
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 18 December 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1286907
This article is part of the Research Topic Improving Outcomes in Diabetic Foot Care - A Worldwide Perspective View all 16 articles
 Paolo Izzo1*
Paolo Izzo1* Claudia De Intinis1
Claudia De Intinis1 Marcello Molle2
Marcello Molle2 Andrea Polistena1
Andrea Polistena1 Simone Sibio1
Simone Sibio1 Massimo Codacci-Pisanelli1
Massimo Codacci-Pisanelli1 Daniele Biacchi1
Daniele Biacchi1 Pierfrancesco Di Cello3
Pierfrancesco Di Cello3 Daniele Santini4
Daniele Santini4 Luciano Izzo1
Luciano Izzo1 Sara Izzo2
Sara Izzo2Background: Diabetes mellitus is a prevalent chronic condition that significantly impacts global health. Diabetic foot complications, such as foot ulcers, pose a substantial burden on individuals with diabetes and can lead to serious consequences, including amputation. Platelet-rich plasma (PRP) has emerged as a promising therapeutic approach for enhancing the healing of diabetic foot ulcers.
Methods: In our study, we treated 12 patients with chronic diabetic ulcers using PRP injections administered at three-week intervals. Our objective was to assess the reduction in wound size and the rate of complete healing at 6 months after the start of the treatment. Additionally, we conducted a comprehensive literature review to contextualize our findings.
Results: Out of the 12 patients, 8 achieved complete healing of their diabetic foot ulcers, while the remaining four showed significant improvement with more than 50% reduction in the initial lesion size. 3 patients developed mild irritation at the inoculation site. These outcomes, combined with the evidence from published studies, highlight the effectiveness of PRP in promoting the healing of diabetic foot ulcers.
Conclusion: In conclusion, our study demonstrates the potential of platelet-rich plasma (PRP) as a successful therapeutic option for enhancing the healing process of chronic diabetic foot ulcers. The favorable outcomes observed, including a high rate of complete healing and significant wound size reduction, underscore the value of PRP treatment in managing this challenging complication. Further research and larger studies may provide additional insights into the mechanisms and long-term benefits of PRP in diabetic wound healing.
Diabetes mellitus is a prevalent chronic condition that poses significant health challenges worldwide (1). Its complications can affect multiple organ systems (causing retinopathy, renal failure and other conditions (2)), with diabetic foot complications being among the most consequential and debilitating. During the lifetime of a diabetic patient, there is approximately a 19% to 34% risk of developing a foot ulcer, with an increasing incidence in recent years due to the growing life expectancy (3). The comorbidities associated with diabetic ulcers are significant, including peripheral neuropathy, peripheral arterial disease, foot ulcers, and infections, which can lead to amputation in up to 20% of cases (4). In recent years, the use of Platelet-Rich Plasma (PRP) has increasingly gained prominence in the treatment of skin lesions and defects, finding applications not only in alopecia and skin rejuvenation but also in surgical wounds (5), scar treatment (6), and ulcers (7). PRP has become an effective tool in the treatment of these conditions, occupying a significant role in the field of dermatology, plastic surgery and wound healing. In this study, we present a series of cases involving the treatment of diabetic foot lesions using platelet-rich plasma (PRP).
We selected 12 patients between 2022 and 2023 (after obtaining informed consent) with diabetic foot ulcers that had not shown signs of healing for at least three months with conventional treatments. In addition to standard wound care (disinfection, debridement, and moist dressings), we administered perilesional platelet-rich plasma (PRP) injections during the initial outpatient visit and at a three-week interval. Patients with unstable hemodynamics due to ischemic heart disease or coagulation disorders, as well as those on anticoagulant and/or non-steroidal anti-inflammatory drug (NSAID) therapy, were excluded. Patients with the following characteristics were also excluded: platelet count below 150,000/mm³, uncontrolled diabetes mellitus (glycated hemoglobin level ≥ 75 mmol/l based on the latest laboratory data obtained within 28 days of enrollment), requirement of ongoing oral corticosteroid therapy (> 20 mg/day of prednisolone or equivalent), a history of malignant tumors with disease-free interval of three years or less and patients who are pregnant or planning to become pregnant, in order to optimize the outcome of the treatment (8). We did not make patient selections based on gender, and we did not recruit patients under the age of 18.
