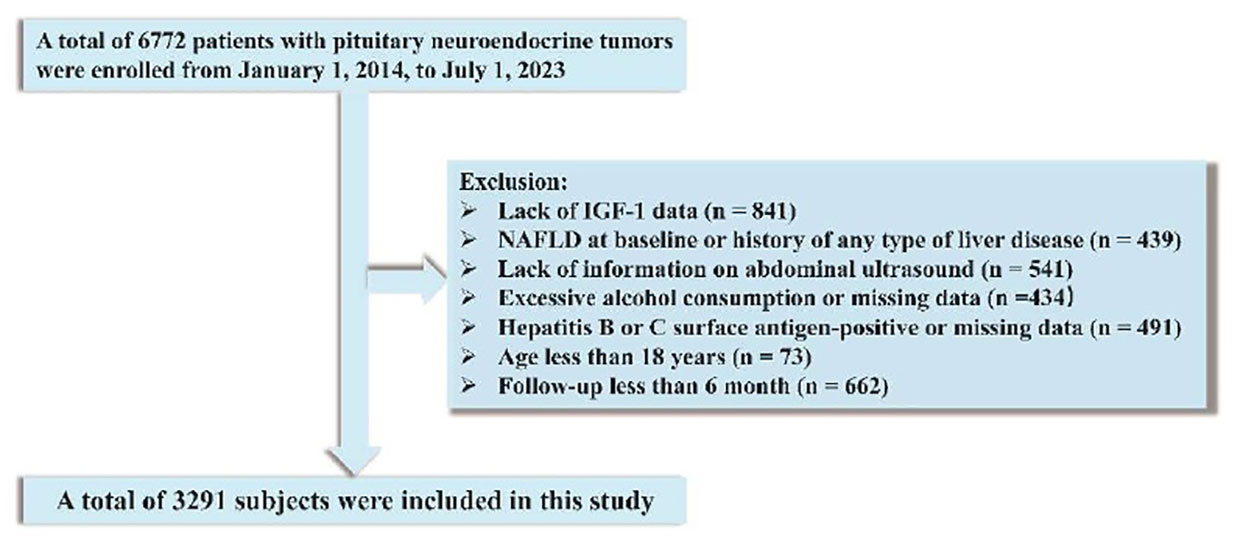
We recruited a total of 6772 patients diagnosed with PitNET in the period from January 1, 2014, to July 1, 2023, at the People’s Hospital of Xinjiang Uygur Autonomous Region. Excluded were patients with no available data on IGF-1 (n = 841), preexisting NAFLD or any type of liver disease (n = 439), lack of information on abdominal ultrasound (n = 541), excessive alcohol consumption or unavailability of data on alcohol consumption (n = 434), hepatitis B or C antigen-positive status or missing data (n = 491), age less than 18 years (n = 73), and follow-up time less than 6 month (n = 662). Ultimately, the final analysis included 3,291 patients after these exclusions (Figure 1). Approval was received from the Ethical Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region (No.KY2023013149). The need for written informed consent was waived owing to the retrospective nature of the study. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline recommendations for reporting our findings (25).
2.2 Data collection
2.2.1 Clinical variables collection
Information on demographics, lifestyle behaviors, and medical history was extracted from the electronic medical records of all study participants. Weight, height, and blood pressure were measured using standard protocols. Details regarding variable definitions are provided in the Supplementary Materials. Medical history was assessed according to International Classification of Diseases, 10th Revision (ICD-10) codes. Medical history included diabetes, hypertension, hyperlipemia, NAFLD, and any type of liver disease.
2.2.2 Lab testing
Participants were fasting for at least eight hours before the blood draw. Fasting blood glucose (FBG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG), uric acid (UA), and hypersensitive C-reactive protein (hs-CRP) were measured in the central laboratory of the People’s Hospital of Xinjiang Uygur Autonomous Region. IGF-1 was quantified using the DPC Immulite 2000 chemiluminescence analyzer (Siemens AG, Munich, Germany) by the immunochemiluminescence method. Serum prolactin is measured by immunometric assays. Adrenal insufficiency had to be established through appropriate stimulation tests (26). Assessment of additional function of the pituitary using baseline measurements of free triiodothyronine, free thyroxine, thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (in males) or estradiol (in females). Details of pituitary function tests are provided in the Supplementary Materials.
2.3 Definition
IGF-1 at baseline was categorized into three groups based on age- and sex-adjusted reference ranges. Reduced IGF-1, namely, IGF-1<-2 standard deviation score (SDS) for sex and age (27). Elevated IGF-1, namely, IGF-1>2SDS for sex and age.
2.4 Follow-up and outcome assessment
Participants were assessed for NAFLD by abdominal ultrasound once a year during follow-up. NAFLD was diagnosed based on the diagnostic criteria established previously (28–30). The diagnostic criteria for NAFLD are provided in the Supplementary Materials.
The follow-up period (measured in months) of each participant was calculated from the baseline date to the earliest of the subsequent dates: occurrence of NAFLD, death, loss to follow-up, or July 1, 2023 (the end of the study’s follow-up), for patients who did not have an endpoint event during follow-up but had their last clinical visit. The outcome was the first occurrence of NAFLD. These outcome events were reviewed and adjudicated centrally by an independent clinical events committee.
2.5 Statistical analysis
IGF-1 at baseline was categorized into three groups according to age- and sex- adjusted reference ranges: normal IGF-1, elevated IGF-1, and reduced IGF-1. The cumulative incidence of NAFLD across different IGF-1 categories was assessed and compared by using Kaplan-Meier plots and log-rank tests. We evaluated collinearity in all models by measuring the variance inflation factor (VIF) and using a pre-set threshold of 5 as an indication of multicollinearity (Supplementary Table 1). Any variables with a VIF above 5 were excluded. Hazard ratios (HRs) and 95% confidence intervals (CIs) for NAFLD were calculated by univariable and multivariable models with Cox regression analysis. IGF-1 was assessed as a categorical and continuous variable. Given a non-normal distribution, IGF-1 was lg10-transformed for the continuous model. The dose-response relationship between lgIGF-1 and the risk of developing NAFLD was plotted in non-adjusted and adjusted models. Concerning the nonlinear relationship, we utilized a two-piecewise linear regression model to investigate the threshold effect of lgIGF-1 on the occurrence of NAFLD, employing a smoothing function. Moreover, we performed subgroup analyses and reported interactions. In addition, sensitivity analyses were performed to ensure the robustness of our findings. Detailed information regarding the statistical analysis can be found in the Supplementary Materials. Statistical tests were performed using R version 4.1.1, and P < 0.05 (two-sided) was considered statistically significant.
