
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 18 April 2024
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1284144
This article is part of the Research TopicCardiovascular Diseases Related to Diabetes and Obesity, volume IVView all 11 articles
Background: Chinese visceral adiposity index (CVAI) is a reliable visceral obesity index, but the association between CVAI and risk of cardiovascular disease (CVD) remains unclear. We explored the associations of CVAI with incident CVD, heart disease, and stroke and compared the predictive power of CVAI with other obesity indices based on a national cohort study.
Methods: The present study included 7,439 participants aged ≥45 years from China Health and Retirement Longitudinal Study (CHARLS). Cox regression models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Restricted cubic splines analyses were adopted to model the dose–response associations. Receiver operator characteristic (ROC) analyses were used to compare the predictive ability of different obesity indices (CVAI, visceral adiposity index [VAI], a body shape index [ABSI], conicity index [CI], waist circumference [WC], and body mass index [BMI]).
Results: During 7 years’ follow‐up, 1,326 incident CVD, 1,032 incident heart disease, and 399 stroke cases were identified. The HRs (95% CI) of CVD, heart disease, and stroke were 1.50 (1.25-1.79), 1.29 (1.05-1.57), and 2.45 (1.74-3.45) for quartile 4 versus quartile 1 in CVAI. Linear associations of CVAI with CVD, heart disease, and stroke were observed (P nonlinear >0.05) and per-standard deviation (SD) increase was associated with 17% (HR 1.17, 1.10-1.24), 12% (1.12, 1.04-1.20), and 31% (1.31, 1.18-1.46) increased risk, respectively. Per-SD increase in CVAI conferred higher risk in participants aged<60 years than those aged ≥60 years (P interaction<0.05). ROC analyses showed that CVAI had higher predictive value than other obesity indices (P<0.05).
Conclusions: CVAI was linearly associated with risk of CVD, heart disease, and stroke and had best performance for predicting incident CVD. Our findings indicate CVAI as a reliable and applicable obesity index to identify higher risk of CVD.
Cardiovascular disease (CVD) is highly prevalent worldwide and its number nearly doubled from 271 million in 1990 to 523 million in 2019 (1, 2). The global trends for disability-adjusted life years and years of life lost also increased significantly and the number of CVD deaths increased steadily by 53.7% from 1990 to 2019 (2, 3). Among the modifiable risk factors for attributable CVD burden, high body mass index (BMI) ranked fifth and could worsen most other CVD risk factors (2, 4, 5). Unfortunately, 50.7% of Chinese adults were overweight or obesity in the most recent national survey (6), which may result in larger CVD burden. However, BMI might not fully capture cardiometabolic risk because of its failure in discriminating adequately between body fat mass and lean tissues or identifying regional body fat distribution (7, 8). Therefore, identifying reliable and applicable obesity indices would benefit to reducing CVD burden among Chinese population.
Recently, Chinese visceral adiposity index (CVAI), like visceral adiposity index (VAI) for Western population, was established to assess visceral adiposity among Chinese population and could predict metabolic disorders incidence well (9, 10). Numerous studies have demonstrated that CVAI has better performance than other obesity indices for predicting hypertension, diabetes, and their comorbidity (11–16). However, whether CVAI also predict incident CVD well among general population remains unclear. Studies based on rural population from a Chinese county or health examination population in a Beijing hospital reported positive associations between CVAI and stroke or coronary heart disease (17, 18), respectively. Another study from a Chinese province showed that CVAI was positively associated with CVD in women but not in men (19). Therefore, prospective cohort studies based on national data are needed to further validate CVAI as a visceral obesity index to predict incident CVD, which would provide additional epidemiological evidence for future CVD prevention.
Our study aimed to explore the dose–response associations of CVAI with CVD, heart disease, and stroke and compare the predictive ability of CVAI with that of other obesity indices (VAI, a body shape index [ABSI], conicity index [CI], waist circumference [WC], and BMI) based on China Health and Retirement Longitudinal Study (CHARLS).
The CHARLS, established in 2011, is an ongoing nationally representative cohort study focusing on adults aged ≥45 years in China. Details of the study design have been described elsewhere (20). In brief, a total of 17,708 participants were recruited from 150 counties of 28 provinces in China by a multistage probability sampling strategy from June 2011 to March 2012 and were followed up every-two years. The protocols of CHARLS were approved by the Biomedical Ethics Review Committee of Peking University and all participants provided signed informed consent before their enrollment.
