- 1Department of Pediatrics, Boston Children’s Hospital and Harvard Medical School, Boston, MA, United States
- 2Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
- 3Center for Depression, Anxiety and Stress Research, McLean Hospital, Belmont, MA, United States
- 4Department of Psychiatry, Boston Children’s Hospital and Harvard Medical School, Boston, MA, United States
- 5Department of Sports Medicine, Harvard Medical School, Boston, MA, United States
- 6Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, MA, United States
- 7Division of Pediatric Endocrinology, Massachusetts General Hospital for Children and Harvard Medical School, Boston, MA, United States
Objective: Studies in estrogen deficiency states such as primary ovarian insufficiency and Turner syndrome suggest that estrogen status may be an important modulator of mood and emotions. In this study we compared depressive and anxiety symptoms between adolescent and young adult female oligo-amenorrheic athletes (AA) and eumenorrheic females (EM), and explored structural, and functional changes in related brain areas during reward processing, a behavioral construct that is altered in depression and anxiety.
Methods: We included (i) 24 AA participating in ≥4 hours/week of aerobic exercise or running ≥20 miles/week for ≥6 months in the preceding year, with lack of menstrual cycles for ≥3 months within at least 6 preceding months of oligo-amenorrhea, OR in premenarchal girls, absence of menses at >15 years), and (ii) 27 EM aged 14-25 years. Participants completed the Beck Depression Inventory-II (BDI-II), State and Trait Anxiety Inventory (STAI), and Mood and Anxiety Symptoms Questionnaire (MASQ). Structural MRI and brain activation during a functional MRI (fMRI) task that probes reward and punishment processing was examined in a subset of 10 AA and 23 EM.
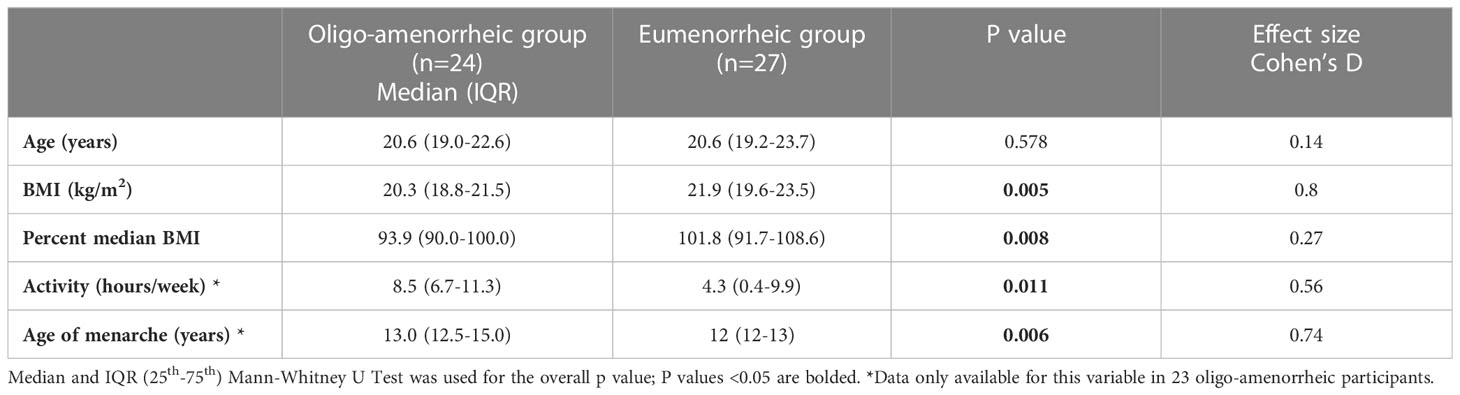
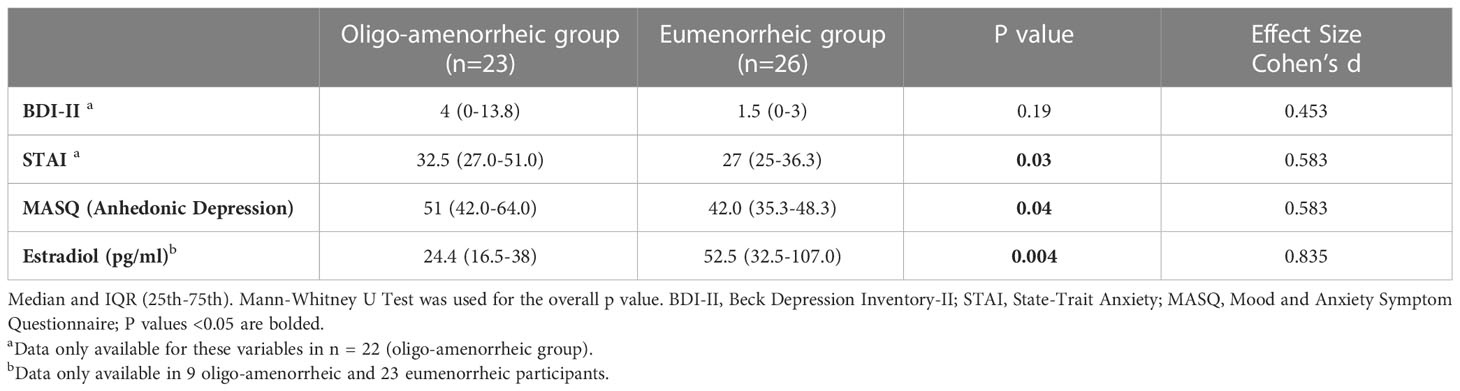
Results: Median (IQR) age and BMI of AA and EM groups were 20.6 (19.0-22.6) vs. 20.6 (19.2-23.7) years, p=0.6 and v 20.3 (18.8-21.5) vs. 21.9 (19.6-23.5) kg/m2, p=0.005, respectively. While groups did not differ for BDI-II scores, AA had higher anhedonic depression MASQ scores (p=0.04), and STAI (p=0.03) scores vs. EM. In the fMRI subset, AA had higher caudate volumes vs. EM [F(1, 29)=9.930, p=0.004]. Lower activation observed in the right caudate during reward anticipation in AA compared with EM (p=0.036) suggests blunted reward processing in the striatum in estrogen deficient states.
