- 1Department of General Surgery, Digestive Disease Hospital, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Department of Breast and Thyroid Surgery, The Third Affiliated Hospital of Zunyi Medical University (The First People’s Hospital of Zunyi), Zunyi, Guizhou, China
Aims: To investigate the potential association between type 2 diabetes (T2D) and hepatocellular carcinoma (HCC) in East Asian populations using Mendelian randomization (MR) analyses.
Methods: Bidirectional Mendelian randomization (MR) studies were conducted using summary statistics from genome-wide association studies (GWAS) related to T2D and HCC. The potential effects of confounders such as chronic hepatitis B, chronic hepatitis C, body mass index, and alcohol intake frequency were corrected using a multivariate MR study. Various MR methods, including the inverse variance weighted (IVW) approach, were used to estimate the associations between T2D and HCC. Sensitivity analysis and assessment of heterogeneity were performed to ensure the robustness of the results.
Results: In the forward MR study, the IVW approach of MR analysis suggested an inverse association between T2D and HCC, with a risk odds ratio of 0.8628 (95% confidence interval [CI], 0.7888–0.9438). Furthermore, even after adjusting for BMI, chronic hepatitis B, and alcohol intake frequency, this study still supports the inverse association between T2D and HCC. Additional MR methods provided further support for this relationship. Sensitivity analysis and assessment of heterogeneity confirmed the robustness of the results. The reverse MR analysis did not show a clear impact of genetic liability to HCC on reduced risk of T2D(OR=0.9788; 95% CI, 0.9061-1.0574).
Conclusion: This study provides evidence of an inverse association between T2D and HCC in East Asian populations using MR analysis. Further studies are warranted to validate these findings.
1 Introduction
Hepatocellular carcinoma (HCC) is the predominant subtype of liver cancer and poses an alarming health challenge. Research estimates indicate that HCC makes up about 75-85% of all liver cancer cases worldwide (1). HCC incidence has exhibited a steady rise over the past few decades, thus intensifying the gravity of public health implications. Type 2 diabetes (T2D) is a metabolic disorder defined by hyperglycemia and insulin resistance (2, 3). It is a significant global health issue affecting millions of individuals worldwide. The incidence of T2D has been persistently increasing and is projected to continue to rise in the foreseeable future (4, 5).
The relationship between T2D and HCC is still a matter of debate. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, along with obesity and alcohol consumption, have been widely established as significant risk factors for the development of HCC (6–9). Moreover, chronic hyperglycemia, insulin resistance, and inflammation, common in T2D, are also believed to contribute to HCC development and progression (10, 11). Although T2D is suggested to be the most important risk factor for HCC (12), considering the possibility of reverse causality, the question of whether diabetes increases the risk of HCC independently of other risk factors has not been definitively answered.
Based on current research, both chronic HCV itself and HCV-associated cirrhosis may increase the risk of subsequent development of T2D, and existing diabetes appears to accelerate the progression of chronic HCV infection to cirrhosis (13, 14). Similarly, hepatitis B is also more prevalent in individuals with T2D, with a higher risk of chronic infection and liver-related complications (15). Excessive alcohol consumption is a well-established risk factor for the development of HCC. In patients with T2D, excessive alcohol consumption has been associated with an increased risk of liver complications and metabolic syndrome (16).Shared risk factors, such as obesity, alcohol consumption, hepatitis B or C infections and other risk factors, further complicate the T2D-HCC relationship (17, 18). Therefore, establishing a causal link remains challenging due to confounding factors and reverse causality.
Mendelian randomization (MR) employs genetic variants as instrumental variables to investigate the causal link between the exposure (such as T2D) and the outcome (such as HCC) (19, 20). This approach can overcome limitations of traditional observational studies, such as confounding and reverse causality. Two types of MR approaches, namely bidirectional MR and multivariable MR, have gained attention for their ability to address confounding factors (21). Bidirectional MR investigates the association between the exposure and an outcome by using genetic variants associated with the exposure as instrumental variables and genetic variants associated with the outcome as instrumental variables (22). This approach helps to explore whether the exposure is a risk factor for outcome or vice versa. Multivariable MR, on the other hand, allows for the adjustment of multiple confounding factors simultaneously (23). By incorporating genetic variants associated with potential confounders, such as obesity or viral hepatitis infections, this approach helps to remove the influence of these factors on the relationship between the exposure and outcome, providing more robust evidence.
Given the high prevalence of T2D and HCC in East Asian populations, understanding the association between these two conditions is crucial for developing effective prevention and treatment strategies.By employing bidirectional MR and multivariable MR, we aims to elucidate the potential causal association between T2D and HCC risk, while also accounting for confounding factors. The findings of this study will contribute to our understanding of the aetiology of HCC in East Asian populations and may have implications for the development of targeted interventions to reduce the burden of T2D and HCC in these populations.
2 Materials and methods
2.1 Study design
See Figure 1 for the study design. In the MR study, summary-level data of genome-wide association analyses on T2D, HCC, and confounding factors including Chronic hepatitis B(CHB), Chronic hepatitis C(CHC), body mass index(BMI), and alcohol intake frequency from published genome-wide association studies (GWASs). We first performed a forward MR study to examine the causative risk of T2D for HCC and corrected for confounders including CHB, CHC, BMI and alcohol intake frequency using multivariable MR analysis. We then performed reverse MR analysis to investigate the relationship between genetic susceptibility to HCC and T2D. The GWAS cited in this article were all approved by the relevant review board. The MR study did not need an ethical permit based on summary-level data.