The present study was conducted based on data from four waves (2011, 2013, 2015, and 2018) in the CHARLS. Of the 17,708 participants, we excluded participants with CVD or its unknown status at baseline (n= 3,118), CVD status at follow-up unknown (n= 1,015), younger than 45 years (n= 564), and missing data for age, BMI, WC, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C; n= 5,572). Finally, a total of 7,439 participants were included for the analysis.
The information about demographic characteristics, lifestyle factors, disease history was collected using standardized questionnaires by asking their age, gender, area, region, educational level, marital status, smoking, drinking, hypertension, diabetes, dyslipidemia, cardiovascular disease, cancer, liver disease, kidney disease, and medical history. Smoking was defined as smoking ≥100 cigarettes in their lifetime and drinking defined as drinking alcohol ≥12 times during the last year. BMI was calculated by dividing weight (kg) by the square of height. Hypertension was defined as systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg and/or use of antihypertensive medication (21).
Blood samples were collected from participants after fasting overnight. Levels of fasting plasma glucose (FPG), total cholesterol (TC), TG, HDL-C, and low-density lipoprotein cholesterol (LDL-C) were measured by enzymatic colorimetric test, hemoglobin A1c measured by boronate affinity HPLC. Elevated TC was defined as TC ≥200 mg/dl. Diabetes was defined as fasting FPG ≥7.0 mmol/L, and/or non-fasting FPG ≥11.1 mmol/L, and/or hemoglobin A1c (HbA1c) ≥6.5%, and/or current treatment with anti-diabetes medication (22). The CVAI, VAI, ABSI, and CI were calculated as follows (9, 10, 23, 24):
Accordant with previous studies (25, 26), incident CVD was assessed by the following standardized questions: “Have you been told by a doctor that you have been diagnosed with a heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems?” or “Have you been told by a doctor that you have been diagnosed with a stroke?”. Participants who reported heart disease or stroke were defined as having CVD.
Continuous data are described as median (interquartile range) and were analyzed by regression analysis to conduct trend tests among participants by quartiles of CVAI. Categorical data are presented as number (percentage) and were analyzed by Cochran-Armitage trend test.
Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CVD, heart disease, and stroke by quartiles of CVAI. We also evaluated the effect of per standard deviation (SD) increase in CVAI through putting the value of CVAI divided by SD into the model. Model 1 was adjusted for age and gender; Model 2 was adjusted for model 1 plus area, region, educational level, marital status, smoking, and drinking; Model 3 was further adjusted for hypertension, diabetes, and TC. Dose–response associations between CVAI and incident CVD, heart disease, and stroke were assessed by restricted cubic splines (RCS) analysis incorporating four knots at the 5th, 35th, 65th, and 90th percentiles, with the knot at 25th percentile of the distribution as the reference. Subgroup analyses for per SD increase in CVAI across gender (men vs. women), age (<60 vs. ≥60 years), hypertension (no vs. yes), diabetes (no vs. yes), and elevated TC (no vs. yes) were conducted and its potential multiplicative interactions were tested. Sensitivity analyses were adapted by excluding participants with cancer, liver, and kidney disease at baseline.
The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the predictive value of CVD, heart disease, and stroke with CVAI, VAI, ABSI, CI, WC, and BMI. The differences among those AUCs were tested with the Z statistic. The RCS curves were modeled by R V.4.2.2, AUCs calculated by Medcalc v9.3, and other analyses involved using SAS V.9.4 (SAS Inst., Cary, NC). The Two-sided P< 0.05 was considered statistically significant.
Of the 7,439 participants included, the median age was 58.13 (13.16) years and 52.74% were women. The baseline characteristics for quartiles of CVAI are presented in Table 1. Participants in higher CVAI quartiles tended to be older, women, northerner, with hypertension, with diabetes, and have higher levels of TG, TC, VAI, ABSI, CI, WC, and BMI (P trend<0.05). The proportions of participants living in rural areas, marriage, smoking, drinking, and level of HDL-C decreased with increasing CVAI quartiles (P trend<0.05).
