- 1Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 2Clinical Research Center of Shaanxi Province for Dental and Maxillofacial Diseases, College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 3College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 4Centre of Stomatology, West China Xiamen Hospital of Sichuan University, Xiamen, China
- 5Bioinspired Engineering and Biomechanics Center (BEBC), Xi’an Jiaotong University, Xi’an, China
Introduction: A potential association between periodontitis and endometriosis has been indicated in previous observational studies. Nevertheless, the causal link between these two disorders has not been clarified.
Methods: Based on publicly available genome-wide association study (GWAS) summary datasets, we conducted a bidirectional Mendelian randomization (MR) study to investigate the relationship between periodontitis and endometriosis and its subtypes. Single nucleotide polymorphisms (SNPs) strongly associated with candidate exposures at the genome-wide significance level (P < 5 × 10−8) were selected as instrumental variables (IVs). The inverse variance-weighted regression (IVW) was performed to estimate the causal effect of periodontitis on endometriosis. We further conducted two sensitivity analyses, MR-Egger and weighted median, to test the validity of our findings. The main results were replicated via data from the UK Biobank. Finally, a reverse MR analysis was performed to evaluate the possibility of reverse causality.
Results: The IVW method suggested that periodontitis was positively associated with endometriosis of the pelvic peritoneum (OR = 1.079, 95% CI = 1.016 to 1.146, P = 0.014). No causal association was indicated between periodontitis and other subtypes of endometriosis. In reversed analyses, no causal association between endometriosis or its subtypes and periodontitis was found.
Conclusions: Our study provided genetic evidence on the causal relationship between periodontitis and endometriosis of the pelvic peritoneum. More studies are necessary to explore the underlying mechanisms.
1 Introduction
Periodontitis is a chronic inflammation characterized by loss of periodontal tissue support and alveolar bone, clinical attachment loss, and bleeding gums (1). An updated study verified that 46% of adults from the United States had periodontitis (2). The oral microbial communities detuning in periodontitis could elicit immune subversion and contribute to diseases in distance (3). Therefore, periodontitis not only affects oral functions but is also associated with some systemic diseases (4). Diseases of the cardiovascular, neurodegenerative, and even reproductive systems have been confirmed to be associated with periodontitis in various observational studies until now (5). However, limited research explored the causal effects between those diseases and periodontitis. Further study of the causality between periodontitis and associated comorbidities will provide novel insights into systemic disease treatment.
Endometriosis, similar to periodontitis, is a chronic inflammatory disease in the reproductive system. It affects 5%–10% of women of reproductive age worldwide and has been defined as a major reason for infertility (6). Endometriosis is divided into three phenotypes based on extrauterine sites where endometrial glands and stroma abnormally present 1) superficial peritoneal lesions (SUPs), 2) ovarian endometriomas (OMAs), and 3) deep infiltrating endometriosis (DIE) (7). SUP penetration is limited to 5 mm under the peritoneal surface layer (8). SUP is the least severe endometriosis with the mildest symptoms. OMA refers to chocolate cysts in the ovary, which contain a dark brown fluid (9). DIE is deemed to be the most terrible subtype with an erosive extent deeper than 5 mm under the peritoneum. Patients with DIE always suffer from severe pain (10). These phenotypes also differ at the cellular level. Single-cell profiling suggested more disorders of hormonal, inflammatory, and immunological signatures in DIE, compared with SUP and OMA (8). Because of the heterogeneity of clinical features and invasive diagnostic methods, there is an average of 6.7 years before definitive diagnosis (11, 12).
When focusing on the relationship between endometriosis and periodontitis, an earlier cross-sectional study (N = 2,664) based on the National Health and Nutrition Examination Survey demonstrated that women with endometriosis had a 57% higher risk of gingivitis and periodontitis than those without [adjusted odds ratio (OR) = 1.57, 95% CI = 1.06, 2.33] (13). A case–control study (N = 50) showed that participants with endometriosis had a higher gingival index than normal, and moderate and severe periodontitis was more common in women with endometriosis compared with normal groups (14). In a word, some observational studies have revealed a potential correlation between periodontitis and endometriosis. However, the issue of whether there exists a causal relationship between periodontitis and endometriosis or its subtypes remains unknown.
Mendelian randomization (MR) is an economical and time-saving option to explore the causal effects. Single-nucleotide polymorphisms (SNPs) are assigned randomly during the formation of a sperm cell. They could be used to reduce reverse causation or confounders and then assess the causal relationship between exposures and outcomes (15, 16). Bidirectional MR is one of the developed MR, which is performed in both ways to reduce misleading estimates from elementary MR (17). Some studies have used bidirectional MR to explore the association direction between periodontitis and different diseases, such as arthritis (18), depression (19), and psoriasis (20).
Here, we performed a bidirectional two-sample MR study between periodontitis and endometriosis. SNPs as instrumental variables (IVs) and their associations with outcomes were selected from relevant genome-wide association studies (GWASs). In addition, we selected three endometriosis subgroup datasets in order to represent SUP, OMA, and DIE, respectively. We aim to provide more genetic evidence to define the relationship between periodontitis and endometriosis.
2 Materials and methods
2.1 Study design
A bidirectional two-sample Mendelian randomization study was utilized to explore the causal association between periodontitis and endometriosis. We also performed a two-sample MR analysis using the dataset from the UK Biobank (UKB) to validate the causality of periodontitis on endometriosis. As shown in Figure 1, the study is based on three vital assumptions: firstly, there are strong associations between exposure and IVs. Secondly, IVs are not associated with the confounders. Thirdly, IVs should totally affect outcomes through exposure.