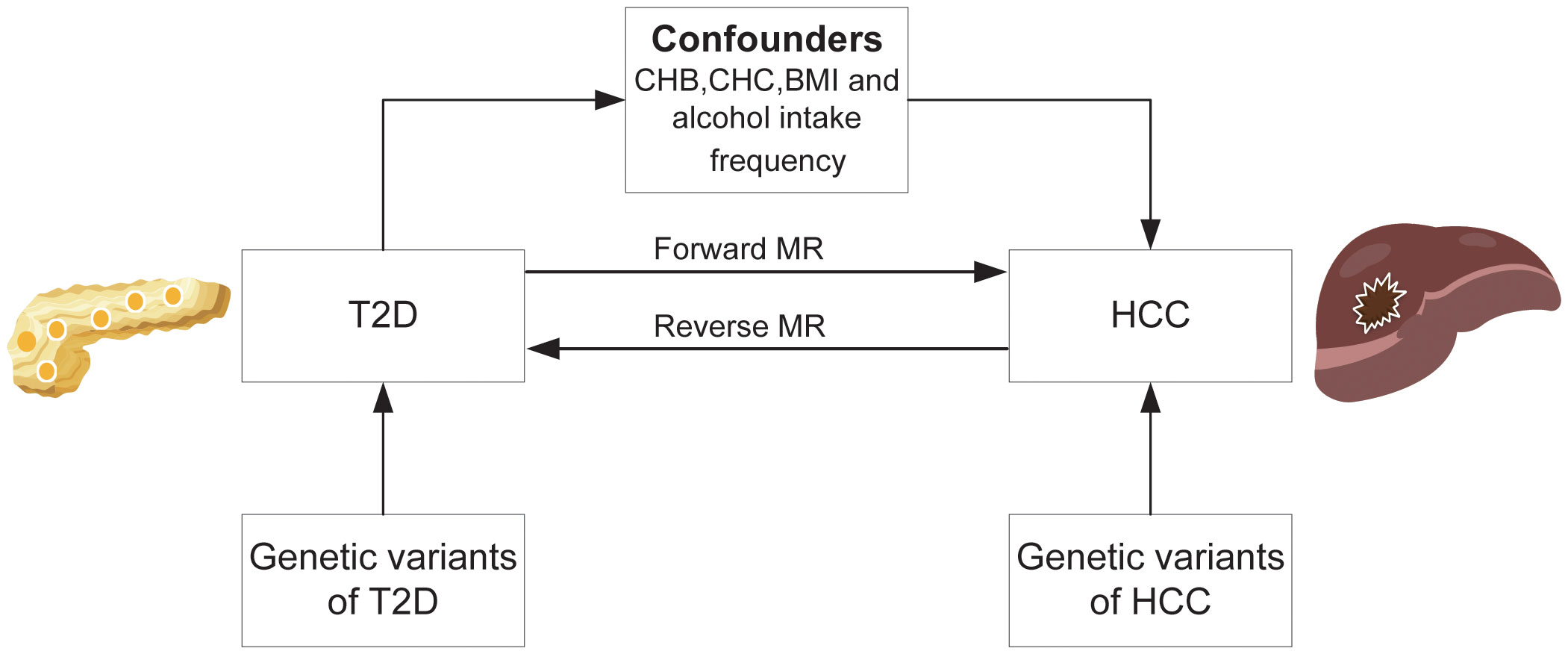
Figure 1 Overall study design based on Mendelian randomization analysis. T2D, type 2 diabetes; HCC, hepatocellular carcinoma; CHB; chronic hepatitis B; CHC, chronic hepatitis C; BMI, body mass index.
2.2 Data sources for T2D
One hundred and seventy-four single-nucleotide polymorphisms (SNPs) associated with T2D were identified at the genome-wide significance threshold (p<5×10-8) from a meta analysis of recently published GWASs including 433,540 individuals of East Asian descent(77,418 cases and 356,122 controls) (24). Sixteen palindromic SNPs (rs10830963, rs11257657, rs256904, rs340875, rs4237150, rs4736999, rs4739515, rs475002, rs6885132, rs7090695, rs7210161, rs7900112, rs8037894, rs838720, rs9368194, rs988748) with intermediate allele frequencies was removed. Finally, one hundred and fifty-eight SNPs without linkage were chosen as instrumental variables for T2D (r2<0.001, window size =10,000 kb). (Supplementary Table 1) Association analyses were adjusted for gender and body mass index(BMI). These instruments have also been used in previous MR studies (25, 26). Additionally,We also used this summary-level data for T2D in the reverse MR analysis.
2.3 Data sources for HCC
Summary-level data for the associations of T2D associated SNPs with HCC were obtained from a meta-analysis of GWAS from Biobank Japan (1,866 cases and 195,745 controls of East Asian descent) (27, 28). Cases in Biobank Japan were defined by International Classification of Disease code ICD10 C. For the reverse MR analysis, three independent SNPs(rs1131500, rs113777417, rs8107030) associated with HCC at the genome-wide significance threshold (P < 5×10–8) were selected as instrumental variables from the Sakaue et al. GWAS(r2<0.01). (Supplementary Table 2) (28) The three SNPs had no significant association with T2D and were used as instrumental variables in the reverse sensitivity analysis.
2.4 Data sources for confounding factors
Four confounding factors, including Chronic hepatitis B(CHB), Chronic hepatitis C(CHC), body mass index(BMI) and alcohol intake frequency, possibly associated with HCC were selected (29). Summary data on these confounding factors are from the second wave of genome-wide association analyses from IEU-OpenGWAS project (https://gwas.mrcieu.ac.uk/), with GWAS ID:bbj-a-99, bbj-a-101, bbj-a-1,and ukb-e-1558_EAS, respectively. To mitigate the effects of racial differences, the populations in these GWAS data related to confounders were all of East Asian ancestry.
