- 1Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore, Pakistan
- 2Department of Pharmacology, Faculty of Pharmaceutical Sciences, Government College University Faisalabad, Faisalabad, Pakistan
The most common cause of infertility and metabolic problems among women of reproductive age is polycystic ovary syndrome (PCOS), a multifaceted disorder. It is an endocrine disorder that occurs in approximately one in seven women. Among these PCOS patients, two thirds will not ovulate on a regular basis and seek treatment for ovulation induction. The symptoms vary in their severity, namely ovulation disorders, excessive androgen levels, or polycystic ovarian morphology. All these symptoms require a therapeutic approach. Many drugs are used to eradicate PCOS symptoms, like metformin, clomiphene citrate, spironolactone, and pioglitazone. Long-term treatment is required to achieve the desired outcome, which is often accompanied by significant adverse reactions. Some herbs and phytochemicals are equally effective for treating PCOS and produce minimal side effects. Recently, herbal products are gaining popularity due to their wide biological activities, safety, availability, and efficacy. The present review covers aetiology, current treatment, pathophysiology, and detailed pre-clinical and clinical studies on plants and phytochemicals that are proven to be useful for the treatment of symptoms associated with PCOS.
Introduction
The Stein–Leventhal syndrome was the initial name for the condition that is now known as polycystic ovary syndrome (PCOS) (1). It is a harmful illness that predominantly affects females and is characterized by an enlargement of the ovaries that are filled with many tiny cysts that are actually immature follicles (2). Ovulation, menstrual abnormalities, infertility, and insulin resistance are all related to PCOS. Acne, hirsutism, and weight gain are all possible symptoms of PCOS (3). As the condition worsens, it culminates in other health problems such as dysfunctional uterine haemorrhage, obesity, Type 2 diabetes, endometrial cancer, high cholesterol, and cardiovascular diseases (4). Modifications to a woman’s lifestyle, as well as pharmacological therapies, are currently used as standard care treatments for PCOS (5). Alterations to one’s nutrition and an increase in physical activity are the desired lifestyle changes for the treatment of PCOS (6). Even though, there are many drugs on the market, the most effective strategy for PCOS is to adjusting one’s lifestyle. Losing weight and exercising are two of the most effective ways to treat PCOS without the risk of negative side effects (7).
The use of oestrogen-progestin combinations, antiandrogens, and hypoglycaemic medications are some examples of pharmacological therapies for PCOS (8). However, their chronic use is associated with the risk of endometrial cancer in women after menopause. This risk seems to increase as the dose and the length of use increase (2). Since ancient times, medicinal plants have had a great deal of potential for treatment and mitigation of acute and chronic diseases, and now, as a result of the research that has been conducted, new medicinal plants that are useful and advantageous are being uncovered (9). Traditional Persian medicine (TPM) with holistic approaches towards human health has utilized many herbal therapies. The French consumed herbal extracts for the eradication of PCOS symptoms (10). Natural polyphenols have been used for decades to manage hormonal problems (11). Many studies, including randomized controlled trials, case studies, and animal experiments, have been conducted on herbal medications because of their fewer side effects and to validate their traditional uses (12).
Method
Different data bases such as Google scholars, Pubmed and Web of Science were searched for phytochemicals and plants pharmacologically validated for the prevention and treatment of PCOS. Key words used for searching included but not limited to “PCOS, “polycystic ovary syndrome”, “phytochemicals used for pcos”, “plants for PCOS”, “herbs for PCOS”, “treatment of PCOS” and “prevention of PCOS”. From the available full text articles, a master list of phytochemicals and plants was first prepared. These research studies were qualitatively evaluated for the positive and negative controls, dose, duration of therapy, method of induction of PCOS, mechanism of action and efficacy of chemical/crude drugs. The names of medicinal plants were confirmed from www.theplantlist.org.
Pathophysiology of PCOS
In spite of the ongoing research for better understanding of the morphology, pathophysiology, and therapeutic methods of PCOS, the underlying aetiology of PCOS is still a mystery (13). A number of factors, including genetics, lifestyle, and individual characteristics, interact to cause PCOS, which in turn causes serious health consequences and a disturbed hormonal and metabolic cycle (12). The menstrual cycle is disturbed at multiple stages in women with PCOS (14). The follicular cells generate an anti-androgen called anti-Mullerian hormone (AMH), which is highly concentrated in primordial and antral follicles, and regulates their progression into the initial level. When the AMH hormone is secreted in excess, it throws off the ovarian follicle growth cycle (15). Infertility may result from immature follicles because they block the sensitivity to follicle-stimulating hormone (FSH), preventing continued follicle development (16). Overproduction of gonadotropin releasing hormone is the second most prevalent symptom of PCOS (17). This is caused by a malfunction in the feedback mechanism of the hypothalamus-hypophysis-ovarian axis (HHOA) (18). On top of that, HHOA causes an abnormally high ratio of blood luteinizing hormone (LH) to FSH (19). Because of the imbalance in the levels of these two hormones, the ovarian theca cells produce an excessive amount of the male hormone androgen (20). It leads to a reduction in the levels of oestradiol and progesterone, which induces gonadotropic hypothalamus cells to produce an increased quantity of GnRH and LH (21). The disorder’s growth and progression are thus largely attributable to this recurrent cycle of dysfunction (22).
The PCOS shows a marked rise in LH to FSH ratio, increased androgen and AMH levels, and insulin resistance (23). A rise in the ratio of LH to FSH levels is characterized by anovulation and irregular periods (24). Increased androgen levels in the body lead to multiple symptoms such as acne, menstrual disturbances, and obesity (25). A large number of primordial follicles are seen with an increase in AMH level (26). Obesity, high blood pressure, and metabolic syndromes are connected with PCOS with increased insulin resistance as shown in Figure 1 (27).
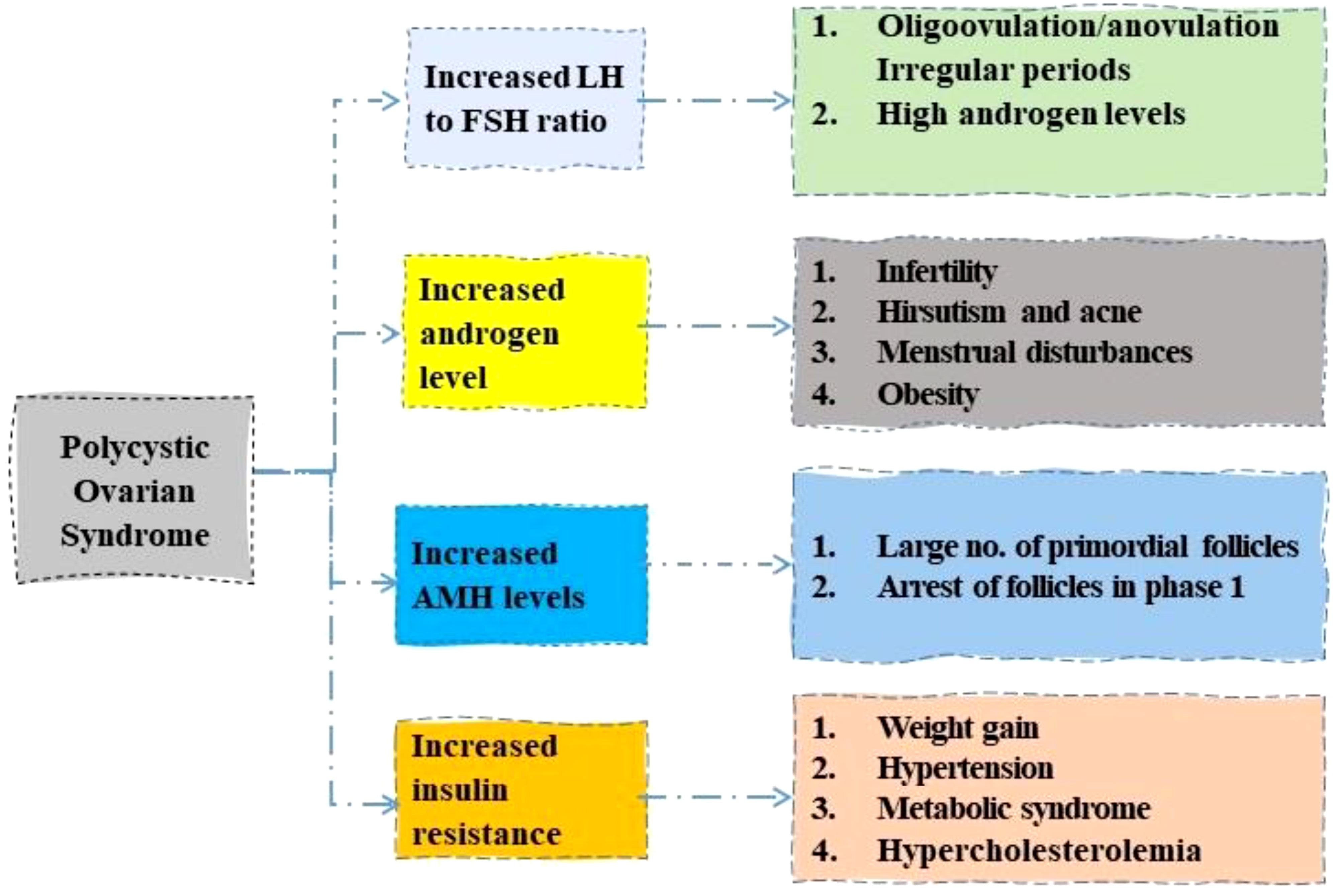
Figure 1 Role of different hormones in development of Polycystic ovary syndrome. LH, luteinizing hormone; FSH, follicle stimulating hormone; AMH, anti-Mullerian hormone.