The product used in the study was obtained using the classical Tube Method (9). Once the blood sample (approximately 30 ml) is collected, it is gently mixed to ensure proper mixing with the anticoagulant (sodium citrate 3.8%) present in the tubes. Subsequently, two cycles of centrifugation are performed. In the first phase, the product is centrifuged at 1500 rpm for 10 minutes, resulting in the separation of the cellular components from the plasma. Using a 10 ml L/L syringe with a 22G needle, all the plasma is aspirated from the six tubes and transferred to the remaining three tubes, which are then subjected to further centrifugation at 5000 rpm for 10 minutes. The final yield of the process is approximately 3 mL of product with a volume ratio of 10:1 and a platelet concentration around ¾ times higher than whole blood (ranging from 1.1 × 106 to 1.7 × 106 platelets/μl).
The treatment of the wound is divided into several phases. Before proceeding with wound disinfection using iodopovidone, it is crucial to assess the presence of bacteria in the wound through a qualitative swab. The presence of an infection represents a negative prognostic factor and can compromise the response to treatment. In case the culture examination is positive, targeted antibiotic therapy will be administered based on the antibiogram. In the second phase, after thorough disinfection, surgical debridement (Wound Bed Preparation) is performed, involving the removal of non-vital tissues until a clean and bleeding wound bed is achieved. Subsequently, a second disinfection is carried out. The actual treatment involves the infiltration of 3 ml of PRP onto the wound bed and along the wound’s edge, using a 1 ml syringe and a 30G needle. At the end of the procedure, the ulcer is covered with a non-adherent dressing, using non-adherent gauzes.
This procedure is repeated once three week when changing the dressing until the healing of the wound or for a maximum of 12 weeks.
The dimensions (based on the greatest diameter) of the lesions were evaluated upon entry into the study and at a three-month interval after the last injection. Additionally, the rate of complete wound healing (defined as the absence of any breaks in the epidermis) was quantified (10). The ulcers were assessed using the PUSH scale (11) at the beginning of the treatment (T0)and at the 6-month follow-up(T6).
We then conducted a literature search on PubMed and Scopus using the following search terms: “PRP AND diabetic foot”, “PRP AND diabetic ulcer”, “Platelet rich plasma AND diabetic foot”, “Platelet rich plasma AND diabetic ulcer”.
Out of the 12 treated patients, 4 were male and 12 were female. The age ranged from 65 to 81 years, with a mean of 76 years and a median of 78 years. The average size (based on the greatest diameter of the lesion) at the beginning of the treatment was 2.17 cm (with a median of 2 cm and a range between 1 cm and 4.4 cm). None of the ulcers treated was gangrenous according to the Wagner Score (see Table 1) (12). Six patients had high blood pressure, and five met the criteria for metabolic syndrome (defined as having three or more of the following five criteria: waist circumference over 40 inches (men) or 35 inches (women), blood pressure over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dl, fasting high-density lipoprotein (HDL) cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women), and fasting blood sugar over 100 mg/dl) (13).
At the end of the treatment, 8 patients achieved complete healing with restitution ad integrum of the skin (with a complete healing rate of 66.67%), while the remaining four subjects experienced partial healing (>50% reduction in size of the lesion), with an average size of 1.1 cm (median 1.1 cm, range 1-1.2 cm) (Table 1) (Figures 1A–C). No major adverse effect or infections of the lesions were observed during the treatment, 3 patients developed mild irritation at the inoculation site.
