
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 16 May 2023
Sec. Cellular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.956772
This article is part of the Research Topic TCM Approaches in Cellular Endocrinology View all 7 articles
Polycystic ovary syndrome (PCOS) is a reproductive endocrine disease characterized by menstrual disorders, infertility, and obesity, often accompanied by insulin resistance and metabolic disorders. The pathogenesis of PCOS is relatively complex and has a certain relationship with endocrine disorders. The increase of androgen and luteinizing hormone (LH) is the main cause of a series of symptoms. Traditional Chinese medicine (TCM) has obvious advantages and significant curative effects in the treatment of this disease. It can effectively reduce the insulin level of PCOS patients, regulate lipid metabolism, and increase ovulation rate and pregnancy rate and has fewer side effects. This article reviews the efficacy and safety of Chinese herbs and other TCM (such as acupuncture) in the treatment of PCOS and its complications in recent years, as well as the effect and mechanism on cellular endocrine, in order to provide a new clinical idea for the treatment of PCOS.
Polycystic ovary syndrome (PCOS) is one of the common endocrine diseases in women of childbearing age, which seriously affects their reproduction, metabolism, and psychology. The clinical manifestations of PCOS are mainly irregular menstruation, hyperandrogenemia (HA), ovulation dysfunction infertility, polycystic ovary, and hormonal changes, which affect patients’ fertility, quality of life, and long-term health. The global prevalence is estimated to range from 4% to 12% due to poor eating habits and unprecedented psychological and social stress (1). In addition, a large number of women with PCOS symptoms are not clearly diagnosed. The prevalence of diabetes in the PCOS population is approximately 26.5%, which significantly reduces the quality of life of patients and imposes a high cost on the healthcare system. It has been reported that the medical-related economic burden of PCOS in the United States is $4.36 billion per year (2). Different pharmaceutical treatments have been proposed for PCOS. However, they also have drawbacks, such as adverse reactions, low patient compliance with long-term medication, low efficacy, and contraindications in some cases (3, 4). Traditional Chinese medicine (TCM), as a complementary and alternative treatment method, is an effective way.
PCOS is an endocrine and metabolic disorder caused by the interaction of heredity and the environment. Its etiology and pathogenesis have not been elucidated completely yet (5). Previous studies have suggested PCOS may be linked to mood and mental disorders (6). Emotional factors can affect the changes of the hypothalamic–pituitary–ovarian axis (HPOA) and cause neuroendocrine changes, resulting in irregular ovarian ovulation and PCOS. Some studies have suggested that the occurrence of PCOS is related to DNA methylation, X chromosome inactivation, and histone modification (7). Some scholars have found that the anti-Müllerian hormone (AMH) is associated with the etiology of PCOS, and the concentration of AMH can predict the ovarian response in ovulation induction (8). In recent years, alterations in intestinal flora have been found to be involved in the pathogenesis of PCOS. It can lead to insulin resistance (IR) and influence androgen metabolism and follicle development (9). Inflammation is also one of the risk factors, and any disturbance of the level of inflammatory factors may lead to the dysfunction of ovarian function (10). In addition, a meta-analysis suggested a significant correlation between PCOS and prostate-specific antigen (PSA), and the role of PSA in patients with PCOS cannot be ignored clinically (11).
There is no disease name of PCOS in ancient Chinese medicine books. According to its clinical manifestations, it can be classified as “irregular menstruation”, “amenorrhea”, or “infertility” category. The treatment mainly focuses on restoring the balance between the kidney, Tiangui, Chongren, and uterus. Tiangui is a sexual stimulant for women, which is essential for women’s menstruation and pregnancy, and its function is similar to neuroendocrine hormones that regulate reproduction. The disturbance of the time, state, and rhythm of Tiangui can lead to female reproductive problems, and ovarian dysfunction in PCOS patients can show Tiangui disorders. TCM has a good curative effect and high safety in the treatment of PCOS, which has attracted more and more attention (12). However, the biological mechanism has not been clearly elucidated, and the treatment protocols are still controversial. This paper reviewed the effects of traditional Chinese as a treatment for PCOS and introduced its potential cellular endocrine mechanism in order to provide a reference for the clinical treatment and research of PCOS.
Acupuncture is an indispensable part of traditional medicine and has been used in China for more than 3,000 years. Acupuncture has clinical efficacy in the treatment of cardiovascular diseases, epilepsy, anxiety, circadian rhythm disorders, PCOS, low reproductive capacity, and autonomic nervous system diseases (13). With the modernization of TCM and its advantages, such as convenient operation and economic and satisfactory results, more and more countries are beginning to apply it to treat and prevent diseases. Acupuncture was also not confined to its original model and gradually evolved into electroacupuncture (EA), warm needle, acupoint insertion, and ear point, and they have been used in the treatment of PCOS (14, 15). Currently, a large number of clinical and animal trials have shown that acupuncture can regulate the function of the HPOA and metabolism in PCOS, promote ovulation, and improve IR and endometrial receptivity (ER) (16).
Acupuncture is a TCM therapy in which very thin metal needles are inserted into specific parts of the body. Acupuncture can control the function of the autonomic nervous system (ANS); it can balance the sympathetic and parasympathetic nerve activity, regulate the adaptive neurotransmitters in related brain regions, and reduce the autonomic response, which has been proved to have a certain curative effect on PCOS patients (17, 18). Professor Shi treated PCOS women with obesity and infertility by acupuncture from the perspective of spleen and kidney function. The pathogenesis was a deficiency of the spleen and kidney as the primary and obstruction of phlegm-dampness and collaterals as the secondary. According to clinical syndrome differentiation, the main acupoints are selected from the meridian, pregnancy meridian, kidney meridian, and spleen and stomach meridian, and the corresponding acupoint prescriptions and techniques are used. The purpose is to tonify the kidney, strengthen the spleen, nourish qi, dissolve phlegm, remove dampness and remove stasis, promote blood circulation to restore menstrual flow, and help pregnancy (19). Professor Shi put forward the theory of “treatment based on syndrome differentiation, equal attention to the nature of acupoints and medicines, similar acupoints and prescriptions, and determination of reinforcing and reducing methods”. It fully embodies the idea of TCM “treatment based on syndrome differentiation” and shows the curative effect of acupoints and acupuncture techniques (19). EA can inhibit the overexpression of AMH and increase the expression level of P450arom in ovarian granulosa cells (GCs), thereby re-establishing the dependence of follicular development on follicle-stimulating hormone (FSH) and improving follicular dysplasia and hyperandrogenemia in PCOS patients with kidney deficiency and phlegm-dampness (20). In addition, acupuncture can improve vascular endothelial function, regulate dyslipidemia, correct IR, and improve endocrine disorders (21). EA has similar effects to metformin (22) and alleviates anxiety and depression in PCOS patients by regulating serum β-endorphin and androgen levels (23).
The acupuncture method of “regulating pregnancy and du-pulse” can improve the menstrual cycle, increase endometrial thickness, promote oocyte growth and follicle development, reduce serum luteinizing hormone (LH) level, improve ovarian function, and increase ovulation rate in PCOS patients (24, 25). Acupuncture can affect the production of β-endorphin, gonadotropin-releasing hormones, ovulation, and the menstrual cycle (26). Cao Y et al. conducted a randomized controlled trial (RCT) on 60 PCOS patients and found that LH/FSH ratio, LH, and total testosterone (TT) were significantly reduced after 12 weeks of acupuncture treatment, as well as significant improvements in body mass index (BMI), menstrual times, and polycystic ovary number (27). Huang S et al. found that personalized acupuncture can improve live birth rates (LBRs) in infertile women with PCOS in a multicenter RCT (28). However, in 2017 and 2018, the Journal of American Medical Association published two trial reports examining the potential effect of acupuncture on improving in vitro fertilization (IVF) or LBR in women with PCOS. The study found that acupuncture did not increase LBR, which may be due to unexpected biases in the study, such as using ineffective control interventions and underestimating the effect of real acupuncture (29). A 1,000-sample RCT trial also showed that acupuncture did not increase the rate of live births in infertile patients (30).
The standard treatment of PCOS includes oral medications, lifestyle changes, and surgery. Pharmacology-based treatments are effective in only 60% of patients. Therefore, acupuncture provides an alternative (14). In women with PCOS and IR, acupuncture was superior to metformin in improving glucose metabolism and had a lower incidence of gastrointestinal adverse effects (31). On the basis of Western medicine treatment of PCOS, adding acupuncture treatment can improve the curative effect and shorten the course of the disease. Letrozole combined with EA and TCM can significantly improve the menstrual cycle and reduce body weight, LH, LH/FSH, testosterone (T), and AMH levels. The therapeutic effect is superior to that of Western medicine alone, and there are no adverse reactions (32), based on the research of Jo J et al. (33). Acupuncture combined with TCM could improve the endocrine level and IR of phlegm-dampness PCOS and reduce miR-29 expression and TCM symptom score (34).
Modern acupuncture treatment of PCOS mainly includes Sanyinjiao (SP 6), Guanyuan (CV 4), Zigong (EX-CA 1), Zhongji (CV 3), and Qihai (CV 6). Points were selected based on the theory of meridians and zang-fu organs, syndrome differentiation, and meridian circulation. In addition, the method of selecting adjacent points was also adopted. The main acupoints are located in the fetal pulse, the spleen meridian of Foot Taiyin, and the stomach meridian of Foot Yangming. In the special acupoints, Zheng Mu, Wu Shu, and Bei Shu are used more. Generally, five to seven acupuncture points are taken (35). Although the mechanism of acupuncture treatment for PCOS has been found in modern medicine, most of the trials have small sample sizes and lack consistency in the selection of acupoints. Larger, long-term randomized controlled trials are needed in the future to provide standardized protocols. A summary of the effect of acupuncture on PCOS outcomes is shown in Table 1.