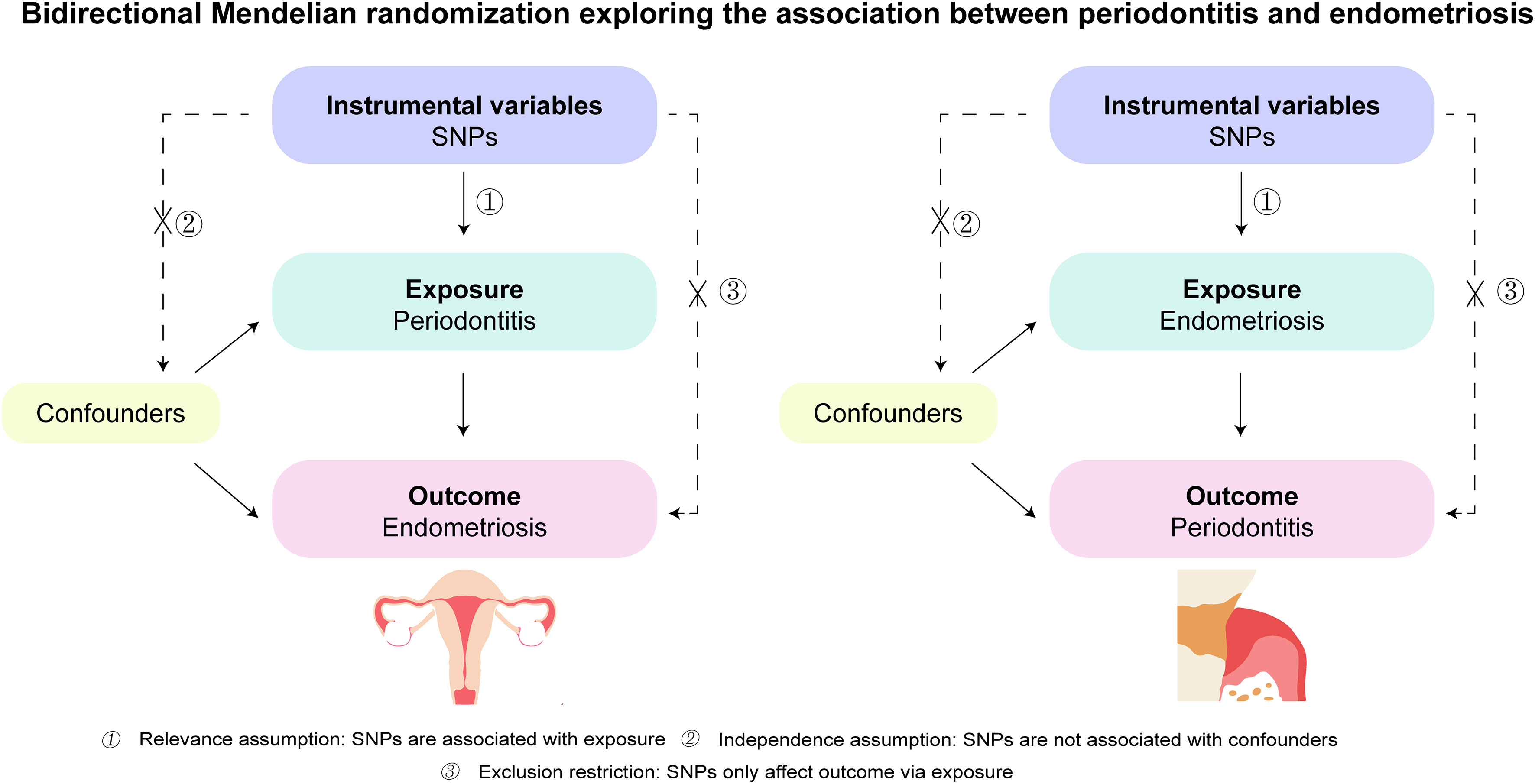
Figure 1 Schematics for the bidirectional Mendelian randomization design. Mendelian randomization requires valid genetic instrumental variants satisfying three assumptions. SNPs, single-nucleotide polymorphisms.
The present study was based on publicly available GWAS summary data, and ethical consent had been obtained in the original studies (21–25). All the data can be used without any restrictions.
2.2 GWAS datasets of periodontitis on the risk of endometriosis
We selected the IVs associated with periodontitis from three published GWAS datasets (23–25). All the participants were Europeans, including Dutch, German, and European-American. SNPs associated with the exposure at the genome-wide significance level (P < 5 × 10−8) were selected.
The datasets of endometriosis and its subgroups were obtained from FinnGen. The FinnGen study was initiated in 2017, which included more than half a million participants (age >18 years) who lived in Finland (26). The FinnGen GWAS summary data were extracted from the MRC-IEU database for endometriosis overall (8,828 cases, 68,969 controls), endometriosis of the ovary (3,231 cases, 68,969 controls), endometriosis of the pelvic peritoneum (2,953 cases, 68,969 controls), and also endometriosis of the rectovaginal septum and vagina (1,360 cases, 68,969 controls). Then, we also chose another dataset of endometriosis from UKB as the validation. UKB is a large prospective study, which aims to investigate the role of genetics, environment, and lifestyle in the causes of leading diseases. Its data come from 500,000 volunteers aged 40–69 in the United Kingdom (27). More details of exposure and outcome datasets are shown in Supplementary Table 1.