3 Results
3.1 Characteristics of study participants
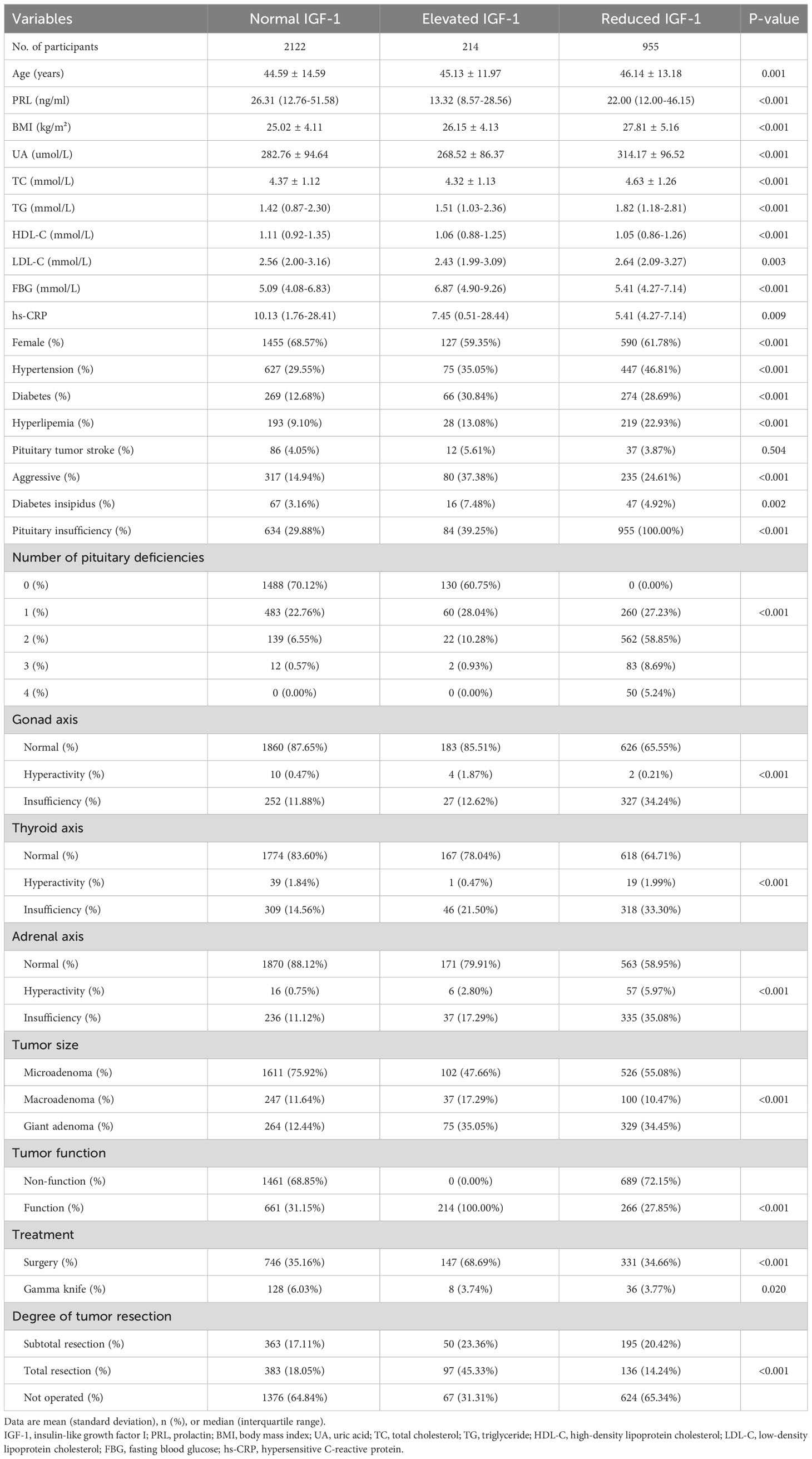
Baseline characteristics of the participants grouped by IGF-1 are shown in Table 1. We divided IGF-1 into three groups according to age- and sex-adjusted reference ranges. Totally, 3291 subjects were enrolled in this cohort study, of whom 2122 (64.5%) were classified as normal IGF-1, 214(6.5%) were classified as elevated IGF-1, and 955(29%) were classified as reduced IGF-1. The mean age of the participants was 45.08 ± 14.05 years, with females accounting for 66%. Compared with patients with normal IGF-1, those with elevated IGF-1 and reduced IGF-1 were more likely to have a higher BMI, UA, TG, FBG, number of pituitary deficiencies, and lower HDL-C. They were also more likely to have hypertension, diabetes, hyperlipemia, diabetes insipidus, aggressive tumors, pituitary insufficiency, gonad axis insufficiency, thyroid axis insufficiency, adrenal axis insufficiency, and a giant adenoma at baseline.
3.2 Relationship between IGF-1 and new-onset NAFLD
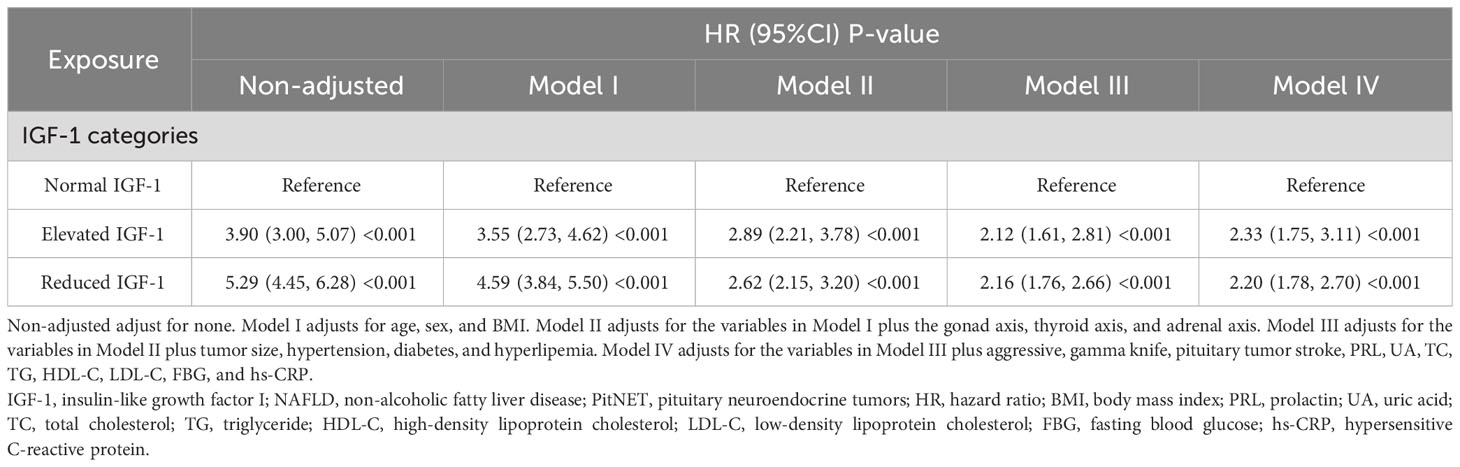
During a median follow-up of 65 months, 698 NAFLD events occurred. Kaplan-Meier curve graphs confirmed that patients in the group with reduced IGF-1 and elevated IGF-1 levels at baseline had a greater likelihood of suffering NAFLD compared to patients in the normal IGF-1 group (log-rank test, P < 0.001) (Figure 2). Table 2 displays associations between IGF-1 levels and the risk of NAFLD. IGF-1 was evaluated as a ranked variable using age- and sex-adjusted reference ranges. In Cox regression analyses, we observed that both reduced and elevated IGF-1 levels were associated with an increased risk of NAFLD in non-adjusted and adjusted models. Compared with participants with normal IGF-1, the HRs of those with elevated and reduced IGF-1 were 2.33 (95% CI 1.75, 3.11) and 2.2 (95% CI 1.78, 2.7) in model IV, respectively.
