- 1Reproductive Medical Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Reproduction and Genetics, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To assess whether progesterone (P) levels on the trigger day during preimplantation genetic testing (PGT) cycles are associated with embryo quality and pregnancy outcomes in the subsequent first frozen-thawed blastocyst transfer (FET) cycle.
Methods: In this retrospective analysis, 504 eligible patients who underwent ICSI followed by frozen-thawed embryo transfer (FET) with preimplantation genetic test (PGT) between December 2014 and December 2019 were recruited. All patients adopted the same protocol, namely, the midluteal, short-acting, gonadotropin-releasing hormone agonist long protocol. The cutoff P values were 0.5 and 1.5 ng/ml when serum P was measured on the day of human chorionic gonadotropin (HCG) administration, and cycles were grouped according to P level on the day of HCG administration. Furthermore, the effect of trigger-day progesterone on embryo quality and the subsequent clinical outcome of FET in this PGT population was evaluated.
Results: In total, 504 PGT cycles were analyzed. There was no significant difference in the number of euploid blastocysts, top-quality blastocysts, euploidy rate, or miscarriage rate among the three groups (P>0.05). The 2PN fertilization rate (80.32% vs. 80.17% vs. 79.07%) and the top-quality blastocyst rate (8.71% vs. 8.24% vs. 7.94%) showed a downward trend with increasing P, and the between-group comparisons showed no significant differences (P>0.05). The clinical pregnancy rate (41.25% vs. 64.79%; P<0.05) and live birth rate (35.00% vs. 54.93%; P<0.05) in subsequent FET cycles were substantially lower in the high-P group than in the P ≤ 0.5 ng/ml group. After adjustments were made for confounding variables, multivariate logistic regression analysis revealed that the high-P group had a lower clinical pregnancy rate (adjusted OR, 0.317; 95% CI, 0.145–0.692; P=0.004) and live birth rate (adjusted OR, 0.352; 95% CI, 0.160–0.773; P=0.009) than the low-P group in subsequent FET cycles, and the differences were significant.
Conclusion(s): This study demonstrates that in the PGT population, elevated P on the trigger day may diminish the top-quality blastocyst rate (although there is no difference in the euploidy rate). Trigger-day P is an important factor influencing clinical outcomes in subsequent FET cycles.
Introduction
With the recent improvements in reproductive medicine theory and laboratory technology, increasing emphasis has been placed on the patient safety assessment during assisted reproductive technology (ART). High levels of progesterone (P) are not unusual in the late follicular period, and the use of gonadotropin-releasing hormone (GnRH) agonists and antagonists is known to lower these P levels. Even so, the incidences of high P among in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles with GnRH agonists and antagonists are reported to range from 13% to 46% (1, 2) and from 9% to 38% (3, 4), respectively. At present, the effect of high P during the late follicular phase on reproductive outcomes is controversial.
In their meta-analysis (5), Venetis et al. concluded that the current evidence does not favor an association between trigger-day P and clinical pregnancy rates. However, recent information highlights the detrimental effect of increased P on the reproductive outcomes of fresh cycles, probably because the supraphysiological levels of P affect endometrial receptivity during controlled ovarian stimulation (1, 6). Based on these findings and the development of vitrification freezing techniques, some experts have recommended a “freeze-all” strategy for these patients. In other words, embryos from fresh cycles with elevated P are frozen and transferred later in a FET cycle. This avoids the effect of controlled ovarian stimulation on the endometrium to a certain extent, but there is no consensus on its effect on embryo quality. Together with endometrial receptivity, embryo quality is a critical factor in embryo implantation (7). Several studies have agreed that P does not affect embryo quality (8–10), and this has been confirmed by data from oocyte donation (6, 11) and FET cycles (8–10). Conversely, some scholars have proposed that top-quality blastocysts represented by morphological grade are affected by high P (12, 13). Furthermore, two large retrospective studies have shown that patients with high P have lower rates of top-quality blastocysts, cumulative live birth, and implantation (14, 15). Clearly, patients with high P on the trigger day may lack top-quality blastocysts.
The rapid development of ART and genetic diagnosis technology has led to PGT. At present, PGT is classified mainly as PGT for aneuploidy (PGT-A), PGT for chromosomal structure rearrangement (PGT-SR), and PGT for monogenetic disorders (PGT-M). Patients with advanced age, recurrent pregnancy loss (RPL), and recurrent implantation failure (RIF) are the key target populations for PGT-A (16); PGT-SR is primarily utilized in the detection of chromosomal diseases, including abnormalities in the number of chromosomes, such as Klinefelter syndrome (47, XXY), and aberrant chromosomal structures such as deletion, duplication, inversion, and translocation (17); PGT-M is generally used to distinguish couples with a high risk of monogenic genetic diseases, such as common fibrocystic diseases, hereditary hemoglobinopathy, Huntington’s disease, and other rare diseases (18). PGT-A provides a relatively accurate assessment of embryo quality (19). Several clinical studies using PGT-A have found that elevated P during the late follicular period is irrelevant to the embryo euploidy rate and pregnancy outcomes (9, 10). However, these findings contradict previously reported studies that high P affects embryo quality, possibly due to the use of different thresholds (12, 15). Additionally, there are limited data assessing the correlation between P on the day of HCG injection and embryo quality, euploidy rates, and pregnancy outcomes in subsequent FET cycles. Therefore, this study aimed to measure serum P levels on the trigger day of fresh cycles and assess the impact of trigger-day P on the top-quality blastocyst rate, the euploidy rate, and clinical outcomes of the subsequent FET in the PGT population, with the aim of providing a reference for clinical work in ART.
Materials and methods
Study design and population
Patients who underwent preimplantation genetic test (PGT) at our center were mostly those with chromosomal translocation and monogenic diseases. This retrospective cohort study was conducted among patients who underwent ICSI/PGT for conception at the First Affiliated Hospital of Zhengzhou University Reproductive Medicine Center from December 2014 to December 2019. We included patients aged 20-40 years who accepted controlled ovarian stimulation with the unified protocol and endometrium with the same hormone replacement. Afterward, they underwent the first cycle of frozen-thawed euploid blastocyst transplantation. Owing to the retrospective nature of the study, informed consent was waived. All operations were carried out in conformity with the applicable rules and regulations.