2.3 GWAS datasets of endometriosis on the risk of periodontitis
The exposure data of endometriosis and its phenotypes were obtained from the same FinnGen study as mentioned above. The outcome data for the risk of periodontitis were obtained from a GWAS meta-analysis conducted by the Gene Lifestyle Interactions in Dental Endpoints (GLIDE) on Europeans (22). Seven primary studies were included in the analysis. Periodontitis was recognized via the Centers for Disease Control and Prevention/American Academy of Periodontology definitions (28), probing depth (29), or even self-reported (30). The details of the primary studies are shown in Supplementary Table 2. In our study, we excluded the data of people with Hispanic/Latino ancestry. Data from European ancestry were collected from the original analysis.
2.4 Selection of instrumental variables
SNPs that were strongly associated with exposure at the genome-wide significance level (P < 5 × 10−8) were selected as IVs. We conducted several quality-control measures to select qualified IVs. First, the independence of SNPs was assessed based on stringent criteria (r2 > 0.001; clumping window < 10,000 kb). Second, we used the PhenoScanner tool to check whether any of the selected SNPs were associated with potential confounders at the outcome. We set the threshold at genome-wide significance (P < 5 × 10−8) when using the PhenoScanner tool. Third, proxy SNPs were not used as IVs if SNPs were not in the 1000G reference panel. In addition, SNPs with a minor allele frequency of less than 0.01 should be excluded to avoid potential bias from the original GWAS due to the low confidence. The R2 and F statistics were also calculated to avoid bias from weak instruments. SNPs were excluded if the F statistics were less than 10.
For the analyses of periodontitis as exposure, we selected five SNPs as valid IVs (Supplementary Table 3). In reversed analyses, during the selection of IVs associated with endometriosis, rs58502716 was not in the 1000G reference panel, and it was removed from the instruments. In addition, SNPs associated with endometriosis of the rectovaginal septum and vagina at a genome-wide significance level of P < 5 × 10−6 were selected in order to obtain enough IVs. rs76109112, rs139869063, rs200290589, and rs117783935 were eliminated as their F statistics were no more than 10. Finally, 10, 10, 5, and 13 SNPs were recognized as IVs associated with endometriosis overall, endometriosis of the ovary, endometriosis of the pelvic peritoneum, and endometriosis of the rectovaginal septum and vagina, respectively (Supplementary Tables 4–7).
2.5 Statistical analysis
The inverse variance-weighted (IVW) method in the random-effects model was applied as a dominating approach to analyze the bidirectional causal relationship between periodontitis and endometriosis. Weighted median, MR-Egger, and simple median were also added as complementary approaches to reduce potential horizontal pleiotropy and bias from the IVW method (31). The weighted median method could generate an unbiased estimate if more than half of the weight from effective IVs (32). MR-Egger is more suitable if the Pintercept < 0.05 because it can provide the estimates after pleiotropy is corrected (33).
To test the validity of our findings, sensitivity analyses were conducted using weighted median and MR-Egger regression. Q-tests were performed in both IVW and MR-Egger regression to assess potential heterogeneity. The MR-Egger intercept was used to assess whether the included SNPs had potential horizontal pleiotropy. Weighted median provides consistent estimates when at least 50% of the information is from valid instrumental variables. Leave-one-out analyses were also utilized to estimate the causality and heterogeneity of the study (34). As for the pleiotropy analysis, the P-value of the MR-Egger intercept less than 0.05 is considered to suggest the pleiotropy of the study (35).
Additionally, the statistical power of each analysis was calculated via mRnd, an online calculator (https://shiny.cnsgenomics.com/mRnd/). All data were analyzed by R (version 3.1.5), combined with the R package “TwoSampleMR.” A P-value < 0.05 was considered to be statistically significant. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (Supplementary Table 8).
3 Results
3.1 The causal effect of periodontitis on endometriosis
The results of MR analyses on the association between periodontitis and the risk of endometriosis are shown in Figure 2. In the subgroup analyses, we observed that periodontitis was positively associated with endometriosis of the pelvic peritoneum (OR = 1.079, 95% CI = 1.016 to 1.146, P = 0.014). The replication of the main results using UKB data supported the null causality of periodontitis on endometriosis (Supplementary Figure 1). No causal association was indicated between periodontitis and other subtypes of endometriosis. Suggestive estimates were observed in weight median, MR-Egger, and simple median methods similarly.