During 7-year’ follow-up, a total of 1,326 incident CVD, 1,032 incident heart disease, and 399 stroke cases were identified. Figure 1 showed positive linear associations of CVAI with CVD (P nonlinear =0.4617), heart disease (P nonlinear =0.3872), and stroke (P nonlinear =0.9046). Risk of CVD, heart disease, and stroke increased across quartiles of CVAI (P trend<0.05; Table 2). In model 3, the multivariable adjusted HRs (95% CIs) for quartiles 2, 3, and 4 were 1.20 (1.00-1.44), 1.32 (1.11-1.58), and 1.50 (1.25-1.79) for CVD, 1.13 (0.92-1.38), 1.23 (1.01-1.50), and 1.29 (1.05-1.57) for heart disease, 1.63 (1.14-2.33), 1.65 (1.16-2.36), and 2.45 (1.74-3.45) for stroke. For per-SD increase in CVAI, the risk increased 17% (HR 1.17, 1.10-1.24) for CVD, 12% (1.12, 1.04-1.20) for heart disease, and 31% (1.31, 1.18-1.46) for stroke. Results of sensitivity analyses by excluding participants with cancer, liver, and kidney disease did not alter (Supplementary Table S1).
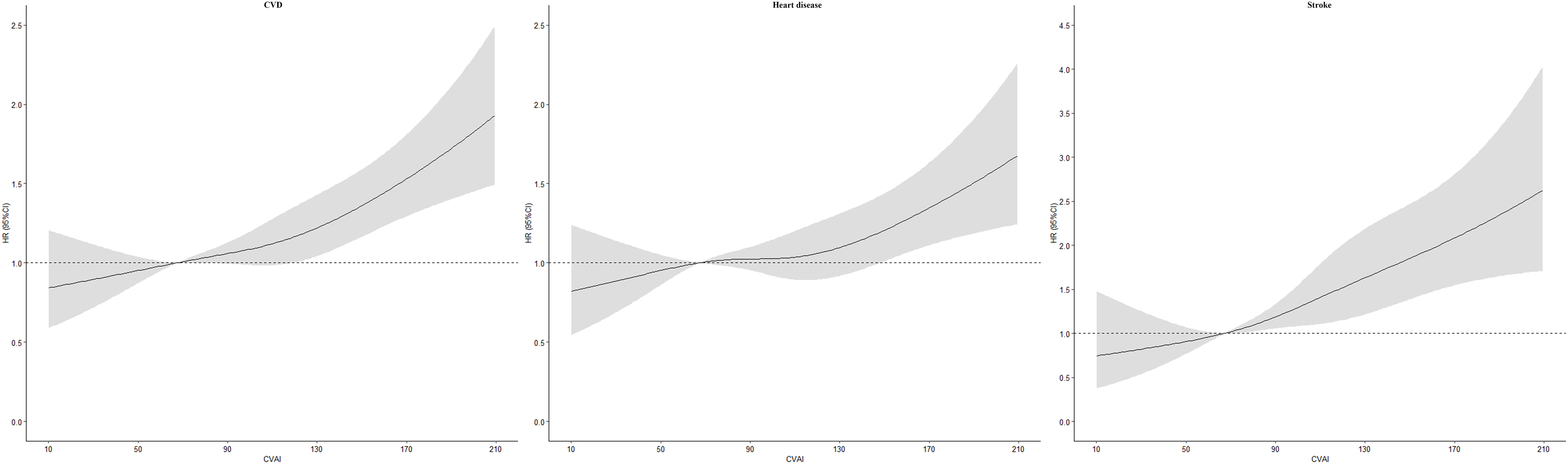
Figure 1 Dose–response association between Chinese visceral adiposity index and risk of cardiovascular disease HR, hazard ratio; CI, confidence interval; CVAI, Chinese visceral adiposity index; CVD, cardiovascular disease. Adjusted for age, gender, area, region, educational level, marital status, smoking, drinking, hypertension, diabetes, and total cholesterol.
We further explored the associations of per SD increase in CVAI with CVD, heart disease, and stroke in different age (<60 years vs ≥60 years), gender (men vs. women), hypertension (no vs. yes), diabetes (no vs. yes), and elevated TC (no vs. yes) groups (Table 3). Subgroup analyses showed that the risk of CVD, heart disease, and stroke were higher for participants aged<60 years than those aged ≥60 years (P interaction<0.05), with HRs (95% CIs) of 1.26 (1.16-1.37) and 1.11 (1.02-1.21) for CVD, 1.17 (1.06-1.29) and 1.10 (1.00-1.21) for heart disease, 1.51 (1.30-1.77) and 1.17 (1.02-1.35) for stroke, respectively. When stratified by gender, hypertension, diabetes, and elevated TC, multiplicative interactions were not observed for CVD, heart disease, and stroke (P interaction >0.05). Sensitivity analyses showed similar results (Supplementary Table S2).