Symptoms and complications of PCOS
The PCOS exhibits its symptoms through various signs that include menstrual irregularities and a number of cysts in the ovary, causing abdominal pain and cramps during periods (28). The physical appearance also shows some abnormal changes in PCOS, such as hirsutism, acne, alopecia, and seborrhoea (29).
Hirsutism
As a result of an increased androgen concentration, the condition known as hirsutism causes an abnormally large amount of terminal hair to grow in areas of the body that are normally bald or have only a small amount of hair growth (30). Androgenic substances such as testosterone, dihydrotestosterone (DHT), dehydroepiandrosterone sulphate (DHEAS), and androstenedione are all released by a female body when it reaches reproductive age (31). Only androgens and DHT have the ability to attach to the androgen receptors, which results in a changed morphology and physiology of the hair follicles (4).
Acne and seborrhoea
The sebaceous glands are a type of tiny gland that secretes oil and are found in males and females both (32). The sebaceous glands are notoriously sensitive to changes in the concentration and secretion of androgens (33). In patients with polycystic ovary syndrome (PCOS), an increased concentration of androgens causes sebaceous glands to be stimulated into producing more sebum, which in turn results in the formation of acne and seborrhea (34). Androgens are to blame for the development of sebocytes, which occurs most frequently in the region of the forehead, chin, and middle back (35). The local bacteria cause an infection of the hair follicle structure and then secrete lipolytic enzymes. This causes triglycerides to break down, which in turn causes a blockage and the deposition of oil and other materials that have been destroyed in the area (4).
Androgenic alopecia
Androgenic alopecia is a condition in which a person experiences continuous hair loss in a discernible pattern as a result of an increased concentration of androgens as well as secretions of these hormones (36). In hair follicles that are vulnerable to the process, testosterone is changed into a compound called dihydrotestosterone (DHT). This compound, then attaches to the androgen receptor as well as the genes that are responsible for the reduction in size of large terminal hair follicles over time (37). This was further supported by the results published by Cela et al., which found that 67% of the women who had alopecia, also had PCOS and an increased level of androgens in their bodies (4).
Onycholysis and onychorrhexis
Onycholysis refers to the process in which the nail plate becomes detached from the nail bed as a result of an onychocorneal band (38). Onychorrhexis, on the other hand, refers to the process in which nails split into lengthwise bridges (39). Although the precise explanation for this result is not entirely clear, a number of studies have found that elevated androgen levels in PCOS patients are associated with more severe cases of onycholysis and onychorrhexis (2).
PCOS and associated disorders
PCOS patients are at higher risk for several other chronic and serious health conditions such as insulin resistance, obesity, Type 2 diabetes mellitus, coronary artery disease, atherosclerosis, hypertension, depression, anxiety and low-level inflammation. PCOS is a metabolic disorder that is greatly aggravated by obesity. Adipokines released by adipocytes cause insulin resistance which causes ovarian androgen production as well as alteration in lipid homeostasis (40). It has now been established that hyperinsulinemia adversely affects preantral follicular leading to ovulatory dysfunction (41). Metabolic changes in PCOS, such as hyperinsulinemia, hyperlipidaemia and obesity, increase the risk of cardiovascular diseases. A huge number of PCOS patients also suffer from anxiety and depression which occur due to distresses caused by symptoms such as hirsutism, obesity and altered physical appearances (42).
Pharmacotherapy for PCOS
Several drugs used for the treatment of PCOS are not devoid of harmful effects on patients (43). The issues that are linked with clomiphene citrate (CC) are also something that should be taken under consideration. These concerns include the potential danger of ovarian cancer, the antiestrogenic effects it has on the endometrium, as well as the issue of the early LH surge (44). After using CC for an extended period of time, more than 12 treatment cycles, there is a possibility of developing ovarian cancer. As a result, this medicine should only be used for a maximum of six cycles (45). Moreover, clomiphene and letrozole do not exhibit anti-estrogenic effects on the endometrium and cervical mucus (46). Dehydration, excessive urination, vomiting, headache, lethargy, gastritis, and ovarian disruption leading to irregular menstruation are some of the side effects that may be caused by using spironolactone (47). Metformin use is linked to a wide range of adverse effects, including but not limited to nausea, vomiting, digestive disturbances, and ketoacidosis (48). Furthermore, in patients with PCOS, metformin therapy must be continued indefinitely because stopping the medication after a period of three months has the potential to reverse all the disease progress (49). A long-term intervention is required for PCOS treatment owing to the significant and severe adverse reactions of currently used drugs, which warrants the search for novel therapies for the chronic management of PCOS (50). For the treatment of PCOS, the use of plant-based medications, which have fewer potential negative side effects, is likely to become the most effective strategy (2) as shown in Figure 2.
Treatment for PCOS-related symptoms
It has been shown that making changes to one’s lifestyle, such as maintaining a healthy weight loss, increasing physical activity, and making adjustments to one’s food, can help in the management of PCOS (2). Spironolactone, metformin, and eflornithine are the drugs that are considered to be the first line of defence in the fight against hirsutism (51). Because they have a low androgenic effect, additional contraceptive pills and their combination of either progestins or norgestimate, desogestrel or drospirenone are frequently used to treat hirsutism (52). Contraceptive pills, spironolactone, acarbose, and rosiglitazone are the four most common medications used to treat menstrual problems (53).
In PCOS, whole ovarian hypertrophy is seen with thickened capsule (54). There is an increased number of sub-capsular follicle cysts (55–57). The main features of PCOS are:
● Increase in ovarian size
● Capsules that are thicker than 100 µm
● An increase in the amount of subcapsular follicular cysts (58).
● A deficiency in the number of corporea lutea or albicantia
● Hyperplasia of the ovarian stroma, as well as fibrosis
● Theca cell luteinization before its time (12).