Conclusion: Athletes with amenorrhea had higher depressive and anxiety symptomatology compared to eumenorrheic young women. Exploratory analyses demonstrated increased caudate volumes and decreased caudate activation during reward processing in athletes with amenorrhea suggesting that estrogen may play a role in reward processing.
1 Introduction
Approximately 11.3% of adolescents in the United States between ages 12-17 years have one episode of major depression in the past year (1) and about 31.9% develop anxiety disorders between the ages of 13-18 years with a higher prevalence in females (2). These disorders are a major cause of disability among adolescents and young adults, and a significant risk factor for suicide, which is the third leading cause of death for adolescents aged 15-19 years (3). Understanding the factors that impact mood and anxiety in adolescents may provide novel direction regarding the management of these disorders.
Emerging evidence indicates that estrogen may play a significant role in modulating mood. In particular, estrogen deficiency states such as menopause and Turner syndrome have been associated with a higher risk of depression and anxiety compared to the general population (4, 5). Exercise without appropriate nutritional fueling can result in low energy availability, even in normal-weight athletes; this disrupts the hypothalamic-pituitary-gonadal (HPG) axis, leading to functional hypothalamic amenorrhea (FHA) and low estrogen levels (6). While numerous reports have demonstrated a positive effect of exercise on depression and anxiety (7, 8), the role of estrogen deficiency resulting from physical activity in young athletes with amenorrhea on mood and anxiety has not been studied in detail. To our knowledge, only one study has evaluated mood issues in athletes with respect to their menstrual status and reported a trend for a higher prevalence of bipolar disorders or major depressive disorders in 23% of runners who had absent menses (amenorrhea) versus 0% runners with regular menses (eumenorrhea) (9). Similarly, an increased prevalence of depressed mood has been reported in FHA similar to that in organic amenorrhea, suggesting that estrogen deficiency might be a common factor in both groups driving mood issues (10, 11). Of relevance, we recently reported improvement of trait anxiety with estrogen replacement in girls with FHA (12). However, studies evaluating depression and anxiety in athletes who have estrogen deficiency and are amenorrheic are limited.
One of the hallmarks of mood disorders is abnormal reward and punishment processing that is often associated with structural and functional changes in reward-related brain regions, most prominently the striatum, including the caudate, putamen and the nucleus accumbens (NAc) (13–15). Of note, the ability to anticipate reward is often blunted in depression and is a core behavioral construct that is frequently tested in functional neuroimaging studies examining depression. In general, these studies have shown reduced brain activation in the caudate while anticipating and receiving a reward among adolescents with depression (16). The anticipatory response to punishment is less well known in depression. However, induced anxiety has been reported to increase striatal activation to both rewards and punishment, suggesting anxiety symptoms may impact incentive processing generally (17). Based on these findings we designed a study to compare depressive and anxiety symptoms in adolescent and young adult athletes with irregular or absent menses (oligo-amenorrhea) versus athletes and non-athletes with regular menses (eumenorrhea). Further, we compared a subset of these subjects with respect to structural (volumetric) features of the striatum as well as functional striatal activation using a monetary incentive paradigm that probes a behavioral construct altered in depression/anxiety. We hypothesized that estrogen deficiency would negate the beneficial effects of exercise, and that compared to eumenorrheic females (EM), female athletes with oligo-amenorrhea (AA) would have greater symptoms of depression and anxiety. Additionally, we hypothesized that oligo-amenorrheic athletes would exhibit structural changes in the striatum (caudate, putamen and NAc) and have altered neural activation during reward/punishment anticipation when compared to the EM group. This novel study is the first of its kind to evaluate the relationship of menstrual status on mood in adolescent and young adult female athletes, and underlying neural correlates.
2 Methods
2.1 Participants
Study participants were recruited by advertising in institutional recruitment forums, sports medicine treatment centers and physician practices. The study included 51 right-handed females between ages 14-25 years with BMI between the 10th-90th percentiles: 24 AA, 14 EA and 13 NA. Participants were grouped by menstrual status into two categories: one with oligo-amenorrhea (AAs only, n=24) and the other with regular menses or eumenorrhea and no medical issues, EM (EA, and NA taken together, n=27). Athletes were defined as those participating in ≥4 hours/week of aerobic exercise or running ≥20 miles/week for a period of ≥6 months in the preceding year. NA did not participate in team sports and exercised <2 hours/week. Oligo-amenorrhea was defined as lack of menstrual periods for ≥3 months within at least 6 preceding months of oligo-amenorrhea, or in premenarchal girls, absence of menses at >15 years. Exclusion criteria included (a) conditions other than excessive exercise causing loss of periods, including pregnancy, polycystic ovary syndrome and other conditions of hyperandrogenism, thyroid dysfunction (unless euthyroid on medications for at least 3 months), primary ovarian insufficiency, and hyperprolactinemia, and (b) any contraindications to estrogen use such as an increased predisposition for thromboembolism, underlying liver disease, and a personal or first-degree family history of an estrogen dependent cancer. All participants (or their parents) provided written informed consent to the protocol, which was approved by our Institutional Review Board. Participants below 18 years also provided signed assent for study participation.
