
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 08 January 2024
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1286391
This article is part of the Research Topic Environmental Threats to Human Reproduction View all 13 articles
Background: Phthalates are ubiquitously used in a variety of products and have an adverse effect on folliculogenesis. However, previous epidemiological studies on the associations between phthalate exposure and antral follicle count (AFC) produced conflicting results. The present study aimed to evaluate the associations between urinary phthalate metabolite concentrations and AFC among women undergoing in vitro fertilization (IVF).
Methods: We collected 525 urine samples and measured 8 phthalate metabolites from IVF patients. Poisson regression models were conducted to evaluate the associations between phthalate metabolite concentrations and AFC. In addition, participants were stratified into a younger group (< 35 years) and an older group (≥ 35 years) to explore the potential effect modification by age. We also performed sensitivity analyses by restricting our analyses to only infertile women diagnosed with tubal factor infertility to test the robustness of the results.
Results: Significant positive associations were observed among urinary MBP, MEOHP and ∑PAEs concentrations and AFC after adjusting for age, BMI, year of study and infertility diagnosis. Compared with women in the first tertile, women in the third tertile of MBP and MEOHP had 7.02% (95% CI: 1.18%, 12.9%) and 8.84% (95% CI: 2.83%, 14.9%) higher AFC, respectively, and women in the second and third tertiles of ∑PAEs had 6.19% (95% CI: 0.37%, 12.0%) and 9.09% (95% CI: 3.22%, 15.0%) higher AFC, respectively. In addition, MBP, MEOHP and ∑PAEs also had significant positive associations with AFC in trend tests for dose-response. In the age-stratified analysis, we found a stronger relationship between phthalate metabolite concentrations and AFC among older women and an inverse association among younger women. We observed similar results in the sensitivity analyses.
Conclusion: We found positive associations between phthalate exposure and AFC, which support the idea that phthalate exposure may accelerate primordial follicle recruitment and lead to higher AFC in women undergoing IVF. More studies are needed to better understand their relationships.
Infertility is an ongoing reproductive health problem around the world, and exposure to environmental contaminants is an important factor (1, 2). Phthalate esters (PAEs) are a group of synthetic compounds that are abundantly used as plasticizers or solvents in a variety of products, such as polyvinylchloride, building and finishing materials, personal care products, cosmetics, toys, food packages, medications and medical devices (3). Phthalates in products can be released into the environment due to nonchemical bonds, and human exposure occurs through inhalation, ingestion and dermal absorption (4). Phthalates are rapidly metabolized into monoesters once they enter the human body, in which they have stronger biological activity (5). The metabolites are excreted mainly via urine and have a half-life less than 24 hours (6), therefore, urinary metabolite concentrations are generally used to represent body phthalate exposure levels (7).
Phthalates are endocrine disruptors characterized mainly by their reproductive and developmental toxicity (8). According to the results of several animal studies, they can disrupt ovarian development, inhibit follicle growth, and impair oocyte maturation and embryo development (9–14). Epidemiological studies found that phthalate exposure was associated with adverse reproductive outcomes, such as decreased oocyte retrieval, mature oocytes, fertilized oocytes, good-quality embryos, clinical pregnancy rate and live birth rate (15–18).
Antral follicle count (AFC) is a critical value used to evaluate women’s fecundity, and is defined as the sum of 2-10 mm follicles in both ovaries as observed on ultrasound in the early follicular phase (menstrual days 2-4) (19). As the earliest acquirable follicle parameter in the reproductive clinic, AFC was routinely measured during the infertility treatment period for ovarian reserve assessment, infertility diagnosis, and treatment strategy determination, and AFC was also found to be associated with reproductive outcomes (20, 21).
Phthalates have been shown to reduce antral follicle number in mice (22) and inhibit antral follicle growth in in vitro culture (23). However, phthalate exposure in humans is complicated and continuous, and the results among human studies are controversial. Messerlian et al. reported that urinary phthalate metabolite concentrations were adversely associated with AFC (24), and Li et al. found both positive and negative associations between phthalate metabolite concentrations in serum and AFC (25). Additionally, we observed positive dose-response associations between urinary phthalate metabolites and AFC in our previous study (26). In mammals, the follicle cycle starts from primordial follicle activation and undergoes a series of developments until atresia or ovulation; thus, more AFC may suggest more follicle recruitment from the beginning. It has been reported that di(2-ethylhexyl) phthalate (DEHP) can accelerate primordial follicle recruitment in mice, leading to a lower proportion of primordial follicles and a higher proportion of developing follicles in ovaries (27–29). In the present study, we enlarged the sample size and reanalyzed the association between phthalate exposure and AFC among women undergoing in vitro fertilization (IVF). We also performed stratified analyses to explore potential effect modification by age and conducted sensitivity analysis to test the strength of our results.
To explore the potential effects of phthalate exposure on reproductive health, women who sought infertility treatment were recruited at two separate times at the Reproductive Medicine Center of Tongji Hospital, Wuhan, China, as described previously: from July to August 2014 (30) and from November to December 2016 (31). Briefly, women aged from 20 to 45 years who were infertile and with indications for IVF or intracytoplasmic sperm injection were eligible. Subjects who had an ovariectomy history, other iatrogenic injuries, or health conditions such as autoimmune diseases, congenital gonadal dysplasia, endocrine diseases and sexually transmitted diseases were excluded. Participants who provided a urine sample for phthalate metabolite detection and had AFC data within a 4-month period before enrollment were included in this study. Information on age, height, weight, smoking status and ethnicity was collected at enrollment. These studies were approved by the Ethics Board of Tongji Hospital, and informed consent was obtained for all participants.