Due to the relatively complex pathogenesis of PCOS, there is no single drug that can control all symptoms. Existing pharmaceutical formulations, such as oral contraceptives (OCs), have been suggested as first-line treatments for menstrual irregularities. However, OCs are not appropriate for pregnant women. Insulin sensitizers can reduce insulin levels and hyperandrogenemia in PCOS patients, but the incidence of gastrointestinal adverse reactions is higher. In China, it is a common practice for TCM to treat gynecological problems and infertility. Current studies have shown that Chinese medicine has a beneficial effect on the treatment of PCOS. The database analysis of the Taiwan National Health Insurance Plan showed that 89.22% of women who were newly diagnosed with PCOS had received TCM treatment (38). Jiawei Xiaoyao Powder and Xiangfu are the most commonly used compound and single medicinal materials, respectively (38). Several studies have shown that TCM has similar safety and clinical effects in the treatment of PCOS (39, 40).
According to the main pathogenesis of PCOS, TCM treatment should focus on tonifying the kidney and simultaneously treating the liver, spleen, and heart. Professor Shi Yin summarized the clinical experience and proposed the method of “staging, classification and sorting” for PCOS treatment. The stage should take the law of follicle development as the core and follow the rule of Yin and Yang. In terms of classification, emphasis is placed on individual treatment according to obesity, wasting, non-obesity, and fertility. In addition, psychological counseling and life adjustment are very important for patients, as the unity of body and mind can improve the curative effect (41).
Kidney nourishment and phlegm removal (KNPR) effectively improved the glucose and lipid metabolism of obese PCOS rats. The levels of fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and free fatty acid (FFA) in serum were decreased, and the expression of high-density lipoprotein (HDL) was increased (42). Chen WJ et al. found that Quyu Huatan Decoction has good clinical efficacy in treating PCOS. It can not only improve the secretion of various sex hormones but also regulate the body’s sugar and lipid metabolism (43). In addition, this method can improve hyperandrogenemia, the number of granulosa cell layers and luteal tissue, and regulate LH/FSH ratio (44). Zhibai Dihuang Decoction (MZBDD) has a dose–effect relationship in the treatment of PCOS hyperandrogenism (45). Pan X et al. found that Bushen Jieyu Tiaochong Formula (BJTF) could improve abnormal follicular dilation in PCOS rats and reduce the levels of free testosterone (FT), LH, and LH/FSH ratio in serum. BJTF can relieve chronic psychological stress behavior and regulate brain monoamine neurotransmitter expression and metabolism. The apoptosis index of GCs, glucose-regulated protein 78 (GRP78), CHOP, and ATF4 was decreased (46).
Yu J et al. showed that Yushi Qinggan Recipe (YQR) could improve the clinical symptoms of PCOS patients, regulate their endocrine levels, and promote ovulation and pregnancy. The levels of LH, LH/FSH, T, FT, dehydroepiandrosterone sulfate (DHEAS), insulin (INS), and insulin area under the curve (IAUC) were all significantly reduced. At the same time, the symptoms of acne, irregular menstruation, irritability, breast distension, dry mouth and bitter mouth, greasy hair/alopecia, and constipation were improved (47). Bushen Huoxue Culuan Prescription can reduce LH level and increase FSH level, improve polycystic ovary, increase the number of follicles, facilitate the development of mature follicles, and improve ovulation rate (48). In addition, TCM can downregulate reactive oxygen species (ROS) protein expression in GCs, correct oxidative stress in vivo, and improve the rate of high-quality embryos (49). Bushen Tiaojing decoction combined with Western medicine can improve endometrial thickness and shape, promote menstrual recovery, induce ovulation, and enhance endometrial permeability. The levels of T, LH, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and leptin (LP) in patients were reduced, and the efficacy was better than in Western medicine alone (50). Bushen Tiaochong Decoction has good efficacy in the treatment of obesity PCOS, which can significantly improve the level of a reproductive endocrine hormone, reduce BMI, and improve endometrial acceptance. It is a safe and promising treatment method (51).
TCM can regulate endocrine and improve menstrual irregularity, ovulation, and pregnancy rate by regulating ovarian hemodynamics, serum hormone levels, and menstruation in PCOS patients (52, 53). TCM’s clinical curative effect is better than that of Western medicine treatment (54–56). A meta-analysis showed that the efficacy of TCM combined with letrozole (LE) in the treatment of PCOS was superior to LE alone in regulating ovulation rate, pregnancy rate, number of mature follicles, endometrial thickness, cervical mucus score, serum FSH, LH, E2, T, and prolactin (PRL) levels (57, 58). On the basis of the control group, Bailingtiaogan decoction can obviously improve the clinical symptoms, increase the endometrial thickness, reduce ovarian volume, and improve the clinical pregnancy rate. FFA and C-reactive protein (CRP) levels were significantly lower than those of the control group; the difference was statistically significant (p < 0.05) (59). Wei A et al. showed that the Dingkun pill combined with clomiphene in the treatment of PCOS with infertility is more effective than clomiphene alone (60). Although the addition of Chinese herbal formulae (CHF) to clomiphene may improve pregnancy rates, there are no relevant data to support live birth rates in subfertile women with PCOS. In addition, the evidence on adverse effects is insufficient to indicate whether CHF is safe. In the future, well-designed, well-conducted randomized controlled trials will be needed, with a special focus on live birth rates and other safety indicators (61). TCM cycle therapy combined with Dian-35 treatment has fewer adverse reactions (62). CHF combined with metformin hydrochloride was superior to Western medicine alone in improving the total effective rate of blood glucose and hormone of PCOS. After treatment, serum FSH, LH, LH/FSH, and T changed significantly. FBG, 2-h postprandial blood glucose (2hPG), and BMI were decreased, and the changes in the treatment group were more significant than those in the control group (p < 0.05) (63). No adverse effects of CHF on liver and kidney function were observed in PCOS patients (64).
More and more studies have proved that IR regulates multiple mediators and pathways and is seriously involved in the pathogenesis and development of PCOS (65, 66). A study by Liang RN et al. showed that Heyan Kuntai Capsule (HYKT) can improve glucose and lipid metabolism disorder, IR, and insulin sensitivity in PCOS patients, and its effect is similar to that of insulin sensitizer. After HYKT treatment, BMI and waist–hip ratio (WHR) decreased. Fasting plasma glucose (FPG), 2hPG, and serum sex hormones, such as LH, LH/FSH, and T, were decreased. TC, TG, LDL, INS, and HOMA-IR in the HYKT group were significantly lower than those in the placebo group, while HDL and insulin sensitivity index (ISI) were higher (67). CHF can improve glycolipid metabolism in non-obese PCOS patients by reducing LH levels and LH/FSH (68). Qiu Z et al. found that after Liuwei Dihuang Pills (LWDH Pills) treatment, ISI returned to normal; serum levels of FSH, E2, and progesterone (P) were significantly increased; and LH and T levels were decreased. Polycystic ovarian changes and follicular atresia were reduced (69). Heqi San also has a similar effect (70). In PCOS patients, Dingkun Pill (DKP) or DKP in combination with Diane-35 resulted in a slight improvement in insulin sensitivity (71). Heat-clearing drugs can obviously improve IR and reduce serum LH, T, and PRL levels and TCM syndrome score, and their efficacy is better than that of metformin. Compared with before treatment, BMI, fasting insulin (FINS), 2-h INS, HOMA-IR, LP, LH, PRL, T, and TCM syndrome scores were significantly decreased, while adiponectin (APN) level was increased, and the differences were statistically significant (p < 0.05) (72). The imbalance of intestinal flora is correlated with the incidence of PCOS. The number of butyric-producing bacteria decreased, and the number of lipopolysaccharide-producing and pro-inflammatory bacteria increased in PCOS patients. Jiawei Qigong pill can increase the diversity of intestinal flora and the number of probiotics and improve the structure of intestinal flora and IR (73).
Oxidative stress and inflammation are related to the occurrence of PCOS (74). Lu C et al. confirmed that Bushen Huatan Formula (BHF) can reduce the inflammatory response and oxidative stress of PCOS. After BHF intervention for three menstrual cycles, serum glycerophosphorylethanolamin (GPEA), creatinine, and creatinine levels were decreased in the normal insulin group (NI) and hyperinsulin group (HI). The changes in phospholipid metabolism were mainly observed in the NI group. In the HI group, lysine, phenol sulfate, and phenylpropofol decreased, while ornithine, proline, and acetylcholine increased (75). Cangfu Daotan Decoction (CFDTT) can regulate lipid metabolism, sex hormone secretion, and inflammatory response; CFDTT intervention reduced serum TC, TG, LDL-C, LH, T, IL-1β, IL-6, and TNF-α levels. The levels of HDL-C, FSH, and E2 were increased in a dose-dependent manner. CFDTT induced the expression of organic anion transporting polypeptides (OATPs) in ovarian and uterine tissues (76). TCM can downregulate the expression of apoptosis-related proteins to repair ovarian lesions and improve cell apoptosis (77). Cangfudaotan decoction (CFD) treatment can improve IR, restore serum hormone levels, inhibit inflammatory cytokines, and reduce ovarian morphological damage in PCOS rats. In GCs of PCOS, the cell viability was improved, and apoptosis was inhibited after CFD treatment (78). Chronic stress induces endocrine disorders, leading to the development of PCOS. Xiao-yao-san (XYS) treatment ameliorated the irregular estrus cycle and abnormal follicular development induced by chronic unpredictable mild stress (CUMS). E2, P, and LH levels were decreased, and granulosa cell apoptosis and autophagy were inhibited. β-Hydroxylase, c-Fos, and norepinephrine (NE) were reduced. XYS can be used as a potential therapeutic strategy, and its beneficial effects are related to the regulation of sympathetic nerve activity (79). A summary of the effect of CHF on PCOS outcomes is shown in Table 2.
In an open-label, one-arm, non-randomized, post-marketing surveillance study, Trigonella foenum-graecum seed extract caused ovarian cysts reduction or dissolution in PCOS patients, 71% of patients returned to normal menstrual cycles after treatment, and 12% became pregnant subsequently (80). Cinnamon can improve the menstrual cycle and ovarian size of patients and significantly reduce serum FPG, INS, HOMA-IR, TC, and LDL levels. Short-term cinnamon supplementation had beneficial effects on metabolism; it may be an effective treatment option for some PCOS women (81–84). Miao M et al. found that Dodder Total Flavone can reduce the expression of androgen receptor (AR) in HPOA of PCOS rats. Compared with the PCOS model rat, the levels of serum T, GnRH, and LH; ovarian index; and LH/FSH ratio were decreased in Dodder Total Flavone high-dose, medium-dose, and low-dose groups, while the levels of E2 and FSH were increased. To some degree, the pathological changes of cortical thickening, collagen, increased follicular atresia, and lutein in ovarian tissues were alleviated (85).