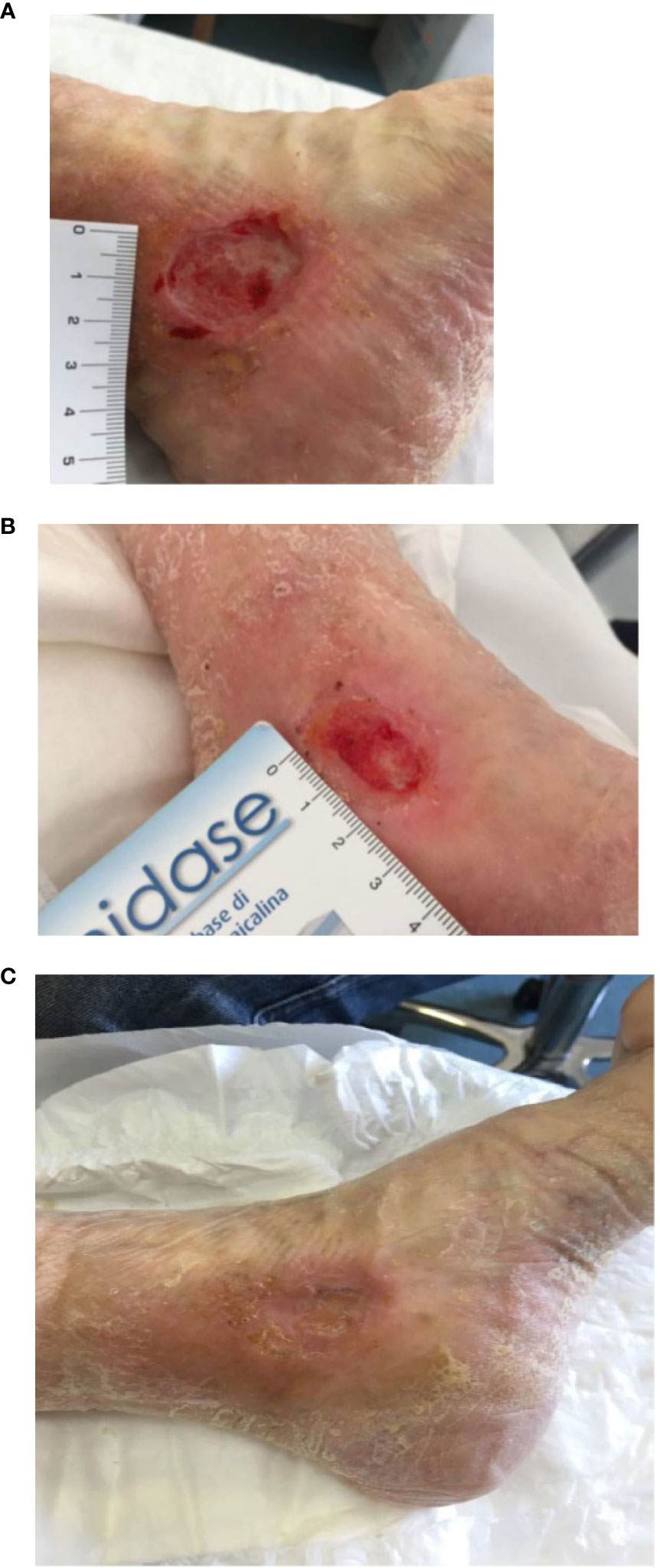
Figure 1 Patient with peri malleolar ulcer before the treatment (A), after the first PRP injection (B) and at the end of the treatment (C).
The use of PRP has been introduced in clinical practice for approximately 20 years (14–19), although the first studies analyzing this technique date back to the 1990s (20–23).
