- 1Department of Scientific Cooperation of Guangxi Academy of Medical Sciences, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China
- 2Department of Scientific Research, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 3Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 4Department of Breast and Thyroid Surgery, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Background: The associations between metabolic dysfunction-associated fatty liver disease (MAFLD) and cancer development, especially extrahepatic cancers, are unknown. The aims of the current study were to investigate the cancer incidence rates of MAFLD and analyze the associations between MAFLD and the development of cancers.
Methods: This historical cohort study included participants who underwent ultrasonographic detection of hepatic steatosis at a tertiary hospital in China from January 2013 to October 2021. MAFLD was diagnosed in accordance with The International Expert Consensus Statement. Cox proportional hazards regression modeling was used to assess the associations between MAFLD and the development of cancers.
Results: Of the 47,801 participants, 16,093 (33.7%) had MAFLD. During the total follow-up of 175,137 person-years (median 3.3 years), the cancer incidence rate in the MAFLD group was higher than that in the non-MAFLD group [473.5 vs. 255.1 per 100,000 person-years; incidence rate ratio 1.86; 95% confidence interval (CI) 1.57–2.19]. After adjustment for age, gender, smoking status, and alcohol status, MAFLD was moderately associated with cancers of the female reproductive system/organs (labium, uterus, cervix, and ovary) [hazard ratio (HR) 2.24; 95% CI 1.09–4.60], thyroid (HR 3.64; 95% CI 1.82–7.30), and bladder (HR 4.19; 95% CI 1.15–15.27) in the total study cohort.
Conclusion: MAFLD was associated with the development of cancers of the female reproductive system/organs (labium, uterus, cervix, and ovary), thyroid, and bladder in the total study cohort.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the main cause of chronic liver disease, with a prevalence of 25.0% worldwide (1) and 29.2% in China (1, 2). The prevalence rates of obesity and diabetes are on the rise. As a result, the prevalence of NAFLD will continue to increase, making NAFLD a growing public health problem (3). NAFLD is a risk factor for morbidity and mortality in liver-related diseases and can cause extrahepatic complications such as metabolic syndrome, type 2 diabetes, cardiovascular disease, and chronic kidney disease (4–7). Previous studies indicated that NAFLD can increase the risk of both intrahepatic and extrahepatic cancers, such as stomach, colorectal, lung, thyroid, and breast (8–11).
In 2020, an international panel of liver disease experts proposed changing the name of NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) and explained the clinical application of this definition in detail (12). The new definition proposes to remove alcohol consumption or the presence of other liver diseases from consideration in the diagnosis of MAFLD. Patients must be diagnosed with hepatic steatosis in addition to being overweight or obese, have type 2 diabetes, or have two or more metabolic risk factors. Therefore, patients with MAFLD may be diagnosed with other chronic liver diseases (12). The definitions of MAFLD and NAFLD are thus not equivalent. In a cohort study from the UK Biobank, which recruited over 500,000 participants aged 40-69 years, the results showed that MAFLD increased cancer risk by approximately 7.0% overall and 59.0% for liver cancer in particular (13). Another cohort study, which also used data from the UK Biobank to explore the association between MAFLD and 24 specific cancers, showed that MAFLD was significantly associated with 10 of the 24 cancers examined, including cancers of the uterus, gallbladder, liver, kidney, thyroid, esophagus, pancreas, bladder, breast, colorectum, and anus (14). Although studies have shown that MAFLD is associated with cancer risk, individual associations with different sites of occurrence have not been conclusively established. The aims of the current study were to assess the incidences of cancer at different sites in patients with MAFLD and compare them with those in a control population.
Materials and methods
Study population and study design
The current historical cohort study included all inpatients diagnosed with MAFLD at the People’s Hospital of Guangxi Zhuang Autonomous Region, China, from January 2013 to October 2021 (registration site http://www.chictr.org.cn/index.aspx; registration number ChiCTR2200058543). Briefly, the study used an advanced medical data management system to manage patients and connected and indexed all diagnosis and treatment records held at the hospital. All the medical information assessed was obtained from the electronic database, including demographic characteristics, medical diagnostic codes, surgical codes, drug prescriptions, and death information. When a patient comes to the clinic, the information is automatically integrated into this system.