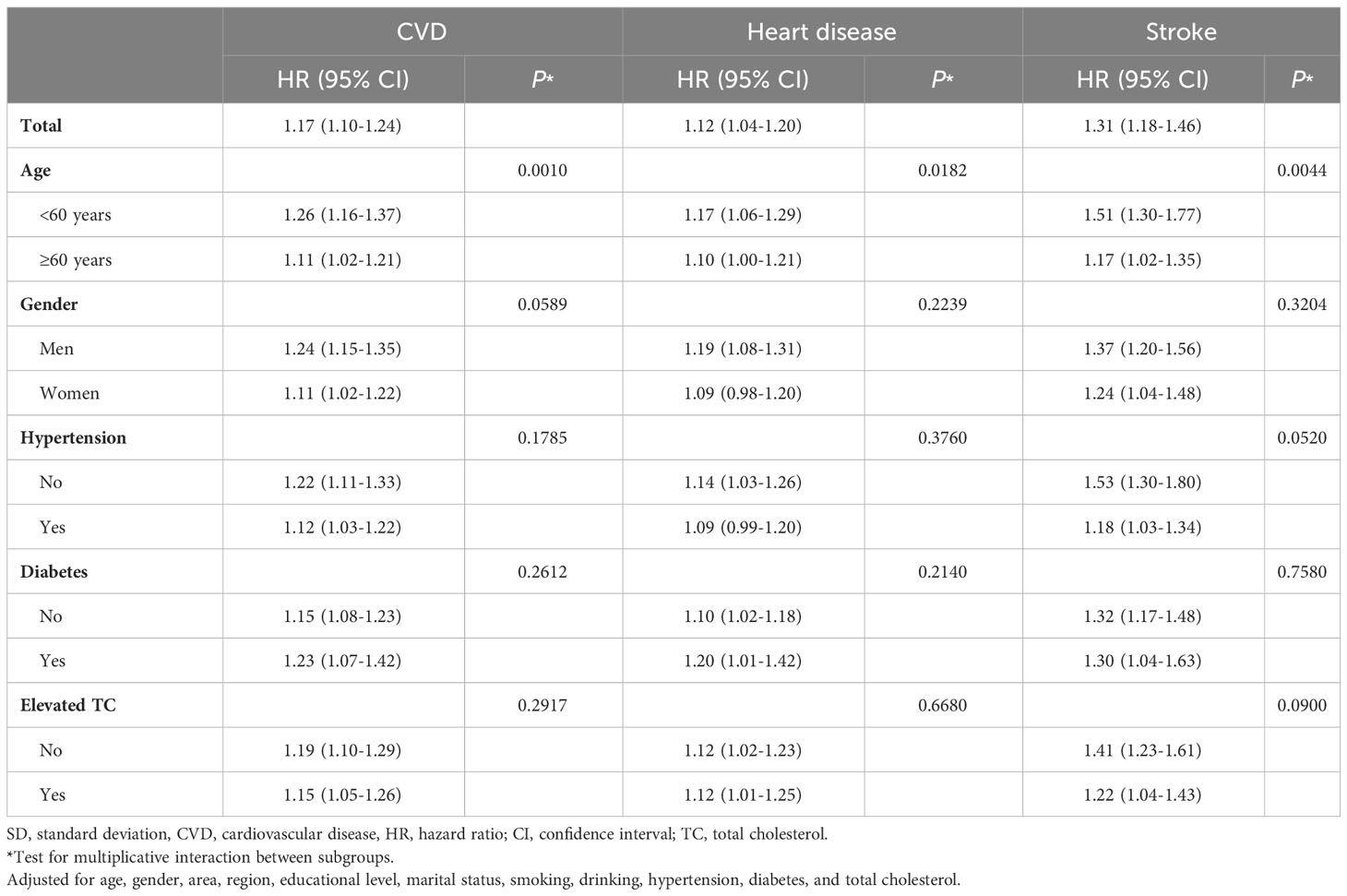
Table 3 Association between per SD increase in Chinese visceral adiposity index and cardiovascular disease.
The ROC curves and AUCs for CVAI, VAI, ABSI, CI, WC, and BMI for predicting CVD, heart disease, and stroke is shown in Figure 2. The AUCs (95% CIs) for CVAI, were 0.602 (0.590-0.613) for CVD, 0.587 (0.576-0.598) for heart disease, 0.631 (0.620-0.642) for stroke, respectively. Among these obesity indices, CVAI had the largest AUC value for CVD, heart disease, and stroke, and the differences were all statistically significant (P< 0.05).
