
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 January 2024
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1305386
This article is part of the Research Topic Novel Treatment Strategies for Thyroid Cancer View all 7 articles
Aims: This study investigates the relationship between the Systemic Inflammatory Response Index (SIRI) and thyroid function.
Methods: Utilizing data from the National Health and Nutrition Examination Survey (NHANES) 2009-2012, we excluded participants lacking SIRI or thyroid function data, those under 20 years, and pregnant individuals. SIRI was determined using blood samples. We conducted weighted multivariate regression and subgroup analyses to discern the independent relationship between SIRI and thyroid function.
Results: The study included 1,641 subjects, with an average age of 47.26±16.77 years, including 48.65% males and 51.35% females. The population was divided into three SIRI-based groups (Q1-Q3). Q3, compared to Q1, exhibited higher age-at-onset, greater male prevalence, and increased levels of FT3, FT4, TT4, leukocytes, and triglycerides. This group also showed a higher incidence of diabetes, hypertension, and smoking. Notably, Q1 had lower LDL and HDL levels. SIRI maintained a positive association with FT4 (β = 0.01, 95% CI = 0.00-0.03, P for trend = 0.0071), TT4 (β = 0.20, 95% CI = 0.10, 0.31, P for trend=0.0001), and TPOAb (β = 8.0, 95% CI = 1.77-14.30, P for trend = 0.0120), indicating that each quartile increase in SIRI corresponded to a 0.01 ng/dL increase in FT4, a 0.2 g/dL increase in TT4, and an 8.03 IU/mL rise in TPOAb. The subgroup analysis suggested the SIRI-thyroid function correlation was influenced by hypertension.
Conclusion: Inflammation may impact the development and progression of thyroid function disorders. Proactive anti-inflammatory treatment might mitigate thyroid abnormalities.
The thyroid gland, the largest endocrine gland in adults, plays a pivotal role in regulating physiological activities (1) and is one of the most important components of the human body. Thyroid hormones (TH), primarily triiodothyronine (T3) and tetraiodothyronine (T4) are crucial for systemic metabolism, neurodevelopment, and energy metabolism, influencing protein, carbohydrate, and lipid metabolism. Recent studies indicate a rising prevalence of thyroid disorders, now second only to diabetes mellitus as a global metabolic disease. These disorders often lead to developmental and cognitive impairments, osteoporosis, and insomnia, significantly impacting patients' physical and mental health (2).
TH comprises both T3 and significant quantities of T4. The active free thyroid hormone in the body consists of free triiodothyronine (Free T3, FT3) and free thyroxine (Free T4, FT4), but when FT3 and FT4 are depleted, total triiodothyronine (Total T3, TT3) and total thyroxine (Total T4, TT4) are converted to FT3 and FT4 in the body, which continues to play the role of TH. Although TH is synthesized in the follicular epithelial cells of the thyroid gland, its synthesis, and secretion depend on the stimulation of thyroid-stimulating hormone (TSH). Thyroid peroxidase antibodies (TPOAb) are found in the microsomes of thyroid cells and are a key enzyme in the synthesis of TH. They work in concert with Thyreoglobulin to iodide L-tyrosine into TH, which is a common autoantibody in the serum of patients with autoimmune thyroid disease. It is seen in autoimmune thyroid diseases such as Hashimoto's thyroiditis (HT) (3).
Inflammation response is a defense response of the organism itself when tissues are damaged or infected by toxins or bacteria, injured by heat or other causes, they go into a normal state of self-protection and damage repair. The System Inflammation Response Index (SIRI) is a new systemic inflammation measure created in 2016 by Qi et al. (4). SIRI (1000cell/uL) = N×M/L, as the calculation method, where N, M, and L are the pretreatment peripheral neutrophil, monocyte, and lymphocyte counts, respectively. Previous studies have concluded that SIRI can be used to differentiate the immune-inflammatory response of the body's three different pathways, and to reflect the body's immune response and inflammation in a more comprehensive way. Subsequently, this index was used to measure the recurrence rate and survival rate after cancer surgery. In addition, the index has been used to measure the degree of brain damage in aneurysmal arachnoiditis (5) hemorrhage, and the development and prognosis of the Corona Virus Disease 2019 (COVID-19).
The relationship between inflammation and thyroid function, though correlated, remains poorly understood. Therefore, our study aims to elucidate the impact of inflammation on thyroid disease development by examining the correlation between SIRI and thyroid function in participants of the National Health and Nutrition Examination Survey (NHANES). This could pave the way for novel strategies in the primary prevention of thyroid diseases. We hypothesized that elevated SIRI is associated with a higher risk of abnormal thyroid function.
Data for this study were sourced from the National Health and Nutrition Examination Survey (NHANES), a biennial cross-sectional study conducted by the Centers for Disease Control and Prevention (U.S.) to assess the health and nutritional status of American citizens. The NHANES study protocol adhered to the Declaration of Helsinki principles and received approval from the Institutional Review Board, with all participants providing written informed consent. Data were accessed from NHANES's public domain (www.example.com; https://www.cdc.gov/nchs/nhanes/, accessed on 12 August 2023), and Figure 1 depicts the participant selection process.