On the day of ovum pick-up surgery, urine samples were collected and transferred to the laboratory immediately, aliquoted and frozen at −80 °C. The concentrations of mono-ethyl phthalate (MEP), mono-methyl phthalate, mono-n-butyl phthalate (MBP), mono(2-ethylhexyl) phthalate (MEHP), mono-benzyl phthalate, mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono-n-octyl phthalate (MOP) were analyzed by high-performance liquid chromatography and tandem mass spectrometry as described in our previous study (26, 32). The limits of detection ranged from 0.01-0.04 μg/L. Values for metabolites with concentrations less than the LOD were assigned with LOD/ . Concentrations of creatinine were measured using clinical chemistry analyzers (17).
AFC, which was measured on days 2-4 of the menstrual cycle by transvaginal ultrasound, and other clinical data (e.g., infertility type, duration of infertility and infertility diagnosis) were abstracted from electronic medical records. Infertility types included primary infertility and secondary infertility. Infertility diagnosis was classified as female factor, male factor, mix factor or unexplained reasons. Female factor infertility included diminished ovarian reserve, tubal factor, ovulatory dysfunction, endometriosis and uterine factor.
Descriptive information of the participants is presented as the number (%) or mean ± standard deviation where appropriate. Phthalate metabolite concentrations are presented as quartiles and geometric means. The molar sum of metabolites of DEHP (∑DEHP) was calculated by the sum of MEHP/278.34, MEOHP/292.34 and MEHHP/294.34. ∑PAEs were also calculated by the molar sum of the concentrations of the eight phthalate metabolites detected in this study. Phthalate metabolite concentrations were standardized by creatinine due to urine dilution and categorized into tertiles (numbered 1, 2, 3 from lowest to highest tertile). We used multivariate generalized linear models with Poisson distribution and log-link function to evaluate the association of metabolite concentrations with AFC by comparing the second and third tertiles to the first (reference category). Tertiles of phthalate metabolite concentrations were also used as continuous variables in models to evaluate the dose-response relationships between metabolites concentrations and AFC. Age (continuous), body mass index (BMI, continuous), year of study (2014 or 2016) and infertility diagnosis (female factor, male factor, mix factor or unexplained) were selected as covariates according to biological relevance or prior knowledge. Race and smoking status were not included as covariates due to the low frequencies of non-Han ethnicity (3.4%) and smokers (4.0%). Age was a critical independent impactor of AFC, to explore its potential modification effect, women were stratified into younger group (< 35 years) and older group (≥ 35 years) before analyses (33, 34). To test the strength of our results, we conducted sensitivity analyses by restricting our analyses to only women who sought infertility treatment due to tubal factors or male factors and had a normal BMI (18.5-24.9 kg/m²). As the results showed that age could modify the associations between phthalate metabolite concentrations and AFC, we further performed re-analyses based on age stratification. Statistical analysis was conducted by SPSS (version 22.0, IBM Co., Armonk, USA).
This study comprised 525 women undergoing IVF who were recruited on two separate occasions, as shown in Table 1. A total of 110 subjects were enrolled in 2014, and 415 were enrolled in 2016. The average (± SD) age and BMI of the study population were 31.1 ± 5.1 years and 21.9 ± 2.7 kg/m², respectively, and most of them were of Han ethnicity (96.6%) and had never smoked (96.0%). The average (± SD) duration of infertility was 3.7 ± 2.9 years, and more than half of the subjects had primary infertility (54.5%) and were diagnosed with female factors (65.6%). The average (± SD) AFC was 13.4 ± 6.8. Most of the tested phthalate metabolites were detected in urine in most of the participants (> 92.6%), except for MOP (26.1%), as shown in Table 2. MBP was the phthalate with the highest level of exposure (median: 187 μg/L), and except for mono-benzyl phthalate (median: 0.16 μg/L), the remaining 5 metabolites had similar concentrations (median: 12.9-18.8 μg/L). MOP was removed from further analyses because of its low detection frequency.
In the Poisson regression models adjusted for age, BMI, year of study and infertility diagnosis, we found that concentrations of MBP, MEOHP and ∑PAEs were positively associated with AFC (Figure 1). Compared with women in the first tertile, women in the third tertile of MBP and MEOHP had a 7.02% (95% CI: 1.18%, 12.9%) and 8.84% (95% CI: 2.83%, 14.9%) increase in AFC, respectively, and women in the second and third tertiles of ∑PAEs had a 6.19% (95% CI: 0.37%, 12.0%) and 9.09% (95% CI: 3.22%, 15.0%) increase in AFC, respectively (Supplementary Table S1). In trend tests by tertile for the dose-response relationship, MBP, MEOHP and ∑PAEs also showed significant positive associations with AFC.