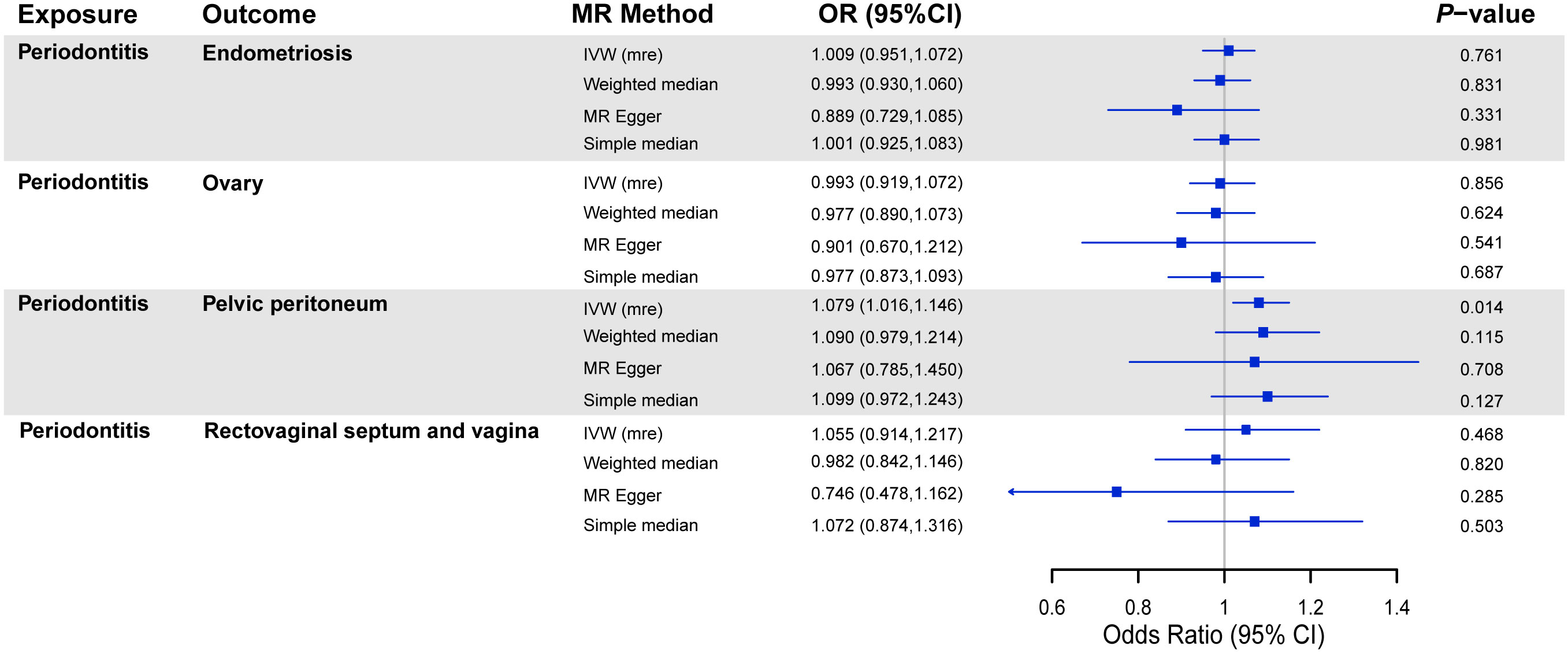
Figure 2 MR analyses on the association between periodontitis and risk of endometriosis. OR, odds ratio; CI, confidence interval; IVW (mre), multiplicative random effect inverse-variance weighted; SNPs, single-nucleotide polymorphisms.
Although other robust MR methods did not manifest a significant association between periodontitis and endometriosis of the pelvic peritoneum, the sensitivity analysis showed no potential horizontal pleiotropy (Table 1). Meanwhile, no heterogeneity existed in the IVW analyses, and none of the SNPs had a distinctive effect on estimates in the leave-one-out analyses (Supplementary Figures 2–10).
3.2 The causal effect of endometriosis on periodontitis
The results of the MR analyses on the association between endometriosis and the risk of periodontitis are shown in Figure 3. There was no causal effect of endometriosis or its subtypes on periodontitis. Consistent estimates were provided through other robust methods. As for the sensitivity analysis, no heterogeneity was found among SNPs, except for endometriosis of the rectovaginal septum and vagina (P = 0.048) (Table 1). In view of this excess heterogeneity, a random-effects analysis was performed as the main method in this study (36). Furthermore, no evidence for pleiotropy was observed via MR-Egger analyses. No pivotal difference emerged in estimates after we singly removed SNP and repeated the MR analysis.
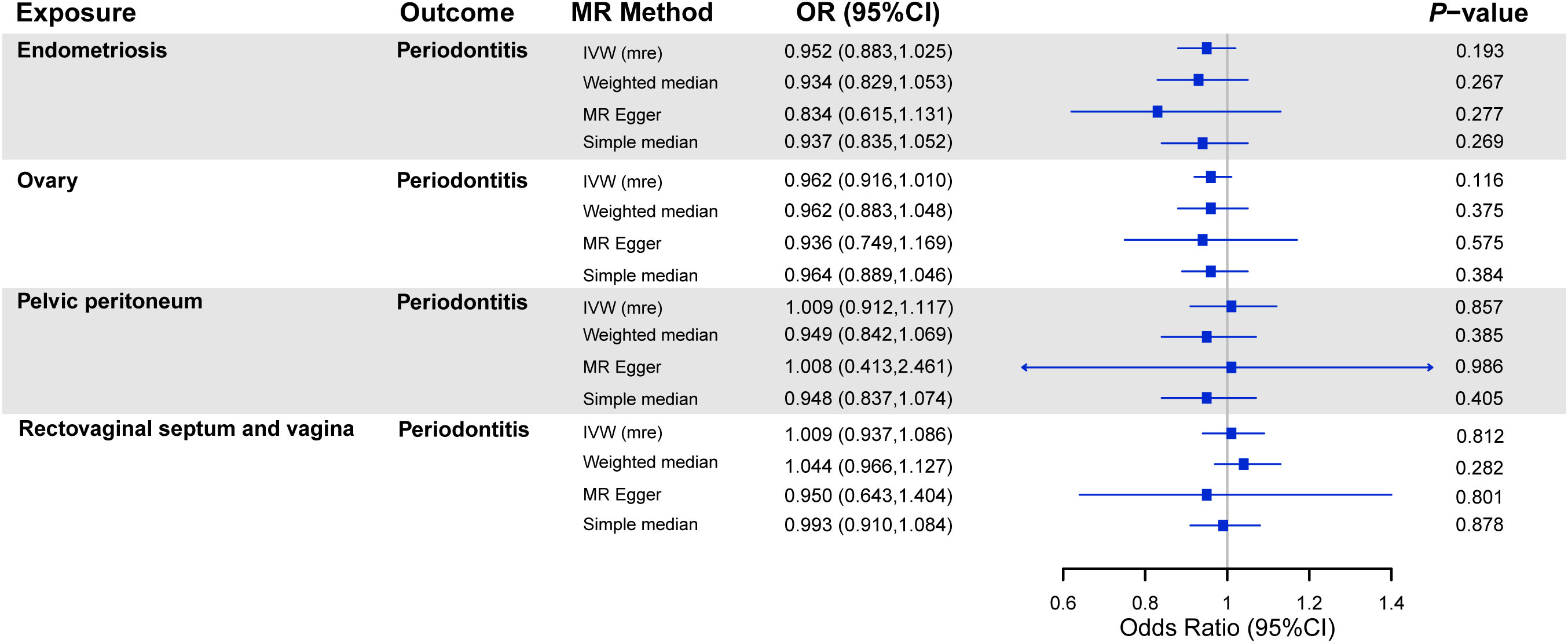
Figure 3 MR analyses on the association between endometriosis and risk of periodontitis. Note: OR, odds ratio; CI, confidence interval; IVW (mre), multiplicative random effect inverse-variance weighted; SNPs, single-nucleotide polymorphisms.
