- 1College of Public Health, Zunyi Medical University, Zunyi, China
- 2Key Laboratory of Maternal & Child Health and Exposure Science of Guizhou Higher Education Institutes, Zunyi, China
- 3Qingdao Municipal Center for Disease Control and Prevention, Qingdao Institute of Prevention Medicine, Qingdao, China
- 4Center for Animal Disease Control and Prevention of Shanghai, Shanghai, China
- 5Center for Animal Disease Control and Prevention of Guizhou Province, Guiyang, China
- 6China Animal Health and Epidemiology Center, Qingdao, China
Background: The prevalence of obesity among women of reproductive age is increasing worldwide, with implications for serious pregnancy complications.
Methods: Following PRISMA guidelines, a systematic search was conducted in both Chinese and English databases up to December 30, 2020. Pregnancy complications and outcomes including gestational diabetes mellitus (GDM), gestational hypertension (GHTN), pre-eclampsia, cesarean section (CS), induction of labor (IOL), and postpartum hemorrhage (PPH) were analyzed. Random-effects or fixed-effects models were utilized to calculate the odds ratio (OR) with 95% confidence intervals (CIs).
Results: Women with overweight and obesity issues exhibited significantly higher risks of GDM (OR, 2.92, 95%CI, 2.18-2.40 and 3.46, 95%CI, 3.05-3.94, respectively) and GHTN (OR, 2.08, 95%CI, 1.72-2.53 and 3.36, 95%CI, 2.81-4.00, respectively) compared to women of normal weight. Pre-eclampsia was also significantly higher in women with overweight or obesity, with ORs of 1.70 (95%CI, 1.44-2.01) and 2.82 (95%CI, 2.66-3.00), respectively. Additionally, mothers with overweight or obesity issues had significantly higher risks of CS (OR, 1.44, 95%CI, 1.41-1.47, and 2.23, 95%CI, 2.08-2.40), IOL (OR, 1.33, 95%CI, 1.30-1.35 and 1.96, 95%CI, 1.85-2.07), and PPH (OR, 1.67, 95%CI, 1.42-1.96 and 1.88, 95%CI, 1.55-2.29).
Conclusion: Women with overweight or obesity issues face increased risks of pregnancy complications and adverse outcomes, indicating dose-dependent effects.
Introduction
The prevalence of obesity is skyrocketing across the globe. According to a study on the Global Burden of Disease, the proportion of adults with a body mass index (BMI) of 25 or greater surged from approximately 29% to 37% in men and from around 30% to 38% in women between 1980 and 2013. Additionally, in 2013, 22.6% of girls in developed countries and 13.4% of girls in developing countries were classified as overweight or obesity (1).
Nowadays, the prevalence of obesity among women of reproductive age is on the rise globally (2). In most developed countries, over half of women of reproductive age are classified as overweight (BMI 25-29.9 kg/m2) or obesity (≧30 kg/m2) (3). It has been estimated that 23.9% of any pregnancy complication was attributable to maternal overweight/obesity (4). M Maternal obesity is associated with a myriad of adverse perinatal outcomes (5), including large for gestational age, macrosomia, preterm birth, and stillbirth (6), Additionally, it can impact delivery outcomes (7), such as cesarean section (CS), induction of labor (IOL), and shoulder dystocia (8), as well as contribute to various pregnancy complications (9), like miscarriage, gestational diabetes mellitus (GDM) (10), etc.
Numerous original studies have extensively examined the impacts of pre-pregnancy BMI on maternal health outcomes (11, 12), Numerous original studies have extensively examined the impacts of pre-pregnancy BMI on maternal health outcomes (13–16). However, these reviews primarily focused on English and French publications and used a limited number of studies to analyze various complications, including maternal, fetal, and neonatal adverse outcomes. Notably, many studies on Chinese women have been published in reputable domestic journals in Chinese (17–19). These studies and their findings may contribute to differences in the relationship between maternal BMI and pregnancy outcomes compared to previous studies. Given the existing literature landscape and the desire for a more focused analysis, this article exclusively examines the relationship between BMI and maternal outcomes. This approach is taken because our previous research has already summarized the relationship between BMI and neonatal or fetal outcomes (5), concentrating solely on maternal outcomes aims to ensure the analysis and discussion are more comprehensive in this paper. Therefore, we conducted a systematic review and meta-analysis to investigate the relationship between maternal pre-pregnancy BMI and the risk of pregnancy complications and outcomes. The pregnancy complications and outcomes evaluated in this meta-analysis include GDM, GHTN, pre-eclampsia, CS, IOL, and PPH.
Materials and methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed for conducting the systematic search. The search encompassed various databases from their earliest available dates up to December 30, 2020. Chinese databases, including China National Knowledge Infrastructure (CNKI), Wiper database (VIP), China Biomedical Literature Database (CBM), and Wanfang database (WF), were searched, along with English databases such as PubMed, Embase, and ISI. The search strategy involved identifying relevant literature using the following terms: (“BMI” or “Body Mass Index” or “obesity” or “overweight” or “underweight” or “Quetelet index”) AND (“pregnancy complications” or “outcomes”). Additionally, efforts were made to include unpublished studies to mitigate publication bias.
Study eligibility
Studies were considered eligible for inclusion in this meta-analysis if they met the following criteria: (1) The study type was observational, including cross-sectional, case-control, or cohort designs. (2) Participants were women, with a measured BMI in the first trimester of pregnancy or at their first antenatal visit. (3) Complete baseline maternal clinical information and pertinent outcome data were available. (4) Participants were singleton pregnant with no pre-existing medical disorders before conception. (5) Studies provided the number of women in each BMI category and reported the occurrence of related adverse outcomes. (6) Exposure groups consisted of women classified as underweight, overweight, or obesity, while the control group comprised women of normal weight. (7) The outcomes of interest were adverse pregnancy complications, which encompassed gestational diabetes mellitus [GDM, defined based on a 75g 2-hour oral glucose tolerance test conducted in the second trimester of pregnancy], gestational hypertension [GHTN, also known as pregnancy-induced hypertension, PIH, defined as diastolic blood pressure ≥90 mm Hg or systolic blood pressure ≥140 mm Hg in the second or third trimester among mothers who had normal blood pressure before pregnancy], and pre-eclampsia [defined as blood pressure ≥140/90 mmHg accompanied by proteinuria], as well as delivery complications, including cesarean section [CS, encompassing both elective and emergency deliveries], induction of labor[IOL, involving the administration of inducing drugs such as prostaglandins or oxytocin to expedite delivery], and post-partum hemorrhage [PPH, defined as blood loss exceeding 500mL within 24 hours after delivery].
Studies were excluded if they involved women with incomplete information on height or weight, or if they did not report any outcomes relevant to the scope of this meta-analysis.
Data extraction and quality assessment
The following information was extracted from each study: author name, country of population, year of publication, study design, the number of women categorized into different BMI levels, pregnancy outcomes, and their occurrence. Data extraction was performed independently by two authors to ensure accuracy, and any inconsistencies were resolved through consensus or consultation with other authors.
The quality assessment of case-control or cohort studies was conducted using the 9-star Newcastle-Ottawa Scale, while the quality of cross-sectional studies was evaluated using the 11-point scale from the Agency for Healthcare Research and Quality (AHRQ). Each study is based on predefined standards, with a score of ≥7 out of 9 or 9 out of 11 indicating high quality. Studies scoring 5-6 out of 9 or 7-8 out of 11 were classified as medium quality, while those with scores <5 out of 9 or <7 out of 11 were considered low quality. Only studies rated as medium or high quality were included in the analysis. The quality assessment was conducted independently by two authors, with any disagreements resolved through consensus.
Statistical analysis
BMI, also known as the Quetelet index, is defined as (weight in kilograms)/(height in meters)2 (20). It has become a universally accepted measure of the degree of overweight or obesity. The World Health Organization (WHO) and the National Institute of Health (NIH) have defined three cutoff points (18.5 kg/m2, 25.0 kg/m2, 30.0 kg/m2), which classify individuals into four groups: underweight, normal weight, overweight and obesity (21).
For this meta-analysis, BMI levels were categorized into these four groups, with normal weight serving as the reference group. The risk of pregnancy complications for individuals in other BMI categories was assessed using odds ratios (OR) with 95% confidence intervals (CIs).
Heterogeneity among studies was assessed using standard chi-square tests and I2 values, with tests carried out in Stata. A random-effects model (REM) was employed in the presence of heterogeneity (I2>50%), while the fixed-effect model (FEM) was utilized otherwise. Sensitivity analysis was conducted to examine potential sources of heterogeneity. Additionally, meta-regression was conducted to identify the sources of the heterogeneity. The funnel plot and Egger’s regression asymmetry test were used to assess the potential publication bias.
Data analysis was conducted using Stata version 11.0 (Stata Corporation, College Station, TX, USA) software. All p-values were two-tailed, and p-value < 0.05 was considered statistically significant.
Results
Search results
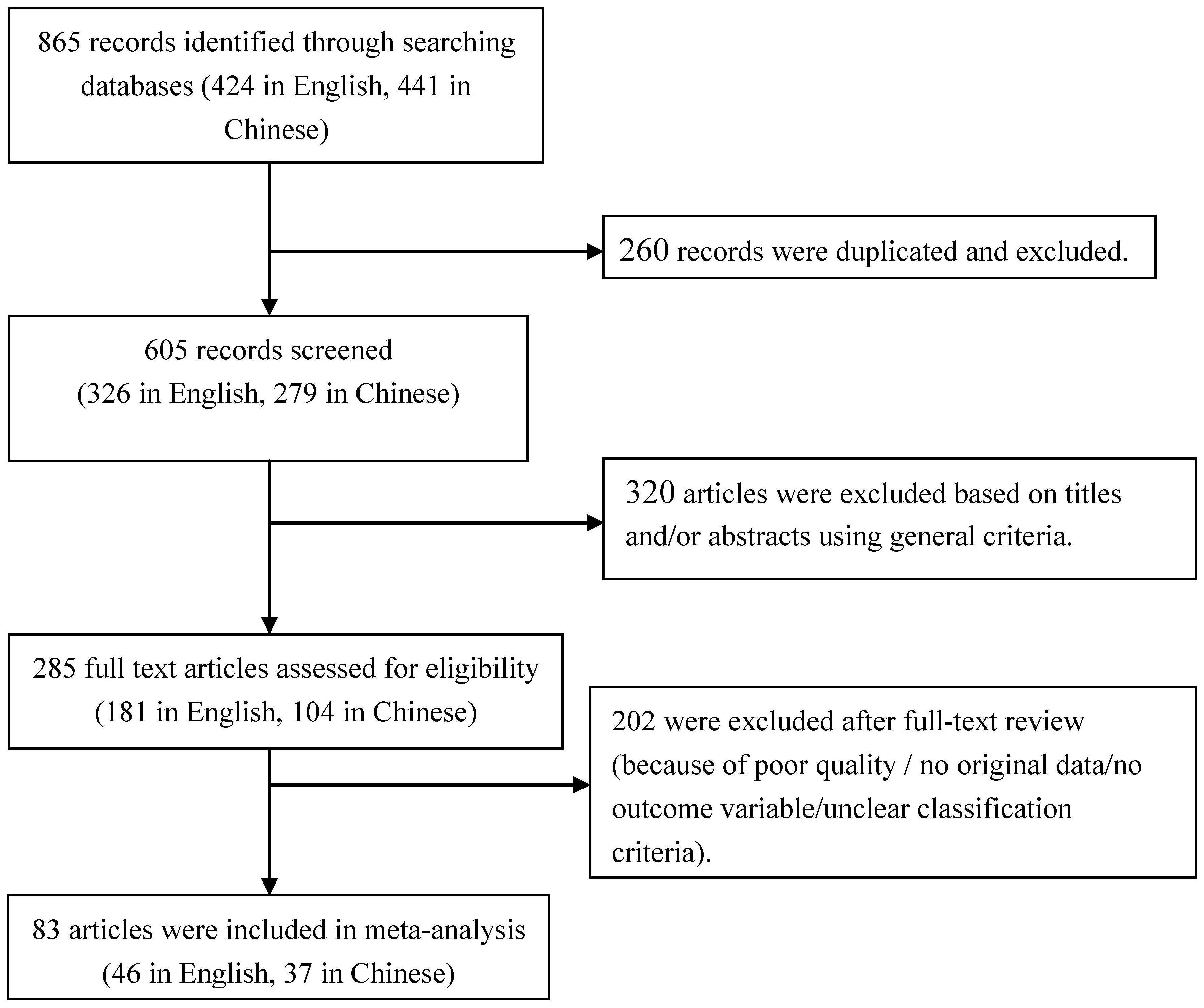
Initially, 865 articles were identified regarding the association between maternal BMI and pregnancy complications. Of these, 441 were found in Chinese databases and 424 in English databases. After excluding 260 duplicated articles, 605 records remained. Subsequently, 285 articles were selected for full-text review after removing 320 pieces based on screening of titles and abstracts. Eventually, 83 studies met all general criteria and were included in the quantitative analysis. A flow diagram illustrating the selection process is provided in Figure 1.