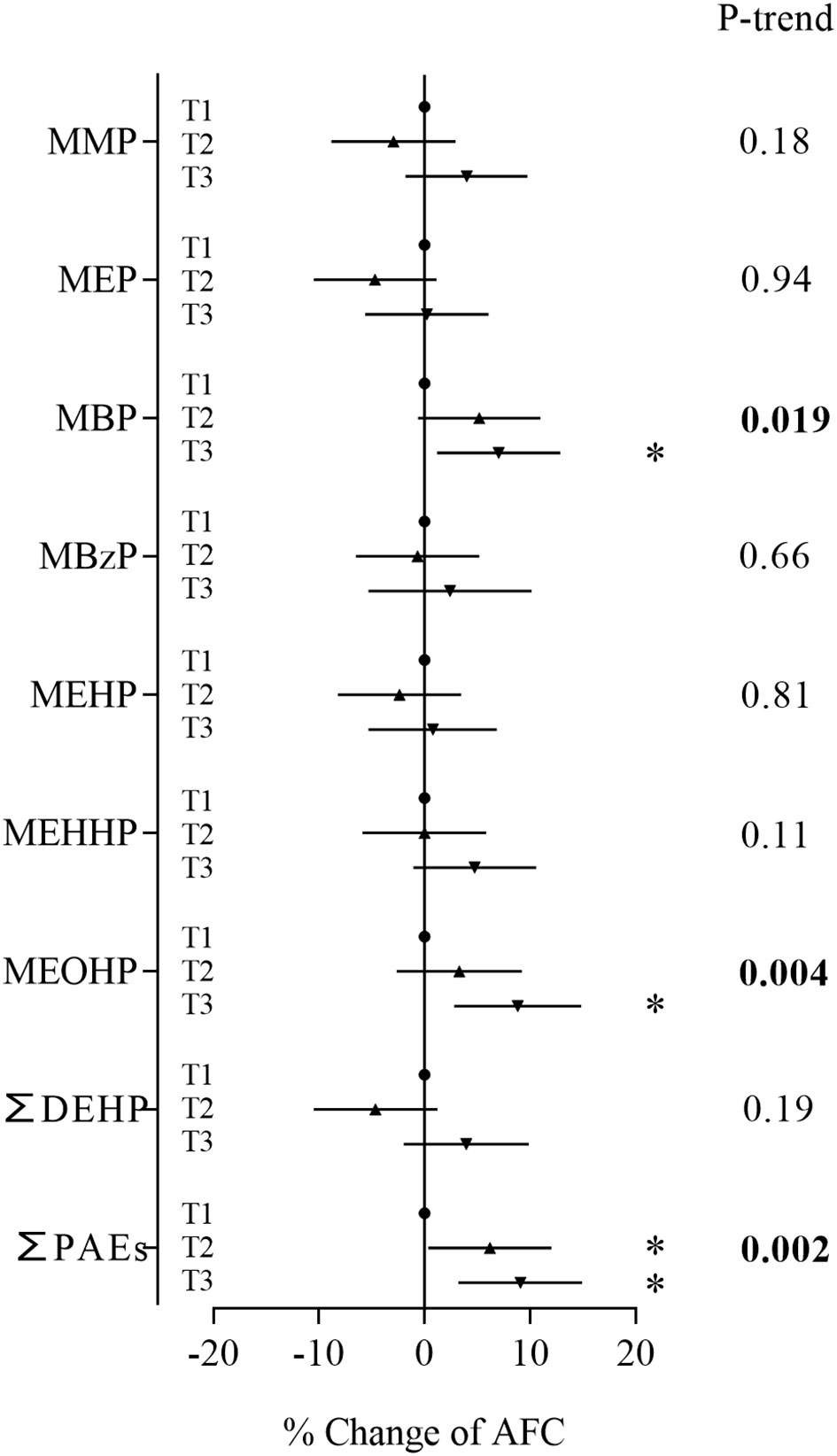
Figure 1 Associations between phthalate metabolite concentrations and AFC. Models were adjusted for age, BMI, year of study and infertility diagnosis. *P < 0.05.
In the age-stratified analysis, we observed a stronger relationship between phthalate metabolite concentrations and AFC among women ≥ 35 years and inverse associations among women less than 35 years (Figure 2). Compared to women in the first tertile, younger women in the second tertile of MEP and ∑DEHP had 6.50% (95% CI: -12.8%, -0.18%) and 7.37% (95% CI: -13.8%, -0.89%) lower AFC, respectively (Supplementary Table S2). However, trend tests for dose-response were not significant. Among older women, subjects in the second tertile of mono-methyl phthalate and ∑PAEs and the third tertile of MEP, MBP, MEHP, MEHHP, MEOHP, ∑DEHP and ∑PAEs had a 15.3% to 39.5% increase in AFC when compared with women in the first tertile (Supplementary Table S3). In addition, MEP, MBP, MEHP, MEHHP, MEOHP, ∑DEHP and ∑PAEs also showed significant trends. To explore whether the discrepancies were caused by differences in phthalate exposure levels, we compared phthalate metabolite concentrations between the two groups. The results showed that younger women and older women had similar phthalate exposure levels (Supplementary Table S4).
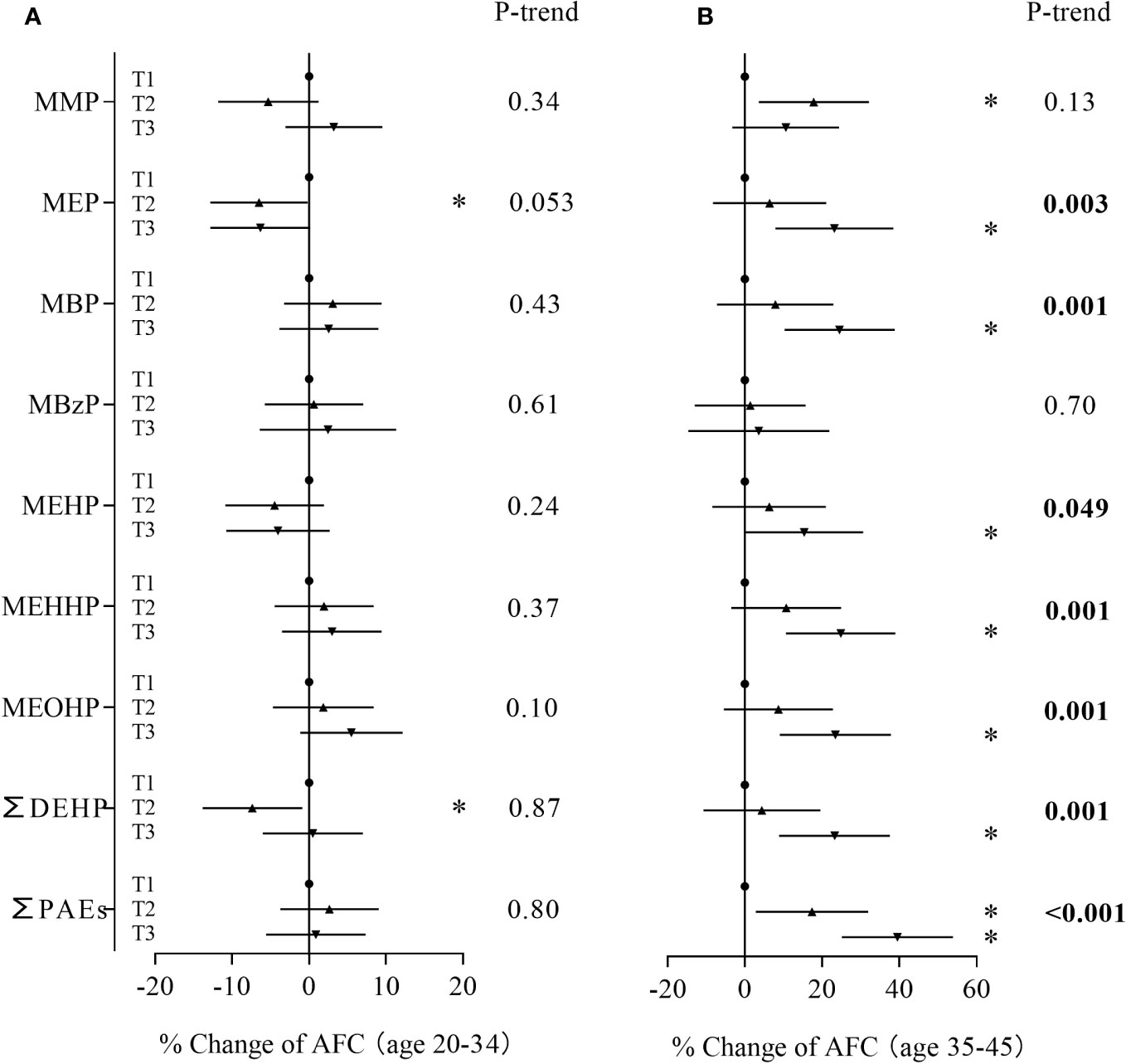
Figure 2 Associations between phthalate metabolite concentrations and AFC stratified by age. (A): women < 35 years, (B): women ≥ 35 years. *P < 0.05.