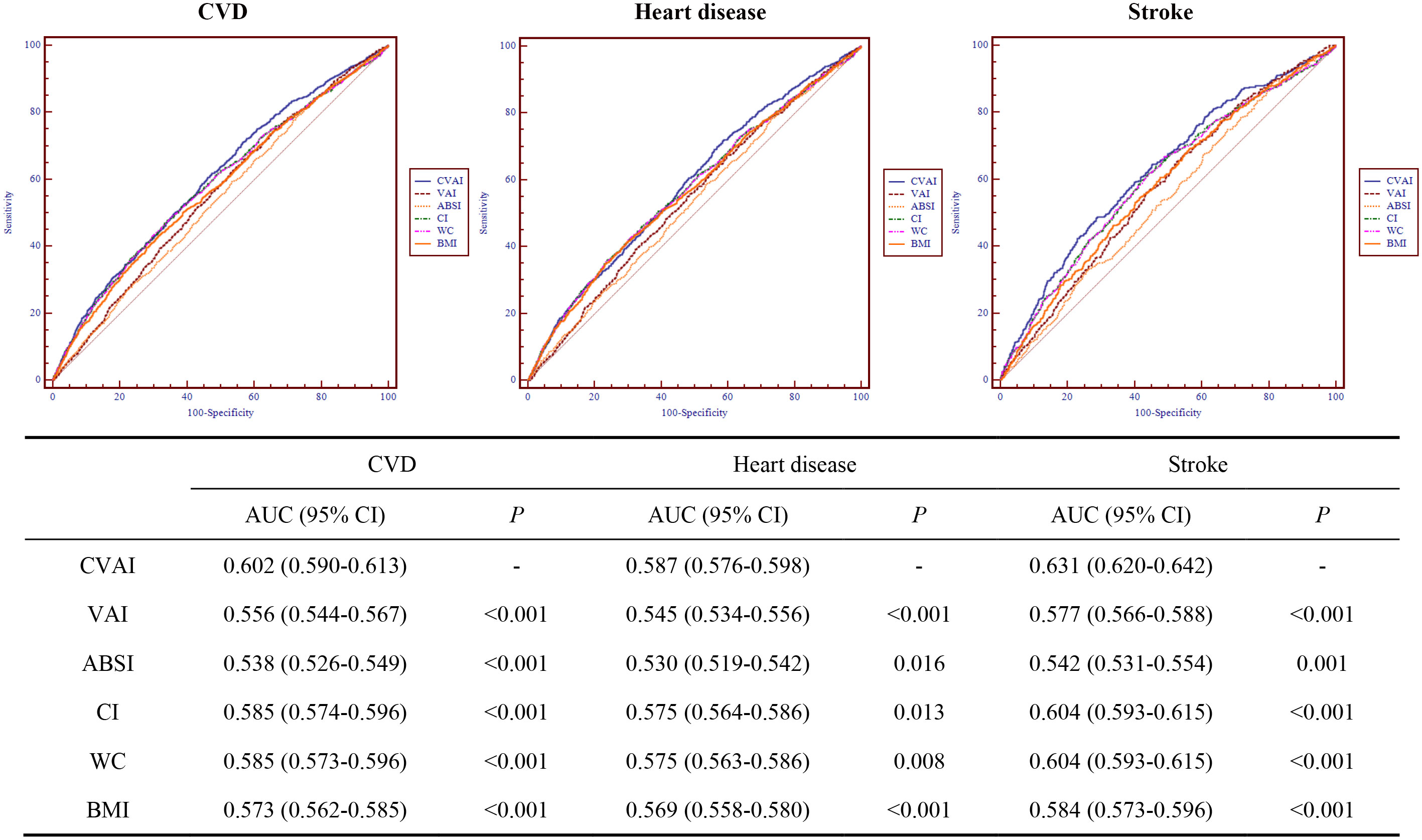
Figure 2 Receiver operating characteristic curves for CVAI, VAI, ABSI, CI, WC, and BMI for predicting cardiovascular disease. CVD, cardiovascular disease; CVAI, Chinese visceral adiposity index; VAI, visceral adiposity index; ABSI, A body shape index; CI, conicity index; WC, waist circumference; BMI, body mass index; AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval.