Our study had a power of > 80% to detect an effect of OR = 1.255 of periodontitis on the risk of endometriosis and a power of > 80% to detect an effect of OR = 1.394 of endometriosis on the risk of periodontitis, on a 5% significance level.
4 Discussion
Periodontitis has been a momentous public health problem. It not only damages oral health but also becomes a potential cause of some systemic conditions (37). Endometriosis is a common chronic disease with several symptoms, including painful menstrual cramps, abdominal pain, and so on (38). The etiology of endometriosis is unclear, so there are still difficulties in treatment. Some observational studies reported the correlation between periodontitis and endometriosis, but the causal effect cannot be affirmed (13, 14, 39). To our knowledge, this was an innovative study to investigate the bidirectional causal effect between periodontitis and endometriosis. Our research showed a potential cause effect of periodontitis on endometriosis of the pelvic peritoneum. In reversed analyses, there was no causal association of endometriosis or its subtypes on the risk of periodontitis.
A promising finding in this study is the causal effect of periodontitis on endometriosis of the pelvic peritoneum, which cast new light on the pathogenesis of endometriosis. Previous research surrounding the etiology of endometriosis of the pelvic peritoneum manifested that cells from the endometrium evade immune surveillance in the peritoneum and contribute to the disease, but other mechanisms are uncertain (40). The cause effect of periodontitis on endometriosis of the pelvic peritoneum might be explained by the mechanisms provided below (and these mechanisms are also summarized in Figure 4):
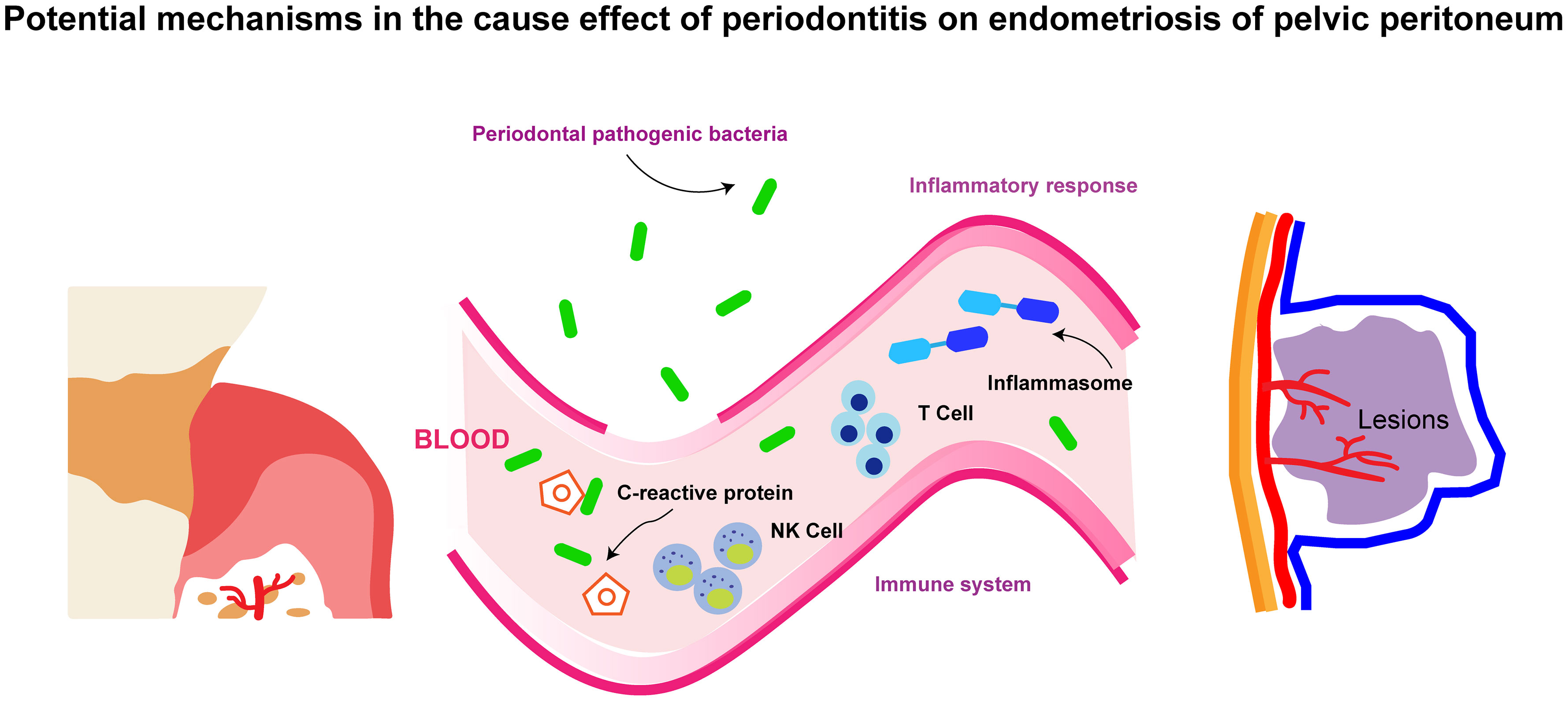
Figure 4 The schematic diagram for the potential mechanisms in the cause effect of periodontitis on endometriosis of pelvic peritoneum.