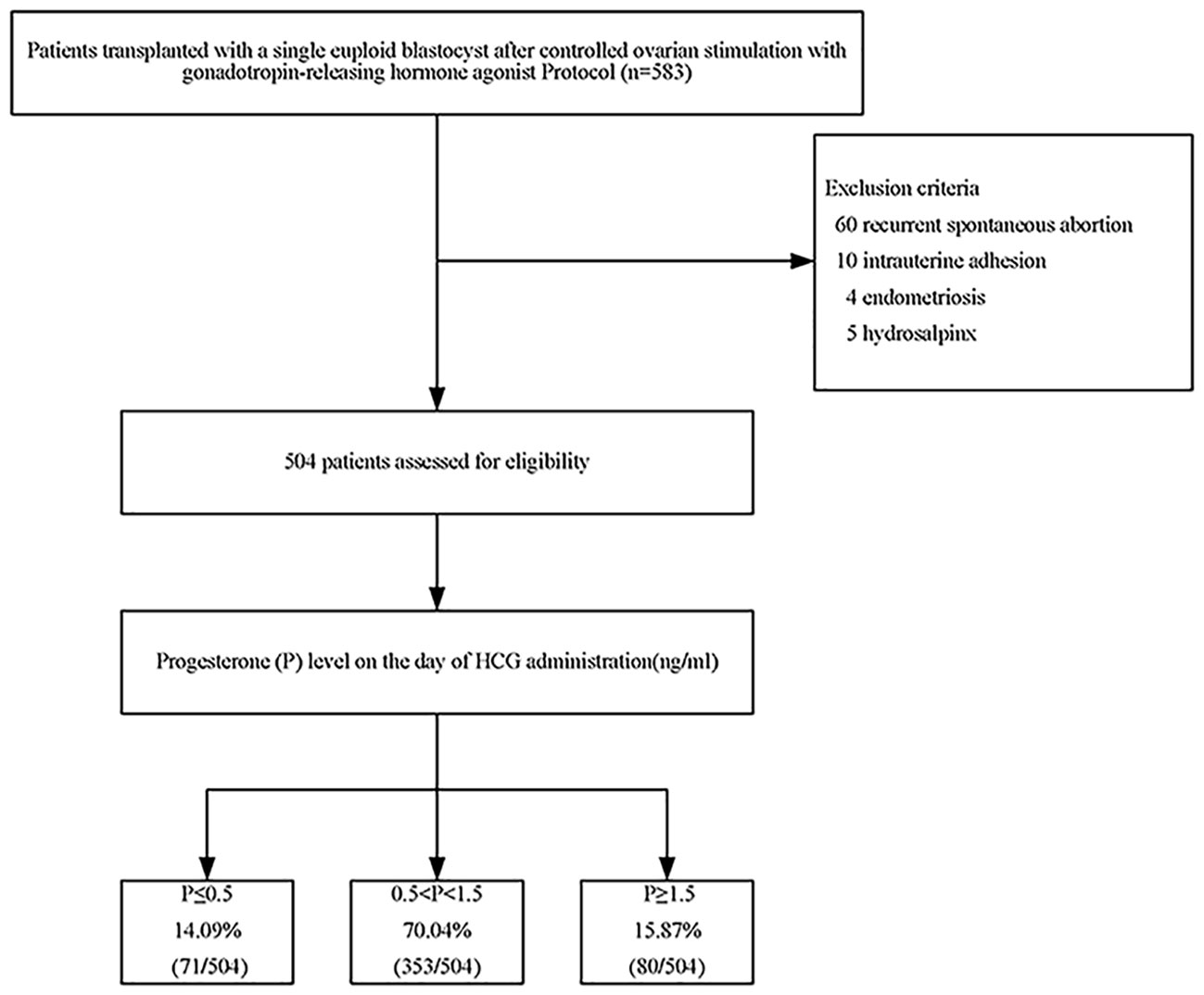
In short, this retrospective study included 583 patients who received ICSI/PGT treatment from December 2014 to December 2019. The patient was excluded if she met any of the following criteria: recurrent spontaneous abortion; intrauterine adhesion; endometriosis; or hydrosalpinx. In the end, 504 eligible patients participated in the trial.
As described in a prior study (20), patients adopted the same protocol, namely, the midluteal, short-acting, gonadotropin-releasing hormone agonist long protocol. The dose of gonadotropin was individually coordinated according to the basic characteristics and responses of each patient. Continuous transvaginal ultrasound scans and serum estradiol (E2) and P were used to track the cycles. Triggering was employed with 250 µg recombinant human chorionic gonadotropin (r-hCG, Merck Serono, Geneva, Switzerland) and 2,000 IU u-HCG (Livzon, Guangzhou, China). Thirty-seven hours later, oocytes were retrieved under the guidance of transvaginal ultrasound.
Laboratory procedures
All oocytes were fertilized by ICSI after 4-6 h. Morphological evaluation of blastocyst-stage embryos was conducted by experienced embryologists under equivalent laboratory conditions according to Gardner and Schoolcraft’s criteria (21). They performed whole genome amplification after trophoblast biopsy of top-quality blastocysts using a laser method. Next, aneuploidy was detected by SNP microarray chip detection technology (SNP array) or next-generation sequencing (NGS) technology for comprehensive chromosome screening (22). Euploid blastocysts with a well-expanded blastocyst cavity (B3-B5 stages), inner cell mass (grade A or B), and trophectoderm (grade A or B) were interpreted as top-quality blastocysts. We stored the blastocysts after biopsy in liquid nitrogen.
FET endometrial preparation
The HRT protocol was adopted for the preparation of the endometrium in all thawing cycles. Subsequently, euploid blastocysts of the highest morphological grade were selected for transfer. The details and operation methods of the protocol have been published previously (23).
P assessment immunoassay
Sex hormone concentrations were evaluated on days 2-4 of the menstrual cycle (before stimulation) and on the trigger day. We recorded the levels of anti-Müllerian hormone (AMH), estradiol (E2), follicle-stimulating hormone (FSH), and progesterone. Serum P was measured on the day of HCG administration using a validated electrochemiluminescence immunoassay (Cobas 12145383). The detection limit and sensitivity of the method were 0.03 ng/mL and 0.15 ng/mL, respectively. The intra-assay and interassay coefficients of variation were 3.0 and 5.5%, respectively. The same detection method was utilized throughout the study and calibrated regularly to reduce unnecessary errors.