In the sensitivity analyses based on women who were diagnosed with tubal factor infertility and infertility due to male factor, MBP, the major contaminant, and sum of PAEs showed positive associations with AFC (Figure 3). Women in the second and third tertiles of MBP and third tertile of ∑PAEs had an AFC range from 9.48% to 12.2% higher than women in the first tertile (Supplementary Table S5). These metabolites also showed consistent significant positive associations with AFC in trend tests. In the sensitivity analyses based on women with normal BMI, MBP and the sum of PAEs still showed positive associations with AFC (Figure 4; Supplementary Table S8). Similarly, stronger relationships were observed between phthalate metabolite concentrations and AFC among women ≥ 35 years than women < 35 years in all sensitivity analyses (Supplementary Tables S6, S7, S9, S10).
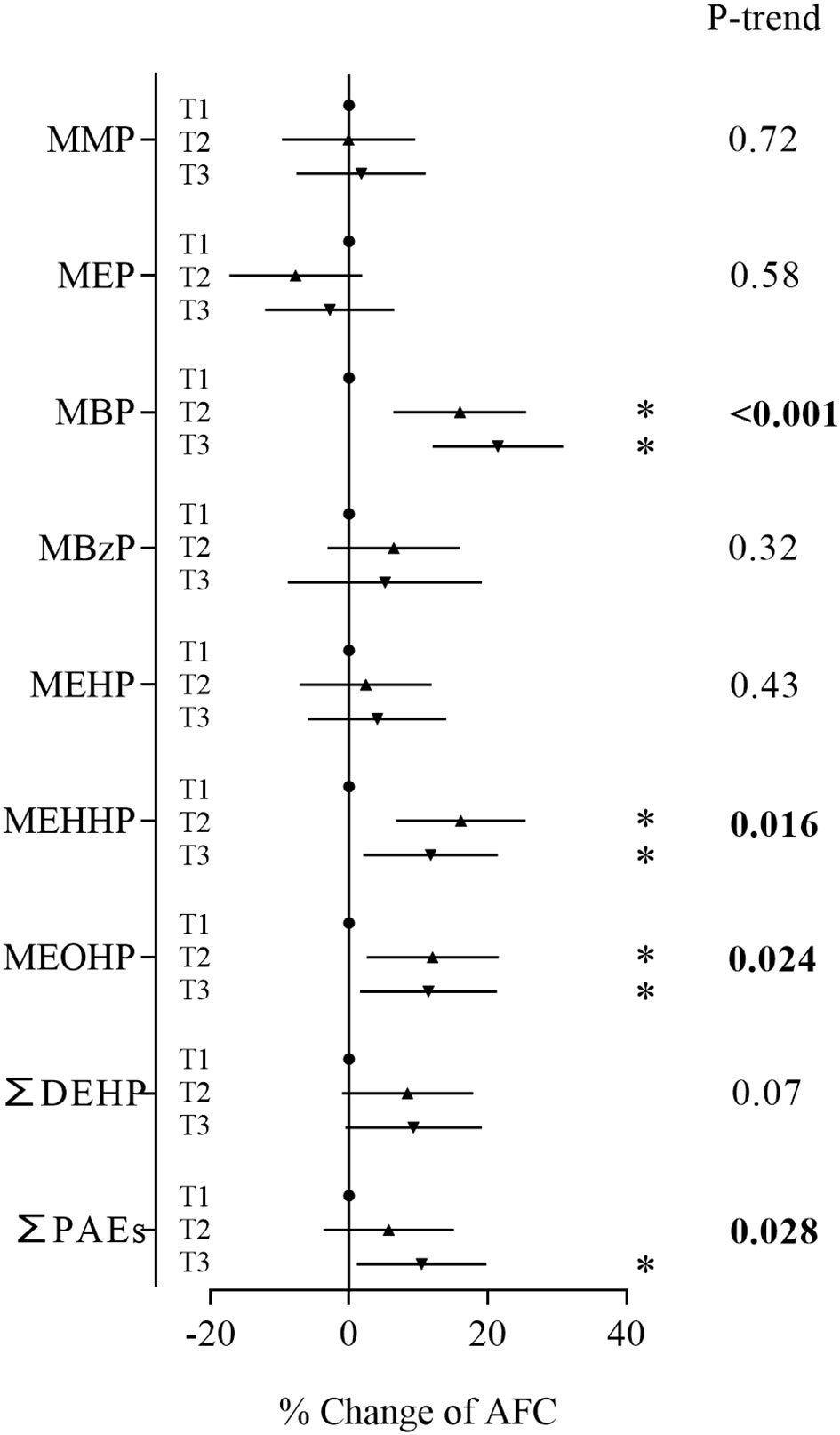
Figure 3 Associations between phthalate metabolite concentrations and AFC based on women diagnosed with tubal factor and infertility due to male factor. *P < 0.05.