4.1 Periodontal pathogenic bacteria
Oral bacteria such as Porphyromonas gingivalis and Fusobacterium nucleatum are prone to transmit into the placenta and induce intrauterine infection (41–43). Similarly, periodontal pathogens might locate in the pelvic peritoneum and then exacerbate endometriosis in situ. Porphyromonas gingivalis could activate peritoneal macrophages and trigger the production of interleukin-1 (IL-1) (44). Subsequent inflammatory cascades, together with increasing IL-1 expression, are pivotal throughout the development of endometriosis in the pelvic peritoneum (45). The frequency of Fusobacterium in endometriosis and endometrial tissues was significantly higher in endometriosis patients than in normal women. Animal studies also demonstrated that Fusobacterium from the oral cavity was a possible source of infecting the uterus through hematogenous transmission (46).
4.2 Inflammatory response
Periodontitis patients usually have a higher count of leukocytes, elevated levels of circulating C-reactive protein, as well as lower levels of hemoglobin and reduced numbers of red blood cells (47). Inflammasome, a multiprotein complex, is also considered as an intermedia. Periodontitis might increase Nlrp7 (a kind of inflammasome) and promote the progression of endometriosis (48). In a word, systemic inflammatory response is conjectured as a mechanism by which periodontitis causes systemic diseases.
4.3 Immune system
Immune dysfunction has been certified as a key cause of endometriosis of the peritoneum. For example, a genomics single-cell RNA-sequencing study first reported that T-cell receptor-positive macrophages, increasing macrophages, and natural killer (NK) dendritic cells existed in the human peritoneal fluid of endometriosis (49). NK cell therapies are also a promising treatment for endometriosis (50). In addition, reducing the immune response induced by P. gingivalis is helpful in alleviating some metabolic diseases associated with periodontitis (51). There is reason to suspect that periodontitis causes endometriosis through autoimmune dysregulation.
Up to now, accepted treatments for endometriosis patients include continuous medical therapies and surgeries to remove pathological tissue (52). However, persistent medications suppress hormone levels and produce side effects, such as hair loss, acne, and mood changes, and operations would lead to inevitable relapse (53). This MR study revealed periodontitis as a novel point to manage endometriosis patients. Targeting a lower risk of periodontitis may therefore improve the prognosis of people with endometriosis of the pelvic peritoneum. In other words, supportive periodontal therapies and routine oral hygiene interventions may produce beneficial effects in treating women with endometriosis of the pelvic peritoneum. In the future, some clinical randomized controlled trials with more samples should be performed to verify the causal relationship. More research is necessary not only to clarify the underlying biological pathways but also to confirm therapeutic targets for endometriosis of the pelvic peritoneum.
The bidirectional MR study has some strengths. Firstly, genetic liability plays a crucial role in the course of periodontitis and endometriosis (18, 38). GWASs allowed larger sample sizes and less bias from population structure. Prevalence rates in the database were almost consistent with those reported in the literature. Hence, MR analysis is suitable for the inference of causality between both (54). Meanwhile, the MR analyses on both sides clarified the direction of causality. Secondly, endometriosis was further subdivided into three subgroups. An interesting finding is that periodontitis might be a cause of endometriosis of the pelvic peritoneum, which has not been realized before.
Some limitations should also be considered. Firstly, IVs had a deficient association with endometriosis of the rectovaginal septum and vagina, since the threshold of P-value was set to 5 × 10−6. Therefore, more convincing evidence is needed to prove the causality between endometriosis of the rectovaginal septum and vagina and periodontitis. Secondly, a weak power was found in each analysis, due to OR close to 1, so more participants were required to detect the causal effects in the future (55).
5 Conclusions
In summary, this bidirectional MR study indicated a positive causal relationship between periodontitis and endometriosis of the pelvic peritoneum in the European population. More epidemiological investigations are indispensable to explore the causality between those two diseases. Further research on the mechanism is also needed in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval had been obtained in all original published studies. The OpenGWAS Database is a publicly available dataset, and GWAS of oral diseases complied with all relevant ethical regulations, including the Declaration of Helsinki, and ethical approval for data collection and analysis was obtained by each study from local boards.