Main outcome measures
The outcomes included various indicators of embryo development and the outcomes of pregnancy. The key results of the study were the clinical pregnancy rate and live birth rate, with other indicators being the euploidy rate and the top-quality blastocyst rate. Clinical pregnancy was defined as one or more gestational sacs detected by ultrasound. Live birth was defined as the delivery of a live infant after 22 weeks of gestation. Miscarriage was defined as a spontaneous abortion of an intrauterine pregnancy before 22 weeks (24). Other indicators were as follows: oocyte maturity rate (number of MII oocytes per oocyte); 2PN fertilization rate (number of 2PN oocytes per MII oocyte); blastocyst formation rate (number of blastocysts formed per cultured); euploidy rate (number of euploid blastocysts per biopsied); and top-quality blastocyst rate (number of top-quality blastocysts per culture).
Statistical analysis
SPSS Statistics for Windows, version 21, was used for statistical analysis. P on the trigger day was regarded as a classified variable and a continuous variable, and patients were categorized into one of the following three groups according to the P level on the trigger day: low P, defined as ≤0.50 ng/ml; medium P, defined as 0.51-1.49 ng/ml; or high P, defined as ≥1.50 ng/ml. Currently, there is no definitive cutoff value for trigger-day P, so these thresholds were chosen according to clinical practice. Studies have demonstrated that a P level above 1.50 ng/mL is the optimal threshold for observing reproductive outcomes (25, 26). However, higher P levels (> 1.5 ng/ml) have been shown to be detrimental not only to endometrial receptivity but also to embryo quality (12, 13). In addition, in a meta-analysis involving more than 55,000 cycles, the thresholds proposed by different studies for hCG-day P have ranged from 0.5 to 3.0 ng/mL, so 0.5 ng/mL was selected as the cutoff value for low P in this study (6). We also summarized each patient’s characteristics. Continuous variables are presented as the mean ± standard deviation or interquartile interval on the basis of whether they followed a normal distribution. For comparisons, Student’s t-test, one-way ANOVA, and Kruskal–Wallis tests were chosen. We use frequencies (percentages) to represent categorical variables and used chi-square tests to analyze differences between groups. We also conducted a univariate logistic analysis to explore the relationships between various variables and pregnancy outcomes. Multivariate logistic regression models were constructed for the crude and adjusted models by calculating the crude odds ratios (ORs) and adjusted ORs with 95% confidence intervals (CIs). Potential confounders were preferential and chosen by relying on ordinary clinical practice, literature, and baseline data, and adjustments were made for these confounding variables in the analysis of changes in each outcome between groups. Adjusted factors included female age at transfer, infertility duration, gravidity, parity, number of miscarriages, body mass index (BMI), basal E2, basal FSH, AMH, genetic category, endometrial thickness, days of embryonic development, E2 level on the day of HCG administration, and Gn total dose. All tests were two-sided, and statistical significance was defined as P < 0.05.
Results
Patient demographics and general characteristics
A total of 583 patients underwent the first FET cycles after PGT. Among them, 60 patients with recurrent miscarriages, 10 with uterine adhesions, 4 with endometriosis, and 5 with hydrosalpinx were excluded. There were 160 patients with reciprocal translocation (31.75%), 86 patients with Robertsonian translocation (17.06%), 77 patients with single-gene disease (15.28%), and 181 patients (35.91%) with different forms of chromosomal abnormalities in this study (e.g., insertion, duplication, deletion, inversion, translocation). In the end, 504 eligible patients entered the study (Figure 1).
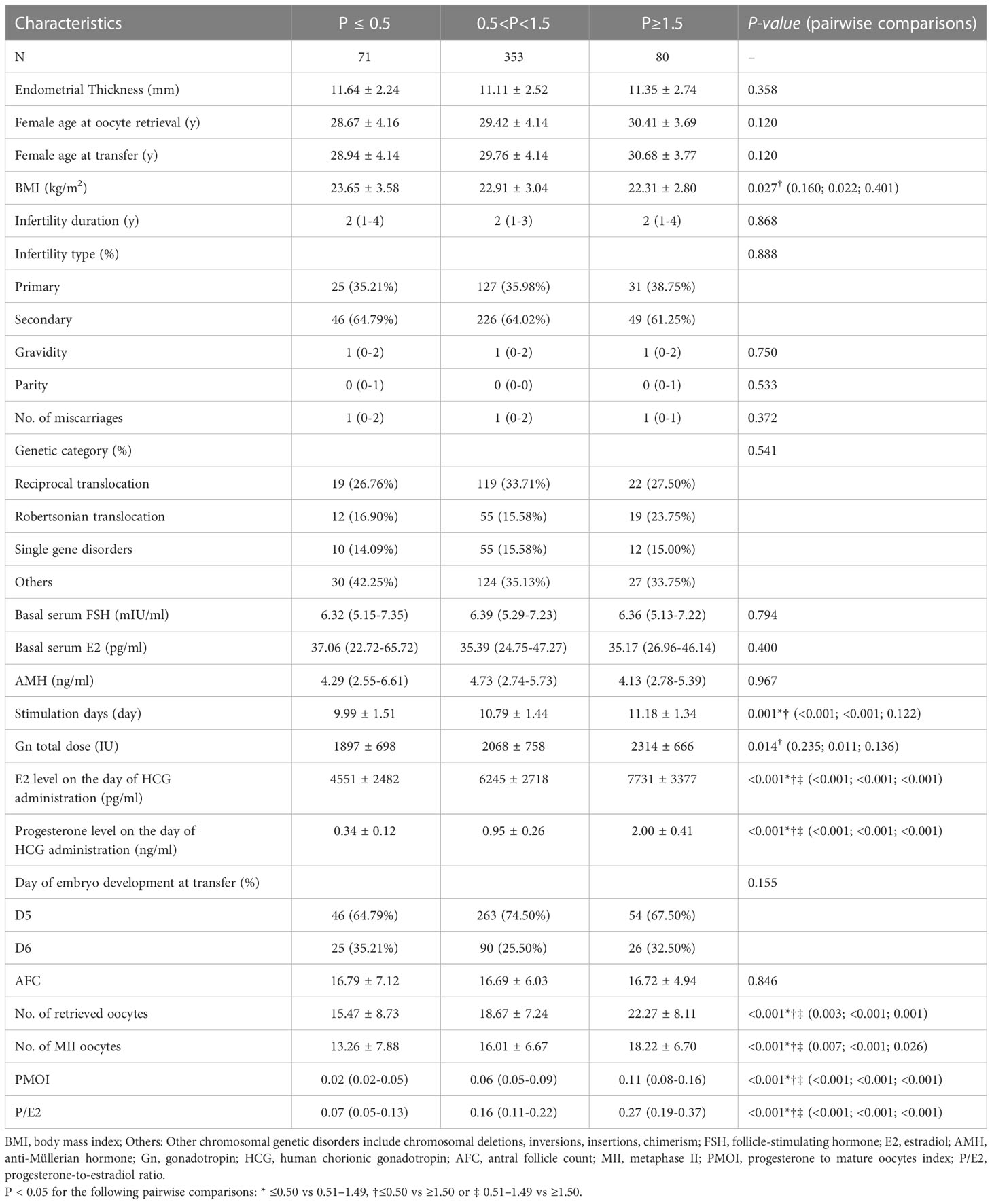
Among them, 71 cases (14.09%) had serum P≤ 0.5 ng/ml, 353 cases (70.04%) had serum 0.5<P<1.5 ng/ml, and 80 cases (15.87%) had serum P≥1.5 ng/ml. There were no significant differences in the following indicators grouped by P on the trigger day: endometrial thickness, years of infertility, infertility type, gravidity, parity, number of miscarriages, genetic category, basal FSH, basal E2, AMH, days of embryonic development, and AFC. Female age increased with increasing P on the trigger day, but there were no significant between-group differences (P>0.05) (Table 1).