2.5 Statistical analysis
Bidirectional MR was employed to investigate the direction of causation between T2D and HCC. In forward MR analysis, The inverse-variance weighted (IVW) was used as the primary method to estimate the associations between exposure (T2D) and outcome (HCC). Risk estimates are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Supplementary analyses were conducted using the MR-Egger, weighted median method, simple mode, and weighted mode (19). The IVW method combines SNP-specific instrumental variable (IV) estimates in a meta-analysis framework, weighting each estimate by the inverse of its variance. This method provides a robust estimate of the causal effect when the IV assumptions hold (30). The weighted median method was employed as an additional analysis approach. It generates reliable estimates as long as more than half of the weights are derived from valid instruments (31). The MR-Egger regression was used to adjust for pleiotropy, which refers to the situation where the IVs affect the outcome (HCC) through pathways other than exposure (T2D). However, it is important to note that this method may have reduced statistical power compared to the IVW method (32). The p-value for the MR-Egger intercept and funnel plots were utilized to assess the directional pleiotropy (32). Leave-one-out sensitivity analysis was performed to evaluate the influence of individual SNPs on HCC. Additionally, MR-Pleiotropy residual sum and outlier (MR-PRESSO) was also used to detect outliers (33). To detect heterogeneity within each SNP, the IVW and MR-Egger methods were utilized (32). The extent of heterogeneity among the SNPs was assessed by analyzing the heterogeneity statistic Q (34). Additionally, To further investigate for potential confounding factors(CHB, CHC, BMI and alcohol intake frequency), multivariable MR was used. The reversed MR was conducted to explore the reverse causation. All analyses were conducted in R software (version 4.3.0) using TwoSampleMR and MR-PRESSO packages (33, 35).
3 Results
3.1 Forward MR analysis
Genetically predicted higher T2D was associated with a decreased risk of HCC (odds ratio per 1-SD increase, 0.8628; 95% confidence interval [CI], 0.7888 to 0.9438; P=.0013). Furthermore, other MR methods also indicated a tendency towards a causal association between T2D and HCC, although not all methods achieved statistical significance (Figures 2, 3). The IVW and MR-Egger methods showed no significant heterogeneity among the SNPs, suggesting that the results are robust (Table 1). Additionally, the leave-one-out method demonstrated that no single SNP was driving the observed associations (Supplementary Figure 1). MR-PRESSO did not identify any outliers (global test p -value: 0.094). The funnel plots, which are basically symmetrical, and further analysis using the MR Egger regression test, did not provide any evidence of pleiotropy (Figure 4). This inverse causality association persisted between T2D and HCC risk after adjustment for a range of potential confounding factors including CHB, BMI and alcohol intake frequency. This trend was still present, although statistically significant differences were not observed after correcting for CHC (Figure 5; Supplementary Tables 3–6).
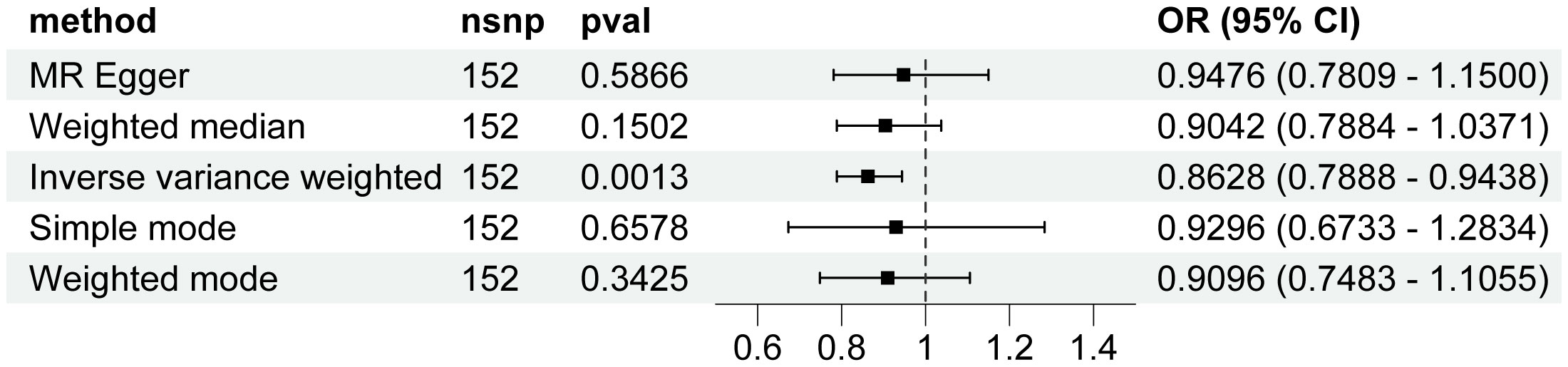
Figure 2 Associations of genetic predisposition to type 2 diabetes with hepatocellular carcinoma. CI, confidence interval; OR, odds ratio; SNPs, single-nucleotide polymorphisms.