Author contributions
BJ: Conceptualization, Data curation, Formal analysis, Writing – original draft. PW: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. PL: Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. YW: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – review & editing. YG: Investigation, Methodology, Software, Writing – review & editing. CW: Investigation, Software, Visualization, Writing – review & editing. YJ: Data curation, Investigation, Methodology, Writing – review & editing. RZ: Funding acquisition, Supervision, Visualization, Writing – review & editing. SD: Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. LN: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research work was supported by the National Natural Science Foundation of China (No.81970981, No.82102221), the Key Research and Development Project of Shaanxi Province (No.2023-YBSF-389), Fundamental Research Funds of Xi’an Jiaotong University for Free Exploration and Innovation-Project for Teacher (XZY012021069), and Project to Enhance the Base of Innovation Ability of Xi’an City-Medical Research (21YXYJ0123).
Acknowledgments
We thank FinnGen, UKB, and MRC-IEU for making the summary data publicly available. We are thankful to the investigators and participants who contributed to those studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1271351/full#supplementary-material
References
1. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. (2018) 45 Suppl 20:S162–s70. doi: 10.1111/jcpe.12946
2. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. (2015) 86:611–22. doi: 10.1902/jop.2015.140520
3. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. (2015) 15:30–44. doi: 10.1038/nri3785
4. Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. BioMed J. (2019) 42:27–35. doi: 10.1016/j.bj.2018.12.001
5. Xu W, Zhou W, Wang H, Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. (2020) 120:45–84. doi: 10.1016/bs.apcsb.2019.12.001
6. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. (2012) 98:511–9. doi: 10.1016/j.fertnstert.2012.06.029
7. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. (2019) 15:666–82. doi: 10.1038/s41574-019-0245-z
8. Shin S, Chung YJ, Moon SW, Choi EJ, Kim MR, Chung YJ, et al. Single-cell profiling identifies distinct hormonal, immunologic, and inflammatory signatures of endometriosis-constituting cells. J Pathol. (2023) 261:323–34. doi: 10.1002/path.6178
9. Hoyle AT, Puckett Y. Endometrioma. StatPearls. Treasure Island (FL): StatPearls Publishing LLC (2023).
10. Chapron C, Pietin-Vialle C, Borghese B, Davy C, Foulot H, Chopin N. Associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. Fertil Steril. (2009) 92:453–7. doi: 10.1016/j.fertnstert.2008.06.003
11. Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. (2011) 96:366–73.e8. doi: 10.1016/j.fertnstert.2011.05.090
12. Koninckx PR, Fernandes R, Ussia A, Schindler L, Wattiez A, Al-Suwaidi S, et al. Pathogenesis based diagnosis and treatment of endometriosis. Front Endocrinol (Lausanne). (2021) 12:745548. doi: 10.3389/fendo.2021.745548
13. Kavoussi SK, West BT, Taylor GW, Lebovic DI. Periodontal disease and endometriosis: analysis of the National Health and Nutrition Examination Survey. Fertil Steril. (2009) 91:335–42. doi: 10.1016/j.fertnstert.2007.12.075
14. Thomas V, Uppoor AS, Pralhad S, Naik DG, Kushtagi P. Towards a common etiopathogenesis: periodontal disease and endometriosis. J Hum Reprod Sci. (2018) 11:269–73. doi: 10.4103/jhrs.JHRS_8_18
15. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/asn.2016010098
16. Ference BA, Holmes MV, Smith GD. Using mendelian randomization to improve the design of randomized trials. Cold Spring Harb Perspect Med. (2021) 11(7):a040980. doi: 10.1101/cshperspect.a040980
17. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
18. Yin KJ, Huang JX, Wang P, Yang XK, Tao SS, Li HM, et al. No genetic causal association between periodontitis and arthritis: A bidirectional two-sample mendelian randomization analysis. Front Immunol. (2022) 13:808832. doi: 10.3389/fimmu.2022.808832
19. Nolde M, Holtfreter B, Kocher T, Alayash Z, Reckelkamm SL, Ehmke B, et al. No bidirectional relationship between depression and periodontitis: A genetic correlation and Mendelian randomization study. Front Immunol. (2022) 13:918404. doi: 10.3389/fimmu.2022.918404
20. Baurecht H, Freuer D, Welker C, Tsoi LC, Elder JT, Ehmke B, et al. Relationship between periodontitis and psoriasis: A two-sample Mendelian randomization study. J Clin Periodontol. (2022) 49:573–9. doi: 10.1111/jcpe.13620
21. Si S, Li J, Tewara MA, Xue F. Genetically determined chronic low-grade inflammation and hundreds of health outcomes in the UK biobank and the finnGen population: A phenome-wide mendelian randomization study. Front Immunol. (2021) 12:720876. doi: 10.3389/fimmu.2021.720876
22. Shungin D, Haworth S, Divaris K, Agler CS, Kamatani Y, Keun Lee M, et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun. (2019) 10:2773. doi: 10.1038/s41467-019-10630-1
23. Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, et al. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. (2010) 19:553–62. doi: 10.1093/hmg/ddp508
24. Munz M, Willenborg C, Richter GM, Jockel-Schneider Y, Graetz C, Staufenbiel I, et al. A genome-wide association study identifies nucleotide variants at SIGLEC5 and DEFA1A3 as risk loci for periodontitis. Hum Mol Genet. (2017) 26:2577–88. doi: 10.1093/hmg/ddx151