2.2 Procedure
Following a screening visit, participants completed questionnaires, had a structural MRI scan and completed a monetary incentive delay task in the scanner. Twenty-three AA and 26 participants from the eumenorrheic group, EM, completed questionnaires to assess their mood. Depressive, anxiety, and anhedonic symptoms were assessed using the Beck Depression Inventory-II (BDI-II) (18), the State-Trait Anxiety Inventory (STAI) (19, 20), and the Mood and Anxiety Symptoms Questionnaire (MASQ) (21) respectively. Imaging data were obtained in 10 AA and 23 EM participants. The majority of EM participants (except for 9) were brought in during the proliferative phase of their cycle, which was determined based on their last menstrual period. Estradiol levels were available for 9 AA and 23 EM participants. Estradiol was assessed by chemiluminescence (Beckman Coulter, Fullerton, CA; sensitivity 20 pg/mL; intraassay CV 2.0-4.2%). We used menstrual status (rather than an estradiol level) as a surrogate of estrogen status given that estradiol levels vary across the menstrual cycle and are dependent on cycle phase, and they do not necessarily reflect the chronicity of estrogen exposure. Further, standard estradiol assays are not always reliable at low levels.
2.3 MRI procedure
Please see the Supplement for detailed information on MRI procedures, data quality assurance, preprocessing, and statistical analysis.
2.4 Monetary incentive delay task
The monetary incentive delay task is designed to measure neural responses to anticipation and receipt of rewards and penalties and has been demonstrated to successfully recruit the striatum in healthy individuals (22). Previous studies using this task have revealed reduced striatal activation to reward anticipation in depressed/anhedonic individuals and increased striatal activation to both reward and punishment in anxious individuals, respectively, compared with healthy adults (17, 23, 24) making it well suited for the present study. At the onset of each trial, participants were presented with a visual cue (0.5 sec) indicating the potential outcome (reward: +$; penalty: –$; no incentive: 0$). After a variable inter-stimulus interval (2.25–3.75 sec), a red target square was briefly presented (0.15 sec). After a second variable delay (2.4–3.9 sec), visual feedback (1.25 sec) based on trial outcome (reward, penalty, or no change) was displayed and a variable intertrial interval ensued (1.5-4.5 sec). Participants were instructed to respond to the square by pressing a button as quickly as possible and that speed determines the probability of success. In order to match task difficulty across participants, the 70th percentile of each participant’s reaction time during a practice session was defined as the individual’s reaction time threshold for success. Practice session was identical to the design described above except that no feedback was displayed. In the reward condition, successful trials were associated with monetary gains ($1.98 to $2.31), whereas unsuccessful trials led to no-change. In the penalty condition, successful trials were associated with no-change, whereas unsuccessful trials were associated with monetary penalties (-$1.85 to -$2.17). No-incentive trials always ended with no-change feedback. The task included five blocks of 24 trials (8 reward, 8 penalty, and 8 no-incentive trials).
2.5 Imaging data acquisition
MRI data were acquired on a 3T scanner (Siemens Medical Systems, Iselin, NJ) equipped with a 32-channel head coil. fMRI data were acquired using a T2*-weighted multiband echo-planar imaging sequence (multiband factor = 3, repetition time = 1.65 sec).
2.6 Imaging data analyses
Both structural and functional images were processed using fMRIPrep 20.2.1 (25, 26), which is based on Nipype 1.5.1 (27, 28). Detailed information is provided in the Supplement.
2.6.1 Structural data analyses
The T1-weighted (T1w) image was intensity bias-corrected and skull-stripped. Brain surfaces were reconstructed using recon-all (FreeSurfer 6.0.1). Intracranial volume (ICV) was calculated to correct for inter-individual differences in total brain size. All estimated volumes of a priori structures were exported to SPSS for statistical analyses.
2.6.2 Functional data analyses
Preprocessing: The following preprocessing steps were performed – skull-strip, susceptibility distortion correction, slice time correction, spatial normalization, spatial smoothing, and automatic removal of motion artifacts using ICA-AROMa (Prium 2015). Additional motion criteria for exclusion were applied to the fMRI data (see Supplement).
First-level Analyses: First five volumes (8.25 sec) were discarded as dummy scans from the beginning of the task and the onset times were adjusted accordingly. First-level of single-subject fMRI data from all runs were implemented using a general linear model (GLM) in SPM. For each run, the following regressors were included in the model-reward cue, penalty cue, no-incentive (neutral) cue, successful reward feedback, unsuccessful reward feedback, successful no penalty feedback, unsuccessful penalty feedback and no-change feedback (no-incentive condition). In addition, mean time-series from the cerebrospinal fluid (CSF) and white matter (WM) masks generated from fmriprep, target, errors (i.e., when the button was pressed before the target presentation) and inter-stimulus intervals (ISIs) were included as covariates of no-interest. Each event was constructed with a hemodynamic response function, modeled using a gamma function, convolved with onset times of events and stimulus duration. A high-pass temporal filter (128 sec/0.008 Hz) was also applied to the model. Contrast maps were constructed for reward anticipation (reward vs. neutral cue) averaged across runs. The analyses were focused only on reward anticipation here due to small sample size. These contrast maps were used in region of interest (ROI)-based statistical analyses to test a priori hypotheses as well as for whole-brain main effects analysis evaluating brain regions affected by the task.
2.7 Statistical analyses
Our primary hypotheses were to investigate the effect of oligo-amenorrhea on depressive and anxiety symptoms. We also explored associated neural correlates of reward and punishment processing in the striatum (specifically caudate, putamen, and nucleus accumbens (NAc)). To this end, we focused our analyses on parsing group differences between those with eumenorrhea (EA + NA) and oligo-amenorrhea (AA). All analyses were performed in SPSS (version 22).
2.7.1 Demographics and clinical questionnaires
Normality of data was determined using the Shapiro-Wilk test. Given that most data were not normally distributed, non-parametric tests (Mann-Whitney U test) were used for comparisons.
2.7.2 Structural data analyses
Striatal volumes for each subject from Freesurfer outputs were exported to SPSS for analyses. To test striatal volumes, a Group (oligo-amenorrheic/eumenorrheic) x ROI (Caudate, Putamen, NAc) x Hemisphere (Left, Right) repeated measures ANOVA was run controlling for age and Intracranial volume (ICV). Significant interactions were followed by individual post-hoc tests.