Characteristics of included studies
These 83 studies, encompassing 1,966,026 women, were published between 1998 and 2019, with sample sizes ranging from 100 to 621,221. The majority of studies were carried out in Asia (n = 53) [China (n = 42), India (n = 5), Korea (n = 2), United Arab Emirates (n = 2), Iran (n = 1), Israel (n = 1)]. Additionally, 16 studies were conducted in Europe [UK (n=6), Denmark (n = 3), Turkey (n=2), Finland (n = 1), Ireland (n = 1), Italy (n = 1), Spain (n = 1), Sweden (n = 1)]. Eight studies were conducted in North America [USA (n=8)], three in Africa [Cameroon (n=1), Nigeria (n=1), Sudan (n=1)], and three in Oceania [Australia (n=3)]. The primary information regarding these studies is summarized in Table 1.
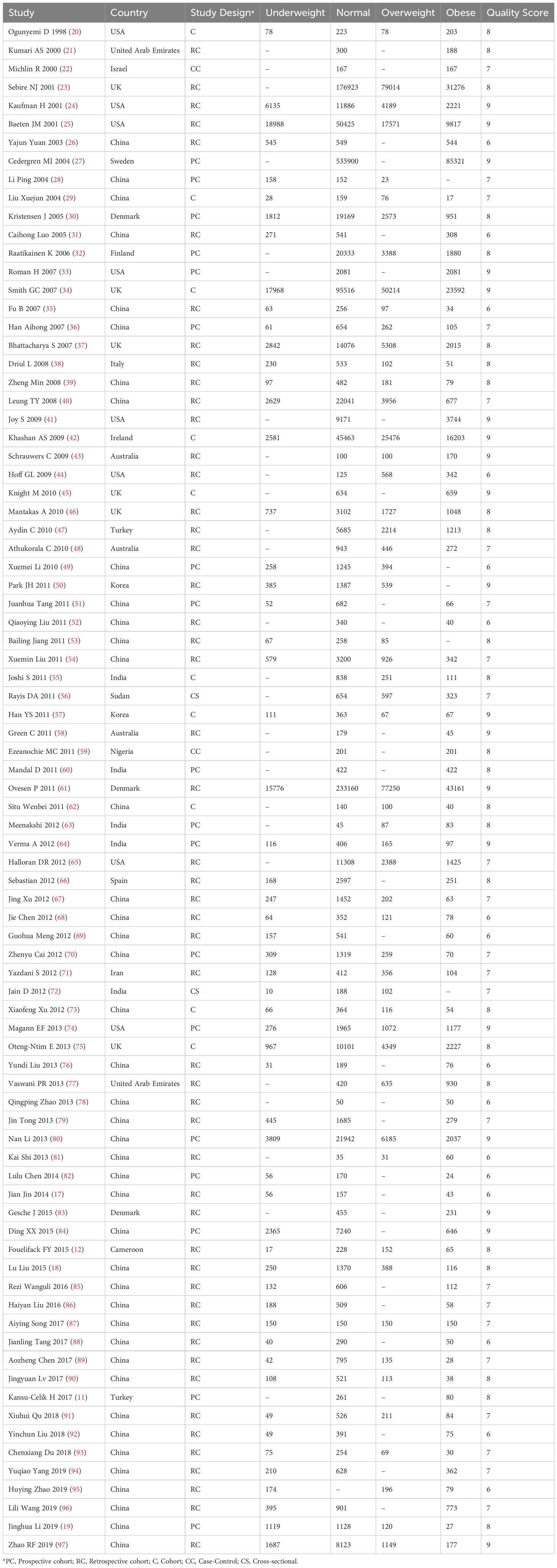
Table 1 Information of articles included in analysis of association between maternal BMI and pregnancy complications.
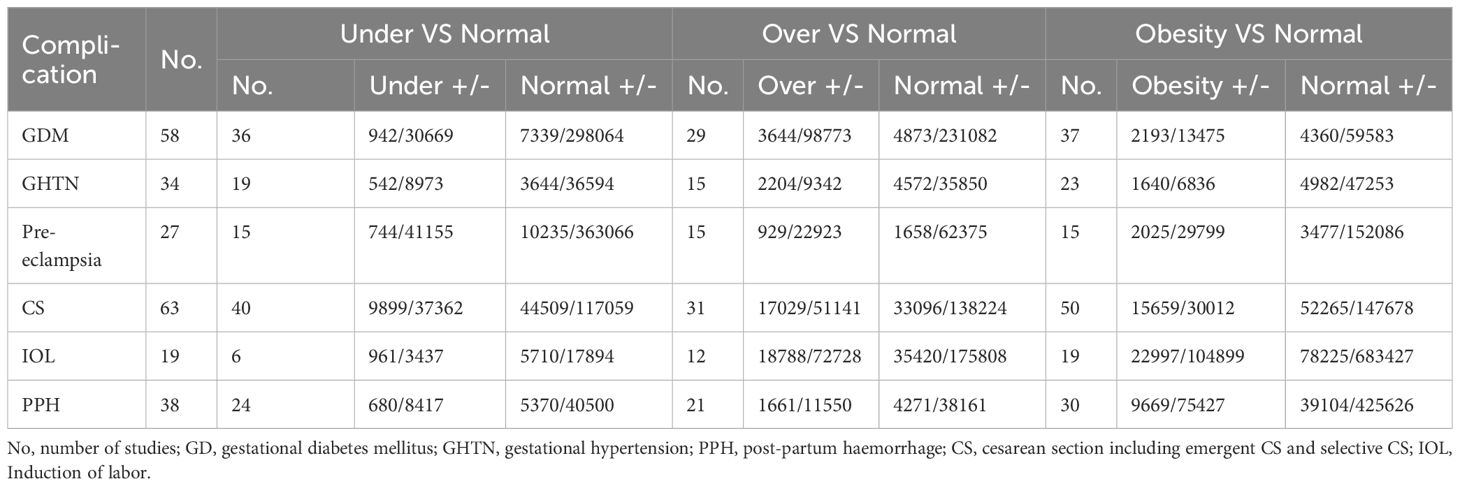
Among the 83 studies, 63 involved CS, 58 involved GDM, 34 involved GHTN, 38 involved PPH, 27 involved pre-eclampsia, and 19 involved IOL. These studies were utilized to assess the risk of pregnancy complications at different BMI levels. The number of studies and individuals involved is presented in Table 2.
Methodological quality
Eighty-one cohort or case-control studies were assessed using the NOS scale and obtained an average score of 7.58 ± 1.05 (Table 1). Among these, 65 were classified as high-quality studies, while 16 were categorized as medium-quality studies. Additionally, two cross-sectional studies were assessed using the AHRQ scale and were deemed of medium quality, each receiving 7 points.