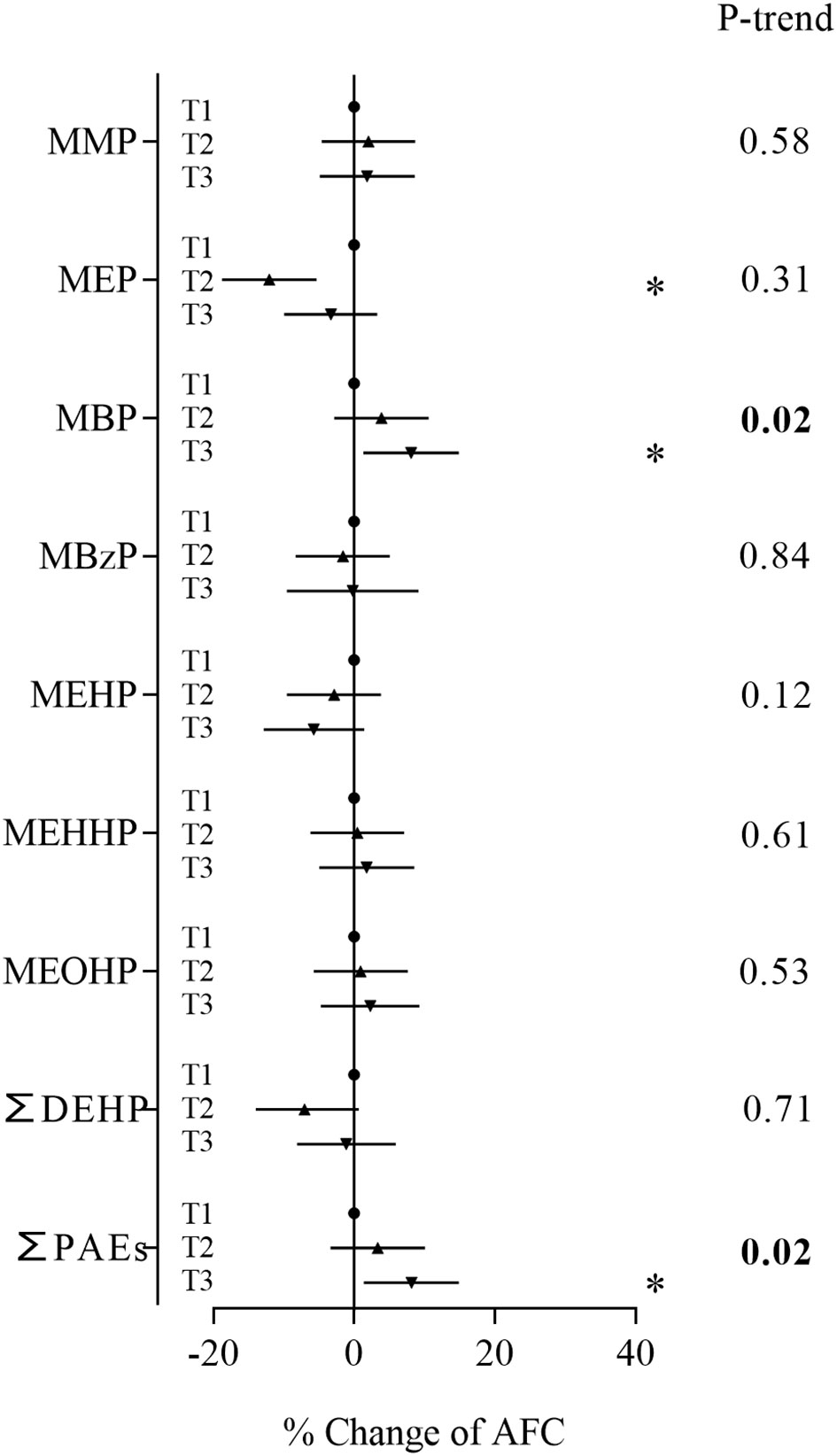
Figure 4 Associations between phthalate metabolite concentrations and AFC based on women with normal BMI. *P < 0.05.
In this study, we found that most of the phthalate metabolites were highly detected in urine samples, and MBP had the highest concentration. The European Food Safety Authorities have recommended the tolerable daily intake for several phthalates, as 10 and 50 μg/kg bw/day for DBP and DEHP, respectively (35). Estimated daily intakes for DBP and DEHP were calculated as reported (36), 58.7% and 10.3% of subjects had higher estimated daily intakes than the tolerable daily intake for DBP and DEHP, respectively, in this study. Compared with previous studies based on 125 non-pregnant women (37) and 946 pregnant women (38) in the same region, the detection rate was similar, and MBP uniformly showed the highest concentration, followed by DEHP metabolites, while the values were higher in our study (median: MBP 187 vs. 62.1 and 41.8 μg/L, MEHHP 18.5 vs. 6.34 and 5.05 μg/L, MEOHP 13.8 vs. 4.74 and 3.99 μg/L, MEHP 13.5 vs. 2.15 and 2.11 μg/L, respectively). This result may indicate an association between phthalate exposure and women with infertility.