2.7.3 Functional data analyses
To investigate the neural correlates of reward and punishment anticipation and to validate the task, a one-sample t-test was conducted across all subjects. Cluster correction at p < 0.05 family-wise error (FWE) with an initial voxel forming threshold of p < 0.001 was utilized. ROI Analyses: To test a priori hypotheses that oligo-amenorrhea would affect reward-related activation in the striatum, we created left and right anatomical NAc, caudate, and putamen ROIs from the FSL Harvard-Oxford Subcortical Atlas using a 40% probability threshold. For each subject, parameter estimates from each of the ROIs were extracted from reward and penalty anticipation contrast maps and were entered into SPSS (version 22). Throughout the analyses, data were inspected for the presence of outliers. Values that exceeded three times the inter-quartile range (the difference between the third and first quartile) of mean parameter estimates were deemed to be outliers and were further investigated to identify if they were due to motion, registration error, or other sources of artifacts. If no problems could be identified and corrected, outlier data points were removed. After inspection for outliers, a repeated measures ANOVA with Group (amenorrheic/eumenorrheic as a between-subject factor and Hemisphere (Right/Left) and Valence (Cue Reward – Cue Neutral, CR_CN/Cue Punishment – Cue Neutral, CP_CN) as within-subject factors was run for each ROI to investigate effects of amenorrhea on reward and punishment anticipation.
3 Results
3.1 Demographics and clinical questionnaires
Table 1 summarizes participant characteristics in the two groups. AA had lower BMI and were significantly older at menarche compared with the eumenorrhea group (t=-2.85, p=0.005; t=2.65, p=0.006, respectively). As expected, AA had higher anxiety (measured by STAI Trait scores, t=2.01, p=0.03) and depressive symptomatology (higher anhedonic depression measured by MASQ scores, t=2.04, p=0.04) and lower estradiol levels (t=-2.02, p=0.004) compared with those with eumenorrhea (see Table 2).
3.2 Structural analyses
Striatal ROI: Group (oligo-amenorrhea, eumenorrhea) x ROI (Caudate, Putamen, NAc) x Hemisphere (Left, Right) repeated measures ANOVA controlling for ICV and age revealed a significant ROI x Group [F(1,2)=4.6, p=0.014, η2=0.14] and a main effect of Group [F(1,29)=5.49, p=0.026, η2=0.16], but no effects of ROI or Hemisphere. Further post-hoc analyses adjusted for Bonferroni correction revealed that AA had higher caudate volumes compared to the eumenorrheic group [F(1, 29)=9.930, p=0.004, η2=0.26]. However, no significant differences were observed for putamen [F(1, 29)=0.28, p>0.5, η2=0.009] or NAc volumes (F(1, 29)=2.81, p=0.11, η2=0.09; see Figure 1].
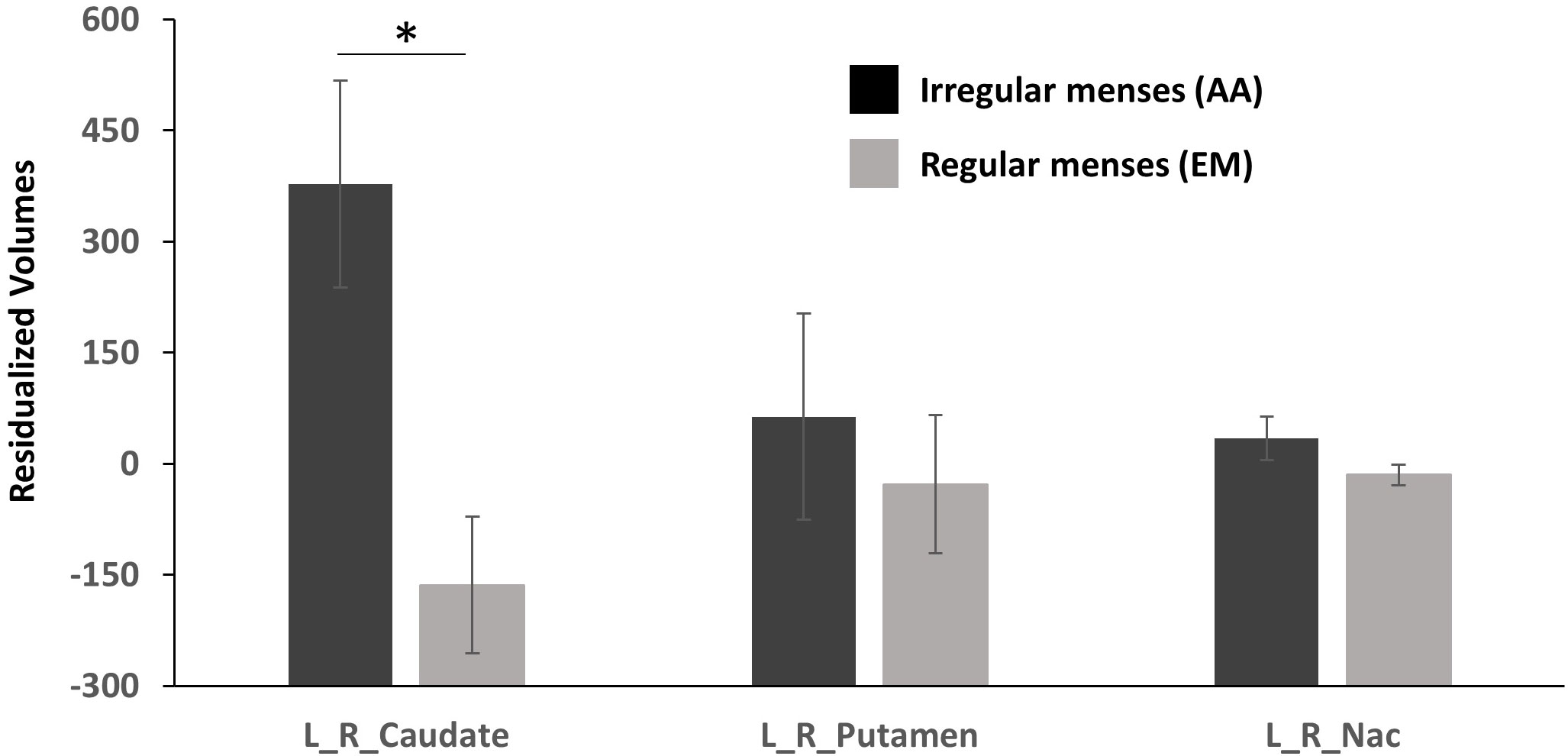
Figure 1 Residualized volumetric (corrected for age and ICV) differences in the caudate, putamen, and nucleus accumbens (NAc) between participants with oligo-amenorrhea and eumenorrhea. Error bars indicate standard error. *p < 0.05.