Maternal pre-pregnancy BMI and the risk of pregnancy complications
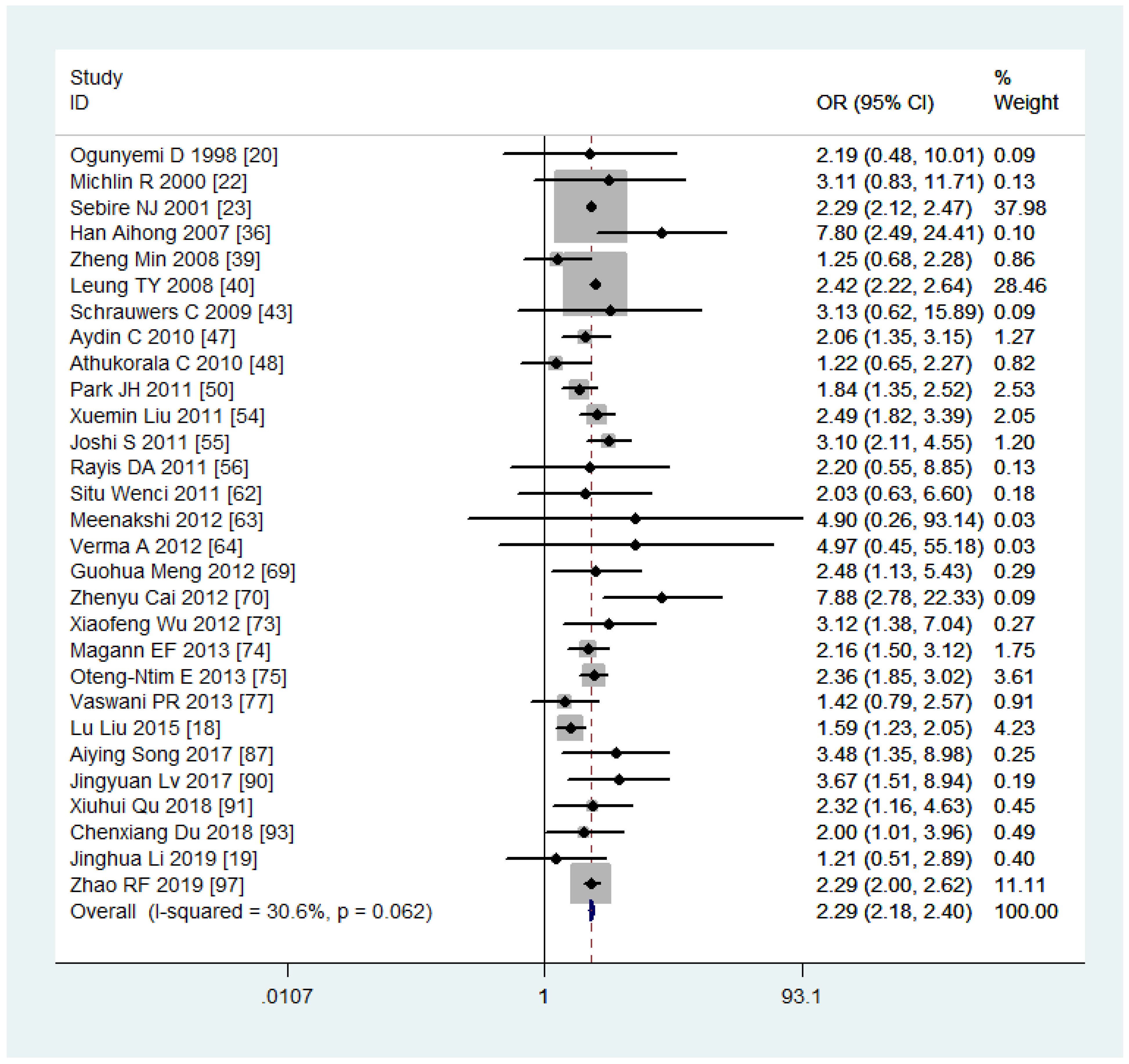
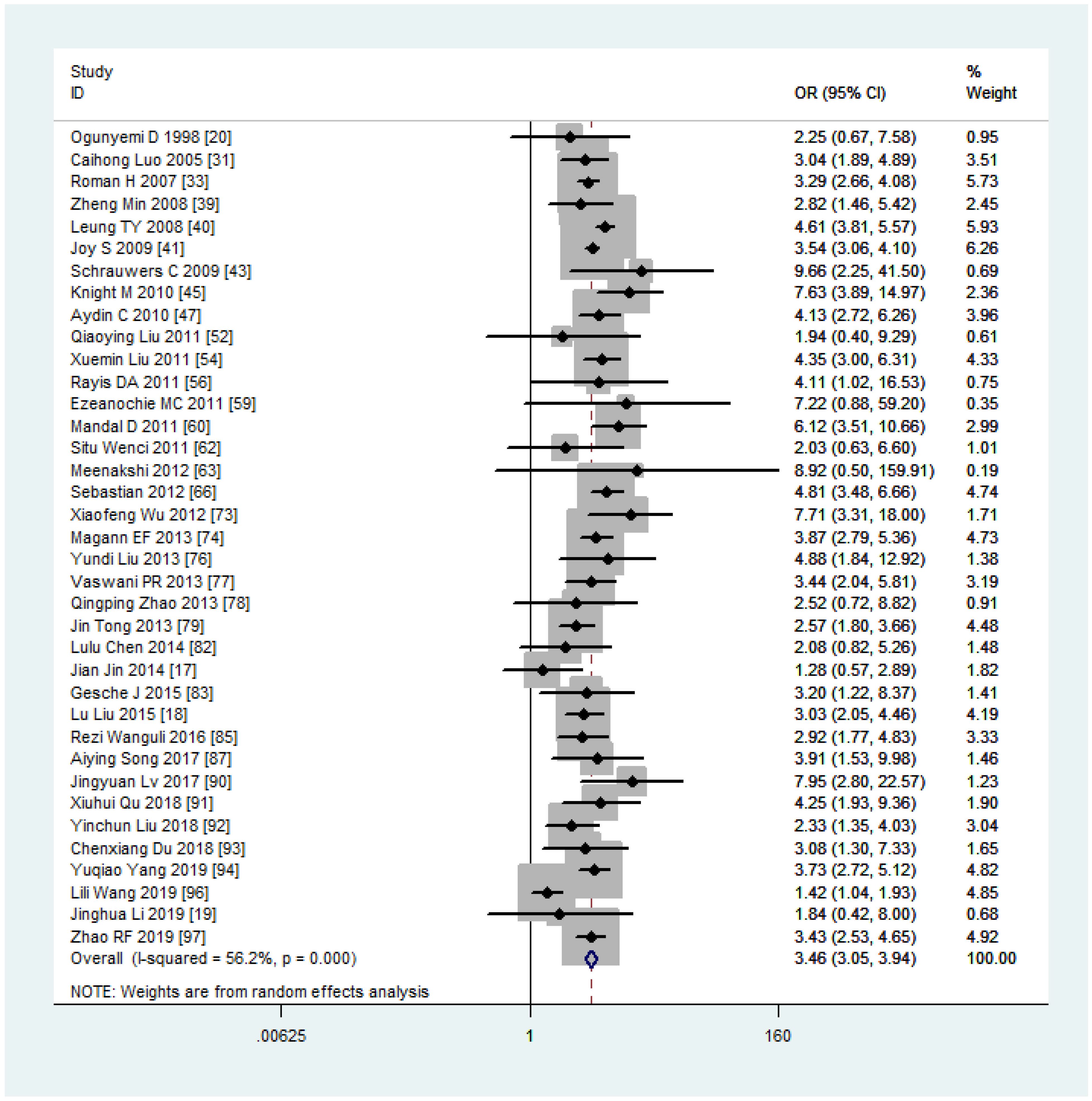
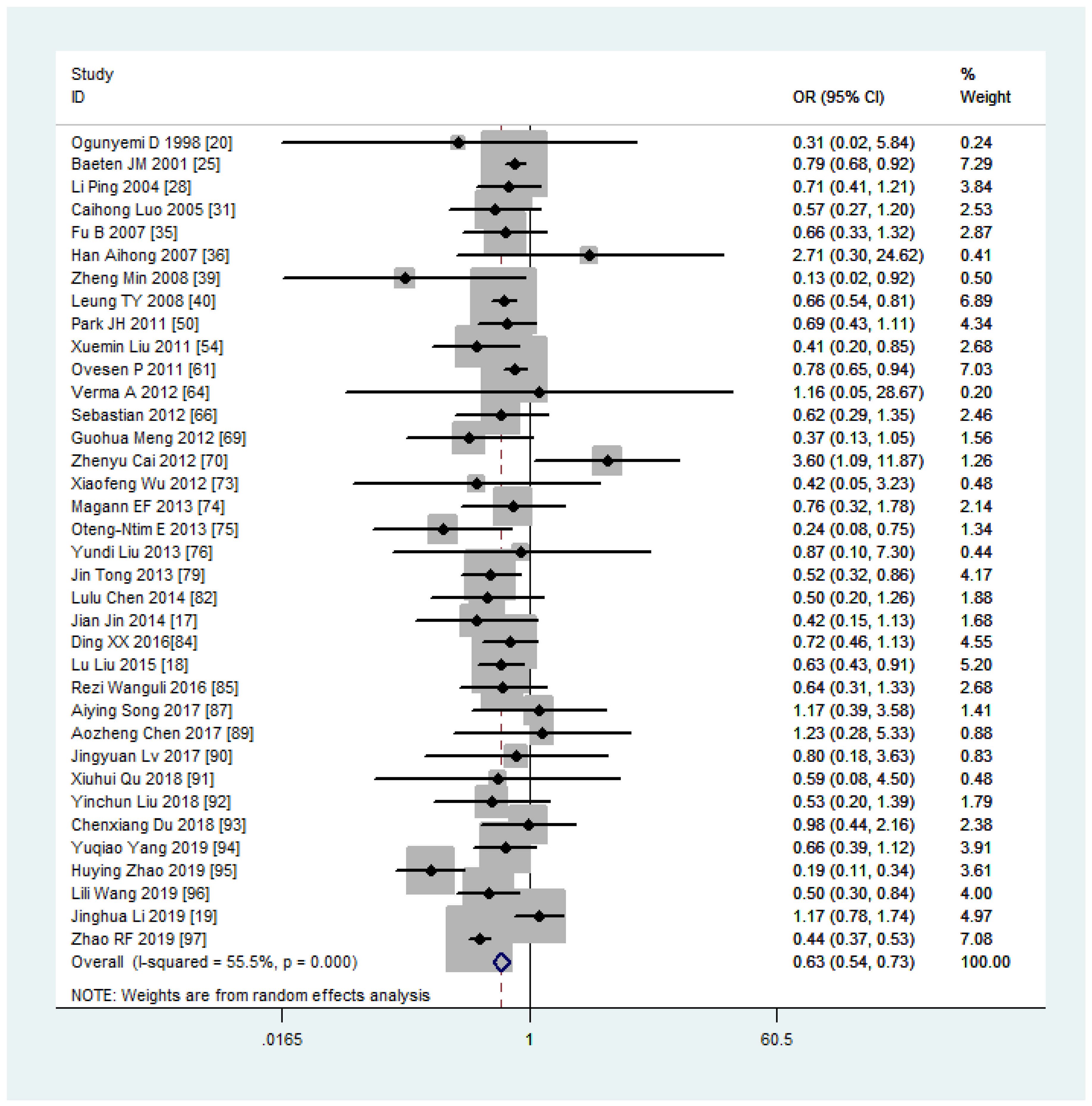
Women with overweight and obesity exhibited a significantly higher risk of GDM (OR, 2.92, 95%CI, 218-2.40 and 3.46, 95%CI, 3.05-3.94, respectively, as shown in Figures 2, 3) and GHTN (OR, 2.08, 95%CI, 1.72-2.53 and 3.36, 95%CI, 2.81-4.00, respectively) compared to women of normal weight. Conversely, when women were underweight, these risks were lower (GDM: OR, 0.63, 95%CI, 0.54-0.73, as depicted in Figure 4; GHTN: OR, 0.64, 95%CI, 0.58-0.71). Pre-eclampsia, a complication of GHTN, was significantly more prevalent in women with overweight and obesity, with ORs of 1.70 (95%CI, 1.44-2.01) and 2.82 (95%CI, 2.66-3.00). On the other hand, pre-eclampsia was significantly lower in women classified as underweight, with an OR of 0.69 (95%CI, 0.64-0.75).