Phytochemicals for PCOS management
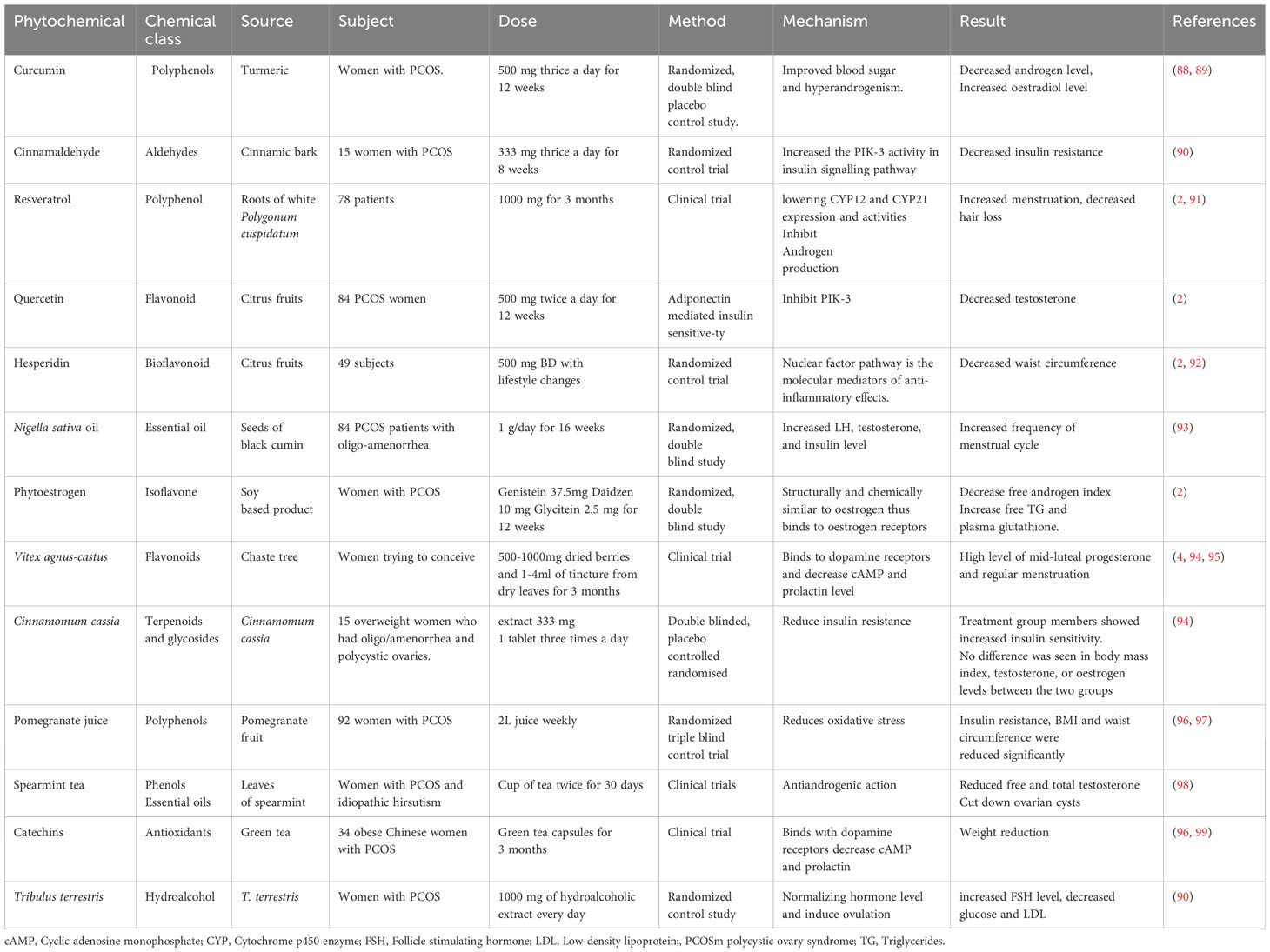
Numerous studies have demonstrated the efficacy of herbal drugs and isolated phytochemicals for the management of PCOS. Several phytochemicals, such as curcumin, rutin and fisetin, regulate the reproductive cycle via reducing testosterone level, and LH to FSH ratio (59). Some phytochemicals such as Naringenin and fisetin also increase the insulin sensitivity and improve the glycaemic control, which contributes to the betterment of PCOS symptoms (60). Some essential oils such as α-thujone are antiandrogenic in nature, so they decrease the level of androgen while improving ovulatory functions (60). Puerin, fisetin, berberine reduce the features of PCOS and associated comorbidities by decreasing oxidative stress (61). Tables 1 and 2 show the sub-clinical and clinical studies carried out on isolated phytochemicals and plants to treat PCOS.

Table 1 Pre-clinical studies on plants and phytochemicals effective against polycystic ovary syndrome.
Polyphenols
Polyphenols are naturally occurring chemicals that serve as a defensive mechanism against a variety of stressors (100). Because of their powerful cardioprotective, antiproliferative, antioxidative, antiapoptotic, and anti-inflammatory capabilities, the significance of such polyhydroxyphenols has significantly increased in recent years (11). Polyphenols have been shown to be effective against a wide variety of diseases, including human cancers, heart problems, cognitive problems, diabetes, metabolic disorders, aging, and inflammation-associated illnesses, as well as PCOS (101). These findings have been reported in a number of studies. Polyphenols are being investigated as a potential treatment PCOS. This is due to the fact that oxidative damage, metabolic, and endocrine abnormalities all play a significant part in the development of PCOS (11). Curcumin and fisetin are two phytoconstituents that have demonstrated a significant reduction in the symptoms of PCOS (66). The lipids and hormonal profiles as well as insulin resistance are improved by these agents. In addition, the activity of enzymes that fight free radicals is increased by these plants (102).
Curcumin
Turmeric contains curcumin, which is biologically active. It possesses a variety of pharmacologic actions and has the potential to be used in patients suffering from PCOS (88). Since, its isolation from Curcuma longa in 1815, the polyphenol curcumin has attracted the interest of researchers all over the world due to its promising biological activities (89). In PCOS patients, curcumin was useful in reducing blood glucose levels, insulin resistance and hyperandrogenism (103). In a controlled double-blind study, PCOS patients were randomly assigned to receive either curcumin (500 mg t.i.d.) or a dummy pill for a period of twelve weeks. Primary outcome indicators included fasting plasma glucose (FPG), fasting insulin (FI), sex hormone levels, and hirsutism. Secondary indicators were anthropometric measurements. Among 72 randomized people, 67 patients finished the trial (104). Once the trial is completed, fasting plasma glucose and DHEA levels had dropped much more in the treated group in comparison to the control group and the placebo (88). A statistically non-significant rise in oestradiol levels of the treatment group was evident as compared to the control group without any adverse events (88). In patients with polycystic ovary syndrome (PCOS), hyperandrogenism and hyperglycaemia may respond favourably to the use of curcumin as a dietary supplement. The findings of this research need to be supported by more extensive studies examining a wider range of dosages over longer time periods (88).
Flavonoids
Large amounts of flavonoids may be able to cure a variety of ailments without causing any severe adverse effects (105). Flavonoids increase the serum levels of FSH, and sharply decrease the serum levels of LH, testosterone and insulin in the PCOS-insulin resistant rats via partly inhibiting the activation of JAK2/STAT3 pathway, partially up-regulating the IL-6 expression and down-regulating the suppressor of cytokine signalling 3 (SOCS3) expression in ovaries of PCOS rats (106). Flavonoids may be natural, citrus, or bioflavonoids (107). Flavonoids extracted from various plants are found to decrease testosterone levels, improve insulin resistance and increase the expression of IL-6 to treat PCOS rats (108). Several major flavonoids are used for PCOS management, namely resveratrol, hesperidin, quercetin, rutin, naringenin, and apigenin (109). These flavonoids decrease the levels of testosterone in the body. They also improve the insulin resistance and decrease the number of cysts in the ovary (110).
Resveratrol
Resveratrol is a plant-derived flavonoid that was initially extracted from the roots of white hellebore in 1940 and in 1960 from the roots of Polygonum cuspidatum, and is currently discovered in approximately seventy different kinds of herbs. Its effectiveness in the treatment of PCOS is well-established (2). It was discovered that resveratrol inhibited the production of CYP17 and CYP21 proteins as well as their enzyme activity to prevent excessive production of androgens in PCOS animals (91). Several research studies demonstrated that resveratrol exhibited PCOS ameliorating effect through decreased LH, LH/FSH, TNF-α and tissue AMH in diseased animals. In addition, resveratrol also increased E2, LH and testosterone, reduced the expression of mTOR and Akt in ovarian tissues to regulate reproductive function in female rats (75, 76). For the purpose of determining whether or not resveratrol is effective in treating PCOS, a clinical trial was carried out on 78 individuals who received 1000 mg of the medication every day for a period of three months. After therapy, the results showed an increased menstruation rate and a decreased rate of hair loss, and the levels of lipids, insulin, and androgens remained the same (2).
Quercetin
Quercetin is an organic flavonoid that is derived from a variety of fruits and vegetables, the majority of which are citrus fruits, such as red grapes, onions, apples, berries, nuts, and seeds (111). Tea is also a good source of quercetin (112). Oxidative stress is thought to be the primary component that leads to PCOS (113). The mechanism of action is an improvement of the NF-κB signalling pathway and a correction of the inflammatory environment of the ovarian tissue (114). PCOS has also been linked to dysfunction of the endocrine glands (115). Beside affecting insulin resistance, quercetin also alters gene expression of GLUT-4 and ER in diabetic pregnant mice to improve embryo development (116). Quercetin also decreased insulin resistance in PCOS rats via decreasing liver glucokinase and hexokinase, and increasing the expression of GLUT-4 and estrogen receptors (73). In women with PCOS, their levels of adiponectin tend to be lower, regardless of their weight (117). Eighty-four PCOS women were given quercetin, and their adiponectin-mediated insulin sensitivity was tested. When compared to placebo, taking quercetin 500 mg twice per day for 12 weeks resulted in a rise in total adiponectin by roughly six percent and an increase in the level of high molecular weight adiponectin by approximately four percent. There was a drop in the level of testosterone, LH and insulin resistance in the quercetin group, which emphasized the function of quercetin in re-modelling the adiponectin-mediated insulin resistance and hormonal level in PCOS women (2).