This prospective cohort study found linear associations of CVAI with CVD, heart disease, and stroke. Compared with quartile 1 of CVAI, the risk of CVD, heart disease, and stroke in quartile 4 increased 50%, 29%, and 145%, respectively. Per-SD increase in CVAI was associated with 17%, 12%, and 31% increased risk of CVD, heart disease, and stroke and confer higher risk in participants aged<60 years than those aged ≥60 years. Finally, we compared the ability of CVAI, VAI, ABSI, CI, WC, and BMI in predicting CVD, heart disease, and stroke and found CVAI as the best predictor among these obesity indices. Our study suggested CVAI as a reliable visceral obesity index for predicting CVD in Chinese adults.
Recent national survey showed that more than half of Chinese adults had overweight or obesity (6). Mounting evidence supported that visceral obesity was more strongly associated with CVD than overall obesity (27–29). Considering higher visceral fat in Asian people than Western people at a given BMI level (30, 31), evaluating visceral obesity would be more helpful for assessing risk of CVD among Chinese people. However, routine access to CT and MRI to assess visceral fat might be not suitable in general clinical practice and large cohort studies; thus, CVAI, constructed based on visceral fat by CT, was developed to evaluate visceral obesity among Chinese population (9). A study from a province in Southwest China study showed CVAI was associated with CVD in women but not in men (19). In our study, we visualized the association of CVAI with CVD and found positive associations in both men and women. Consistent with our findings, Zhao et al. found CVAI associated with stroke based on rural population of a Chinese county (17). We additionally found linear association between CVAI and stroke using national data, which was in line with Zhang et al. using logistic model (32). Similar to our findings, a study based on examination population in a Beijing hospital also found positive associations between CVAI and coronary heart disease in both men and women (18). Previous studies also reported that CVAI was positively associated with carotid atherosclerosis or carotid plaque (33, 34), which verified the rationality of the association between CVAI and CVD. Besides, we further explored the interaction of age, gender, hypertension, diabetes, and elevated TC on the associations of CVAI with CVD, heart disease, and stroke. Subgroup analyses showed participants aged<60 years had higher risk of CVD, heart disease, and stroke than those aged ≥60 years, which may be explained by longer duration of exposure to excess visceral fat.
Our results indicated that CVAI had the best ability to predict incident CVD, heart disease, and stroke among these obesity indices (CVAI, VAI, ABSI, CI, WC, and BMI). Previous studies also showed that CVAI was a better obesity indicator for coronary heart disease incidence than VAI, WC, and BMI and for stroke than other insulin resistance indices (17, 18). Besides, CVAI also had larger predictive value for cardiovascular events than VAI, WC, and BMI in Chinese diabetic patients (35, 36). A potential explanation for those findings may be that CVAI could evaluate visceral fat better in Chinese population (9). Contrast with measurement of visceral fat by CT and MRI (29, 37), CVAI, based on age, BMI, WC, TG, and HDL-C, are available and reliable to assess obesity in routine clinical practice and large cohort studies. Identifying participants with higher risk of CVD and then implementing lifestyle interventions may benefit to lower CVD risk.
Several potential mechanisms were suggested to explain the association between visceral obesity and CVD. Visceral adiposity promotes systemic and vascular inflammation by increasing production of interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein, which lead to atherogenesis and subsequently incident CVD (38–40). Besides, visceral adiposity disturbs the renin–angiotensinogen system and produces excessive reactive oxygen species and reactive nitrogen species, and then induce oxidative stress, which could be presented as oxidized low-density lipoprotein, 8-hydroxylated deoxyguanosine, malondialdehyde, thioredoxin, and advanced oxidation protein products; the oxidative stress would induce a vicious cycle of endothelial dysfunction, inflammation, fibroblast proliferation and affect cerebral arteries through stenosis and occlusion, leading to CVD incidence (38, 41–44). Also, visceral obesity causes insulin resistance through increasing the production of inflammatory markers and adipocytokines and reducing that of adiponectin, which result in CVD incidence (45–47).
The present study comprehensively explored the associations of CVAI with CVD, heart disease, and stroke and compared the predictive ability of CVAI with other obesity indices (VAI, ABSI, CI, WC, and BMI) based on a national prospective cohort study. The findings are reliable and can be generalized to Chinese or other Asian people. Some limitations should be noted. First, we did not evaluate the effect of dynamic change in CVAI on CVD. Second, we failed to adjust for residual confounders, such as diet, air pollution, etc. Third, our study did not enable conclusions causal despite the prospective cohort design.