Evaluations of differences between groups
The results indicated that the total Gn stimulation days and cumulative dose were significantly higher in the high-P group at different P levels. The between-group comparisons among the three groups revealed that with increasing P on the trigger day, there was a significant upward trend in peak E2 levels, the number of oocytes retrieved, MII oocytes, PMOI, and P/E2, and there were significant differences in all between-group comparisons (Table 1).
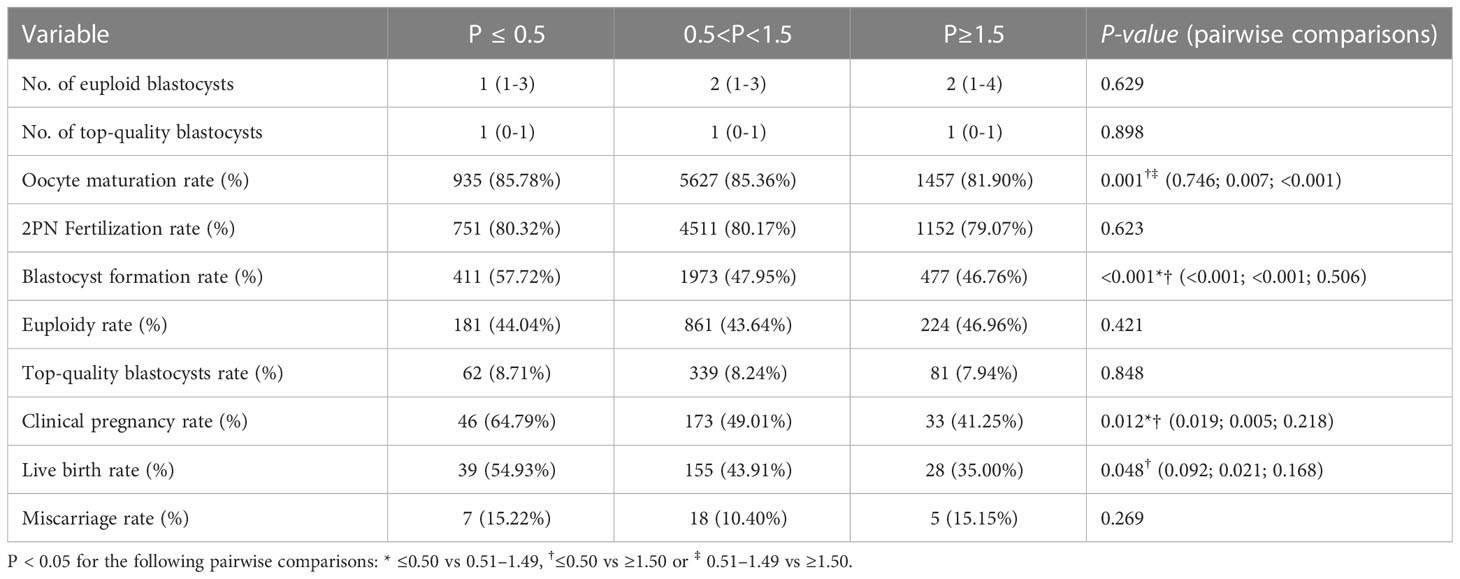
As implied in Table 2, the three groups had statistically comparable numbers of euploid blastocysts, top-quality blastocysts, euploidy rates, and miscarriage rates (P>0.05). The between-group comparisons showed that the oocyte maturity rate and blastocyst formation rate decreased gradually with increasing P on the trigger day. Specifically, the oocyte maturity rate was significantly higher in the low-P and medium-P groups than in the high-P group (85.78% vs. 81.90%, P=0.007, 85.36% vs. 81.90%, P<0.001), and the blastocyst formation rates in the medium- and high-P groups were significantly lower than that in the low-P group (47.95% vs. 57.72%, P<0.001, 46.76% vs. 57.72%, P<0.001). Additionally, we noticed that the 2PN fertilization rate (80.32% vs. 80.17% vs. 79.07%) and top-quality blastocyst rate (8.71% vs. 8.24% vs. 7.94%) decreased with increasing P, although there were no significant differences between groups (P>0.05). Regarding the pregnancy rates of the different subgroups, the clinical pregnancy rate was significantly higher in the low-P group than in the medium-P (64.79% vs. 49.01%, P=0.019) and high-P groups (64.79% vs. 41.25%, P=0.005), albeit the difference between the medium- and high-P groups was not significant (P>0.05). The difference in live birth rate between the low- and high-P groups was also significant (54.93% vs. 35.00%, P=0.021), but that between the low- and medium-P groups or between the medium- and high-P groups was non-significant (P > 0.05). Interestingly, the clinical pregnancy rate (64.79% vs. 49.01% vs. 41.25%) and live birth rate (54.93% vs. 43.91% vs. 35.00%) appeared to drop linearly with increasing P.