In the present study, 251,825 patients undergoing liver ultrasound during hospitalization were selected. Those who had a follow-up time at our hospital of <1 year (n = 142,309), lacked information on body mass index (BMI) (n = 5,662), or whose cancer events occurred within 1 year of follow-up (n = 100) were excluded. Patients with a previous history of discharge diagnosis of liver disease, viral hepatitis, kidney disease, malignant tumor, organ transplantation, or radiation therapy were also excluded (n = 38,701). The inclusion and exclusion details are shown in Supplementary Table 1. Ultimately, 47,801 patients were analyzed: 16,093 in the MAFLD group and 31,708 in the non-MAFLD group (Supplementary Figure 1). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki (6th revision, 2008) and was approved by the Ethics Committees of the People’s Hospital of Guangxi Zhuang Autonomous Region, China. Individual informed consent was not obtained in this study because we analyzed anonymized electronic medical records data as aggregates, with no individual health data available.
Ascertainment of MAFLD
MAFLD was diagnosed based on abdominal ultrasonography evidence of fatty liver in accordance with the Asia-Pacific Guidelines (15). MAFLD can be diagnosed if fatty liver is diagnosed via abdominal ultrasonography and one of the following three conditions exists: 1) overweight or obesity (BMI ≥ 23), 2) diagnosed type 2 diabetes mellitus, or 3) BMI <23 and at least two metabolic risk abnormalities including a) waist circumference ≥90/80 cm, b) blood pressure ≥130/85 mmHg or specific drug treatment, c) plasma triglycerides ≥150 mg/dl (≥1.70 mmol/L) or specific drug treatment, d) plasma HDL-cholesterol <40 mg/dl (<1.0 mmol/L) for men and <50 mg/dl (<1.3 mmol/L) for women or specific drug treatment, e) prediabetes, f) homeostasis model assessment of insulin resistance score ≥2.5 (not included in this study), and (g) plasma high-sensitivity C-reactive protein level >2 mg/L (12) (not included in this study).
Cancer assessment and covariates
All participants were followed prospectively until death, last medical visit, or December 2022. The International Classification of Diseases–Tenth Revision (ICD-10) codes were used to identify incident cancers (C00–C99). Coding details are shown in Supplementary Table 2. In order to minimize spurious diagnoses, all complete medical records of each individual with C00–C99 codes were reviewed by a physician. The covariates assessed in the study included BMI, diabetes mellitus, hypertension, dyslipidemia, and alcohol and smoking status at baseline. BMI was stratified into normal weight (<23) and overweight and obese (≥23). Hypertension was defined as a history of hypertension, the use of antihypertensive medications, or a systolic blood pressure ≥140 mmHg and a diastolic blood pressure ≥90 mmHg. Diabetes was defined as a history of diabetes, taking hypoglycemic drugs, or fasting blood glucose ≥7.0 mmol/L, hBA1c ≥6.5. Dyslipidemia was defined as the use of lipid-lowering agents, LDL-cholesterol >100 mg/dl, or triglycerides >150 mg/dl. All laboratory tests were conducted at the People’s Hospital of Guangxi Zhuang Autonomous Region, China.
Statistical analysis
Continuous variable baseline data from MAFLD patients and non-MAFLD patients were compared using the Wilcoxon test, and categorical baseline data were compared using the card method. The incidence rates of cancers were calculated by dividing the total number of newly diagnosed cancers by the total number of person-years contributed by people at risk during the follow-up time. Poisson regression modeling was used to estimate the incidence rate ratio (IRR) of cancer progression in the two groups. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationships between MAFLD and cancer incidence. Covariate selection was based on a backward selection procedure and other potential confounders identified in the literature (14). In the multivariable analyses, age, gender, smoking status, and alcohol status were adjusted. Stratified analysis by gender was conducted because cancer risks differed between the genders. All reported p-values are two-tailed, and p <0.05 was considered statistically significant. SPSS 18 software (IBM Corp., Armonk, NY, USA) and R software (version 3.3.2, http://www.r-project.org) were used for the statistical analyses.