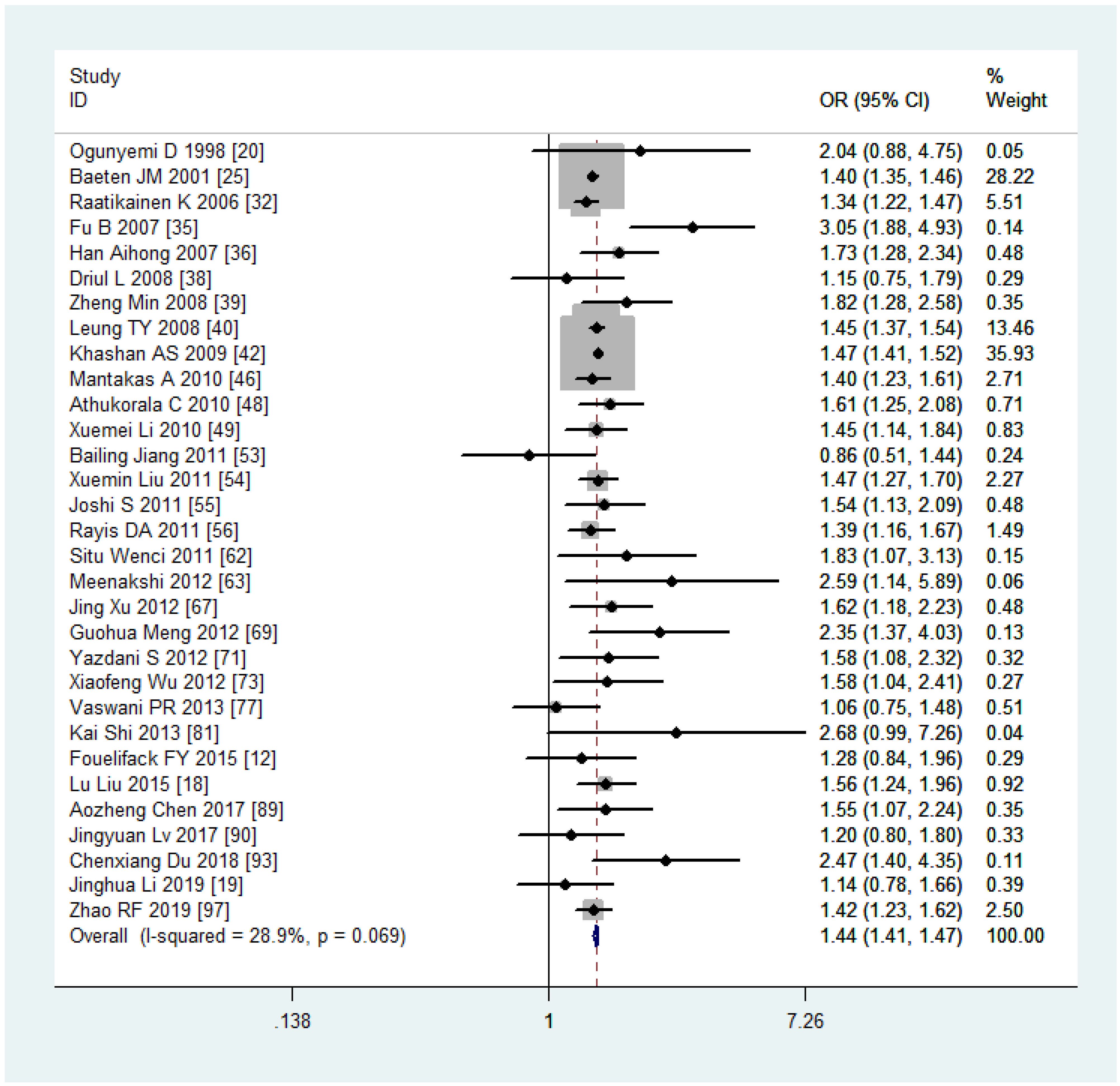
Analyzing the mode of delivery in mothers with overweight and obesity revealed a significantly higher risk of CS (OR, 1.44, 95%CI, 1.41-1.47, as depicted in Figure 5, and 2.23, 95%CI, 2.08-2.40) and IOL (OR, 1.33, 95%CI, 1.30-1.35 and 1.96, 95%CI, 1.85-2.07), respectively. In contrast, mothers classified as underweight had a significantly lower risk of CS (OR, 0.75, 95%CI, 0.73-0.77). Women with overweight and obesity had higher odds of PPH (OR, 1.67, 95%CI, 1.42-1.96 and 1.88, 95%CI, 1.55-2.29) while being underweight was associated with lower odds (0.67, 95%CI, 0.62-0.73). The ORs, heterogeneity, and selected pooling models are presented in Table 3.
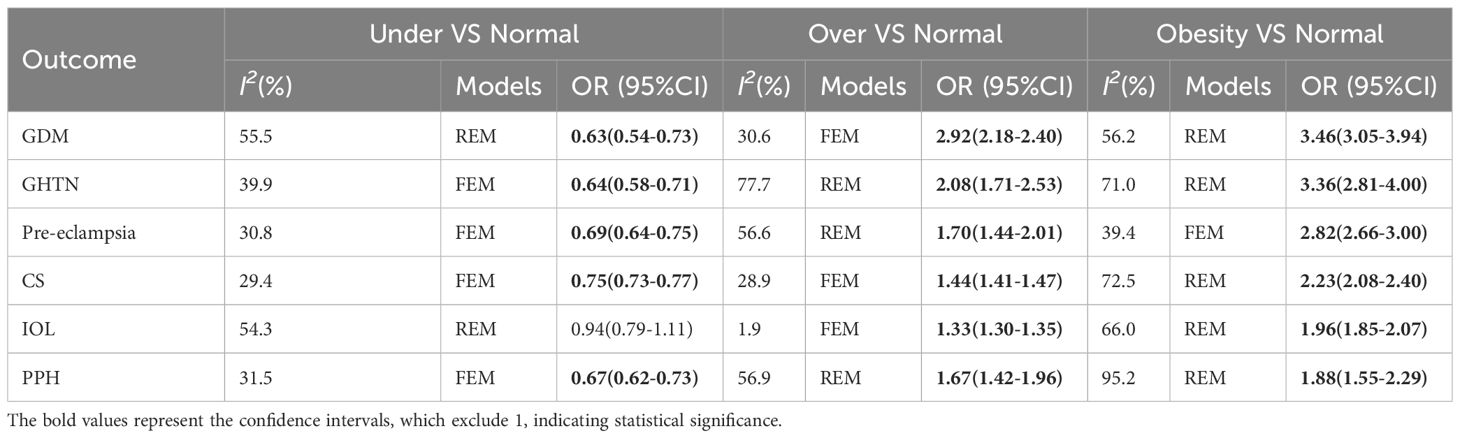
Table 3 The associations between maternal pre-pregnancy BMI and pregnancy complications and outcomes.
Sensitivity analysis
Sensitivity analyses were performed following the summary effect of each of the six outcomes, particularly when significant heterogeneities (I2 > 50%) were observed. In such cases, influential studies were identified and excluded to reassess the combined effect. For example, in the analysis comparing women who were overweight versus those with normal weight in the CS variable, the original I2 value was greater than 76.4%. After removing three articles, the value dropped to 28.9%. The results of sensitivity analyses are shown in Figure 6A. As indicated in Table 3, the majority of results exhibited low or moderate heterogeneity, except for the comparison of obesity versus normal in PPH. For this outcome, sensitivity analyses were illustrated in Figure 6B.
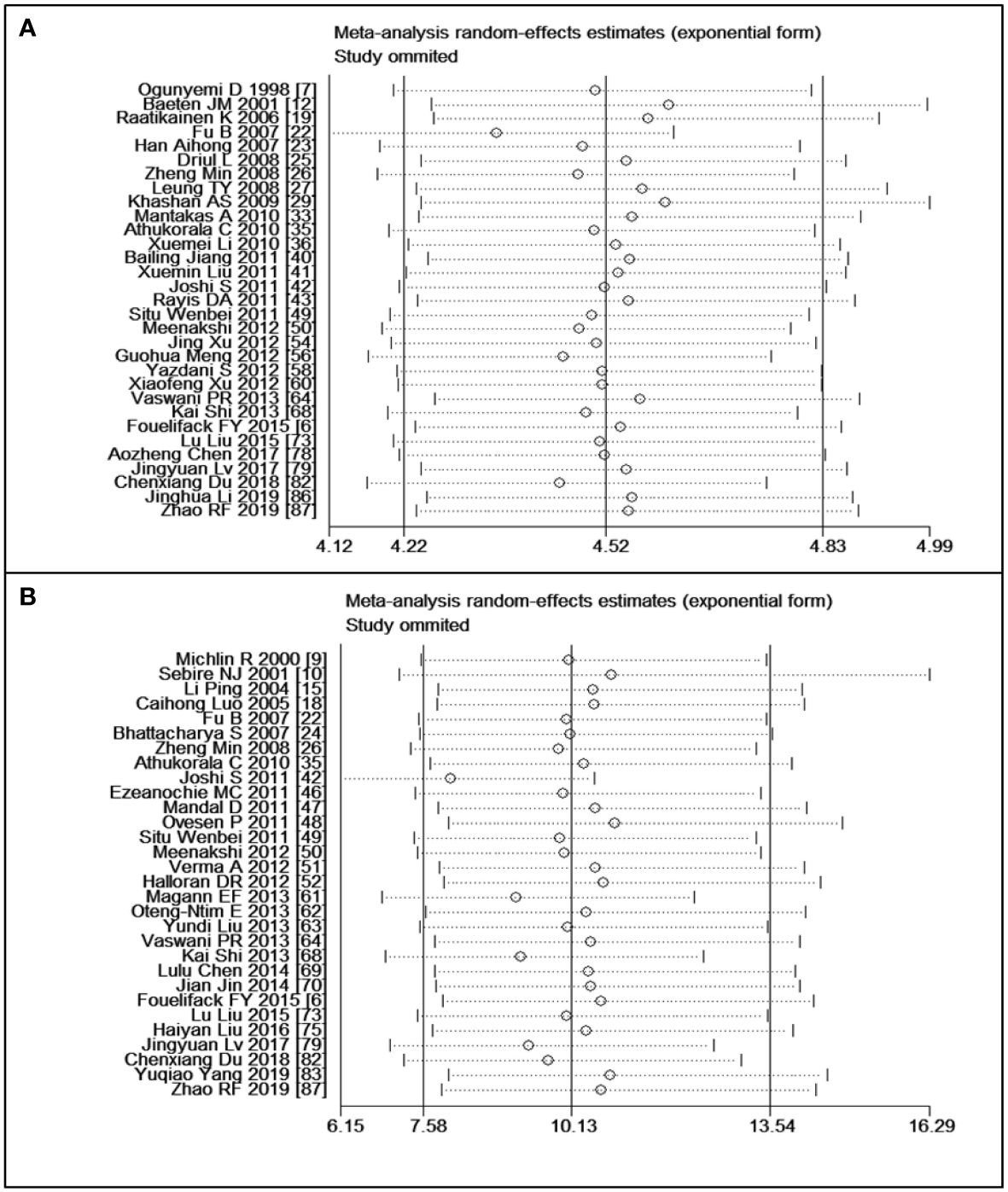
Figure 6 Sensitivity analyses: (A) overweight versus normal weight of cesarean section, (B) obesity versus normal weight of postpartum haemorrhage.
Sub-group analysis and meta-regression
Due to the high heterogeneity observed in PPH among mothers with obesity, we conducted subgroup and meta-regression analyses to explore the source of this heterogeneity. Among the 30 studies included in the analysis, 16 were conducted in China, and 22 were conducted in Asia. Therefore, we performed subgroup analyses and meta-regression using the country and continent of study to assess their potential contributions to the observed heterogeneity in the studies.