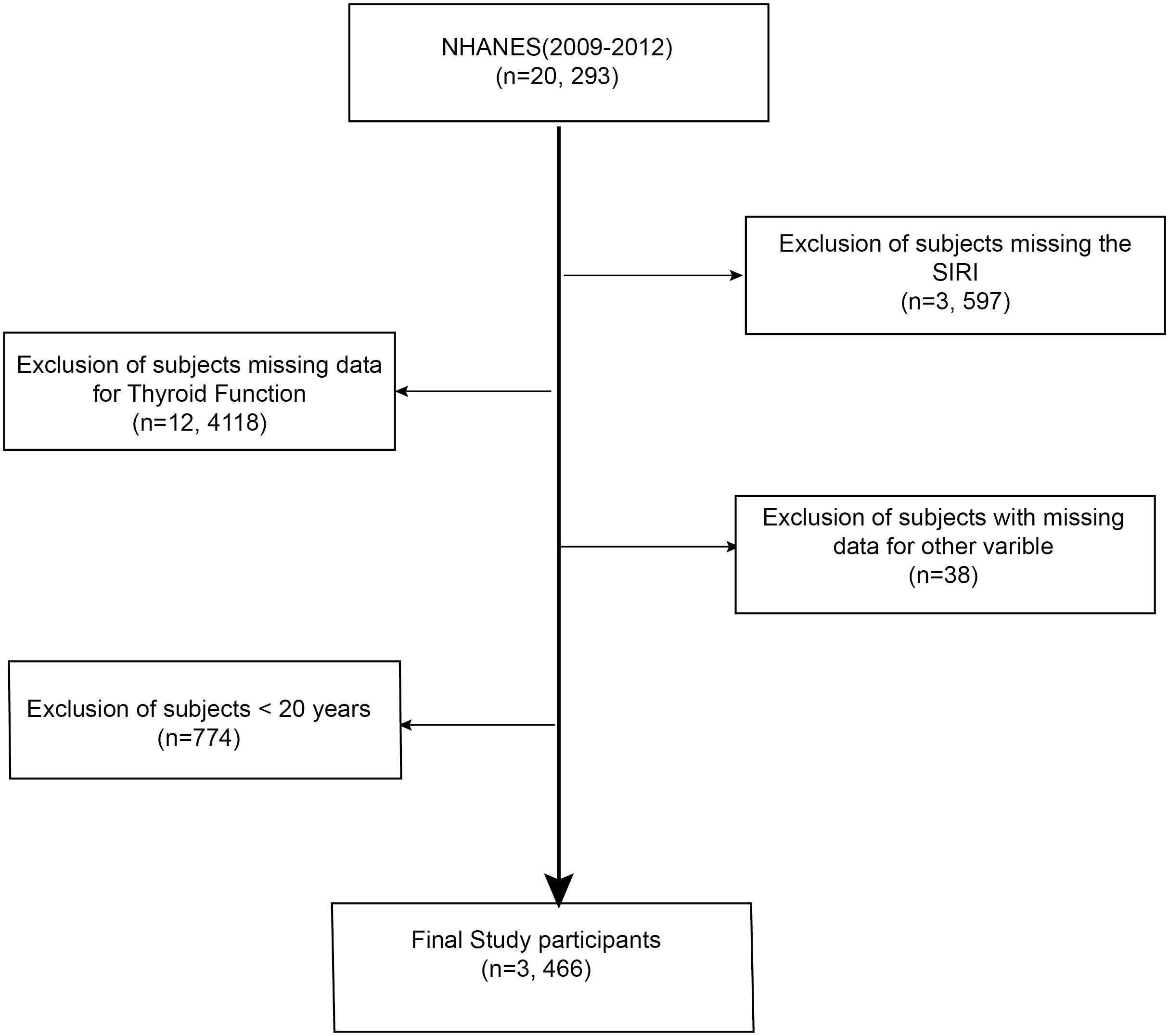
Figure 1 Selection process for the study sample based on the 2009-2012 National Health and Nutrition Examination Survey (NHANES).
This research utilized data from the 2009-2012 NHANES survey cycle, which encompassed comprehensive data on thyroid function, the System Inflammation Response Index (SIRI), and demographic information. The final study cohort, after excluding individuals with incomplete SIRI or thyroid function data, those under 20 years of age, and pregnant women, comprised 1,641 participants. All participants consented to the study, and the Ethical Review Board of the National Centre for Health Statistics authorized the research.
All blood specimens (neutrophil count, monocyte count, and lymphocyte count) were collected at Mobile Examination Centers (MECs). The procedures for quality assurance and control of these specimens adhered to the guidelines outlined in the NHANES Laboratory Procedures Manual, which can be accessed at https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/CBC_F_met_HE.pdf.
The SIRI was defined as follows: SIRI (1000cell/uL) = N×M/L, where N, M, and L are the pretreatment peripheral neutrophil, monocyte, and lymphocyte counts, respectively (4).
This study involved the analysis of serum samples for thyroid function indicators including FT3, FT4, TSH, TPOAb, TT3, and TT4. The methods for serum specimen collection and processing are elaborately described in the National Health and Nutrition Examination Survey (NHANES) Laboratory Procedures Manual (available at: wwwn.cdc.gov/nchs/nhanes/2007-2008/THYROD_E.htm).