We found that urinary MBP, MEOHP and ∑PAEs concentrations were positively associated with AFC, and most of the relationships remained in sensitivity analyses, which suggests the robustness of our results. In line with a previous study, Li et al. reported that the serum concentration of MEHHP was associated with an increased AFC among 297 IVF women (25). However, in a study of 215 women who sought infertility care, Messerlian et al. found a nonlinear inverse association between urinary DEHP metabolite concentrations (MEHP, MEOHP, MEHHP and MECPP) and AFC (24). These discrepancies may be attributed to differences in demographic characteristics (e.g., age, BMI, race, etc.). Furthermore, phthalate exposure status varied between studies. In Messerlian’s study, MEP showed the highest concentration, while the concentration of MBP was relatively low (SG-adjusted median: 54.2 and 12.8 μg/L, respectively), which was in contrast with our data (median: 14.6 and 187 μg/L, respectively). The overall metabolite concentrations were low in Li’s study (median: MEP 2.19 μg/L, MBP 4.17 μg/L). In addition, phthalate may exert toxicity with a nonmonotonic dose-response effect or even a U-shape effect (39).
Primordial follicles, which are composed of a prophase-arrested oocyte enclosed by a single layer of flattened pre-granulosa cells, form at approximately 15-22 weeks of gestation and are complete by 6 months after birth in the human ovary (40). Their fates were to remain dormant, awaken and develop, or die directly during dormancy (41). Only a few of the dormant primordial follicles are activated under the regulation of intercellular and intracellular signals. Once awakened, primordial follicles join the follicle growing pool; most of them undergo atresia during development, and a small proportion undergo consecutive development through primary follicles, secondary follicles, antral follicles, preovulatory follicles and ovulation (42, 43). Therefore, a greater AFC indicates that more primordial follicles were awakened originally. In support of our findings, it has been reported that DEHP accelerates primordial follicle recruitment by decreasing the percentage of primordial follicles and increasing the percentage of developing follicles in postnatal mice (27) and in adult mice (28). Exposure to di-n-butyl phthalate, the prototype of MBP, promotes the depletion of follicular follicles by accelerating primordial follicle recruitment in rats (29). Additionally, in an in vitro culture neonatal ovary model, Hannon et al. revealed that MEHP could directly accelerate primordial follicle recruitment by over activating PI3K signals (44).
The number of primordial follicles in the ovary determines the ovarian reserve (40). Any factors that accelerate primordial follicle recruitment will promote the depletion of ovarian reserve and shorten the reproductive life span of women. It has been reported that phthalate exposure was associated with the risk of premature ovarian failure (45). On the other hand, although a higher AFC was observed in women with higher urinary phthalate metabolite concentrations in this study, follicle development competence may be affected. Previous studies found that phthalate could increase oocyte oxidative stress, disrupt the cell cycle, impair meiotic competence, induce oocyte and granulosa cell apoptosis, inhibit oocyte maturation, cause epigenetic alterations, disrupt DNA damage repair gene expression, etc., which may contribute to adverse reproductive outcomes (10, 46–50).
Age was a critical independent risk factor for female fecundity and was associated with adverse IVF outcomes (e.g., lower oocyte retrieval, pregnancy rate, delivery rate, etc.) (34, 51). In age stratification analyses based on all subjects and in sensitivity analyses, we found that the associations between phthalate metabolite concentrations and AFC were modified by age, which is in line with a previous study (24). In women with advanced age, the ovary may be more vulnerable to external hazardous factors such as phthalates. The risk resistance ability and repair capacity decline with ovarian ageing, such as mitochondrial DNA instability and dysfunction, disturbance of antioxidant signaling and increase in oxidative damage, DNA damage, aneuploidy, epigenetic alteration, microenvironmental alteration (52–54). Moreover, exposure duration is also an important factor. It has been widely reported that women are exposed to phthalate from the fetal period to childhood and then adulthood (38, 55, 56), and long-term exposure is more likely to lead to adverse consequences.
In summary, our results showed that phthalate metabolite concentrations in urine were positively associated with AFC among women undergoing IVF. However, this study still has some limitations. (1) The AFC was measured when women attended the infertility clinic for ovarian reserve assessment, while urine samples were collected the on day of surgery, which was up to 4 months after AFC measurement, and the median interval between AFC measurement and sample collection was 72 days. Humans are continuously exposed to phthalates, and it has been reported that a spot urine sample was a moderate predictor of the 4-month exposure level of phthalates (57). (2) The study population was enrolled from a reproductive center, and they had higher phthalate exposure levels than the normal population as described above; therefore, these findings may not be applicable to the general population. (3) It is important to note that phthalate exposure is just one of the factors that can influence reproductive health and fertility. Lifestyle, genetics, and other environmental factors also play a role, we cannot rule out the influences from other factors in this study. (4) We found positive associations between phthalate exposure and AFC, and while the causation cannot be determined, more studies are needed to better understand their relationships.
The results support the idea that phthalate exposure could accelerate primordial follicle recruitment and promote the depletion of ovarian reserve. Our research fills the gap between animal studies showing that phthalates accelerate primordial follicle recruitment and epidemiological research showing that phthalates are associated with premature ovarian failure. Considering the high exposure frequency and level of phthalates in infertile women, more studies are needed to determine the effect of phthalate on ovarian reserve and folliculogenesis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Ethics Board of Tongji Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YY: Formal Analysis, Investigation, Methodology, Writing – original draft. YD: Data curation, Investigation, Resources, Writing – review & editing. NG: Investigation, Resources, Writing – review & editing. FL: Supervision, Writing – review & editing. TD: Investigation, Methodology, Writing – review & editing. YL: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 81771654, 81571508).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1286391/full#supplementary-material
AFC, Antral follicle count; AMH, Anti-Müllerian hormone; BMI, Body mass index; DEHP, Di(2-ethylhexyl) phthalate; IVF, In vitro fertilization; MEHP, Mono(2-ethylhexyl) phthalate; MEHHP, Mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, Mono(2-ethyl-5-oxohexyl) phthalate; MEP, Mono-ethyl phthalate; MBP, Mono-n-butyl phthalate; MOP, Mono-n-octyl phthalate; PAEs, Phthalate esters.
1. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod update. (2015) 21(4):411–26. doi: 10.1093/humupd/dmv016
2. Gallo A. Reprotoxic impact of environment, diet, and behavior. Int J Environ Res Public Health (2022) 19(3):1303. doi: 10.3390/ijerph19031303
3. Zhang YJ, Guo JL, Xue JC, Bai CL, Guo Y. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment. Environ pollution (2021) 291:118106. doi: 10.1016/j.envpol.2021.118106
4. Wang YX, Liu C, Chen YJ, Chen HG, Yang P, Wang P, et al. Predictors and correlations of phthalate metabolite concentrations in urine and seminal plasma among reproductive-aged men. Environ Res (2018) 161:336–44. doi: 10.1016/j.envres.2017.11.027
5. Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res (2007) 51(7):899–911. doi: 10.1002/mnfr.200600243
6. Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics (2019) 7(2):21. doi: 10.3390/toxics7020021
7. Guo Y, Wu Q, Kannan K. Phthalate metabolites in urine from China, and implications for human exposures. Environ Int (2011) 37(5):893–8. doi: 10.1016/j.envint.2011.03.005
8. Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, et al. A global assessment of phthalates burden and related links to health effects. Environ Int (2016) 97:212–36. doi: 10.1016/j.envint.2016.09.013
9. Zhang Y, Mu X, Gao R, Geng Y, Liu X, Chen X, et al. Foetal-neonatal exposure of Di (2-ethylhexyl) phthalate disrupts ovarian development in mice by inducing autophagy. J hazard mater (2018) 358:101–12. doi: 10.1016/j.jhazmat.2018.06.042
10. Li FP, Zhou JL, Guo AW, Liu Y, Zhang F, Xu BH, et al. Di(n-butyl) phthalate exposure impairs meiotic competence and development of mouse oocyte. Environ pollution (2019) 246:597–607. doi: 10.1016/j.envpol.2018.12.077
11. Rasmussen LM, Sen N, Vera JC, Liu X, Craig ZR. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol reproduct (2017) 96(5):1105–17. doi: 10.1095/biolreprod.116.144691
12. Liu JC, Lai FN, Li L, Sun XF, Cheng SF, Ge W, et al. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis (2017) 8(8):e2966. doi: 10.1038/cddis.2017.350
13. Chu D-P, Tian S, Sun D-G, Hao C-J, Xia H-F, Ma X. Corrigendum to: Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reproduct Fertil Dev (2014) 26(3):491. doi: 10.1071/RD12178_CO
14. Zhou L, Feng W, Mao Y, Chen Y, Zhang X. Nanoengineered sonosensitive platelets for synergistically augmented sonodynamic tumor therapy by glutamine deprivation and cascading thrombosis. Bioactive mater (2023) 24:26–36. doi: 10.1016/j.bioactmat.2022.11.020
15. Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, et al. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int (2018) 111:23–31. doi: 10.1016/j.envint.2017.11.011
16. Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health perspectives (2016) 124(6):831–9. doi: 10.1289/ehp.1509760
17. Deng T, Du Y, Wang Y, Teng X, Hua X, Yuan X, et al. The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: A prospective single-center study. Ecotoxicol Environ safe (2020) 188:109884. doi: 10.1016/j.ecoenv.2019.109884
18. Mínguez-Alarcón L, Messerlian C, Bellavia A, Gaskins AJ, Chiu YH, Ford JB, et al. Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ Int (2019) 126:355–62. doi: 10.1016/j.envint.2019.02.025
19. Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J obstet gynecol (2017) 217(2):129–40. doi: 10.1016/j.ajog.2017.02.027
20. Holte J, Brodin T, Berglund L, Hadziosmanovic N, Olovsson M, Bergh T. Antral follicle counts are strongly associated with live-birth rates after assisted reproduction, with superior treatment outcome in women with polycystic ovaries. Fertil steril (2011) 96(3):594–9. doi: 10.1016/j.fertnstert.2011.06.071
21. Liao S, Xiong J, Tu H, Hu C, Pan W, Geng Y, et al. Prediction of in vitro fertilization outcome at different antral follicle count thresholds combined with female age, female cause of infertility, and ovarian response in a prospective cohort of 8269 women. Medicine (2019) 98(41):e17470. doi: 10.1097/MD.0000000000017470
22. Sen N, Liu X, Craig ZR. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod toxicol (2015) 53:15–22. doi: 10.1016/j.reprotox.2015.02.012
23. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol sciences: an Off J Soc Toxicol (2017) 156(1):217–29. doi: 10.1093/toxsci/kfw245
24. Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum reproduct (2016) 31(1):75–83. doi: 10.1093/humrep/dev292
25. Li Y, Yao Y, Xiao N, Liu Y, Du Y, Liu M, et al. The association of serum phthalate metabolites with biomarkers of ovarian reserve in women of childbearing age. Ecotoxicol Environ safe (2022) 242:113909. doi: 10.1016/j.ecoenv.2022.113909
26. Du YY, Guo N, Wang YX, Hua X, Deng TR, Teng XM, et al. Urinary phthalate metabolites in relation to serum anti-Mullerian hormone and inhibin B levels among women from a fertility center: a retrospective analysis. Reprod Health (2018) 15(1):33. doi: 10.1186/s12978-018-0469-8
27. Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, et al. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol mutagenesis. (2013) 54(5):354–61. doi: 10.1002/em.21776
28. Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol reproduct (2014) 90(6):136. doi: 10.1095/biolreprod.114.119032
29. Tran DN, Jung EM, Yoo YM, Ahn C, Kang HY, Choi KC, et al. Depletion of follicles accelerated by combined exposure to phthalates and 4-vinylcyclohexene diepoxide, leading to premature ovarian failure in rats. Reprod toxicol (2018) 80:60–7. doi: 10.1016/j.reprotox.2018.06.071
30. Du YY, Fang YL, Wang YX, Zeng Q, Guo N, Zhao H, et al. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod toxicol (2016) 61:142–50. doi: 10.1016/j.reprotox.2016.04.005
31. Yao YC, Liu C, Wu LJ, Yuan XQ, Du YY, Li NJ, et al. Associations between medication use and phthalate metabolites in urine and follicular fluid among women undergoing in vitro fertilization. Ecotoxicol Environ safe (2021) 215:112174. doi: 10.1016/j.ecoenv.2021.112174
32. Yao YC, Du YY, Wang YX, Deng TR, Liu C, Teng XM, et al. Predictors of phthalate metabolites in urine and follicular fluid and correlations between urine and follicular fluid phthalate metabolite concentrations among women undergoing in vitro fertilization. Environ Res (2020) 184:109295. doi: 10.1016/j.envres.2020.109295
33. Vitagliano A, Paffoni A, Viganò P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil steril (2023) 120(2):251–65. doi: 10.1016/j.fertnstert.2023.02.036
34. Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET). Sci China Life Sci (2012) 55(8):694–8. doi: 10.1007/s11427-012-4357-0
35. Journal E. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials 2019 . Available at: https://www.efsa.europa.eu/en/efsajournal/pub/5838.