Our study showed that CVAI was linearly associated with risk of CVD, heart disease, and stroke and CVAI had the best predictive ability among these obesity indices. The findings support CVAI as a suitable visceral obesity index for predicting CVD in Chinese or other Asian adults because of the availability of its components in large prospective studies and routine clinical practice.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: This analysis uses data or information from the Harmonized CHARLS dataset and Codebook, Version D as of June 2021 developed by the Gateway to Global Aging Data. The development of the Harmonized CHARLS was funded by the National Institute on Aging (R01 AG030153, RC2 AG036619, R03 AG043052). For more information, please refer to https://g2aging.org/.
The studies involving humans were approved by public databases-China Health and Retirement Longitudinal Study (CHARLS). Besides, the protocols of CHARLS were approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. QH: Data curation, Writing – review & editing. ZL: Data curation, Investigation, Writing – review & editing. XZ: Data curation, Methodology, Writing – review & editing. LY: Conceptualization, Resources, Writing – review & editing. LK: Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant no. 82103935), Henan Province Science and Technology Project (grant no. 232102310497), and Young Key Teacher Funding Program of Huanghuai University.
We thank all our colleagues for their excellent data arrangement and analysis. Besides, we thank all participators of CHARLS for their excellent data collection. This analysis uses data or information from the Harmonized CHARLS dataset and Codebook, Version D as of June 2021 developed by the Gateway to Global Aging Data. We would also like to thank Minghui Han for his contribution to the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1284144/full#supplementary-material
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
4. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation (2021) 143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973
5. Li JJ, Liu HH, Li S. Landscape of cardiometabolic risk factors in Chinese population: a narrative review. Cardiovasc Diabetol (2022) 21(1):113. doi: 10.1186/s12933-022-01551-3
6. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):373–92. doi: 10.1016/S2213-8587(21)00045-0
7. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) (2010) 34(5):791–9. doi: 10.1038/ijo.2010.5
8. Nevill AM, Stewart AD, Olds T, Holder R. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol (2006) 129(1):151–6. doi: 10.1002/ajpa.20262
9. Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep (2016) 6:38214. doi: 10.1038/srep38214
10. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care (2010) 33(4):920–2. doi: 10.2337/dc09-1825
11. Han M, Qie R, Li Q, Liu L, Huang S, Wu X. Chinese visceral adiposity index, a novel indicator of visceral obesity for assessing the risk of incident hypertension in a prospective cohort study. Br J Nutr (2021) 126(4):612–20. doi: 10.1017/S0007114520004298
12. Li Y, Yu D, Yang Y, Cheng X, Piao W, Guo Q. Comparison of several adiposity indexes in predicting hypertension among Chinese adults: data from China nutrition and health surveillance (2015-2017). Nutrients (2023) 15(9):2146. doi: 10.3390/nu15092146
13. Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y. Chinese visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev (2021) 37(2):e3370. doi: 10.1002/dmrr.3370
14. Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metab Res Rev (2018) 34(7):e3048. doi: 10.1002/dmrr.3048
15. Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients (2019) 11(7):1580. doi: 10.3390/nu11071580
16. Ren Y, Cheng L, Qie R, Han M, Kong L, Yan W. Dose-response association of Chinese visceral adiposity index with comorbidity of hypertension and diabetes mellitus among elderly people. Front Endocrinol (Lausanne) (2023) 14:1187381. doi: 10.3389/fendo.2023.1187381
17. Zhao Y, Zhang J, Chen C, Qin P, Zhang M, Shi X. Comparison of six surrogate insulin resistance indexes for predicting the risk of incident stroke: The Rural Chinese Cohort Study. Diabetes Metab Res Rev (2022) 38(7):e3567. doi: 10.1002/dmrr.3567
18. Xie Y, Zhang Y, Qin P, Ping Z, Wang C, Peng X. The association between Chinese Visceral Adipose Index and coronary heart disease: A cohort study in China. Nutr Metab Cardiovasc Dis (2022) 32(3):550–9. doi: 10.1016/j.numecd.2021.10.020