Hesperidin
Hesperidin is a bioflavonoid, initially extracted from the innermost layer of orange peel. It can be found in high concentrations in a wide range of citrus fruits, the most notable of which are lemon, lime, orange, and grapefruit (118). Hesperidin exhibits multiple activities, including anti-inflammatory, anti-microbial, and anti-diabetic effects (119). It appears that signalling pathways, specifically the nuclear factor pathway, are the molecular mediators of anti-inflammatory effects. However, the exact mechanism is not known (92). In a previous in-vitro study, it increased the maturation and growth of preantral follicles of mice to improve fertilization and embryo development via upregulating the expression of PCNA, FSH-R and Bcl-2, and downregulating Bax gene (70).
Isoquinoline alkaloids
Some isoquinoline alkaloids alleviate insulin resistance and activate the insulin signalling pathway. Berberine is a major member of this family (120). It promotes the utilization of glucose and reduces the level of serum androgen. It improves sex hormone binding globulin levels (121). As an isoquinoline alkaloid, berberine is the main effective component of this class, as a multi-target, multi-path plant constituent that interferes with the development of PCOS and related comorbidities with a few adverse reactions (122). It was found that berberine reduced the expression of toll-like receptor 4 (TLR4), Src family tyrosine kinase (LYN), phatidylinositol 3-kinase (PI3K), NF-κB, TNF-α, IL-1, and caspase-3, which was accompanied by a reduction in cell apoptosis, which pointed to the potential significance of berberine in the therapy of PCOS (123).
Phytoestrogens
Phytoestrogens are substances that naturally occur in plants. They have a similar chemical structure to our own body’s oestrogen and are able to bind to the same receptors as that of oestrogen (124). Phytoestrogens have shown both estrogenic and anti-estrogenic effects (125). Isoflavones are the most widely studied phytoestrogens. These are abundantly found in soybeans, legumes, berries, grains, nuts, and wine. Resveratrol, found in fruits, berries, red wine, chocolate, and peanuts, is believed to be responsible for some of the health benefits that include improved hormonal profile, insulin resistance, and antioxidant effects (126).
Phytoestrogens are isoflavones commonly found in soya products. There is evidence that phytoestrogens like Biochanin A, Daidzein, Genistein, formononetin, and Puerarin can help in the management of PCOS symptoms (2). PCOS women experience a disruption in the process of the production of androgen as well as its metabolic process and oestrogen, which causes an increase in the concentrations of androstenedione, testosterone, and dehydroepiandrosterone in their serum (2). Phytoestrogens have molecular structures and sizes nearly identical to oestrogens such as 17-estradiol and diethylstilboestrol. As a result, these attach to oestrogen receptors and have anti-estrogenic effects, helping PCOS patients with hormonal imbalances (2). A randomized, double-blind, and placebo-controlled trial was carried out to demonstrate the effectiveness of soy-isoflavone and phytoestrogen against PCOS. Phytoestrogens consisting of 37.5 mg genistein, 10 mg daidzein, and 2.5 mg glycitein were given to PCOS patients for a period of 12 weeks. A considerable drop was seen in both the free androgen index, serum triglyceride, and insulin levels as a result of therapy with phytoestrogens. Additionally, a significant rise in plasma glutathione level and a fall in monoaldehyde level pointed towards a favourable effect of phytoestrogens on the management of PCOS (2).
Catechins
Green tea is derived from the plant Camellia sinensis and contains a high concentration of catechins along with several minerals and vitamins (127). It has several health benefits, including but not limited to effectiveness against diabetes, insulin resistance, and obesity (96). A total of thirty-four obese Chinese PCOS patients were selected at random to receive either treatment with green tea capsules or a placebo for a period of three months. After receiving therapy, each group’s anthropometric measurements and biochemical and hormonal profiles were compared to those taken before treatment. After treatment, little but noticeable weight loss among those who had consumed green tea without any noticeable variation in hormone levels (99). Various preclinical studies on individual phytochemicals and plants effective against PCOS are shown in Table 2.
Polyunsaturated and dietary short-chain fatty acid
Previous studies have shown that a decline in carnitine, a metabolic intermediate of fatty acids, causes oocyte maturation and regulates energy metabolism and transport of fatty acids (128). Polyunsaturated fatty acids, such as omega-3 and α-linolenic acid play important therapeutic role in PCOS through reduction of inflammation and oxidative stress, and normalizing hormonal irregularities (129). In addition, short-chain fatty acids including butyric acid, are usual breakdown products of dietary fibres by gut microbes, which affect cellular functions such as apoptosis, proliferation and adiposity by G-protein-coupled and other receptors. Butyric acid inhibited PCOS symptoms through reducing insulin resistance, ovarian inflammatory cytokines and gut microbiota (65). Butyric acid treatment of human granulosa tumour cells ameliorated lipopolysaccharide-induced apoptosis, inflammatory cascade and oxidative damage. In addition, intraperitoneal administration of butyric acid exhibited a decline in follicular count, LH, testosterone and insulin level, and increased FSH and estradiol level in obese PCOS mice (65).
Plants to treat PCOS
Several medicinal and dietary herbs and plants have been investigated for the management of PCOS (130). Several plants such as Vitex agnus, Cinnamomum genus, pomegranate, Tribulus terrestris, Mentha species and Nigella sativa have been investigated for their sub-clinical and clinical effectiveness to manage and treat PCOS symptoms.
Vitex agnus-castus
The Vitex agnus-castus (VAC) plant is a shrub or a small tree. The flavonoids make up the majority of VAC components. In vitro research has demonstrated that the flavonoids such as castidin, quercetagetin, and isovitexin have an effect on oestrogen receptors (95). VAC has been shown to be efficacious in both pre-clinical and clinical study for the reduction of prolactin levels, the improvement of the menstrual cycle, and the treatment of infertility. Compounds in VAC binds to dopamine type 2 (DA-2) receptors in the CNS, decreasing cAMP and prolactin release (94). In previous research, women who had been trying to conceive for six to thirty-six months were given a supplement containing VAC (4). It is recommended to take between 500 and 1000 mg of dried berries and 1–4 ml of a tincture made from dried plants. After three months, the supplementation group showed significantly higher levels of mid-luteal progesterone and regular menstrual periods, in contrast to the placebo group, which showed no significant changes. 14 of the 53 women who took the supplement became pregnant, compared to only 4 of the placebo group’s 40 women who did not become pregnant (4).
Cinnamomum genus
As a condiment and aromatic plant, cinnamon is widely used. Its bark and leaves are used to make cinnamon oil. Its major bioactive components include polyphenols and cinnamaldehyde (131). It has been proven that the PCOS-treating properties of Cinnamomum zeylanicum include both reproductive and metabolic benefits (90). It was observed that cinnamonaldehyde had decreased the blood glucose through upregulating the expression of the GLUT4 gene. Cinnamaldehyde also increased the antioxidant response of reactive oxygen species produced in hyperglycaemia so as to safeguard pancreatic beta cells (132). PCOS patients receiving an extract of cinnamon three times a day for eight weeks experienced an improvement in their insulin sensitivity (133). The process for reducing insulin resistance involves increasing glucose utilization and potentiating the insulin signalling pathway via phosphatidylinositol 3-kinase (PI-3 kinase) at the post-receptor level. Recent clinical trial findings show that menstrual disruption in women with PCOS may benefit from cinnamon supplementation of 1500 mg/kg for 6 months. It is thought to be due to insulin resistance that improves and reduces menstrual irregularities (90).
The Cinnamomum cassia (Chinese cinnamon) tree is a tropical evergreen tree that is fragrant. Terpenoids, phenylpropanoids, and glycosides are the primary chemical components found in Cinnamomum cassia (134). A placebo-controlled and randomized experiment was carried out for a total of eight weeks. This study was carried out on 15 obese women who were also suffering from oligomenorrhea or amenorrhea and PCOS. Participants received either 333 mg of Cinnamomum cassia extract or placebo in a tablet three times per day for the entire duration of the study. Insulin sensitivity was significantly improved in the therapy group. There was no significant difference between the two groups in terms of BMI, testosterone levels, or oestradiol levels (94).
Pomegranate juice
The majority of the phytoconstituents found in pomegranate fruit are polyphenols (96). 92 women with PCOS participated in a parallel, randomized, and triple-blinded study. Three treatment groups, each consisting of twenty-three patients, were given two litres per week of either symbiotic pomegranate juice (SPJ), pomegranate juice, or symbiotic beverage (SB). The patients in the control group were given two litres of a placebo beverage per week. At the conclusion of the research project, 86 patients were examined. Insulin resistance, BMI, weight, and waist circumference were decreased significantly in the treatment groups. Both the SPJ and SB groups demonstrated a considerable reduction in their testosterone levels. Any noticeable difference in the FPG, LH, or FSH levels between any of the groups was not evident (97).