3.3 Functional monetary incentive delay analyses
Two participants (two EA) were excluded as they had >10% of trials classified as motion outliers based on motion criteria mentioned in the Data Quality section in the Supplement. Whole brain analysis of reward and punishment anticipation across all subjects was conducted to validate the task. Consistent with other studies, significant activations were observed in the bilateral striatum, mid-cingulate and orbitofrontal cortex (see Supplement Figures S1, S2 and Tables S1, S2).
3.3.1 Caudate
Group (oligo-amenorrhea, eumenorrhea) x Hemisphere (Left, Right) x Valence (CR_CN/CP_CN) repeated measures ANOVA in the caudate revealed a significant Group x Hemisphere effect [F(1,29)=5.61, p=0.025, η2=0.16). Post-hoc analyses adjusted for Bonferroni correction revealed that this effect was mainly driven by differences in the right caudate, with lower activation observed in the AA group (p=0.036, η2=0.14) during both reward and punishment anticipation. No significant differences were observed in the left caudate (p=0.29, η2=0.04; Figure 2).
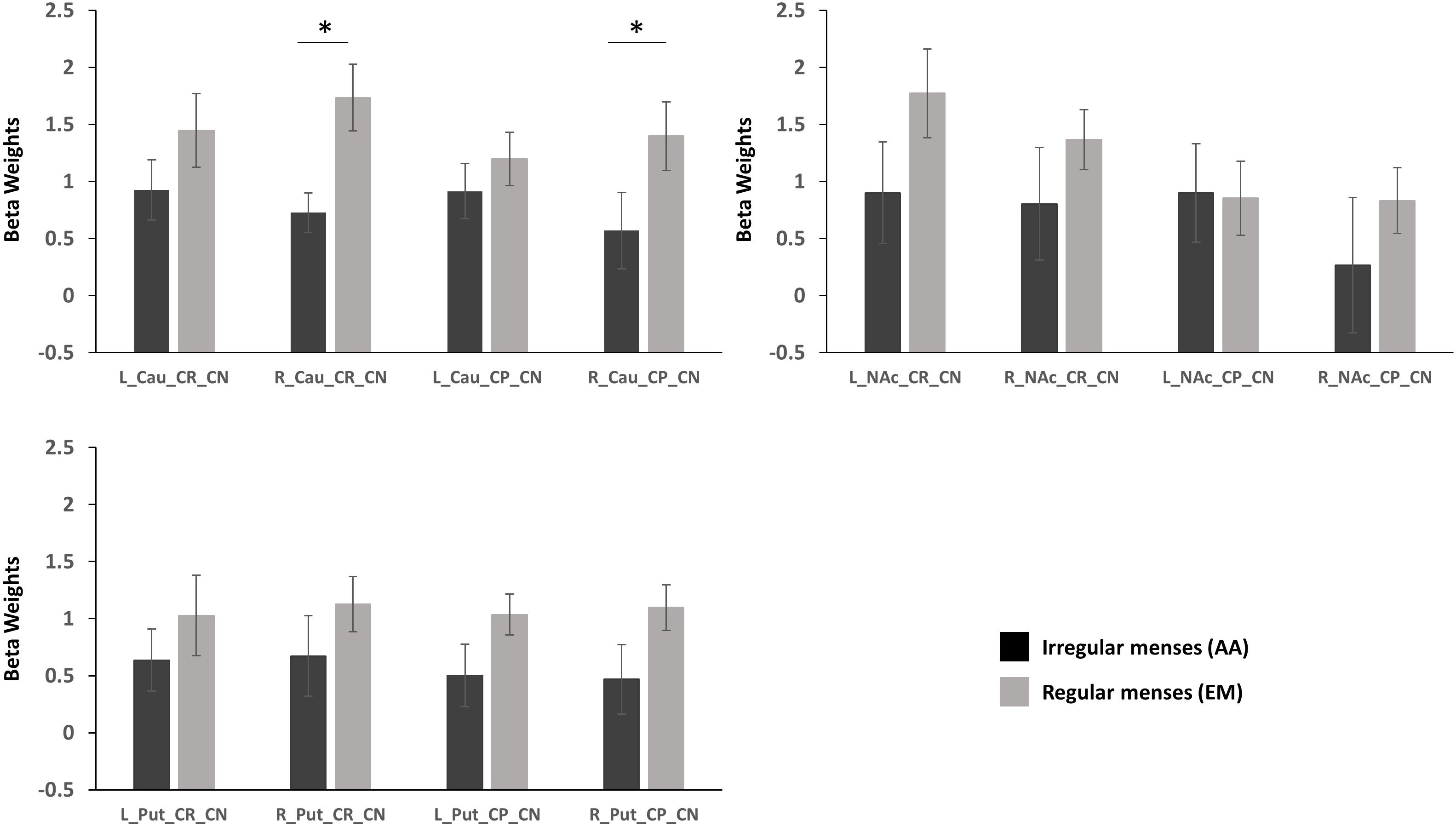
Figure 2 Standardized parameter estimates extracted from functional ROIs during reward anticipation (Cue Reward – Cue Neutral, CR_CN) and punishment activation (Cue Punishment – Cue Neutral, CP_CN) in the caudate (Cau), putamen (Put), and nucleus accumbens (NAc) in participants with oligo-amenorrhea and eumenorrhea. Error bars indicate standard error. *p < 0.05.