36. Yu Y, Peng M, Liu Y, Ma J, Wang N, Ma S, et al. Co-exposure to polycyclic aromatic hydrocarbons and phthalates and their associations with oxidative stress damage in school children from South China. J hazard mater (2021) 401:123390. doi: 10.1016/j.jhazmat.2020.123390
37. Li J, Zhao H, Xia W, Zhou Y, Xu S, Cai Z. Nine phthalate metabolites in human urine for the comparison of health risk between population groups with different water consumptions. Sci total environment (2019) 649:1532–40. doi: 10.1016/j.scitotenv.2018.08.294
38. Li J, Xia W, Wu C, Zhao H, Zhou Y, Wei J, et al. Variations of phthalate exposure and metabolism over three trimesters. Environ pollution (2019) 251:137–45. doi: 10.1016/j.envpol.2019.04.085
39. Rowdhwal SSS, Chen J. Toxic effects of di-2-ethylhexyl phthalate: an overview. BioMed Res Int (2018) 2018:1750368. doi: 10.1155/2018/1750368
40. Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod (2010) 25(12):2944–54. doi: 10.1093/humrep/deq275
41. Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. Results problems Cell different (2016) 58:167–90. doi: 10.1007/978-3-319-31973-5_7
42. Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod update. (2015) 21(6):779–86. doi: 10.1093/humupd/dmv037
43. Ford EA, Beckett EL, Roman SD, McLaughlin EA, Sutherland JM. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction (2020) 159(1):R15–r29. doi: 10.1530/REP-19-0201
44. Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol reproduct (2015) 92(5):120. doi: 10.1095/biolreprod.115.129148
45. Cao M, Pan W, Shen X, Li C, Zhou J, Liu J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere (2020) 242:125206. doi: 10.1016/j.chemosphere.2019.125206
46. Nilsson EE, Sadler-Riggleman I, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenet (2018) 4(2):dvy016. doi: 10.1093/eep/dvy016
47. Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front endocrinol (2015) 6:8. doi: 10.3389/fendo.2015.00008
48. Zhang T, Shen W, De Felici M, Zhang XF. Di(2-ethylhexyl)phthalate: Adverse effects on folliculogenesis that cannot be neglected. Environ Mol mutagenesis. (2016) 57(8):579–88. doi: 10.1002/em.22037
49. Liu X, Craig ZR. Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovarydagger. Biol reproduct (2019) 101(4):854–67. doi: 10.1093/biolre/ioz122
50. Zhou L, Lyu J, Liu F, Su Y, Feng L, Zhang X. Immunogenic PANoptosis-initiated cancer sono-immune reediting nanotherapy by iteratively boosting cancer immunity cycle. Adv mater (Deerfield Beach Fla) (2023):e2305361. doi: 10.1002/adma.202305361
51. Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open (2022) 2022(3):hoac022. European Ivf Monitoring Consortium ftESoHR, Embryology. doi: 10.1093/hropen/hoac022
52. Tesarik J, Galan-Lazaro M, Mendoza-Tesarik R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci (2021) 22(3):1371. doi: 10.3390/ijms22031371
53. Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction (2021) 162(2):R19–r33. doi: 10.1530/REP-21-0022
54. Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol (2021) 236(12):7966–83. doi: 10.1002/jcp.30468
55. Wen HJ, Sie L, Su PH, Chuang CJ, Chen HY, Sun CW, et al. Prenatal and childhood exposure to phthalate diesters and sex steroid hormones in 2-, 5-, 8-, and 11-year-old children: A pilot study of the Taiwan Maternal and Infant Cohort Study. J Epidemiol (2017) 27(11):516–23. doi: 10.1016/j.je.2016.10.009
56. Gao D, Li Z, Wang H, Liang H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci total environment (2018) 645:1400–9. doi: 10.1016/j.scitotenv.2018.07.093
Keywords: phthalate, urine, antral follicle count, infertile women, in vitro fertilization
Citation: Yao Y, Du Y, Guo N, Liu F, Deng T and Li Y (2024) Associations between urinary phthalate concentrations and antral follicle count among women undergoing in vitro fertilization. Front. Endocrinol. 14:1286391. doi: 10.3389/fendo.2023.1286391
Received: 31 August 2023; Accepted: 15 December 2023;
Published: 08 January 2024.
Edited by:
Roland Eghoghosoa Akhigbe, Ladoke Akintola University of Technology, NigeriaReviewed by:
Luísa Correia-Sá, Chemistry and Technology Network (REQUIMTE), PortugalCopyright © 2024 Yao, Du, Guo, Liu, Deng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufeng Li, eXVmZW5nbGk2NEB0amgudGptdS5lZHUuY24=; Taoran Deng, ZGVuZ3RyQHRqaC50am11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.