19. Wang Y, Zhao X, Chen Y, Yao Y, Zhang Y, Wang N. Visceral adiposity measures are strongly associated with cardiovascular disease among female participants in Southwest China: A population-based prospective study. Front Endocrinol (Lausanne) (2022) 13:969753. doi: 10.3389/fendo.2022.969753
20. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol (2014) 43(1):61–8. doi: 10.1093/ije/dys203
21. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension (2003) 42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
22. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev (2019) 35(6):e3158. doi: 10.1002/dmrr.3158
23. Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev (2018) 19(5):737–59. doi: 10.1111/obr.12666
24. Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol (1991) 44(9):955–6. doi: 10.1016/0895-4356(91)90059-I
25. Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
26. Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open (2019) 2(12):e1916591. doi: 10.1001/jamanetworkopen.2019.16591
27. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol (2013) 62(10):921–5. doi: 10.1016/j.jacc.2013.06.027
28. Mongraw-Chaffin M, Allison MA, Burke GL, Criqui MH, Matsushita K, Ouyang P, et al. CT-derived body fat distribution and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab (2017) 102(11):4173–83. doi: 10.1210/jc.2017-01113
29. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol (2019) 7(9):715–25. doi: 10.1016/S2213-8587(19)30084-1
30. Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr (2012) 96(4):714–26. doi: 10.3945/ajcn.112.035758
31. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev (2002) 3(3):141–6. doi: 10.1046/j.1467-789X.2002.00065.x
32. Zhang H, Zhan Q, Dong F, Gao X, Zeng F, Yao J, et al. Associations of Chinese visceral adiposity index and new-onset stroke in middle-aged and older Chinese adults: an observational study. Lipids Health Dis (2023) 22(1):74. doi: 10.1186/s12944-023-01843-x
33. Wang X, Si Z, Wang H, Meng R, Lu H, Zhao Z, et al. Association of Chinese visceral adiposity index and carotid atherosclerosis in steelworkers: A cross-sectional study. Nutrients (2023) 15(4):1023. doi: 10.3390/nu15041023
34. Bi H, Zhang Y, Qin P, Wang C, Peng X, Chen H, et al. Association of Chinese visceral adiposity index and its dynamic change with risk of carotid plaque in a large cohort in China. J Am Heart Assoc (2022) 11(1):e022633. doi: 10.1161/JAHA.121.022633
35. Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol (2022) 21(1):225. doi: 10.1186/s12933-022-01670-x
36. Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol (2020) 19(1):118. doi: 10.1186/s12933-020-01095-4
37. Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care (2018) 21(5):360–5. doi: 10.1097/MCO.0000000000000485
38. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci (2022) 23(2):786. doi: 10.3390/ijms23020786
39. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol (2009) 6(6):399–409. doi: 10.1038/nrcardio.2009.55
40. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature (2006) 444(7121):875–80. doi: 10.1038/nature05487
41. Yan Q, Liu S, Sun Y, Chen C, Yang S, Lin M, et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J Transl Med (2023) 21(1):519. doi: 10.1186/s12967-023-04361-7
42. Micle O, Muresan M, Antal L, Dobjanschi L, Antonescu A, Vicas L, et al. Correlation between reactive oxygen species and homocysteine levels in normal pregnancy. FARMACIA (2011) 59(2).
43. Zaha DC, Pop NO, Pantiş C, Mekeres F. Clinicopathological evaluation of Moyamoya disease. Case report and review of literature. Romanian J Military Med (2020) CXXIII(2).
44. Shaito A, Aramouni K, Assaf R, Parenti A, Orekhov A, Yazbi AE, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci (Landmark Ed) (2022) 27(3):105. doi: 10.31083/j.fbl2703105
45. Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am (2011) 95(5):875–92. doi: 10.1016/j.mcna.2011.06.002
46. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res (2020) 126(11):1549–64. doi: 10.1161/CIRCRESAHA.119.315896
Keywords: Chinese visceral adiposity index, cardiovascular disease, dose-response association, receiver operating characteristic, prospective cohort study
Citation: Ren Y, Hu Q, Li Z, Zhang X, Yang L and Kong L (2024) Dose–response association between Chinese visceral adiposity index and cardiovascular disease: a national prospective cohort study. Front. Endocrinol. 15:1284144. doi: 10.3389/fendo.2024.1284144
Received: 28 August 2023; Accepted: 26 January 2024;
Published: 18 April 2024.
Edited by:
Lu Cai, University of Louisville, United StatesCopyright © 2024 Ren, Hu, Li, Zhang, Yang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongcheng Ren, cmVueW9uZ2NoZW5nQGh1YW5naHVhaS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.