3.3.2 Nucleus accumbens
A Group x Valence x Hemisphere interaction was observed [F(1,29)=4.7, p=0.038, η2=0.14], but further post-hoc analyses revealed no significant effects.
3.3.3 Putamen
No main effects or interactions were observed in the putamen during reward or punishment anticipation.
3.4 Sensitivity analyses
To ensure that structural and functional differences between oligo-amenorrheic and eumenorrheic individuals were not driven by depression/anxiety, physical activity, we repeated the analyses controlling for these variables.
Controlling for depression and anxiety scores: Anhedonic depression and anxiety scores data were not available for 1 oligo-amenorrheic and 1 eumenorrheic individual. The higher caudate volume and lower caudate activation to reward/punishment anticipation observed in AA compared to the EM group remained significant even after controlling for anhedonic depression/anxiety scores [Caudate volume - F(1, 25)=7.234, p=0.013, η2=0.22; Caudate functional activation – F(1, 25)=4.778, p=0.038, η2=0.16].
Controlling for physical activity (Average hours/week for the past year): Physical activity data were not available for 1 oligo-amenorrheic individual. AA had higher caudate volumes compared to the EM group after controlling for physical activity [F(1, 27)=6.060, p=0.021, η2=0.18]. However, after controlling for physical activity, the decreased activation in the right caudate during reward and punishment anticipation in AA reached significance only at a trend level [F(1,270 = 3.76, p = 0.063, η2=0.12], suggesting physical activity may play a small role in this effect.
Sensitivity analyses for age at menarche (as age at menarche was different between groups) and BMI (as a proxy for nutritional status) are provided in the Supplement.
4 Discussion
Our study adds to the sparse literature on mood and anxiety and related structural and functional reward-related changes in oligo-amenorrheic athletes. Consistent with our hypothesis that estrogen deficiency is associated with mood and anxiety alterations; depressive and anxiety symptoms were significantly higher in female athletes with oligo-amenorrhea compared to young women with eumenorrhea. Further, bilateral caudate volume was higher in AA compared with the eumenorrheic group even after adjusting for age and intracranial volume. We also found that AA had decreased activation in the right caudate during anticipation of reward and punishment in the monetary incentive delay task, suggesting that estrogen status may play a role in modulating mood and anxiety. These findings persisted even after we controlled for possible confounders.
Effects of gonadal hormones on mood have been demonstrated in ovariectomized mice, who show symptoms of increased anxiety and reluctance to venture into the center of an open area to seek out food (29) as well as depressive behavior (30). Interestingly, such behaviors normalize after estrogen replacement (30, 31). Women with estrogen deficiency (e.g., post-menopause, Turner syndrome, primary ovarian insufficiency) have a higher prevalence of mood alterations and anxiety compared with the general population (4, 5, 32). Further, functional imaging studies in normally menstruating adult women have described a correlation between estradiol levels and blood oxygen level dependent (BOLD) signal activation in the amygdala-hippocampal region following a reward, indicating that gonadal steroids may play a role in modulation of reward processing (33). Importantly, in a prior study, we showed that hypogonadal adolescent girls with anorexia nervosa (AN) and FHA have an improvement in trait anxiety following estrogen replacement (12).
Consistent with these reports, in the current study, AA exhibited higher scores for anhedonic depression and anxiety compared to the eumenorrheic group. Athletic amenorrhea is a form of FHA, which is characterized by increased depressive symptoms and difficulty dealing with stressors. These individuals tend to display dysfunctional attitudes such as perfectionistic behavior, extra attention to the judgments of others and unrealistic expectations compared to eumenorrheic individuals (10, 11). In one study of 21 healthy controls, 18 amenorrheic women with AN, and 13 normal-weight women with FHA, women with AN and FHA had higher scores for anxiety and depressive symptomology based on the Hamilton Rating Scale for Anxiety (HAM-A) and Depression (HAM-D) than the healthy controls (34). It is important to note that, although depressive and anxiety symptoms were higher in the group with oligo-amenorrhea, mean scores in our study did not reach the level of clinical significance for a diagnosis of anxiety or depression. Exercise, by virtue of being a mood elevator, may have offered some protection to these individuals.
The finding that AA demonstrated increased volume of the caudate region compared to eumenorrheic participants suggests that estrogen may play an important role in volume reductions in specific brain areas during puberty. A longitudinal pediatric neuroimaging study by Giedd et al. indicated that gray matter in the frontal lobe increased linearly during pre-adolescence with maximum size noted before puberty, followed by a decline post-adolescence resulting in a net decrease in volume. Gray matter volume in the parietal lobes followed a similar pattern for changes in volume, except that the slopes were steeper pre- and post-adolescence. This structural change in both lobes peaked one year earlier in females than males, suggesting that maturational volume reductions in these regions may be potentially driven by the influence of estrogen (35). Of note, Lepage et al. evaluated developmental brain morphology in adolescents with Turner syndrome (TS), a condition characterized by a lack of endogenous estrogen. This study compared 30 girls with TS with 21 age-matched healthy female controls, and showed that hypoestrogenic girls with TS have significantly higher caudate volume (similar to findings in athletes with oligo-amenorrhea in our study) suggesting that this may result from delayed maturational involution linked to the lack of estrogen during adolescence (36).