Berberine is a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids. It is found in plants such as Hydrastis canadensis, Berberis vulgaris, Coptis chinensis, Xanthorhiza simplicissima, and Eschscholzia californica. It has obvious anti-inflammatory and antioxidant effects in vitro. In PCOS animals, berberine has neuroprotective and cardiovascular effects; the effects of lowering lipids and improving IR have been clearly demonstrated in the RCT. In addition, preliminary clinical evidence suggests that berberine can reduce endothelial cell inflammation and improve vascular health (86). Berberine has the same effect as metformin in alleviating IR and improving glucolipid metabolism and reproductive endocrine status (87), and it is also beneficial to the reproductive rate of PCOS infertile patients (88). C. chinensis has the function of clearing heat and detoxifying, reversing pathological damage of PCOS ovarian tissue, and regulating the expression of inflammatory factors (89). Quercetin can reduce the levels of INS, IL-1β, IL-6, TNF-α, and NF-κB nuclear translocation. It has a good therapeutic effect on PCOS rats (90). Curcumin decreased BMI, FPG, INS, and IR significantly. In addition, taking curcumin can upregulate peroxisome proliferator-activated receptor gamma (PPAR-gamma) gene, PGC1 α gene, low-density lipoprotein receptor (LDLR) gene expression, and Gpx enzyme activity. Moreover, curcumin can effectively reduce oxidative stress-related complications and increased insulin sensitivity in PCOS patients (92–94).
Chinese herbal medicine has certain benefits in improving IR. Salvia officinalis extract can reduce the BMI and systolic blood pressure of PCOS patients and improve insulin resistance indicators (95). Soy isoflavones for 12 weeks in PCOS women significantly improved IR, hormone status, TC, and oxidative stress biomarkers (96). Marjoram herb significantly reduces INS and HOMA-IR levels. Marjoram improves insulin sensitivity and reduces adrenal androgen levels in PCOS women (97). A summary of this section is shown in Table 3, a summary of the effect of single herb or herbal extract on PCOS is shown in Table 4.
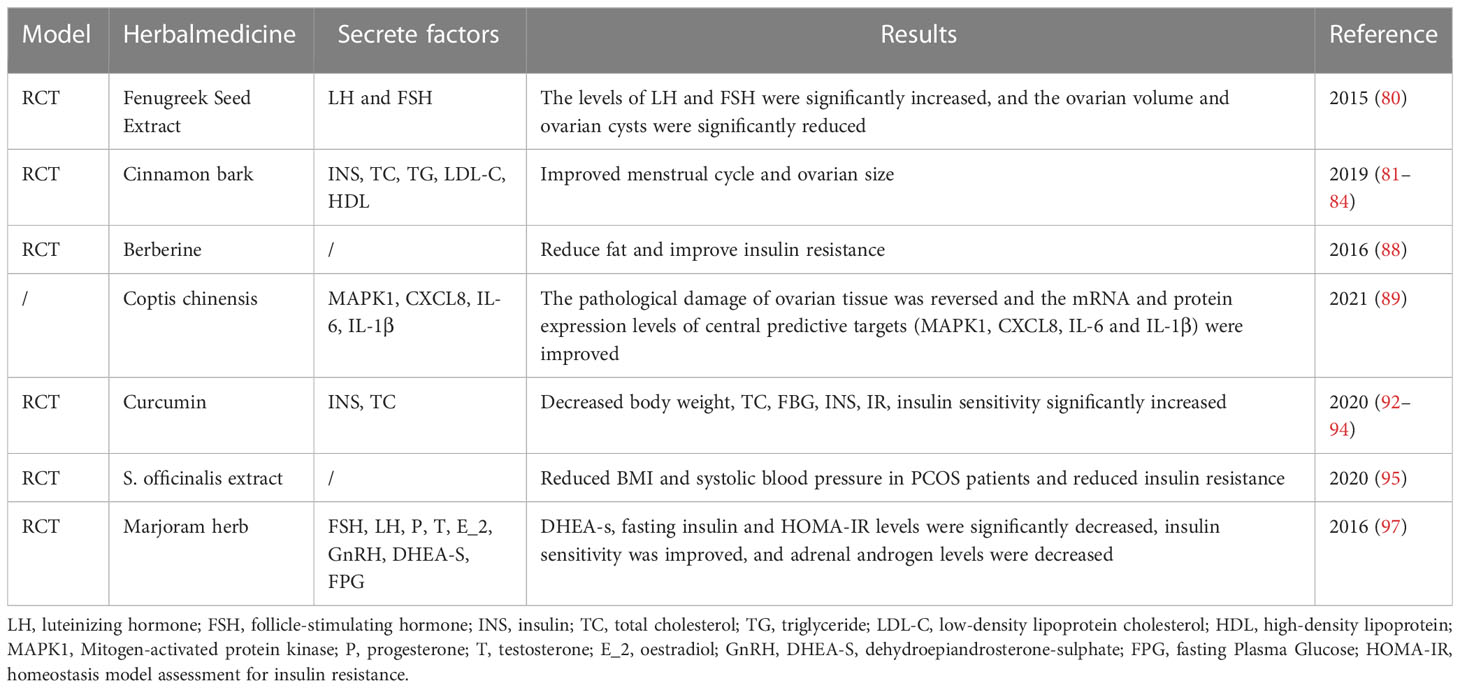
Table 4 Summary of a randomized study on the effect of single herb or herbal extract on PCOS outcomes.
Qigong and Tai Chi are moderate-intensity exercises that can be traced back to ancient China. They can alter the autonomic nervous system, restore dynamic balance, reduce the stress associated with the HPOA, and regulate the balance of the autonomic nervous system to parasympathetic innervation. The effect of Qigong and Tai Chi on emotion regulation may be related to the regulation of the prefrontal cortex, limbic system, and striatum (98). It has been reported that Qigong and Tai Chi have a certain positive effect on the treatment of diseases. Qigong training can improve blood glucose status, and Tai Chi has a good effect on reducing blood glucose and glycosylated hemoglobin levels in patients with type 2 diabetes (99, 100). Tai Chi is better than brisk walking at reducing cardiovascular risk factors and improving psychosocial health (101). Baduanjin is a potentially effective choice for PCOS patients to improve their biomedical and psychosocial health (102).
Shao C et al. selected 80 PCOS patients with phlegm block as the study cohort and randomly divided them into a study group (RG) and a control group (CG). CG was treated with TCM decoction based on syndrome differentiation. RG was treated with Guijiaosan paste at Shenque point on the basis of CG treatment. Compared with the CG group, the BMI, endocrine function, IRI, and TCM syndrome scores were significantly improved in the RG group (p < 0.05) (36). Acupoint catgut embedding therapy combined with TCM is beneficial to improve blood glucose, blood lipid levels, and the pregnancy rate of obese PCOS patients with infertility (37). For patients with simple obesity secondary hyperlipidemia, Taijiquan combined with auricular point sticking treatment can show an obvious synergistic therapeutic effect (103). Combined treatment of acupuncture, moxibustion, and drugs can effectively improve endometrial receptivity and uterine artery blood flow in PCOS patients with kidney deficiency and blood stasis and increase pregnancy rate. The therapeutic effect of HOXA10 is better than that of Western medicine alone. Its mechanism may be related to the regulation of serum HOXA10 expression (104).
PCOS is the most common endocrine and metabolic disorder in women of childbearing age. TCM is based on the concept of holism, using multi-system regulation and multi-target action to treat PCOS. A large number of clinical practices have confirmed that TCM can effectively improve the symptoms and signs of patients, the ovarian microenvironment, the sympathetic nervous system, the endocrine system, and the disorder of glucose and lipid metabolism. There are various methods of TCM to treat this disease, from syndrome differentiation and classic prescription plus or minus treatment to single Chinese medicine, acupuncture, and moxibustion, etc.; the clinical efficacy is remarkable; the adverse reactions are small. In addition, the TCM formulation is flexible and can adjust the medication based on the basic prescription for different patients. The TCM formulation can comprehensively adjust the symptoms of patients in various directions and improve the psychological state of patients.
PCOS is characterized by increased androgens and follicular stagnation, possibly due to an imbalance between AMH and FSH (20). EA has been reported to normalize FSH and AMH levels in granulosa cells by reducing overexpression of AMH, thereby improving follicular stasis in PCOS rats (105). Shi Y et al. used low-frequency EA at acupoints (CV-3 and CV-4) to treat the PCOS rat model. In the EA group, 80% of rats had recovered estrous cycle, improved ovarian morphology, reduced testosterone level, and increased peripheral blood E2 and P450arom levels. The expression of AMH and AMH type II receptors decreased, while the expression of FSH receptors increased (105). Animal experiments conducted by Xu G et al. showed that EA stimulation of CV4, SP6, and ST36 could reduce the number of immature follicles, LH/FSH, and serum AMH levels (106). A recent animal study showed that acupuncture improves ovulation disorder by downregulating LncMEG3 expression, inhibiting PI3K/AKT/mTOR pathway, and reducing granulosa autophagy (107). Another animal study showed that EA can upregulate the number of preovulatory follicles and corpus luteum by increasing the innervation of blood vessels (108).
Bushen Cupailuan Decoction can significantly improve the expression of aromatase mRNA and protein in GCs of PCOS model rats, which may be one of the mechanisms by which Bushen Cupailuan Decoction promotes follicular development and release (109). Baling Tiaogan Decoction can significantly improve clinical symptoms, increase endometrial thickness, reduce ovarian volume, and improve the clinical pregnancy rate. The mechanism may be related to the decrease of serum FFA and CRP levels and the increase of serum β-EP levels (59). The transcript levels of gonadotropin receptors (FSH receptor (FSHR) and LH), steroid receptors (Pgr and Esr1), and steroid proenzymes (Cyp19a1, Hsd3b1, Hsdl7a1, and Cyp11a1) are changed in the ovaries of PCOS rats. CHF ameliorates PCOS symptoms by improving the dysregulation of steroidal hormones and steroid enzymes in PCOS rats (110).