The study accounted for various covariates potentially influencing thyroid function: age, gender (male, female), educational attainment (below high school, high school or equivalent, college graduate or above), marital status (married or not), and race/ethnicity (Mexican American, non-Hispanic White, non-Hispanic Black, and others). Diabetes mellitus was defined as any self-reported diagnosis of diabetes mellitus or self-reported use of insulin or antidiabetic medication or fasting blood glucose greater than 7.0 mmol/L or glycated hemoglobin greater than 6%. Hypertension was defined as any self-reported diagnosis of hypertension or self-reported use of blood pressure-lowering medication or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg. Body Mass Index (BMI) was determined from the measured weight and height. Serum biochemistry analyses included total High-Density Lipoprotein (HDL) cholesterol, total Low-Density Lipoprotein (LDL) cholesterol, and triglycerides.
Statistical analyses in this study were conducted using R packages (The R Foundation: http://www.r-project.org;version3.4.3) and Empower (R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, Massachusetts). Given the complex multi-stage sampling design of the National Health and Nutrition Examination Survey (NHANES), this study utilized MEC exam weights (WTMEC4YR, WTMEC2YR) for analysis. In this study, categorical variables were expressed as weighted percentages, while continuous variables were expressed as weighted means and standard deviations. Differences in characteristics between groups were tested by chi-square test (for categorical variables) and one-way ANOVA (for continuous variables). All statistical tests were two-sided, and a P value< 0.05 was considered statistically significant.
Furthermore, the correlation between the SIRI and thyroid function was analyzed using weighted multivariate logistic regression models. SIRI was treated as a categorical variable in quartiles. Three models were employed: Model 1, without covariate adjustment; Model 2, adjusting for gender, age, and race; and Model 3, further adjusting for education level and marital status. To visually depict the relationship between SIRI and thyroid function, smoothed curve fitting was used. In these plots, the central line represents the effect size, while the surrounding area indicates the 95% CI. P value <0.05 was deemed statistically significant.
The study's baseline characteristics are detailed in Table 1, which outlines the demographic and laboratory parameters of the participants. The study encompassed 3,466 individuals, with an average age of 47.26 ± 16.77 years, comprising 48.65% males and 51.35% females. Participants were stratified into three groups according to the tertiles of the Systemic Immune-Inflammation Index (SIRI): the first tertile group (Q1, N=1,130), the second tertile group (Q2, N=1,150), and the third tertile group (Q3, N=1,185). Notably, the Q3 group, compared to Q1, demonstrated a higher mean age, a greater male proportion, and elevated levels of FT3, FT4, TT4,WBC, and triglycerides. This group also showed a higher prevalence of diabetes, hypertension, and smoking. In contrast, lower levels of LDL and HDL were observed in the Q1 group (all P < 0.05).
Table 2 presents the results of the univariate analyses. In our study population, thyroid function showed no association with race. However, a significant positive correlation was observed between and marital status (β = 0.08, 95% CI = 0.05, 0.10), as well as WBC (β = 0.01, 95% CI = 0.00, 0.01), negatively correlated with age (β = -0.01, 95% CI = 0.01, 0.00) and gender (β = -0.09, 95% CI = -0.21, -0.16).
Regarding FT4, a significant positive correlation was noted with age (β = 0.00, 95% CI = 0.00, 0.00) and the SIRI (β = 0.01, 95% CI = 0.01, 0.02), while a negative correlation was observed with education level (β = -0.02, 95% CI = -0.03, -0.0). TSH and TPOAb both demonstrated a positive correlation with gender (TSH: β = 0.33, 95% CI = 0.08, 0.58; TPOAb: β = 18.12, 95% CI = 11.83, 24.40), with TSH also positively correlating with age (β = 0.01, 95% CI = 0.00, 0.02). TT3 showed a negative correlation with both age (β = -0.38, 95% CI = -0.42, 0.34) and gender (β = -2.07, 95% CI = -3.65, -0.49), while positively correlating with marital status (β = 3.21, 95% CI = 1.63, 4.79) and WBC (β = 0.91, 95% CI = 0.54, 1.27). Finally, TT4 exhibited a positive association with age (β = 0.01, 95% CI = 0.00, 0.01), gender (β = 0.49, 95% CI=0.39, 0.60), WBC (β = 0.08, 95% CI = 0.06, 0.11), and SIRI (β = 0.10, 95% CI = 0.03,0.17), but a negative correlation with education level (β = -0.47, 95% CI = -0.62, -0.33).
Table 3 presents the results of a weighted multivariate linear regression analysis, examining the relationship between the SIRI and thyroid function indicators. The results of the study showed that SIRI was significantly and positively correlated with FT4 and TT4 in thyroid function (FT4: Model 1, β = 0.01, 95% CI = 0.01, 0.02; Model 2, β = 0.01, 95% CI = 0.01, 0.02; Model 3, β = 0.01, 95% CI = 0.01, 0.02; TT4: Model 1, β =0.1, 95% CI = 0.03, 0.17; Model 2, β =0.16, 95% CI = 0.09, 0.23; Model 3, β =0.15, 95% CI = 0.08, 0.22), but there was no statistically significant relationship with FT3, TSH, TgAb, and TT3. Subsequently, we transformed SIRI into quartile categorical variables, and in the fully adjusted model (Model 3) we found that SIRI was associated with FT4 (β = 0.01, 95% CI = 0.00, 0.03, P for trend = 0.0071), TT4 (β = 0.20, 95% CI = 0.10, 0.31, P for trend = 0.0001), and TPOAb (β = 8.03, 95% CI = 1.77, 14.30, P for trend = 0.012) remained positively correlated with each other. This suggests that an increase in SIRI per quartile was associated with an increase in FT4 of 0.01ng/dL, an increase in TT4 of 0.2µg/dL, and an elevation in TPOAb of 8.03IU/mL. No statistically significant differences were found among SIRI and FT3, TSH, and TT3 even in the SIRI quartiles.