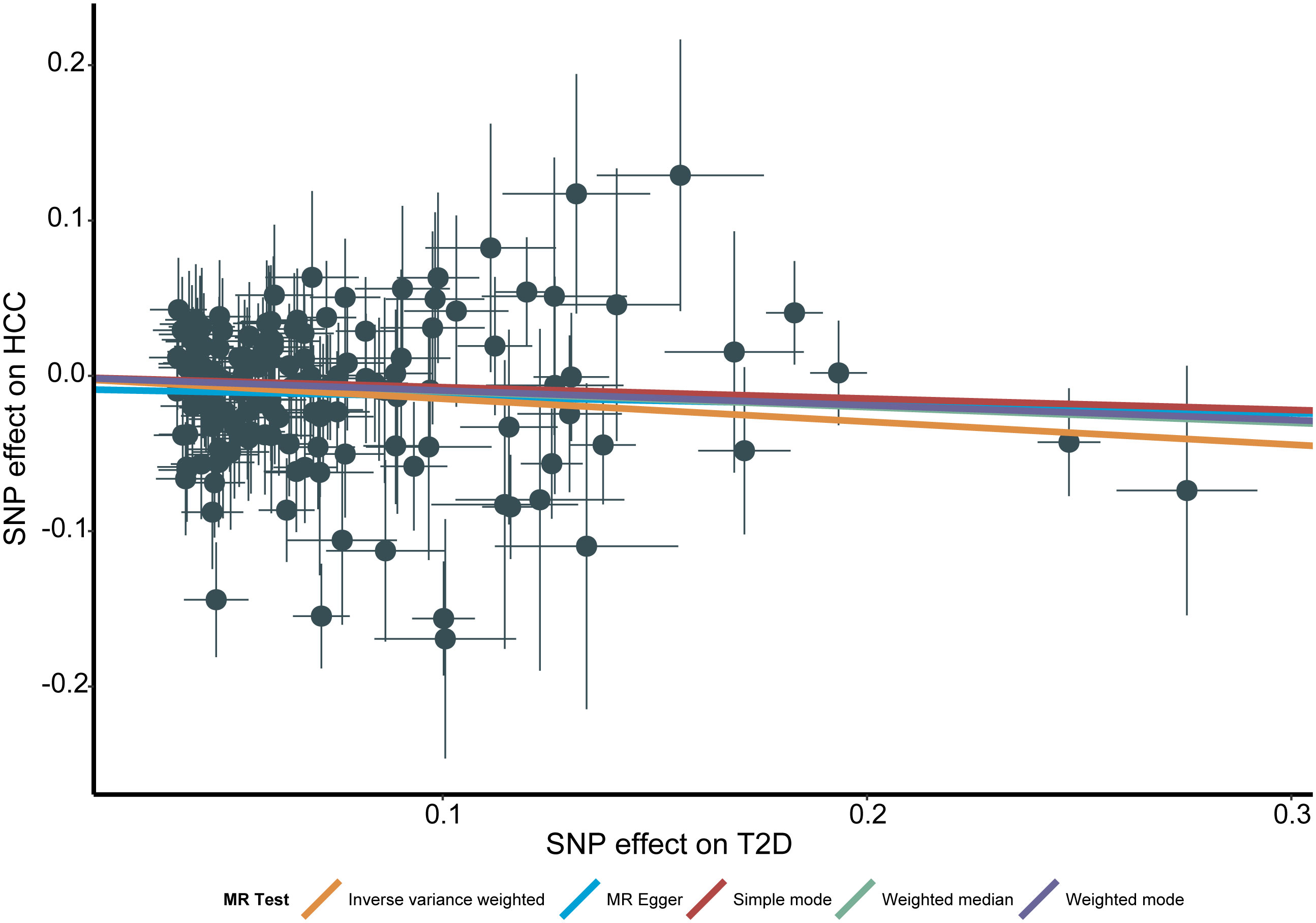
Figure 3 Scatter plot for the associations between type 2 diabetes and the risk of hepatocellular carcinoma. T2D, type 2 diabetes; HCC, hepatocellular carcinoma; MR, Mendelian randomization; SNP, Single nucleotide polymorphism.
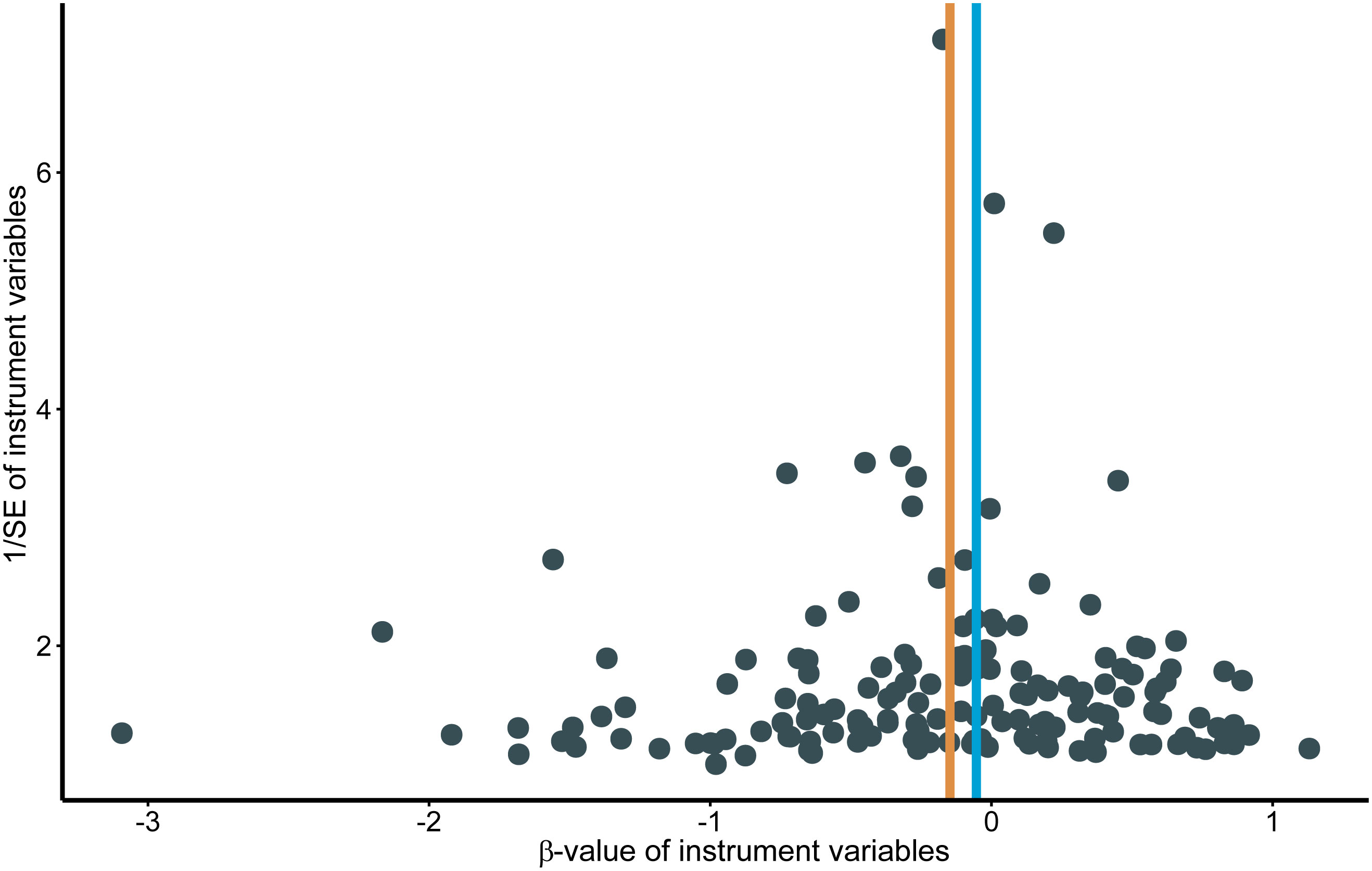
Figure 4 Funnel plots for effects of type 2 diabetes on hepatocellular carcinoma. MR, Mendelian randomization.
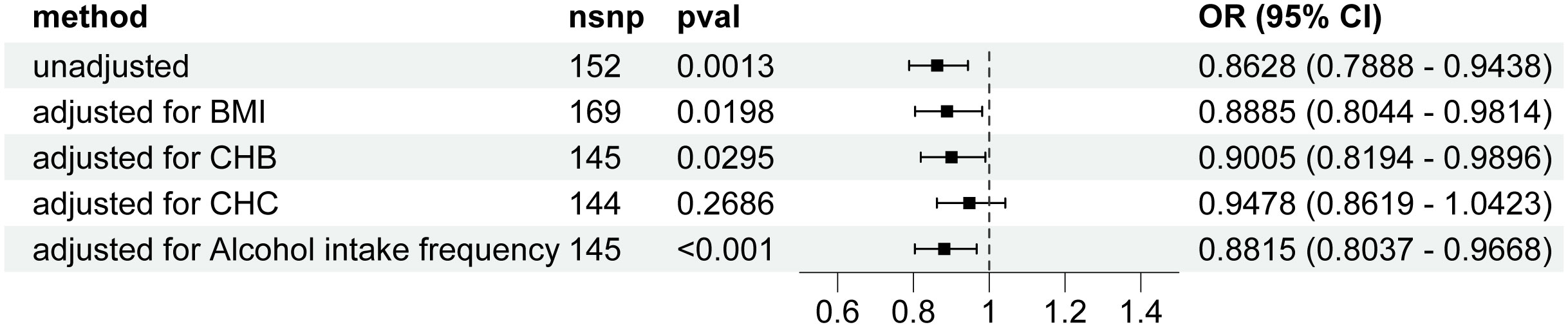
Figure 5 Forest plot for the Mendelian randomization studies investigating the effect of type 2 diabetes on hepatocellular carcinoma using the IVW method after correction for confounders. OR, odds ratio; CI, confidence interval; SNP, Single nucleotide polymorphism; CHB; chronic hepatitis B; CHC, chronic hepatitis C; BMI, body mass index; IVW, inverse variance weighted.
3.2 Reverse MR analysis
In the Reverse MR Analysis study, the IVW method did not show a association between HCC and T2D risk. The change of HCC was 0.9788 (95% CI, 0.9061 to 1.0574; P=0.5876) for a 1-unit increase in the log-transformed odds ratio of T2D (Figure 6). The inconsistent result was also observed in other MR methods (Figure 6). Horizontal pleiotropy was not found using the MR-Egger regression analysis (P=0.2278). The Q-test of heterogeneity by MR Egger was not statistically significant(P=0.6067).