After the intervention of cryptotanshinone, the estrous cycle, body weight, ovarian coefficient, and ovarian morphology of PCOS model rats were improved, and the serum levels of T, androstenedione (A2), LH, and sex hormone-binding globulin (SHBG) were reversed. It may be related to the downregulation of CYP17 and AR gene expression (111). Dodder Total Flavone may regulate the secretion of estrogen and androgen and affect the HPOA to protect PCOS model rats (85).
PLA2G4A protein is essential in the pathogenesis of PCOS and diabetes. Acupuncture treatment can significantly downregulate the level of mir-32-3p, which regulates the expression of PLA2G4A protein (112). EA intervention can reduce PCOS-like symptoms in rats, and DHEA exposure-induced skeletal muscle autophagy deficits were improved. EA ameliorates IR, mitochondrial dysfunction, and endoplasmic reticulum stress by inactivating the mTOR/4E-BP1 signaling pathway (113). KNPR can effectively improve the glucose and lipid metabolism of obese female PCOS rats and increase the serum APN expression, the average density of AdipoR2 in ovarian GC membrane, and the average density of p38MAPK mRNA and FATP1 in GC cells. KNPK treatment of PCOS may be related to the activation of the APN/p38MAPK signaling pathway (42). The pharmacological effects of Zishen Yutai Pills (ZSYTP) on PCOS may be related to the HIF-1 signaling pathway, IR, and gene expression such as RNA splicing (114). ETAQC can upregulate the expression of p-AKT and GLUT4 in ovarian GCs and improve the degree of IR in ovarian tissue, thereby improving the quality of IVF-ET in PCOS patients (115).
PI3K/Akt signaling is one of the classical insulin signaling pathways; PCOS rats showed abnormal IR and PI3K/Akt signal transduction (116). Heqi San could restore serum hormone levels and ovarian morphological lesions and improve IR. Heqi San can change the expression levels of key factors of the PI3K/APT pathway, including p-ERK, p-AKT, p-GSK3β, IRS-1, PTEN, and GLTU4. Bioinformatics analysis showed that rno-miR144-3p, rno-miR-30c-2-3p, rno-miR-486, rno-miR-3586-3p, and rno-miR-146b-5p may play key roles in the occurrence of PCOS (70, 70). The study of Wang LH et al. showed that the mRNA expression and protein level of insulin receptor substrate 1 (IRS-1) and PI3Kp85α were significantly decreased in model group rats. Shouwu Jiangqi Decoction (SJD) can enhance the expression of IRS-1 and PI3K (91). Irs-1 Ser307 plays a key role in insulin signaling (117). LWDH Pills can significantly downregulate the phosphorylation level of IRS-1 (S307) in PCOS-IR rats and upregulate the phosphorylation levels of PI3Kp85 alpha, Akt, and FoxO1a and the mRNA levels of FSHR and Cyp19a1. IR can be improved by regulating PI3K/Akt signaling pathway (69).
Berberine could restore HOMA-IR and ISI values to normal levels and enhance GLUT4 expression. After berberine treatment, ovarian morphology also returned to normal. Zhang N et al. found that berberine may reduce the PCOS pathology and IR value through PI3K/AKT activation and MAPK pathway inhibition of GLUT4 upregulation (118). Berberine significantly decreased the expression of mTOR mRNA and increased the expression of IRS-1 mRNA in GCs. Berberine enhances insulin sensitivity by modulating the IRS-1 signaling pathway and mammalian target of rapamycin (mTOR) in patients with PCOS (119).
Sterol regulatory Element Binding protein-1 (SREBP1) expression increased in PCOS rats, and overexpression of SREBP1 inhibited the phosphorylation of insulin receptor β and AKT in primary GCs; these can aggravate the mitochondrial dysfunction and oxidative stress. EA treatment inhibits SREBP1 expression by inducing the activation of the AMP-activated protein kinase (AMPK) signaling pathway in PCOS-like rats. EA intervention ameliorates IR, mitochondrial dysfunction, and oxidative stress by regulating lipid metabolism regulator SREBP1, thus alleviating PCOS-like symptoms (120). Sirtuin 3 (SIRT3) expression was significantly decreased in GCs of PCOS patients (121). Bu-Shen-Tian-Jing Formula effectively alleviated the pathogenesis of PCOS by improving oxidative stress and glucose metabolism via mitochondrial SIRT3 and the insulin-induced PI3K/AKT signaling pathway (122).
Cangfu Daotan Decoction can reduce ROS protein expression in PCOS ovarian granulosa cells, correct oxidative stress in vivo, and improve the rate of high-quality embryos. Network pharmacologic analysis revealed the AGE-RAGE signaling pathway, endocrine resistance, IL-17 signaling pathway, prolactin signaling pathway, and hypoxia-inducible factor 1 (HIF-1) signaling pathway in diabetic complications. In addition, PI3K-Akt, IR, Toll-like receptors, MAPK, and AGE-RAGE were associated with PCOS (123). Erxian decoction (EXD) was used to treat PCOS. PI3K-Akt, insulin resistance, toll-like receptor, MAPK, and AGE-RAGE were associated with EXD treatment of PCOS (124).
Chronic inflammation is accompanied by the occurrence and progression of PCOS. The expression levels of IL-6 and TNF-α in PCOS rats are significantly increased, and the Compound Malt Pill (CMP) treatment can reduce the expressions of IL-6 and TNF-α (125). Zhu Y et al. found that the Guizhi Fuling pill could improve the IR of PCOS patients by regulating intestinal flora and controlling inflammatory response (9, 9). TCM could inhibit the expression of serum LPS and TLR4, thus inhibiting the activation of NF-κB signaling-mediated inflammatory response in ovarian tissue (126).
Elevated levels of serum inflammatory cytokines IL-17a, IL-1Ra, and IL-6 lead to long-term subclinical inflammation in women. Abnormal changes in inflammatory factors can disrupt a woman’s normal ovulation and fertilization system, leading to PCOS, which is characterized by thinner periods and abnormal ovulation. TCM can effectively reduce oxidative stress-related complications in PCOS patients (92, 93). C. chinensis plays a therapeutic role in PCOS by regulating mRNA and protein expression levels of MAPK1, CXCL8, IL-6, and IL-1β (89). Quercetin significantly reduced the levels of insulin, IL-1β, IL-6, and TNF-α in blood and NF-κB nuclear translocation in GCs. This may be related to the inhibition of toll-like receptor/NF-κB signaling pathway and the improvement of the inflammatory microenvironment in ovarian tissue of PCOS rats (90).
Wang C et al. found that CFD could improve IR, restore serum hormone levels, inhibit inflammatory cytokines, and reduce ovarian morphological damage and apoptosis in PCOS rats. It was related to the regulation of gene expression mediated by the IGF-1-PI3K/Akt-Bax/Bcl-2 pathway (78). BJTF may improve reproductive endocrine homeostasis and promote follicular development and ovulation by inhibiting the PERK-ATF4-CHOP signaling pathway. In addition, the expression of GRP78 was downregulated, which delayed the apoptosis of ovarian GCs mediated by ER stress. Moreover, BJTF can improve the behavioral performance of rats by regulating brain monoamine neurotransmitters (46). BSZYD can repair ovarian lesions and improve apoptosis through the ERα-mediated PI3K/AKT/mTOR pathway (77).
A summary of the mechanism is shown in Figure 1.
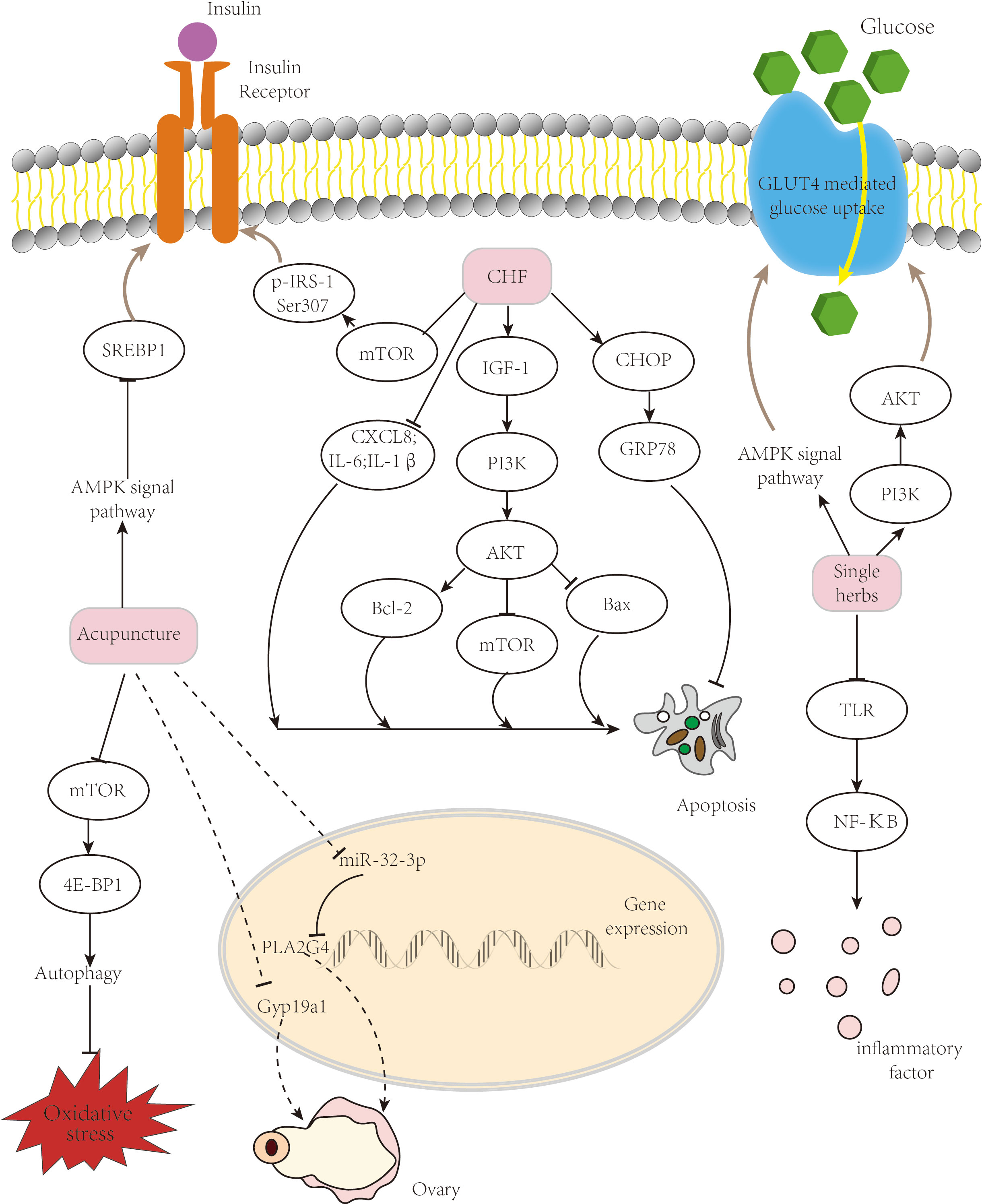
Figure 1 Mechanism of TCM treatment of PCOS. TCM ameliorates insulin resistance and improves follicular development via several pathways. TCM, traditional Chinese medicine; PCOS, polycystic ovary syndrome.