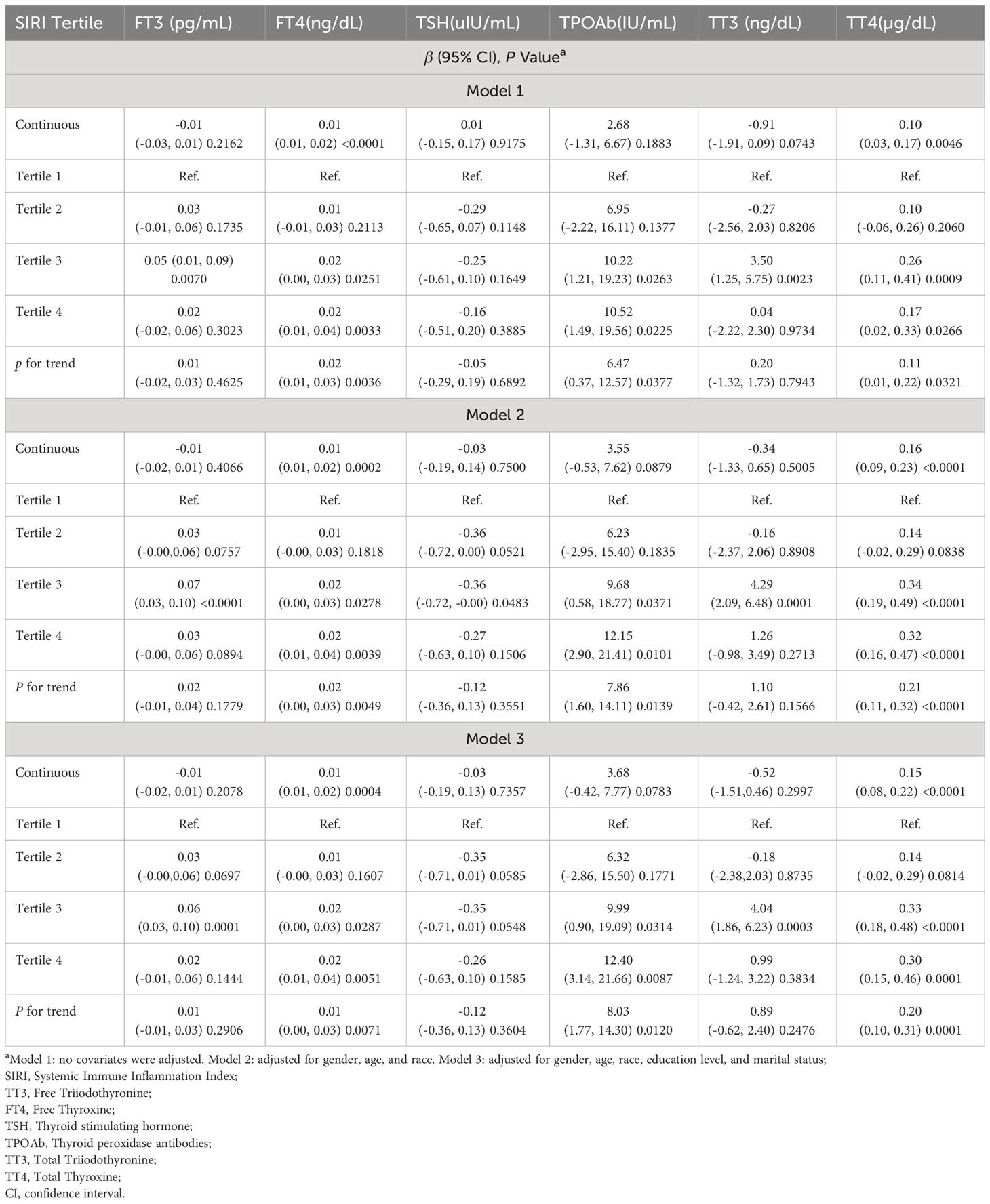
Table 3 Association between SIRI and thyroid function among U.S. adults in NHANES from 2009-2012, weighted.
Table 4 and Figure 2 further elucidate the association between adjusted SIRI and thyroid function parameters. Utilizing a generalized additive model and restricted cubic spline (RCS) curves, which accounted for relevant confounding factors, a nonlinear association was discerned between SIRI and FT3, TT3, and TT4. Critical breakpoints (K) were determined for each hormone: 1.17 for FT3, 1.19 for TT3, and 1.18 for TT4. On the left side of these breakpoints, a positive correlation existed between SIRI and the hormones (FT3: β = 0.09, 95% CI = 0.04, 0.14, P for trend = 0.0003; TT3: β = -3.10, 95% CI = -4.47, -1.73, P for trend <0.0001; TT4: β = 0.26, 95% CI=0.05, 0.46, P for trend = 0.0138). Conversely, on the right side of the breakpoints, a significant negative correlation was observed between SIRI and FT3 and TT3, but not with TT4 (FT3: β = -0.04, 95% CI = -0.06, -0.02, P for trend = 0.0001; TT3: β = 5.58, 95% CI = 2.62, 8.55, P for trend = 0.0002; TT4: β = 0.05, 95% CI = -0.05, 0.14, P for trend = 0.3344). The log-likelihood ratio tests yielded P about <0.001, <0.001, and 0.106, respectively.