Results
Baseline characteristics of the study participants
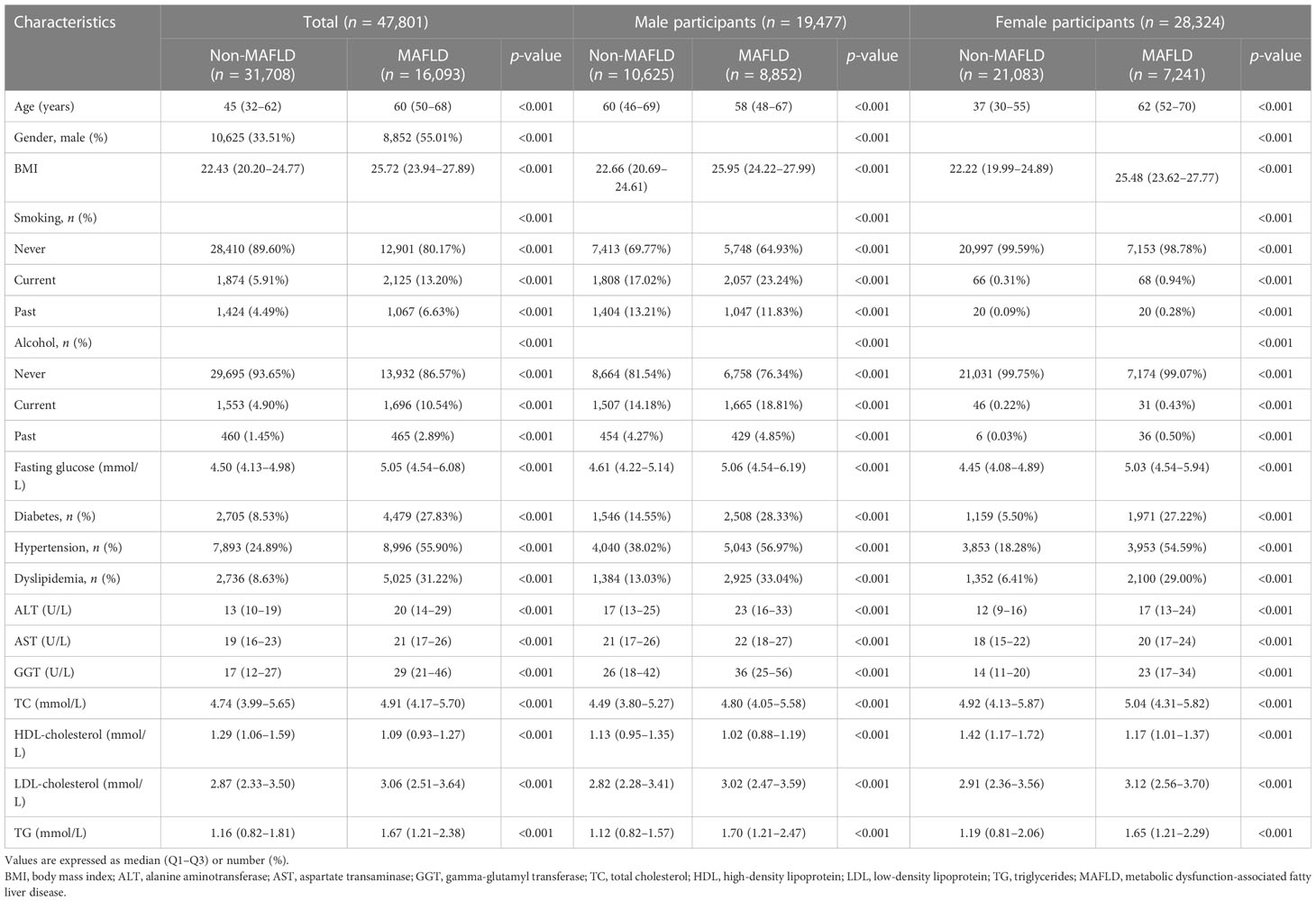
The study included 47,801 participants after the exclusion and inclusion criteria were applied. The prevalence of MAFLD was 33.7% (n = 16,093). Participants in the MAFLD group were older, more likely to be male, more likely to smoke, more likely to be diabetic, and more likely to be hypertensive and/or have dyslipidemia. The levels of fasting glucose, total cholesterol, serum alanine aminotransferase, and gamma-glutamyl transferase were higher in the participants with MAFLD. The baseline characteristics of the study participants are summarized in Table 1.