PCOS is an endocrine syndrome with a relatively complex etiology and pathogenesis and various clinical manifestations. There are some disadvantages of Western medicine in the treatment of this disease, such as numerous side effects (127), and TCM is relatively safe in improving the symptoms of endocrine and metabolic disorders in PCOS patients. The advantage of TCM treatment of PCOS lies in syndrome differentiation, in-depth analysis of the primary and secondary relationship of pathogenic factors in different stages, grasping individual characteristics, and closely following the syndrome type to establish an individualized treatment plan and can achieve good clinical efficacy. TCM treatment of PCOS based on syndrome differentiation has made some progress in clinical research. This article describes the mechanism of TCM treatment of PCOS, including correcting endocrine hormone disorder, gene expression, and regulatory factors; improving IR; correcting lipid metabolism disorder; and improving pregnancy outcomes (128, 129).
TCM has been shown to be effective in treating PCOS. However, so far, the understanding of the etiology, pathogenesis, and treatment of TCM is not uniform, and TCM treatment still lacks evidence-based medical evidence. The proposed prescription drugs are mainly based on the clinical experience of doctors. A set of standard treatment plans has not been formed, and further research, exploration, and summary are needed. In addition, TCM focuses on overall efficacy and clinical safety but lacks accurate analysis and monitoring. At present, there are not enough studies on the pharmacodynamics and toxicology mechanism of TCM, and the active ingredients of the drug are unclear. Based on the advantages and disadvantages of TCM treatment of PCOS, new technologies and means can be adopted in the future to screen out the pharmacoactive components, identify the bioactive compounds, predict their related targets, and clarify the molecular mechanism of action and toxicological effects of Chinese herbal medicine in order to improve the curative effect of TCM treatment and make it the preferred treatment method.
HC, CD, and ZM have contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
This work was supported by the COVID-19 Emergency Response Project of Shanghai Sixth People's Hospital in 2022 (ynxg202218), Shanghai Sixth People's Hospital Medical Service Level Improvement Project Clinical Medical Technical Backbone Team Cultivation Project (20220213); Three-year Action Plan (2021-2023) of Shanghai Municipality for Further Accelerating the Inheritance, Innovation, and Development of Traditional Chinese Medicine [grant number: ZY(2021-2023)-0205-04]; Construction of East China Area and Municipal TCM Specialist Disease Alliance [grant number: ZY(2021-2023)-0302]; Provincial General Project of Innovation and Entrepreneurship training Program for College students in Heilongjiang Province (S202210228075).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Azziz R. Polycystic ovary syndrome. Obstet Gynecol (2018) 132(2):321–36. doi: 10.1097/aog.0000000000002698
2. Ding T, Hardiman PJ, Petersen I, Baio G. Incidence and prevalence of diabetes and cost of illness analysis of polycystic ovary syndrome: a Bayesian modelling study. Hum Reprod (2018) 33(7):1299–306. doi: 10.1093/humrep/dey093
3. Gadalla MA, Norman RJ, Tay CT, Hiam DS, Melder A, Pundir J, et al. Medical and surgical treatment of reproductive outcomes in polycystic ovary syndrome: an overview of systematic reviews. Int J Fertil Steril (2020) 13(4):257–70. doi: 10.22074/ijfs.2020.5608
4. Yang S, Gu YY, Jing F, Yu CX, Guan QB. The effect of statins on levels of dehydroepiandrosterone (Dhea) in women with polycystic ovary syndrome: a systematic review and meta-analysis. Med Sci Monitor (2019) 25:590–7. doi: 10.12659/msm.914128
5. Tian Y, Zhao H, Chen HT, Peng YQ, Cui LL, Du YZ, et al. Variants in fshb are associated with polycystic ovary syndrome and luteinizing hormone level in han Chinese women. J Clin Endocrinol Metab (2016) 101(5):2178–84. doi: 10.1210/jc.2015-3776
6. Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine (2018) 62(2):318–25. doi: 10.1007/s12020-018-1692-3
7. Rawat K, Sandhu A, Gautam V, Saha PK, Saha L. Role of genomic DNA methylation in pcos pathogenesis: a systematic review and meta-analysis involving case-controlled clinical studies. Mol Hum Reprod (2022) 28(8):gaac024. doi: 10.1093/molehr/gaac024
8. Vagios S, James KE, Sacha CR, Hsu JY, Dimitriadis I, Bormann CL, et al. A patient-specific model combining antimullerian hormone and body mass index as a predictor of polycystic ovary syndrome and other oligo-anovulation disorders. Fertil Steril (2021) 115(1):229–37. doi: 10.1016/j.fertnstert.2020.07.023
9. Zhu Y, Li Y, Liu M, Hu XD, Zhu HQ. Guizhi fuling wan, Chinese herbal medicine, ameliorates insulin sensitivity in pcos model rats with insulin resistance Via remodeling intestinal homeostasis. Front Endocrinol (2020) 11:575. doi: 10.3389/fendo.2020.00575
10. Velez LM, Seldin M, Motta AB. Inflammation and reproductive function in women with polycystic ovary syndrome†. Biol Reprod (2021) 104(6):1205–17. doi: 10.1093/biolre/ioab050
11. Wu ZH, Tang Y, Niu X, Pu FF, Xiao XY, Kong W. Prostatic-specific antigen (Psa) levels in patients with polycystic ovary syndrome (Pcos): a meta-analysis. J Ovarian Res (2019) 12(1):94. doi: 10.1186/s13048-019-0569-2
12. Shen W, Jin B, Pan Y, Han Y, You T, Zhang Z, et al. The effects of traditional Chinese medicine-associated complementary and alternative medicine on women with polycystic ovary syndrome. Evid Based Complement Alternat Med (2021) 2021:6619597. doi: 10.1155/2021/6619597
13. Qu F, Li R, Sun W, Lin G, Zhang R, Yang J, et al. Use of electroacupuncture and transcutaneous electrical acupoint stimulation in reproductive medicine: a group consensus. J Zhejiang Univ-SCI B (2017) 18(3):186–93. doi: 10.1631/jzus.B1600437
14. Zhang HL, Li D, Li R, Huo ZJ, Qiao J. Treatment of polycystic ovary syndrome with acupuncture. Acupunct Med (2018) 36(4):269. doi: 10.1136/acupmed-2017-011570
15. Sheng JL, Jin XQ, Zhu JF, Chen YD, Liu X. The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and meta-analysis. Evidence-Based Complementary Altern Med (2019) 2019:12. doi: 10.1155/2019/9714313
16. Jia L-Y, Feng J-X, Li J-L, Liu F-Y, L-z X, Luo S-J, et al. The complementary and alternative medicine for polycystic ovary syndrome: a review of clinical application and mechanism. Evidence-Based Complementary Altern Med (2021) 2021:5555315. doi: 10.1155/2021/5555315
17. Li YW, Li W, Wang ST, Gong YN, Dou BM, Lyu ZX, et al. The autonomic nervous system: a potential link to the efficacy of acupuncture. Front Neurosci (2022) 16:1038945. doi: 10.3389/fnins.2022.1038945
18. Yu CC, Kong LH, Ma CY, Shen F, Yao GJ, Xiong Y, et al. The rules of acupoint-selection of acupuncture for polycystic ovary syndrome based on data mining. World J Acupunct-Moxibustion (2016) 26(3):73–8. doi: 10.1016/s1003-5257(17)30071-5
19. Guo S, Hu XY, Gao YL. [Professor shi yin's experience in treatment of obese polycystic ovary syndrome based on spleen and kidney functions]. Zhongguo Zhen Jiu (2021) 41(4):429–32. doi: 10.13703/j.0255-2930.20200225-0004
20. Zhao QY, Sun Y, Zhou J, Gao YL, Ma GZ, Hu ZH, et al. Effectiveness of herb-partitioned moxibustion combined with electroacupuncture on polycystic ovary syndrome in patients with symptom pattern of kidney deficiency and phlegm-dampne. J Tradit Chin Med (2021) 41(6):985–93. doi: 10.19852/j.cnki.jtcm.2021.06.017
21. Zhang JX, You XM, Yang J, Liu Y, Huang S, Lin QP, et al. [Effect of acupuncture on vascular endothelial function in patients of polycystic ovary syndrome with different glucose tolerance status]. Zhongguo Zhen Jiu (2021) 41(2):155–60. doi: 10.13703/j.0255-2930.20200110-k0007
22. Yu LQ, Cao LY, Xie J, Shi Y, Zhou LY, He TF, et al. [Efficacy and mechanism of electroacupuncture on insulin resistant polycystic ovary syndrome]. Zhongguo Zhen Jiu (2020) 40(4):379–83. doi: 10.13703/j.0255-2930.20190903-k0003
23. Zhang HL, Huo ZJ, Wang HN, Wang W, Chang CQ, Shi L, et al. [Acupuncture ameliorates negative emotion in pcos patients: a randomized controlled trial]. Zhongguo Zhen Jiu (2020) 40(4):385–90. doi: 10.13703/j.0255-2930.20191231-k0005
24. Zhuo Y, Wu J, Lin W, Pi M, Chen P, Yang Z. [the "Regulating conception-governor vessel" acupuncture method for infertility of polycystic ovarian syndrome]. Zhongguo Zhen Jiu (2016) 36(12):1237–41. doi: 10.13703/j.0255-2930.2016.12.002
25. Budihastuti UR, Melinawati E, Sulistyowati S, Nurwati I. Electroacupuncture effect on polycystic ovary syndrome to improve oocytes' growth. Med acupuncture (2019) 31(6):379–83. doi: 10.1089/acu.2019.1354
26. Chen H, Lim CED. The efficacy of using acupuncture in managing polycystic ovarian syndrome. Curr Opin Obstet Gynecol (2019) 31(6):428–32. doi: 10.1097/gco.0000000000000582