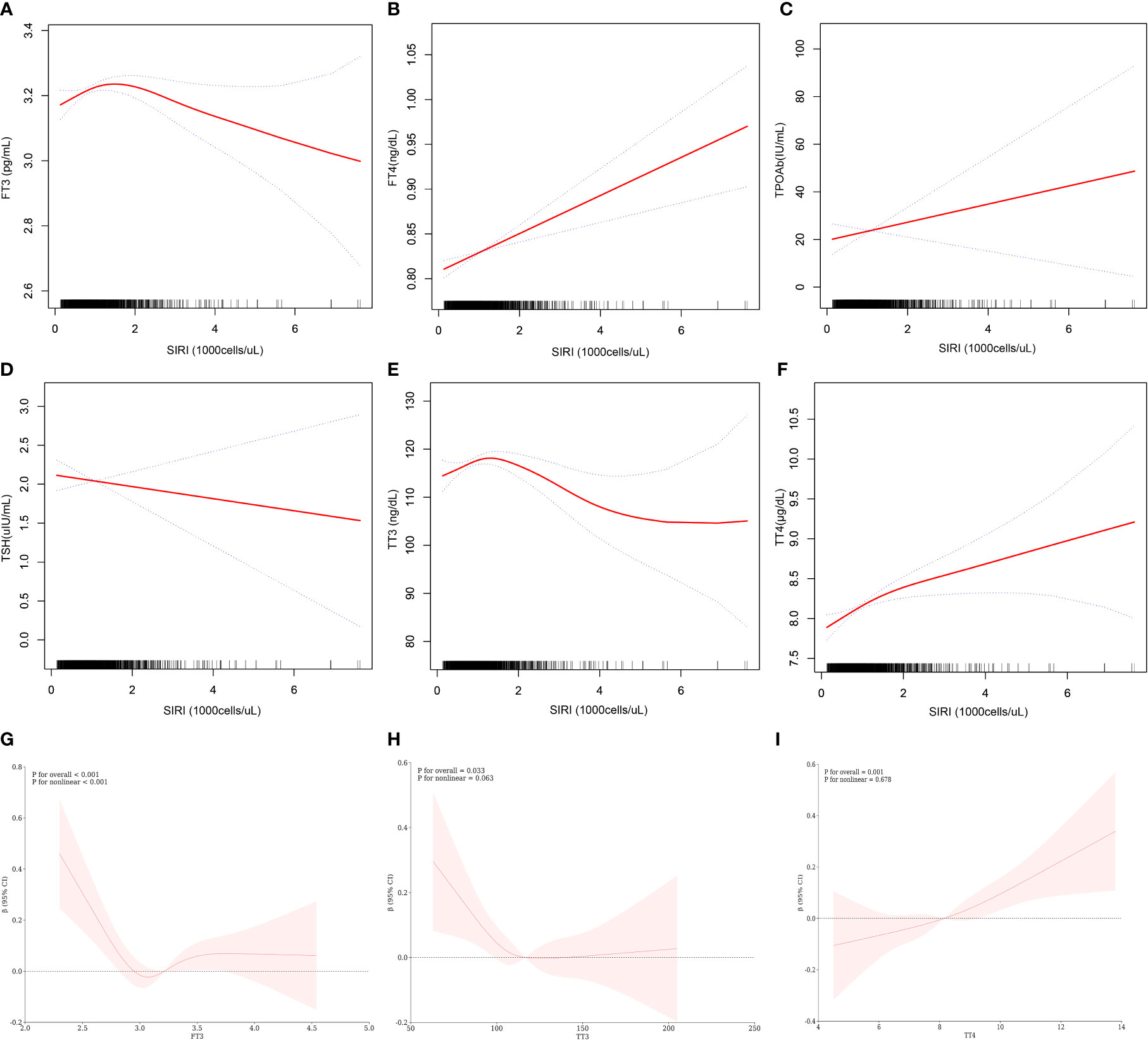
Figure 2 Relationship between SIRI and thyroid function. (A–F) is a curve-fit plot of SIRI versus thyroid function (FT3, FT4, TPOAb, TSH, TT3, and TT4). (G–I) is a restricted cubic spline plot of SIRI versus FT3, TT3, and TT4.
Figure 3 presents subgroup analyses examining the relationship between the SIRI and thyroid function. To ascertain if SIRI’s association with thyroid function is consistent across the general population and to identify distinct subgroups, we conducted analyses stratified by age, gender, BMI, and hypertension, along with interaction tests. Our findings reveal significant interactions of SIRI with FT4 in different age groups, with TT4 in the context of hypertension, and with TPOAb across age groups (all P for trend <0.05). However, no notable differences were observed in terms of gender and BMI. Thus, our study indicates that SIRI’s correlation with thyroid function varies with hypertension status and may be relevant for individuals without hypertension. Importantly, our interaction tests did not reveal any significant differences associated with gender and BMI, indicating that these factors do not influence the relationship between SIRI and thyroid function.