Incidence rates of cancer
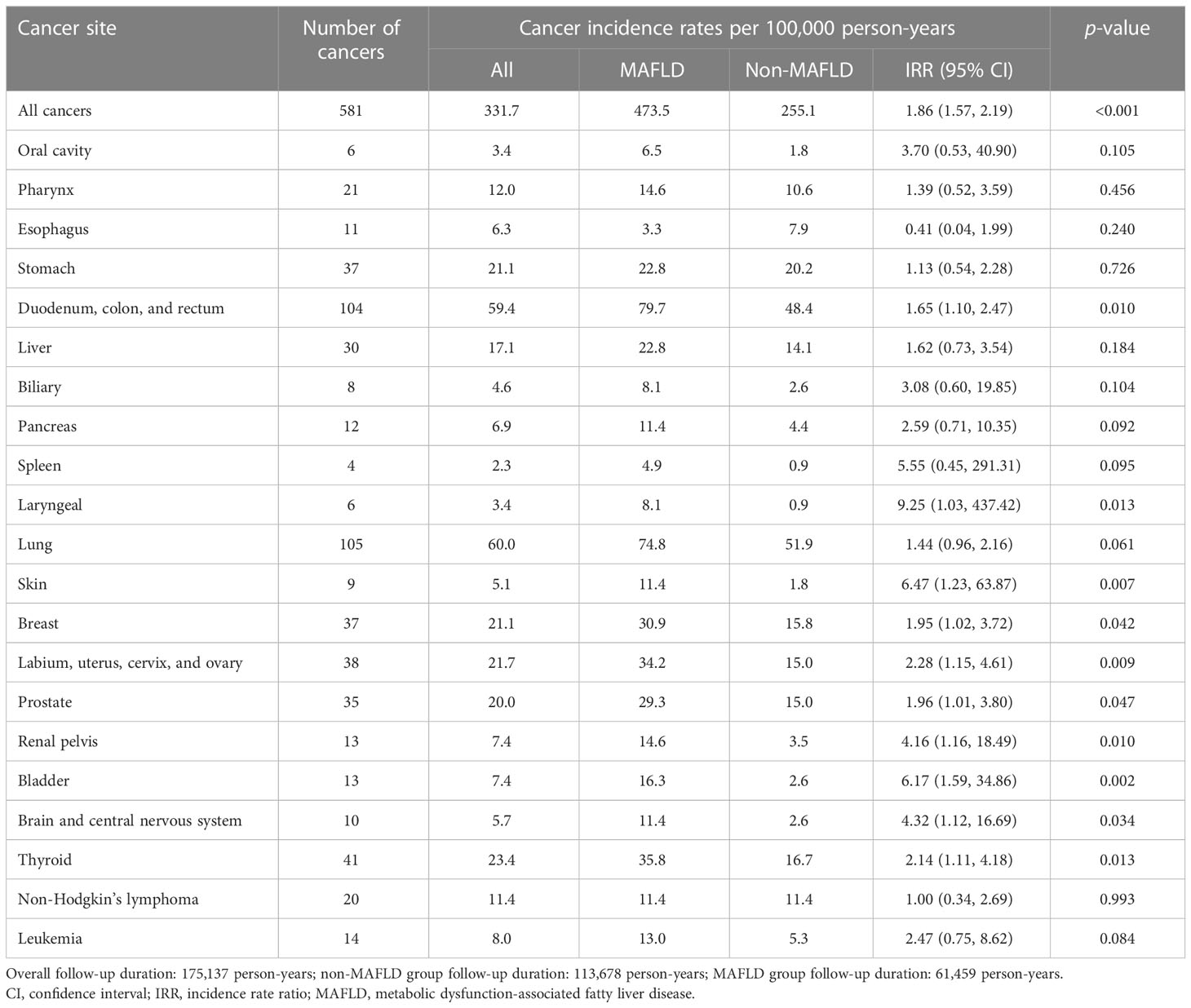
The follow-up period (median 3.3 years, interquartile range 2.0–5.1 years) included 175,137 person-years of follow-up. Malignancies were newly diagnosed in 291 participants (1.81%) with MAFLD and 290 participants (0.91%) without MAFLD. In the MAFLD group, the overall cancer incidence rate was 473.5 (95% CI 420.6–531.1) per 100,000 person-years, which was significantly higher than that in the non-MAFLD group which was 255.1 (95% CI 226.6–286.2) per 100,000 person-years (IRR 1.86, 95% CI 1.57–2.19) (Table 2).
In the MAFLD group, the incidence rates of nine specific cancers were significantly higher than those in the non-MAFLD group, including cancers of the duodenum, colon, and rectum (IRR 1.65, 95% CI 1.10–2.47); breast (IRR 1.95, 95% CI 1.02–3.72); female reproductive system/organs (labium, uterus, cervix, and ovary) (IRR 2.28, 95% CI 1.15–4.61); prostate (IRR 1.96, 95% CI 1.01–3.80); thyroid (IRR 2.14, 95% CI 1.11–4.18); renal pelvis (IRR 4.16, 95% CI 1.16–18.49); bladder (IRR 6.17, 95% CI 1.59–34.86); and brain and central nervous system (IRR 4.32, 95% CI 1.12–16.69) (Table 2).