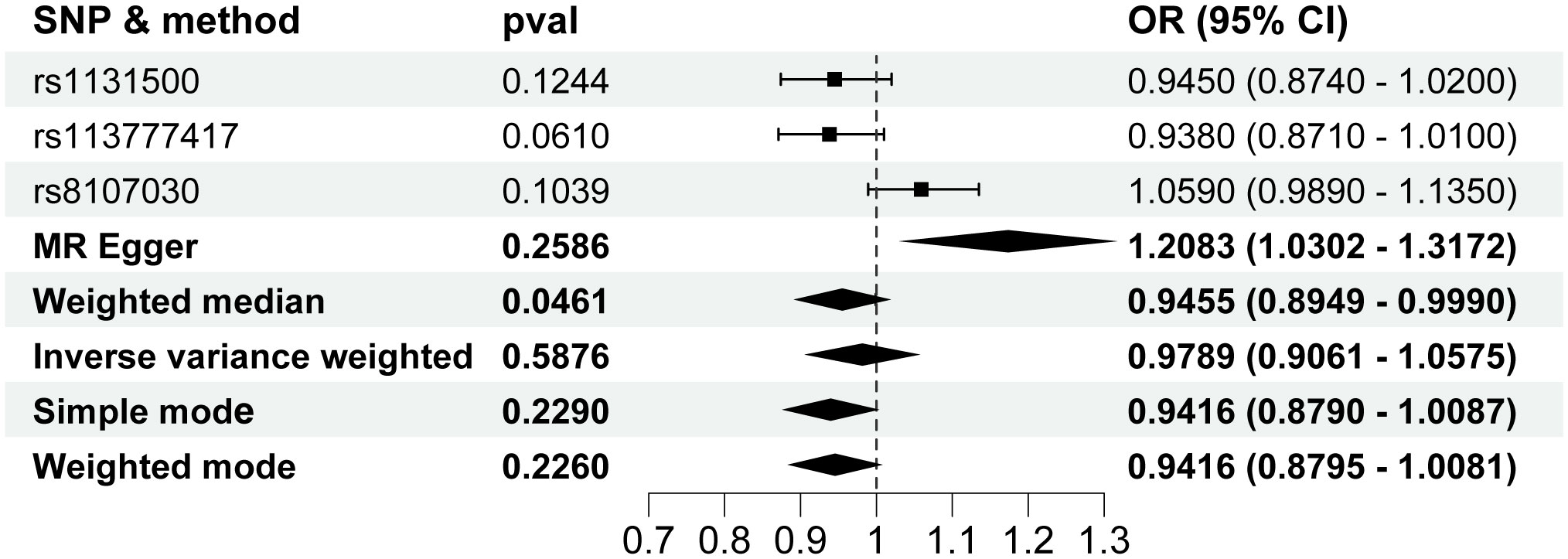
Figure 6 Forest plot for the Mendelian randomization studies investigating the effect of hepatocellular carcinoma on type 2 diabetes. OR, odds ratio; SNP, single-nucleotide polymorphisms.
4 Discussion
Our MR analysis suggested inverse genetic correlations of T2D with HCC. Forward MR analysis indicated that genetically predicted T2D were robustly associated with a decreased risk of HCC in East Asian descent. However, the data from the reverse MR analysis supporting an impact of HCC on T2D were limited.
The result in our study is inconsistent with the findings of other studies that have shown an association between T2D and a higher risk of liver cancer. For example, In a study of 1.57 million adults enrolled in 14 prospective US-based studies, it was found that T2D was associated with a higher risk of liver cancer. The finding was consistent after correcting for viral hepatitis infection (36). This also accords with a study from Spain, which showed that T2D is a risk factor for HCC development (37). Another study investigates the link between T2D and HCC incidence in African Americans and whether it varies by race. Using data from the Southern Community Cohort Study, researchers followed low-income participants aged 40-79 with and without diabetes. Results indicate that T2D significantly increases the risk of HCC in White/European Americans, but less so in Black/African Americans. Additionally, controlling for T2D lessens the higher risk of HCC among African Americans, suggesting a stronger link between diabetes and liver cancer in White/European Americans. These findings may indicate distinct mechanisms and impacts of T2D on HCC in African Americans versus White/European Americans (38). A possible explanation for this difference with our MR results might be that the subjects in these studies were all European populations. In contrast, our study was limited to East Asian populations. Ethnographic discrepancies may be the main reason for the differences in results.
T2D has been widely reported as an independent risk factor for the risk of HCC (39, 40), and this risk can be reduced with aggressive interventions (41–44). For example, a study conducted in Taiwan found that the use of H1-antihistamines (AHs) was associated with a reduced risk of HCC in T2D patients without hepatitis B virus (HBV) or hepatitis C virus (HCV) infection in a dose-dependent manner (41). Another study found that adherence to a T2D prevention diet, which includes a healthy balance of glycemic index, cereal fibre, polyunsaturated to saturated fats, trans fat, sugar-sweetened beverages, nuts, coffee, and red and processed meats, was associated with a lower risk of HCC among US men and women (42). In patients with both T2D and chronic HCV infection, the use of dipeptidyl peptidase 4 inhibitors (DPP-4 inhibitors), a class of oral antidiabetic drugs, was associated with a lower risk of HCC (43). A two-centre study in a developing country identified several risk factors for HCC in T2D patients, including Chinese and Malay ethnicities interacting with viral hepatitis, weight loss, abdominal pain/discomfort, alcohol consumption, fatty liver, low platelet count, raised alanine transaminase, and alkaline phosphatase levels. The use of statins was found to reduce the risk of HCC by 63% in these patients (44). Our MR study conducted in East Asian populations is consistent with these findings, suggesting that patients with T2D may help reduce this HCC risk. However, it is important to note that the numerous confounding factors may have influenced the interpretation of the results. Our analysis provides more solid evidence than the previous study because our study includes various sensitivity methods to preclude the possibility of bias from horizontal pleiotropy to eliminate causality between T2D and HCC. Furthermore, the causal association persisted after adjustment for potential confounding factors including BMI,CHB and alcohol intake frequency. After adjusting for CHC, the previously observed significant differences were no longer present. This could potentially be attributed to the genetic characteristics of East Asian populations, which have their own unique genetic features and patterns of gene variation. In this population, certain genes may be associated with susceptibility or resistance to HCV infection, but after adjustment, the shared genetic background among East Asians may diminish the strength of this association or mask it entirely.