The objective of this study was to evaluate the association between the SIRI and thyroid function in a population of US adults. In our cross-sectional analysis of 1,641 participants, we discovered a significant positive correlation between SIRI and both FT4) and TTT4. However, no significant correlation was found between SIRI and TSH. Adjustments for all covariates revealed elevated levels of FT4, TT4, and TPOAb in the highest quartile of SIRI (Tertile 4) compared to the lowest (Tertile 1). Additionally, we employed Generalised Additive Models and Restricted Cubic Splines to further delineate the SIRI-thyroid function relationship. Our findings indicated a nonlinear association between SIRI and FT3, TT3, and TT4. Notably, the nature of the relationship varied across the breakpoint; SIRI was positively correlated with FT3, TT3, and TT4 on the left side of the breakpoint, whereas on the right side, it was negatively correlated with FT3 and TT3. Still, the correlation with TT4 was not statistically significant. Finally, the interaction term analysis suggested that gender and BMI did not significantly influence these associations.
This study is the first to investigate the correlation between SIRI and thyroid function risk. Previous research has established connections between thyroid function and various clinicopathological factors. Chen S et al. observed a negative relationship between the Healthy Eating Index (HEI) and levels of FT3 and TT3. Additionally, they found a strong association between the Dietary Inflammatory Index (DII) with increased FT3 and TT4 levels, suggesting DII’s utility in evaluating dietary impacts on inflammatory potential (6). Anti-inflammatory diets, such as DASH and Mediterranean, have been noted to lower systemic inflammation levels (7, 8). Research indicates that gut flora and dietary fiber modulate inflammatory states via inter-organ signaling, thus impacting pathophysiological aspects of obesity, diabetes, and dyslipidemia (9, 10). Leonardo et al. demonstrated that pro-inflammatory diets can lead to intestinal imbalance, bacterial overgrowth, increased intestinal permeability, and oxidative stress. This was evidenced by examining the faces of HT patients, suggesting that such an inflammatory response could accelerate HT development and support the notion of a thyroid-gut axis (6). GlycA, a glycoprotein biomarker, indicates systemic inflammation by measuring acute phase reactants and various glycosylated proteins. Research by Nilay Yukse et al. found that patients with hypothyroidism, regardless of treatment status, exhibited elevated GlycA levels, indicative of low-grade systemic inflammation. Similarly, Nizamutdinova IT identified a strong link between hyperthyroidism, inflammatory responses, apoptosis, and activation of hypertrophy-related proteins. Hyperthyroidism triggers NF-kB activation, escalating NO, lipid peroxidation, and free radical production. This results in a marked increase in TNF-a, IL-1b, IL-4, IL-6, and IL-10 levels (5, 11). A meta-analysis further confirmed significantly higher serum CRP levels in hypothyroid patients (12), corroborating previous findings (13). It has been demonstrated that obesity-induced adipose tissue expansion provides many internal signals that may trigger an inflammatory response (e.g. adipocyte death, hypoxia, and mechanical stress). A cohort study verified that overweight and obesity are linked to elevated free T3 levels. Altered thyroid function in these patients increases energy expenditure, aiding in weight loss and preventing further weight gain. Consequently, TSH and FT3 normalization post-weight loss may contribute to the challenges in maintaining weight loss (14). Given the association between inflammation, abnormal thyroid function, and the reliability of SIRI as an inflammation indicator, a positive correlation between SIRI and thyroid function appears likely.
Prior epidemiological studies indicate that hypertension, obesity, and gender significantly impact thyroid function. Ladan Mehran et al. found that hypertension diminishes TH sensitivity (15). Research has also linked obesity to a heightened risk of hypothyroidism (16), possibly due to obesity’s nature as a chronic, low-grade inflammatory state. In this state, inflammatory markers such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α), produced by overloaded adipose tissue, increase (17). Estrogen, a key growth factor for thyroid cells, promotes growth through genomic and non-genomic pathways via the membrane-bound estrogen receptor. This receptor is associated with the MAPK and PI3K tyrosine kinase signaling pathways (18), potentially explaining the higher prevalence of thyroid diseases in women compared to men. According to our subgroup analysis, there was a significant SIRI with FT4 in age (P for interaction = 0.0221), SIRI with TT4 in hypertension (P for interaction = 0.368), and SIRI with TPOAb in age (P for interaction = 0.0221). These findings suggest that the relationship between the SIRI and thyroid function is influenced by hypertension. Hyperthyroidism has been linked to increased cardiovascular disease risk and endothelial dysfunction (19). It may also lead to secondary systolic hypertension through elevated cardiac output and increased renin, angiotensin, and aldosterone levels. Notably, a significant positive correlation between SIRI and FT4, TT4, and TPOAb persists in nonhypertensive subgroups, underscoring its relevance in this population.