Rodent studies indicate that estrogen has dopamine agonist actions and promotes dopamine secretion in the striatum and thereby improves reward sensitivity (37, 38). Functional neuroimaging studies in humans have yielded similar findings. During the mid-follicular phase of the menstrual cycle, estradiol levels positively correlate with brain activity in the amygdala and hippocampal complex during reward anticipation (33). In a more recent study, Macoveneau et al. examined reward processing in 58 women randomized to GnRH analogues (which block sex steroid production) and placebo (39). Compared to participants on placebo, the GnRH analogue group showed reduced activation in the amygdala to monetary gains suggesting that sex steroids may modulate reward processing (39). Of note, these participants also had increased depressive symptoms. Similarly, estrogen replacement in perimenopausal women resulted in increased response in the striatum during reward anticipation relative to placebo (40). Previous studies have also demonstrated hypoactivation to gains as well as penalties in patients with depression compared with healthy individuals (24). Our finding of decreased activation in the caudate regions for reward and punishment anticipation (functional MRI task) is in line with these findings and with our hypothesis that estrogen deficiency may blunt the response to reward processing.
The main limitation of our study is the small number of participants with MRI scans. This resulted in the eumenorrheic athlete and non-athlete groups being combined, as they both have regular menses (and also because these two groups were similar for STAI and MASQ scores). The significance of reduced caudate activation in AA vs EM (p=0.025) did not survive multiple corrections applied for 3 ROIs (p = 0.05/3 = 0.017), possible driven by small sample size. However, it is important to note that our results are characterized by moderate effect sizes. A few participants could not be included due to technical difficulties with MRI processing and image acquisition, and it will be important to replicate our findings in a larger sample. The second limitation relates to the relatively normal scores of anhedonic depression and anxiety in study participants, indicating no increase in a clinical diagnosis of depression or anxiety, notwithstanding higher mean STAI and MASQ anhedonic depression scores in oligo-amenorrheic vs. eumenorrheic women. Thus, higher mean scores on these questionnaires do not necessarily translate to a clinical diagnosis of anxiety or depression (known to be associated with structural brain changes). This was further corroborated by our sensitivity analyses that showed the significances for caudate functional and structural alterations persisted even after controlling for anhedonic depression and anxiety scores. Similarly, controlling for physical activity and menarchal age did not change our results for structural differences between groups. However, the reduced caudate activation in the AA group was significant only at a trend level after controlling for physical activity, suggesting that exercise, by virtue of being a mood elevator, may have offered some (albeit a small) protection to these individuals. The lack of significance may have been due to the small sample size in our exploratory analyses. Lack of data regarding caloric intake and the use estrogen status based on menstrual status (as estradiol levels were not available in all individuals and do not necessarily reflect the chronicity of estrogen exposure as they are cycle phase dependent) are other limitations that need to be addressed in future studies. Although our data provide important preliminary evidence that estrogen status may play a role in brain structure and function, we cannot determine causality as these are cross sectional studies. These findings underscore the need to identify young athletes with estrogen deficiency and to evaluate the implications of these findings on psychiatric endpoints. From a clinical perspective, identifying comorbidities related to estrogen status may help propose early interventions involving improved diet, reduced activity and psychological counseling and may help improve outcomes.
5 Conclusion
Oligo-amenorrheic athletes have greater anxiety and depressive symptoms compared with young women with eumenorrhea. Oligo-amenorrheic athletes also have increased caudate volumes, which may be related to delayed maturational decrease in cortical volumes seen in estrogen deficiency states. In addition, relative to eumenorrheic athletes and non-athletes, oligo-amenorrheic athletes demonstrate blunted responses to rewards. These findings require validation in larger studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board MGH. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
PK and SN analyzed the data, CB, PK and SN interpreted the data. CB, PK, SN, and MM were contributors in writing the manuscript. FP, KA, KE, DP, and MM edited and reviewed and edited the manuscript. CB and MM conceptualized and obtained the funding for the study. All authors read and approved the final manuscript.
Funding
MGH Pilot feasibility study (CB), Pediatric endocrine Society Clinical Scholar Award (CB), K24 HD071843 (MM), R0I HD060827 (MM), DP was partially supported by National Institute of Mental Health (NIMH) grant R37 MH068376. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. A grant from the Wellcome Leap and an honorarium from the American Psychological Association for editorial work.
Conflict of interest
Over the past 3 years, DP has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutcis former BlackThorn Therapeutics, Neurocrine Bioscences, Neuroscience Software, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, and Takeda Pharmaceuticals; honoraria from the Psychonomic Society for editorial work and Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. In addition, he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics former BlackThorn Therapeutics, and Neuroscience Software. Over the past 3 years, MM has received consulting fees from Sanofi and Abbvie, and served on the scientific advisory board for Abbvie and Ipsen. No funding from these entities was used to support the current work, and all views expressed are solely those of the author.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.976050/full#supplementary-material
References
1. Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics (2016) 138(6). doi: 10.1542/peds.2016-1878
2. Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the national comorbidity survey replication–adolescent supplement (NCS-a). J Am Acad Child Adolesc Psychiatry (2010) 49(10):980–9. doi: 10.1016/j.jaac.2010.05.017