Association between progesterone levels and pregnancy outcomes
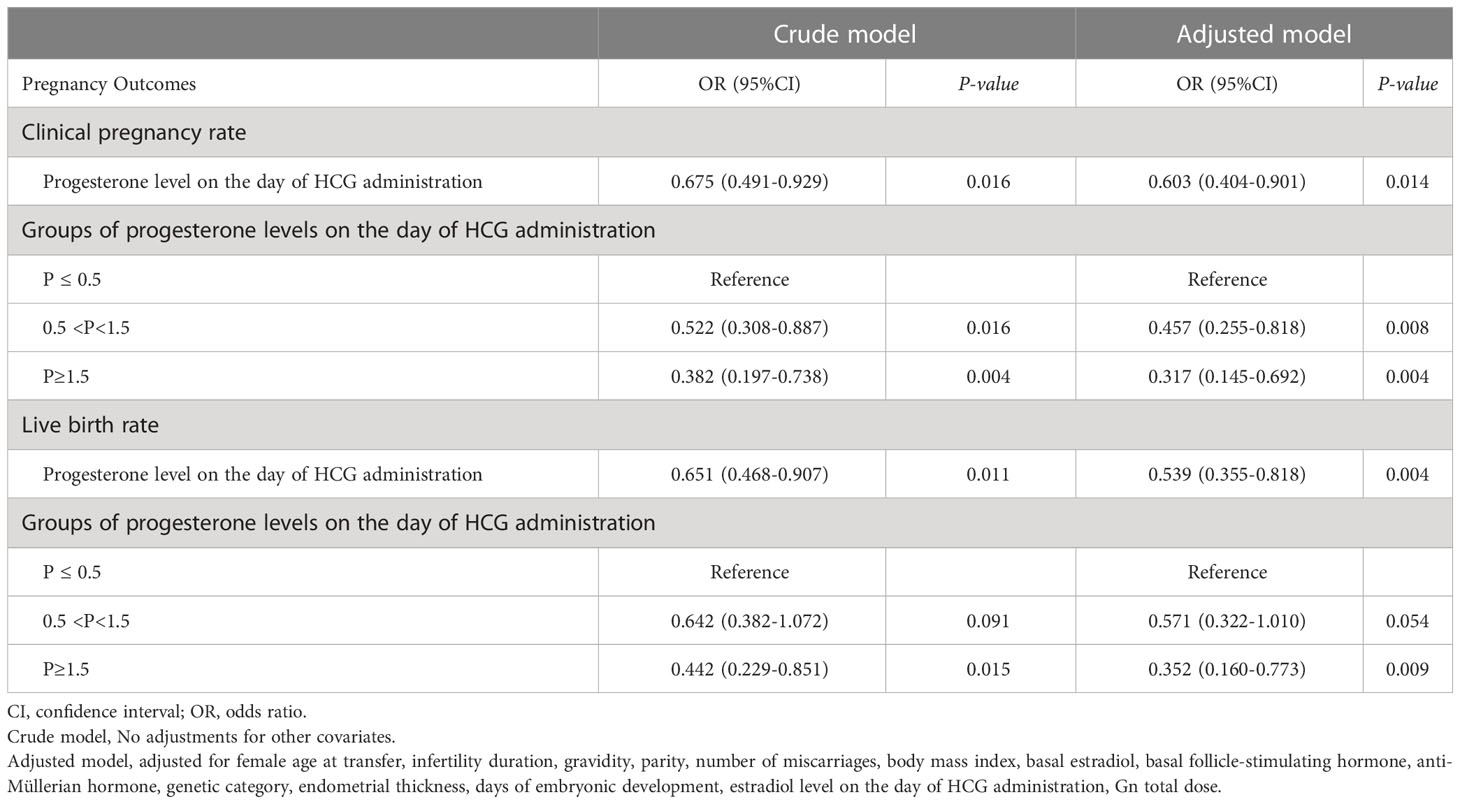
The initial, univariate regression analysis showed that age, gravidity, number of miscarriages, P on the trigger day, days of embryonic development, and number of euploid blastocysts were the associated factors affecting pregnancy outcomes (P<0.05) (Table 3). Subsequently, we utilized a logistic regression model to evaluate the relationship between P on the trigger day and pregnancy outcomes. In an unadjusted analysis of the pregnancy outcomes in our cohort, patients were subdivided into three groups according to P at the time of triggering. With the low-P group as the reference, P was found to be significantly associated with the clinical pregnancy rate and live birth rate (P<0.05). To avoid potential deviation in the results and to further estimate the effects of confounding factors and P on pregnancy outcomes, we adjusted for female age at transfer, infertility duration, gravidity, parity, number of miscarriages, BMI, basal E2, basal FSH, AMH, genetic category, endometrial thickness, days of embryonic development, E2 on the trigger day, and Gn total dose. In the fully adjusted model, with trigger-day P used as a categorical variable, a considerably decreased clinical pregnancy rate for subsequent FET cycles was observed in the medium- and high-P groups compared with the low-P group (adjusted OR, 0.457; 95% CI, 0.255–0.818; P=0.008; adjusted OR, 0.317; 95% CI, 0.145–0.692; P=0.004). Regarding the live birth rate, with the low-P group used as the reference, the high-P group showed a declining trend in live birth rate in subsequent FET cycles, and the difference was significant (adjusted OR, 0.352; 95% CI, 0.160-0.773; P=0.009). Additionally, the difference in the live birth rate between the medium-P and low-P groups was not significant (adjusted OR, 0.571; 95% CI, 0.322-1.010; P=0.054). Furthermore, when trigger-day P was a continuous variable, a significant correlation (P<0.05) was reported between P and the clinical pregnancy rate and live birth rate in the subsequent FET cycles regardless of whether the model was adjusted (P<0.05) (Table 4).