With respect to gender, the incidence rate of thyroid cancer in the male participants in the MAFLD group was significantly higher than that in the non-MAFLD group (IRR 3.89, 95% CI 1.07–14.14), but in the female participants, the incidence rates of thyroid cancer did not differ significantly in the two groups. In the female participants, the MAFLD group had significantly higher incidence rates of cancers of the stomach (IRR 3.13, 95% CI 1.05–9.30), lung (IRR 3.01, 95% CI 1.54–5.91), skin (IRR 10.72, 95% CI 1.20–95.88), breast (IRR 2.99, 95% CI 1.56–5.76), and female reproductive system/organs (labium, uterus, cervix, and ovary) (IRR 3.31, 95% CI 1.75–6.27) (Supplementary Table 3). The cumulative incidence of all cancers is shown in Supplementary Figure 2.
Association between MAFLD and cancer risk
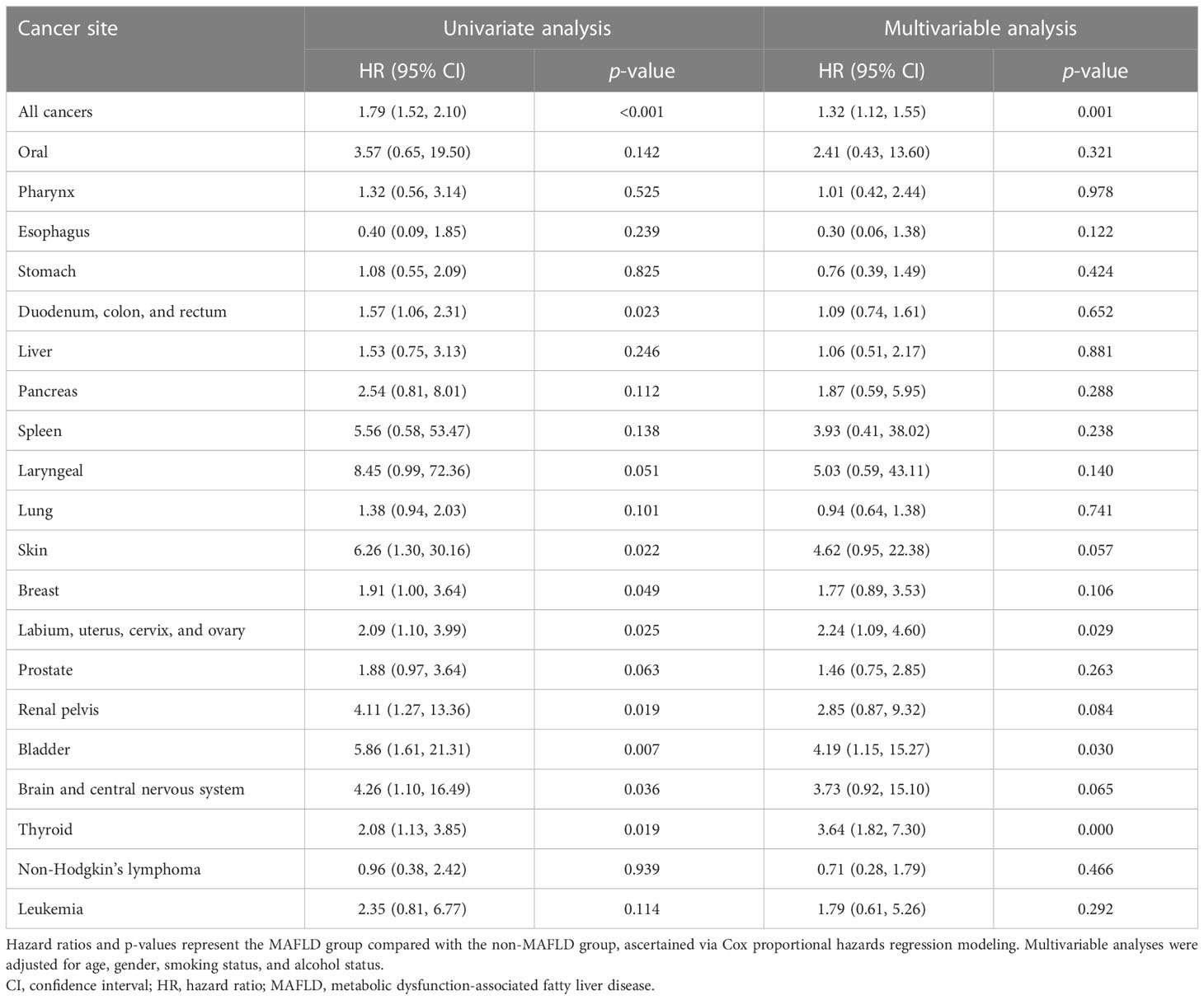
In the univariate analysis, participants in the MAFLD group had a higher risk of all cancers than those in the non-MAFLD group (HR 1.79, 95% CI 1.52–2.10), and the result was consistent after adjustment for age, gender, smoking status, and alcohol status (HR 1.35, 95% CI 1.15–1.60) (Table 3). At different cancer sites, MAFLD was significantly associated with cancers of the female reproductive organ (labium, uterus, cervix, and ovary) (HR 2.24, 95% CI 1.09–4.60), thyroid (HR 3.64, 95% CI 1.82–7.30), and bladder (HR 4.19, 95% CI 1.15–15.27) in the total study cohort. In the subgroup analysis by gender, the results were consistent, with the exception of bladder cancer (Supplementary Table 4).