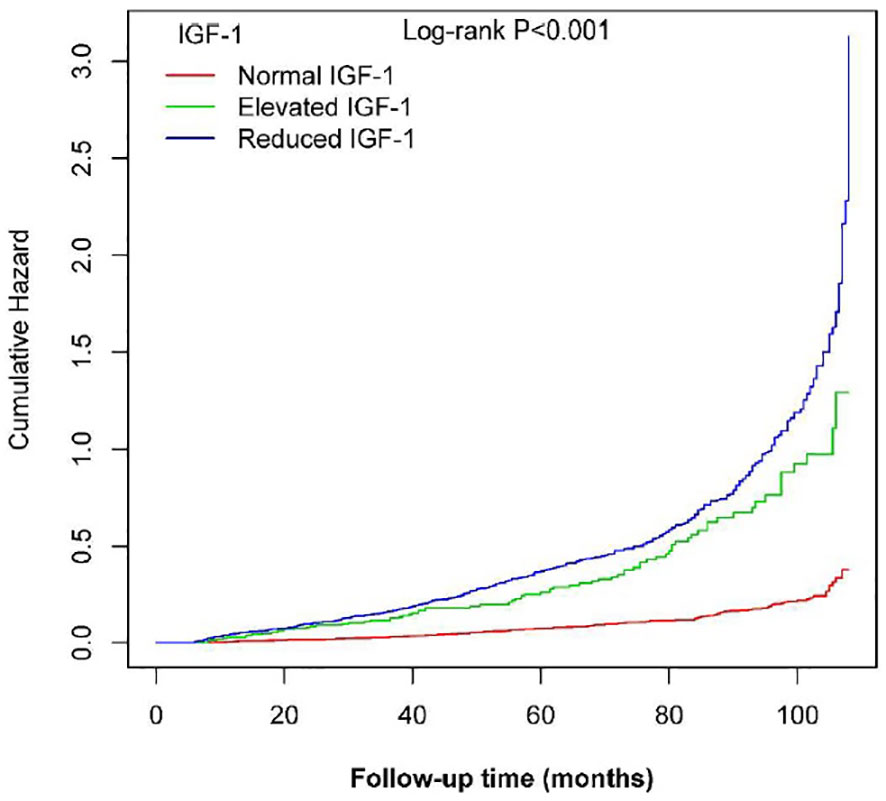
Figure 2 Kaplan-Meier curves showing the cumulative hazards of new-onset NAFLD based on categories in PitNET patients.
3.3 Sensitivity analysis
We carried out the following sensitivity analyses. To minimize the reverse causation, we excluded instances that occurred within the first 2 years of follow-up, and the results remained consistent (Supplementary Table 2). Also, the relationship of IGF-1 with the risk of NAFLD development remained robust after removing patients with aggressive PitNET (Supplementary Table 3). Furthermore, reduced IGF-1 and elevated IGF-1 were still associated with a significantly higher risk of NAFLD after excluding patients with giant PitNET (Supplementary Table 4). Additionally, in patients with PitNET who underwent surgery, the association of IGF-1 with the risk of new-onset NAFLD was also observed (Supplementary Table 5). Likewise, the results remained similar after considering death as a competing risk (Supplementary Table 6). These sensitivity analyses did not materially alter the risk estimates.
3.4 Dose-response and threshold effect analysis
The dose-response and threshold effects between IGF-1 levels and the risk of NAFLD in PitNET patients are shown in Table 3 and Figure 3. The association between IGF-1 levels and NAFLD rates followed a U-shaped curve, with both reduced and elevated IGF-1 levels significantly correlating with higher rates of NAFLD. To elucidate the nonlinear relationship, a recursive algorithm and two-piecewise linear analysis were utilized to identify the inflection point of lgIGF-1, which was determined to be 2.35 ng/ml (log likelihood ratio test, P< 0.001). This U-shaped relationship between IGF-1 levels and the risk of NAFLD persisted even after adjustments in multiple models. For lgIGF-1 levels below 2.35 ng/ml, the HR for NAFLD was 0.24 (95% CI 0.15-0.37) in model IV. Conversely, for lgIGF-1 levels above 2.35 ng/ml, the HR for NAFLD was 7.8 (95% CI 4.01-15.17) in model IV.