Discussion
The findings of this study showed that trigger-day P was independent of the embryo euploidy rate in the PGT population, that the rate of top-quality blastocysts decreased gradually with rising trigger-day P, and that trigger-day P is an important factor influencing clinical outcomes in subsequent FET cycles.
Our data showed that in the PGT population, the progesterone level of the late follicular phase in fresh cycles was irrelevant to the embryo euploidy rate. These findings were consistent with previously reported studies in which trigger-day P had no relationship with euploidy rates (8–10). Moreover, a robust multivariate regression analysis was conducted in this single-center study to account for the impact of various confounders on pregnancy outcomes. Increasing trigger-day P was thought to be critical in lowering the clinical pregnancy rate and live birth rate. Similarly, in a study of patients who underwent FET, Pal et al. confirmed that high P on the day of HCG was related to the decreased success rate of FET (27). However, these results contradicted previous studies on late-follicular P levels and FET results (1, 6, 9). The current study obtained different results from other studies regarding the relationship of elevated P with embryo quality and pregnancy outcomes, and this may be due to differences in the P cutoff value and detection methods used. For example, Bosch et al. (25) observed by trend analysis that when the serum P level on the trigger day was 1.5 ng/ml, it reached the critical threshold level at which P would have a negative effect on pregnancy outcomes. Racca et al. (12) concluded that the trigger-day P 1.5 ng/ml group had considerably decreased embryo and blastocyst utilization. Similarly, S. Santos (28) et al. reported that P levels below 0.5 ng/ml on the day of HCG administration had a detrimental effect on live birth. Various studies have used different analytical methods to determine specific thresholds for elevated P. As a result, the final results obtained by applying different cutoff values were inconsistent. On the other hand, the population was composed mainly of patients who underwent PGT, most of whom had chromosomal translocations or single-gene disorders. The heterogeneity of this population itself also explains the difference from other results.
Racca et al. (29) discovered that on the trigger day, the mean number of top-quality blastocysts was lower in the P≥1.5 ng/ml group than in the P<1.5 ng/ml group. Two large retrospective studies (14, 15) also indicated that high P was relevant to a reduced rate of top-quality blastocysts. Furthermore, Vanni et al. (13) believed that when P levels reached or approached 1.5 ng/ml (>1.49), they could be considered an early warning sign of a decline in the top-quality blastocyst rate. The present study also found that the rate of top-quality blastocysts decreased gradually with rising P, although the differences among the three groups were not significant. This also indirectly implied that high P may have an impact on the top-quality blastocyst rate. Animal studies have shown that decreased follicular P can improve oocyte development in vitro (30). Furthermore, Valbuena et al. found that high E2 can be harmful to cleavage-stage embryos (31). The high P on the trigger day was frequently accompanied by high E2, according to findings from this study and earlier ones (6, 25, 32). Regrettably, it is unknown whether superphysiological levels of hormones will hinder the quality of human embryos. In a study published in 2021, Tokgoz et al. (33) revealed that embryo transfer at the blastocyst stage in the fresh cycle could improve pregnancy outcomes, even though the P level on the trigger day was higher than 0.85 ng/mL. Indeed, it is unclear whether blastocyst transfer can partially offset the negative effect on pregnancy outcome of elevated P on the trigger day. Since the mechanism by which high P on the trigger day impacts embryo quality is currently unknown, it is difficult to assume that trigger-day P has a residual influence on pregnancy outcomes in subsequent FET cycles by affecting blastocyst quality. Moreover, there are few studies evaluating whether low P on the trigger day disrupts pregnancy outcomes or embryo quality in subsequent FET cycles. Only one study reported the effect of P<0.8 ng/ml on euploid embryos and reproductive outcomes in subsequent FET cycles (34). Nonetheless, a large-scale meta-analysis of over 55,000 cycles noted that P was significantly and inversely associated with pregnancy outcomes when the P level reached 0.8 ng/ml or above in the late-follicular phase (6). Therefore, a different cutoff value was selected for our study based on S. Santos et al. and Arvis et al. In fresh-embryo transfer cycles, they noticed that low P (< 0.5 ng/ml) on the day of HCG administration significantly decreased live birth rates (28, 35). However, in our research, when P was <0.5 ng/ml, neither the embryo euploidy rates nor pregnancy outcomes of subsequent FET cycles were affected. This indicates that low P in the late follicular phase will not have an impact on embryo quality. However, it may lead to changes in endometrial receptivity and thus affect pregnancy outcomes in fresh cycles. Moreover, the underlying mechanism for the high P on the trigger day during ART is not clear. Studies have shown that three main aspects contributing to high P include the number of follicles, the gonadotropin dose and actions on granulosa cells, and the role of luteinizing hormone stimulation on follicular membrane cells (36). In our research, we also noticed higher gonadotropin doses, estradiol concentrations, and retrieved and mature oocytes in the high-P group.
Our study precisely investigated the influence of low P on the embryo euploidy rate and pregnancy outcomes in subsequent FET cycles and discovered that low P was not linked to the embryo quality or pregnancy outcomes in subsequent first FET cycles. Additionally, a unified ovarian stimulation and endometrial preparation protocol was implemented in all patients. The research involved only patients who underwent single euploid blastocyst transfer. Furthermore, a standardized protocol and the application of multiple regression logistic analysis models were used to weaken the effects of potential confounding factors and ensure the reliability of the results.
However, due to the limitations of this retrospective study, there are inevitable deviations even though the effects of potential confounders were minimized. First, PGT patients in our center comprise mainly chromosomal translocations and single-gene disorders, and the results of this study cannot be extrapolated to the general infertile population. Second, it is evident from the present study that there is a gradual increase in female age with increasing P on the trigger day, and there is no denying the influence of female age on pregnancy outcomes (37). Third, there is no definitive cutoff value for trigger-day P. P assays and their cutoff values vary among studies in different centers and may vary depending on the assay method. Finally, studies have shown that the duration of P elevation also affects pregnancy outcomes (38, 39), but we did not separately analyze patients with different durations of P elevation.