The underlying mechanism driving the positive correlation between the SIRI and thyroid hormones FT4, TT4, and TPOAb remains elusive. However, existing research sheds light on this association. It’s established that thyroid diseases prompt alterations in TH and related regulatory hormones. For instance, hyperthyroidism results from an excessive release of TH (T3 and T4), leading to protein, lipid, and DNA damage due to stress, increased basal metabolic rate, and oxygen consumption in body tissues (20). HT stems from an autoimmune attack on thyroid tissue, characterized by lymphocytic infiltration, fibrosis, and interstitial atrophy. RET/PTC rearrangements, implicated in HT, activate pathways that induce both HT and the expression of genes like CXCL5 and CXC chemokine receptor 2 (CXCR2), linked to inflammation and tumor invasion (21). Furthermore, Galdiero (22) et al. observed that in thyroid cancer patients, neutrophils upregulate CD 11b and CD 66b, correlating tumor size. Collectively, these findings strongly suggest a link between thyroid dysfunction and inflammation. SIRI emerges as a comprehensive marker of the body’s inflammatory and immune response, surpassing traditional parameters like the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in predictive capability. This extends to tumor prognosis, cardiovascular disease, chronic obstructive pulmonary disease, and COVID-19. Yuxiu Yang et al.’s study with 1527 patients with moderate coronary artery stenosis exemplifies SIRI’s superior performance over conventional cardiovascular risk models (23). Multiple studies corroborate these findings, highlighting SIRI’s reliability and potential applications. In our study, accounting for confounding factors, we identified a positive correlation between FT4, TT4, and TPOAb, indicating SIRI’s role in the pathogenesis and progression of thyroid disorders. Thus, monitoring SIRI levels could provide essential insights for therapeutic decision-making and tracking disease progression in thyroid diseases. This study paves the way for further investigation and underscores the role of inflammation in thyroid disorders.
The robust sample size and thorough adjustment for covariates enhance the credibility and representativeness of our study. Nevertheless, it is not without limitations. The cross-sectional design precludes causal inference, necessitating future prospective studies with larger cohorts to clarify causality. Our reliance on data from a public dataset, NHANES, meant we could not modify the study design. While we accounted for several covariates, potential confounders, such as medication use (e.g., steroids), could still have influenced the outcomes. Unfortunately, NHANES did not collect data on these variables, restricting our ability to incorporate them into our analysis.
Furthermore, as NHANES is a U.S.-based study, our evaluation was confined to the association between SIRI and thyroid function in the American adult population. The limitation to a single-country sample with restricted ethnic diversity hinders the generalizability of our findings. In summary, while our study indicates a correlation between elevated SIRI levels and increased FT4, TT4, and TPOAb levels, and a nonlinear relationship among FT3, TT3, and TT4, further research is essential for validation.
The studies involving human participants were reviewed and approved by the ethics review board of the National Center for Health Statistics.
The patients/participants provided their written informed consent to participate in this study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YZ: Writing – original draft, Data curation, Investigation. BW: Writing – review & editing, Conceptualization, Methodology. WH: Writing – review & editing, Supervision, Validation. BY: Writing – review & editing, Formal Analysis, Investigation. JC: Writing – review & editing, Validation. FA: Writing – review & editing, Validation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Qilu Chinese Medicine Advantage Specialist cluster Project (project number: YWC2022ZKJQ0003), Explored the mechanism of promoting mesenteric vascular repair in the treatment of Crohn’s enteritis by adding flavor to Si-Miao Yong’an Tang based on TGF-β1/Smads signaling pathway(project number: M-2023305) and Shandong University of Traditional Chinese Medicine Hospital "Young Famous Traditional Chinese Medicine Practitioners" Cultivation Program in 2023.
We would like to thank all the participants for their participation and contribution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NHANES, National Health and Nutritional Examination Survey; CI, Confidence interval; SIRI, System Inflammation Response Index; NLR, Neutrophil to Lymphocyte Ratio; PLR, Platelet to Lymphocyte Ratio; OR, Odds ratio; HT, Hashimoto’s thyroiditis; BMI, Body Mass Index; TT3, Free Triiodothyronine; FT4, Free Thyroxine; TSH, Thyroid stimulating hormone; TPOAb, Thyroid peroxidase antibodies; TT3, Total Triiodothyronine; TT4, Total Thyroxine; WBC, White blood cell count; HEI, Healthy Eating Index; DII, Dietary Inflammatory Index.