The subgroup analysis revealed that the risk of PPH was lower among mothers with obesity in China (OR, 1.77, 95% CI, 1.32-2.36, with I2 = 44.4%) compared to other countries (OR, 1.97, 95% CI, 1.51-2.56, with I2 = 97.8%) (Figure 7). When stratified by continent of study, mothers with obesity had a higher risk of PPH in Asia (OR, 1.92, 95% CI, 1.40-2.62, with I2 = 63.8%) compared to non-Asia regions (OR, 1.86, 95% CI, 1.38-2.50, with I2 = 98.7%) (Figure 8). Although the heterogeneity was lower in the China or Asia subgroup analyses by countries or continent, respectively, the OR value of the Asian group was greater than that of China. This difference may be attributed to one study conducted in India among the six articles in Asia but not in China, with a reported OR value of 7.06 (55), thereby elevating the overall OR value in Asia. Meta-regression analysis indicated that neither the country nor the continent of study was the source of heterogeneity, with p-values of 0.502 and 0.416, respectively. It is plausible that other factors such as the age and parity of pregnant women, sample size, season of pregnancy, or environmental factors may contribute to the observed significant heterogeneity.
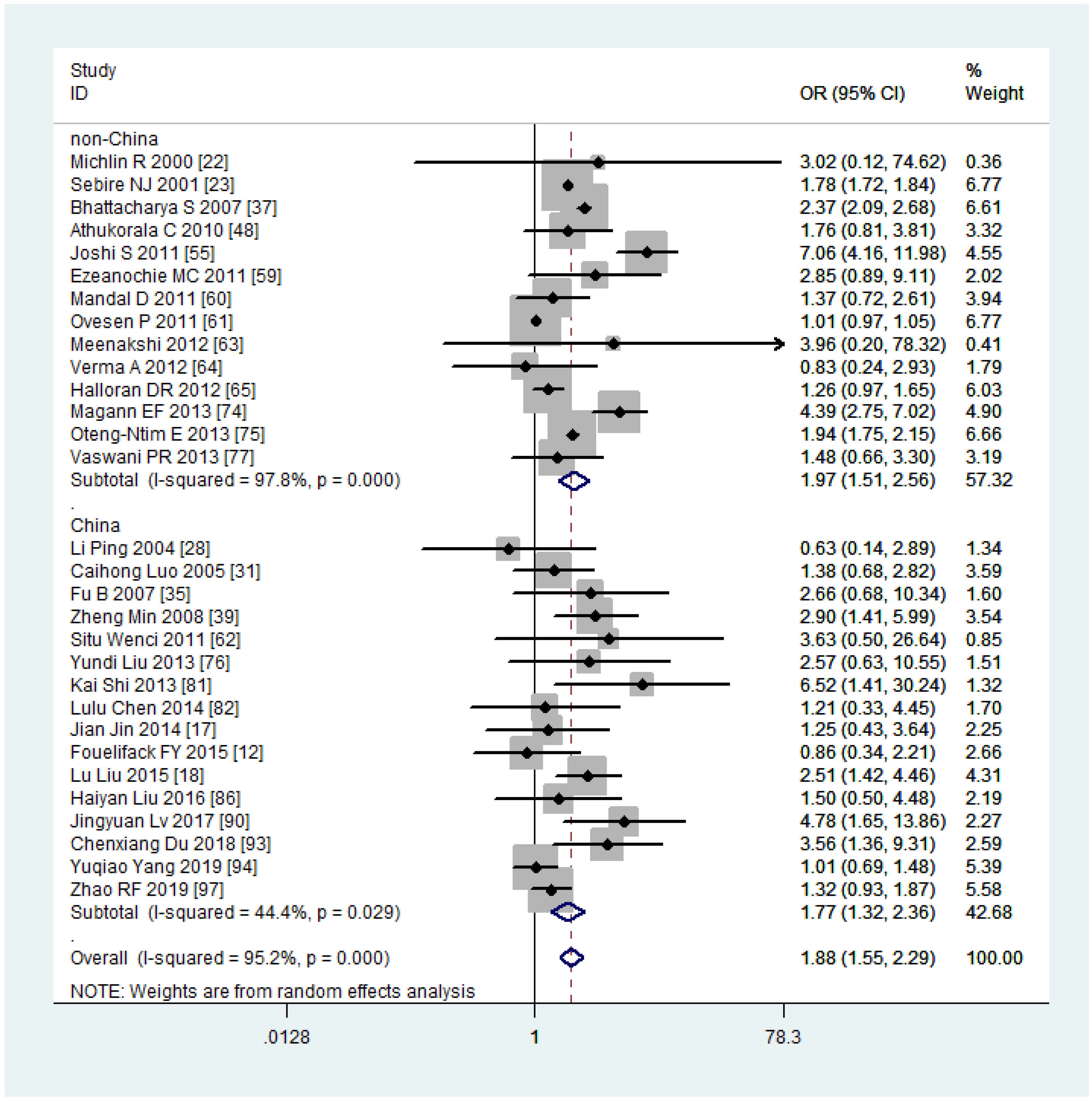
Figure 7 Sub-group analysis (China VS non-China) of Postpartum haemorrhage for overweight compared with normal weight.
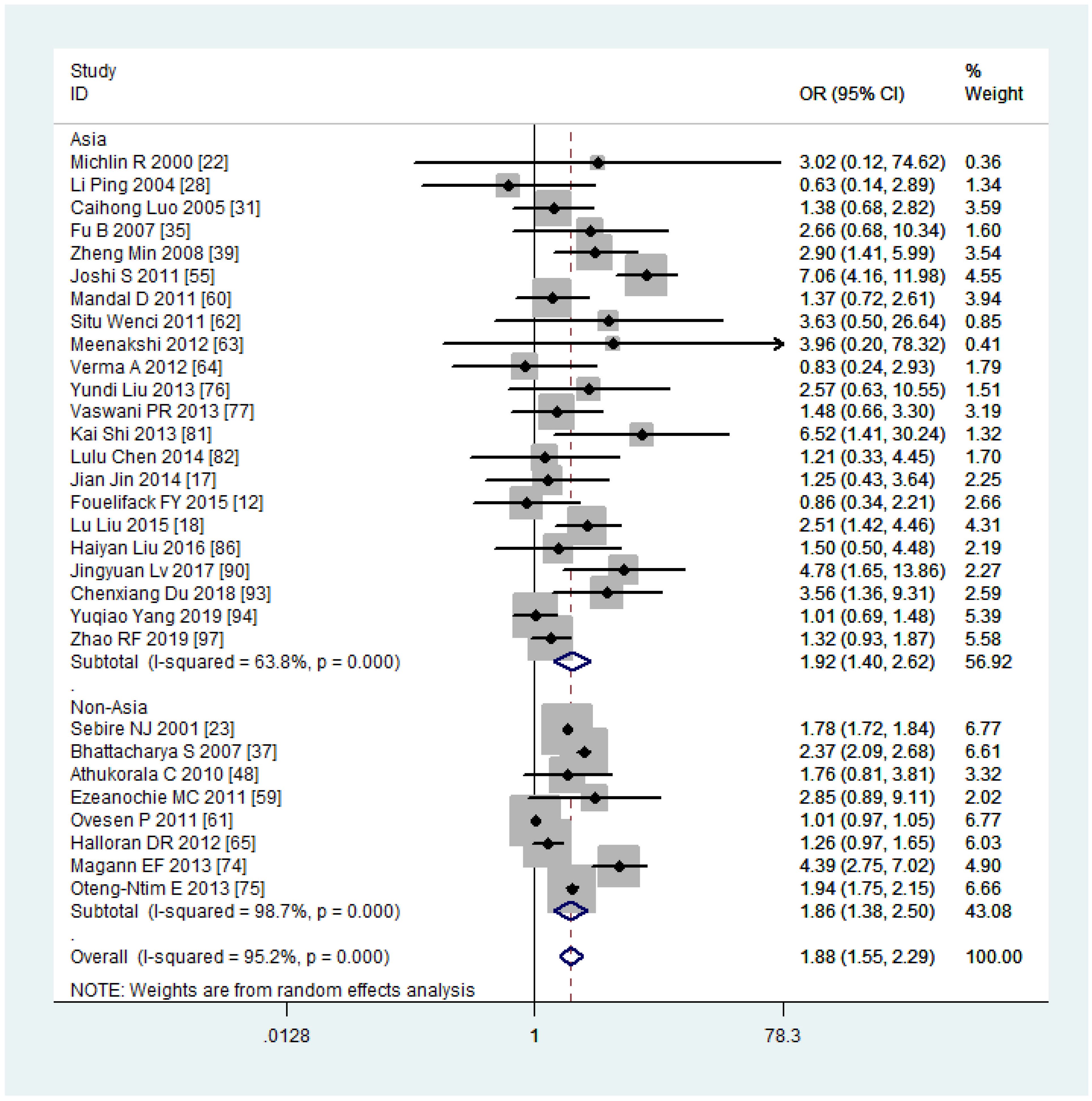
Figure 8 Sub-group analysis (Asia VS non-Asia) of Postpartum haemorrhage for overweight compared with normal weight.
Despite all studies being observational, among them there are two case-control studies and two cross-sectional studies, we attempted to perform subgroup analysis based on the study design. Results indicate that for the CS outcome, the OR values of case-control or cross-sectional studies have slightly decreased compared to the previous total results, yet the 95% confidence interval has significantly widened. Conversely, for cohort studies, there is minimal difference between the results and the total results. Due to the limited number of articles by case-control and cross-sectional study designs for other outcomes, subgroup analysis was not conducted.
Publication bias evaluation
The funnel plots and Egger’s test results indicated no significant publication bias (P > 0.05) across the 18 results of the six pregnancy outcomes. A funnel plot for CS, which encompassed the largest number of studies, is provided in Figure 9.