Tribulus terrestris
In women with PCOS, the floral parts and fruits of Tribulus terrestris have been shown to increase the frequency of ovulation and decrease the size of ovarian cysts (135). The treatment with 10 mg of a hydroalcoholic extract of T. terrestris normalized the menstrual irregularity as well as hormonal changes. Additionally, the ovarian cysts were effectively eliminated and the normal function of the ovary was restored (136). In PCOS, the postulated mechanism of T. terrestris was the normalization of the hormonal balance and the induction of ovulation through antiestrogenic action. In a randomized control study, women who took 1000 mg of hydroalcoholic T. terrestris extract every day showed promising hypoglycaemic effects. Treated patients also had less total cholesterol and low-density lipoproteins, which showed therapeutic effectiveness of this crude drug against obesity-induced PCOS (90).
Mentha species
Mentha spicata (commonly known as Spearmint) and Mentha piperita (known as peppermint) are among the most widely studied species of Lamiaceae family for the treatment of PCOS (137). Both species have been evaluated for different reproductive health problems in women such as PCOS, amenorrhea and dysmenorrhea. These species have two major bioactive components; essential oils and phenols (138). Studies on spearmint extract demonstrated that it improved PCOS symptoms and ovarian histology against estradiol and letrozole induced PCOS disease models (139). Spearmint oil also increased follicular development at 150 and 300 mg/kg dose in PCOS rats through decreasing ovarian cysts, atretic follicles and testosterone level (140). Mentha piperita herbal tea (40 g/L) demonstrated the efficacy to treat PCOS in letrozole induced PCOS in rats and moderated letrozole induced fibrosis in ovary by decreasing estradiol level (139). Clinical trials showed that drinking spearmint tea (5 g in 250 mL water) twice a day for 30 days reduced testosterone levels (both free and total), and raised levels of LH and FSH. It is possible to draw the conclusion that Mentha spicata is a useful antiandrogenic treatment in patients diagnosed with PCOS because it lowers both free and total androgen levels and cuts down on the number of ovarian cysts (98).
Nigella sativa
Nigella sativa, also known as black cumin or black seeds, has been widely documented for its potential to treat PCOS. A previous study demonstrated that seeds extract of nigella improved the maturation of oocytes isolated from PCOS mice by inhibiting the expression of COX-2 and oxidative stress (87). The efficacy of Nigella sativa oil was later confirmed in PCOS patients exhibiting oligo-amenorrhea. Nigella oil increased menstrual regularity in PCOS patients by increasing LH, testosterone and insulin level in PCOS patients (93). Thymoquinone, an essential oil found in Nigella, showed its effectiveness against mifepristone induced PCOS in rats by decreasing autophagy through up-regulation of androgen receptors and proinflammatory cytokines, and up-regulation of aromatase enzyme (77).
Adverse effects and toxicity of phytochemicals
Use of phytochemicals and plant extracts is not devoid of adverse effects. It is found that long-term use of phytoestrogens such as genistein, disrupts the pituitary-hypothalamus axis resulting in long-term inhibition of endogenous steroid production (141). It is well-established that oestrogens increase the risk of breast cancer, early onset of maturity, and gravidity in exposed individuals. However, the association of these adverse effects with phytochemicals is still to address. Some phytochemicals, such as genistein can worsen hypertension due to their vasopressin action (142). Abdominal pain, diarrhoea and headache are common adverse effects of phytochemical therapy. Phytochemicals and plant extracts used against PCOS need further investigation related to their safety in human (143).
Conclusion and future prospective
Recently, the incidence of PCOS is increasing due to genetic, environmental, and intrinsic individual factors, the management of which poses a significant hurdle to better quality of life and reproductive health in female individuals. Currently available medications focus on regulating the menstrual cycle, obesity, and insulin resistance. Several isolated phytochemicals such as curcumin, berberine, rutin, resveratrol, quercetin, hesperidin, biochanin, apigenin, fisetin, diazene, genistein, formononetin, and puerin have shown potential to treat PCOS in preclinical studies. Moreover, curcumin, cinnamaldehyde, resveratrol, quercetin, hesperidin, phytoestrogen, Vitex agnus-castus, Cinnamomum cassia, pomegranate juice, spearmint tea, catechins, Nigella sativa and Tribulus terrestris have demonstrated their efficacy to treat symptoms in PCOS patients.
These isolated phytochemicals and herbal drug formulations may serve as alternatives to metformin and clomiphene citrate for PCOS management as well as be used as adjuvants to conventional therapy for synergistic effect. Therefore, further large-scale clinical studies must be carried out to evaluate the dosage and duration of therapy with these phytochemicals and herbal drugs individually and in combination with other drugs to treat PCOS. The main problem with using these phytochemicals is the limited knowledge regarding their safety profile, quality, and efficacy. Hence, there is an extensive need to emphasize research regarding the efficacy, safety, and metabolomic profiling of these herbal drugs to achieve therapeutic outcomes and assure the quality of products.
Author contributions
SM: Data curation, Writing – original draft, Writing – review & editing. SS: Investigation, Writing – original draft, Writing – review & editing. AS: Investigation, Writing – original draft, Writing – review & editing. MK: Conceptualization, Writing – original draft, Writing – review & editing. AK: Formal analysis, Writing – original draft, Writing – review & editing. MA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Azziz R. Polycystic ovary syndrome: what's in a name? J Clin Endocrinol Metab (2014) 99(4):1142–5. doi: 10.1210/jc.2013-3996
2. Joshi M, Shankar R, Pathak K, Yadav RJPR. Polycystic ovarian syndrome: A review covering phytoconstituents for its outstrip management. Pharmacol Res-Modern Chin Med (2021) 1:100011. doi: 10.1016/j.prmcm.2021.100011
3. Jahan S, Munir F, Razak S, Mehboob A, Ain QU, Ullah H, et al. Ameliorative effects of rutin against metabolic, biochemical and hormonal disturbances in polycystic ovary syndrome in rats. J Ovarian Res (2016) 9(1):1–9. doi: 10.1186/s13048-016-0295-y
4. Goswami PK, Khale A, Ogale S. Natural remedies for polycystic ovarian syndrome (PCOS): a review. Int J Pharm Phytopharmacological Res (2012) 1(6):396–402.
6. Kidson W. Polycystic ovary syndrome: a new direction in treatment. Med J Aust (1998) 169(10):537–40. doi: 10.5694/j.1326-5377.1998.tb123402.x
7. Harwood K, Vuguin P, DiMartino-Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Horm Res Paediatr (2007) 68(5):209–17. doi: 10.1159/000101538
8. Yildiz BO. Recent advances in the treatment of polycystic ovary syndrome. Expert Opin Investig Drugs (2004) 13(10):1295–305. doi: 10.1517/13543784.13.10.1295
9. Matthews HB, Lucier GW, Fisher KD. Medicinal herbs in the United States: research needs. Environ Health Perspect (1999) 107(10):773–8. doi: 10.1289/ehp.99107773
10. Bahramsoltani R, Farzaei MH, Rahimi R. Herbal remedies for atherosclerosis: From back to the future. Cardiovasc Dis (2017) 1:188. doi: 10.2174/9781681084893117010007
11. Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MH. Polyphenols: Natural compounds with promising potential in treating polycystic ovary syndrome. Reprod Biol (2021) 21(2):100500. doi: 10.1016/j.repbio.2021.100500
12. Khanage SG, Subhash TY, Bhaiyyasaheb IR. Herbal drugs for the treatment of Polycystic ovary syndrome (PCOS) and its complications. Pharm Res (2019) 2(1):5–13.
13. Walters K, Bertoldo M, Handelsman DJBP, Endocrinology RC. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab (2018) 32(3):271–81. doi: 10.1016/j.beem.2018.03.008
14. Chang RJ. A practical approach to the diagnosis of polycystic ovary syndrome. Am J Obstet Gynecol (2004) 191(3):713–7. doi: 10.1016/j.ajog.2004.04.045
15. Campbell BK, Clinton M, Webb R. The role of anti-Müllerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology (2012) 153(9):4533–43. doi: 10.1210/en.2012-1158
16. Visser JA, de Jong FH, Laven JS, Themmen APJR. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction (2006) 131(1):1–9.
17. Ashraf S, Nabi M, Rashid F, Amin SJEJoMHG. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt J Med Hum Genet (2019) 20(1):1–10. doi: 10.1186/s43042-019-0031-4
18. Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum Reprod (2021) 36(9):2421–8. doi: 10.1093/humrep/deab181
19. Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med (2014) 2014:719050. doi: 10.1155/2014/719050
20. Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol (2018) 182:27–36. doi: 10.1016/j.jsbmb.2018.04.008
21. Casper RF, Mitwally MF. Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab (2006) 91(3):760–71. doi: 10.1210/jc.2005-1923
22. Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of Anti-Müllerian Hormone in pathophysiology, diagnosis and treatment of Polycystic Ovary Syndrome: a review. Reprod Biol Endocrinol (2015) 13(1):1–10. doi: 10.1186/s12958-015-0134-9
23. Crisosto N, de Guevara AL, Echiburú B, Maliqueo M, Cavada G, Codner E, et al. Higher luteinizing hormone levels associated with antimüllerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil Steril (2019) 111(2):381–8.
24. Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HGJM. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause (2008) 15(6):1139–44. doi: 10.1097/gme.0b013e3181735687
25. De Leo V, La Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. J Endocr Rev (2003) 24(5):633–67. doi: 10.1210/er.2002-0015
26. Schmidt KL, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Müllerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol (2005) 234(1-2):87–93. doi: 10.1016/j.mce.2004.12.010
27. Anderson P, Critchley J, Chan J, Cockram C, Lee Z, Thomas G, et al. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes (2001) 25(12):1782–8. doi: 10.1038/sj.ijo.0801837
28. Darby A. Symptoms associated with polycystic ovarian syndrome (PCOS). Embryo Project Encyclopedia (2017) 13:1–5.
29. Lindholm Å, Andersson L, Eliasson M, Bixo M, Sundström-Poromaa I. Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynaecol Obstet (2008) 102(1):39–43. doi: 10.1016/j.ijgo.2008.01.023
30. Abinaya S, Siva D, Sabitha R, Achiraman SJR. An overview of hyperandrogenism in PCOS and the prospective underlying factors. Res J Life Sci Bioinform Pharmac Chem Sci (2019) 1(5):179–86.
31. Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol (2001) 22(3):185–212. doi: 10.1006/frne.2001.0216
32. Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol Regul Integr Comp Physiol (1987) 252(1):R166–80. doi: 10.1152/ajpregu.1987.252.1.R166
33. Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. J Dermatol Ther (2006) 19(4):210–23. doi: 10.1111/j.1529-8019.2006.00077.x
34. Arentz S, Smith CA, Abbott J, Bensoussan A. Perceptions and experiences of lifestyle interventions in women with polycystic ovary syndrome (PCOS), as a management strategy for symptoms of PCOS. BMC Women’s Health (2021) 21(1):1–8. doi: 10.1186/s12905-021-01252-1
35. Plewig G, Melnik B, Chen W. Acne-mimicking diseases. In: Plewig and Kligman´ s Acne and Rosacea. Springer (2019). p. 299–410.
36. Katzer T, Leite Junior A, Beck R, da Silva C. Physiopathology and current treatments of androgenetic alopecia: going beyond androgens and anti-androgens. Dermatol Ther (2019) 32(5). doi: 10.1111/dth.13059
37. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol (2004) 18(5):671–83. doi: 10.1016/j.bpobgyn.2004.05.001
38. Piraccini BM, Alessandrini A, Starace M. Onychoscopy: dermoscopy of the nails. J Dermatol Clin (2018) 36(4):431–8. doi: 10.1016/j.det.2018.05.010
39. Verma S, Rani S, Yadav A. An emanation of nail lacquer in the management of nail disorders: a comprehensive review. World J Pharm Res (2020) 10(1):870–98.
40. Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights: Reprod Health (2019) 13:1179558119874042. doi: 10.1177/1179558119874042
41. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
42. Kolhe JV, Chhipa AS, Butani S, Chavda V, Patel SS. PCOS and depression: Common links and potential targets. Reprod Sci (2021) p:1–18.
43. Hugar AL, Kanjikar AP, Londonkar RL. Polycystic ovary syndrome (PCOS)-A mini review. J Gynecol (2018) 3(1):000148.
44. Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case–control study. Clin Endocrinol (2012) 77(6):898–904. doi: 10.1111/j.1365-2265.2012.04443.x
45. Fiedler K, Ludwig M. Use of clomiphene citrate in in vitro fertilization (IVF) and IVF/intracytoplasmic sperm injection cycles. Fertil Steril (2003) 80(6):1521–3. doi: 10.1016/S0015-0282(03)02208-8
46. He D, Jiang F. Meta-analysis of letrozole versus clomiphene citrate in polycystic ovary syndrome. Reprod Biomed Online (2011) 23(1):91–6. doi: 10.1016/j.rbmo.2011.03.024
47. Armanini D, Andrisani A, Bordin L, Sabbadin C. Spironolactone in the treatment of polycystic ovary syndrome. Expert Opin Pharmacother (2016) 17(13):1713–5. doi: 10.1080/14656566.2016.1215430
48. Duleba AJ. Medical management of metabolic dysfunction in PCOS. Steroids (2012) 77(4):306–11. doi: 10.1016/j.steroids.2011.11.014
49. Palomba S, Falbo A, Zullo F, Orio JR. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev (2009) 30(1):1–50. doi: 10.1210/er.2008-0030
50. Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab (2017) 28(3):186–98. doi: 10.1016/j.tem.2016.11.008
51. Yahya Abdelrahman M, et al. Polycystic ovary syndrome in adolescents. American family physician (2010) 6(2):108–22. doi: 10.2174/157340410791321309
52. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician (2016) 94(2):106–13.
53. Tripathy S, Roy C, Banerjee A, Sciences A. Polycystic ovary syndrome: its genetics and treatments: polycystic ovary syndrome: its genetics and treatments. Indian J Physiol Allied Sci (2020) 72(1):1–12.
54. Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res (2004) 35(2):103–8. doi: 10.1016/j.arcmed.2003.10.005
55. Timpatanapong P, Rojanasakul A. Hormonal profiles and prevalence of polycystic ovary syndrome in women with acne. J Dermatol (1997) 24(4):223–9. doi: 10.1111/j.1346-8138.1997.tb02778.x
56. Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Human Reprod (2011) 26(3):594–603. doi: 10.1093/humrep/deq357
57. Hafez E, Hafez B. Folliculogenesis, egg maturation, and ovulation. (2000), 68–81. doi: 10.1002/9781119265306.ch5
58. Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update (2021) 27(3):584–618. doi: 10.1093/humupd/dmaa051
59. Kandasamy V, Balasundaram U. Caesalpinia bonduc (L.) Roxb. As a promising source of pharmacological compounds to treat Poly Cystic Ovary Syndrome (PCOS): A review. J Ethnopharmacol (2021) 279:114375. doi: 10.1016/j.jep.2021.114375
60. Zeng L-H, Rana S, Hussain L, Asif M, Mehmood MH, Imran I, et al. Polycystic ovary syndrome: a disorder of reproductive age, its pathogenesis, and a discussion on the emerging role of herbal remedies. Frontiers Pharmacol (2022) 13. doi: 10.3389/fphar.2022.874914
61. Ansari RM. Potential use of durian fruit (Durio zibenthinus Linn) as an adjunct to treat infertility in polycystic ovarian syndrome. J Integr Med (2016) 14(1):22–8. doi: 10.1016/S2095-4964(16)60240-6
62. Hong Y, Yin Y, Tan Y, Hong K, Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Med Sci Monitor (2019) 25:395. doi: 10.12659/MSM.912341
63. Nynca A, Swigonska S, Piasecka J, Kolomycka A, Kaminska B, Radziewicz-Pigiel M, et al. Biochanin A affects steroidogenesis and estrogen receptor-β expressionin porcine granulosa cells. Theriogenology (2013) 80(7):821–8. doi: 10.1016/j.theriogenology.2013.07.009
64. Shen H-R, Xu X, Li X-L. Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis. Reprod Biol Endocrinol (2021) 19(1):1–11. doi: 10.1186/s12958-020-00684-y
65. Liu K, He X, Huang J, Yu S, Cui M, Gao M, et al. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clin Epigenet (2023) 15(1):86. doi: 10.1186/s13148-023-01487-9
66. Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pacific J Reprod (2016) 5(2):116–22. doi: 10.1016/j.apjr.2016.01.006
67. Medigović IM, Živanović JB, Ajdžanović VZ, Nikolić-Kokić AL, Stanković SD, Trifunović SL, et al. Effects of soy phytoestrogens on pituitary-ovarian function in middle-aged female rats. Endocrine (2015) 50(3):764–76. doi: 10.1007/s12020-015-0691-x
68. Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MH. Ameliorative effects of fisetin in letrozole-induced rat model of polycystic ovary syndrome. J Steroid Biochem Mol Biol (2021) 213:105954. doi: 10.1016/j.jsbmb.2021.105954
69. Rajaei S, Alihemmati A, Abedelahi A. Antioxidant effect of genistein on ovarian tissue morphology, oxidant and antioxidant activity in rats with induced polycystic ovary syndrome. Int J Reprod BioMed (2019) 17(1):11. doi: 10.18502/ijrm.v17i1.3816
70. Shoorei H, Banimohammad M, Kebria MM, Afshar M, Taheri MM, Shokoohi M, et al. Hesperidin improves the follicular development in 3D culture of isolated preantral ovarian follicles of mice. Exp Biol Med (2019) 244(5):352–61. doi: 10.1177/1535370219831615
71. YanCun L, GuanRong T, CongHui H. Effect of puerarin injection on insulin resistance and reproductive hormones of rats with polycystic ovary syndrome. Maternal Child Health Care China (2015) 30(25):4372–4.