27. Cao Y, Chen H, Zhao D, Zhang L, Yu X, Zhou X, et al. The efficacy of tung's acupuncture for sex hormones in polycystic ovary syndrome: a randomized controlled trial. Complement Ther Med (2019) 44:182–8. doi: 10.1016/j.ctim.2019.04.016
28. Huang S, Hu M, Ng EHY, Stener-Victorin E, Zheng Y, Wen Q, et al. A multicenter randomized trial of personalized acupuncture, fixed acupuncture, letrozole, and placebo letrozole on live birth in infertile women with polycystic ovary syndrome. Trials (2020) 21(1):239. doi: 10.1186/s13063-020-4154-1
29. Gu S, Fan AY. Controversial conclusions from two randomized controlled trials for acupuncture's effects on polycystic ovary syndrome or in vitro fertilization support. J Integr Med (2020) 18(2):89–91. doi: 10.1016/j.joim.2020.01.007
30. Wu X-K, Stener-Victorin E, Kuang H-Y, Ma H-L, Gao J-S, Xie L-Z, et al. Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA (2017) 317(24):2502–14. doi: 10.1001/jama.2017.7217
31. Wen Q, Hu M, Lai M, Li J, Hu Z, Quan K, et al. Effect of acupuncture and metformin on insulin sensitivity in women with polycystic ovary syndrome and insulin resistance: a three-armed randomized controlled trial. Hum Reprod (2022) 37(3):542–52. doi: 10.1093/humrep/deab272
32. Yin Y, Zhang Y, Zhang H, Jiang D, Guo G. [Clinical therapeutic effects of acupuncture combined with Chinese herbal medicine on infertility of polycystic ovary syndrome in the patients with ovulation induction with letrozole]. Zhongguo Zhen Jiu (2018) 38(1):27–32. doi: 10.13703/j.0255-2930.2018.01.006
33. Jo J, Lee YJ, Lee H. Acupuncture for polycystic ovarian syndrome: a systematic review and meta-analysis. Medicine (2017) 96(23):10. doi: 10.1097/md.0000000000007066
34. Yang J, Liu Y, Huang J, Xu J, You X, Lin Q, et al. [Acupuncture and Chinese medicine of artificial cycle therapy for insulin resistance of polycystic ovary syndrome with phlegm damp type and its mechanism]. Zhongguo Zhen Jiu (2017) 37(11):1163–8. doi: 10.13703/j.0255-2930.2017.11.007
35. Jin C, Pang R, Xu L, Wu Z, Zhao J. Clinical rules for acupoint selection and prescription composition in treatment of polycystic ovary syndrome with acupuncture. Zhongguo zhen jiu = Chin acupuncture moxibustion (2015) 35(6):625–30. doi: 10.13703/j.0255-2930.2015.06.032
36. Shao C, Dong W, Zhang H. Application of guijiaosan shenque acupoint paste can improve the scores of obesity, endocrine and tcm symptoms in treating obese polycystic ovary syndrome. Am J Transl Res (2021) 13(9):10694–702.
37. Qin WM, Zhao K, Yang HY. Effect of acupoint catgut embedding therapy combined with Chinese medicine for nourishing the kidneys and promoting blood circulation and improving blood glucose and lipid levels as well as the pregnancy rate in obese pcos patients with infertility. Exp Ther Med (2016) 12(5):2909–14. doi: 10.3892/etm.2016.3715
38. Liao WT, Chiang JH, Li CJ, Lee MT, Su CC, Yen HR. Investigation on the use of traditional Chinese medicine for polycystic ovary syndrome in a nationwide prescription database in Taiwan. J Clin Med (2018) 7(7):15. doi: 10.3390/jcm7070179
39. Lai L, Flower A, Prescott P, Wing T, Moore M, Lewith G. Standardised versus individualised multiherb Chinese herbal medicine for oligomenorrhoea and amenorrhoea in polycystic ovary syndrome: a randomised feasibility and pilot study in the uk. BMJ Open (2017) 7(2):12. doi: 10.1136/bmjopen-2016-011709
40. Arentz S, Smith CA, Abbott J, Fahey P, Cheema BS, Bensoussan A. Combined lifestyle and herbal medicine in overweight women with polycystic ovary syndrome (Pcos): a randomized controlled trial. Phytother Res (2017) 31(9):1330–40. doi: 10.1002/ptr.5858
41. Shang H, Yu L, Sun J, Shi Y. [Professor shi yin's experience of staging, classification and sorting method for polycystic ovary syndrome]. Zhongguo Zhen Jiu (2016) 36(12):1296–301. doi: 10.13703/j.0255-2930.2016.12.019
42. Zhang C, Xu X, Huang Y, Li W, Deng D, Luo F. The method of kidney nourishing and phlegm removing method by the apn signal pathway regulating the mechanism research of the glucose and lipid metabolism disorder in obese pcos rats. Lishizhen Med Materia Med Res (2018) 29(5):1025–8.
43. Chen W-J, Wang F-F. Effect of quyu huatan decoction on lipid metabolism and hormone levels of patients with polycystic ovary syndrome. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin materia Med (2016) 41(3):532–5. doi: 10.4268/cjcmm20160329
44. Chen X. Effect of removing blood stasis and phlegm method to the comprehensive sex hormone and ovarian morphology in rats with polycystic ovary syndrome. Chin J Basic Med Traditional Chin Med (2015) 21(12):1514. doi: 10.19945/j.cnki.issn.1006-3250.2015.12.016
45. Zhao YM, Zheng DX, Cheng R, Xu X, Lian FM, Tong XL, et al. Dose-effect analysis of treatment by modified zhibaidihuang decoction on polycystic ovary syndrome hyperandrogenism. J Tradit Chin Med (2018) 38(2):280–6.
46. Pan X, Liu Y, Liu L, Pang B, Sun Z, Guan S, et al. Bushen jieyu tiaochong formula reduces apoptosis of granulosa cells via the perk-Atf4-Chop signaling pathway in a rat model of polycystic ovary syndrome with chronic stress. J Ethnopharmacol (2021) 292:114923. doi: 10.1016/j.jep.2021.114923
47. Yu J, Liu L, Zhai D, Zhang D, Chen W, Yao R, et al. The effects of yushi qinggan recipe in treating polycystic ovary syndrome with dampness-heat of gan channel: a randomized controlled trial. Chin J Integrated Traditional Western Med (2019) 39(3):282–7.
48. Zhou X, Liu Y, Yue W, Wang Q, Wang Y. Clinical effects of bushen huoxue culuan prescription in the treatment of kidney and blood stasis due to polycystic ovary syndrome. Chin J Clin Pharmacol (2017) 33(9):786–9. doi: 10.13699/j.cnki.1001-6821.2017.09.005
49. Liang Y, Q-h T, Mu Y-x, Du H-l. Effects of cangfu congxian decoction on oxidative stress in polycystic ovary syndrome patients. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin J integrated traditional Western Med (2016) 36(6):685–9.
50. Du J. Effect of bushen tiaojing decoction in promoting ovulation among polycystic ovarian syndrome barrenness patients. Chin J Exp Traditional Med Formulae (2015) 21(16):171–4. doi: 10.13422/j.cnki.syfjx.2015160171
51. Ma R, Wang X, Zhu X. Clinical efficacy of bushen tiaochong decoction in obesity patients with polycystic ovary syndrome and effect on endometrial receptivity. Chin J Exp Traditional Med Formulae (2018) 24(5):188–92. doi: 10.13422/j.cnki.syfjx.2018050188
52. Wang N, Yang Y, Wang H, Yang C. Clinical research of menshi xiaonang yin combined with tanshinone capsules in the treatment of kidney deficiency and phlegm stasis type polycystic ovary syndrome. Lishizhen Med Materia Med Res (2018) 29(9):2200–3.
53. Luo P, Tang F, He M, Li C. Efficacy of yangyinshugan capsule on ovarian hemodynamics and pregnancy outcomes in patients with polycystic ovarian syndrome complicated with infertility. Pharmacol Clinics Chin Materia Med (2018) 34(2):109–12. doi: 10.13412/j.cnki.zyyl.2018.02.029
54. Liu Y, Song C, Bao H, Xu H, Long S, Quan X, et al. Dihuang wan combined with xionggui erchen tang on hyperandrogenism and polycystic ovary syndrome with syndrome of kidney deficiency and blood stasis. Chin J Exp Traditional Med Formulae (2018) 24(18):180–5. doi: 10.13422/j.cnki.syfjx.20181830
55. Li J, Zheng R, Lin Z, Hu F, Lin Y, Zeng G, et al. Impact of Chinese herbal medicine on glucolipid metabolic outcomes in women with polycystic ovary syndrome: a systematic review and meta-analysis. Evid Based Complement Alternat Med (2022) 2022:3245663. doi: 10.1155/2022/3245663
56. Shayan A, Masoumi SZ, Shobeiri F, Tohidi S, Khalili A. Comparing the effects of agnugol and metformin on oligomenorrhea in patients with polycystic ovary syndrome: a randomized clinical trial. J Clin Diagn Res (2016) 10(12):Qc13–qc6. doi: 10.7860/jcdr/2016/22584.9040
57. Tang X, Huang Q, Wang C, Zhang D, Dong S, Yu C. Kuntai capsule combined with letrozole on gonadal hormone levels and ovarian function in patients with pcos: a systematic review and meta-analysis. Front Endocrinol (Lausanne) (2021) 12:789909. doi: 10.3389/fendo.2021.789909
58. Ma QW, Tan Y. Effectiveness of Co-treatment with traditional Chinese medicine and letrozole for polycystic ovary syndrome: a meta-analysis. J Integr Med (2017) 15(2):95–101. doi: 10.1016/s2095-4964(17)60320-0
59. Wang C, Li Y. Bailing tiaogan decoction in treating infertilitas feminis of liver qi stagnation type polycystic ovary syndrome. Chin J Exp Traditional Med Formulae (2016) 22(13):165–8. doi: 10.13422/j.cnki.syfjx.2016130165
60. Wei A, Xiaohui D, Song Y. Clinical efficacy observation of dingkun pills combined with clomiphene in the treatment of polycystic ovary syndrome with infertility. Chineses J Pract Gynecol Obstetrics (2018) 34(4):444–7. doi: 10.19538/j.fk2018040123
61. Zhou K, Zhang J, Xu L, Lim CED. Chinese Herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Systematic Rev (2021) 6(6):CD007535. doi: 10.1002/14651858.CD007535.pub4
62. Xu B, Li M, Chen C, Luo Y. Effects of tcm periodic therapy on sex hormone, glucose and lipid metabolism of patients with polycystic ovary syndrome. Chin J Inf Traditional Chin Med (2016) 23(1):35–8.