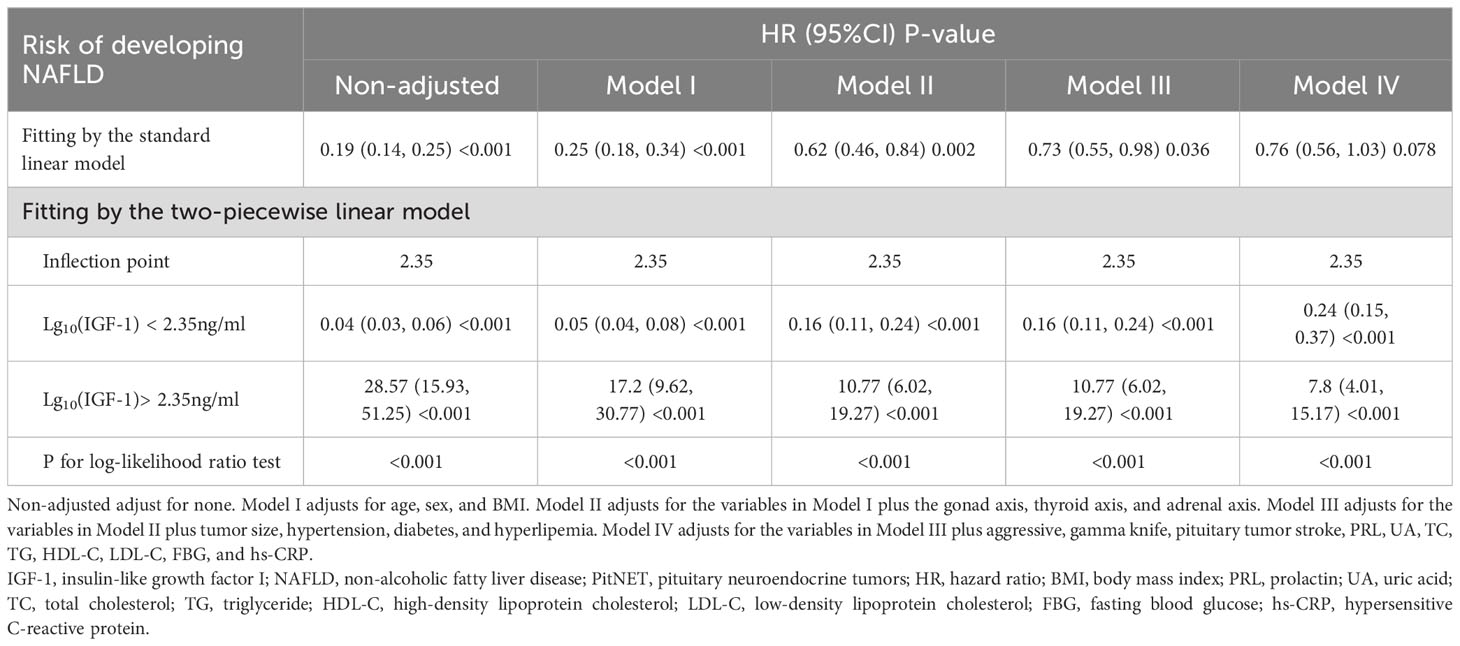
Table 3 Threshold effect analyses of lg10(IGF-1) on the risk of new-onset NAFLD using two-piecewise regression models.
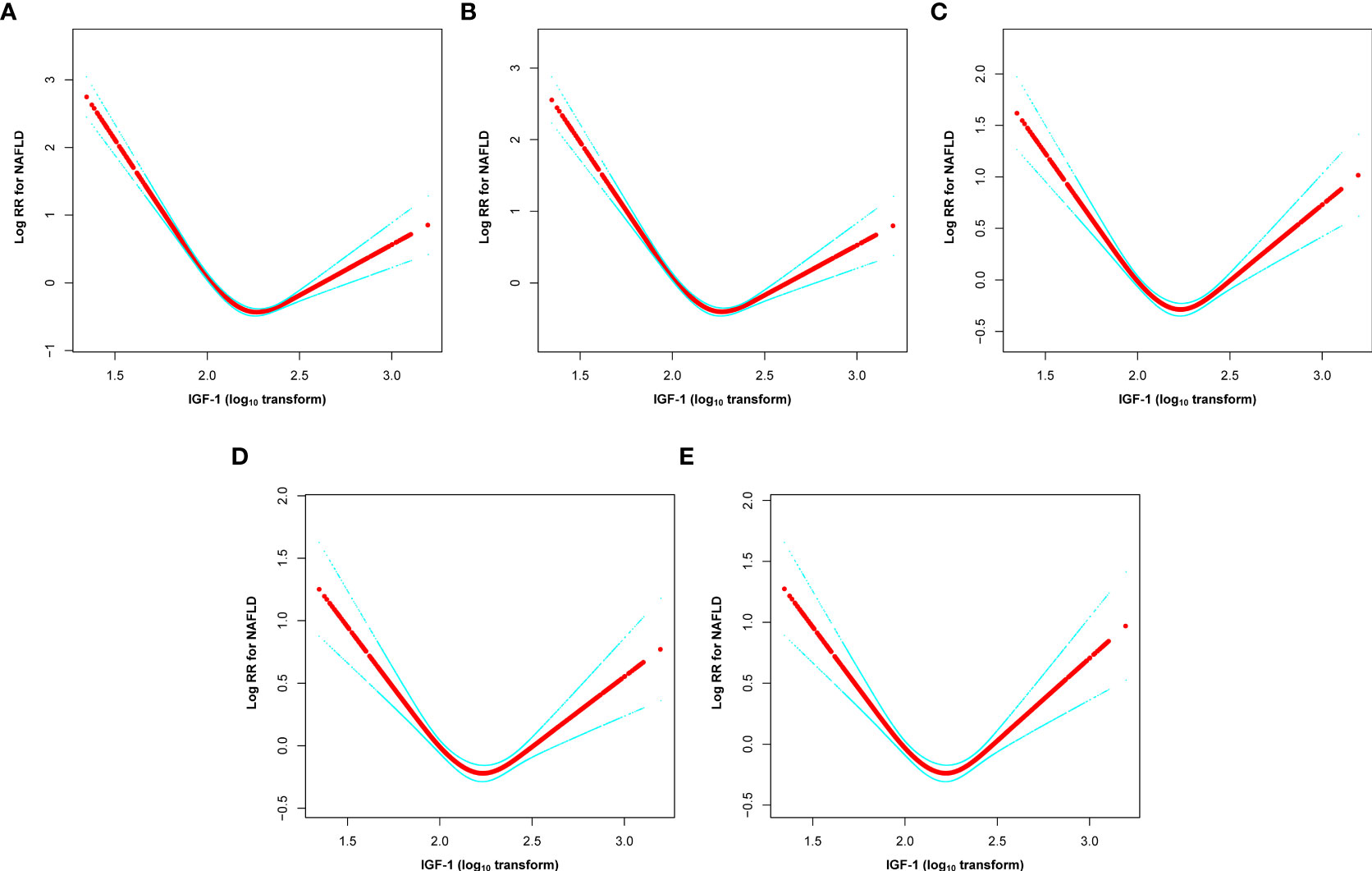
Figure 3 U-shaped relationship between lgIGF-1 and the risk of new-onset NAFLD in PitNET patients. (A) non-adjusted, (B) model I, (C) model II, (D) model III, and (E) model IV. Non-adjusted adjust for none. Model I adjusts for age, sex, and BMI. Model II adjusts for the variables in Model I plus the gonad axis, thyroid axis, and adrenal axis. Model III adjusts for the variables in Model II plus tumor size, hypertension, diabetes, and hyperlipemia. Model IV adjusts for the variables in Model III plus aggressive, gamma knife, pituitary tumor stroke, PRL, UA, TC, TG, HDL-C, LDL-C, FBG, and hs-CRP. IGF-1, insulin-like growth factor I; NAFLD, non-alcoholic fatty liver disease; PitNET, pituitary neuroendocrine tumors; HR, hazard ratio; BMI, body mass index; PRL, prolactin; UA, uric acid; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; hs-CRP, hypersensitive C-reactive protein.