Our study did not show a clear impact of genetic liability to HCC on reduced T2D risk. Genetic liability to HCC and risk of developing T2D were inconsistent in our MR approach. In addition, there are currently no reports in the literature for HCC and T2D risk.Thus, our inverse MR analysis neither negates nor confirms the effect of HCC on T2D.
Our study has several strengths. First, We conducted a bidirectional MR study to clarify the causal inference between T2D and HCC. This design can significantly reduce the risk of causal inference errors caused by genetic bias and yield more reliable conclusions. Second, The association between T2D and HCC was further verified after adjusting for confounding factors that have been shown to have a close relationship with the development of HCC. The characteristics of our study design make the results particularly robust. In addition, we limited our analysis to East Asian populations. This reduces bias due to differences in population structure.
There are some limitations of this study that should be considered. Firstly, our MR analysis was limited to individuals of East Asian ancestry due to the availability of relevant genetic data. Due to regional and ethnic differences, these results may not be fully applicable to other populations. Secondly, despite controlling for confounding factors such as BMI, CHB, CHC and alcohol intake frequency, there may still be unaccounted confounders that could influence the study results, including age, gender, family history, and chronic liver diseases (such as non-alcoholic fatty liver disease and cirrhosis). Further research could explore additional potential confounding variables. Thirdly, the study relied on existing data resources, and there may be limitations in data acquisition and measurement regarding the specific relationship between T2D and HCC occurrence. Future studies could consider using more accurate and detailed data collection methods. Additionally, while the study utilized MR analysis to infer a inverse association between T2D and HCC risk, further research is needed to validate and confirm causality. Other study designs such as longitudinal studies or experimental research may provide stronger evidence of causality.
In conclusion, Our study, based on GWAS data, suggests that East Asians with genetic susceptibility to T2D are less susceptible to HCC. The findings may have important implications for the prevention and clinical management of these conditions. Whether HCC has a causal effect on reducing T2D warrants more study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
JH: Conceptualization, Software, Writing – original draft, Writing – review & editing. YX: Data curation, Software, Writing – review & editing. XC: Writing – review & editing. JY: Writing – review & editing. LZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JH is supported by the Department of Science and Technology of Guizhou Province (No. Qiankehe Foundation - ZK[2023] General 485). LZ is supported by the National Natural Science Foundation of China (NSFC) (No. 81960125) and the Department of Science and Technology of Guizhou Province (No. Qiankehe Foundation [2020] 1Y302).
Acknowledgments
We would like to express our gratitude to the researchers for sharing their data on the BioBank Japan project, as well as data from the IEU Open GWAS Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1308561/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Zanella MT, Kohlmann OJ, Ribeiro AB. Treatment of obesity hypertension and diabetes syndrome. Hypertension (2001) 38:705–08. doi: 10.1161/01.hyp.38.3.705
3. Reaven GM. Banting lecture 1988. Role insulin resistance Hum disease Diabetes (1988) 37:1595–607. doi: 10.2337/diab.37.12.1595
4. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet (2022) 400:1803–20. doi: 10.1016/S0140-6736(22)01655-5
5. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet (2017) 389:2239–51. doi: 10.1016/S0140-6736(17)30058-2
6. Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell (2019) 179:561–77. doi: 10.1016/j.cell.2019.08.052
7. Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer (2013) 13:123–35. doi: 10.1038/nrc3449
8. Sun B, Zhang L, Xiang D, Li Q, Ren Y, Cao Y, et al. The effect of alcohol consumption in unresectable hepatocellular carcinoma with transarterial chemoembolization. J Oncol (2022) 2022:7062105. doi: 10.1155/2022/7062105
9. Becattini B, Breasson L, Sardi C, Zani F, Solinas G. PI3Kgamma promotes obesity-associated hepatocellular carcinoma by regulating metabolism and inflammation. Jhep Rep (2021) 3:100359. doi: 10.1016/j.jhepr.2021.100359
10. Yang X, Wang Y, Luk AO, So WY, Ma RC, Kong AP, et al. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocr Relat Cancer (2013) 20:161–71. doi: 10.1530/ERC-12-0290
11. Shi T, Kobara H, Oura K, Masaki T. Mechanisms underlying hepatocellular carcinoma progression in patients with type 2 diabetes. J Hepatocell Carcinoma (2021) 8:45–55. doi: 10.2147/JHC.S274933
12. Simon TG, King LY, Chong DQ, Nguyen LH, Ma Y, VoPham T, et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology (2018) 67:1797–806. doi: 10.1002/hep.29660
13. Fabrizi F, Dixit V, Messa P. Hepatitis C virus and mortality among patients on dialysis: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol (2019) 43:244–54. doi: 10.1016/j.clinre.2018.10.009
14. Stepanova M, Lam B, Younossi Y, Srishord MK, Younossi ZM. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. J Viral Hepat (2012) 19:341–45. doi: 10.1111/j.1365-2893.2011.01554.x
15. Terrault NA, Lok A, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (2018) 67:1560–99. doi: 10.1002/hep.29800