63. Diao Y, Yan H, Huang Y, Qi L. A clinical observation on treating polycystic ovary syndrome with insulin resistance and obesity patients by integrated Chinese and Western medicine. Chin J Basic Med Traditional Chin Med (2017) 23(8):1170–3. doi: 10.19945/j.cnki.issn.1006-3250.2017.08.044
64. Liu X, Xu X, Zheng D, Wen S, Liu R, Cheng R, et al. Treatment of pcos hyperandrogenism patients with yin deficiency induced fire hyperactivity syndrome by modified zhibai dihuang decoction. Chin J Integrated Traditional Western Med (2018) 38(1):29–32.
65. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
66. Moghetti P, Tosi F. Insulin resistance and pcos: chicken or egg? J Endocrinol Invest (2021) 44(2):233–44. doi: 10.1007/s40618-020-01351-0
67. Liang RN, Liu Z, Li PS, Fan P, Xu L, Sun XY, et al. Kuntai capsules improve glucolipid metabolism in patients with polycystic ovary syndrome a randomized, double-blind, placebo-controlled trial. Medicine (2019) 98(39):5. doi: 10.1097/md.0000000000016788
68. Sun X, Ding C, Yang X, Zhan X, Sun X, Ding CF, et al. Effects of bushen tiaogan recipe on glucolipid metabolism in non-obese patients with polycystic ovary syndrome. Chin J Integrated Traditional Western Med (2017) 37(5):530–3.
69. Qiu Z, Dong J, Xue C, Li X, Liu K, Liu B, et al. Liuwei dihuang pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through Pi3k/Akt signaling pathway. J Ethnopharmacol (2020) 250:111965. doi: 10.1016/j.jep.2019.111965
70. Zhao HX, Zhou DC, Chen Y, Liu DL, Chu SF, Zhang SM. Beneficial effects of heqi San on rat model of polycystic ovary syndrome through the Pi3k/Akt pathway. Daru-Journal Pharm Sci (2017) 25:12. doi: 10.1186/s40199-017-0188-7
71. Deng Y, Xue W, Wang YF, Liu XH, Zhu SY, Ma X, et al. Insulin resistance in polycystic ovary syndrome improved by Chinese medicine dingkun pill (???): a randomized controlled clinical trial. Chin J Integr Med (2019) 25(4):246–51. doi: 10.1007/s11655-018-2947-1
72. Zhang T. Effect of qingre yangyin recipe on endocrine and metabolism of polycystic ovary syndrome patients. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin J integrated traditional Western Med (2015) 35(10):1175–80.
73. Zhang N, Li C, Guo Y, Wu HC. Study on the intervention effect of qi gong wan prescription on patients with phlegm-dampness syndrome of polycystic ovary syndrome based on intestinal flora. Evid Based Complement Alternat Med (2020) 2020:6389034. doi: 10.1155/2020/6389034
74. Shokrpour M, Asemi Z. The effects of magnesium and vitamin e Co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. Biol Trace Elem Res (2019) 191(1):54–60. doi: 10.1007/s12011-018-1602-9
75. Lu C, Zhao X, Li Y, Li Y, Yuan C, Xu F, et al. Serum metabolomics study of traditional Chinese medicine formula intervention to polycystic ovary syndrome. J Pharm BioMed Anal (2016) 120:127–33. doi: 10.1016/j.jpba.2015.12.020
76. Yi WM, Li X, Chen K, Zhu M, Cai XH, Pan AZ. Effects of cangfu daotan decoction on obese polycystic ovary syndrome and its mechanism. Steroids (2021) 165:8. doi: 10.1016/j.steroids.2020.108740
77. Jiang X, Yuan Y, Shi M, Zhang S, Sui M, Zhou H. Bu-Shen-Zhu-Yun decoction inhibits granulosa cell apoptosis in rat polycystic ovary syndrome through estrogen receptor α-mediated Pi3k/Akt/Mtor pathway. J Ethnopharmacol (2021) 288:114862. doi: 10.1016/j.jep.2021.114862
78. Wang C, Ding C, Hua Z, Chen C, Yu J. Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome Via igf-1-Pi3k/Akt-Bax/Bcl-2 pathway. Mediators Inflammation (2020) 2020:8865647. doi: 10.1155/2020/8865647
79. Sun HY, Li Q, Liu YY, Wei XH, Pan CS, Fan JY, et al. Xiao-Yao-San, a Chinese medicine formula, ameliorates chronic unpredictable mild stress induced polycystic ovary in rat. Front Physiol (2017) 8:729. doi: 10.3389/fphys.2017.00729
80. Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, et al. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, furocyst (Tm)) in polycystic ovary syndrome (Pcos). Int J Med Sci (2015) 12(10):825–31. doi: 10.7150/ijms.13024
81. Khan AA, Begum W. Efficacy of darchini in the management of polycystic ovarian syndrome: a randomized clinical study. J Herb Med (2019) 15:5. doi: 10.1016/j.hermed.2018.11.005
82. Hajimonfarednejad M, Nimrouzi M, Heydari M, Zarshenas MM, Raee MJ, Jahromi BN. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: a randomized double-blind placebo controlled clinical trial. Phytother Res (2018) 32(2):276–83. doi: 10.1002/ptr.5970
83. Borzoei A, Rafraf M, Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac J Clin Nutr (2018) 27(3):556–63. doi: 10.6133/apjcn.062017.02
84. Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol (2014) 211(5):6. doi: 10.1016/j.ajog.2014.05.009
85. Miao M, Peng M, Yan X, Zhu Z, Bai M, Wei Z, et al. Effect of dodder total flavone on polycystic ovary syndrome rat models induced by letrozole. Chin J Exp Traditional Med Formulae (2019) 25(16):17–23. doi: 10.13422/j.cnki.syfjx.20191607
86. Cicero AFG, Baggioni A. Berberine and its role in chronic disease. In: Gupta SC, Prasad S, Aggarwal BB, editors. Anti-inflammatory nutraceuticals and chronic diseases, vol. 928 . Cham: Springer International Publishing Ag (2016). p. 27–45. Advances in Experimental Medicine and Biology.
87. Li MF, Zhou XM, Li XL. The effect of berberine on polycystic ovary syndrome patients with insulin resistance (Pcos-ir): a meta-analysis and systematic review. Evidence-Based Complementary Altern Med (2018) 2018:8. doi: 10.1155/2018/2532935
88. Wu XK, Wang YY, Liu JP, Liang RN, Xue HY, Ma HX, et al. Randomized controlled trial of letrozole, berberine, or a combination for infertility in the polycystic ovary syndrome. Fertil Steril (2016) 106(3):757. doi: 10.1016/j.fertnstert.2016.05.022
89. Ma JX, Ye M, Ma K, Zhou K, Zhang Y, Wang X, et al. Network pharmacology-based strategy for predicting active ingredients and potential targets of coptis chinensis franchin polycystic ovary syndrome. Evid Based Complement Alternat Med (2021) 2021:6651307. doi: 10.1155/2021/6651307
90. Wang Z, Zhai D, Zhang D, Bai L, Yao R, Yu J, et al. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci (2017) 24(5):682–90. doi: 10.1177/1933719116667218
91. Wang LH, Wang X, Yu XZ, Xu WT. Potent therapeutic effects of shouwu jiangqi decoction on polycystic ovary syndrome with insulin resistance in rats. Chin J Integr Med (2016) 22(2):116–23. doi: 10.1007/s11655-015-2147-9
92. Jamilian M, Foroozanfard F, Kavossian E, Aghadavod E, Shafabakhsh R, Hoseini A, et al. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN (2020) 36:128–33. doi: 10.1016/j.clnesp.2020.01.005
93. Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of curcumin supplementation on oxidative stress, sirtuin-1 and peroxisome proliferator activated receptor gamma coactivator 1 alpha gene expression in polycystic ovarian syndrome (Pcos) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr-Clin Res Rev (2020) 14(2):77–82. doi: 10.1016/j.dsx.2020.01.002
94. Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-crp levels in Overweight/Obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med (2019) 47:7. doi: 10.1016/j.ctim.2019.102201
95. Amini L, Mojab F, Jahanfar S, Sepidarkish M, Raoofi Z, Maleki-Hajiagha A. Efficacy of salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: a double-blinded placebo-controlled clinical trial. Complement Ther Med (2020) 48:7. doi: 10.1016/j.ctim.2019.102245
96. Jamilian M, Asemi Z. The effects of soy isoflavones on metabolic status of patients with polycystic ovary syndrome. J Clin Endocrinol Metab (2016) 101(9):3386–94. doi: 10.1210/jc.2016-1762
97. Haj-Husein I, Tukan S, Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J Hum Nutr Diet (2016) 29(1):105–11. doi: 10.1111/jhn.12290
98. Yeung A, Chan JSM, Cheung JC, Zou L. Qigong and tai-chi for mood regulation. Focus (Am Psychiatr Publ) (2018) 16(1):40–7. doi: 10.1176/appi.focus.20170042
99. Ding M, Wang CY, Dong XS, Yi XR. The effects of qigong on type 2 diabetes mellitus: a systematic review and meta-analysis. Evidence-Based Complementary Altern Med (2018) 2018:8. doi: 10.1155/2018/8182938
100. Chao MY, Wang CY, Dong XS, Ding M. The effects of tai chi on type 2 diabetes mellitus: a meta-analysis. J Diabetes Res (2018) 2018:9. doi: 10.1155/2018/7350567
101. Chan AWK, Chair SY, Lee DTF, Leung DYP, Sit JWH, Cheng HY, et al. Tai chi exercise is more effective than brisk walking in reducing cardiovascular disease risk factors among adults with hypertension: a randomised controlled trial. Int J Nurs Stud (2018) 88:44–52. doi: 10.1016/j.ijnurstu.2018.08.009
102. Yu XF, Chau JPC, Huo LT. The effectiveness of traditional Chinese medicine-based lifestyle interventions on biomedical, psychosocial, and behavioral outcomes in individuals with type 2 diabetes: a systematic review with meta-analysis. Int J Nurs Stud (2017) 80:165–80. doi: 10.1016/j.ijnutstu.2018.01.009
103. Song QH, Yuan YD, Jiao C, Zhu XM. Curative effect of tai chi exercise in combination with auricular plaster therapy on improving obesity patient with secondary hyperlipidemia. Int J Clin Exp Med (2015) 8(11):21386–92.