Discussion
In this retrospective cohort study, the relationships between MAFLD and different cancer types were analyzed. MAFLD was associated with higher incidence rates of cancers, particularly extrahepatic-specific cancers of the bladder, thyroid, and female reproductive system/organs (labium, uterus, cervix, and ovary). As MAFLD is a new definition, cohort studies on the relationship between MAFLD and cancer are scarce, with only two cohort studies from the UK Biobank database. The UK Biobank is a large-scale, population-based prospective cohort study that recruited over 0.5 million participants aged 40-69 years in 2006-2010 and combined extensive measurements of baseline data and genotype data with linked national medical records for longitudinal follow-up (13). One study selected 423,252 participants who were diagnosed with MAFLD. During the median follow-up of 8.2 years, compared with participants without MAFLD, those with MAFLD had a multivariate-adjusted HR of 1.07 (95% CI 1.05–1.10) for all cancers and 1.59 (95% CI 1.28, 1.98) for liver cancer (13). The other study included 352,911 participants, with 37.2% diagnosed as MAFLD, and during the median follow-up of 8.2 years, compared with non-MAFLD, MAFLD was significantly associated with 10 of the 24 examined cancers, including corpus uteri (HR = 2.36, 95% CI 1.99–2.80), gallbladder (2.20, 1.14–4.23), liver (1.81, 1.43–2.28), kidney (1.77, 1.49–2.11), thyroid (1.69, 1.20–2.38), esophagus (1.48, 1.25–1.76), pancreas (1.31, 1.10–1.56), bladder (1.26, 1.11–1.43), breast (1.19, 1.11–1.27), and colorectal and anus cancers (1.14, 1.06–1.23) (14). The results of this study are mostly consistent with the two UK Biobank cohort studies, except for liver cancer. In this present study, there was no significant relationship between MAFLD and liver cancer. The discrepancy may be related to differences in study populations in the two studies. The UK Biobank study recruited a representative sample of the general population based on ethnic and sociodemographic data, with an equal proportion of men and women and a balanced population between the ages of 40 and 69 years over a 5-year period (16, 17), whereas our study data were derived from a hospital patient population.
MAFLD is defined differently from NAFLD, which excludes liver-related diseases. The exclusion of other causes such as drug-induced hepatitis, viral hepatitis, alcoholism, and other liver diseases is not a requirement for MAFLD (18). The definition of MAFLD covers the systemic risk associated with fatty liver disease, whereas NAFLD focuses on liver-related factors. Therefore, given the strong association between MAFLD and the established risk factors for these diseases, it is not surprising that MAFLD is associated with an increased risk of both intrahepatic and extrahepatic events (13). Currently, the definition of MAFLD is somewhat controversial in the field of liver disease (19, 20). A meta-analysis showed that MAFLD was not a replacement for NAFLD and that there were significant differences in the prevalence and risk factors between them (21). MAFLD was proposed as an alternative definition in an effort to improve people’s awareness, especially primary care doctors, and to better summarize metabolic dysfunction (22).
In the current study, MAFLD was moderately associated with cancers of the female reproductive system/organs (labium, uterus, cervix, and ovary), bladder, and thyroid in the total study cohort. MAFLD increases the risks of obesity, diabetes, and hypertension, in turn increasing the burden of metabolism-related diseases (21). In the present study, the MAFLD group had a higher median BMI than the non-MAFLD group, and the prevalence rates of diabetes, hypertension, and dyslipidemia were also higher in the MAFLD group. Studies on hypertension and cancer indicate that patients with hypertension have a significantly higher risk of cancer than non-hypertensive patients, particularly with respect to colorectal cancer and breast cancer (23–25). In a two-sample Mendelian randomization study, there were causal detrimental effects of type 2 diabetes on cancer of the uterus, kidney, pancreas, and lung (26). Dyslipidemia and obesity are evidently risk factors for most cancer types (27, 28). This may explain why the cancer incidence rate was significantly higher in the MAFLD group in the current study. The precise pathophysiological mechanisms that link MAFLD and cancers are unknown, and further studies are needed to elucidate these links. Moreover, there may be significant implications for cancer screening and surveillance strategies in MAFLD patients given the growing number of patients with MAFLD.