16. Choi S, Kim K, Lee JK, Choi JY, Shin A, Park SK, et al. Association between change in alcohol consumption and metabolic syndrome: analysis from the health examinees study. Diabetes Metab J (2019) 43:615–26. doi: 10.4093/dmj.2018.0128
17. Ajmera V, Cepin S, Tesfai K, Hofflich H, Cadman K, Lopez S, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol (2023) 78:471–78. doi: 10.1016/j.jhep.2022.11.010
18. Li MY, Li TT, Li KJ, Zhou C. Type 2 diabetes mellitus characteristics affect hepatocellular carcinoma development in chronic hepatitis B patients with cirrhosis. World J Clin cases (2023) 11:1009–18. doi: 10.12998/wjcc.v11.i5.1009
19. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods (2019) 10:486–96. doi: 10.1002/jrsm.1346
20. Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafo MR, et al. Mendelian randomization. Nat Rev Methods Primers (2022) 2:6. doi: 10.1038/s43586-021-00092-5
21. Luykx JJ, Lin BD. Are psychiatric disorders risk factors for COVID-19 susceptibility and severity? a two-sample, bidirectional, univariable, and multivariable Mendelian Randomization study. Transl Psychiatry (2021) 11:210. doi: 10.1038/s41398-021-01325-7
22. Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample mendelian randomization study. JAMA Psychiatry (2019) 76:399–408. doi: 10.1001/jamapsychiatry.2018.4175
23. Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
24. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature (2020) 582:240–45. doi: 10.1038/s41586-020-2263-3
25. Zhang W, Zhang L, Zhu J, Xiao C, Cui H, Yang C, et al. Additional evidence for the relationship between type 2 diabetes mellitus and stroke through observational and genetic analyses. Diabetes (2023) 72:1671–81. doi: 10.2337/db22-0954
26. Zhang H, Xiu X, Yang Y, Yang Y, Zhao H. Identification of putative causal relationships between type 2 diabetes and blood-based biomarkers in east asians by mendelian randomization. Am J Epidemiol (2022) 191:1867–76. doi: 10.1093/aje/kwac118
27. Nagai A, Hirata M, Kamatani Y, Muto K, Matsuda K, Kiyohara Y, et al. Overview of the BioBank Japan Project: Study design and profile. J Epidemiol (2017) 27:S2–08. doi: 10.1016/j.je.2016.12.005
28. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
29. Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin (2010) 31:1409–20. doi: 10.1038/aps.2010.142
30. Ye T, Shao J, Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann Stat (2021) 49:2079–100. doi: 10.1214/20-AOS2027
31. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol (2017) 46:1734–39. doi: 10.1093/ije/dyx034
32. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
33. Verbanck M, Chen C, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50:693–98. doi: 10.1038/s41588-018-0099-7
34. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods (2006) 11:193. doi: 10.1037/1082-989X.11.2.193
35. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408. doi: 10.7554/eLife.34408
36. Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane FL, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. Adults. Cancer Res (2016) 76:6076–83. doi: 10.1158/0008-5472.CAN-16-0787
37. Qi Y, Fan L, Ran D, Xu J, Wang Y, Wu J, et al. Main risk factors of type 2 diabetes mellitus with nonalcoholic fatty liver disease and hepatocellular carcinoma. J Oncol (2021) 2021:7764817. doi: 10.1155/2021/7764817
38. Conway R, Sudenga S, McClain D, Blot WJ. Diabetes and liver cancer risk: A stronger effect in Whites than Blacks? J Diabetes Complications (2021) 35:107816. doi: 10.1016/j.jdiacomp.2020.107816
39. Yang WS, Shu XO, Gao J, Li HL, Cai H, Yang G, et al. Prospective evaluation of type 2 diabetes mellitus on the risk of primary liver cancer in Chinese men and women. Ann Oncol (2013) 24:1679–85. doi: 10.1093/annonc/mdt017
40. Cao LL, Han Y, Pei L, Yue ZH, Liu BY, Cui JW, et al. A serum metabolite classifier for the early detection of type 2 diabetes mellitus-positive hepatocellular cancer. Metabolites (2022) 12:610. doi: 10.3390/metabo12070610
41. Wu SY, Chen WM, Chen YC, Chiang MF, Lee MC, Soong RS. Effects of H1-Antihistamines on hepatocellular carcinoma risk in patients with type 2 diabetes mellitus. Diabetes Metab (2023) 49:101393. doi: 10.1016/j.diabet.2022.101393
42. Luo X, Sui J, Yang W, Sun Q, Ma Y, Simon TG, et al. Type 2 diabetes prevention diet and hepatocellular carcinoma risk in US men and women. Am J Gastroenterol (2019) 114:1870–77. doi: 10.14309/ajg.0000000000000450
43. Hsu WH, Sue SP, Liang HL, Tseng CW, Lin HC, Wen WL, et al. Dipeptidyl peptidase 4 inhibitors decrease the risk of hepatocellular carcinoma in patients with chronic hepatitis C infection and type 2 diabetes mellitus: A nationwide study in Taiwan. Front Public Health (2021) 9:711723. doi: 10.3389/fpubh.2021.711723
Keywords: type 2 diabetes, hepatocellular carcinoma, Mendelian randomization, East Asian populations, association
Citation: Huo J, Xu Y, Chen X, Yu J and Zhao L (2024) Inverse association between type 2 diabetes and hepatocellular carcinoma in East Asian populations. Front. Endocrinol. 14:1308561. doi: 10.3389/fendo.2023.1308561
Received: 06 October 2023; Accepted: 11 December 2023;
Published: 03 January 2024.
Edited by:
Qiang Huo, Nanjing Jiangbei Hospital, ChinaReviewed by:
Aaron Balasingam Koenig, INOVA Health System, United StatesFei Wu, Shandong First Medical University, China
Ajit Venniyoor, The Royal Hospital, Oman
Copyright © 2024 Huo, Xu, Chen, Yu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijin Zhao, bGlqaW4uemhhb0B6bXUuZWR1LmNu
†ORCID: Lijin Zhao, orcid.org/0000-0002-7741-5074
 Jinlong Huo
Jinlong Huo Yaxuan Xu1
Yaxuan Xu1 Xingqi Chen
Xingqi Chen Lijin Zhao
Lijin Zhao