25. Munz M, Richter GM, Loos BG, Jepsen S, Divaris K, Offenbacher S, et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur J Hum Genet. (2019) 27:102–13. doi: 10.1038/s41431-018-0265-5
26. Yang F, Hu T, Cui H. Serum urate and heart failure: a bidirectional Mendelian randomization study. Eur J Prev Cardiol. (2022) 29:1570–8. doi: 10.1093/eurjpc/zwac100
27. Peakman TC, Elliott P. The UK Biobank sample handling and storage validation studies. Int J Epidemiol. (2008) 37 Suppl 1:i2–6. doi: 10.1093/ije/dyn019
28. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. (2007) 78:1387–99. doi: 10.1902/jop.2007.060264
29. Polk DE, Weyant RJ, Crout RJ, McNeil DW, Tarter RE, Thomas JG, et al. Study protocol of the Center for Oral Health Research in Appalachia (COHRA) etiology study. BMC Oral Health. (2008) 8:18. doi: 10.1186/1472-6831-8-18
30. Yu YH, Steffensen B, Ridker PM, Buring JE, Chasman DI. Candidate loci shared among periodontal disease, diabetes and bone density. Front Endocrinol (Lausanne). (2022) 13:1016373. doi: 10.3389/fendo.2022.1016373
31. Huang D, Lin S, He J, Wang Q, Zhan Y. Association between COVID-19 and telomere length: A bidirectional Mendelian randomization study. J Med Virol. (2022) 94:5345–53. doi: 10.1002/jmv.28008
32. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
33. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
34. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
35. Li Y, Liu H, Ye S, Zhang B, Li X, Yuan J, et al. The effects of coagulation factors on the risk of endometriosis: a Mendelian randomization study. BMC Med. (2023) 21:195. doi: 10.1186/s12916-023-02881-z
36. Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. (2020) 11:597. doi: 10.1038/s41467-020-14389-8
37. D'Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. (2018) 6:954–65. doi: 10.1016/S2213-8587(18)30038-X
38. Peiris AN, Chaljub E, Medlock D. Endometriosis. Jama. (2018) 320:2608. doi: 10.1001/jama.2018.17953
39. MaChado V, Lopes J, Patrão M, Botelho J, Proença L, Mendes JJ. Validity of the association between periodontitis and female infertility conditions: a concise review. Reproduction. (2020) 160:R41–r54. doi: 10.1530/rep-20-0176
40. Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. (2021) 184:2807–24. doi: 10.1016/j.cell.2021.04.041
41. Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. (2010) 78:1789–96. doi: 10.1128/iai.01395-09
42. Thomas C, Timofeeva I, Bouchoucha E, Canceill T, Champion C, Groussolles M, et al. Oral and periodontal assessment at the first trimester of pregnancy: The PERISCOPE longitudinal study. Acta Obstet Gynecol Scand. (2023) 102:669–80. doi: 10.1111/aogs.14529
43. Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. (2009) 88:575–8. doi: 10.1177/0022034509338032
44. Hanazawa S, Murakami Y, Hirose K, Amano S, Ohmori Y, Higuchi H, et al. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. (1991) 59:1972–7. doi: 10.1128/iai.59.6.1972-1977.1991
45. Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. (2013) 19:558–69. doi: 10.1093/humupd/dmt024
46. Muraoka A, Suzuki M, Hamaguchi T, Watanabe S, Iijima K, Murofushi Y, et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci Transl Med. (2023) 15:eadd1531. doi: 10.1126/scitranslmed.add1531
47. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. (2005) 76:2106–15. doi: 10.1902/jop.2005.76.11-S.2106
48. Bullon P, Navarro JM. Inflammasome as a key pathogenic mechanism in endometriosis. Curr Drug Targets. (2017) 18:997–1002. doi: 10.2174/1389450117666160709013850
49. Zou G, Wang J, Xu X, Xu P, Zhu L, Yu Q, et al. Cell subtypes and immune dysfunction in peritoneal fluid of endometriosis revealed by single-cell RNA-sequencing. Cell Biosci. (2021) 11:98. doi: 10.1186/s13578-021-00613-5
50. Hoogstad-van Evert J, Paap R, Nap A, van der Molen R. The promises of natural killer cell therapy in endometriosis. Int J Mol Sci. (2022) 23(10):5539. doi: 10.3390/ijms23105539
51. Blasco-Baque V, Garidou L, Pomié C, Escoula Q, Loubieres P, Le Gall-David S, et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut (2017) 66(5):872–85. doi: 10.1136/gutjnl-2015-309897
52. Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. (2018) 131:557–71. doi: 10.1097/aog.0000000000002469
53. Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. (2009) 15:441–61. doi: 10.1093/humupd/dmp007
54. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
Keywords: causal associations, inflammation, bacterial spread, infertility, periodontal diseases
Citation: Jin B, Wang P, Liu P, Wang Y, Guo Y, Wang C, Jia Y, Zou R, Dong S and Niu L (2024) Association between periodontitis and endometriosis: a bidirectional Mendelian randomization study. Front. Endocrinol. 15:1271351. doi: 10.3389/fendo.2024.1271351
Received: 02 August 2023; Accepted: 06 February 2024;
Published: 29 February 2024.
Edited by:
Achmad Kemal Harzif, University of Indonesia, IndonesiaReviewed by:
Zhen Tan, Peking University, ChinaMarcos Edgar Herkenhoff, University of São Paulo, Brazil
Copyright © 2024 Jin, Wang, Liu, Wang, Guo, Wang, Jia, Zou, Dong and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaojie Dong, ZG9uZ3NoYW9qaWVAbWFpbC54anR1LmVkdS5jbg==; Lin Niu, bml1bGluQHhqdHUuZWR1LmNu
 Bilun Jin
Bilun Jin Pengfei Wang4
Pengfei Wang4 Yijie Wang
Yijie Wang