3.5 Stratification analysis and tests for interaction
We conducted stratified analyses to assess the relationship between IGF-1 and the incidence of NAFLD in various subgroups (Figure 4). Subgroup tests revealed that sex had an interaction effect on the relationship between IGF-1 and NAFLD (P interaction < 0.001). In contrast, the association between IGF-1 and incidence of NAFLD was not significantly modified by age, BMI and tumor function (P interactions > 0.05).
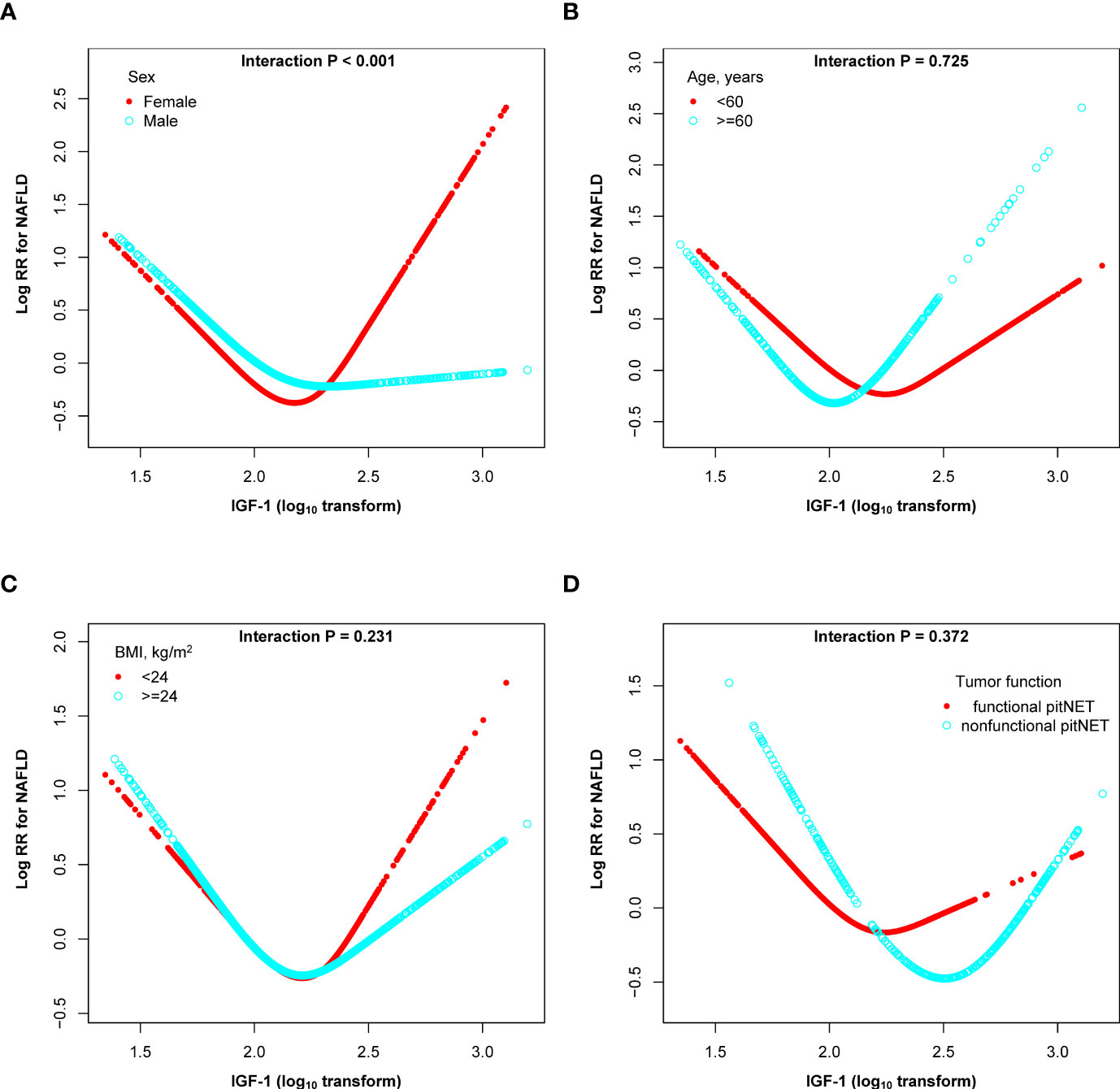
Figure 4 Subgroup analyses of the association between the lgIGF-1 and the risk of NAFLD in PitNET patients. (A) subgroup analyses stratified by sex; (B) subgroup analyses stratified by sex; (C) subgroup analyses stratified by BMI; (D) subgroup analyses stratified by tumor function. Adjusted, if not stratified, for age, sex, BMI, gonad axis, thyroid axis, adrenal axis, tumor size, hypertension, diabetes, hyperlipemia, aggressive, gamma knife, pituitary tumor stroke, PRL, UA, TC, TG, HDL-C, LDL-C, FBG, and hs-CRP.
4 Discussion
In this study, we found a U-shaped relationship between IGF-1 and the risk of new-onset NAFLD in patients with PitNET. After adjusting for potential confounders, both reduced and elevated IGF-1 levels were strongly associated with an increased risk of NAFLD compared with patients with normal IGF-1 levels. These findings remained robust in sensitivity analyses and were consistent across all subgroups.