104. Yang J, Lin QP, You XM, Zhang JX, Liu Y, Huang J, et al. [Effect of combined treatment with acupuncture, moxibustion and medication on endometrial receptivity and expression of serum Hoxa10 in polycystic ovary syndrome of kidney deficiency and blood stagnation]. Zhongguo Zhen Jiu (2020) 40(11):1154–8. doi: 10.13703/j.0255-2930.20191022-k0006
105. Shi Y, Li L, Zhou J, Sun J, Chen L, Zhao J, et al. Efficacy of electroacupuncture in regulating the imbalance of amh and fsh to improve follicle development and hyperandrogenism in pcos rats. BioMed Pharmacother (2019) 113:108687. doi: 10.1016/j.biopha.2019.108687
106. Xu G, Zhang A-D, Wang X, Liu J-D, Feng J-W, Chen Y-L. [Effect of electroacupuncture at different acupoints on follicle development and related factors in serum and ovary tissues of pcos rats]. Zhen Ci Yan Jiu (2019) 44(10):740–6. doi: 10.13702/j.1000-0607.190041
107. Chen X, Tang H, Liang Y, Wu P, Xie L, Ding Y, et al. Acupuncture regulates the autophagy of ovarian granulosa cells in polycystic ovarian syndrome ovulation disorder by inhibiting the Pi3k/Akt/Mtor pathway through Lncmeg3. BioMed Pharmacother (2021) 144:112288. doi: 10.1016/j.biopha.2021.112288
108. Tong X, Liu Y, Xu X, Shi J, Hu W, Ma T, et al. Ovarian innervation coupling with vascularity: the role of electro-acupuncture in follicular maturation in a rat model of polycystic ovary syndrome. Front Physiol (2020) 11:474. doi: 10.3389/fphys.2020.00474
109. Guo Y, Tan Y, Zou Y. Effects of bushen cupailuan decotion on granular cell aromatase in polycystic ovary syndrome model rats. J Nanjing Univ Traditional Chin Med (2016) 32(1):38–40. doi: 10.14148/j.issn.1672-0482.2016.0038
110. Yang H, Lee YH, Lee SR, Kaya P, Hong EJ, Lee HW. Traditional medicine (Mahuang-tang) improves ovarian dysfunction and the regulation of steroidogenic genes in letrozole-induced pcos rats. J Ethnopharmacol (2020) 248:9. doi: 10.1016/j.jep.2019.112300
111. Yu J, Wang Z, Zhou L, Sun S, Huang L, Chai Z, et al. Study of cryptotanshinone on improving hyperandrogenism of polycystic ovary syndrome Via down-regulating the expression of gene Cyp17 and ar. China J Traditional Chin Med Pharm (2014) 29(5):1699–705.
112. Wu J, Chen X. Acupuncture therapy protects pcos patients with diabetes by regulating mir-32-3p/Pla2g4a pathway. Am J Transl Res (2021) 13(8):8819–32.
113. Peng Y, Guo LY, Gu AX, Shi BB, Ren YK, Cong J, et al. Electroacupuncture alleviates polycystic ovary syndrome-like symptoms through improving insulin resistance, mitochondrial dysfunction, and endoplasmic reticulum stress Via enhancing autophagy in rats. Mol Med (2020) 26(1):13. doi: 10.1186/s10020-020-00198-8
114. Chen Y, Chai X, Zhao Y, Yang X, Zhong C, Feng Y. Investigation of the mechanism of zishen yutai pills on polycystic ovary syndrome: a network pharmacology and molecular docking approach. Evid Based Complement Alternat Med (2021) 2021:6843828. doi: 10.1155/2021/6843828
115. Lian F, Zhang S, Sun Z, Yu Y, Xiang S. Effects of erzhi tiangui and qigong compound on the akt-Glut4 insulin signal pathway of polycystic ovarian syndrome and ivf-et outcomes. Chin J Integrated Traditional Western Med (2018) 38(4):410–4.
116. Xu J, Wang X, Ji F, Huang L, Zheng L, Yang J, et al. Effect of tanzhixiao recipe on Pi3k/Akt pathway in pcos-ir model rats. Chin J Exp Traditional Med Formulae (2016) 22(24):156–60. doi: 10.13422/j.cnki.syfjx.2016240156
117. Xie Y, Huang D, Li Q, Lu F, Xu L, Zou X, et al. [Effects of bushen tongmai recipe on expression of irs-1 Ser307 in polycystic ovarian syndrome rats accompanying with insulin resistance]. Zhongguo Zhong Yao Za Zhi (2010) 35(5):635–8. doi: 10.4268/cjcmm20100521
118. Zhang N, Liu XY, Zhuang LL, Liu XM, Zhao HS, Shan YH, et al. Berberine decreases insulin resistance in a pcos rats by improving Glut4: dual regulation of the Pi3k/Akt and mapk pathways. Regul Toxicol Pharmacol (2020) 110:6. doi: 10.1016/j.yrtph.2019.104544
119. Kuang HY, Duan YW, Li D, Xu YW, Ai WX, Li W, et al. The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome. PloS One (2020) 15(8):14. doi: 10.1371/journal.pone.0235404
120. Peng Y, Yang XM, Luo X, Liu CH, Cao X, Wang HY, et al. Novel mechanisms underlying anti-polycystic ovary like syndrome effects of electroacupuncture in rats: suppressing Srebp1 to mitigate insulin resistance, mitochondrial dysfunction and oxidative stress. Biol Res (2020) 53(1):13. doi: 10.1186/s40659-020-00317-z
121. Zhang Q, Ren J, Wang F, Pan M, Cui L, Li M, et al. Mitochondrial and glucose metabolic dysfunctions in granulosa cells induce impaired oocytes of polycystic ovary syndrome through sirtuin 3. Free Radic Biol Med (2022) 187:1–16. doi: 10.1016/j.freeradbiomed.2022.05.010
122. Zhang Q, Ren J, Wang F, Li M, Pan M, Zhang H, et al. Chinese Herbal medicine alleviates the pathogenesis of polycystic ovary syndrome by improving oxidative stress and glucose metabolism Via mitochondrial sirtuin 3 signaling. Phytomedicine (2023) 109:154556. doi: 10.1016/j.phymed.2022.154556
123. Xu WT, Tang MY, Wang JH, Wang LH. Identification of the active constituents and significant pathways of cangfu daotan decoction for the treatment of pcos based on network pharmacology. Evidence-Based Complementary Altern Med (2020) 2020:15. doi: 10.1155/2020/4086864
124. Liu L, Du B, Zhang H, Guo X, Zhou Z, Xiu A, et al. A network pharmacology approach to explore the mechanisms of erxian decoction in polycystic ovary syndrome. Chin Med (2018) 13:46. doi: 10.1186/s13020-018-0201-1
125. Lan N, Wang S, Qiu W, Chen R. Effects of compound malt pill on the expression of il-6,Tnf-Alpha in pcos model rats. Pharmacol Clinics Chin Materia Med (2016) 32(3):136–40. doi: 10.13412/j.cnki.zyyl.2016.03.036
126. Wang Y, Xiao H, Liu Y, Tong Q, Yu Y, Qi B, et al. Effects of bu shen hua zhuo formula on the Lps/Tlr4 pathway and gut microbiota in rats with letrozole-induced polycystic ovary syndrome. Front Endocrinol (Lausanne) (2022) 13:891297. doi: 10.3389/fendo.2022.891297
127. Morgante G, Massaro MG, Di Sabatino A, Cappelli V, De Leo V. Therapeutic approach for metabolic disorders and infertility in women with pcos. Gynecol Endocrinol (2018) 34(1):4–9. doi: 10.1080/09513590.2017.1370644
128. Jazani AM, Azgomi HND, Azgomi AND, Azgomi RND. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (Pcos). Daru-Journal Pharm Sci (2019) 27(2):863–77. doi: 10.1007/s40199-019-00312-0
Keywords: polycystic ovary syndrome, TCM, cellular endocrinology, mechanism of action, metabolic disorders
Citation: Chen H, Deng C, Meng Z and Meng S (2023) Effects of TCM on polycystic ovary syndrome and its cellular endocrine mechanism. Front. Endocrinol. 14:956772. doi: 10.3389/fendo.2023.956772
Received: 30 May 2022; Accepted: 03 April 2023;
Published: 16 May 2023.
Edited by:
Xiaohua Li, Seventh People’s Hospital of Shanghai, ChinaReviewed by:
Hongli Zhang, Seventh People’s Hospital of Shanghai, ChinaCopyright © 2023 Chen, Deng, Meng and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengxi Meng, bWVuZ3N4MTYzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.