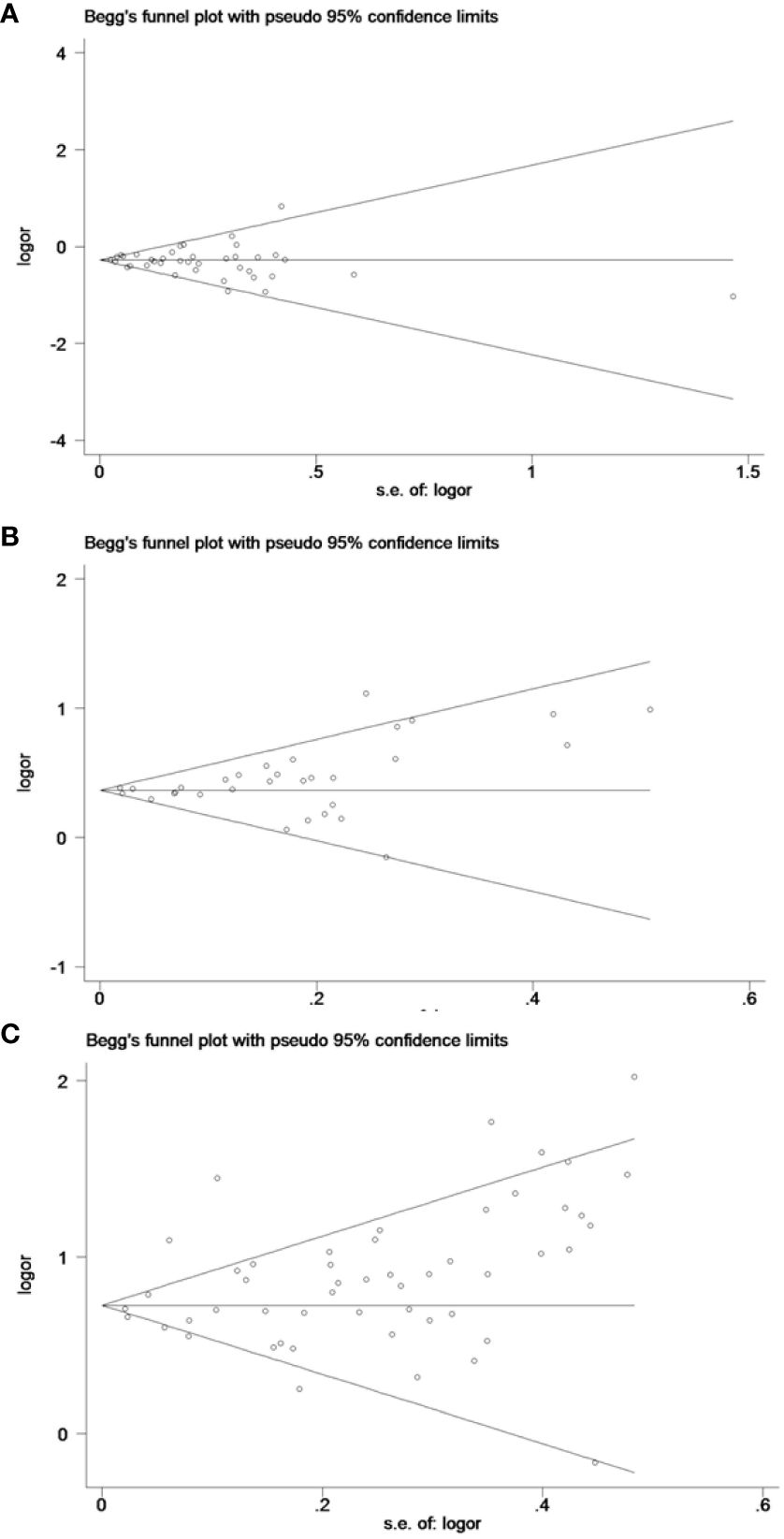
Figure 9 The funnel plots for cesarean section: (A) underweight versus normal weight, (B) overweight versus normal weight, and (C) obese versus normal weight.
Discussion
Main findings
This study provided a quantitative estimation of the risk of adverse pregnancy complications among mothers with varying BMI levels. It was found that mothers who were diagnosed as overweight or obesity faced a significantly higher risk of pregnancy complications, including GDM, GHTN, and pre-eclampsia. Additionally, they were at a heightened risk of adverse pregnancy outcomes such as CS, IOL, and PPH. Moreover, a dose-dependent relationship was observed, indicating an increased risk as the BMI levels rose.
Strengths and limitations
While previous systematic reviews and meta-analyses have explored the association between maternal body mass index and maternal health outcomes (13–16), each has its unique approach and findings. Three reviews among them only included 49 (13), 22 (14) and 13 (15) studies, respectively, a limited number in the quantitative synthesis. Furthermore, they all focused on the impact of maternal pre-pregnancy body mass index on maternal, fetal, and neonatal adverse outcomes, which results in less relevant literature on maternal outcomes. For example, the meta-analysis published in 2008 (13), which included 49 articles, only focused on hemorrhage and infection outcomes of pregnant women, with 3-4 studies. In another meta-analysis published in 2019 (15), only five and seven articles were used to analyze the relationship between gestational diabetes, gestational hypertension, and maternal pre-pregnancy BMI. The most extensive review to date, published in 2021 (16), included 86 studies and evaluated the relationship between maternal, fetal, and neonatal adverse outcomes and maternal pre-pregnancy body mass index, a broader range of outcomes. However, while examining almost the same number of studies, our review offers a distinct perspective by including a more diverse range of studies. Particularly, we included more articles from Asia, Africa, Europe, and North America providing a more comprehensive understanding of the global landscape of maternal health outcomes related to BMI. Thus, our findings complement existing literature and offer valuable insights into the worldwide situation.
Our study employed rigorous methodology, conducting comprehensive literature searches and applying stringent inclusion criteria, resulting in the inclusion of 83 articles for quantitative synthesis. The quality of included studies was assessed using the NOS tool for 81 cohort or case-control studies and ARHQ for cross-sectional studies, ensuring methodological robustness. Notably, our analysis revealed medium to low levels of heterogeneity between studies, and the relatively narrow confidence intervals further strengthened the reliability of our findings. These methodological strengths enabled us to draw firm conclusions from our meta-analysis.
There are several limitations to acknowledge in this meta-analysis. Firstly, the majority of included studies relied on pre-pregnancy BMI, with only a small portion using first-trimester BMI. While this discrepancy could potentially impact our meta-analysis results, previous stratified analyses have suggested that the difference may not be statistically significant (14). Secondly, our analysis focused solely on the association between maternal pre-pregnancy BMI and pregnancy outcomes, overlooking the effect of gestational weight gain (GWG). Given that approximately half of reproductive-age women have overweight or obesity issues and are at higher risk of substantial weight gain during pregnancy, the omission of GWG could be a limitation. Indeed, studies have shown that excessive GWG is associated with an increased risk of GHTN, PPH, and CS compared to women with normal weight gain (98). Several recently published meta-analyses (99) have focused on the association between GWG and maternal and infant outcomes. Combining these findings with our results could provide a more comprehensive understanding of the factors influencing pregnancy outcomes. Thirdly, the lack of detailed parity or age data across BMI groups in most studies may introduce bias into the pooled risk estimates. However, many included studies accounted for parity or age imbalances among BMI groups through adjustments during data analysis, mitigating potential biases to some extent. Fourthly, the outcome of CS encompassed both emergent and selective CS, with unclear distinctions provided in many included articles. Considering previous studies indicating that pregnant women with obesity or overweight have a higher likelihood of choosing selective CS over emergent CS (15), this ambiguity could affect our findings. Lastly, despite we have conducted subgroup analyses on certain factors regarding the outcomes of PPH or CS, the absence of subgroup analyses based on regions, study design types, or relevant environmental factors related to other outcomes of interest may constrain the generalizability of our results.
Interpretation
In our paper, pregnant women with overweight and obesity faced an increased risk of developing GDM, with ORs 2.20 (95% CI, 2.02-2.39) and 3.46 (95% CI, 3.05-3.94), respectively. These findings presented narrower confidence intervals compared to a previous meta-analysis based on the PubMed database and 20 articles conducted in 2007 (10), where the ORs were reported as 2.14 (95% CI, 1.82-2.53) and 3.56 (95% CI, 3.05-4.21). Markedly, our meta-analysis, including 58 studies on GDM, demonstrated low to medium between-study heterogeneities (I2 = 54.3%, 33.4%, 56.2%), contributing to the narrowed 95% CI of the ORs. Additionally, research has indicated (100) that the cumulative incidence of type 2 diabetes increased significantly in the first 5 years postpartum due to elevated fasting glucose levels during pregnancy. Therefore, targeting pregnant mothers with elevated glucose levels may represent a more effective approach to diabetes prevention.
In our analysis of 34 studies, it was evident that mothers with overweight and obesity faced an elevated risk of GHTN. While an earlier study suggested that obesity might not independently contribute to pregnancy-induced hypertensive disorders (101), our findings underscored the disparities in GHTN risks across various BMI levels, potentially attributed to higher booking blood pressure among women with obesity (102). Controlling appropriate pre-pregnancy weight could be a preventive measure for GHTN, highlighting the importance of further research to elucidate its underlying mechanisms.
An evident dose-dependent effect was observed concerning maternal pre-pregnancy BMI and the likelihood of delivery by CS. Women with overweight and obesity have a higher propensity to opt for CS (containing emergent CS and selective CS) or IOL, a finding consistent with several previous meta-analyses (16, 103). The heightened risk of CS in women with higher BMI may be attributed to various factors. Firstly, increased BMI could contribute to labor induction failure (104), a potentially leading to CS instead of IOL (103). Moreover, factors such as fetal macrosomia, labor dystocia due to increased pelvic soft tissue, and other complications might further predispose women with higher BMI to CS (61). Furthermore, considering other adverse pregnancy complications like gestational hypertension, gestational diabetes, and fetal complications such as stillbirth or admission to the neonatal intensive care unit (6), these factors could contribute to the increased CS rate observed in women with obesity.
In contrast to women with overweight and obesity, those classified as underweight exhibited a protective effect against GDM, GHTN, pre-eclampsia, CS, and PPH. However, it’s important to note that being underweight during pregnancy may carry its own set of risks, such as an increased likelihood of preterm birth and delivering an SGA or LBW baby (5). Therefore, it is imperative to establish appropriate clinical guidelines and implement public health interventions aimed at managing the weight of pregnant women, whether they are classified as obesity or underweight, in order to safeguard the health of both mothers and their babies.
Conclusion
Our analysis provides a quantitative estimation of the detrimental effects of pre-pregnancy overweight and obesity on maternal complications and delivery outcomes. Future studies should strive to explore more effective strategies to mitigate the growing threat of overweight and obesity among pregnant women.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Writing – original draft, Funding acquisition, Conceptualization, Resources. ML: Writing – original draft, Data curation, Software, Visualization. YY: Writing – original draft, Software, Formal analysis. LX: Writing – review & editing. RZ: Writing – review & editing. CL: Writing – review & editing, Supervision. PL: Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Guizhou Science and Technology Plan Project (QKHJC-ZK[2023]-Key057, QKHZC[2021]-Normal163, and QKHZC[2020]-1Y037), Special project of new academic seedling cultivation and innovation exploration of Zunyi Medical University (Guizhou science cooperation platform talents [2018]5772–070), Scientific Research Program of Guizhou Provincial Department of Education (QJJ [2023] 019) and Science & Technology Program of Guizhou Province (QKHPTRC-CXTD[2022]014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1280692/full#supplementary-material
Abbreviations
BMI, body mass index; GDM, gestational diabetes mellitus; GHTN, gestational hypertension; CS, cesarean section; IOL, induction of labor; PPH, postpartum hemorrhage.