From the early studies, the efficacy of using plasma derivatives has demonstrated significant effectiveness in accelerating recovery time and wound healing speed. Patients treated with plasma derivatives showed a weekly healing rate of 0.4 cm2 compared to 0.13 cm2 in non-treated patients (22). In 2009, a systematic review conducted in Brazil (24) analyzed 18 studies, including 7 randomized clinical trials. The review highlighted a significant effect on the healing process (95% CI odds ratio 2.94-20.31). However, it also noted a lack of consistency in the type of treatment and protocol used, as well as variability in the type of dressings and study design (25). This variability among the studies has made it difficult to establish a unified standard in this field of research and reflects the considerable challenges in implementing standardized protocols for the treatment of difficult wounds. During the same period, a prospective randomized trial conducted in Greece (26) compared the healing of large difficult wounds (>2.5 cm in any one dimension) using a protease-modulating matrix with or without the addition of platelet-derived growth factors. The study did not find statistically significant differences in accelerating the healing process between the two groups. Another interesting application of PRP was tested in an Italian study in 2009 (27), where PRP was used as an adjuvant to autologous adipose tissue grafting in the treatment of lower limb ulcers and cervical facial defects. This combined treatment demonstrated efficacy both in vitro, where it increased the survival of adipose cells, and in vivo, with complete healing of all lower limb lesions achieved in an average of 9.7 weeks. A study conducted in 2015 (28) evaluated the use of PRP in the treatment of diabetic foot ulcers in patients with lower limb vascular diseases. The study included 72 patients, 30 of whom had Critical Limb Ischemia (CLI). The patients were treated for a period of 24 months, and the results showed a reduction in ulcer area of >90% in 52 patients and a limb salvage rate of 89% (100% in patients without CLI, 73% in patients with CLI). These findings demonstrated the effectiveness of PRP even in challenging patients with multiple comorbidities. Results similar to those have been obtained from a Japanese study (29). During the same period, other studies have demonstrated an improvement in the healing process through the use of PRP in diabetic foot ulcers (30–35). Other studies, on the other hand, have considered the antibacterial effect of PRP (30) to reduce the incidence of superinfections in the lesions (36, 37). Recently, new methods have been devised to deliver the growth factors in PRP, such as in a 2020 Chinese study where an injectable hydrogel with Platelet-Rich Plasma release was developed (38). Other studies have evaluated the efficacy of PRP treatment based on the administration vehicle, and they have found that the most effective mode of administration remains perilesional injection. Indeed, in a study from 2023, patients treated with topical PRP gel showed slower healing rates compared to those treated with perilesional injections (39).
The action of PRP in wound healing appears to be caused by the presence of numerous growth factors (such as TGF-B, PDGF, FGF, and ECGF), chemical mediators like histamine and serotonin, and proteins such as CTAP-3 and PF4, with effects on angiogenesis and the activation of extracellular matrix (ECM) cells. The existence of these growth factors, particularly when VEGF and FGF-2 are concurrently present (40), triggers the development of new blood vessels at the injection site, as observed in both animal models (41) and in vivo studies (42). Additionally, the presence of VEGF seems to enhance blood flow (42). Additionally, PRP has antibacterial properties. These combined mechanisms are the basis for PRP’s action in wound healing (43–45).
Platelet-rich plasma (PRP) has been widely used in the treatment of diabetic foot and associated wounds, demonstrating effectiveness and utility over time, along with a reduced rate of complications. Recent biotechnological advancements are opening new avenues in its delivery and the development of combined therapies, thus leading to increasingly exciting prospects in regenerative medicine.
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
PI: Writing – original draft, Writing – review & editing. CD: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. AP: Data curation, Writing – original draft. SS: Data curation, Writing – original draft. MC: Data curation, Writing – review & editing. DB: Methodology, Writing – review & editing. PD: Data curation, Writing – original draft. DS: Conceptualization, Writing – review & editing. LI: Methodology, Writing – review & editing. SI: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol (2020) 18(2):104–9. doi: 10.2174/1570161117666190405165911
2. Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med (2017) 110(3):104–9. doi: 10.1177/0141076816688346
3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med (2017) 376(24):2367–75. doi: 10.1056/NEJMra1615439
4. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care (2023) 46(1):209–21. doi: 10.2337/dci22-0043
5. Bolton L. Platelet-rich plasma: optimal use in surgical wounds. Wounds (2021) 33(8):219–21. doi: 10.25270/wnds/2021.219221
6. Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg (2011) 37(7):931–8. doi: 10.1111/j.1524-4725.2011.01999.x
7. Helmy Y, Farouk N, Ali Dahy A, Abu-Elsoud A, Fouad Khattab R, Elshahat Mohammed S, et al. Objective assessment of Platelet-Rich Plasma (PRP) potentiality in the treatment of Chronic leg Ulcer: RCT on 80 patients with Venous ulcer. J Cosmet Dermatol (2021) 20(10):3257–63. doi: 10.1111/jocd.14138
8. Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep (2018) 8(1):1513. doi: 10.1038/s41598-018-19419-6
9. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. J Cutan Aesthet Surg (2014) 7(4):189–97. doi: 10.4103/0974-2077.150734
10. Singh K, Yadav VB, Yadav U, Nath G, Srivastava A, Zamboni P, et al. Evaluation of biogenic nanosilver-acticoat for wound healing: A tri-modal in silico, in vitro and in vivo study. Colloids Surf A Physicochem Eng Asp (2023) 670:131575. doi: 10.1016/j.colsurfa.2023.131575
11. Gardner SE, Hillis SL, Frantz RA. A prospective study of the PUSH tool in diabetic foot ulcers. J Wound Ostomy Continence Nurs (2011) 38(4):385–93. doi: 10.1097/WON.0b013e31821e4dbd
12. Wagner FW Jr. A classification and treatment program for diabetic, neuropathic, and dysvascular foot problems. A.A.O.S. Instr Course Lect (1979) 27:143–65.
13. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech (2009) 2(5-6):231–7. doi: 10.1242/dmm.001180
14. Driver VR, Hanft J, Fylling CP, Beriou JM. Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage (2006) 52(6):68–70, 72, 74 passim.
15. Yuan N, Wang C, Wang Y, Yu T, Long Y, Zhang X, et al. [Preparation of autologous platelet-rich gel for diabetic refractory dermal ulcer and growth factors analysis from it]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi (2008) 22(4):468–71.
16. Dougherty EJ. An evidence-based model comparing the cost-effectiveness of platelet-rich plasma gel to alternative therapies for patients with nonhealing diabetic foot ulcers. Adv Skin Wound Care (2008) 21(12):568–75. doi: 10.1097/01.ASW.0000323589.27605.71
17. Saldalamacchia G, Lapice E, Cuomo V, De Feo E, D'Agostino E, Rivellese AA, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis (2004) 14(6):395–6. doi: 10.1016/s0939-4753(04)80029-2
18. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care (2001) 24:483–8. doi: 10.2337/diacare.24.3.483
19. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci (2004) 30:145–51. doi: 10.1016/j.transci.2004.01.004
20. Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet (1990) 170(1):56–60.
21. Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg (1991) 14(4):526–32; discussion 532-6. doi: 10.1016/0741-5214(91)90247-R
22. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care (1992) 15(11):1598–604. doi: 10.2337/diacare.15.11.1598
23. Atri SC, Misra J, Bisht D, Misra K. Use of homologous platelet factors in achieving total healing of recalcitrant skin ulcers. Surgery (1990) 108(3):508–12.
24. Villela DL, Santos VL. Evidence on the use of platelet-rich plasma for diabetic ulcer: a systematic review. Growth Factors (2010) 28(2):111–6. doi: 10.3109/08977190903468185
25. Singh AV, Gemmati D, Kanase A, Pandey I, Misra V, Kishore V, et al. Nanobiomaterials for vascular biology and wound management: A review. Veins Lymphatics (2018) 7(1). doi: 10.4081/vl.2018.7196
26. Kakagia DD, Kazakos KJ, Xarchas KC, Karanikas M, Georgiadis GS, Tripsiannis G, et al. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Its Complications (2007) 21(6):387–91. doi: 10.1016/j.jdiacomp.2007.03.006
27. Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C: Methods (2009) 15(4):625–34. doi: 10.1089/ten.tec.2008.0518
28. Kontopodis N, Tavlas E, Papadopoulos G, Pantidis D, Kafetzakis A, Chalkiadakis G, et al. Effectiveness of platelet-rich plasma to enhance healing of diabetic foot ulcers in patients with concomitant peripheral arterial disease and critical limb ischemia. Int J Low Extrem Wounds (2016) 15(1):45–51. doi: 10.1177/1534734615575829
29. Sakata J, Sasaki S, Handa K, Uchino T, Sasaki T, Higashita R, et al. A retrospective, longitudinal study to evaluate healing lower extremity wounds in patients with diabetes mellitus and ischemia using standard protocols of care and platelet-rich plasma gel in a Japanese wound care program. Ostomy Wound Manage (2012) 58(4):36–49.
30. Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J (2011) 8(3):307–12. doi: 10.1111/j.1742-481X.2011.00797.x
31. Motolese A, Vignati F, Antelmi A, Saturni V. Effectiveness of platelet-rich plasma in healing necrobiosis lipoidica diabeticorum ulcers. Clin Exp Dermatol (2015) 40(1):39–41. doi: 10.1111/ced.12474
32. Perez-Zabala E, Basterretxea A, Larrazabal A, Perez-Del-Pecho K, Rubio-Azpeitia E, Andia I. Biological approach for the management of non-healing diabetic foot ulcers. J Tissue Viability (2016) 25(2):157–63. doi: 10.1016/j.jtv.2016.03.003
33. Löndahl M, Tarnow L, Karlsmark T, Lundquist R, Nielsen AM, Michelsen M, et al. Use of an autologous leucocyte and platelet-rich fibrin patch on hard-to-heal DFUs: a pilot study. J Wound Care (2015) 24(4):172–4, 176-8. doi: 10.12968/jowc.2015.24.4.172
34. Jaseem M, Alungal S, Dhiyaneswaran, Shamsudeen J. Effectiveness of autologous PRP therapy in chronic nonhealing ulcer: A 2-year retrospective descriptive study. J Family Med Prim Care (2020) 9(6):2818–22. doi: 10.4103/jfmpc.jfmpc_177_20
35. Ullah A, Jawaid SI, Qureshi PNAA, Siddiqui T, Nasim K, Kumar K, et al. Effectiveness of injected platelet-rich plasma in the treatment of diabetic foot ulcer disease. Cureus (2022) 14(8):e28292. doi: 10.7759/cureus.28292
36. Deng W, Boey J, Chen B, Byun S, Lew E, Liang Z, et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care (2016) 25(7):393–7. doi: 10.12968/jowc.2016.25.7.393
37. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg (2017) 38:206–11. doi: 10.1016/j.avsg.2016.04.023
38. Qian Z, Wang H, Bai Y, Wang Y, Tao L, Wei Y, et al. Improving chronic diabetic wound healing through an injectable and self-healing hydrogel with platelet-rich plasma release. ACS Appl Mater Interfaces (2020) 12(50):55659–74. doi: 10.1021/acsami.0c17142
39. Rani DA, Khanna S, Mishra SP, Kumar S. A comparative evaluation of topical application versus perilesional injection of platelet-rich plasma in diabetic foot ulcer. Int J Low Extrem Wounds (2023) 24:15347346231176727. doi: 10.1177/15347346231176727
40. Brill A, Elinav H, Varon D. Differencial role of platelet granular mediators in angiogenesis. Cardiovasc Res (2004) 63:226–35. doi: 10.1016/j.cardiores.2004.04.012
41. Yokota K, Ishida O, Sunagawa T, Suzuki O, Nakamae A, Ochi M. Platelet-rich plasma accelerated surgical angio-genesis in vascular-implanted necrotic bone: an experimental study in rabbits. Acta Orthop (2008) 79(1):106–10. doi: 10.1080/17453670710014842
42. Suzuki O, Bishop AT, Sunagawa T, Katsube K, Friedrich PF. VEGF Promoted surgical angiogenesis in necrotic bone. Microsurgery (2004) 24:85–91. doi: 10.1002/micr.10190
43. Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, Cattini L, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy (2014) 16(9):1294–304. doi: 10.1016/j.jcyt.2014.06.003
44. Shao S, Pan R, Chen Y. Autologous platelet-rich plasma for diabetic foot ulcer. Trends Endocrinol Metab (2020) 31(12):885–90. doi: 10.1016/j.tem.2020.10.003
Keywords: PRP, diabetic foot, wound care, ulcer, case series, literature review
Citation: Izzo P, De Intinis C, Molle M, Polistena A, Sibio S, Codacci-Pisanelli M, Biacchi D, Di Cello P, Santini D, Izzo L and Izzo S (2023) Case report: The use of PRP in the treatment of diabetic foot: case series and a review of the literature. Front. Endocrinol. 14:1286907. doi: 10.3389/fendo.2023.1286907
Received: 01 September 2023; Accepted: 28 November 2023;
Published: 18 December 2023.
Edited by:
José Luis Lázaro Martínez, Complutense University of Madrid, SpainReviewed by:
Ajay Vikram Singh, Federal Institute for Risk Assessment (BfR), GermanyCopyright © 2023 Izzo, De Intinis, Molle, Polistena, Sibio, Codacci-Pisanelli, Biacchi, Di Cello, Santini, Izzo and Izzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Izzo, bHVjaWFuby5penpvQHVuaXJvbWExLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.