Previous studies have confirmed that IGF-1 levels are reduced in obese populations and that the relative deficiency of GH and IGF-1 in obese patients promotes the development of NAFLD (27, 31). Randomized controlled trials have demonstrated that recombinant human growth hormone (rhGH) replacement therapy significantly improves hepatic steatosis in obese adolescents and adults (22). In a study by Nishizawa et al, the incidence of NAFLD was higher in GHD patients than in controls, and hepatic steatosis and fibrosis were reduced 6 months after rhGH therapy (32). A cross-sectional study suggested that IGF-1 deficiency was positively associated with NAFLD (defined using a validated hepatic steatosis index) in patients with PitNET (33). However, another study did not find an association between IGF-1 deficiency and NAFLD (estimated by calculating the fatty liver index) in patients with non-functioning PitNET (34). It is noteworthy that the two studies defined NAFLD differently. In our current study, NAFLD was defined using abdominal ultrasonography. Besides, the cross-sectional designs of these studies hindered the evaluation of causality between IGF-1 deficiency and NAFLD.
IGF-1 deficiency is associated with dyslipidemia, visceral obesity, weight gain, and insulin resistance (23), all of which are involved in the progression of NAFLD (35–37). In the presence of IGF-1 deficiency, multiple mechanisms contribute to the development of NAFLD. Firstly, previous studies have shown an association between IGF-1 deficiency, liver dysfunction and hyperlipidemia (38). Furthermore, recent studies have confirmed that rhGH treatment improves lipid metabolism in obese children (39, 40). In our study, we also found higher levels of blood lipids in the group with reduced IGF-1 levels. Secondly, excess lipid accumulation in the liver plays a crucial role in hepatic steatosis (41, 42). IGF-1, as the effector hormone of GH, mediates its protective role in the pathogenesis of NAFLD by regulating cholesterol transport and regulating the adipogenic pathway (43). Furthermore, IGF-1 is responsible for regulating lipolysis and anti-inflammatory responses (44). Deficiency of GH or IGF-1 leads to the absence of feedback loops in the hypothalamic-pituitary-adipose axis (45). Thirdly, deficiency of the GH/IGF-1 system produces leptin resistance, which leads to bulimia, obesity and subsequent insulin resistance, all of which contribute to early and aggressive hepatic steatosis (46). Lastly, the regulation of metabolic, immune and hepatic stellate cell function by IGF-1 is crucial in preventing the development of NAFLD (43). Patients with pituitary tumors often experience hypopituitarism due to the occupational effects of the tumor (16), with GH/IGF system involvement being the most common (17). Overall, these prior studies suggest that maintaining optimal IGF-1 levels through GH replacement therapy may be an effective treatment option for GHD patients with liver impairment and associated steatosis and fibrosis.
In our study, elevated IGF-1 was also an established risk factor for NAFLD. Functional GH-secreting pituitary adenomas, also known as acromegaly, are characterized by increased levels of growth hormone and its target hormone, IGF-1 (47). According to guidelines, IGF-1 is recommended as a screening and diagnostic tool for acromegaly (48). As indicated by multiple studies, there is a correlation between acromegaly and metabolic disorders, such as hypertension, diabetes, and dyslipidemia (49). While glucose and lipid abnormalities, as well as metabolic disorders, are frequently observed in patients with acromegaly, most cases can be effectively managed by normalizing IGF-1 levels (50–52). However, the prevalence of NAFLD in patients suffering from acromegaly and the correlation between elevated IGF-1 levels and NAFLD have not been investigated. In our current study, we observed that individuals with elevated IGF-1 levels had a 2fold higher risk of NAFLD compared to those with normal IGF-1 levels, after adjusting for covariates. This suggests that controlling IGF-1 levels within the standard range can decrease the incidence of NAFLD in individuals diagnosed with acromegaly. Achieving biochemical remission is one of the therapeutic goals for patients with acromegaly, and guidelines recommend maintaining serum IGF-1 within the sex- and age-specific normal ranges (53). Studies have shown that biochemical control of acromegaly can normalize mortality rates (49).
Our findings suggest that reduced and elevated IGF-1 levels are associated with an increased risk of new-onset NAFLD. This further emphasizes the importance of maintaining IGF-1 in an optimal range to prevent NAFLD in patients with PitNET. Furthermore, the dose-response and threshold effect analyses presented in our study propose a potential cut-off point that could guide the establishment of targeted IGF-1 level management in patients with PitNET. However, this proposed threshold warrants validation through additional prospective research. In the future, exploring the association between IGF-1 levels and the risk of NAFLD in a broader demographic could validate the necessity for IGF-1 level screening in populations vulnerable to NAFLD, potentially leading to more precise identification of individuals at increased risk for the condition. The potential use of GH, IGF-1 modulators, or other agents that directly target the IGF-1 axis as therapeutic options for NAFLD remains an intriguing avenue for future exploration. Additionally, examining the relationship between IGF-1 and NAFLD through genetic, molecular, and environmental lenses could yield novel preventive and management strategies for NAFLD. The public health significance of these findings is profound, given the rising incidence of NAFLD in the global population.
5 Strengths and limitations
There are several strengths in our study, including the large sample size, long follow-up time, low loss to follow-up rate, and strict statistical analysis. Despite these strengths, some limitations of this study are important to note. Firstly, the patient population was collected retrospectively from a single institution. Additional studies of a larger patient cohort are clearly needed to validate and refine these findings. However, it is worth noting that PitNET is not a common disease, making it challenging to conduct prospective studies on a large scale. Secondly, we cannot rule out the possibility of some residual confounding despite a comprehensive adjustment of baseline characteristics. Thirdly, causality cannot be confirmed in this study due to its retrospective design. However, to mitigate the potential impact of reverse causality, patients with preexisting NAFLD were excluded from the study. Fourth, the levels of IGF-1 reported in this study are based on a single measurement taken at baseline, which may not adequately reflect the dynamic changes that occur during long-term follow-up. Fifth, based on the instability of GH and the large number of missing GH data, our study did not identify the association between GH and NAFLD.
6 Conclusion
In summary, in our cohort study, there was a U-shaped trend between IGF-1 and new-onset NAFLD in patients with PitNET. Both elevated and reduced levels of IGF-1 were associated with an increased risk of NAFLD. It is suggested that individualized, comprehensive treatment of PitNET might reduce the occurrence of comorbidities. Thus, further studies are necessary to confirm our findings.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Some or all of the datasets generated and/or analyzed during the current study are not publicly available but can be obtained from the corresponding author upon reasonable request. Requests to access these datasets should be directed toZ3VvemV5YW5nQDEyNi5jb20=.
Ethics statement
The studies involving humans were approved by Ethical Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because as a retrospective cohort study, ethical approval is not necessary.