References
1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/s0140-6736(14)60460-8
2. Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. (2016) 4:1025–36. doi: 10.1016/s2213-8587(16)30217-0
3. Kaplan SA. Re: National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. J Urol. (2011) 186:1982–3. doi: 10.1016/j.juro.2011.07.061
4. Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. Bjog. (2019) 126:984–95. doi: 10.1111/1471-0528.15661
5. Liu P, Xu L, Wang Y, Zhang Y, Du Y, Sun Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev. (2016) 17:1091–102. doi: 10.1111/obr.12455
6. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. Jama. (2014) 311:1536–46. doi: 10.1001/jama.2014.2269
7. Ellis JA, Brown CM, Barger B, Carlson NS. Influence of maternal obesity on labor induction: A systematic review and meta-analysis. J Midwifery Womens Health. (2019) 64:55–67. doi: 10.1111/jmwh.12935
8. Ramachenderan J, Bradford J, McLean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol. (2008) 48:228–35. doi: 10.1111/j.1479-828X.2008.00860.x
9. Lim CC, Mahmood T. Obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. (2015) 29:309–19. doi: 10.1016/j.bpobgyn.2014.10.008
10. Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. (2007) 30:2070–6. doi: 10.2337/dc06-2559a
11. Kansu-Celik H, Kisa Karakaya B, Guzel AI, Tasci Y, Erkaya S. To evaluate the effect of pre-pregnancy body mass index on maternal and perinatal outcomes among adolescent pregnant women. J Matern Fetal Neonatal Med. (2017) 30:1574–8. doi: 10.1080/14767058.2016.1214122
12. Fouelifack FY, Fouedjio JH, Fouogue JT, Sando Z, Fouelifa LD, Mbu RE. Associations of body mass index and gestational weight gain with term pregnancy outcomes in urban Cameroon: a retrospective cohort study in a tertiary hospital. BMC Res Notes. (2015) 8:806. doi: 10.1186/s13104-015-1765-9
13. Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. (2008) 9:635–83. doi: 10.1111/j.1467-789X.2008.00511.x
14. Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, Bilano V, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. (2015) 16:758–70. doi: 10.1111/obr.12293
15. D'Souza R, Horyn I, Pavalagantharajah S, Zaffar N, Jacob CE. Maternal body mass index and pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol MFM. (2019) 1:100041. doi: 10.1016/j.ajogmf.2019.100041
16. Vats H, Saxena R, Sachdeva MP, Walia GK, Gupta V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obes Res Clin Pract. (2021) 15:536–45. doi: 10.1016/j.orcp.2021.10.005
17. Jin J. The relationship between preconceptional body mass index and gestational growth and maternal and infant outcomes (in Chinese). Chin J Women Children Health. (2014) 5:69–70. doi: 10.19757/j.cnki.issn1674-7763.2014.06.021
18. Liu L, Hong ZX, Wang J, Ding BJ, Bi YX. Effects of pre-pregnancy BMI and peri-gestational weight gain on pregnancy outcomes (in Chinese). Chin J Woman Child Health Res. (2015) 26:1139–42. doi: 10.3969/j.issn.1673-5293.2015.06.011
19. Li JH, Yang L, Chen Q, Liu J, He Y. A prospective cohort study on the effects of prepregnancy body mass index and gestational weight gain on pregnancy complications and pregnancy outcomes(in Chinese). Chin J Obstetrics Gynecol. (2019) 54:184–8. doi: 10.3760/cma.j.issn.0529-567x.2019.03.008
20. Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J Matern Fetal Med. (1998) 7:190–3. doi: 10.1002/(sici)1520-6661(199807/08)7:4<190::Aid-mfm5>3.0.Co;2-d
21. Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynaecol Obstet. (2001) 73:101–7. doi: 10.1016/s0020-7292(00)00391-x
22. Michlin R, Oettinger M, Odeh M, Khoury S, Ophir E, Barak M, et al. Maternal obesity and pregnancy outcome. Isr Med Assoc J. (2000) 2:10–3.
23. Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. (2001) 25:1175–82. doi: 10.1038/sj.ijo.0801670
24. Kaufman HK, Jacques DL, Coleman SK. Ganzeier G Prepregnancy body mass index and pregnancy outcome. Obstetrics Gynecol. (2001) 97:S71.
25. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. (2001) 91:436–40. doi: 10.2105/ajph.91.3.436
26. Yuan YJ, Cao WQ, Wang JP, Yang XF, Huang M. Effects of body mass index on high-risk pregnancy and pregnancy outcome(in Chinese). Maternal Child Health Care China. (2003) 10):26–7.
27. Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. (2004) 103:219–24. doi: 10.1097/01.Aog.0000107291.46159.00
28. Li P, Luo LM, Wu QK. Relevance of body mass index with complication and outcome of pregnancy(in Chinese). Shanghai Med J. (2004) 11):840–2. doi: 10.3969/j.issn.0253-9934.2004.11.016
29. Liu XJ, Zhang CX. The relationshops between the pregestational weight and the abnormal pregnancy result(in Chinese). J Shandong First Med Univ Shandong Acad Med Sci. (2004) 05):403–5. doi: 10.3969/j.issn.1004-7115.2004.05.009
30. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. Bjog. (2005) 112:403–8. doi: 10.1111/j.1471-0528.2005.00437.x
31. Luo CH. A probe into the relationship between abnormal boby mass index and outcome of pregnancy(in Chinese). Guangxi Med J. (2005) 09):1343–5. doi: 10.3969/j.issn.0253-4304.2005.09.018
32. Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obes (Silver Spring). (2006) 14:165–71. doi: 10.1038/oby.2006.20
33. Roman H, Robillard PY, Hulsey TC, Laffitte A, Kouteich K, Marpeau L, et al. Obstetrical and neonatal outcomes in obese women. West Indian Med J. (2007) 56:421–6.
34. Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. (2007) 97:157–62. doi: 10.2105/ajph.2005.074294
35. Fu B. A clinical research of effect of pre-pregnancy body mass index and maternal weight gain on pregnancy outcome(in Chinese). Maternal Child Health Care China. (2007) 08):1024–6. doi: 10.3969/j.issn.1001-4411.2007.08.017
36. Han AH. The effect of prepregnancy body mass index and pregnancy weight gain on pregnancy outcome(in Chinese). Maternal Child Health Care China. (2007) 32):4534–5. doi: 10.3969/j.issn.1001-4411.2007.32.017
37. Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. (2007) 7:168. doi: 10.1186/1471-2458-7-168
38. Driul L, Cacciaguerra G, Citossi A, Martina MD, Peressini L, Marchesoni D. Prepregnancy body mass index and adverse pregnancy outcomes. Arch Gynecol Obstet. (2008) 278:23–6. doi: 10.1007/s00404-007-0524-0
39. Zheng M, Yao MY, Tan FM. The effect to before pregnancy body mass index on pregnancy outcome in nulliparous women(in Chinese). J Mudanjiang Med Univ. (2008) 29:27–30. doi: 10.13799/j.cnki.mdjyxyxb.2008.06.034
40. Leung TY, Leung TN, Sahota DS, Chan OK, Chan LW, Fung TY, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. Bjog. (2008) 115:1529–37. doi: 10.1111/j.1471-0528.2008.01931.x
41. Joy S, Istwan N, Rhea D, Desch C, Stanziano G. The impact of maternal obesity on the incidence of adverse pregnancy outcomes in high-risk term pregnancies. Am J Perinatol. (2009) 26:345–9. doi: 10.1055/s-0028-1110084
42. Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol. (2009) 24:697–705. doi: 10.1007/s10654-009-9375-2
43. Schrauwers C, Dekker G. Maternal and perinatal outcome in obese pregnant patients. J Matern Fetal Neonatal Med. (2009) 22:218–26. doi: 10.1080/14767050902801652
44. Hoff GL, Cai J, Okah FA, Dew PC. Pre-pregnancy overweight status between successive pregnancies and pregnancy outcomes. J Womens Health (Larchmt). (2009) 18:1413–7. doi: 10.1089/jwh.2008.1290
45. Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. (2010) 115:989–97. doi: 10.1097/AOG.0b013e3181da8f09
46. Mantakas A, Farrell T. The influence of increasing BMI in nulliparous women on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. (2010) 153:43–6. doi: 10.1016/j.ejogrb.2010.06.021
47. Aydin C, Baloglu A, Yavuzcan A, Inci A. The effect of body mass index value during labor on pregnancy outcomes in Turkish population (obesity and pregnancy outcomes). Arch Gynecol Obstet. (2010) 281:49–54. doi: 10.1007/s00404-009-1060-x
48. Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. (2010) 10:56. doi: 10.1186/1471-2393-10-56
49. Li XM. The effect of maternal body mass index and pregnancy weight gain on pregnancy outcome(in Chinese). Chin J Woman Child Health Res. (2010) 21:596–597,600. doi: 10.3969/j.issn.1673-5293.2010.05.0010
50. Park JH, Lee BE, Park HS, Ha EH, Lee SW, Kim YJ. Association between pre-pregnancy body mass index and socioeconomic status and impact on pregnancy outcomes in Korea. J Obstet Gynaecol Res. (2011) 37:138–45. doi: 10.1111/j.1447-0756.2010.01332.x
51. Tang JH. The effect of body mass index on the pregnancy outcome of primiparas(in Chinese). Compr Clin Pract China. (2011) 27:1219–21. doi: 10.3760/cma.j.issn.1008-6315.2011.11.035
52. Liu QY, Gan XH, Jiang H. The effect of pre-pregnancy body mass index (BMI) and pregnancy weight gain on pregnancy outcome(in Chinese). J Qiqihar Med Univ. (2011) 32:2094–5. doi: 10.3969/j.issn.1002-1256.2011.13.023
53. Jiang BL. Study on the correlation between prepregnancy body mass index and pregnancy weight gain and pregnancy outcome. J Military Surgeon Southwest China. (2011) 13:668–9. doi: 10.3969/j.issn.1672-7193.2011.04.047
54. Liu XM, Chen ZY, Wang GX, Wang W, Xi Q, Du J. Effects of pre-pregnancy BMI and increasing range of BMI during pregnancy on pregnancy outcome(in Chinese). Maternal Child Health Care China. (2010) 25:3084–7.
55. Joshi S, Unni J, Vijay S, Khanijo V, Gupte N, Divate U. Obesity and pregnancy outcome in a private tertiary hospital in India. Int J Gynaecol Obstet. (2011) 114:82–3. doi: 10.1016/j.ijgo.2011.01.020
56. Rayis DA, Abbaker AO, Salih Y, Adam I. Obesity and pregnancy outcome in Khartoum, Sudan. Int J Gynaecol Obstet. (2011) 113:160–1. doi: 10.1016/j.ijgo.2010.12.008
57. Han YS, Ha EH, Park HS, Kim YJ, Lee SS. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. Int J Obes (Lond). (2011) 35:570–7. doi: 10.1038/ijo.2010.162
58. Green C, Shaker D. Impact of morbid obesity on the mode of delivery and obstetric outcome in nulliparous singleton pregnancy and the implications for rural maternity services. Aust N Z J Obstet Gynaecol. (2011) 51:172–4. doi: 10.1111/j.1479-828X.2010.01271.x
59. Ezeanochie MC, Ande AB, Olagbuji BN. Maternal obesity in early pregnancy and subsequent pregnancy outcome in a Nigerian population. Afr J Reprod Health. (2011) 15:55–9.
60. Mandal D, Manda S, Rakshi A, Dey RP, Biswas SC, Banerjee A. Maternal obesity and pregnancy outcome: a prospective analysis. J Assoc Physicians India. (2011) 59:486–9.
61. Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. (2011) 118:305–12. doi: 10.1097/AOG.0b013e3182245d49
62. C. SW, Zhang YX, Wu YY, Xu YH. Clinical study of pregnancy and pregnancy outcome in women with simple obesity(in Chinese). J Med Inf. (2011) 24:5397–8.