In summary, we used an accurate PGT/FET cycle model to determine the relationship of trigger-day P with embryo quality and subsequent pregnancy outcomes. The results showed that in the single vitrified frozen euploid blastocyst transfer cycle, trigger-day P was irrelevant to the embryo euploidy rate. However, elevated P may reduce the rate of top-quality blastocysts. Trigger-day P is an important factor influencing pregnancy outcomes in subsequent FET cycles. Since this study includes all PGT patients subjected to the short-acting, gonadotropin-releasing hormone agonist long protocol, it remains unclear whether findings can be extrapolated to populations using other protocols or to the general infertile population. On the other hand, in view of the small sample size included in the low-P and high-P groups in this study, the existence of bias cannot be ruled out, and the lack of subsequent multicycle follow-up makes it impossible to explain the impact of high P on prognosis. Further studies and randomized clinical trials with larger sample sizes are advisable. In conclusion, we should treat this result with caution. In daily clinical practice, each center needs to evaluate its P threshold before performing FET in patients showing high P on the trigger day to obtain better outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YZ, JL and YC contributed to the study design, data analysis and manuscript preparation. HS, ZB, FW and BS handled patient recruitment and data collection. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by grant 31970799 from the National Natural Science Foundation of China.
Acknowledgments
The authors are grateful for the professional manuscript services of American Journal Experts in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y, et al. Effect of hcg-day serum progesterone and oestradiol concentrations on pregnancy outcomes in gnrh agonist cycles. Reprod BioMed Online (2012) 24(5):511–20. doi: 10.1016/j.rbmo.2012.02.003
2. Ubaldi F, Camus M, Smitz J, Bennink HC, Van Steirteghem A, Devroey P. Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (Gnrh-a) and recombinant follicle-stimulating hormone (Fsh) and gnrh-a and urinary fsh. Fertility Sterility (1996) 66(2):275–80. doi: 10.1016/s0015-0282(16)58453-2
3. Bosch E, Valencia I, Escudero E, Crespo J, Simon C, Remohi J, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril (2003) 80(6):1444–9. doi: 10.1016/j.fertnstert.2003.07.002
4. Ochsenkuhn R, Arzberger A, von Schonfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: A retrospective study with 2,555 fresh embryo transfers. Fertil Steril (2012) 98(2):347–54. doi: 10.1016/j.fertnstert.2012.04.041
5. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? a systematic review and meta-analysis. Hum Reprod Update (2007) 13(4):343–55. doi: 10.1093/humupd/dmm007
6. Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after ivf: A systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update (2013) 19(5):433–57. doi: 10.1093/humupd/dmt014
7. Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update (2006) 12(6):731–46. doi: 10.1093/humupd/dml004
8. Hernandez-Nieto C, Lee JA, Alkon T, Luna-Rojas M, Klein J, Copperman AB, et al. Premature luteinization in the era of pgt-a: Embryonic reproductive potential is not affected by elevated progesterone levels during ovarian hyperstimulation. Fertility Sterility (2019) 112(3):e29. doi: 10.1016/j.fertnstert.2019.07.209
9. Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, et al. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod (2020) 35(8):1889–99. doi: 10.1093/humrep/deaa123
10. Neves AR, Santos-Ribeiro S, Garcí. Martínez S, Soares S, García-Velasco JA, Garrido N, et al. P–615 the effect of late-follicular phase progesterone rise on embryo ploidy, embryo quality and cumulative live birth rates following a freeze-only strategy. Hum Reprod (2021) 36(Supplement_1):i416–417. doi: 10.1093/humrep/deab130.614
11. Racca A, De Munck N, Santos-Ribeiro S, Drakopoulos P, Errazuriz J, Galvao A, et al. Do we need to measure progesterone in oocyte donation cycles? a retrospective analysis evaluating cumulative live birth rates and embryo quality. Hum Reprod (2020) 35(1):167–74. doi: 10.1093/humrep/dez238
12. Racca A, Santos-Ribeiro S, De Munck N, Mackens S, Drakopoulos P, Camus M, et al. Impact of late-follicular phase elevated serum progesterone on cumulative live birth rates: Is there a deleterious effect on embryo quality? Hum Reprod (2018) 33(5):860–8. doi: 10.1093/humrep/dey031
13. Vanni VS, Somigliana E, Reschini M, Pagliardini L, Marotta E, Faulisi S, et al. Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in gnrh antagonist Ivf/Icsi cycles. PloS One (2017) 12(5):e0176482. doi: 10.1371/journal.pone.0176482
14. Bu Z, Zhao F, Wang K, Guo Y, Su Y, Zhai J, et al. Serum progesterone elevation adversely affects cumulative live birth rate in different ovarian responders during in vitro fertilization and embryo transfer: A Large retrospective study. PloS One (2014) 9(6):e100011. doi: 10.1371/journal.pone.0100011
15. Yang Y, Liu B, Wu G, Yang J. Exploration of the value of progesterone and Progesterone/Estradiol ratio on the hcg trigger day in predicting pregnancy outcomes of pcos patients undergoing Ivf/Icsi: A retrospective cohort study. Reprod Biol Endocrinol (2021) 19(1):184. doi: 10.1186/s12958-021-00862-6
16. Patrizio P, Shoham G, Shoham Z, Leong M, Barad DH, Gleicher N. Worldwide live births following the transfer of chromosomally “Abnormal” embryos after Pgt/A: Results of a worldwide web-based survey. J Assist Reprod Genet (2019) 36(8):1599–607. doi: 10.1007/s10815-019-01510-0
17. Garcia-Pascual CM, Navarro-Sanchez L, Navarro R, Martinez L, Jimenez J, Rodrigo L, et al. Optimized ngs approach for detection of aneuploidies and mosaicism in pgt-a and imbalances in pgt-Sr. Genes (Basel) (2020) 11(7):724. doi: 10.3390/genes11070724
18. De Rycke M, Berckmoes V. Preimplantation genetic testing for monogenic disorders. Genes (Basel) (2020) 11(8):871. doi: 10.3390/genes11080871