72. Jahan S, Abid A, Khalid S, Afsar T, Shaheen G, Almajwal A, et al. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: a histological and a biochemical study. J Ovarian Res (2018) 11(1):1–10. doi: 10.1186/s13048-018-0400-5
73. Neisy A, Zal F, Seghatoleslam A, Alaee S. Amelioration by quercetin of insulin resistance and uterine GLUT4 and ERα gene expression in rats with polycystic ovary syndrome (PCOS). Reproduction Fertil Dev (2019) 31(2):315–23. doi: 10.1071/RD18222
74. Zhou Y-Y, He C-H, Lan H, Dong Z-W, Wu Y-Q, Song J-L. Rhamnocitrin decreases fibrosis of ovarian granulosa cells by regulating the activation of the PPARγ/NF-κB/TGF-β1/Smad2/3 signaling pathway mediated by Wisp2. Ann Trans Med (2022) 10(14):2–37. doi: 10.21037/atm-22-2496
75. Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res (2018) 11(1):55. doi: 10.1186/s13048-018-0427-7
76. Chen M, He C, Zhu K, Chen Z, Meng Z, Jiang X, et al. Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication. Theranostics (2022) 12(2):782. doi: 10.7150/thno.67167
77. Saha P, Kumar S, Datta K, Tyagi RK. Upsurge in autophagy, associated with mifepristone-treated polycystic ovarian condition, is reversed upon thymoquinone treatment. J Steroid Biochem Mol Biol (2021) 208:105823. doi: 10.1016/j.jsbmb.2021.105823
78. Mushtaq F, Akhtar MF, Saleem A, Sharif A, Akhtar B, Askary AE, et al. Berberis aristata DC Extract Counteracts the High Fat Diet-Induced Reproductive Toxicity in Female Wistar Rats via Modulating Oxidative Stress and Resistance to Leptin and Insulin. Endocr Metab Immune Disord-Drug Targets (2022) 22(14):1390–402. doi: 10.2174/1871530322666220429125241
79. Mehraban M, Jelodar G, Rahmanifar F. A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J Ovarian Res (2020) 13(1):1–8. doi: 10.1186/s13048-020-00633-8
80. Ayyadurai T, Moola AK, Mohan PK, Thiruppathi SK, Shanmugam A, Bollipo Diana RK. Ameliorative effects of Guilandina bonduc L. aqueous seed extract on letrozole induced polycystic ovary syndrome in female wistar albino rats. Adv Traditional Med (2022) 22(4):885–903.
81. Suriyakalaa U, Ramachandran R, Doulathunnisa JA, Aseervatham SB, Sankarganesh D, Kamalakkannan S, et al. Upregulation of Cyp19a1 and PPAR-γ in ovarian steroidogenic pathway by Ficus religiosa: A potential cure for polycystic ovary syndrome. J Ethnopharmacol (2021) 267:113540. doi: 10.1016/j.jep.2020.113540
82. Khaled N, El-Bahy AA, Radwan R, Handoussa H, AbdelMaksoud S. Ocimum kilimandscharicum L. restores ovarian functions in letrozole - induced Polycystic Ovary Syndrome (PCOS) in rats: Comparison with metformin. Life Sci (2019) 232:116640. doi: 10.1016/j.lfs.2019.116640
83. Pyun B-J, Yang H, Sohn E, Yu SY, Lee D, Jung DH, et al. Tetragonia tetragonioides (Pall.) kuntze regulates androgen production in a letrozole-induced polycystic ovary syndrome model. Molecules (2018) 23(5):1173.
84. Shoaib M, Saleem A, Zeb A, Khan MI, Akhtar M. Chemical Characterization and Ameliorating Effect of Centratherum anthelminticum Extract against Polycystic Ovary Syndrome in Wistar Rats. Int J Endocrinol (2023) 2023:4978562. doi: 10.1155/2023/4978562
85. Küpeli Akkol E, İlhan M, Ayşe Demirel M, Keleş H, Tümen I, Süntar İ. Thuja occidentalis L. and its active compound, α-thujone: Promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J Ethnopharmacol (2015) 168:25–30. doi: 10.1016/j.jep.2015.03.029
86. Gao Y, Mo S, Cao H, Zhi Y, Ma X, Huang Z, et al. The efficacy and mechanism of Angelica sinensis (Oliv.) Diels root aqueous extract based on RNA sequencing and 16S rDNA sequencing in alleviating polycystic ovary syndrome. Phytomedicine (2023) 120:155013. doi: 10.1016/j.phymed.2023.155013
87. Eini F, Joharchi K, Kutenaei MA, Mousavi P. Improvement in the epigenetic modification and development competence in PCOS mice oocytes by hydro-alcoholic extract of Nigella sativa during in-vitro maturation: An experimental study. Int J Reprod BioMed (2020) 18(9):733. doi: 10.18502/ijrm.v13i9.7668
88. Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine (2021) 80:153395. doi: 10.1016/j.phymed.2020.153395
89. Giordano A, Tommonaro G. Curcumin and cancer. Cur Cancer (2019) 11(10):2376. doi: 10.3390/nu11102376
90. Pachiappan S, Ramalingam K, Balasubramanian A. A review on phytomedicine and their mechanism of action on PCOS. Int J Curr Res Rev (2020) 12(23):81. doi: 10.31782/IJCRR.2020.122322
91. Marti N, Bouchoucha N, Sauter K-S, Flück CE. Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PloS one (2017) 12(3):. doi: 10.1371/journal.pone.0174224
92. Tejada S, Pinya S, Martorell M, Capó X, Tur JA, Pons A, et al. Potential anti-inflammatory effects of hesperidin from the genus citrus. Curr Med Chem (2018) 25(37):4929–45. doi: 10.2174/0929867324666170718104412
93. Naeimi SA, Hajimehdipoor H, Saber S. Comparing the effect of Nigella sativa oil soft gel and placebo on oligomenorrhea, amenorrhea and laboratory characteristics in patients with polycystic ovarian syndrome, a randomized clinical trial. Res J Pharmacognosy (2020) 7(1):49–59.
94. Arentz S, Smith CA, Abbott J, Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. Complementary Altern Medicines (2014) 14(1):1–19. doi: 10.1186/1472-6882-14-511
95. Zahid H, Rizwani GH, Ishaqe S. Phytopharmacological review on Vitex agnus-castus: a potential medicinal plant. Chin Herbal Medicines (2016) 8(1):24–9. doi: 10.1016/S1674-6384(16)60004-7
96. Chitra V, Dhivya DP. Role of herbal in management of polycystic ovarian syndrome and its associated symptoms. Int J Herbal Medicines (2017) 5(5):125–31.
97. Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari M-H, Amooee S, Barati-Boldaji RJN, Metabolism, et al, et al. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutrition, Metabolism and Cardiovascular Diseases (2019) 29(2):201–8. doi: 10.1016/j.numecd.2018.07.002
98. Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, Delibaş N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res (2007) 21(5):444–7. doi: 10.1002/ptr.2074
99. Chan CC, Koo MW, Ng EH, Tang O-S, Yeung WS, Ho P. Effects of Chinese green tea on weight and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Investig (2006) 13(1):63–8.
100. Rezayian M, Niknam V, Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol Rep (2019) 6:1309–13. doi: 10.1016/j.toxrep.2019.10.001
101. Jabczyk M, Nowak J, Hudzik B, Zubelewicz-Szkodzińska B. Curcumin in metabolic health and disease. Nutrients (2021) 13(12):4440. doi: 10.3390/nu13124440
103. Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac J Reprod (2016) 5(2):116–22. doi: 10.1016/j.apjr.2016.01.006
104. Kamal DAM, Salamt N, Yusuf ANM, Kashim MIAM, Mokhtar MH. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients (2021) 13(9):3126. doi: 10.3390/nu13093126
105. Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol (1983) 32(7):1141–8. doi: 10.1016/0006-2952(83)90262-9
106. Darabi P, Khazali H, Mehrabani Natanzi M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecological Endocrinol (2020) 36(7):582–7. doi: 10.1080/09513590.2019.1706084
107. Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nut Res (1998) 18(12):1995–2018. doi: 10.1016/S0271-5317(98)00169-9
108. Zhou Y, Lv L, Liu Q, Song J. Total flavonoids extracted from Nervilia Fordii function in polycystic ovary syndrome through IL-6 mediated JAK2/STAT3 signaling pathway. Bioscience Rep (2019) 39(1):1–15. doi: 10.1042/BSR20181380
109. Ożarowski M, Karpiński TM. Extracts and flavonoids of Passiflora species as promising anti-inflammatory and antioxidant substances. Curr Pharm Des (2021) 27(22):2582–604. doi: 10.2174/1381612826666200526150113
110. Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, Jafari-Vayghan H, Alizadeh M, Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res (2020) 13(1):1–10. doi: 10.1186/s13048-020-0616-z
111. Tereschuk ML, Baigorí MD, De Figueroa LI, Abdala LR. Flavonoids from Argentine Tagetes (Asteraceae) with antimicrobial activity. In: Public health microbiology. Springer (2004). p. 317–30.