The strength of the present study is that it is the first large cohort study to explore the association between MAFLD and cancer in western China. However, there are some limitations to this study. First, the participants of this study were derived from a hospital patient population, so the potential selection bias was unavoidable. Second, fatty liver was diagnosed using abdominal ultrasound rather than liver biopsy. Ultrasound has limited sensitivity, and it could result in steatosis with less than 20% of fatty liver individuals that cannot be detected (29, 30). However, due to its invasive nature, liver biopsy is not feasible in large population-based studies. Third, the follow-up time of this study was short (median 3.3. years), leading to outcome events that could not be observed. In addition, the small numbers of some site-specific cancers may have resulted in the instability of the results. Thus, a longer follow-up is required to verify the results. Fourth, the parameters used to assess the diagnosis of MAFLD, such as waist circumference, insulin, oral glucose tolerance test, and hsCRP, are missing in this study. This may have resulted in some MAFLD cases being missed and MAFLD may have misclassification in some participants. So, we will construct a long-term follow-up of a prospective cohort study that has complete baseline data to avoid this bias. Fifth, cancer cases were identified by ICD-10 codes, which may be associated with misclassification or underreporting. Sixth, the pathological severity of hepatic steatosis was not collected in this study. Lastly, metabolic abnormalities may change with the state of the participants, so the metabolic state at baseline may not accurately reflect the true metabolism of the individual.
Conclusion
In this retrospective cohort study, MAFLD was associated with an increased risk of cancer. The study suggests that multidisciplinary assessment is required and attention should be paid to the development of malignancy in patients with MAFLD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was performed in accordance with the principles outlined in the Declaration of Helsinki and approved by the People’s Hospital of Guangxi Zhuang Autonomous Region Ethics Committee and the Institutional Review Board. Individual informed consent was not obtained in this study because we analyzed anonymized electronic medical records data as aggregates, with no individual health data available.
Author contributions
SW and JY conceived and designed the study and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Major Project of Science and Technology of Guangxi Zhuang Autonomous Region (grant number Guike-AA22096018) and the National Natural Science Foundation of China (grant number 82160589).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.985858/full#supplementary-material
References
1. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. (2019) 69(6):2672–82. doi: 10.1002/hep.30251
2. Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: A systematic review and meta-analysis. Hepatology. (2019) 70(4):1119–33. doi: 10.1002/hep.30702
3. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, united kingdom, and united states for the period 2016-2030. J Hepatol (2018) 69(4):896–904. doi: 10.1016/j.jhep.2018.05.036
4. Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol (2009) 104(4):861–7. doi: 10.1038/ajg.2009.67
5. Han JE, Shin HB, Ahn YH, Cho HJ, Cheong JY, Park B, et al. Relationship between the dynamics of non-alcoholic fatty liver disease and incident diabetes mellitus. Sci Rep (2022) 12(1):2538. doi: 10.1038/s41598-022-06205-8
6. Kouvari M, Chrysohoou C, Skoumas J, Pitsavos C, Panagiotakos DB, Mantzoros CS. Investigators as. the presence of NAFLD influences the transition of metabolically healthy to metabolically unhealthy obesity and the ten-year cardiovascular disease risk: A population-based cohort study. Metabolism. (2022) 128:154893. doi: 10.1016/j.metabol.2021.154893
7. Zhang M, Lin S, Wang MF, Huang JF, Liu SY, Wu SM, et al. Association between NAFLD and risk of prevalent chronic kidney disease: why there is a difference between east and west? BMC Gastroenterol (2020) 20(1):139. doi: 10.1186/s12876-020-01278-z
8. Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut (2021) 71(4):778–88. doi: 10.1136/gutjnl-2021-324191
9. Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - a longitudinal cohort study. J Hepatol (2019) 71(6):1229–36. doi: 10.1016/j.jhep.2019.08.018