Author contributions
YG: Methodology, Writing – review & editing. YH: Methodology, Writing – original draft. CY: Methodology, Writing – review & editing. MA: Software, Writing – review & editing. JM: Data curation, Writing – review & editing. ZJ: Project administration, Validation, Writing – review & editing. AT: Formal analysis, Methodology, Writing – review & editing. HA: Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Specialized Program for Precision Medicine Research of the People’s Hospital of Xinjiang Uygur Autonomous Region (20220307).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1290007/full#supplementary-material
References
1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of nafld and nash: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
2. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of nafld. Hepatology (2020) 72(5):1605–16. doi: 10.1002/hep.31173
3. Tillman EJ, Rolph T. Fgf21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol (Lausanne) (2020) 11:601290. doi: 10.3389/fendo.2020.601290
4. Kundu D, Kennedy L, Zhou T, Ekser B, Meadows V, Sybenga A, et al. P16 ink4a drives nonalcoholic fatty liver disease phenotypes in high fat diet fed mice through biliary E2f1/foxo1/igf-1 signaling. Hepatology (2023) 78(1):243–57. doi: 10.1097/HEP.0000000000000307
5. Yang B, Lu L, Zhou D, Fan W, Barbier-Torres L, Steggerda J, et al. Regulatory network and interplay of hepatokines, stellakines, myokines and adipokines in nonalcoholic fatty liver diseases and nonalcoholic steatohepatitis. Front Endocrinol (Lausanne) (2022) 13:1007944. doi: 10.3389/fendo.2022.1007944
6. Carter JK, Friedman SL. Hepatic stellate cell-immune interactions in nash. Front Endocrinol (Lausanne) (2022) 13:867940. doi: 10.3389/fendo.2022.867940
7. Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol (2016) 78:181–205. doi: 10.1146/annurev-physiol-021115-105331
8. Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H, et al. Advancements in the treatment of non-alcoholic fatty liver disease (Nafld). Front Endocrinol (Lausanne) (2022) 13:1087260. doi: 10.3389/fendo.2022.1087260
9. Martinez-Arranz I, Bruzzone C, Noureddin M, Gil-Redondo R, Minchole I, Bizkarguenaga M, et al. Metabolic subtypes of patients with nafld exhibit distinctive cardiovascular risk profiles. Hepatology (2022) 76(4):1121–34. doi: 10.1002/hep.32427
10. Targher G, Byrne CD, Tilg H. Nafld and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut (2020) 69(9):1691–705. doi: 10.1136/gutjnl-2020-320622
11. Molitch ME. Diagnosis and treatment of pituitary adenomas: A review. JAMA (2017) 317(5):516–24. doi: 10.1001/jama.2016.19699
12. Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: A review. JAMA (2023) 329(16):1386–98. doi: 10.1001/jama.2023.5444
13. Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med (2020) 382(10):937–50. doi: 10.1056/NEJMra1810772
14. Pappachan JM, Raskauskiene D, Kutty VR, Clayton RN. Excess mortality associated with hypopituitarism in adults: A meta-analysis of observational studies. J Clin Endocrinol Metab (2015) 100(4):1405–11. doi: 10.1210/jc.2014-3787
15. Bolfi F, Neves AF, Boguszewski CL, Nunes-Nogueira VS. Mortality in acromegaly decreased in the last decade: A systematic review and meta-analysis. Eur J Endocrinol (2018) 179(1):59–71. doi: 10.1530/EJE-18-0255
16. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am (2008) 37(1):151–71. doi: 10.1016/j.ecl.2007.10.011
17. Cordeiro D, Xu Z, Mehta GU, Ding D, Vance ML, Kano H, et al. Hypopituitarism after gamma knife radiosurgery for pituitary adenomas: A multicenter, international study. J Neurosurg (2018). doi: 10.3171/2018.5.JNS18509
18. Lim CT, Korbonits M. Update on the clinicopathology of pituitary adenomas. Endocr Pract (2018) 24(5):473–88. doi: 10.4158/EP-2018-0034
19. Yuan XX, Zhu HJ, Pan H, Chen S, Liu ZY, Li Y, et al. Clinical characteristics of non-alcoholic fatty liver disease in chinese adult hypopituitary patients. World J Gastroenterol (2019) 25(14):1741–52. doi: 10.3748/wjg.v25.i14.1741
20. Savastano S, Di Somma C, Pizza G, De Rosa A, Nedi V, Rossi A, et al. Liver-spleen axis, insulin-like growth factor-(Igf)-I axis and fat mass in overweight/obese females. J Transl Med (2011) 9:136. doi: 10.1186/1479-5876-9-136
21. Ma IL, Stanley TL. Growth hormone and nonalcoholic fatty liver disease. Immunometabolism (2023) 5(3):1742. doi: 10.1097/in9.0000000000000030
22. Xue J, Liang S, Ma J, Xiao Y. Effect of growth hormone therapy on liver enzyme and other cardiometabolic risk factors in boys with obesity and nonalcoholic fatty liver disease. BMC Endocr Disord (2022) 22(1):49. doi: 10.1186/s12902-022-00967-y
23. Matsumoto R, Fukuoka H, Iguchi G, Nishizawa H, Bando H, Suda K, et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm IGF Res (2014) 24(5):174–9. doi: 10.1016/j.ghir.2014.07.002
24. Luo X, Jiang X, Li J, Bai Y, Li Z, Wei P, et al. Insulin-like growth factor-1 attenuates oxidative stress-induced hepatocyte premature senescence in liver fibrogenesis via regulating nuclear P53-progerin interaction. Cell Death Dis (2019) 10(6):451. doi: 10.1038/s41419-019-1670-6
25. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (Strobe) statement: guidelines for reporting observational studies. Lancet (2007) 370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X
26. Husebye ES, Pearce SH, Krone NP, Kämpe O. Adrenal insufficiency. Lancet (2021) 397(10274):613–29. doi: 10.1016/s0140-6736(21)00136-7
27. Rufinatscha K, Ress C, Folie S, Haas S, Salzmann K, Moser P, et al. Metabolic effects of reduced growth hormone action in fatty liver disease. Hepatol Int (2018) 12(5):474–81. doi: 10.1007/s12072-018-9893-7
28. Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis (2008) 9(2):108–12. doi: 10.1111/j.1751-2980.2008.00331.x
29. Cai X, Gao J, Hu J, Wen W, Zhu Q, Wang M, et al. Dose-response associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese chinese population: retrospective evidence from a population-based cohort study. Dis Markers (2022) 2022:4930355. doi: 10.1155/2022/4930355