112. Jeszka-Skowron M, Krawczyk M, Zgoła-Grześkowiak A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: Influence of citric acid addition on extraction of metals. J Food Compost Anal (2015) 40:70–7. doi: 10.1016/j.jfca.2014.12.015
113. Jamilian H, Jamilian M, Samimi M, Afshar Ebrahimi F, Rahimi M, Bahmani F, et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Gynecol Endocrinol (2017) 33(6):442–7. doi: 10.1080/09513590.2017.1290071
114. Wang Z, Zhai D, Zhang D, Bai L, Yao R, Yu J, et al. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci (2017) 24(5):682–90. doi: 10.1177/1933719116667218
115. Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord (2015) 16(4):365–71. doi: 10.1007/s11154-016-9326-7
116. Bolouki A, Zal F, Alaee S. Ameliorative effects of quercetin on the preimplantation embryos development in diabetic pregnant mice. J Obstet Gynaecol Res (2020) 46(5):736–44. doi: 10.1111/jog.14219
117. Mirza SS, Shafique K, Shaikh AR, Khan NA, Anwar Qureshi M. Association between circulating adiponectin levels and polycystic ovarian syndrome. J Ovarian Res (2014) 7(1):1–7. doi: 10.1186/1757-2215-7-18
118. Pyrzynska K. Hesperidin: A review on extraction methods, stability and biological activities. Nutrients (2022) 14(12):2387. doi: 10.3390/nu14122387
119. Prasathkumar M, Anisha S, Dhrisya C, Becky R, Sadhasivam S. Therapeutic and pharmacological efficacy of selective Indian medicinal plants–a review. Phytomedicine Plus (2021) 1(2):100029. doi: 10.1016/j.phyplu.2021.100029
120. Singh S, Pathak N, Fatima E, Negi AS. Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur J Med Chem (2021) 226:113839. doi: 10.1016/j.ejmech.2021.113839
121. Zhang S-w, Zhou J, Gober H-J, Leung WT, Wang L. Effect and mechanism of berberine against polycystic ovary syndrome. Biomedicine & Pharmacotherapy (2021) 138:111468. doi: 10.1016/j.biopha.2021.111468
122. Ortiz-Flores AE, Luque-Ramírez M, Escobar-Morreale HF. Pharmacotherapeutic management of comorbid polycystic ovary syndrome and diabetes. Expert Opin Pharmacother (2018) 19(17):1915–26. doi: 10.1080/14656566.2018.1528231
123. Wang Y, Fu X, Xu J, Wang Q, Kuang H. Systems pharmacology to investigate the interaction of berberine and other drugs in treating polycystic ovary syndrome. Sci Rep (2016) 6(1):1–10. doi: 10.1038/srep28089
124. Jungbauer A, Medjakovic S. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol (2014) 139:277–89. doi: 10.1016/j.jsbmb.2012.12.009
125. Khorchani MJ, Zal F, Neisy A. The phytoestrogen, quercetin, in serum, uterus and ovary as a potential treatment for dehydroepiandrosterone-induced polycystic ovary syndrome in the rat. Reproduction Fertil Dev (2020) 32(3):313–21. doi: 10.1071/RD19072
126. Romualdi D, Costantini B, Campagna G, Lanzone A, Guido M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril (2008) 90(5):1826–33. doi: 10.1016/j.fertnstert.2007.09.020
127. Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients (2019) 11(2):474. doi: 10.3390/nu11020474
128. Amini M, Bahmani F, Foroozanfard F, Vahedpoor Z, Ghaderi A, Taghizadeh M, et al. The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosomatic Obstet Gynecol (2020) p:1–9.
129. Shahnazi V, Zaree M, Nouri M, Mehrzad-Sadaghiani M, Fayezi S, Darabi M, et al. Influence of ω-3 fatty acid eicosapentaenoic acid on IGF-1 and COX-2 gene expression in granulosa cells of PCOS women. Iranian J Reprod Med (2015) 13(2):71.
130. Moini Jazani A, Nasimi Doost Azgomi H, Nasimi Doost Azgomi A, Nasimi Doost Azgomi R. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). DARU J Pharm Sci (2019) 27(2):863–77. doi: 10.1007/s40199-019-00312-0
131. Ribeiro-Santos R, Andrade M, Madella D, Martinazzo AP, Moura LdAG, de Melo NR, et al. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci Technol (2017) 62:154–69. doi: 10.1016/j.tifs.2017.02.011
132. Guo X, Sun W, Huang L, Wu L, Hou Y, Qin L, et al. Effect of cinnamaldehyde on glucose metabolism and vessel function. Int Med J Exp Clin Res (2017) 23:3844. doi: 10.12659/MSM.906027
133. Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol (2014) 211(5):487.e1–6. doi: 10.1016/j.ajog.2014.05.009
134. Zhang C, Fan L, Fan S, Wang J, Luo T, Tang Y, et al. Cinnamomum cassia Presl: a review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules (2019) 24(19):3473. doi: 10.3390/molecules24193473
135. Rao SK. An insight on polycystic Ovary syndrome (PCOS) and use of herbal medicines as alternative treatment. In: Treating Endocrine and Metabolic Disorders With Herbal Medicines. IGI Global (2021). p. 125–63.
136. Saiyed A, Jahan N, Makbul SAA, Ansari M, Bano H, Habib SH. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on letrozole induced polycystic ovarian syndrome in rats. Integr Med Res (2016) 5(4):293–300. doi: 10.1016/j.imr.2016.10.002
137. Sharafieh G, Salmanifarzaneh F, Gharbi N, Sarvestani FM, Rahmanzad F, Razlighi MR, et al. Histological and molecular evaluation of Mentha arvensis extract on a polycystic ovary syndrome rat model. JBRA Assisted Reprod (2023) 27(2):247.
138. Çam M, Dinç Işıklı M, Yüksel E, Alaşalvar H, Başyiğit B. Application of pressurized water extraction and spray drying techniques to produce soluble spearmint tea. J Food Meas Charact (2018) 12(3):1927–34.
139. Amoura M, Lotfy ZH, Neveen E, Khloud A. Potential effects of Mentha piperita (peppermint) on Letrozole-induced polycystic ovarian syndrome in female albino rat. Int J (2015) 3(10):211–26.
140. Ataabadi MS, Alaee S, Bagheri MJ, Bahmanpoor S. Role of essential oil of Mentha spicata (Spearmint) in addressing reverse hormonal and folliculogenesis disturbances in a polycystic ovarian syndrome in a rat model. Advanced Pharm Bull (2017) 7(4):651.
141. Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr (1997) 127(2):263–9.
142. Scallet AC, Wofford M, Meredith JC, Allaben WT, Ferguson SA. Dietary exposure to genistein increases vasopressin but does not alter β-endorphin in the rat hypothalamus. Toxicological Sci (2003) 72(2):296–300. doi: 10.1093/toxsci/kfg029
Keywords: phytochemical, herbal therapy, PCOS, infertility, oxidative stress, inflammation
Citation: Malik S, Saeed S, Saleem A, Khan MI, Khan A and Akhtar MF (2024) Alternative treatment of polycystic ovary syndrome: pre-clinical and clinical basis for using plant-based drugs. Front. Endocrinol. 14:1294406. doi: 10.3389/fendo.2023.1294406
Received: 14 September 2023; Accepted: 14 December 2023;
Published: 11 January 2024.
Edited by:
Senthilkumar Palaniappan, Karpagam Academy of Higher Education, IndiaReviewed by:
Arnab Banerjee, Birla Institute of Technology and Science, IndiaSanaz Alaeejahromi, Shiraz University of Medical Sciences, Iran
Copyright © 2024 Malik, Saeed, Saleem, Khan, Khan and Akhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Furqan Akhtar, ZnVycWFuLnBoYXJtYWNpc3RAZ21haWwuY29t
 Sidra Malik
Sidra Malik Ammara Saleem
Ammara Saleem Muhammad Imran Khan
Muhammad Imran Khan Aslam Khan
Aslam Khan Muhammad Furqan Akhtar
Muhammad Furqan Akhtar