19. Scott RT, Miller KA, Olivares R, Su J, Fratterelli JL, Treff NR. Microarray based 24 chromosome preimplantation genetic diagnosis (Mpgd) is highly predictive of the reproductive potential of human embryos: A prospective blinded non-selection trial. Fertility Sterility (2008) 90:S22–S3. doi: 10.1016/j.fertnstert.2008.07.438
20. Li G, Wu Y, Niu W, Xu J, Hu L, Shi H, et al. Analysis of the number of euploid embryos in preimplantation genetic testing cycles with early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol. Front Endocrinol (Lausanne) (2020) 11:424. doi: 10.3389/fendo.2020.00424
21. Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol (1999) 11(3):307–11. doi: 10.1097/00001703-199906000-00013
22. Shi D, Xu J, Niu W, Liu Y, Shi H, Yao G, et al. Live births following preimplantation genetic testing for dynamic mutation diseases by karyomapping: A report of three cases. J Assist Reprod Genet (2020) 37(3):539–48. doi: 10.1007/s10815-020-01718-5
23. Ziqi J, Hao S, Manman L, Zhiqin B, Mingzhu H, Yile Z. Endometrial thickness changes after progesterone administration do not affect the pregnancy outcomes of frozen-thawed euploid blastocyst transfer: A retrospective cohort study. Fertility Sterility (2021) 116(6):1502–1512. doi: 10.1016/j.fertnstert.2021.08.008
24. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril (2017) 108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005
25. Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: Analysis of over 4000 cycles. Hum Reprod (2010) 25(8):2092–100. doi: 10.1093/humrep/deq125
26. Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In't Veld P, Schuit F, et al. Progesterone rise on hcg day in gnrh Antagonist/Rfsh stimulated cycles affects endometrial gene expression. Reprod BioMed Online (2011) 22(3):263–71. doi: 10.1016/j.rbmo.2010.11.002
27. Pal L, Kovacs P, Witt B, Jindal S, Santoro N, Barad D. Postthaw blastomere survival is predictive of the success of frozen-thawed embryo transfer cycles. Fertil Steril (2004) 82(4):821–6. doi: 10.1016/j.fertnstert.2004.02.136
28. Santos-Ribeiro S, Polyzos NP, Haentjens P, Smitz J, Camus M, Tournaye H, et al. Live birth rates after ivf are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum Reprod (2014) 29(8):1698–705. doi: 10.1093/humrep/deu151
29. Racca A, Vanni VS, Somigliana E, Reschini M, Viganò P, Santos-Ribeiro S, et al. Is a freeze-all policy the optimal solution to circumvent the effect of late follicular elevated progesterone? a multicentric matched-control retrospective study analysing cumulative live birth rate in 942 non-elective freeze-all cycles. Hum Reprod (2021) 36(9):2463–72. doi: 10.1093/humrep/deab160
30. Urrego R, Herrera-Puerta E, Chavarria NA, Camargo O, Wrenzycki C, Rodriguez-Osorio N. Follicular progesterone concentrations and messenger RNA expression of mater and Oct-4 in immature bovine oocytes as predictors of developmental competence. Theriogenology (2015) 83(7):1179–87. doi: 10.1016/j.theriogenology.2014.12.024
31. Valbuena D, Martin J, de Pablo JL, Remohı́ J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertility Sterility (2001) 76(5):962–8. doi: 10.1016/s0015-0282(01)02018-0
32. Healy MW, Yamasaki M, Patounakis G, Richter KS, Devine K, DeCherney AH, et al. The slow growing embryo and premature progesterone elevation: Compounding factors for embryo-endometrial asynchrony. Hum Reprod (2017) 32(2):362–7. doi: 10.1093/humrep/dew296
33. Tokgoz VY, Tekin AB. Serum progesterone level above 0.85 Ng/Ml and Progesterone/Estradiol ratio may be useful predictors for replacing cleavage-stage with blastocyst-stage embryo transfer in fresh Ivf/Icsi cycles without premature progesterone elevation. Arch Gynecol Obstet (2022) 305(4):1011–9. doi: 10.1007/s00404-021-06304-3
34. Boynukalin FK, Yarkiner Z, Gultomruk M, Turgut NE, Ecemis S, Findikli N, et al. Elevation of progesterone on the trigger day exerts no carryover effect on live birth in freeze-all cycles. Gynecol Endocrinol (2021) 37(4):367–71. doi: 10.1080/09513590.2020.1786510
35. Arvis P, Lehert P, Guivarc'h-Leveque A. Both high and low hcg day progesterone concentrations negatively affect live birth rates in Ivf/Icsi cycles. Reprod BioMed Online (2019) 39(5):852–9. doi: 10.1016/j.rbmo.2019.07.001
36. Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod BioMed Online (2010) 21(4):446–9. doi: 10.1016/j.rbmo.2010.05.018
37. Tsai YR, Huang FJ, Lin PY, Kung FT, Lin YJ, Lin YC, et al. Progesterone elevation on the day of human chorionic gonadotropin administration is not the only factor determining outcomes of in vitro fertilization. Fertil Steril (2015) 103(1):106–11. doi: 10.1016/j.fertnstert.2014.10.019
38. Lee VC, Li RH, Chai J, Yeung TW, Yeung WS, Ho PC, et al. Effect of preovulatory progesterone elevation and duration of progesterone elevation on the pregnancy rate of frozen-thawed embryo transfer in natural cycles. Fertil Steril (2014) 101(5):1288–93. doi: 10.1016/j.fertnstert.2014.01.040
Keywords: preimplantation genetic testing, trigger-day progesterone, euploid blastocyst transfer, embryo quality, pregnancy outcome
Citation: Li J, Cui Y, Shi H, Bu Z, Wang F, Sun B and Zhang Y (2023) Effects of trigger-day progesterone in the preimplantation genetic testing cycle on the embryo quality and pregnancy outcomes of the subsequent first frozen-thawed blastocyst transfer. Front. Endocrinol. 14:990971. doi: 10.3389/fendo.2023.990971
Received: 11 July 2022; Accepted: 23 February 2023;
Published: 06 March 2023.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Xiuxian Zhu, Shanghai First Maternity and Infant Hospital, ChinaYavuz Tokgöz, Eskişehir Osmangazi University, Türkiye
Copyright © 2023 Li, Cui, Shi, Bu, Wang, Sun and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yile Zhang, bHVuYTAyMDk5NkAxMjYuY29t
†These authors have contributed equally to this work
 Jingdi Li
Jingdi Li Yueyue Cui1,2†
Yueyue Cui1,2† Hao Shi
Hao Shi Zhiqin Bu
Zhiqin Bu Bo Sun
Bo Sun Yile Zhang
Yile Zhang