30. Wu J, Guo J, Wang A, Zhang Y, Wu S, Liu Y, et al. Nonalcoholic fatty liver disease and risk of intracerebral hemorrhage. Nutr Metab Cardiovasc Dis (2022) 32(11):2561–7. doi: 10.1016/j.numecd.2022.08.010
31. Dichtel LE, Corey KE, Misdraji J, Bredella MA, Schorr M, Osganian SA, et al. The association between igf-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin Transl Gastroenterol (2017) 8(1):e217. doi: 10.1038/ctg.2016.72
32. Nishizawa H, Iguchi G, Murawaki A, Fukuoka H, Hayashi Y, Kaji H, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with gh deficiency and the impact of gh replacement therapy. Eur J Endocrinol (2012) 167(1):67–74. doi: 10.1530/EJE-12-0252
33. Hwang YA, Lee HW, Ahn SH, Lee EJ, Ku CR, Kim SU. Positive association between nonalcoholic fatty liver disease and growth hormone deficiency in patients with nonfunctioning pituitary adenoma. Front Endocrinol (Lausanne) (2022) 13:1057769. doi: 10.3389/fendo.2022.1057769
34. Auer MK, Stalla GK, Stieg MR. Investigating the role of cortisol and growth hormone in fatty liver development: fatty liver index in patients with pituitary adenomas. Pituitary (2016) 19(5):461–71. doi: 10.1007/s11102-016-0726-1
35. Cai X, Aierken X, Ahmat A, Cao Y, Zhu Q, Wu T, et al. A nomogram model based on noninvasive bioindicators to predict 3-year risk of nonalcoholic fatty liver in nonobese mainland chinese: A prospective cohort study. BioMed Res Int (2020) 2020:1–12. doi: 10.1155/2020/8852198
36. Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne) (2023) 14:1149239. doi: 10.3389/fendo.2023.1149239
37. Friesen CS, Hosey-Cojocari C, Chan SS, Csanaky IL, Wagner JB, Sweeney BR, et al. Efficacy of weight reduction on pediatric nonalcoholic fatty liver disease: opportunities to improve treatment outcomes through pharmacotherapy. Front Endocrinol (Lausanne) (2021) 12:663351. doi: 10.3389/fendo.2021.663351
38. Kaji H, Sakurai T, Iguchi G, Murata M, Kishimoto M, Yoshioka S, et al. Adult growth hormone deficiency in Japan: results of investigation by questionnaire. Endocr J (2002) 49(6):597–604. doi: 10.1507/endocrj.49.597
39. Liang S, Xue J, Li G. Effects of recombinant human growth hormone administration on cardiovascular risk factors in obese children with relative growth hormone deficiency. Lipids Health Dis (2018) 17(1):66. doi: 10.1186/s12944-018-0721-9
40. Wu J, Zhao F, Zhang Y, Xue J, Kuang J, Jin Z, et al. Effect of one-year growth hormone therapy on cardiometabolic risk factors in boys with obesity. BioMed Res Int (2020) 2020:2308124. doi: 10.1155/2020/2308124
41. Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int (2021) 15(1):21–35. doi: 10.1007/s12072-020-10121-2
42. Pan CS, Weiss JJ, Fourman LT, Buckless C, Branch KL, Lee H, et al. Effect of recombinant human growth hormone on liver fat content in young adults with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) (2021) 94(2):183–92. doi: 10.1111/cen.14344
43. Cristin L, Montini A, Martinino A, Scarano Pereira JP, Giovinazzo F, Agnes S. The role of growth hormone and insulin growth factor 1 in the development of non-alcoholic steato-hepatitis: A systematic review. Cells (2023) 12(4):6. doi: 10.3390/cells12040517
44. Gancheva S, Kahl S, Herder C, Strassburger K, Sarabhai T, Pafili K, et al. Metabolic surgery-induced changes of the growth hormone system relate to improved adipose tissue function. Int J Obes (Lond) (2023) 47(6):505–11. doi: 10.1038/s41366-023-01292-7
45. Bruno C, Vergani E, Giusti M, Oliva A, Cipolla C, Pitocco D, et al. The "Adipo-cerebral" Dialogue in childhood obesity: focus on growth and puberty. Physiopathological and nutritional aspects. Nutrients (2021) 13(10). doi: 10.3390/nu13103434
46. Lohr H, Hess S, Pereira MMA, Reinoss P, Leibold S, Schenkel C, et al. Diet-induced growth is regulated via acquired leptin resistance and engages a pomc-somatostatin-growth hormone circuit. Cell Rep (2018) 23(6):1728–41. doi: 10.1016/j.celrep.2018.04.018
47. Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S. Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol (2022) 10(11):804–26. doi: 10.1016/S2213-8587(22)00244-3
48. Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, et al. Multidisciplinary management of acromegaly: A consensus. Rev Endocr Metab Disord (2020) 21(4):667–78. doi: 10.1007/s11154-020-09588-z
49. Hong S, Kim KS, Han K, Park CY. Acromegaly and cardiovascular outcomes: A cohort study. Eur Heart J (2022) 43(15):1491–9. doi: 10.1093/eurheartj/ehab822
50. Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev (2019) 40(1):268–332. doi: 10.1210/er.2018-00115
51. Caron PJ, Petersenn S, Houchard A, Sert C, Bevan JS, Group PS. Glucose and lipid levels with lanreotide autogel 120 mg in treatment-naive patients with acromegaly: data from the primarys study. Clin Endocrinol (Oxf) (2017) 86(4):541–51. doi: 10.1111/cen.13285
52. Rochette C, Graillon T, Albarel F, Morange I, Dufour H, Brue T, et al. Increased risk of persistent glucose disorders after control of acromegaly. J Endocr Soc (2017) 1(12):1531–9. doi: 10.1210/js.2017-00334
Keywords: U-shaped, insulin-like growth factor I, nonalcoholic fatty liver disease, pituitary neuroendocrine tumors, cohort study
Citation: Hu Y, Yuan C, Abdulnaimu M, Memetmin J, Jie Z, Tuhuti A, Abudueini H and Guo Y (2024) U-Shaped relationship of insulin-like growth factor I and incidence of nonalcoholic fatty liver in patients with pituitary neuroendocrine tumors: a cohort study. Front. Endocrinol. 15:1290007. doi: 10.3389/fendo.2024.1290007
Received: 06 September 2023; Accepted: 17 January 2024;
Published: 02 February 2024.
Edited by:
Xiang'En Shi, Capital Medical University, ChinaReviewed by:
Neethi Dasu, Jefferson University Hospitals, United StatesSimona Galoiu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Hu, Yuan, Abdulnaimu, Memetmin, Jie, Tuhuti, Abudueini and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanying Guo, Z3VvemV5YW5nQDEyNi5jb20=
 Yan Hu1
Yan Hu1 Yanying Guo
Yanying Guo