3. Statistics, N.C.f.H. Adolescent health (2019). Available at: https://www.cdc.gov/nchs/fastats/adolescent-health.htm.
4. Harlow BL, Cramer DW, Annis KM. Association of medically treated depression and age at natural menopause. Am J Epidemiol (1995) 141(12):1170–6. doi: 10.1093/oxfordjournals.aje.a117390
5. Cardoso G, Daly R, Haq NA, Hanton L, Rubinow DR, Bondy CA, et al. Current and lifetime psychiatric illness in women with turner syndrome. Gynecol Endocrinol (2004) 19(6):313–9. doi: 10.1080/09513590400021227
6. Weiss Kelly AK, Hecht S, Council On Sports M, Fitness. The female athlete triad. Pediatrics (2016) 138(2). doi: 10.1542/peds.2016-0922
7. Strohle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) (2009) 116(6):777–84. doi: 10.1007/s00702-008-0092-x
8. Wegner M, et al. Effects of exercise on anxiety and depression disorders: review of meta- analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets (2014) 13(6):1002–14. doi: 10.2174/1871527313666140612102841
9. Gadpaille WJ, Sanborn CF, Wagner WW Jr. Athletic amenorrhea, major affective disorders, and eating disorders. Am J Psychiatry (1987) 144(7):939–42. doi: 10.1176/ajp.144.7.939
10. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril (2001) 76(2):310–6. doi: 10.1016/S0015-0282(01)01921-5
11. Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril (1993) 60(3):486–92. doi: 10.1016/S0015-0282(16)56165-2
12. Misra M, Katzman DK, Estella NM, Eddy KT, Weigel T, Goldstein MA, et al. Impact of physiologic estrogen replacement on anxiety symptoms, body shape perception, and eating attitudes in adolescent girls with anorexia nervosa: data from a randomized controlled trial. J Clin Psychiatry (2013) 74(8):e765–71. doi: 10.4088/JCP.13m08365
13. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci (2013) 14(9):609–25. doi: 10.1038/nrn3381
14. Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am (2009) 32(3):549–75. doi: 10.1016/j.psc.2009.05.004
15. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther (2018) 24(11):994–1003. doi: 10.1111/cns.12835
16. Keren H, O'Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry (2018) 175(11):1111–20. doi: 10.1176/appi.ajp.2018.17101124
17. Gorka AX, Fuchs B, Grillon C, Ernst M. Impact of induced anxiety on neural responses to monetary incentives. Soc Cognit Affect Neurosci (2018) 13(11):1111–9. doi: 10.1093/scan/nsy082
18. Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation (1996).
19. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Press: Palo Alto, CA: Consulting Psychologists (1983).
20. Spielberger CD. State-trait anxiety inventory: bibliography. 2nd ed. Press: Palo Alto, CA: Consulting Psychologists (1989).
21. Watson D, et al. Testing a tripartite model: II. exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol (1995) 104(1):15–25.
22. Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage (2000) 12(1):20–7. doi: 10.1006/nimg.2000.0593
23. Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry (2009) 166(6):702–10. doi: 10.1176/appi.ajp.2008.08081201
24. Admon R, Nickerson LD, Dillon DG, Holmes AJ, Bogdan R, Kumar P, et al. Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med (2015) 45(1):121–31. doi: 10.1017/S0033291714001123
25. Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods (2019) 16(1):111–6. doi: 10.1038/s41592-018-0235-4
26. Esteban O, Blair R, Christopher J, Markiewicz SL, Berleant C, Moodie FM. Ayse ilkay isik FMRIPrep. Software Zenodo (2018).
27. Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform (2011) 5:13. doi: 10.3389/fninf.2011.00013
28. Gorgolewski KJ, Esteban O, Christopher J, Markiewicz EZ. (2018). M.P.N. David Gage Ellis, Dorota Jarecka, “Nipype. Software. Zenodo.
29. Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci USA (1998) 95(23):13941–6. doi: 10.1073/pnas.95.23.13941
30. Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, et al. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci Lett (2014) 573:13–8. doi: 10.1016/j.neulet.2014.04.053
31. Diz-Chaves Y, Kwiatkowska-Naqvi A, Von Hulst H, Pernia O, Carrero P, Garcia-Segura LM. Behavioral effects of estradiol therapy in ovariectomized rats depend on the age when the treatment is initiated. Exp Gerontol (2012) 47(1):93–9. doi: 10.1016/j.exger.2011.10.008
32. Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, et al. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J Clin Endocrinol Metab (2011) 96(2):E278–87. doi: 10.1210/jc.2010-0613
33. Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA (2007) 104(7):2465–70. doi: 10.1073/pnas.0605569104
34. Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab (2009) 94(12):4710–6. doi: 10.1210/jc.2009-1046
35. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci (1999) 2(10):861–3. doi: 10.1038/13158
36. Lepage JF, Mazaika PK, Hong DS, Raman M, Reiss AL. Cortical brain morphology in young, estrogen-naive, and adolescent, estrogen-treated girls with turner syndrome. Cereb Cortex (2013) 23(9):2159–68. doi: 10.1093/cercor/bhs195
37. Esther DK. Estradiol and the reward system in humans. Curr Opin Behav Sci (2018) 23:58–64. doi: 10.1016/j.cobeha.2018.03.010
38. Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse (1998) 29(4):379–91. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M
39. Macoveanu J, Henningsson S, Pinborg A, Jensen P, Knudsen GM, Frokjaer VG, et al. Sex-steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology (2016) 41(4):1057–65. doi: 10.1038/npp.2015.236
Keywords: estradiol, adolescent, anxiety, depression, reward and punishment processing
Citation: Baskaran C, Kumar P, Plessow F, Nimmala S, Ackerman KE, Eddy KT, Pizzagalli DA and Misra M (2023) Depressive and anxiety symptoms, and neural correlates of reward and punishment anticipation in female athletes with amenorrhea. Front. Endocrinol. 14:976050. doi: 10.3389/fendo.2023.976050
Received: 22 June 2022; Accepted: 28 April 2023;
Published: 18 May 2023.
Edited by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceReviewed by:
Osamu Yoshino, University of Yamanashi, JapanEmily Kraus, Stanford Healthcare, United States
Copyright © 2023 Baskaran, Kumar, Plessow, Nimmala, Ackerman, Eddy, Pizzagalli and Misra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charumathi Baskaran, Y2hhcnVtYXRoaS5iYXNrYXJhbkBjaGlsZHJlbnMuaGFydmFyZC5lZHU=
†These authors have contributed equally to this work and share first authorship
 Charumathi Baskaran
Charumathi Baskaran Poornima Kumar
Poornima Kumar Franziska Plessow
Franziska Plessow Supritha Nimmala2
Supritha Nimmala2 Kathryn E. Ackerman
Kathryn E. Ackerman Kamryn T. Eddy
Kamryn T. Eddy Diego A. Pizzagalli
Diego A. Pizzagalli Madhusmita Misra
Madhusmita Misra