63. Meenakshi, Srivastava R, Sharma NR, Kushwaha KP, Aditya V. Obstetric behavior and pregnancy outcome in overweight and obese women: maternal and fetal complications and risks in relation to maternal overweight and obesity. J Obstet Gynaecol India. (2012) 62:276–80. doi: 10.1007/s13224-012-0215-z
64. Verma A, Shrimali L. Maternal body mass index and pregnancy outcome. J Clin Diagn Res. (2012) 6:1531–3. doi: 10.7860/jcdr/2012/4508.2551
65. Halloran DR, Marshall NE, Kunovich RM, Caughey AB. Obesity trends and perinatal outcomes in black and white teenagers. Am J Obstet Gynecol. (2012) 207:492.e1–7. doi: 10.1016/j.ajog.2012.09.023
66. Sebastián Manzanares G, Angel Santalla H, Irene Vico Z, López Criado MS, Alicia Pineda L, José Luis Gallo V. Abnormal maternal body mass index and obstetric and neonatal outcome. J Matern Fetal Neonatal Med. (2012) 25:308–12. doi: 10.3109/14767058.2011.575905
67. Xu J, Chen X, Wang Y. Influence of body mass index index increase in nulliparous women pregnancy outcome(in Chinese). Clin Focus. (2012) 27:1393–5.
68. Chen J. Study on the relationship between maternal body mass index and adverse pregnancy outcome(in Chinese). J Hebei Med Univ. (2012) 33:948–9. doi: 10.3969/j.issn.1007-3205.2012.08.029
69. Meng GH, Zhou L. Pre-pregnancy weight and weight gain during pregnancy have an influence on pregnancy outcome(in Chinese). Anhui Med Pharm J. (2012) 16:778–80. doi: 10.3969/j.issn.1009-6469.2012.06.025
70. Cai ZY, Jia Z, Liang XJ. Relationship between prepregnancy body mass index, pregnancy weight gain and pregnancy outcome(in Chinese). J Aerospace Med. (2012) 23:952–4. doi: 10.3969/j.issn.2095-1434.2012.08.034
71. Yazdani S, Yosofniyapasha Y, Nasab BH, Mojaveri MH, Bouzari Z. Effect of maternal body mass index on pregnancy outcome and newborn weight. BMC Res Notes. (2012) 5:34. doi: 10.1186/1756-0500-5-34
72. Jain D, Khuteta R, Chaturvedi V, Khuteta S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies: observational study. J Obstet Gynaecol India. (2012) 62:429–31. doi: 10.1007/s13224-012-0225-x
73. Wu XF. Relationship between weight before pregnancy, weight gain during pregnancy and pregnancy outcome(in Chinese). China Pract Med. (2012) 7:270–1. doi: 10.14163/j.cnki.11-5547/r.2012.15.183
74. Magann EF, Doherty DA, Sandlin AT, Chauhan SP, Morrison JC. The effects of an increasing gradient of maternal obesity on pregnancy outcomes. Aust N Z J Obstet Gynaecol. (2013) 53:250–7. doi: 10.1111/ajo.12047
75. Oteng-Ntim E, Kopeika J, Seed P, Wandiembe S, Doyle P. Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PloS One. (2013) 8:e53749. doi: 10.1371/journal.pone.0053749
76. Liu YD. Relationship between BMI increase and pregnancy outcome in early pregnancy (in Chinese). J Qiqihar Med Coll. (2013) 34:49–50. doi: 10.3969/j.issn.1002-1256.2013.01.031
77. Vaswani PR, Balachandran L. Pregnancy outcomes in a population with high prevalence of obesity: How bad is it? Clin Epidemiol Global Health. (2013) 1:5–11. doi: 10.1016/j.cegh.2012.11.006
78. Zhao QP. Analysis of the influence of prepregnancy obesity and pregnancy weight gain on pregnancy outcome (in Chinese). China Health Industry. (2013) 10:171–2. doi: 10.16659/j.cnki.1672-5654.2013.19.094
79. Tong J, Gu N, Li J, Xu CC, Yang L, Zhu ZH, et al. Effects of prepregnancy body mass index and pregnancy weight gain on pregnancy outcome(in Chinese). Chin J Perinatal Med. (2013) 16:561–5. doi: 10.3760/cma.j.issn.1007-9408.2013.09.014
80. Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PloS One. (2013) 8:e82310. doi: 10.1371/journal.pone.0082310
81. Shi K, Gu AW, Qiang JP. Effect of pre-pregnancy body mass index on pregnancy outcome(in Chinese). J Ningxia Med Univ. (2013) 35:1173–4. doi: 10.16050/j.cnki.issn1674-6309.2013.10.042
82. Chen LL, Liu J, Kang Y, Liu J, He SF. Relationship between prepregnancy body mass index and pregnancy growth and maternal and neonatal outcome(in Chinese). Chongqing Med. (2014) 43:1178–80. doi: 10.3969/j.issn.1671-8348.2014.10.008
83. Gesche J, Nilas L. Pregnancy outcome according to pre-pregnancy body mass index and gestational weight gain. Int J Gynaecol Obstet. (2015) 129:240–3. doi: 10.1016/j.ijgo.2014.12.013
84. Ding XX, Xu SJ, Hao JH, Huang K, Su PY, Tao FB. Maternal pre-pregnancy BMI and adverse pregnancy outcomes among Chinese women: Results from the C-ABCS. J Obstet Gynaecol. (2016) 36:328–32. doi: 10.3109/01443615.2015.1050652
85. Rezi Wanguli ADL, Rezia ABLT, Chen PP, Zhang SY, Li LX. Prepregnancy weight, pregnancy weight gain and the relationship between age and pregnancy outcome(in Chinese). Maternal Child Health Care China. (2016) 31:78–80. doi: 10.7620/zgfybj.j.issn.1001-4411.2016.01.32
86. Liu HY, Cai YY, Xu K, Xu YJ, Zhou JM, Long FM, et al. Effect of pre-pregnancy BMI and gestational weight gain of primiparas on delivery way and pregnancy outcomes(in Chinese). J Guizhou Med Univ. (2016) 41:37–40+44. doi: 10.19367/j.cnki.1000-2707.2016.01.011
87. Song AY. Maternal weight before and during pregnancy and its relationship with obstetric complications and pregnancy outcomes(in Chinese). Clin Res Pract. (2017) 2:124–5. doi: 10.19347/j.cnki.2096-1413.201723061
88. Tang JL. Correlation analysis of pregnancy BMI and BMI increased during pregnancy and pregnancy outcome (in chinese). Electronic J Pract Gynecological Endocrinol (2017) 4(25):47–9. doi: 10.3969/j.issn.2095-8803.2017.25.027
89. Chen AZ, Qin JL, Li Y, Zhao PY, Wang L, Cheng JJ. Impact of pre-pregnancy BMI on obstetric complications and pregnancy outcomes (in chinese). J Tongji University(Medical Science) (2017) 38(02):114–9. doi: 10.16118/j.1008-0392.2017.02.024
90. Lv JY, Guan YQ. The clinical analysis for pre-pregnancy overweight, gestational hypertension and the outcome of maternal and child after pregnancy (in chinese). Heilongjiang Med Pharm (2017) 40(04):139–41. doi: 10.3969/j.issn.1008-0104.2017.04.065
91. Qu XH. Study on the correlation between prepregnancy body mass index and pregnancy weight gain and pregnancy outcome (in Chinese). World Latest Med Inf (2018) 18(81):54–5. doi: 10.19613/j.cnki.1671-3141.2018.81.035
92. Liu YC, Zhu LY, Huang YC, Ouyang LZ, Huang XL, Zhang Q. Effects of different pre pregnancy body mass index and maternal body mass changes on pregnancy outcomes (in chinese). Jilin Med J (2018) 39(03):423–5. doi: 10.3969/j.issn.1004-0412.2018.03.009
93. Du CX, Liu H, Li QY, Zhang Y, Cchen M, Ru XC, et al. Impact of pre-pregnancy BMI and gestational weight gain on obstetric complications and pregnancy outcomes of hami uygur women (in Chinese). Int Med Health Guidance News (2018) 24(19):2948–51. doi: 10.3760/cma.j.issn.1007-1245.2018.19.0187-
94. Yang YQ, Dong XF. Effect of BMI and weight gain on complications and outcomes before pregnancy (in chinese). Xinjiang Med J (2019) 49(11):1101–4.
95. Zhao HY, Pan SL, Liu YL, Zheng WT, Li DJ. Effects of pre-pregnancy overweight/obesity on obstetric outcomes and labor duration (in chinese). Prog Obstetrics Gynecology (2019) 28(06):416–9. doi: 10.13283/j.cnki.xdfckjz.2019.06.003
96. Wang LL, Tian Y, Lu LP. Effects of pre-pregnancy body mass index and pregnancy weight gain on the incidence and pregnancy outcome of gestational diabetes mellitus (in chinese). Maternal Child Health Care China (2019) 34(01):62–4. doi: 10.7620/ZGFYBJ.J.ISSN.1001-4411.2019.01.21
97. Zhao RF, Zhou L, Zhang WY. Identifying appropriate pre-pregnancy body mass index classification to improve pregnancy outcomes in women of childbearing age in beijing, china: a retrospective cohort study. Asia Pac J Clin Nutr (2019) 28(3):567–76. doi: 10.6133/apjcn.201909_28(3).0016
98. Kominiarek MA, Saade G, Mele L, Bailit J, Reddy UM, Wapner RJ, et al. Association between gestational weight gain and perinatal outcomes. Obstet Gynecol (2018) 132(4):875–81. doi: 10.1097/aog.0000000000002854
99. Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med (2018) 16(1):153. doi: 10.1186/s12916-018-1128-1
100. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care (2002) 25(10):1862–8. doi: 10.2337/diacare.25.10.1862
101. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol (2002) 42(1):29–34. doi: 10.1111/j.0004-8666.2002.00035.x
102. Ohkuchi A, Iwasaki R, Suzuki H, Hirashima C, Takahashi K, Usui R, et al. Normal and high-normal blood pressures, but not body mass index, are risk factors for the subsequent occurrence of both preeclampsia and gestational hypertension: a retrospective cohort study. Hypertens Res (2006) 29(3):161–7. doi: 10.1291/hypres.29.161
103. Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. Diabetes Endocrine Pregnancy Outcome Study Toronto. Qjm (2001) 94(7):347–56. doi: 10.1093/qjmed/94.7.347
Keywords: maternal, body mass index, obesity, pregnancy outcomes, complications
Citation: Zhang Y, Lu M, Yi Y, Xia L, Zhang R, Li C and Liu P (2024) Influence of maternal body mass index on pregnancy complications and outcomes: a systematic review and meta-analysis. Front. Endocrinol. 15:1280692. doi: 10.3389/fendo.2024.1280692
Received: 21 August 2023; Accepted: 13 May 2024;
Published: 04 June 2024.
Edited by:
Eytan R. Barnea, BioIncept, LLC, United StatesReviewed by:
Fang Liu, Inner Mongolia Medical University, ChinaPatricia Canto, National Autonomous University of Mexico, Mexico
Copyright © 2024 Zhang, Lu, Yi, Xia, Zhang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Liu, bGl1cGluZ0BjYWhlYy5jbg==; Chao Li, bGljaGFvQGNhaGVjLmNu
†These authors have contributed equally to this work
 Yi Zhang
Yi Zhang Mei Lu
Mei Lu Ying Yi3†
Ying Yi3† Ping Liu
Ping Liu