10. Simon TG, Roelstraete B, Sharma R, Khalili H, Hagstrom H, Ludvigsson JF. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: A population-based cohort study. Hepatology. (2021) 74(5):2410–23. doi: 10.1002/hep.31845
11. Wang Z, Zhao X, Chen S, Wang Y, Cao L, Liao W, et al. Associations between nonalcoholic fatty liver disease and cancers in a Large cohort in China. Clin Gastroenterol Hepatol (2021) 19(4):788–796e4. doi: 10.1016/j.cgh.2020.05.009
12. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.03.039
13. Liu Z, Suo C, Shi O, Lin C, Zhao R, Yuan H, et al. The health impact of MAFLD, a novel disease cluster of NAFLD, is amplified by the integrated effect of fatty liver disease-related genetic variants. Clin Gastroenterol Hepatol (2022) 20(4):e855–75. doi: 10.1016/j.cgh.2020.12.033
14. Liu Z, Lin C, Suo C, Zhao R, Jin L, Zhang T, et al. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metabolism. (2022) 127:154955. doi: 10.1016/j.metabol.2021.154955
15. Farrell GC, Chitturi S, Lau GK, Sollano JD. Asia-Pacific working party on n. guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-pacific region: executive summary. J Gastroenterol Hepatol (2007) 22(6):775–7. doi: 10.1111/j.1440-1746.2007.05002.x
16. Ollier W, Sprosen T, Peakman T. UK Biobank: from concept to reality. Pharmacogenomics. (2005) 6(6):639–46. doi: 10.2217/14622416.6.6.639
17. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
18. Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, et al. Metabolic associated fatty liver disease (MAFLD) increases the risk of systemic complications and mortality. a meta-analysis and systematic review of 12,620,736 individuals. Endocr Pract (2022). 28(7):667–72. doi: 10.1016/j.eprac.2022.03.016
19. Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: Implications of a premature change in terminology. Hepatology. (2021) 73(3):1194–8. doi: 10.1002/hep.31420
20. Spearman CW, Desalegn H, Ocama P, Awuku YA, Ojo O, Elsahhar M, et al. The sub-Saharan Africa position statement on the redefinition of fatty liver disease: From NAFLD to MAFLD. J Hepatol (2021) 74(5):1256–8. doi: 10.1016/j.jhep.2021.01.015
21. Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol (2021) 74(5):1254–6. doi: 10.1016/j.cgh.2021.11.038
22. Fouad Y, Gomaa A, Semida N, Ghany WA, Attia D. Change from NAFLD to MAFLD increases the awareness of fatty liver disease in primary care physicians and specialists. J Hepatol (2021) 74(5):1254–6. doi: 10.1016/j.jhep.2020.12.035
23. Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep (2017) 7:44877. doi: 10.1038/srep44877
24. Sionakidis A, McCallum L, Padmanabhan S. Unravelling the tangled web of hypertension and cancer. Clin Sci (Lond). (2021) 135(13):1609–25. doi: 10.1042/CS20200307
25. Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. (2021) 70(6):1147–54. doi: 10.1136/gutjnl-2020-321661
26. Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is type 2 diabetes causally associated with cancer risk? evidence from a two-sample mendelian randomization study. Diabetes. (2020) 69(7):1588–96. doi: 10.2337/db20-0084
27. Parra-Soto S, Cowley ES, Rezende LFM, Ferreccio C, Mathers JC, Pell JP, et al. Associations of six adiposity-related markers with incidence and mortality from 24 cancers-findings from the UK biobank prospective cohort study. BMC Med (2021) 19(1):7. doi: 10.1186/s12916-020-01848-8
28. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. . BMJ (2015) 350:g7607. doi: 10.1136/bmj.g7607
29. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol (2009) 51(6):1061–7. doi: 10.1016/j.jhep.2009.09.001
Keywords: MAFLD, cancer, metabolic dysfunction, fatty liver disease, incidence rate
Citation: Wei S, Hao Y, Dong X, Huang J, Huang K, Xie Y, Liu H, Wei C, Xu J, Huang W, Dong L and Yang J (2023) The relationship between metabolic dysfunction-associated fatty liver disease and the incidence rate of extrahepatic cancer. Front. Endocrinol. 14:985858. doi: 10.3389/fendo.2023.985858
Received: 04 July 2022; Accepted: 08 February 2023;
Published: 20 February 2023.
Edited by:
Amelia Kekeletso Ranotsi, Maluti Adventist College, LesothoReviewed by:
Kristin Morrill, University of Arizona, United StatesXueli Zhang, Shanghai Cancer Institute, China
Jun Ma, Fudan University, China
Copyright © 2023 Wei, Hao, Dong, Huang, Huang, Xie, Liu, Wei, Xu, Huang, Dong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianrong Yang, Z3h5YW5namlhbnJvbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Suosu Wei
Suosu Wei Yanrong Hao2†
Yanrong Hao2† Xiaofeng Dong
Xiaofeng Dong Jianrong Yang
Jianrong Yang