1. Mannaa FA, Abdel-Wahhab KG, Hassan LK, Taher RF, Morsy FA, Fadl NN. Influence of Melissa officinalis methanolic extract on hyperthyroidism in a rat mode. Egypt Pharmaceut J (2022) 20:134–44. doi: 10.4103/epj.epj_60_20
2. Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis (2010) 5:17. doi: 10.1186/1750-1172-5-17
3. Choi S, Kim MJ, Park YJ, Kim S, Choi K, Cheon GJ, et al. Thyroxine-binding globulin, peripheral deiodinase activity, and thyroid autoantibody status in the association of phthalates and phenolic compounds with thyroid hormones in the adult population. Environ Int (2020) 140:105783. doi: 10.1016/j.envint.2020.105783
4. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122(14):2158–67. doi: 10.1002/cncr.30057
5. Li S, Xu H, Wang W, Gao H, Li H, Zhang S, et al. The systemic inflammation response index predicts survival and recurrence in patients with resectable pancreatic ductal adenocarcinoma. Cancer Manag Res (2019) 11:3327–37. doi: 10.2147/CMAR.S197911
6. Chen S, Peng Y, Zhang H, Zou Y. Relationship between thyroid function and dietary inflammatory index in Hashimoto thyroiditis patients. Med (Baltimore) (2023) 102(46):e35951. doi: 10.1097/MD.0000000000035951
7. Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The dietary approaches to stop hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: A randomized cross-over clinical trial. Ann Nutr Metab (2014) 64:20–7. doi: 10.1159/000358341
8. Zupo R, Castellana F, Panza F, Lampignano L, Murro I, Di Noia C, et al. Adherence to a mediterranean diet and thyroid function in obesity: a cross-sectional Apulian survey. Nutrients. (2020) 12(10):3173. doi: 10.3390/nu12103173
9. Kim TH, Ko J, Kim BR, Shin DY, Lee EJ, Yoon JS. Serum selenium levels in patients with graves disease: Associations with clinical activity and severity in a retrospective case-control study. Korean J Ophthalmol: KJO (2022) 36(1):36–43. doi: 10.3341/kjo.2021.0146
10. Kvícala J, Havelka J, Nímec J, Zeman V. Selenium and rubidium changes in subjects with pathologically altered thyroid. Biol Trace Elem Res (1992) 32:253–8. doi: 10.1007/BF02784608
11. Galvao S, Bensenor IM, Blaha MJ, Jones S, Toth PP, Santos RD, et al. GlycA as a novel biomarker of systemic inflammation in hypothyroidism. Thyroid. (2023) 33(10):1171–81. doi: 10.1089/thy.2023.0070
12. Tellechea ML. Meta-analytic evidence for increased low-grade systemic inflammation and oxidative stress in hypothyroid patients. Can levothyroxine replacement therapy mitigate the burden? Endocrine. (2021) 72(1):62–71. doi: 10.1007/s12020-020-02484-1
13. Grey A, Mitnick MA, Shapses S, Ellison A, Gundberg C, Insogna K. Circulating levels of interleukin-6 and tumor necrosis factor-alpha are elevated in primary hyperparathyroidism and correlate with markers of bone resorption–a clinical research center study. J Clin Endocrinol Metab (1996) 81:3450–4. doi: 10.1210/jcem.81.10.8855783
14. Witkowska-Sędek E, Kucharska A, Rumińska M, Pyrżak B. Thyroid dysfunction in obese and overweight children. Endokrynol Pol (2017) 68(1):54–60. doi: 10.5603/EP.2017.0007
15. Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab (2022) 107(1):167–76. doi: 10.1210/clinem/dgab646
16. Song RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA. The impact of obesity on thyroid autoimmunity and dysfunction: A systematic review and meta-analysis. Front Immunol (2019) 10:2349. doi: 10.3389/fimmu.2019.02349
17. Fontenelle LC, Feitosa MM, Severo JS, Freitas TE, Morais JB, Torres-Leal FL, et al. Thyroid function in human obesity: underlying mechanisms. Horm Metab Res (2016) 48(12):787–94. doi: 10.1055/s-0042-121421
18. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer (2014) 21(5):T273–83. doi: 10.1530/ERC-14-0053
19. Neves JS, Vale C, von Hafe M, Borges-Canha M, Leite AR, Almeida-Coelho J, et al. Thyroid hormones and modulation of diastolic function: a promising target for heart failure with preserved ejection fraction. Ther Adv Endocrinol Metab (2020) 11. doi: 10.1177/2042018820958331
20. Costilla M, Delbono RM, Klecha A, Cremaschi GA, Arcos MLB. Oxidative stress produced by hyperthyroidism status induces the antioxidant enzyme transcription through the activation of the nrf-2 factor in lymphoid tissues of balb/c mice. Oxid Med Cell Longev (2019) 2019:7471890. doi: 10.1155/2019/7471890
21. Cui D, Zhao Y, Xu J. Activation of CXCL5-CXCR2 axis promotes proliferation and accelerates G1 to S phase transition of papillary thyroid carcinoma cells and activates JNK and p38 pathways. Cancer Biol Ther (2019) 20(5):608–16. doi: 10.1080/15384047.2018.1539289
22. Galdiero MR, Varricchi G, Loffredo S, Bellevicine C, Lansione T, Ferrara AL, et al. Potential involvement of neutrophils in human thyroid cancer. PloS One (2018) 13(6):e0199740. doi: 10.1371/journal.pone.0199740
Keywords: thyroid hormone, systemic inflammatory response index, thyroid function, positive correlation, national health and nutrition examination survey
Citation: Zhai Y, Wang B, Han W, Yu B, Ci J and An F (2024) Correlation between systemic inflammatory response index and thyroid function: 2009-2012 NHANES results. Front. Endocrinol. 14:1305386. doi: 10.3389/fendo.2023.1305386
Received: 01 October 2023; Accepted: 27 December 2023;
Published: 22 January 2024.
Edited by:
Malgorzata Gabriela Wasniewska, University of Messina, ItalyReviewed by:
Giorgia Pepe, University of Messina, ItalyCopyright © 2024 Zhai, Wang, Han, Yu, Ci and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjun Wang, bTE1NjYyNzcxNTI2QDE2My5jb20=; a2V5YW42MjM2QDE2My5jb20=
†ORCID: Benjun Wang, orcid.org/0009-0006-2170-5300
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.